Abstract
Objective
Current data are limited on the course of type 1 diabetes (T1D) in children and adolescents through the first few years of diabetes. The Pediatric Diabetes Consortium T1D New Onset (NeOn) Study was undertaken to prospectively assess natural history and clinical outcomes in children treated at 7 US diabetes centers from the time of diagnosis. This paper describes clinical outcomes in the T1D NeOn cohort during the first 3 years post-diagnosis.
Results
1048 participants (mean age 9.2 years, 49% female, 65% non-Hispanic White) were enrolled between July 2009 and April 2011. Mean HbA1c (±SD) was 7.2% (55 mmol/mol) at 3 months, followed by a progressive rise to 8.4% (68 mmol/mol) at 36 months post-diagnosis, with only 30% of participants achieving target HbA1c<7.5% (58 mmol/mol). The percentage of participants in partial remission estimated by insulin dose adjusted HbA1c [HbA1c % + (4 × insulin dose unit/kg/24 h)] ≤9 sharply declined from 23% at 12 months to 7% at 36 months. The percentage of participants developing diabetic ketoacidosis (DKA) was 1% in the first year after diagnosis, increasing to 6% in year 2 and year 3.
Conclusions
These results demonstrate the gradual decline in glycemic control due to waning residual endogenous insulin secretion with increasing duration of T1D in children and adolescents. These data indicate the need to translate recent advances in automated insulin delivery, new insulin analogs and adjunctive pharmacologic agents into novel treatment strategies to maintain optimal glycemic control even early in the course of T1D.
Keywords: diabetes, children, type 1, HbA1c or glycemic control
Introduction
It has long been recognized that optimal metabolic control is much easier to achieve during the partial remission (or honeymoon) period in youth with new-onset T1D than later in the course of the disease. Moreover, the Diabetes Control and Complications Trial (DCCT) demonstrated that intensive treatment of T1D could slow the loss of residual β-cell function, resulting in lower HbA1c levels and a reduced risk of severe hypoglycemia (1). The recent DirecNet/TrialNet Metabolic Control Study (2, 3) and the European Pediatric Onset Study (4) indicate that the highest c-peptide values and lowest HbA1c levels are observed in youth with newly diagnosed T1D during the first 6 months of treatment, whereas c-peptide levels begin to decline and HbA1c levels increase over the subsequent 6 months (2-6).
Outside of the relatively small numbers of participants enrolled in clinical trials of early use of insulin pumps and continuous glucose monitors, the time course and consequences of loss of residual β-cell function in youth with new onset T1D in clinical practice settings has not been well established. A clear understanding of the waning of the honeymoon phase of T1D in children and adolescents would facilitate the design of clinical trials of new therapies aimed at preserving residual β-cell function, as well as enhancing vigilance in diabetes management during this critical phase of transition (7). In order to fill gaps in knowledge regarding the natural history of the honeymoon phase of T1D in children and adolescents, the Pediatric Diabetes Consortium (PDC) enrolled pediatric patients who had been newly diagnosed with T1D into the New Onset (NeOn) Study (5). Clinical outcomes in the NeOn cohort during the first year of treatment were similar to those in recent clinical trials, in that the lowest HbA1c levels were observed between 3-6 months post-diagnosis, with a progressive rise in HbA1c values at 9 and 12 months (5). In this paper, we describe the results of follow-up evaluations in the PDC T1D NeOn Study cohort after the first year following diagnosis through up to 3 years of follow up.
Methods
Patients
The PDC enrolled 1,048 patients diagnosed with T1D between July 2009 and April 2011. The protocol was approved by the Institutional Review Board (IRB) at each of the 7 participating pediatric diabetes treatment centers in the US. To be eligible for enrollment in the study, patients had to be <19 years of age and managed at one of the 7 participating PDC centers within 3 months of diagnosis. Informed consent was obtained from participants 18 years of age and from parents of those less than 18 years of age; assent was obtained from participants <18 years of age as required by local IRB regulations. A detailed description of PDC, the design of the study, and data collection methods have been published previously (5).
Data Collection
Demographic, socioeconomic and clinical data were collected from medical records and from interviews with the participant and/or parent. Follow-up visits, including screening and diagnosis of thyroid and celiac disease screening and diagnosis were completed per usual care and all visits were entered in the standardized electronic case report forms for the study. Severe hypoglycemic (SH) events were defined as episodes of hypoglycemia that required the assistance of another person to treat with oral carbohydrate, intravenous glucose, or intramuscular glucagon, due to altered consciousness or seizure. Diabetic ketoacidosis (DKA) events were defined by the DCCT criteria of pH <7.3 or HCO3 <15mEq/l with hyperglycemia and treatment in a healthcare facility. HbA1c levels were measured by the DCA immunoassay method (Siemens Health Care) at all of the centers. The Insulin Dose Adjusted HbA1c (IDAA1c) is a validated measure to determine beta cell reserve in patients with T1D. IDAA1c values ≤9.0 correspond to a predicted stimulated C-peptide >300 pmol/l and it was defined as HbA1c % + [4 × insulin dose (units per kg per 24 h)] (8).
Statistical Analyses
Clinical characteristics of the participants were presented at the following time points: diagnosis (0-14 days), 3 months (1.5-4.5 months), 6 months (4.5-7.5 months), 12 months (10.5-15 months), 24 months (21-27 months) and 36 months (33-39 months) from diagnosis. A 3-year completer was defined as a participant who had a visit between 33-39 months from diagnosis. Body mass index (BMI) was calculated from the height and weight measurements closest to each target time point. BMI percentile and standard deviation score for age and gender were calculated using the 2000 CDC population growth chart data (9).
Descriptive statistics included means, SD, median, interquartile range, and proportions when appropriate. Correlations between frequency of self-monitoring of blood glucose (SMBG) and HbA1c were computed using Spearman correlation, and correlations for HbA1c at different visits were assessed by Pearson correlation. Repeated measures least square models were used to assess whether HbA1c and IDAA1c differed by DKA and SH status over time. Interaction between DKA/SH status with visit time was also evaluated. Since the difference in HbA1c between DKA and no DKA subgroups was different by visit, t-test was used to compare HbA1c between DKA vs. no DKA at each visit, and the results were presented separately by visit. A repeated measures least square model was also used to assess the association between HbA1c and obesity. Life-table method was used to calculate cumulative event rate for comorbidities. Statistical analyses were conducted using SAS 9.4 statistical software (SAS Institute Inc., Cary, North Carolina).
Results
The mean age of the cohort at diagnosis was 9.2 years (range 0.7 to18.8 years); 49% were female; 65% were non-Hispanic White, 21% were Hispanic and 8% were non-Hispanic Black (Table 1). The majority of participants had health insurance, parents with a college degree or above, and a family income greater than $50,000. A comparison of baseline factors in 3-year completers vs. non-completers is shown in Supplemental Table 1, and clinical outcome measures among 3-year completers are reported in Supplemental Table 2.
Table 1.
Characteristics at Diagnosis (N=1048a)
| # | % | |
|---|---|---|
| Age | ||
| <5 years | 199 | 19% |
| 5–<12 years | 568 | 54% |
| 12–<19years | 281 | 27% |
| Mean ± SD | 9.2 ± 4.2 | |
| Range | 0.7 – 18.8 | |
| Gender | ||
| Female | 513 | 49% |
| Race/Ethnicity | ||
| Non-Hispanic White | 671 | 65% |
| Hispanic or Latino | 216 | 21% |
| Black/African American | 81 | 8% |
| Other/Multiple Race | 59 | 6% |
| Health Insurance | ||
| Private | 681 | 66% |
| Children's Health Plan or Other Government | 302 | 29% |
| Military | 19 | 2% |
| None | 23 | 2% |
| Parent Education | ||
| High School or Less | 291 | 34% |
| Associate Degree | 121 | 14% |
| BS/BA | 245 | 29% |
| MS/MA/Professional Degree | 202 | 24% |
| Family Income | ||
| <$25,000 | 101 | 14% |
| $25,000-$49,999 | 133 | 19% |
| $50,000-$74,999 | 112 | 16% |
| $75,000-$99,999 | 101 | 14% |
| ≥$100,000 | 255 | 36% |
Number of participants with missing data: race/ethnicity (21), health insurance (23), parent education (189), and family income (346).
Glycemic Control of T1D (Table 2 and Figure 1)
Table 2.
Clinical Characteristics by Completed Follow-up Visit
| Diagnosis | 3 Months | 6 Months | 12 Months | 24 Months | 36 Months | |
|---|---|---|---|---|---|---|
| N=1048b | N=841b | N=838b | N=897b | N=822b | N=607b | |
| Pump Use | 12 (1%) | 76 (9%) | 141 (17%) | 309 (34%) | 376 (46%) | 293 (48%) |
| N=784b | N=786b | N=847b | N=481b | N=356b | ||
| CGM Use | NA | 2% | 3% | 2% | 4% | 4% |
| N=507b | N=495b | N=517b | N=417b | N=322b | ||
| Self Blood Glucose Monitoring (# tests / day) median (IQR) | NA | 5 (4, 6) | 5 (4, 6) | 5 (4, 6) | 5 (4, 7) | 5 (4, 7) |
| N=980b | N=788b | N=789b | N=859b | N=789b | N=579b | |
| HbA1c (% [mmol/mol]) mean ± SD | 11.5 ± 2.3 [102 ± 25] | 7.2 ± 1.1 [55 ± 12] | 7.3 ± 1.4 [56 ± 15] | 7.8 ± 1.5 [62 ± 16] | 8.1 ± 1.4 [65 ± 16] | 8.4 ± 1.6 [68 ± 18] |
| Age <5 years | 10.5 ± 2.0 [91 ± 21] | 7.8 ± 1.1 [62 ± 12] | 7.9 ± 1.2 [62 ± 14] | 7.9 ± 1.1 [62 ± 12] | 7.9 ± 1.0 [63 ± 11] | 8.0 ± 1.2 [64 ± 13] |
| 5–<12 years | 11.5 ± 2.3 [102 ± 25] | 7.1 ± 1.0 [54 ± 11] | 7.2 ± 1.2 [55 ± 13] | 7.8 ± 1.3 [62 ± 14] | 8.1 ± 1.4 [65 ± 15] | 8.4 ± 1.5 [68 ± 17] |
| 12–<19 years | 12.1 ± 2.4 [109 ± 27] | 7.0 ± 1.2 [53 ± 13] | 7.0 ± 1.7 [53 ± 18] | 7.7 ± 2.0 [61 ± 21] | 8.1 ± 1.8 [65 ± 20] | 8.6 ± 2.0 [71 ± 22] |
| HbA1c<7.5% (58 mmol/mol) | 3% | 61% | 61% | 45% | 34% | 30% |
| N=747b | N=611b | N=708b | N=741b | N=539b | ||
| HbA1c Change (% [mmol/mol]) mean ± SD | NA | −4.3 ± 2.5 [−47 ± 28] | +0.1 ± 1.1 [+1 ± 12] | +0.5 ± 1.2 [+5 ± 13] | +0.3 ± 1.1 [+4 ± 12] | +0.2 ± 1.1 [+2 ± 12] |
| N=691b | N=713b | N=795b | N=442b | N=316b | ||
| Total Insulin (u/day/kg) mean ± SD | NA | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.7 ± 0.3 | 0.8 ± 0.4 | 1.0 ± 0.6 |
| Basal | NA | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.4 ± 0.3 | 0.4 ± 0.2 |
| Bolus | NA | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.4 ± 0.3 | 0.4 ± 0.4 | 0.6 ± 0.5 |
| N=669b | N=678b | N=767b | N=421b | N=296b | ||
| IDAA1c (%) mean ± SD | NA | 9.3 ± 1.8 | 9.4 ± 2.1 | 10.5 ± 2.2 | 11.3 ± 2.4 | 12.2 ± 2.9 |
| IDAA1c≤9.0% | NA | 49% | 48% | 23% | 12% | 7% |
| Age<5 years | NA | 35% | 31% | 22% | 13% | 10% |
| 5–<12 years | NA | 52% | 52% | 21% | 11% | 3% |
| 12–<19 years | NA | 53% | 54% | 28% | 11% | 14% |
| N=473b | N=581b | N=376b | N=188b | |||
| IDAA1c Change (%) mean ± SD | NA | NA | +0.2 ± 1.6 | +0.9 ± 1.7 | +0.9 ± 2.0 | +1.2 ± 2.8 |
| N=1044b | N=727b | N=584b | ||||
| SH in prior yeara | NA | NA | NA | 2% | 3% | 4% |
| # SH events >1 in prior yeara | NA | NA | NA | <1% | <1% | 1% |
| DKA reported in prior yeara | NA | NA | NA | 1%c | 6% | 6% |
| # DKA events >1 in prior yeara | NA | NA | NA | <1%c | 1% | 1% |
Participant/parent was asked at the visit whether an event had occurred in the previous 12 months.
N varies due to missing data at the particular time point.
DKA events at diagnosis were excluded.
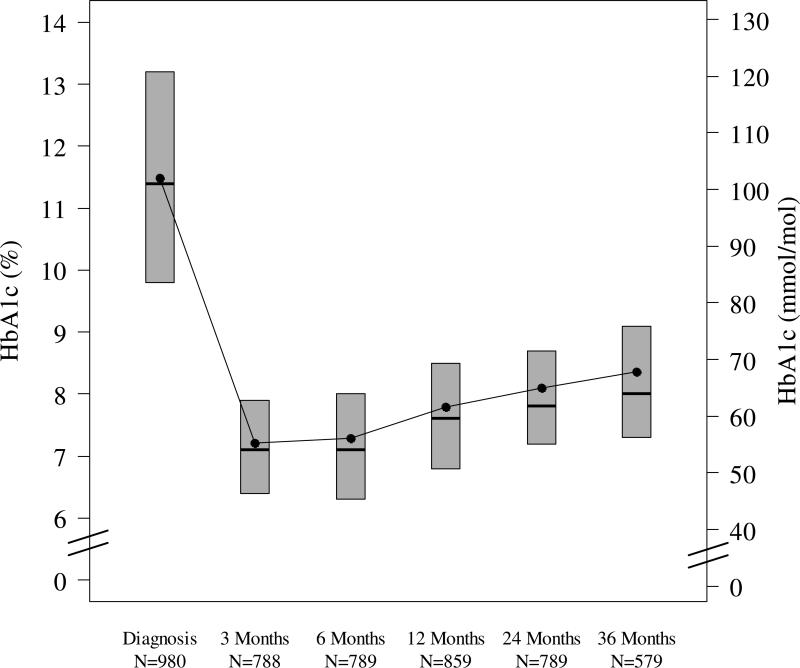
Figure 1. HbA1c by T1D Duration.
The bottom and the top of each box plot denote the 25th and 75th percentiles. Horizontal line inside each box plot denotes the median, and the dot denotes the mean.
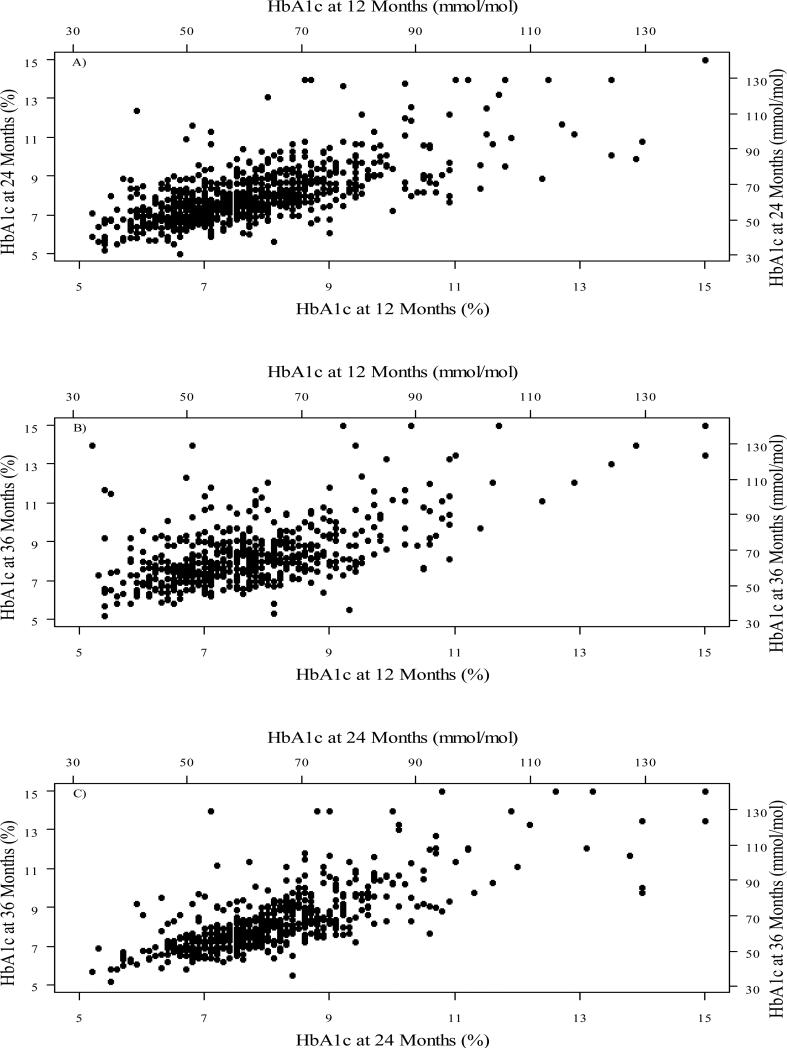
Nadir mean HbA1c (±SD) was 7.2 (±1.1) to 7.3 (±1.4) % [55 (±12) to 56 (±15) mmol/mol] at 3 and 6 months, followed by a progressive rise to 8.4 (±1.6) % [68 (±18) mmol/mol] at 36 months post-diagnosis. The highest HbA1c levels at 36 months were seen in adolescents (Table 2). Sixty-one percent of participants achieved target HbA1c levels <7.5% (58 mmol/mol) at 3 and 6 months but this fell to only 30% by 36 months. HbA1c levels were significantly correlated among different visits (Figure 3), with Pearson correlation coefficient (95% confidence interval) of 0.68 (0.64, 0.71), 0.56 (0.50, 0.62) and 0.74 (0.69, 0.77) for HbA1c at 24 months vs. 12 months, 36 months vs. 12 months and 36 months vs. 24 months, respectively.
Figure 3. Scatter Plots for HbA1c at Different Visits.
A) HbA1c at 24 months vs. 12 months; B) HbA1c at 36 months vs. 12 months; C) HbA1c at 36 months vs. 24 months.
Diabetes Management
An insulin pump was being used by 46% of participants at 24 months and 48% at 36 months. Only 2-4% of participants reported using a continuous glucose monitor between 6 and 36 months. The median self-reported number of self-monitored blood glucose (SMBG) tests per day remained consistent at 5 per day through 36 months (Table 2). There was a significant association between HbA1c and the daily number of SMBG tests with a correlation that increased from 12 months to 36 months (r = −0.27, −0.24, −0.33 for 12, 24 and 36 months respectively, all p-values<0.001).
IDDA1c
As shown in Table 2, there also was a progressive increase in IDAA1c that reflected the increases in HbA1c values and total daily insulin doses over the course of the study. Consequently, the percentage of participants who were in partial remission, as indicated by IDAA1c values ≤9.0, fell from 49% at 3 months to 23% at 12 months and to only 7% at 36 months, respectively. The rate of decline in C-peptide estimated by the IDAA1c was lower in younger participants.
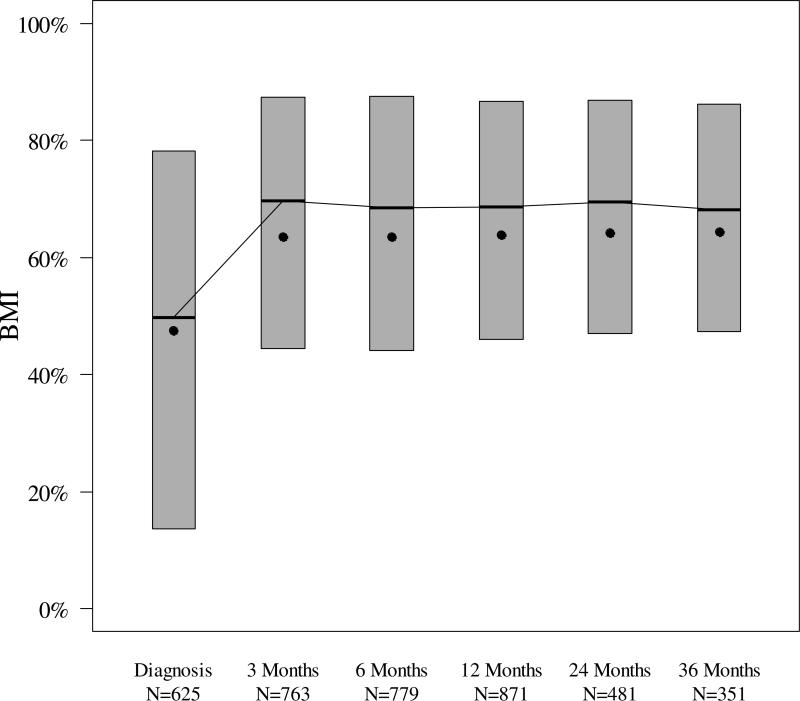
BMI
The median (interquartile range) BMI percentile was 50% (14–78%) at the time of diagnosis, increased to 69% (44–88%) at 6 months, and then remained relatively constant throughout the first three years of T1D (Figure 2). BMI percentile was inversely associated with HbA1c (P<0.001) after adjusting for visit time.
Figure 2. BMI Percentile by T1D Duration.
The bottom and the top of each box plot denote the 25th and 75th percentiles. Horizontal line inside each box denotes the median, and the dot denotes the mean.
Severe Hypoglycemia and Diabetic Ketoacidosis
During the first year of T1D, SH episodes were reported by 2% of participants. By 24 and 36 months, the percentages of participants with SH episodes in the prior 12 months were 3% and 4%, respectively. The percentage of participants who reported more than one episode of SH within the prior 12 months was only 1% at 36 months. DKA occurred in 1% of participants during the first year of T1D, not including episodes at presentation. By 24 and 36 months, the percentages of participants with DKA in the prior 12 months increased considerably to 6% and 6% (Table 2). Mean HbA1c levels were significantly higher among those with a DKA event than those without a DKA event at 12 months, 24 months and 36 months (all p-values<0.001), with mean A1c 9.4 ± 1.6% vs. 7.8 ± 1.4% (79 ± 17 mmol/mol vs. 61 ± 16 mmol/mol), 9.3 ± 2.3% vs. 8.0 ± 1.4% (79 ± 25 mmol/mol vs. 64 ± 15 mmol/mol) and 9.3 ± 1.9% vs. 8.3 ± 1.6% (79 ± 21 mmol/mol vs. 67 ± 17 mmol/mol) among the DKA vs. no DKA group at 12, 24, and 36 months respectively. Those participants with a DKA event also had significantly higher IDAA1c than those without a DKA event after adjusting for visit time (P=0.002). There was no significant difference in either HbA1c or IDAA1c levels between those participants with a SH event and those without a SH event after adjusting for visits (P=0.18 and 0.64, respectively).
Comorbidities diagnosed after initial screening evaluation at diagnosis
As shown in Table 3, the cumulative rate for diagnosis of thyroid disease slightly increased from 2% during the first year after diagnosis to 3% after three years. While only <1% of participants had been diagnosed with psychiatric disorders and 2% had been diagnosed with celiac disease during the first year of diabetes, the cumulative event rate of psychiatric disorders and celiac disease increased to 2% and 5% respectively after three years of T1D duration.
Table 3.
Comorbidities after T1D Diagnosis (N=1044a)
| Diabetes Duration, years | Thyroid diseasesb | Celiac disease | Psychiatric disorderc | |||
|---|---|---|---|---|---|---|
| N | Cumulative Event Rate | N | Cumulative Event Rate | N | Cumulative Event Rate | |
| 1 | 16 | 2% | 19 | 2% | 7 | <1% |
| 2 | 7 | 2% | 14 | 3% | 10 | 2% |
| 3 | 5 | 3% | 11 | 5% | 3 | 2% |
| Total | 28 | 44 | 20 | |||
Excludes 4 participants with no visit available after enrollment
Includes Graves' disease, Hashimoto's and any other thyroiditis
Includes bipolar, anxiety, depression, disruptive behavior, central pain syndrome
Discussion
An important finding of this study is that the rise in HbA1c levels, increases in total daily insulin doses and waning of the partial remission period that were observed between 6 and 12 months after the diagnosis of T1D in this cohort (5) continued for the next 2 years at slower rates, but accompanied with a steep increase in number of episodes of DKA. While the pattern of glycemic control changes, namely the timing of nadir HbA1c and the trajectory of rise in HbA1c, is similar to those reported during the first 12 months of new onset patients in the DirecNet-TrialNet Metabolic Control Study (2, 3) and during 24 months of follow-up of the Pediatric Onset Study in Europe (4), HbA1c levels were higher in the NeOn Study cohort at each time point. These differences may be due to the difference in the frequency and intensity of contact with participants in clinical trials versus patients in clinical practice, but could also be due to differences in patients who participate in clinical trials versus those who do not. It is noteworthy that patients and caregivers responded to the increasing challenges in managing their diabetes by maintaining a high frequency of blood glucose monitoring and increasing use of insulin pumps over time.
While c-peptide responses to mixed meal tolerance tests were not measured in the NeOn Study, the IDAA1C developed and validated by Mortensen and colleagues, was used as a surrogate measure of residual β-cell function (8). Those investigators demonstrated that an IDAA1C value ≤9.0 predicts peak meal-stimulated c-peptide levels that are almost always >300 pmol/l. Similar c-peptide responses can be seen in youth with T1D with IDAA1C values between 9.0 and 10.0, albeit less frequently. Although there is data supporting the use of IDAA1c, the IDAA1c has some limitations. The data were derived from a non-US population with a different genetic background. The stimulated C-peptide cut-off level as a measure of residual β-cell function was even higher than the cut-off level that was used to define absolute insulin deficiency in the DCCT cohort (C-peptide <200pmol/L) despite the fact that, unlike the DCCT patient population, subjects younger than 13 years of age were enrolled into the study by Mortensen. Hence, the IDAA1c might not be applicable to assess the β-cell reserve for younger patients who tend to have lower C-Peptide levels as compared to older people with new onset T1D. Moreover, the DPV partial remission analysis demonstrated younger children are significantly less likely to experience partial remission (10). The data points on the regression analysis graph published in Mortensen's paper depict many subjects without partial remission (8) who have stimulated C-peptide levels >300pg/mL. The possible underestimation of partial remission and a faster rate of increase in older versus younger subjects could be driven by the higher insulin dose requirement per kilogram weight commonly observed in adolescent patients during puberty (11). Our finding that the proportion of participants with IDDA1c values ≥9.0 was as high as 23% at 12 months but fell to 12% and 7% at 24 and 36 months suggests that future β-cell preservation studies may have to be extended to 24-36 months in order to detect differences in C-peptide responses between experimental and control groups.
It was somewhat surprising that the reported frequency of SH episodes did not increase with waning of the partial remission phase during the first 3 years of diabetes treatment. One possible explanation is that the IDAA1c might not be a sensitive measure to determine the risk for severe hypoglycemia in this patient population. Unlike SH, the number of episodes of DKA did rise in parallel to the rise in HbA1c levels, a finding similar to that in pediatric patients with longer diabetes duration enrolled in the T1D Exchange Clinic Registry (12).
T1D Exchange Registry data indicate that youth with T1D have not been spared from the epidemic of obesity that afflicts so many non-diabetic children (13). Thus, the initial increase in BMI to >60th percentile at 6 months is likely due to the regain of weight lost prior to the diagnosis of diabetes, whereas, the lack of any substantial change in BMI after 6 months is evidence against a major effect of insulin treatment itself.
It is not uncommon for associated autoimmune diseases to develop after the diagnosis of T1D, and this explains the increasing prevalence of thyroid and celiac disease over time. The increasing prevalence of psychiatric disorders probably represents the increasing age of the cohort as participants entered adolescence, increased awareness of mental health issues (14) as well as the longer duration of the disease.
There are a few limitations of the PDC NeOn Study. The study was not designed as a population-based study, since all of the participants enrolled into the study were being followed in academic pediatrics diabetes centers within 3 months of diagnosis. While this factor raises a concern about representativeness of our findings, the majority of pediatric patients with T1D in the U.S. are managed by pediatric diabetologists as common practice, unlike the adult patient population whose care is often shared by specialists and primary care givers (15). Maximum amount of possible follow up was incomplete for 29% of the cohort; however, baseline characteristics appeared quite similar between those with complete versus incomplete follow up, suggesting that this is not a source of bias.
The findings of the NeOn Study underscore the importance of maintaining a commitment by patients and parents of children with T1D to intensive insulin therapy during the transition period out of the partial remission phase in order to keep HbA1c levels within the target range. Unfortunately, only about 30% of participants were able achieve this goal after the first 2 years of T1D despite their high median income and well educated background. The percentage of pediatric patients who are within target glycemic range has been reported to be even lower, based on a recently published database study (16). Nevertheless, improvements in accuracy and ease of use of CGM devices and the introduction of integrated sensor-augmented pump systems provide new and relatively untapped opportunities for achieving optimal glycemic control in a larger proportion of youth with T1D (17), including those recently diagnosed. As the DCCT has shown, getting a larger proportion of patients to target HbA1c levels as early in the course of the disease as possible will have long lasting benefits in reducing the risk of future vascular complications (1, 18).
Supplementary Material
Acknowledgements
The Pediatric Diabetes Consortium and its activities are supported by the Jaeb Center for Health Research Foundation through an unrestricted grant from NovoNordisk. The University of Michigan Consortium center is supported by the Michigan Diabetes Research and Training Center from the National Institute of Diabetes and Digestive and Kidney Diseases (DK020572). E.C., P.C., and K.R. researched data, contributed to discussion, wrote manuscript, and reviewed/edited manuscript. W.T., G.K., R.B., R.G., C.K., J.S, J.L., and M.R. researched data, contributed to discussion, and reviewed/edited manuscript. R.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. W. Tamborlane is a paid consultant for AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk, Sanofi, and Takeda. J. Lee is a paid consultant for Verily. R. Beck and R. Gal have grant funding from Novo Nordisk and Boehringer Ingelheim.
The Pediatric Diabetes Consortium Publications Committee:
The following comprises a listing of the Pediatric Diabetes Consortium Publications Committee members: William Tamborlane, MD, Yale University, New Haven, CT; Georgeanna Klingensmith, MD, Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, Aurora, CO; Mark A. Clements, MD, PhD, Children's Mercy Kansas City, Kansas City, MO; Kenneth Copeland, MD, University of Oklahoma College of Medicine, Oklahoma City, OK; Tamara S. Hannon, MD, Indiana University School of Medicine, Indianapolis, IN; Rubina Heptulla, MD, Albert Einstein College of Medicine, Bronx, NY; Joane Less, MBA, University of Oklahoma College of Medicine, Oklahoma City, OK; Ashley Shoemaker, MD, Vanderbilt University School of Medicine, Nashville, TN; Jamie Wood, MD, University Hospitals, Cleveland, OH.
The Pediatric Diabetes Consortium Study Group:
Clinical Centers: (Listed clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators.) (1) Baylor College of Medicine, Houston, TX: Morey Haymond, MD (PI); Fida Bacha, MD (I); Maria J. Redondo, MD, PhD (I); Elizabeth Johnson (C); Andrene McDonald (C); Mariam Pontifies (C); (2) Children's Hospital of Los Angeles, Los Angeles, CA: Jamie Wood, MD (PI, Dr. Wood is now at University Hospitals, Cleveland, OH); Brian Ichihara (C); Megan Lipton, MA (C); Courtney Engle (C); (3) Stanford University, Stanford, CA: Avni Shah, MD (PI); Bruce Buckingham, MD (I); Liana Hsu (C); (4) Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, Aurora, CO: Georgeanna J. Klingensmith, MD (PI); Heidi Haro (C); Katherine Manseau (C); (5) University of Florida, Gainesville, FL: Desmond Schatz, MD (PI); Janet Silverstein, MD (I); Michael J. Haller, MD (I); Jamie Thomas (C); (6) Yale University, New Haven, CT: William V. Tamborlane, MD (PI); Michelle Van Name, MD (I); Eda Cengiz, MD, MHS (I); Amy Steffen (C); Jennifer Finnegan (C); Elvira Duran (C); (7) University of Michigan, Ann Arbor, MI: Joyce M. Lee, MD, MPH (PI); Emily Hirschfeld (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Lindsey C. Beaulieu, MA; Peiyao Cheng, MPH; Robin L. Gal, MSPH; Craig Kollman, PhD; TJ Mouse; Katrina J. Ruedy, MSPH.
Footnotes
E. Cengiz, P. Cheng, K. Ruedy, C. Kollman, G. Klingensmith, J. Silverstein, and M. Redondo report no conflict of interest.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham BA, Beck RW, Ruedy KJ, et al. The effects of inpatient hybrid closed-loop therapy initiated within 1 week of type 1 diabetes diagnosis. Diabetes Technol Ther. 2013;15:401–8. doi: 10.1089/dia.2013.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckingham B, Beck RW, Ruedy KJ, et al. Effectiveness of early intensive therapy on beta-cell preservation in type 1 diabetes. Diabetes Care. 2013;36:4030–5. doi: 10.2337/dc13-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kordonouri O, Hartmann R, Pankowska E, et al. Sensor augmented pump therapy from onset of type 1 diabetes: late follow-up results of the Pediatric Onset Study. Pediatr Diabetes. 2012;13:515–8. doi: 10.1111/j.1399-5448.2012.00863.x. [DOI] [PubMed] [Google Scholar]
- 5.Cengiz E, Connor CG, Ruedy KJ, et al. Pediatric diabetes consortium T1D New Onset (NeOn) study: Clinical outcomes during the first year following diagnosis. Pediatric Diabetes. 2014;15:287–93. doi: 10.1111/pedi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cengiz E, Sherr JL, Erkin-Cakmak A, et al. A bridge to insulin pump therapy: twice-daily regimen with NPH and detemir insulins during initial treatment of youth with type 1 diabetes mellitus. Endocr Pract. 2011;17:862–6. doi: 10.4158/EP11031.OR. [DOI] [PubMed] [Google Scholar]
- 7.Couper JJ, Haller MJ, Ziegler AG, Knip M, Ludvigsson J, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2014. Phases of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2014;15(Suppl 20):18–25. doi: 10.1111/pedi.12188. [DOI] [PubMed] [Google Scholar]
- 8.Mortensen HB, Hougaard P, Swift P, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32:1384–90. doi: 10.2337/dc08-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuczmarski R, Ogden C, Grummer-Strawn L, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 10.Nagl K, Hermann JM, Plamper M, et al. Factors contributing to partial remission in type 1 diabetes: analysis based on the insulin dose-adjusted HbA1c in 3657 children and adolescents from Germany and Austria. Pediatr Diabetes. 2016 doi: 10.1111/pedi.12413. [DOI] [PubMed] [Google Scholar]
- 11.Wiegand S, Raile K, Reinehr T, et al. Daily insulin requirement of children and adolescents with type 1 diabetes: effect of age, gender, body mass index and mode of therapy. Eur J Endocrinol. 2008;158:543–9. doi: 10.1530/EJE-07-0904. [DOI] [PubMed] [Google Scholar]
- 12.Cengiz E, Xing D, Wong JC, et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange clinic registry. Pediatr Diabetes. 2013;14:447–54. doi: 10.1111/pedi.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood JR, Miller KM, Maahs DM, et al. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36:2035–7. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritsch SL, Overton MW, Robbins DR. The interface of child mental health and juvenile diabetes mellitus. Psychiatr Clin North Am. 2015;38:59–76. doi: 10.1016/j.psc.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Paris CA, Imperatore G, Klingensmith G, et al. Predictors of Insulin Regimens and Impact on Outcomes in Youth with Type 1 Diabetes: The SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155:183–9. doi: 10.1016/j.jpeds.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 16.Miller KM, Foster NC, Beck RW, et al. Current State of Type 1 Diabetes Treatment in the U.S.: Updated Data From the T1D Exchange Clinic Registry. Diabetes Care. 2015;38:971–8. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 17.Cengiz E, Sherr JL, Weinzimer SA, Tamborlane WV. New-generation diabetes management: glucose sensor-augmented insulin pump therapy. Expert Rev Med Devices. 2011;8:449–58. doi: 10.1586/erd.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polak JF, Backlund JY, Cleary PA, et al. Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2011;60:607–13. doi: 10.2337/db10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.