Abstract
The cannabinoid CB1 receptor is a G protein-coupled receptor and plays an important role in many biological processes and physiological functions. A variety of CB1 receptor agonists and antagonists, including endocannabinoids, phytocannabinoids and synthetic cannabinoids have been discovered or developed over the past 20 years. In 2005 it was discovered that the CB1 receptor contains allosteric site(s) which can be recognized by small molecules, or allosteric modulators. A number of CB1 receptor allosteric modulators, both positive and negative, have since been reported and importantly, they display pharmacological characteristics that are distinct from those of orthosteric agonists and antagonists. Given the psychoactive effects commonly associated with CB1 receptor agonists and antagonists/inverse agonists, allosteric modulation may offer an alternate approach to attain potential therapeutic benefits while avoiding inherent side effects of orthosteric ligands. This review details the complex pharmacological profiles of these allosteric modulators, their structure-activity relationships, and efforts in elucidating binding modes and mechanisms of actions of reported CB1 allosteric modulators. The ultimate development of CB1 receptor allosteric ligands could potentially lead to improved therapies for CB1-mediated neurological disorders.
Keywords: Allosteric modulation, CB1 receptor, PAM (Positive Allosteric Modulator (PAM), NAM (Negative Allosteric Modulator), Structure-activity relationship (SAR)
1. INTRODUCTION
Cannabinoid research originated from the study of pharmacological effects of the plant constituents isolated from marijuana (Cannabis sativa). The plant contains at least sixty ‘cannabinoids’, a term referring to chemicals unique to Cannabis. Among these, (−)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) is the principal constituent that produces the main psychoactive effects of marijuana.1 The receptors which interact with Δ9-THC were discovered and cloned in 1990s and were named CB1 and CB2 receptors.2–4 Subsequently, the endogenous cannabinoids, anandamide and 2-arachidonoylglycerol (2-AG) were identified.5,6 Hence, currently identified components of the cannabinoid system consist of two well-characterized G protein-coupled receptors (GPCRs), CB1 and CB2, a family of membrane phospholipid-derived endogenous ligands known as endocannabinoids, and functional proteins involved in the synthesis, transport, and inactivation of these endogenous ligands.7 Several other receptors such as the recently discovered GPR55 receptor have been shown to recognize certain cannabinoids, but questions remain if they truly belong to the cannabinoid family.8 The ubiquitous CB1 receptor is densely expressed in the brain and mediates inhibition of neurotransmitter release,3,9 whereas CB2 is predominantly found in immune cells and modulates cell migration and cytokine release.4,9 The CB1 receptor is able to couple to all three types of G proteins (Gi/o, Gs, and Gq) although Gαi/o is the prominent one and its signaling has been extensively reviewed previously.10–13
The CB1 receptor has been shown to play important roles in a variety of functions such as pain, learning and memory, analgesia, appetite and feeding behaviors, anxiety, and depression.14–16 The modulation of the CB1 receptor or both CB1 and CB2 receptors has been utilized successfully for therapeutic benefits. Currently, cannabinoid compounds are licensed under three brand names: Sativex® (Δ9-THC and cannabidiol) as adjunctive treatment for symptomatic relief of neuropathic pain in adults with multiple sclerosis, and Marinol® (Δ9-THC or dronabinol) and Cesamet® (nabilone, a synthetic analog of Δ9-THC) for the suppression of nausea and vomiting provoked by cancer therapy and as appetite stimulants in AIDS patients who experience excessive weight loss. In addition to these proven therapeutic uses, cannabinoid agonists also hold therapeutic promise in other conditions such as Alzheimer’s disease, anxiety disorders, Tourette’s syndrome, tardive dyskinesia, amyotrophic lateral sclerosis, gastrointestinal disorders, inhibition of angiogenesis and growth of tumors, hypertension, hemorrhagic and cardiogenic shock, atherosclerosis, and glaucoma.17,18
CB1 antagonists/ inverse agonists have been suggested to have potential therapeutic uses for drug dependence, impaired fertility in some women, stroke, hypotension associated with endotoxemic shock triggered by advanced liver cirrhosis, and intestinal hypomotility in paralytic ileus.17 The CB1-selective antagonist/inverse agonist SR141716A (rimonabant, Acomplia®) was licensed in UK in 2006 for obesity treatment and related metabolic risk factors, although it was subsequently withdrawn due to an associated risk of suicidal ideation. Therefore, clinical usage of FDA-approved cannabinoid drugs is usually reserved for extreme conditions due to the psychoactivity associated with cannabinoid agonists or anxiogenic and depressive effects of antagonists.19,20
It has recently been reported that the CB1 receptor contains allosteric binding site(s) which are topologically distinct from the orthosteric site. The first CB1 allosteric modulators, Org27569, Org27759 and Org29647, were discovered in 2005,21 sparking interest in discovering additional allosteric ligands. Since then, a variety of modulators for the CB1 receptor have been reported. While only a few CB2 allosteric modulators have been reported so far,22 an increasing number of allosteric modulators of the CB1 receptor that display interesting activities has been discovered. Some of these compounds have been studied more extensively, such as the Org compounds, PSNCBAM-1, and ZCZ011; others may require further studies to confirm their allosteric effects.
According to the mode of actions, allosteric modulators can in general be classified as positive allosteric modulators (PAMs) or negative allosteric modulators (NAMs), positively or negatively modulate the affinity and/or efficacy of the orthosteric agonists, respectively.23 Based on an operational model of allosterism, cooperativity factor α denotes the cooperativity effect on the binding affinity, whereas modulation factor β is used to quantify the effect on the efficacy of the orthosteric ligands.24,25 A composite cooperativity parameter αβ (or logαβ) is normally used to incorporate the modulation of both affinity and efficacy. A modulator is considered a NAM if αβ<1 and a PAM if αβ>1. Hypothetically, pure allosteric modulators possess no basal activity in the absence of orthosteric ligands. However, there have also been reports of allosteric agonists and allosteric inverse agonists, allosteric modulators that exert their effects by binding to a receptor site distinct from the orthosteric site and do not require the presence of an orthosteric ligand.26
Compared to orthosteric ligands, allosteric modulators offer several unique advantages: 1) Allosteric modulators provide selective spatial and temporal signaling, exerting their effects only in the presence of orthosteric ligands such as endocannabinoids which are transiently released on demand and removed from their sites of action by cellular uptake. Thus allosteric modulators do not have long lasting enhancing/blocking effect like exogenous orthosteric ligands or allosteric agonists/antagonists. 2) GPCR allosteric binding sites are often less conserved than orthosteric sites, thus allosteric ligands have a greater potential for receptor subtype specificity. 3) The effect of allosteric modulators is saturable because of their dependence on endogenous ligands for signaling.27,28 Given the side effects associated with CB1 receptor orthosteric agonists and antagonists, allosteric modulators may offer a much needed alternate approach to target cannabinoid receptors for therapeutic benefits.
GPCR allosteric modulators are well known to be both pathway and probe dependent, in which the same allosteric ligand exhibits diverse effects in various signaling pathways or display varied extent and direction (positive, neutral, or negative) of interaction with orthosteric ligands dependent upon the specific orthosteric ligand.25,29 For example, allosteric modulators of the glucagon-like peptide 1 (GLP1) receptor can substantially enhance cAMP production and β arrestin recruitment, while having little or no effect on intracellular calcium mobilization or ERK1/2 phosphorylation.30,31 As will be discussed below, CB1 receptor allosteric modulators (e.g. Org27569), like GLP1 and many other GPCRs, have been reported to be both signaling pathway and probe dependent. The ability allosteric modulators to modulate selected signaling pathways introduces an additional opportunity for fine-tuning intracellular signaling with allosteric modulators.32
In this review, we will summarize the ongoing efforts to develop CB1 allosteric modulators, including discussion of their complex, sometimes contradictory pharmacology, their structure-activity relationships (SARs), their mechanisms of action and their potential therapeutic applications.
2. NEGATIVE ALLOSTERIC MODULATORS (NAMS) OF THE CB1 REEPTOR
Figure 1 depicts the structures of reported NAMs of the CB1 receptor.
Figure 1.
Structures of reported CB1 receptor NAMs.
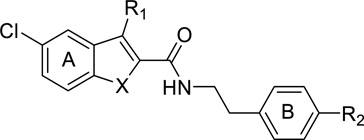
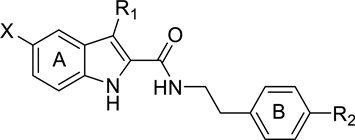
A. Organon compounds (Indole-2-carboxamides)
The first CB1 allosteric modulators ever identified were three compounds developed by Organon, Org27569, Org27759 and Org29647 (Figure 1).21 These compounds are 5-substituted indoles with a carboxamide functionality at the 2-position. Among them, Org27569 has been extensively studied in a variety of in vitro assays, and more recently in several behavioral models. Interestingly, these Org compounds possessed a complex profile: they displayed positive binding cooperativity with the orthosteric agonist CP55940 for CB1 receptor binding as would PAMs, but like NAMs, reduced the efficacy of the agonists like NAMs in a number of in vitro functional assays.
a. Pharmacological studies of Org compounds
In equilibrium binding assays, all three Org compounds produced a significant, but saturable, increase in [3H]CP55940 specific binding and an incomplete saturable reduction of [3H]SR141716A specific binding to mouse brain membranes.21 Of the three compounds, Org27569 produced the most marked effect in the binding of both radiolabeled agonist and antagonist. In dissociation kinetic studies, the three compounds dose-dependently reduced both slow and fast radioligand dissociation rate constants with no significant effect on the percentage of each phase. In contrast to its strong negative cooperativity with [3H]SR141716A, Org27569 had close to neutral cooperativity with cannabinoid agonists such as HU-210, WIN55212-2, Δ9-THC, methanandamide, anandamide, and 2-AG,33,34 displaying the first sign of probe dependence.
The activity of the Org compounds, particularly Org27569, in a number of functional assays has been examined employing various cannabinoid agonists. All three Org compounds antagonized agonist-induced signaling by inhibiting [35S]GTPγS binding with a significant reduction in the Emax value for both CP55940 and anandamide.21,34 However, Org27569 was significantly less effective as an inhibitor of WIN55212-2 as compared with CP55940 in both brain membranes and hCB1R cell membranes. While Org 27569 alone had no significant effect on [35S]GTPγS binding at concentrations up to 10 µM in mouse brain membranes, it behaved as a weak inverse agonist at the same concentrations in hCB1R-CHO cells,34 exhibiting system dependence. In addition, a concentration-dependence was present in reducing the basal level of [35S]GTPγS binding in these HEK293 cells; however, this effect was only significant at high concentrations (e.g. 10 µM).35 Together, these findings suggest Org27569 behaves as a NAM and weak inverse agonist in the [35S]GTPγS.
CB1 agonists are known to inhibit forskolin-stimulated cAMP production and this process is Gαi-mediated.36 Org27569 significantly reduced inhibition of cAMP signaling by CP55940, although it was less effective as an inhibitor of WIN55212-2-mediated inhibition. In pertussis toxin (PTX) pretreated hCB1R cells where the CB1-Gi protein is uncoupled, CP55940 did not inhibit but rather stimulated the production of cAMP mediated by the Gs protein, an effect which was abolished by Org27569.34 In the AlphaScreen cAMP assay, Org27569 completely abolished the inhibition effects of all tested agonists (2-AG, anandamide, methanandamide, Δ9-THC, WIN55212-2, CP55940, HU-210) on cAMP formation.33 More recently, Cawston et al. studied the real-time accumulation of cAMP, activation and desensitization of potassium channel-mediated cellular hyperpolarization and CB1 internalization in HEK293 and AtT20 cells expressing haemagglutinin-tagged human and rat CB1 using BRET CAMYEL assay.37 In these studies, unlike SR141716A, Org27569 did not immediately inhibit CP55940-induced cAMP accumulation, but did so only 9 min after the allosteric modulator treatment. The authors proposed that Org27569 produced a change of CB1 receptor into a constitutively inactive conformation (i.e., NAM effect), resulting in more rapid desensitization and reduced internalization of CB1 receptor, in agreement with previous results from Fay et al. and Ahn et al.35,38 In a subsequent study, Cawston et al. demonstrated that Org27569 enhanced CB1-mediated peak hyperpolarization (i.e., PAM effect), which may be a result of biased allosteric modulation differentially affecting the two pathways measured.39
Activation of the CB1 receptor also results in signaling through Gq/11 proteins to increase intracellular Ca2+ concentration.40,41 Using CHO cells engineered to overexpress both CB1 receptors and the promiscuous G protein Gα16, Nguyen et al. showed that the agonist CP55940 dose-dependently induced intracellular calcium mobilization. Org27569, Org27759 and Org29647 decreased the Emax of CP55940 in a dose-dependent manner, consistent with a negative allosteric mechanism.42
In an AlphaScreen pERK1/2 assay, Org27569 completely abolished the response to HU-210 and CP55940, consistent with its NAM effects in other functional assays. However, Org27569 showed no significant effect on activation of pERK1/2 by anandamide, methanandamide, Δ9-THC, and only partially inhibited 2-AG- and WIN55212-2-induced pERK1/2 activation.33–35 The effect of Org27569 alone on ERK1/2 phosphorylation is inconsistent. Ahn and colleagues first reported that Org27569 enhanced ERK1/2 phosphorylation, and as treatment with the Gi/o inhibitor PTX did not affect this Org27569-induced ERK1/2 phosphorylation, it was suggested that the Org27569-activated ERK1/2 signaling pathway is Gi-independent.35 However, Ballie et al. found that Org27569 alone acted as an agonist of Gαs signaling, but also was a weak partial agonist of Gαi signaling in pERK assays.34 In contrast, Khajehali et al. reported that Org27569 alone did not affect pERK1/2 signaling.33 Several factors could have contributed to this perceived discrepancy such as receptor expression levels, where receptors have a greater tendency to couple to certain pathways (e.g. ERK) in high expressing systems but not in lower expressing systems. Moreover, the allosteric effects of Org27569 have been suggested to be somewhat time-dependent,37 whereas the cannabinoid-mediated pERK1/2 has been known to be transient in nature,33 and therefore the time at which the response is measured may influence the results thus creating perceived bias.
In addition to the G protein-dependent signaling pathways, Org27569 was also shown to inhibit CP55940- and WIN55212-2-induced β-arrestin recruitment.34,35 Ahn et al. employed siRNA technology to demonstrate that the two β-arrestin subtypes played different roles in Org27569-dependent CB1 signaling pathways.43 β-Arrestin 2 plays a critical role in CB1 receptor internalization while β-arrestin 1 is required for kinase activation in MEK1/2-ERK1/2 and Src pathways. No effect of β-arrestin siRNAs on Org27569-induced Akt phosphorylation was observed. Org27569 treatment increased phosphorylation of ERK1/2, MEK1/2, and Src but did not alter Akt phosphorylation in both HEK293 cells and rat hippocampal neurons.43 Org27569 alone has no effect on β-arrestin recruitment.34
In the mouse vas deferens assay, Org compounds by themselves neither inhibited nor enhanced electrically evoked contractions, suggesting that they are neither allosteric agonists nor inverse agonists. In the presence of Org compounds, the maximal agonist effect of WIN55212-2 to inhibit the electrically evoked contractions of the mouse vas deferens was significantly reduced, indicating an insurmountable mode of antagonism.21
Straiker et al. recently studied the “first generation” synthetic and endogenous NAMs and PAMs in synaptic transmission in cultured autaptic hippocampal neurons.44 This model, unlike the commonly used over-expression system, is a neuronal model system that utilizes endogenously synthesized endocannabinoids (e.g. 2-AG). The authors found that Org27569 attenuated 2-AG-mediated depolarization induced suppression of excitation (DSE), but did not directly inhibit the CB1 receptor on its own, consistent with the behavior of a NAM.
Finally, Org27569 may also exert its NAM effect through alteration of CB1 neuronal membrane localization. While cholesterol allowed enrichment of CB1 receptors at the axon where endocannabinoid pathway effectors are mainly localized, Org27569 drove CB1 receptors close to the soma.45 These results suggest a dual mechanism for Org27569 allosteric modulation including both enhancement of CB1 receptor affinity for orthosteric ligands and topological control of CB1 neuronal membrane localization.
In summary, Org27569 displays a complex pharmacological profile that is both pathway- and probe-dependent. In the presence of the orthosteric agonist CP55940, Org27569 increases the proportion of orthosteric high affinity sites, inhibits Gpp(NH)p induced binding/uncoupling, and leads to a variety of (signal modulating) effects. These functional effects include inhibition of orthosteric agonist-induced Gαi, cAMP inhibition, GTPγS binding, orthosteric agonist-induced Gαs mediated cAMP production, orthosteric agonist-induced β-arrestin recruitment (PTX insensitive), and enhancement of orthosteric agonist-induced Gαi mediated pERK production.34 The presence and magnitude of each of these effects is highly dependent on the orthosteric ligands utilized. In the absence of an orthosteric ligand, Org27569 promotes the activation of Gαs (cAMP, PTX insensitive), causes a decrease in basal [35S]GTPγS binding (allosteric inverse agonism) while having no effect on β arrestin turnover. The effect of Org27569 alone on ERK1/2 phosphorylation is still in debate.
b. Structure-activity relationship studies on Org27569
Since the discovery of the Org compounds, several structure-activity relationship studies have been performed on the indole-2-carboxamide template. Piscitelli et al. reported that replacing the amide group by an ester group completely abolished any enhancing effect on [3H]CP55940 binding to the CB1 receptor (e.g 5 and 7, Table I).46 Instead, these ester analogs inhibited [3H]CP55940 binding, in contrast to the enhancing effect of their carboxamide counterparts, although only at high concentrations. Both the 5-chloro and an alkyl group at position 3 on the indole ring A were beneficial but not crucial for the stimulation of [3H]CP55940 binding to CB1 receptor. Optimal substituents at the para position of the phenyl ring B include piperidinyl, nitro, and dimethylamino groups. None of the tested compounds displayed significant effect on [3H]CP55940 binding to the CB2 receptor.
Table I.
Structural modification effects of Org27569 on [3H]CP55940 binding affinity from Di Marzo and Silvestri’s laboratories.36
BS: % of binding stimulation; BI: % of binding inhibition at 10 µM.
Cawston et al. used a real-time kinetic BRET CAMYEL assay to characterize cAMP signaling of these structurally-related indole-2-carboxamide analogs developed by Di Marzo and Silvestri’s laboratories.39 They discovered that some of the compounds including Org27569 displayed a delay in inhibiting CP55940-mediated cAMP inhibition, whereas some (e.g. 7) acted immediately. The authors suggested that these latter compounds might work through the same mechanism as those that showed the signaling delay, but cAMP signaling inhibition appears to be immediate simply due to a rapid rate of desensitization. Alternatively, the latter compounds might act through an alternative mechanism, i.e., by simply stabilizing the off state of the receptor. However, the hyperpolarization was not altered by either group of the compounds. In addition, several compounds were shown to modulate cAMP signaling through the CB2 receptor. Taken together, these results suggest that slight structural changes may result in biased signaling through the allosteric site by differentially affecting the two pathways measured (desensitization vs. hyperpolarization).
Mahmoud and coworkers found that linear alkyl chains at the C3 position of the indole ring A were preferred, as presence of an aromatic or a aliphatic cyclic ring such as phenyl, benzyl, cyclochexyl, cyclohexylmethyl either abolished or significantly reduced the CB1 modulatory activity (Table II).47 Among the four C3-alkyl analogs examined, n-C5H11 displayed the highest allostery (cooperativity factor α values 17.6 and 6.95 respectively for compound 11a and Org27569). The activity was eliminated when the ethylene linker between the indole ring A and the phenyl ring B was shortened to methylene while the presence of an additional hydrophilic group such as hydroxymethylene reduced the binding affinity. Replacing the piperidinyl substituent on the phenyl ring B with an acyclic dimethylamino group led to compound 11j which exhibited the most robust allostery (KB = 167.3 nM; α = 16.55). Changing the indole scaffold to benzofuran (13b) resulted in a significant loss of binding affinity though the cooperativity with the orthosteric ligand [3H]CP55940 was markedly enhanced. The potent analogs in this study demonstrated a concentration-dependent inhibitory effect on CP55940-induced GTPγS binding. The laboratory extended the study further by investigating the length of the alkyl chain at C3 of the indole ring A from ethyl to n-nonanyl. The n-pentyl analog was the most robust ligand with good affinity and cooperativity while the n-hexyl analog (compound 12f) had the best potency, but displayed significant reduction in binding cooperativity. However, a correlation between the length of the side chain and binding affinity or cooperativity was not observed. Among the few tested substituents at C5 and C6 positions of the indole ring A, a chloro group at C5 remains the optimal substituent.
Table II.
Structural modification effects of Org27569 on [3H]CP55940 binding affinity and cooperativity from Kendall and Lu’s laboratories.37,39
When the nitrogen of the indole ring A was methylated, the resulting compound still enhanced [3H]CP55940 binding, although at a much higher concentration (ICAM-a: KB = 5778 nM, α = 11.9).48 Compound 11a exhibited a significant decrease in inverse agonist [3H]SR141716A binding (KB = 931 nM, α = 0.11) and inhibited CP55940-induced [35S]GTPγS binding in a concentration-dependent manner. 11a alone did not alter the time course of ERK1/2 phosphorylation though the level of pERK1/2 was increased in its presence compared to the stimulation produced by CP55940 alone. It was also demonstrated that β-arrestin 1, but not β-arrestin 2 played a role in 11a-induced ERK1/2 phosphorylation.48
Focusing on the phenyl ring B, Nguyen and colleagues reported that the activity of the dialkylamino analogs increased from methyl to ethyl and then declined as the side chain was lengthened further, implying a limited space at this region of the CB1 binding pocket (Table III).42 Acylated analogs were completely devoid of any activity. Substitutions at the 2- and 3- positions of the phenyl ring B were also explored and it appeared that each position preferred different substituents (e.g. 28 vs. 29). Replacing the flexible ethylene linker between the amide bond and the phenyl ring B by cyclic or aromatic rings resulted in a complete loss of activity. A fluoro could be used as a replacement of chloro at C5 position of the indole ring A and an alkyl substituent at C3 was not necessary (e.g. 43). The most potent analog possessed a chloro at C5, no substituent at C3 of the indole ring A and diethylamino substituent at C4 of the phenyl ring B (45). None of the examined compounds displayed significant CB2 inhibition or agonist activity on their own at either receptor.
Table III.
Structural modification effects of Org27569 on inhibiting CP55940 induced Ca2+ mobilization from Zhang’s laboratory.33
Greig and colleagues filed a patent application on a series of indole-2-sulfonamides (Figure 2).49 It was disclosed that the ethylene linker could be replaced by a methylene moiety in this series, in contrast to the indole-2-carboxamide series. In fact, in the presence of methylene linker, when the N-piperidinyl substituent at the C4 position of the phenyl ring B was replaced by bi-aromatic rings, there was a significant gain in potency, resulting in single digit nanomolar IC50 values. The chloro at C5 position of the indole ring A could be eliminated or replaced with a bromo. The ethyl group at the C3 position was found to be unnecessary for activity, similar to in the carboxamide series. Unlike the carboxamide series, these sulfonamides did not increase the binding of the endogenous ligand, but reduced the activation of the CB1-mediated β-arrestin signaling pathway.
Figure 2.
Indole-2-sulfonamides as CB1 allosteric modulators and their IC50 values in the PathHunter™ β-arrestin assay.
In summary, these SAR studies revealed that the indole scaffold was optimal for CB1 allosteric modulation (Figure 3).47 An electron-withdrawing group like chloro or fluoro at C5 of the indole was beneficial though not necessary for CB1 activity.42,46,50 The C3 position of the indole ring preferred no substitution or a short linear alkyl chain no longer than n-pentyl.42,47,48,50 The dimethylamino or diethylamino substituents showed superior potency to N-piperidinyl group at the para position on the phenyl ring and were preferred for the activity.42,46,50 Moving the N-dialkylamino substituent from para to meta position reduced activity while other tested substituents appeared to prefer other positions, suggesting that the meta position had a smaller steric tolerance than the para position.42 An ethylene linker between the phenyl ring and the carboxamide moiety was essential for the activity as shortening it to methylene or replacing it with more hydrophilic or cyclic linkers abolished or decreased the activity.42,47
Figure 3.
Summary of SAR of the indole-2-carboxamide scaffold.
So far, most of the SAR studies focused on exploring substitution pattern on the indole or phenyl ring. While these modifications only resulted in analogs with modest improvement on potency (IC50 or EC50), they provide valuable SAR information that will guide future medicinal chemistry campaigns. Moreover, the pharmacokinetics of these analogs has yet to be studied. More rigorous alterations such as scaffold hopping may lead to novel chemotypes that possess not only enhanced potency but also improved physiochemical properties.
c. Allosteric binding site determination
Because there are currently no high resolution crystallographic or NMR structures of the CB1 receptor, the binding modes of CB1 ligands have mainly been studied by molecular modeling, mutagenesis and mass spectrometry. In this section, the receptor residues are numbered according to the Ballesteros-Weinstein system.51 They are presented as AX.YZB or a shorter form. A and B are one letter symbols of the original and mutated amino acid residues respectively. X represents the number of the transmembrane domain. Y indicates the position of the residue in relation to the most highly conserved class A residue in each transmembrane helix and Z is the absolute number in the whole receptor protein sequence. Loop residues are numbered according to the protein amino acid number due to little conservation within GPCRs.
Iliff et al. presented the development of CHARMM force field parameters for Org27569 for future modeling studies.52 Fay et al. reported that the C98-C107 disulfide bond at the membrane proximal region of the CB1 receptor can alter the efficacy of Org27569.38 Mutation of cysteine to alanine or reduction of the sulfide bond increased the positive binding cooperativity of Org27569. Ahn and coworkers reported that the impact of Org27569 on [3H]CP55940 was most marked on the inactive T210A CB1 mutant and less pronounced on the constitutively active T210I receptor, implying that TM3 may be involved in the binding pocket of Org27569.35 Through [35S]GTPγS binding assays and through experiments involving electrostatic interaction with the nitrogen on the piperidine ring of Org27569 by mutation, Shore and colleagues demonstrated that K3.28192 residue had an important role and proposed an allosteric binding site that partially overlaps with the orthosteric site.53 The authors suggested that at this binding site, Org27569 promoted an intermediate conformation of the CB1 receptor that affected receptor activation by sterically blocking the movements of the second extracellular loop, preventing a key electrostatic interaction between the third extracellular loop residue K373 and D2.63176, and hindering movements of TMH6.
In efforts to identify the Org27569 binding site, a number of covalent and photoactivatable probes have been developed (Figure 4). Stornaiuolo and coworkers attached an prop-2-yn-1-yl moiety to the nitrogen of the indole ring of Org27569.45 Mass spectrometry was used to determine that this probe linked covalently to either S2.45 or S3.42 in the TM1-4 region corresponding to a Cholesterol Consensus Motif. In silico simulation suggested that Org27569 binding elicits a TM3 displacement, leading to enhanced CP55940 binding affinity.
Figure 4.
Structures of Org27569-based covalent probes.
Another probe GAT100 was developed by Kulkarni et al. by replacing the chloro at C5 of the indole ring with a thioisocyanate group (Figure 4), a known warhead with the potential to bind covalently with cysteine groups of proteins.54 The probe increased the [3H]CP55940 binding level after repeated washings, implying that it remained bound to the CB1 receptor covalently. This probe was demonstrated subsequently to be a NAM of CP55940, 2-AG, and anandamide with higher potencies than Org27569 and PSNCBAM-1 for β-arrestin 1 recruitment, PLCβ3 and ERK1/2 phosphorylation, cAMP accumulation, and CB1 receptor internalization. A mass spectrometry study would be useful to confirm if C7.38382 is the residue that binds to the isothiocynate warhead as implicated by the computational docking study.55
Qiao et al. recently synthesized several compounds with photoactivatable groups (benzophenone and azide moieties) at various positions of Org27569 in order to probe the binding site(s) upon radiation (Figure 4). These compounds were found to have similar pharmacological properties to their lead compounds in radioligand and GTPγS binding assays.56 The authors did not report binding site detection with these compounds.
While still in early developmental stages, these probes together with molecule modeling and mutagenesis studies will certainly assist in the elucidation of the allosteric binding site(s) of the CB1 receptor.
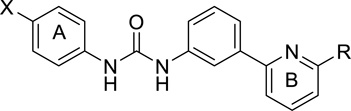
B. PSNCBAM-1 (1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea)
Another CB1 receptor NAM, PSNCBAM-1, was discovered by Prosidion Limited in 2007 after screening a proprietary small molecule library using a human CB1 receptor expressing yeast reporter-based assay (Figure 1).57 This molecule is based on a diaryl urea scaffold, with a high structural similarity to the Org compounds. PSNCBAM-1 showed a pharmacological profile similar to Org27569, increasing CB1 agonist binding but decreasing their functional responses in several in vitro assays. In the only in vivo study reported so far, PSNCBAM-1 decreased food intake and body weight in an acute feeding model. 57
a. Pharmacological studies of PSNCBAM-1
In radioligand binding experiments, PSNCBAM-1 dose-dependently enhanced [3H]CP55940 binding, while reducing [3H]SR141716A binding, showing the same profile as Org27569. Unlike Org27569 which showed no effect on [3H]WIN55212-2 binding, PSNCBAM-1 had a small but significant effect on the specific binding of [3H]WIN55212-2 in mouse brain membranes and hCB1R cells.34 PSNCBAM-1 appeared to influence only orthosteric ligand maximal occupancy (Emax) rather than affinity (pEC50). Kinetic studies showed that PSNCBAM-1 appeared to increase the proportion of high-affinity [3H]CP55940 binding while not greatly affecting its dissociation rate. Fay et al. reported that the C98-C107 disulfide bond at the membrane proximal region of the CB1 receptor can alter the efficacy of PSNCBAM-1, in a similar manner as that of Org27569.38 Reduction of the sulfide bond resulted in enhanced PSNCBAM-1 binding.
It was also shown that PSNCBAM-1 reversed [35S]GTPγS binding stimulated by CP55940 or AEA in both hCB1 HEK cells and rat cerebellar membranes. 34,57,58 While PSNCBAM-1 did not significantly affect the EC50 value of CP55940, it reduced CP55940 efficacy by approximately 50%, suggesting that PSNCBAM-1 was acting as a noncompetitive antagonist. Wang et al. reported that PSNCBAM-1 noncompetitively inhibited [35S]GTPγS stimulation by two agonists, CP55940 and WIN55212-2, while only slightly affecting agonist potency in isolated murine cerebellar membranes.58 Similarly to Org27569, PSNCBAM-1 displayed both probe and cell type-dependence, showing greater potency against CP55940 than WIN55212-2 in inhibition of agonist-induced [35S]GTPγS stimulation.58 PSNCBAM-1 alone showed weak inverse agonism, producing a partial reduction in [35S]GTPγS binding in HEK293-hCB1 cell membranes. However, PSNCBAM-1 had no effect on constitutively active hCB1 yeast cells, indicating that it did not behave as an inverse agonist in this assay. Finally, PSNCBAM-1 had no effect on CP55940-stimulated [35S]GTPγS binding in hCB2 HEK293 cells, confirming its selectivity for the CB1 over the CB2 receptor.57
PSNCBAM-1 at 10µM completely reversed the inhibition of forskolin-stimulated cAMP accumulation induced by CP55940 and AEA, but only partially at 1 µM.34,57 PSNCBAM-1 did not inhibit CP55940-induced cAMP accumulation, suggesting that PSNCBAM-1 shares the same mechanism of action as Org27569. Using real-time cAMP BRET measurement, Cawston et al. found that PSNCBAM-1, similar to Org27569, displayed a concentration-dependent time delay in antagonizing the agonist-induced inhibition of cAMP.37 As this delay did not appear to be caused by drug solubility or a switch in signaling pathways from Gαi to Gαs, the authors proposed that PSNCBAM-1, like Org27569, induced the CB1 receptor into a constitutively inactive conformation, making it desensitize more rapidly while reducing its internalization, resulting in enhanced agonist binding.
Like Org27569, PSNCBAM-1 dose-dependently reduced the Emax values of both CP55940 and WIN55212-2 in PathHunter β-arrestin assays while having no effect on the pEC50 of these two orthosteric agonists. It also displayed probe-dependence, appearing as a significantly less potent inhibitor of WIN55212-2 as compared with CP55940.34 Wang et al. reported that in the CB1 agonist-mediated modulation of inhibitory transmission in the cerebellum using electrophysiology, 10 µM PSNCBAM-1 abolished the inhibitory effect of 5 µM CP55940 but not that of 5 µM WIN55212-2.58 Overall, PSNCBAM-1 demonstrated agonist-dependent effects in the tested assays. While PSNCBAM-1 did not reverse the reduction of WIN55212-2-inhibited inhibitory neurotransmission like the CB1 antagonist/inverse agonist AM251, pretreatment with PSNCBAM-1 attenuated the elevated miniature inhibitory postsynaptic currents (mIPSC) frequency caused by AM251. In addition, PSNCBAM-1 was shown to lack modulatory effects on GABAB receptor pathways at IN-PC synapses, which may be beneficial in reducing elevated inhibitory transmission by CB1 antagonists/ inverse agonists.
In the neuronal model reported by Straiker et al, PSNCBAM-1, like Org27569, attenuated 2-AG-mediated depolarization induced suppression of excitation (DSE) but did not directly inhibit the CB1 receptor, as expected with negative allosteric modulators.44 Of all the CB1 modulators tested, PSNCBAM-1 was the most efficacious in producing this effect.
In summary, PSNCBAM-1 showed a pharmacological profile similar to Org27569, where it behaved as a PAM in binding but as a NAM in several functional assays. Similarly, PSNCBAM-1 also showed pathway and probe dependence, dose-dependently antagonizing stimulation of hCB1 receptor signaling elicited by CP55940, WIN55212-2, AEA, and 2-AG with different potencies. Compared to Org27569, PSNCBAM-1 has been less studied. For example, the effects of PSNCBAM-1 on ERK phosphorylation have not been investigated, either alone or with an agonist.
b. Structure-activity relationship studies on PSNCBAM-1
German et al. described the first SAR study on PSNCBAM-1, demonstrating that alkyl substitution at the 2-aminopyridine moiety and electron deficient aromatic groups such as a cyano at the 4-chlorophenyl position are important for CB1 allosteric modulation in calcium mobilization and binding assays (Table IV).59 Focusing on the pyridine B region, the authors found that increasing the size of the substituents both in the ring expanded and ring opened series resulted in lower potency, suggesting that there was a limited space in the binding pocket. A cyclic ring was not required as the most potent analog examined was the N,N-dimethylamino derivative (compound 11, IC50 27.4 nM). Converting the basic amino group to the neutral amide reduced the activity by around 8-fold, implying that a basic center is preferred at this region. Regarding the substitution on the phenyl ring A, the CB1 allosteric modulation was found to be strongly influenced by the electron density of ring A. Electron-withdrawing groups provided more potent ligands compared to analogs with electron-donating substituents. The best compounds were the 4-fluoro and 4-cyano analogs (compounds 27 and 29, IC50 32 nM and 33 nM respectively). Moving the 4-chloro on the phenyl ring A to other positions resulted in reduced potency, especially compounds with a substituent at the 2-position which showed no activity up to 10 µM. Replacing the phenyl ring A with the electron-deficient pyridine ring slightly reduced the activity while bigger neutral naphthyl rings completely abolished the activity. None of the reported compounds displayed antagonism at the CB2 receptor or agonism activity at either cannabinoid receptor. Compounds 11 and 29 dose-dependently lowered the top of the agonist curve of CP55940 in the calcium mobilization assay, confirming their allosteric characteristics. These compounds also increased the [3H]CP55940 binding level in a similar manner to PSNCBAM-1.
Table IV.
Representative compounds and their effects on allosteric modulation reported by Zhang’s laboratory.47
Data disclosed in another patent application by Thakur et al. were in good agreement with German and coworkers (Table V),60 indicating that compounds having comparable activities in the cAMP and β-arrestin assays to the PSNCBAM-1 contain electron-withdrawing groups such as trifluoromethyl, iodo and fluoro in place of chloro at the 4-position on the phenyl ring A. 3-Substituted analogs were slightly less potent whereas the 2-substituted isomers were generally inactive except for the small fluoro substituent. A long chain substituent at the 3-position significantly reduced the activity in most cases. The results from the two assays are generally in agreement with each other. Changing the urea functional group to other spacers such as carbamate, methylated ureas, cyclic ureas, four- and five-membered rings has not provided any good analogs yet. On the pyridine ring B, replacing the N-pyrrolidinyl ring by a piperazinyl ring yielded a compound having similar activities to PSNCBAM-1 in both assays.
Table V.
Examples of active compounds revealed in the patent application by Thakur et al.48
The SAR findings from both studies are summarized in Figure 5.
Figure 5.
Summary of SAR of the PSNCBAM-1 Scaffold.
The similarities between the Org and PSNABAM-1 series are evident with some common SARs. On the left-hand side, both the 5-position of indole on Org27569 and the para position of the phenyl A on PSNCBAM-1 both favor a halogen for activity. On the right hand side, tertiary amine groups such as dialkylamino, pyrrolidyl, or piperidyl are preferred for both series. The urea moiety of PSNCBAM-1 offers two hydrogen bond donors and three hydrogen bond acceptors similar to the combined pharmacophore of carboxamide and the nitrogen center on the indole ring of Org27569. These two compounds have similar size and length and the distance between the indole ring A and the phenyl ring B of Org27569 is approximately the same as the distance between ring A and B of PSNCBAM-1. It is interesting to see if these two series bind to the same receptor binding site.
C. Peptide endocannabinoids (Pepcans)
In 2012, Bauer et al identified an α-hemoglobin-derived dodecapeptide (RVDPVNFKLLSH, pepcan-12), which exists in abundance in mouse brain, as the first endogenous CB1 negative allosteric modulator.61 Pepcan-12 reduced binding levels of both [3H]CP55940 and [3H]WIN55212-2 in the radiolabeled binding assay, and it decreased the dissociation rate constant of [3H]CP55940 in kinetic experiments. The saturable but incomplete binding reduction indicated that pepcan-12 acted as a negative allosteric modulator rather than a competitive antagonist of the CB1 receptor. It should be noted that Gomes and coworkers previously reported pepcan-12 as a CB1 agonist based on their binding and functional studies.62 The C-terminal fluorescence-labeled pepcan-12 derivative was shown to bind selectively to CB1 and CB2 receptors, but not to GPR55 or TRPV1 receptors. In addition, it exhibited potent negative allosteric modulation of WIN55212-2- and 2-AG-induced cAMP accumulation, HU-210-induced [35S]GTPγS binding, and CB1 receptor internalization. Pepcan-12 alone elicited no effect on cAMP production and [35S]GTPγS binding while behaving as a partial agonist in the CB1 receptor internalization assay. These studies were limited to no more than 1 µM of pepcan-12 due to its tendency to form aggregates at higher concentrations.
In line with its classification as a CB1 receptor NAM, Straiker et al. found that pepcan-12 has no direct effect on EPSCs (excitatory postsynaptic current) but modestly inhibits CB1 responses in their neuronal model.44 It is noteworthy that pepcan-12 may act as a potent PAM at CB2 receptor.63
Dvorácskó et al.64 recently reported a truncated hemopressin peptide (PVNFKFL, Hp(1–7)), a related family member of pepcan-12, could act as an allosteric ligand or indirect regulator of the endocannabinoid system rather than an endogenous ligand as postulated previously. The radiolabeled Hp(1–7) could only be displaced by unlabeled peptide counterparts but not by the orthosteric agonist JWH018 or orthosteric inverse agonist AM251, indicating that Hp(1–7) binds to a different site compared to these orthosteric ligands. However, Hp(1–7) was found to bind to both wild type and CB1 knockout mouse brain membrane homogenate and displayed similar effects in these two types of membrane homogenate in the [35S]GTPγS assay, implying that its main target protein is not CB1 receptor.
D. Pregnenolone
Vallée et al. in 2014 reported that pregnenolone, an endogenous steroid hormone, acted as an endogenous CB1 allosteric inhibitor, as it reduced Δ9-THC-induced pERK1/2 activation in human CB1-CHO cells.65 It did not affect equilibrium binding of [3H]CP55940 and [3H]WIN55212-2. In contrast, Khajehali et al. did not observe any effect of pregnenolone on WIN55212-2-inhibited pERK activation.33 This discrepancy implies that pregnenolone displays probe dependence in pERK activation.33 Khajehali et al. also reported a concentration-dependent displacement of [3H]SR141716A by pregnenolone; however, it could not be concluded if pregnenolone behaved as a competitive or allosteric ligand due to an incomplete displacement of [3H]SR141716A by pregnenolone at the maximum concentration that could be used in the assay. Lastly, pregnenolone at 1 µM did not have any effect on 2-AG signaling in autaptic hippocampal neurons.44
E. Fenofibrate
Fenofibrate, a PPAR agonist, was previously reported as a CB2 receptor agonist using the PathHunter™ β-arrestin recruitment assay.66 Recently, Priestley et al. discovered that fenofibrate acted like a CB1 NAM at high concentrations.67 At 10–100 µM, fenofibrate increased the dissociation rate constant for [3H]CP55940 and reduced the maximal response to CP55490. Fenofibrate displayed an atypical bell-shaped concentration-response curve in several functional assays. For instance, fenofibrate alone enhanced [35S]GTPγS binding, increased total ERK expression and β-arrestin recruitment up to around 10 µM, and exhibited reverse effects at higher concentrations. Based on results from both competition and kinetic dissociation binding assays, the authors proposed that fenofibrate acted as a negative allosteric modulator of CB1 receptor at higher concentrations and as an agonist at lower concentrations. But given the extremely high concentrations used in these studies, the observed effects could be non-specific. In addition, fenofibrate was able to inhibit electrically evoked contractions in guinea pig ileum like CP55940, although the inhibition was incomplete relative to CP55940.
F. Cannabidiol
(−)-Cannabidiol (CBD), one of the major constituents of marijuana (Cannabis sativa), displays none of the psychotropic effects of Δ9-THC, yet possesses some interesting pharmacological effects on its own.68,69 In addition, CBD has been shown to attenuate some of the unwanted effects of Δ9-THC70 and antagonizes agonist activities of WIN55212-2 and CP55940 in the mouse isolated vas deferens at submicromolar KB values (120 nM and 34 nM respectively). Insurmountable antagonism of CBD on α1-adrenoceptor agonists also has been reported. 71–75 Because these effects occurred at CBD concentrations well below its CB1 receptor binding affinity (Ki values: 4 to more than 10 µM), they were thought to be mainly CB1 receptor-independent.76
Recently, Laprairie and colleagues77 reported that CBD (at concentrations below 1 µM) acted as a NAM, as it reduced the efficacy and potency of 2-AG and Δ9-THC on PLCβ, ERK, β-arrestin 2 recruitment and CB1 internalization with cooperativity coefficient for ligand efficacy of less than 1. At higher concentrations, CBD behaved as a weak partial agonist in these functional assays. Whether the in vitro CB1 NAM effect of CBD plays any role in contributing to its in vivo effects remains undetermined.
The reported NAMs and their pharmacological effects in a variety of bioassays are summarized in Table VI.
Table VI.
Summary of reported NAMs and their pharmacological effects
| Ligand | Assay | Pharmacological effects | References |
|---|---|---|---|
| Org27569 | Radioligand binding |
↑ [3H]CP55490 binding ↓ [3H]SR141716A binding No effect on [3H]WIN55212- 2 binding |
Price et al., 200521 Ahn et al., 201235 Baillie et al., 201334 |
| Calcium | ↓ agonist-induced Ca2+ mobilization |
Nguyen et al, 201542 |
|
| GTPγS | ↓ agonist induced [35S]GTPγS binding ↓ [35S]GTPγS binding alone |
Price et al., 200521 Ahn et al., 201235 Baillie et al., 201334 |
|
| cAMP | ↓ simulation (Gαs-mediated) and inhibition (Gαi-mediated) by agonists Alone ↑ cAMP production |
Baillie et al, 201334 Cawston et al., 201337 Khajehali et al., 201533 |
|
| ERK | ↓ ERK phosphorylation activated by CP55940 and HU- 210; partial inhibition on 2-AG and WIN55212-2-induced pERK No effect on ERK phosphorylation activated by anandamide, methanandamide, Δ9-THC In debate if ↑ ERK phosphorylation alone |
Ahn et al., 201235 Baillie et al., 201334 Khajehali et al., 201533 |
|
| β-Arrestin | ↓ agonist-induced β-arrestin recruitment Alone had no effect |
Ahn et al., 201235 Ahn et al., 201343 Baillie et al, 201334 |
|
| Report gene | ↓ reporter gene activity by CP55940 |
Price et al., 200521 | |
| Mouse vas deferens |
↓ agonist effects | Price et al., 200521 | |
| Synaptic transmission in hippocampal neurons |
↓ 2-AG-mediated suppression of excitation |
Straiker et al., 201544 |
|
| PSNCBAM-1 | Radioligand binding |
↑ [3H]CP55490 binding Little effect on [3H]WIN55212-2 binding ↓ [3H]SR141716A binding |
Horswill et al., 200757 Baillie et al., 201334 |
| Calcium | ↓ agonist-induced Ca2+ mobilization |
German et al.,201459 |
|
| GTPγS | ↓ agonist-induced [35S]GTPγS binding ↓ [35S]GTPγS binding alone |
Horswill et al., 200757 Wang et al., 201158 |
|
| cAMP | ↓ agonist-induced inhibition | Horswill et al., 200757 Baillie et al., 201334 Cawston et al., 201337 |
|
| β-Arrestin | ↓ agonist-induced β-arrestin recruitment Alone had no effect |
Baillie et al., 201334 | |
| Yeast reporter | ↓ agonist effects (CP55940, WIN55212-2, AEA, 2-AG) |
Horswill et al., 200757 |
|
| Synaptic transmission in hippocampal neurons |
↓ 2-AG-mediated suppression of excitation |
Straiker et al., 201544 |
|
| Pepcan-12 | Radioligand binding |
↓ [3H]CP55490 and [3H]WIN55212-2 binding |
Bauer et al, 201261 |
| cAMP | ↓ agonist-induced inhibition Alone had no effect |
||
| GTPγS | ↓ agonist induced [35S]GTPγS binding Alone had no effect |
||
| CB1 receptor internalization |
↓ receptor internalization Alone acted as partial agonist |
||
| Synaptic transmission in hippocampal neurons |
↓ 2-AG-mediated suppression of excitation |
Straiker et al., 201544 |
|
| Pregnenolone | Radioligand binding |
No effect on [3H]CP55490 and [3H]WIN55212-2 binding ↓ [3H]SR141716A binding |
Vallée et al, 201465 Khajehali et al., 201533 |
| ERK phosphorylation |
↓ THC-induced ERK phosphorylation No effect on WIN55212-2- inhibited ERK phosphorylation |
||
| Synaptic transmission in hippocampal neurons |
No effect on 2-AG-mediated suppression of excitation |
Straiker et al., 201544 |
|
| Fenofibrate | Radioligand binding |
↓ [3H]CP55940 binding at 10- 100 µM |
Priestley et al., 201567 |
| Total ERK | Alone ↑ total ERK level up 10 µM and ↓ total ERK level at more than 10 µM |
||
| GTPγS | Alone ↑ [35S]GTPγS binding up to 1-10 µM and ↓ [35S]GTPγS binding at more than 10 µM. Effects were reversed by antagonist AM251 dose- dependently. |
||
| β-Arrestin | Alone ↑ β-arrestin recruitment up 10 µM and ↓ β-arrestin recruitment at more than 10 µM |
||
| Cannabidiol | ERK phosphorylation |
↓ agonist-induced ERK Phosphorylation |
Laprairie et al., 201577 |
| β-Arrestin 2 | ↓ β-arrestin 2 recruitment | ||
| CB1 receptor internalization |
↓ agonist-induced receptor internalization |
3. POSITIVE ALLOSTERIC MODULATORS (PAMS) OF THE CB1 RECEPTOR
Structures of reported PAMs are depicted in figure 6.
Figure 6.
Structures of reported CB1 receptor PAMs.
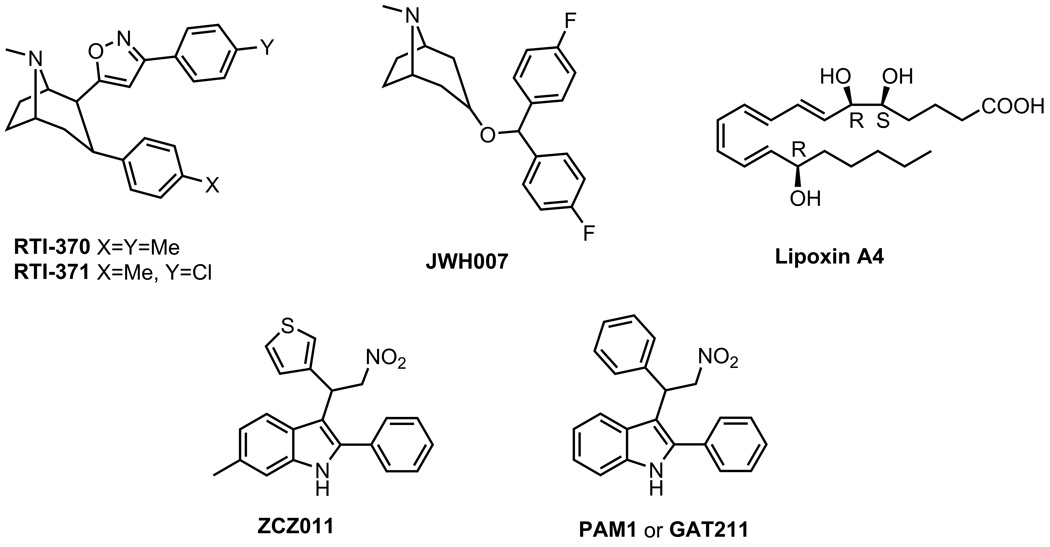
A. Tropane derivatives (RTI-371)
Navarro et al. identified several selective dopamine transporter inhibitors as CB1 PAMs in a calcium mobilization-based assay.78 RTI-370, RTI-371, and JHW007 displayed concentration-dependent increases in the Emax of CP55940 activity without any effect on the agonist potency. In the absence of CP55940, none of these compounds displayed any measureable activity up to 10 µM, implying that they exerted their effects via positive allosteric modulation.
B. Lipoxin A4
Pamplona et al. in 2012 reported the anti-inflammatory lipid lipoxin A4 as an endogenous allosteric enhancer of the CB1 receptor.79 Lipoxin A4 itself had low binding affinity at the CB1 receptor, but enhanced the binding of radiolabeled cannabinoid agonists [3H]CP55940 and [3H]WIN55212-2 to the CB1 receptor and potentiated the displacement of [3H]SR141716A by AEA. Addition of lipoxin A4 slowed down the kinetic displacement of [3H]CP55940 by an excessive amount of WIN55212, confirming the allosteric modulatory mechanism of lipoxin A4. However, it did not displace [3H]SR141716A at the orthosteric binding site33 nor did it alter endocannabinoid metabolism in a separate study.79 In addition, lipoxin A4 enhanced the potency of AEA in decreasing forskolin-induced cAMP in CB1-transfected HEK cells according to one study79, but showed no effects in another.33 Finally, costimulation with AEA and lipoxin A4 resulted in a decrease in G protein binding in the GTPγS assay, as would NAMs.79
Lately, Straiker and colleagues found that lipoxin A4 antagonized CB1 signaling in their neuronal model in the presence of 2-AG but had not effect on EPSCs on its own, implying that lipoxin A4 acted as a NAM with 2-AG as the orthosteric ligand.44 This observation illustrates the probe dependent effect in which lipoxin A4 acted as PAM with AEA and NAM with 2-AG or biased signaling for different pathways investigated in these two studies.
C. ZCZ011
Recently, Ignatowska-Jankowska et al. reported a novel synthetic blood-brain barrier permeable small molecule ZCZ011 with interesting pharmacological properties.80 ZCZ011 demonstrated a concentration-dependent increase in [3H]CP55940 and [3H]WIN55212-2 specific binding while decreasing the specific binding of the CB1 receptor orthosteric inverse agonist [3H]SR141716A in equilibrium binding assays. In a saturation binding assay, ZCZ011 increased the maximum binding level of [3H]CP55940 without significantly affecting binding affinity Kd. Hence, its profile is consistent with characteristics of a PAM. A higher Bmax value is the result of ZCZ011 as a PAM promoting the formation of ternary complexes (orthosteric agonist/receptor/G-protein), resulting in an increased number of available receptors for the orthosteric ligand to bind to.
ZCZ011 enhanced both AEA- and CP55940-stimulated [35S]GTPγS binding to mouse brain membranes without affecting the potency of these two orthosteric agonists. ZCZ011 displayed positive cooperation with cAMP inhibition of AEA at 1 µM concentration, but not at the lower concentration of 100 nM. ZCZ011 dose-dependently enhanced AEA-stimulated β-arrestin recruitment and ERK phosphorylation. These results are consistent with ZCZ011 being a PAM. Alone, ZCZ011 acted as an agonist in inhibiting forskolin-stimulated cAMP production, increased maximal stimulation of β-arrestin recruitment, and enhanced CP55940-induced ERK1/2 phosphorylation only at a concentration higher than 1 µM.
Crystal structures of four related indoles were previously reported by Kerr et al (Figure 7).81 Of these four indoles, compounds III and IV had moderate CB1 agonism effect while compounds I and II were inactive. In all four crystals, the phenyl ring was positioned on the same side with the indole core while the substituents Y and Z occupied the other side. These preliminary results implied that a 2-nitroethyl moiety at the C3 position and a phenyl group at C2 position of the indole template were critical for CB1 receptor enhancing activity while position 5 could tolerate different substituents.
Figure 7.
Four PAMs based on the indole scaffold with solved crystal structures.81
D. PAM1 or GAT211
A recent patent disclosure from Thakur and Kulkarni detailed a number of indole derivatives structurally similar to ZCZ011 (Figure 8).82 The compounds were characterized in vitro by the DiscoveRx HitHunter cAMP XS+ assay. The (S)-isomer was more potent than its (R)-counterpart (EC50 370 nM and 1597 nM respectively). A 5-chloro substituent on the indole ring A was not favored. Replacing the 3-(2-nitroethyl) substituent on the indole ring A with the 3-nitrophenyl group completely abolished activity. Alkylation of the nitrogen of the indole ring A by a phenyl group provided an analog with moderate potency. The phenyl ring B could be replaced by other aromatic rings such as 2-pyridinyl and interestingly, in this case, the (R)-isomer was slightly more potent than its (S)-isomer. Extending the phenyl ring B with a 4-piperidinyl group, but not another 4-phenyl ring retained the activity. A bulkier group such as naphthyl in place of the phenyl ring C slightly decreased the activity (EC50 744 nM). Halogen substitution on the phenyl ring C was tolerated.
Figure 8.
Examples of potent analogs disclosed in the patent application by Thakur and Kulkarni.82
The currently reported CB1 receptor PAMs and their pharmacological effects are summarized in Table VII.
Table VII.
Summary of reported PAMs and their pharmacological effects.
| Ligand | Assay | Pharmacological effects | References |
|---|---|---|---|
| Tropane derivatives |
Calcium | ↑ agonist-induced Ca2+ mobilization |
Navarro et al, 200978 |
| Lipoxin A4 | Radioligand binding |
↑ [3H]CP55940 and [3H]WIN55212-2 ↑ displacement of [3H]SR141716A by AEA No effect on [3H]SR141716A binding alone |
Pamplona et al, 201279 |
| cAMP | ↑ AEA-inhibited cAMP production |
||
| GTPγS | No effect on AEA-induced [35S]GTPγS binding |
||
| Synaptic transmission in hippocampal neurons |
No effect on 2-AG- mediated suppression of excitation |
Straiker et al., 201544 |
|
| ZCZ011 | Radioligand binding |
↑ [3H]CP55940 and [3H]WIN55212-2 binding ↓ [3H]SR141716A binding |
Ignatowska- Jankowska et al., 201580 |
| ERK phosphorylation |
↑ AEA and CP55940- induced ERK phosphorylation Alone had no effect |
||
| cAMP | ↑ AEA-inhibited cAMP production Alone: ↑ cAMP production |
||
| GTPγS | ↑ agonist-induced [35S]GTPγS binding |
||
| β-arrestin | ↑ agonist-induced β- arrestin recruitment |
4. IN VIVO EFFECTS OF CB1 RECEPTOR ALLOSTERIC MODULATORS
In vivo cannabinoid effects are typically evaluated by cannabinoid tetrad which is a battery of four tests measuring spontaneous locomotion activity, antinociception, rectal temperature, and catalepsy in animals following drug injection. Other meaningful yet more complicated and time-consuming tests include drug discrimination, self-administration/conditioned place preference, tolerance and dependence.
Surprisingly, the interesting in vitro CB1 allosteric modulation of the first generation of CB1 NAMs, Org27569 and PSNCBAM-1, may not translate directly to in vivo efficacy. Gamage et al. reported that Org27569 reduced food consumption in both CB1-deficient and wild-type mice while SR141716A reduced food intake in wild-type mice only.83 While detected in mouse brain (4 µg/mg) and blood (3.7 mg/ml) 1 hour after a 30 mg/kg intraperitoneal administration, Org27569 did not block antinociceptive, cataleptic, or hypothermic actions of the orthosteric agonists such as anandamide, CP55940, and Δ9-THC. In addition, it did not alter the discriminative stimulus effects of or substitute for anandamide or Δ9-THC in FAAH-deficient and wild-type mice in the drug discrimination paradigm. The authors concluded that the anorexic effect of Org27569 may occur independently of the CB1 receptor.
Another independent study by Ding et al. at the same time reported that while Org27569 did not significantly alter the body temperature, it markedly attenuated the hypothermic effect of CP55940 and anandamide especially between 60–120 minutes after drug administration in rats.84 However, in sharp contrast with SR141716A, pretreatment with Org27569 did not significantly block the cataleptic effect and antinociception of CP55940. Org27569 alone did not elicit increased grooming and scratching behaviors nor did it modify these behaviors induced by SR141716A. In another study by the same group, it was shown that pretreatment with Org27569 significantly attenuated both cue- and drug-induced reinstatement of cocaine- and methamphetamine-seeking behavior like SR141716A, highlighting the potential of Org27569 in treatment of drug addiction; although the mechanisms for these behaviors remain unknown.85 Nevertheless, because Org27569 showed high pharmacological selectivity for CB1 receptor against over 40 GPCRs, including those commonly associated with drug addiction, when tested in the NIMH Psychoactive Drug Screening Program (PDSP),85 it was concluded that these in vivo effects were most likely mediated by CB1 receptors.
Similarly, PSNCBAM-1, like the inverse agonist SR141617A, significantly reduced food intake and decreased body weight in feeding studies. No adverse effects on animal behavior or obvious signs of toxicity were observed during this in vivo experiment. 57 However, no other studies have been conducted on PSNCBAM-1 to date. Like Org27569, PSNCBAM-1 possessed high selectivity for CB1 receptor against over 40 GPCRs in PDSP (unpublished data). The other NAM, pregnenolone, was shown to inhibit hypoactivity, antinociception, hypothermia, catalepsy, food intake and memory impairment caused by Δ9-THC and block the effects of Δ9-THC on glutamate and dopamine release. 65
On the other hand, PAMs such as lipoxin A4, ZCZ011, and GAT211 all displayed altered cannabinoid agonist-induced in vivo effects. Cannabinoid tetrad effects were observed in mice upon intracerebroventricular injection of lipoxin A4. These cannabimimetic effects were prevented with the CB1 receptor antagonist SR141716A, and were not observed in CB1 knockout mice. Lipoxin A4 significantly potentiated the cataleptic effect of AEA, but not 2-AG or CP55940.33,79 Moreover, lipoxin A4 demonstrated a CB1 receptor-dependent protective effect against β-amyloid (1–40)-induced spatial memory impairment in mice, which was fully reversed by CB1 antagonist SR141716A.79
While ZCZ011 was found to enter mouse brain at around 100–120 ng/g after 45 to 87 min post intraperitoneal injections of 40 mg/kg,86 it did not have any psychoactive effects in mice when administered alone. ZCZ011 significantly augmented the antinociceptive, cataleptic, and hypothermic effects of CP55940. ZCZ011 also enhanced AEA-induced hypothermia, but did not affect AEA’s antinociceptive or cataleptic effects. In addition, it did not alter the antinociceptive effect of 1 mg/kg nicotine. In the drug discrimination assay, ZCZ011 significantly increased the potency of the discriminative stimulus effects of AEA in FAAH knockout mice. ZCZ011 completely reversed nociceptive behavior in both well-established neuropathic and inflammatory pain models. The antinociceptive effect of ZCZ011 was demonstrated to be resistant to tolerance and was prevented by the CB1 receptor antagonist SR141716 but not by the CB2 receptor antagonist SR144528. The latter results indicate that ZCZ011 acted through a CB1 receptor-mediated mechanism of action.80
Compounds PAM-1 and PAM-3 alone or in combination with Δ9-THC caused a more pronounced hypothermia effect than Δ9-THC alone. PAM-3 was found to potentiate the analgesic effect of Δ9-THC. Compound PAM-1 did not elevate the extracellular dopamine level in NAc shell like the orthosteric agonist Δ9-THC. 82
The in vivo effects of current CB1 allosteric modulators are summarized in Table VIII.
Table VIII.
In vivo effects of CB1 allosteric modulators.
| Ligand | In vivo pharmacological effects | References |
|---|---|---|
| Org27569 | ↓ food intake No effect on the agonist-induced antinociception, catalepsy ↓ CP55940-induced hypothermic in rats but not in mice Did not increase grooming and scratching behaviors. No effect in drug discrimination test ↓ cue and drug-induced reinstatement of cocaine- and methamphetamine- seeking behavior |
Gamage et al., 201483 Ding et al., 201484 Jing et al, 201485 |
| PSNCBAM-1 | ↓ food intake and body weight | Horswill et al., 200757 |
| Pregnenolone | ↓ THC-induced hypoactivity, antinociception, hypothermia, catalepsy, food intake and memory impairment ↓ THC effect on glutamate and dopamine release |
Vallée et al., 201465 |
| Lipoxin A4 | Cannabinoid tetrad effects ↑ cataleptic effect of AEA |
Pamplona et al., 201279 |
| ZCZ011 | No psychoactive effects alone ↑ CP55940-induced antinociceptive, cataleptic, hypothermic effects ↑ potency of AEA in drug discrimination assay |
Ignatowska- Jankowska et al, 201580 |
| PAM1/3 | ↑ hypothermia ↑ analgesic effect of Δ9-THC No effect on extracellular dopamine level |
Thakur et al., 201482 |
5. PERSPECTIVES AND CONCLUSIONS
Recent years have witnessed major advances in the discovery of novel CB1 receptor allosteric modulators and in our understanding of their mechanisms of actions in vitro and their behaviors in vivo. This is extremely exciting, particularly at a time when the once promising CB1 receptor antagonists/inverse agonists such as SR141716 have been either withdrawn from market or discontinued from development due to the associated risk of depression and suicidal ideation. The fact that NAMs exert their effects through the endogenous ligands by fine tuning their signaling, instead of entirely blocking the receptor function with exogenous cannabinoids, provides an exciting alternative to traditional orthosteric antagonists/inverse agonists for CB1 mediated disorders. Similarly, PAMs such as the first extensively characterized ZCZ011 would offer a novel strategy to modulate CB1 receptor for therapeutic benefits without the CNS side effects that are characteristic of direct CB1 receptor agonism.
The pharmacological profiles of some of the allosteric modulators are complex and not thoroughly understood at the moment. One of the most extensively studied Org27569 have been evaluated in a variety of in vitro bioassays and more recently in vivo assays; however, the results are inconsistent so far. For instance, Org27569 has been reported to be a weak enhancer of CP55940-stimulated ERK1/2, an inhibitor of pERK1/2 activation, a pERK1/2 allosteric agonist, and as having no effect on its own on ERK1/2 signaling. While this may be related to time dependence of ERK signaling and receptor expression level, it may also result from the fact that CB1 receptors couple to all three G proteins (Gαi, Gαs and Gαq) and display functional selectivity.33 Indeed, allosteric modulator effects on CB1 signaling have been found to be markedly diverse, not only by affecting different signaling pathways, but also by being differentially responsive to various molecular probes. A good example would be that Org27560 blocked cAMP inhibition induced by all cannabinoid orthosteric agonists tested, but had little or no effect on ERK1/2 phosphorylation mediated by certain orthosteric agonists.33
While probe dependence may not be important in physiological systems that have only one endogenous ligand for the receptor, many GPCRs bind and respond to multiple endogenous ligands, such as the CB1 receptor which binds to both AEA and 2-AG. Importantly, these endogenous ligands may mediate different or even opposing effects. It has been demonstrated in vivo that an elevated anandamide level impaired CB1 receptor-mediated long term potentiation, learning and memory while 2-AG enhanced these processes.87,88 Therefore, it would be therapeutically useful to develop an allosteric modulator drug that selectively targets only one endogenous ligand but not both. Further, a CB1 receptor allosteric ligand that potentiates the action of 2-AG while antagonizing that of anandamide would have potential therapeutic value for learning and memory preservation.
The signaling pathway dependence observed with some allosteric modulators could provide an alternative and exciting opportunity to selectively target certain cannabinoid signaling pathways for the development of CB1 receptor-specific treatments for numerous disorders. Org27569 alone enhanced ERK1/2 phosphorylation while showing little or no effects in several other in vitro functional assays.34,35 It has been previously reported that the CB1-activated ERK signaling cascade was a key mediator of several forms of cocaine-induced synaptic plasticity, suggesting its involvement in addiction.89 Thus a CB1 receptor allosteric modulator that selectively modulates pERK signaling could be useful as therapeutics for conditions such as addiction. Moreover, the pathway dependence could be useful to develop allosteric modulators that do not enhance or inhibit pathways responsible for the unwanted effects commonly associated with cannabinoid agonists (psychoactivity) or CB1 antagonist/inverse agonist SR141716 (depression and suicidal ideation). However, the specific pathways that are responsible for the untoward effects are not yet clear. Therefore, it is important to thoroughly assess effects of the modulator in various signaling pathways.
Interestingly, the Org compounds and PSNCBAM-1 have shown no or reduced inverse agonist effects as compared to SR141716.21,57 Inverse agonism has been suggested to be responsible for the adverse profiles with CB1 antagonists/inverse agonists, as it disrupts the basal tone of endocannabinoids that is crucial for a variety of cellular processes.90 In fact, GPCR inverse agonists are known to exert long-lasting effects on receptor function that might have additional clinical ramifications.91 None of the Org compounds displayed reduction (or enhancement) of CB1 receptor constitutive activity in the mouse vas deferens contraction assay.21 Similarly, PSNCBAM-1 was reported to have no effect on constitutive activity in hCB1 yeast reporter assays, and it produced significantly lower inverse efficacy than SR141716 in the [35S]GTPγS assay.57 These results are supported by the recent findings where Ahn and co-workers, using CB1 receptor mutants, demonstrated that Org27569 induces a CB1 receptor state that is characterized by enhanced agonist affinity and decreased inverse agonist affinity.35 Therefore, the availability of modulators with reduced inverse agonism will help further evaluate this theory.
Many of the studies conducted to date are based on engineered systems with receptor over-expression, which may affect the coupling of the receptors to differential G proteins, thus affecting the signaling in a manner that may not translate into native tissues or animals. While the importance of these in vitro systems should never be discounted, they only reveal what the modulators can do, not necessarily what they will do in native systems. Recently, the activity of several modulators (Org27569, PSNCBAM-1 and pepcan-12) were confirmed in a neuronal model of synaptic transmission of endocannabinoids by measuring AEA or 2-AG mediated depolarization induced suppression of excitation while others showed no effects (pregnenolone, pepcan-12 and lipoxin A4).35,44 These results represent the first steps and underline the importance of more in-depth studies in physiologically native systems to better understand the signaling of these interesting CB1 allosteric modulators.
Two of the most studied allosteric modulators to date are Org27569 and PSNCBAM-1. Aside from the structural resemblance between the NAMs Org27569 and PSNCBAM-1, Org27569 shares the same indole core with PAMs ZCZ011 and GAT211. Structural modifications of substituents at the 2 and 3 positions of the indole template resulted in opposite CB1 receptor modulatory effects in NAM Org27569 and PAM ZCZ011 or GAT211. This observation raises some questions on the binding interactions with the receptor. Do these allosteric modulators and their analogs bind to the same or distinct binding sites on the CB1 receptor? If there are multiple orthosteric sites, could this multiplicity contribute to the probe-dependence property? One theory by Kenakin and Christopoulos attributes the biased agonism to the ligand preference for different subsets of receptor states, with each stabilized state being able to couple to its own preferred intracellular signaling responses.92 Importantly, these results clearly demonstrate that the allosteric properties can be fine-tuned with appropriate structural modifications, thus potentially resulting in preference for differential pathways, or biased signaling.
Further SAR studies will be beneficial for subsequent development to improve not only potency but selectivity and pharmacokinetic properties as well. There is limited SAR information on these scaffolds. Most of current efforts were focused at positions 3 and 5 of the indole ring A and position 4 of the phenol ring B or Org27569, knowledge on electronic and steric requirements at other positions is still lacking. Likewise, there are only two SAR studies on the PSNCBAM-1 scaffold and little data on ZCZ011 template. The urea moiety of PSNCBAM-1 locks the molecule into a rigid conformation, impacting solubility. ZCZ011 has a chiral center, thus generating two stereoisomers. These isomers may have distinct pharmacological properties as seen in the case of PAM1/GAT211. This gives rise to questions of which isomer is the active or more potent allosteric modulator and if they are metabolized differently in vivo. Therefore, improved tool compounds are certainly needed to support future studies of cannabinoid pathways. Armed with SAR information, researchers will be better equipped to attach substituents at appropriate positions to achieve their purposes such as hydrophilic groups to improve solubility, modifying structures responsible for undesired pharmacokinetic properties, or warheads for covalent binding probes.
Pharmacokinetic properties of these allosteric modulators such as Org27569 could obscure their expected cannabinoid mimetic effects. Even though Org27569 was found in blood and brain after i.p. administration, its poor solubility is well documented and its permeability is yet to be studied. Therefore, pharmacokinetic studies are required to assess if the ligand can reach the site of action at a sufficient concentration to exert its cannabinoid effects. PSNCBAM-1 and the lipophilic pregnenolone encounter the same solubility issue. These compounds need to be dissolved in vegetable oil, detergents, and organic solvents (ethanol or dimethyl sulfoxide) before diluted by warm saline and used immediately for animal dosing. The availability of allosteric modulators with improved pharmacokinetic properties compared to the current ones would certainly help elucidating the in vivo effects of allosteric modulators.
Despite their great promise, we should always keep challenges of the allosteric modulation strategy in consideration. Firstly, the probe dependence should be carefully noted in interpreting data from primary in vitro screen for CB1 allosteric modulators as different cell types and/or use of different orthosteric probes may give varied, even opposing results. Hence, results from second-messenger screens (i.e. cAMP, intracellular calcium levels) should be confirmed with more comprehensive and downstream signals (i.e. ERK) to give a more complete assessment of ligand properties and their ranking priorities for drug development. Secondly, the less conserved allosteric sites which contribute to subtype selectivity could result in significant species diversity. It poses a problem in conventional drug screening in which new compounds are typically screened against recombinant human receptors overexpressed in cell lines followed by in vivo rodent models for therapeutic treatment in human. Thirdly, neurodegenerative diseases often lead to loss of neurons or neuronal functions, resulting in decreased availability of endogenous agonist. Thus, a PAM which acts by enhanced agonist binding (α effect) will progressively lose efficacy over the course of the disease. Lastly, SARs of PAMs for some GPCRs appear to be “flat” compared to those of orthosteric ligands, therefore requiring extended maneuvers in drug design.93
We are still far away from fully understanding the complexity of cannabinoid system and the allosteric mechanisms. As such, the observed in vitro mechanisms may not be easily extrapolated to expected in vivo effects. We have witnessed this discrepancy with the NAM Org27569 which displays convincing in vitro pharmacological effects, yet lacks the typical in vivo cannabinoid effects. While it remains possible that some of the in vivo effects are non-CB1 receptor mediated, the observed discrepancy may also be the result of the complex biased signaling produced by allosteric modulators at the CB1 receptor, particularly where an in vivo effect is dependent upon a certain signaling pathway. Another factor that should be carefully considered in performing in vivo studies is the time dependence of these allosteric modulators.33 A perceived negative effects may have resulted from the transient nature of the allosteric modulators where a broad observation window is required. Together, these underscore the importance of more extensive studies required to understand this gap in pharmacological translation.
Until allosteric sites are well defined and gold standard allosteric modulators are found, we have few pharmacological tools to study the pathways, verify specificity of candidate allosterism, and assign ligand classification. As crystallographic and NMR studies of CB1 receptor are not available at present, we only have limited insight into sites of actions for ligand-receptor complex. Mutagenesis studies up to date only indicate that the mutations alter the activation state of CB1 receptor, thus affecting the binding of Org27569. They do not confirm that the mutated residues belong to the allosteric binding site. As a NAM, Org27569 could prefer to bind to an inactive receptor. An allosteric modulator might also interfere with the transport of the orthosteric ligand through the lipid bilayer, influencing the formation of orthosteric ligand-receptor complex. The use of covalent probes has its own challenges. Even though the probe has high structural similarity with the original allosteric modulator, it could covalently bind to a completely distinct site due to its chemically reactive warhead. Computational stimulations have shed a light into elucidation of binding sites and visualization of ligand transport and metabolism within the CB1 receptor.53,94–96 Complementary experimental data is required to confirm the results proposed by in silico studies.
An emerging drug development strategy is the design of compounds with multiple modes of actions to achieve synergistic effects. For example, inhibition of fatty acid amide hydrolase increases the availability of endocannabinoids, thus enhancing the effect of PAMs. Another strategy that has been explored for other GPCRs is designing bitopic or dualsteric ligands which bind to both allosteric and orthosteric sites. The orthosteric interaction promotes receptor binding and receptor activation while the allosteric interaction provides receptor subtype-selectivity and targets preferred intracellular signaling pathway activation. 97–101 As allosteric binding sites of CB1 receptors are currently not well-characterized, the design of this class of compounds is more challenging compared to the more advanced structural insight of other GPCRs such as the muscarinic acetylcholine receptor. These dualsteric ligands could display either orthosteric or allosteric properties under different conditions.102,103
Finally, it should be pointed out that the allosteric approach has recently seen much clinical success and several allosteric modulators have been advanced to the market as therapeutics, demonstrating that allosteric modulation can be a safe and therapeutically relevant approach to targeting GPCRs.23,104 The first such drug was cinacalcet (Sensipar/Mimpara; Amgen), a PAM of the calcium sensing receptor (CasR, a member of the GPCR C family), is used to treat hyperparathyroidism.105 More recently, Maraviroc (Celsentri/selzentry; Pfizer), a NAM of the CCR5 receptor (a member of the GPCR A family), has been approved for the treatment of HIV.106
In conclusion, the cannabinoid system remains an important drug target for a variety of diseases and allosteric modulation offers a novel approach to target the CB1 receptor for therapeutic benefits. In particular, the biased signaling of allosteric modulators may provide unique advantages in avoiding adverse reactions associated with the majority of orthosteric agonists and antagonists. Encouragingly, the availability of allosteric modulators with diverse structural scaffolds and their extensive pharmacological studies allows a clearer understanding of the biochemical and behavioral properties of these first generation synthetic and endogenous CB1 allosteric modulators. However, much work is still required across many fields in order to fully take advantage of the CB1 receptor allosterism and develop therapeutic agents to manipulate CB1 receptor signaling via the allosteric site(s).
Acknowledgments
This work was supported in part by National Institute on Drug Abuse, National Institutes of Health, USA (grants DA040693, DA032837 and DA003672), and by the National Natural Science Foundation of China (81473089).
REFERENCES
- 1.Kumar RN, Chambers WA, Pertwee RG. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia. 2001;56:1059–1068. doi: 10.1046/j.1365-2044.2001.02269.x. [DOI] [PubMed] [Google Scholar]
- 2.Devane WA, Dysarz FAI, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 3.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 4.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 5.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 6.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 7.De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141(5):765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasperi V, Dainese E, Oddi S, Sabatucci A, Maccarrone M. GPR55 and its interaction with membrane lipids: comparison with other endocannabinoid-binding receptors. Curr Med Chem. 2013;20(1):64–78. [PubMed] [Google Scholar]
- 9.Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc B Biol Sci. 2012;367:3353–3363. doi: 10.1098/rstb.2011.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlett AC. Cannabinoid receptor signaling. Handbook of Experimental Pharmacology. 2005;168:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- 11.Demuth DG, Molleman A. Cannabinoid signaling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 12.Howlett AC, Breivogel CSSRC, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- 14.Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 15.Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol. 2002;12:324–330. doi: 10.1016/s0959-4388(02)00328-8. [DOI] [PubMed] [Google Scholar]
- 17.Pertwee RG, Thomas A. In: Therapeutic applications for agents that act at CB1 and CB2 receptors. Reggio PH, editor. The cannabinoid Receptors: Humana Press; 2009. pp. 361–392. [Google Scholar]
- 18.Pacher P, Bátkai S, Kunos G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- 20.Jones D. End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discov. 2008;7(12):961–962. doi: 10.1038/nrd2775. [DOI] [PubMed] [Google Scholar]
- 21.Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, McLean A, McIntosh L, Goodwin G, Walker G, Westwood P, Marrs J, Thomson F, Cowley P, Christopoulos A, Pertwee RG, Ross RA. Allosteric Modulation of the Cannabinoid CB1 Receptor. Mol Pharmacol. 2005;68(5):1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- 22.Pandey P, Roy KK, Doerksen RJ. Identification and characterization of allosteric site(s) for dihydrogambogic acid (DHGA) and trans-β-caryophyllene (TBC) as cannabinoid CB2 allosteric modulators. ACS National Meeting & Exposition. 2015 [Google Scholar]
- 23.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8(1):41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenakin T. Gaddum Memorial Lecture 2014: receptors as an evolving concept: from switches to biased microprocessors. Br J Pharmacol. 2015;172:4238–4253. doi: 10.1111/bph.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wootten D, Christopoulos A, Sexton PM. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov. 2013;12:630–644. doi: 10.1038/nrd4052. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol Sci. 2007;28(8):366–373. doi: 10.1016/j.tips.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 28.Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol. 2008;3:530–541. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 29.Valant C, Felder CC, Sexton PM, Christopoulos A. Probe Dependence in the Allosteric Modulation of a G ProteinCoupled Receptor: Implications for Detection and Validation of Allosteric Ligand Effects. Mol Pharmacol. 2012;81(1):41–52. doi: 10.1124/mol.111.074872. [DOI] [PubMed] [Google Scholar]
- 30.Koole C, Wootten D, Simms J, Valant C, Sridhar R, Woodman OL, Miller LJ, Summers RJ, Christopoulos A, Sexton PM. Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: implications for drug screening. Mol Pharmacol. 2010;78(3):456–465. doi: 10.1124/mol.110.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wootten D, Savage EE, Valant C, May LT, Sloop KW, Ficorilli J, Showalter AD, Willard FS, Christopoulos A, Sexton PM. Allosteric modulation of endogenous metabolites as an avenue for drug discovery. Mol Pharmacol. 2012;82(2):281–290. doi: 10.1124/mol.112.079319. [DOI] [PubMed] [Google Scholar]
- 32.Leach K, Sexton PM, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci. 2007;28(8):382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Khajehali E, Malone DT, Glass M, Sexton PM, Christopoulos A, Leach K. Biased agonism and biased allosteric modulation at the CB1 cannabinoid receptor. Mol Pharmacol. 2015;88(2):368–379. doi: 10.1124/mol.115.099192. [DOI] [PubMed] [Google Scholar]
- 34.Baillie GL, Horswill JG, Anavi-Goffer S, Reggio PH, Bolognini D, Abood ME, McAllister S, Strange PG, Stephens GJ, Pertwee RG, Ross RA. CB1 Receptor Allosteric Modulators Display Both Agonist and Signaling Pathway Specificity. Mol Pharmacol. 2013;83(2):322–338. doi: 10.1124/mol.112.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn KHM, Mariam M, Kendall Debra A. Allosteric Modulator ORG27569 Induces CB1 Cannabinoid Receptor High Affinity Agonist Binding State, Receptor Internalization, and Gi Protein-independent ERK1/2 Kinase Activation. J Biol Chem. 2012;287(14):12070–12082. doi: 10.1074/jbc.M111.316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17(14):5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cawston EER, William J, Breen Courtney M, Grimsey Natasha L, Connor Mark, Glass Michelle. Real-time characterization of cannabinoid receptor 1 (CB1) allosteric modulators reveals novel mechanism of action. Br J Pharmacol. 2013;170(4):893–907. doi: 10.1111/bph.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fay JFF, David L. The Membrane Proximal Region of the Cannabinoid Receptor CB1 N Terminus Can Allosterically Modulate Ligand Affinity. Biochemistry. 2013;52(46):8286–8294. doi: 10.1021/bi400842k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cawston EE, Connor M, Di Marzo V, Silvestri R, Glass M. Distinct Temporal Fingerprint for Cyclic Adenosine Monophosphate (cAMP) Signaling of Indole-2-carboxamides as Allosteric Modulators of the Cannabinoid Receptors. J Med Chem. 2015;58(15):5979–5988. doi: 10.1021/acs.jmedchem.5b00579. [DOI] [PubMed] [Google Scholar]
- 40.Lauckner JE, Hille B, Mackie K. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Nat Acad Sci USA. 2005;102(52):19144–19149. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntosh BT, Hudson B, Yegorova S, Jollimore CA, Kelly ME. Agonist-dependent cannabinoid receptor signalling in human trabecular meshwork cells. Br J Pharmacol. 2007;152(7):1111–1120. doi: 10.1038/sj.bjp.0707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen TG Nadezhda, Decker Ann M, Li Jun-Xu, Wiley Jenny L, Thomas Brian F, Kenakin Terry P, Zhang Yanan. Structure-activity relationships of substituted 1H–indole-2-carboxamides as CB1 receptor allosteric modulators. Bioorg Med Chem. 2015;23(9):2195–2203. doi: 10.1016/j.bmc.2015.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn KHM, Mariam M, Shim Joong-Youn, Kendall Debra A. Distinct Roles of β-Arrestin 1 and β-Arrestin 2 in ORG27569-induced Biased Signaling and Internalization of the Cannabinoid Receptor 1 (CB1) J Biol Chem. 2013;288(14):9790–9800. doi: 10.1074/jbc.M112.438804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Straiker A, Mitjavila J, Yin D, Gibson A, Mackie K. Aiming for allosterism: Evaluation of allosteric modulators of CB1 in a neuronal model. Pharmacol Res. 2015;99:370–376. doi: 10.1016/j.phrs.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stornaiuolo M, Bruno A, Botta L, Regina GL, Cosconati S, Silvestri R, Marinelli L, Novellino E. Endogenous vs Exogenous Allosteric Modulators in GPCRs: A dispute for shuttling CB1 among different membrane microenvironments. Sci Rep. 2015;5:15433. doi: 10.1038/srep15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piscitelli FL Alessia, La Regina Giuseppe, Coluccia Antonio, Morera Ludovica, Allara Marco, Novellino Ettore, Di Marzo Vincenzo, Silvestri Romano. Indole-2-carboxamides as Allosteric Modulators of the Cannabinoid CB1 Receptor. J Med Chem. 2012;55(11):5627–5631. doi: 10.1021/jm201485c. [DOI] [PubMed] [Google Scholar]
- 47.Mahmoud MMA, Hamed I, Ahn Kwang H, Damaraju Aparna, Samala Sushma, Pulipati Venkata K, Kolluru Srikanth, Kendall Debra A, Lu Dai. Structure-Activity Relationship Study of Indole-2-carboxamides Identifies a Potent Allosteric Modulator for the Cannabinoid Receptor 1 (CB1) J Med Chem. 2013;56(20):7965–7975. doi: 10.1021/jm4009828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn KH, Mahmoud MM, Samala S, Lu D, Kendall DA. Profiling two indole-2-carboxamides for allosteric modulation of the CB1 receptor. J Neurochem. 2013;124(5):584–589. doi: 10.1111/jnc.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greig IR, Ross RA, Pertwee RG, Trembleau L, Abdelrahman M, Baillie GL. The University Court of the University of Aberdeen, assignee. N-(Arylalkyl)-1H–indole-2-sulfonic acid amide compounds and their therapeutic use as cannabinoid allosteric modulators. Greater Britain patent WO 2012/117216 A1. 2012 [Google Scholar]
- 50.Khurana LA, Hamed I, Olszewska Teresa, Ahn Kwang H, Damaraju Aparna, Kendall Debra A, Lu Dai. Optimization of Chemical Functionalities of Indole-2-carboxamides To Improve Allosteric Parameters for the Cannabinoid Receptor 1 (CB1) J Med Chem. 2014;57(7):3040–3052. doi: 10.1021/jm5000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballesteros JA, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 52.Hadley AIliff, Diane LLynch, Evangelia Kotsikorou, Reggio PH. Parameterization of Org27569: An allosteric modulator of the cannabinoid CB1 G protein-coupled receptor. J Comput Chem. 2011;32(10):2119–2126. doi: 10.1002/jcc.21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shore DMB, Gemma L, Hurst Dow H, Navas Frank, III, Seltzman Herbert H, Marcu Jahan P, Abood Mary E, Ross Ruth A, Reggio Patricia H. Allosteric Modulation of a Cannabinoid G Protein-coupled Receptor. J Biol Chem. 2014;289(9):5828–5845. doi: 10.1074/jbc.M113.478495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulkarni PM, Kulkarni AR, Korde A, Tichkule RB, Laprairie RB, Denovan-Wright EM, Zhou H, Janero DR, Zvonok N, Makriyannis A, Cascio MG, Pertwee RG, Thakur GA. Novel Electrophilic and Photoaffinity Covalent Probes for Mapping the Cannabinoid 1 Receptor Allosteric Site(s) J Med Chem. 2016;59(1):44–60. doi: 10.1021/acs.jmedchem.5b01303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laprairie RB, Kulkarni AR, Kulkarni PM, Hurst DP, Lynch D, Reggio PH, Janero DR, Pertwee RG, Stevenson LA, Kelly MEM, Denovan-Wright EM, Thakur GA. Mapping Cannabinoid 1 Receptor Allosteric Site(s): Critical Molecular Determinant and Signaling Profile of GAT100, a Novel, Potent, and Irreversibly Binding Probe. ACS Chem Neurosci. 2016;7(6):776–798. doi: 10.1021/acschemneuro.6b00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiao C-J, Ali HI, Ahn KH, Kolluru S, Debra A Kendall, Lu D. Synthesis and biological evaluation of indole-2-carboxamides bearing photoactivatable functionalities as novel allosteric modulators for the cannabinoid CB1 receptor. Eur J Med Chem. 2016 doi: 10.1016/j.ejmech.2016.05.044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horswill J, Bali U, Shaaban S, Keily J, Jeevaratnam P, Babbs A, Reynet C, In PWK. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br J Pharmacol. 2007;152:805–814. doi: 10.1038/sj.bjp.0707347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Horswill JG, Whalley BJ, Stephens GJ. Effects of the Allosteric Antagonist 1-(4-Chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea (PSNCBAM-1) on CB1 Receptor Modulation in the Cerebellum. Mol Pharmacol. 2011;79(4):758–767. doi: 10.1124/mol.110.068197. [DOI] [PubMed] [Google Scholar]
- 59.German ND, Ann M, Gilmour Brian P, Gay Elaine A, Wiley Jenny L, Thomas Brian F, Zhang Yanan. Diarylureas as Allosteric Modulators of the Cannabinoid CB1 Receptor: Structure-Activity Relationship Studies on 1-(4-Chlorophenyl)-3-{3-[6-(pyrrolidin-1-yl)pyridin-2-yl]phenyl}urea (PSNCBAM-1) J Med Chem. 2014;57(18):7758–7769. doi: 10.1021/jm501042u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thakur GAT Ritesh B, Kulkarni Pushkar Mukund, Kulkarni Abhijit Raghunath Northeastern University, assignee. Allosteric modulators of the cannabinoid 1 receptor. United States patent WO 2015/027160 A2. 2015 [Google Scholar]
- 61.Bauer MC Andrea, Tamborrini Marco, Eisen David, Lerner Raissa, Lutz Beat, Poetz Oliver, Pluschke Gerd, Gertsch Juerg. Identification and Quantification of a New Family of Peptide Endocannabinoids (Pepcans) Showing Negative Allosteric Modulation at CB1 Receptors. J Biol Chem. 2012;287(44):36944–36967. doi: 10.1074/jbc.M112.382481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, Heimann AS, Ferro ES, Scarlata S, Fricker LD, Devi LA. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009;23:3020–3029. doi: 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrucci V, Chicca A, Viveros-Paredes J, Gertsch J. Peptide endocannabinoids (Pepcans) are PAMs of CB2 receptors and involved in the innate immune response. 24th annual symposiums on the cannabinoids. 2014:62. [Google Scholar]
- 64.Dvorácskó S, Tömböly C, Berkecz R, Keresztes A. Investigation of receptor binding and functional characteristics of hemopressin(1–7) Neuropeptides. 2016 doi: 10.1016/j.npep.2016.02.001. in press. [DOI] [PubMed] [Google Scholar]
- 65.Vallée M, Vitiello S, Bellocchio L, Hébert-Chatelain E, Monlezun S, Martin-Garcia E, Kasanetz F, Gemma L Baillie, Panin F, Cathala A, Roullot-Lacarrière V, Fabre S, Hurst DP, Lynch DL, Shore DM, Deroche-Gamonet V, Spampinato U, Revest J-M, Maldonado R, Reggio PH, Ross RA, Marsicano G, Piazza PV. Pregnenolone Can Protect the Brain from Cannabis Intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGuinness D, Malikzay A, Visconti R, Lin K, Bayne M, Monsma F, Lunn CA. Characterizing cannabinoid CB2 receptor ligands using DiscoveRx PathHunter beta-arrestin assay. J Biomol Screen. 2009;14:49–58. doi: 10.1177/1087057108327329. [DOI] [PubMed] [Google Scholar]
- 67.Priestley RSN Sarah A, Alexander Stephen PH, Kendall David A. A potential role for cannabinoid receptors in the therapeutic action of fenofibrate. FASEB J. 2015;29(4):1446–1455. doi: 10.1096/fj.14-263053. [DOI] [PubMed] [Google Scholar]
- 68.Martin-Santos R, Crippa J, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farré M, Zuardi A, McGuire P. Acute effects of a single, oral dose of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18(32):4966–4979. doi: 10.2174/138161212802884780. [DOI] [PubMed] [Google Scholar]
- 69.Fernández-Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, Martínez-Orgado J. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75(2):323–333. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of Δ9-tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–177. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- 71.Pertwee RG, Ross RA, Craib SJ, Thomas A. (−)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur J Pharmacol. 2002;456:99–106. doi: 10.1016/s0014-2999(02)02624-9. [DOI] [PubMed] [Google Scholar]
- 72.Ryan D, Drysdale AJ, Pertwee RG, Platt B. Interactions of cannabidiol with endocannabinoid signalling in hippocampal tissue. Eur J Neurosci. 2007;25:2093–2102. doi: 10.1111/j.1460-9568.2007.05448.x. [DOI] [PubMed] [Google Scholar]
- 73.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- 76.Laprairie RB, Bagher AM, Kelly MEM, Dupré DJ, Denovan-Wright EM. Type 1 Cannabinoid Receptor Ligands Display Functional Selectivity in a Cell Culture Model of Striatal Medium Spiny Projection Neurons. J Biol Chem. 2014;289(36):24845–24862. doi: 10.1074/jbc.M114.557025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the type 1 cannabinoid receptor. Br J Pharmacol. 2015;172(20):4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navarro HA, Howard JL, Pollard GT, Carroll1 FI. Positive allosteric modulation of the human cannabinoid (CB1) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol. 2009;156:1178–1184. doi: 10.1111/j.1476-5381.2009.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pamplona FA, Ferreira J, Octávio Menezes de Lima J, Duarte FS, Bento AF, Forner S, Villarinho JG, Bellocchio L, Wotjak CT, Lerner R, Monory K, Lutz B, Canetti C, Matias I, Calixto JB, Giovanni Marsicano, Guimarães MZP, Takahashi RN. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Nat Acad Sci USA. 2012;109(51):21134–31139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, Damaj IM, Poklis J, Wiley JL, Zanda M, Zanato C, Greig IR, Lichtman AH, Ross RA. A Cannabinoid CB1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacol. 2015;40(13):2948–2959. doi: 10.1038/npp.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kerr JR, Trembleau L, Storey JMD, Wardella JL, Harrison WTA. Crystal structures of four indole derivatives as possible cannabinoid allosteric antagonists. Acta Crystallogr E Crystallogr Commun. 2015;71:654–659. doi: 10.1107/S2056989015008476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thakur GA, Kulkarni PM. Northeastern University, assignee. Allosteric modulators of CB1 cannabinoid receptors. United States patent US 2015/0005346 A1. 2015 [Google Scholar]
- 83.Gamage TF, Ignatowska-Jankowska BM, Wiley JL, Abdelrahman M, Trembleau L, Greig IR, Thakur GA, Tichkule R, Poklis J, Ross RA, Pertwee RG, Lichtman AH. In-vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569. Behav Pharmacol. 2014;25(2):182–185. doi: 10.1097/FBP.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding YQ Yanyan, Jing Li, Thorn David A, Zhang Yanan, Li Jun-Xu. Behavioral effects of the cannabinoid CB1 receptor allosteric modulator ORG27569 in rats. Pharmacol Res Perspect. 2014;2(6) doi: 10.1002/prp2.69. e00069/00061-e00069/00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jing LQ Yanan, Zhang Yanyan, Li Jun-Xu. Effects of the cannabinoid CB1 receptor allosteric modulator ORG 27569 on reinstatement of cocaine- and methamphetamine-seeking behavior in rats. Drug Alcohol Depen. 2014;143:251–256. doi: 10.1016/j.drugalcdep.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poklis JL, Clay DJ, Ignatowska-Jankowska BM, Zanato C, Ross RA, Greig IR, Abdullah RA, Mustafa MA, Lichtman AH, Poklis A. HPLC-MS-MS Determination of ZCZ-011, A Novel Pharmacological Tool for Investigation of the Cannabinoid Receptor in Mouse Brain Using Clean Screen FAStColumn Extraction. J Anal Toxicol. 2015;39:353–358. doi: 10.1093/jat/bkv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Basavarajappa BS, Nagre NN, Xie S, Subbanna S. Elevation of endogenous anandamide impairs LTP, learning, and memory through CB1 receptor signaling in mice. Hippocampus. 2014;24(7):808–818. doi: 10.1002/hipo.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan B, Zhong P, Sun D, Liu Q-S. Extracellular Signal-Regulated Kinase Signaling in the Ventral Tegmental Area Mediates Cocaine-Induced Synaptic Plasticity and Rewarding Effects. J Neurosci. 2011;31(31):11244–11255. doi: 10.1523/JNEUROSCI.1040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bergman J, Delatte MS, Paronis CA, Vemuri K, Thakur GA, Makriyannis A. Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol Behav. 2008;93(4-5):666–670. doi: 10.1016/j.physbeh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Greasley PJ, Clapham JC. Inverse agonism or neutral antagonism at G-protein coupled receptors: A medicinal chemistry challenge worth pursuing? Eur J Pharmacol. 2006;553:1–9. doi: 10.1016/j.ejphar.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 92.Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- 93.Hopkins CR, Lindsley CW, Niswender CM. mGluR4-positive allosteric modulation as potential treatment for Parkinson’s disease. Future Med Chem. 2009;1(3):501–513. doi: 10.4155/fmc.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tiburu EK, Tyukhtenko S, Zhou H, Janero DR, Struppe J, Makriyannis A. Human Cannabinoid 1 GPCR C-Terminal Domain Interacts with Bilayer Phospholipids to Modulate the Structure of its Membrane Environment. AAPS J. 2011;13(1):92–98. doi: 10.1208/s12248-010-9244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hurst DP, Grossfield A, Lynch DL, Feller S, Romo TD, Gawrisch K, Pitman MC, Reggio PH. A Lipid Pathway for Ligand Binding Is Necessary for a Cannabinoid G Protein-coupled Receptor. J Biol Chem. 2010;285:17954–17964. doi: 10.1074/jbc.M109.041590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lynch DL, Reggio PH. Molecular Dynamics Simulations of the Endocannabinoid N-Arachidonoylethanolamine (Anandamide) in a Phospholipid Bilayer: Probing Structure and Dynamics. J Med Chem. 2005;48(15):4824–4833. doi: 10.1021/jm058185d. [DOI] [PubMed] [Google Scholar]
- 97.Kamal M, Jockers R. Bitopic ligands: all-in-one orthosteric and allosteric. F1000 Biol Rep. 2009;1:77. doi: 10.3410/B1-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davie BJ, Christopoulos A, Scammells PJ. Development of M1 mAChR Allosteric and Bitopic Ligands: Prospective Therapeutics for the Treatment of Cognitive Deficits. ACS Chem Neurosci. 2013;4(7):1026–1048. doi: 10.1021/cn400086m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valant C, Sexton P, Christopoulos A. Orthosteric/allosteric bitopic ligands: going hybrid at GPCRs. Mol Interv. 2009;9(3):125–135. doi: 10.1124/mi.9.3.6. [DOI] [PubMed] [Google Scholar]
- 100.Valant C, Lane JR, Sexton PM, Christopoulos A. The Best of Both Worlds? Bitopic Orthosteric/Allosteric Ligands of G Protein-Coupled Receptors. Annu Rev Pharmacol Toxicol. 2012;52:153–178. doi: 10.1146/annurev-pharmtox-010611-134514. [DOI] [PubMed] [Google Scholar]
- 101.Mohr K, Tränkle C, Kostenis E, Barocelli E, Amici MD, Holzgrabe U. Rational design of dualsteric GPCR ligands: quests and promise. Br J Pharmacol. 2010;159(5):997–1008. doi: 10.1111/j.1476-5381.2009.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lane JR, Donthamsetti P, Shonberg J, Draper-Joyce CJ, Dentry S, Michino M, Shi L, López L, Scammells PJ, Capuano B, Sexton PM, Javitch JA, Christopoulos A. A new mechanism of allostery in a G protein-coupled receptor dimer. Nat Chem Biol. 2014;10:745–752. doi: 10.1038/nchembio.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maggio R, Scarselli M, Capannolo M, Millan MJ. Novel dimensions of D3 receptor function: Focus on heterodimerisation, transactivation and allosteric modulation. Eur Neuropsychopharmacol. 2015;25:1470–1479. doi: 10.1016/j.euroneuro.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 104.Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem Biol. 2008;3(9):530–541. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 105.Harrington PE, Fotsch C. Calcium sensing receptor activators: calcimimetics. Curr Med Chem. 2007;14(28):3027–3034. doi: 10.2174/092986707782794096. [DOI] [PubMed] [Google Scholar]
- 106.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49(11):4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]