Abstract
The aim of this study was to evaluate the effect of growth hormone (GH) in the maintenance of the ovarian primordial follicle reserve. Ovaries from 16 mo old GH-deficient Ames Dwarf (df/df) and Normal (N/df) mice were used. A subgroup of df/df and N mice received GH or saline injections for six weeks starting at 14 mo of age. In addition, ovaries from 12 mo old mice overexpressing bovine GH (bGH) and controls were used. df/df mice had higher number of primordial and total follicles than N/df mice (p<0.05), while GH treatment decreased follicle counts in both genotypes (p<0.05). In addition, bGH mice had lower number of primordial and total follicles than the controls (p<0.05). pFoxO3a levels were higher in mice treated with GH and in bGH mice (p<0.05) when comparing with age match controls. These results indicate that increased circulating GH is associated with a reduced ovarian primordial follicle reserve and increased pFoxO3a content in oocytes.
Keywords: ovarian aging, primordial follicle, ovarian follicular reserve, GH, IGF, FOXO
1. Introduction
Ames Dwarf mice (df/df) have a defective Prop1 (Prophet of Pit1) gene, which impairs anterior pituitary gland development, resulting in deficient growth hormone (GH) secretion (Andersen et al., 1995; Miquet et al., 2005). As a result df/df mice have very low levels of circulating insulin-like growth factor I (IGF-I), are smaller and live around 30 to 65% longer than Normal littermates (N/df) (Brown-Borg et al., 1996; Chandrashekar and Bartke, 1993). Interestingly, early life short-term treatment of df/df mice with exogenous GH can reduce the longevity, insulin sensitivity and cellular stress resistance (Masternak et al., 2010; Panici et al., 2010). In contrast, transgenic mice overexpressing bovine GH (bGH) have elevated plasma levels of GH, resulting in increased circulating IGF-I levels and adult body size (Blackburn et al., 1997; Wolf et al., 1993). The lifespan of bGH mice is approximately 50% shorter than for normal littermates (Wolf et al., 1993). Collectively, this evidence points to an important role of the GH/IGF-I axis in the aging process, which seems to be dependent on the stage of life and maturation at which it occurs.
A progressive decline and depletion of the ovarian primordial follicle reserve is the main determinant of the age at the onset of menopause (Faddy et al., 1992; te Velde et al., 1998). Concomitant with the reduction in the number of follicles, the quality of the remaining oocytes generally decreases with age (Faddy et al., 1992; Hirshfield, 1994). The size of the primordial follicle reserve decreases about 10 fold from the ages of 0.5 to 1.5 y in female mice (Kevenaar et al., 2006) and is already depleted in 2.5 y old females (Słuczanowska-Gł0105;bowska et al., 2013). It is well known that a functional GH/IGF-I axis is important for the normal ovarian function (Bachelot et al., 2002; Chandrashekar et al., 2004; Zaczek et al., 2002). However, young female mice with a disrupted GH receptor gene (GHRKO), which also have lower serum IGF-I and increased longevity (Coschigano et al., 2003), have increased number of primordial follicles (Slot et al., 2006), and still have ovarian activity at older ages, when normal mice have exhausted the ovarian follicular reserve (Sluczanowska-Glabowska et al., 2012). GH and GHR deficient mice share several characteristics with mice subjected to calorie restriction (CR), including reduction of serum levels of IGF-I and insulin (Bonkowski et al., 2006; Bonkowski et al., 2009). Not surprisingly, it has been shown that mice subjected to CR also have an increased ovarian primordial follicle reserve (Li et al., 2011; Li et al., 2015; Xiang et al., 2012). Despite that, no studies to date have quantified the ovarian reserve in df/df and bGH mice, which would enhance the present understanding of the role of the GH/IGF-I axis in regulating the rate of primordial follicle depletion.
The activation of the transcription factor Forkhead Box O3a (FoxO3a) is an essential step for the activation of the primordial ovarian reserve irreversible growth (Castrillon et al., 2003; John et al., 2007). FoxO3a is a downstream effector of the phosphoinositide 3-kinase (Pi3k)/protein kinase B (Akt1) signaling and its pathway (John et al., 2008). Hyperphosphorylation of FoxO3a results in its nuclear exclusion, culminating in the global activation of primordial follicles and premature ovarian failure (Castrillon et al., 2003). We have previously shown that oocytes enclosed in primordial/primary follicles from df/df mice have lower levels of pFoxO3a (Schneider et al., 2014). The reduced activation of the FoxO pathway by both insulin and IGF-I seems to have a central role in the extended longevity phenotype observed in the df/df and GHRKO mice (Bartke, 2008). Therefore, oocyte FoXO3a phosphorylation can be also reduced and represent a link between somatic and ovarian aging.
Based on this evidence, the aim of this study was to evaluate the ovarian primordial follicle reserve, as well as the activation of the FoxO3a transcription factor in aged df/df mice, treated with GH or saline in adult life, and in bGH mice.
2. Materials and Methods
2.1 Animals and treatments
For these experiments six groups of female mice were used. Four groups consisted of Ames dwarf (Prop-1df, df/df, n=12) and their normal littermates (N/df, n=12) mice, between 16 and 18 mo old, receiving GH (n=6 for df/df, and n=6 for N/df mice) or saline injections (n=6 for df/df, and n=6 for N/df mice). The other two groups consisted of 10 to 12 mo old bGH (n=6) and normal mice (N, n=6). Mice were maintained under temperature (22 ± 2°C) and humidity (40–60%) controlled conditions. All experiments were approved by the Ethics Committee for Animal Experimentation from the University of Southern Illinois, IL, USA. Mice treated with GH received recombinant porcine GH subcutaneous injections (4 µg/g of body weight; Alpharma, Inc., Victoria, Australia) twice daily, beginning at 14 mo of age during 6 wk. Control mice received saline injections in the same way as GH treated mice. After 6 wk of treatment, mice were kept two more weeks in the same conditions until euthanasia. The GH treatment used in the current study was proven effective for increasing serum IGF-I concentrations and body weight gain in previous studies using the same dose and treatment length in young (1 mo old) (Louis et al., 2010; Masternak et al., 2010), middle age (5 mo old) (Gesing et al., 2014) and old mice (16 mo old) (Louis et al., 2010). Body weights were measured before the first GH injection and at the end of the 6 wk treatment to confirm effectiveness of the treatment.
2.2 Tissue collection and processing
The mice were anesthetized and euthanized after fasting for 12 h and the pair of ovaries was collected, dissected from surrounding adipose tissue and placed in 10% formalin buffered solution. After that, the ovaries were removed from the formalin solution, dehydrated in alcohol, cleared in xylene and embedded individually in Paraplast Plus (Sigma Chemical Company®, St. Louis, MO, USA). One ovary of the pair was then serially sectioned at 5µm using a semi-automated rotary microtome (RM2245, Leica Biosystems, San Diego, CA, USA). Sampling started at the beginning of the visible area of the ovarian surface until the end of the structure, and every 6th section was selected and placed on a standard histological slide for staining and counting (adapted from Myers et al. (2004)). Intermediate sections were randomly selected for immunohistochemistry analysis using slides impregnated with 3% organosilane (Sigma Chemical Company®, St. Louis, MO, USA) in ethanol.
2.3 Morphological classification and follicle counting
The slides were dried at 56°C for 24 h, stained with hematoxylin-eosin, and mounted with coverslips and synthetic resin (Sigma Chemical Company®, St. Louis, MO, USA). Images of the ovarian sections were captured with a digital camera coupled to a microscope (Nikon Eclipse E200, Nikon Corporation, Japan) using the 10 and 40× objectives, assisted by the software Moticam 5.0 (Motic, Hong Kong, China).
Only follicles containing an oocyte with clearly visible nucleus were counted in each slide. Follicle classification was based on Myers et al. (2004). Follicles were classified as primordial (oocyte surrounded by a single layer of flattened granulosa cells), in transition (oocyte surrounded by a layer of flattened granulosa cells and at least one cuboid granulosa cell), primary (oocyte surrounded by a single layer of cuboidal granulosa cells), secondary (oocyte surrounded by two or more layers of cuboid granulosa cells without a visible antrum) and tertiary (follicles with a clearly defined antral space and a with multiple layers of granulosa cells around the oocyte). To estimate actual follicle quantity the number of follicles in each category was multiplied by six to account for the section sampling and by two to account for the fact that only one ovary of the pair was used.
2.4 Immunohistochemistry analysis
For immunohistochemistry analysis, the ovarian samples were deparaffinized with xylene and rehydrated with graded alcohols. The primary polyclonal antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and diluted in 1.5% BSA solution. The anti-FoxO3a (FKHRL1 antibody; N16, SC-101683-goat (IgG)) and anti-phosphorylated FoxO3a (pFoxO3a; p-FKHRL1 antibody; Ser 253, SC-101683-rabbit (IgG)) antibodies were used at a final dilution of 1:50. Blockage of the endogenous peroxidase activity was achieved with hydrogen peroxide blocking solution (Spring Bioscience, Pleasanton, CA, USA), while the antigen recovery was performed in humid heat, during 3 min after the boiling point, in citrate solution at pH 6.0. Non-specific background staining was reduced by covering the tissue sections that received protein block (Spring Bioscience, Pleasanton, CA, USA). Thereafter, slides were incubated overnight with the primary antibody in a humid chamber at 4°C. The slides with pFoxO3a and FoxO3a antibodies were instilled with secondary antibodies Reveal Polyvalente HRP® Kit (Spring Bioscience, Pleasanton, CA, USA) and Dako LSAB®2 System-HRP (Dako Corporation, Carpinteria, CA, USA), respectively. The slides were incubated at room temperature with 3,3'-diaminobenzidine (DAB-K3468, DAKO Corporation, Carpinteria, CA, USA), counterstained with Mayer's hemalum solution (Merck, Darmstadt, Germany) and mounted with coverslips and synthetic resin (Sigma Chemical Company®, St. Louis, MO, USA). The images of the follicles were captured by a digital camera coupled to a microscope (Nikon Eclipse E200, Nikon Corporation, Japan) using a 40X objective assisted by the software Moticam 5.0 (Motic®, Hong Kong, China). Only oocytes enclosed in follicles classified as primordial/in transition (n=108; n=18/group) and primary (n=108; n=18/group) were used. Protein quantification was performed by image analysis software (Image J®) and the most common value (the mode) of each area (oocyte) was registered by the 32-bit histogram application, using a scale ranging from 0 (the greatest staining intensity) to 255 (no staining) that was converted to a scale from 0 (no staining) to 4 (greatest staining) (Moreira et al., 2013; Schneider et al., 2014).
2.5 Statistical analysis
All statistical analyzes were performed using Graphpad Prism 5 (Graphpad Inc., La Jolla, CA, USA). Two-way ANOVA was used for comparing the number and size of follicles and immunostaining between df/df and N/df mice (effect of the genotype, treatment with GH and the interaction). T-test was performed for comparing the number and size of follicles and immunostaining between bGH and normal mice. A P value lower than 0.05 was considered as statistically significant, and between 0.05 and 0.10 as a trend.
3. Results
GH treatment increased body weight gain in both N and df/df mice (P<0.05) at the end of the 6 wk treatment (Suppl. Figure 1). However, body weight did not change (P>0.05) in Saline treated mice during the same period (Suppl. Figure 1), therefore confirming the effectiveness of the treatment applied in the current study.
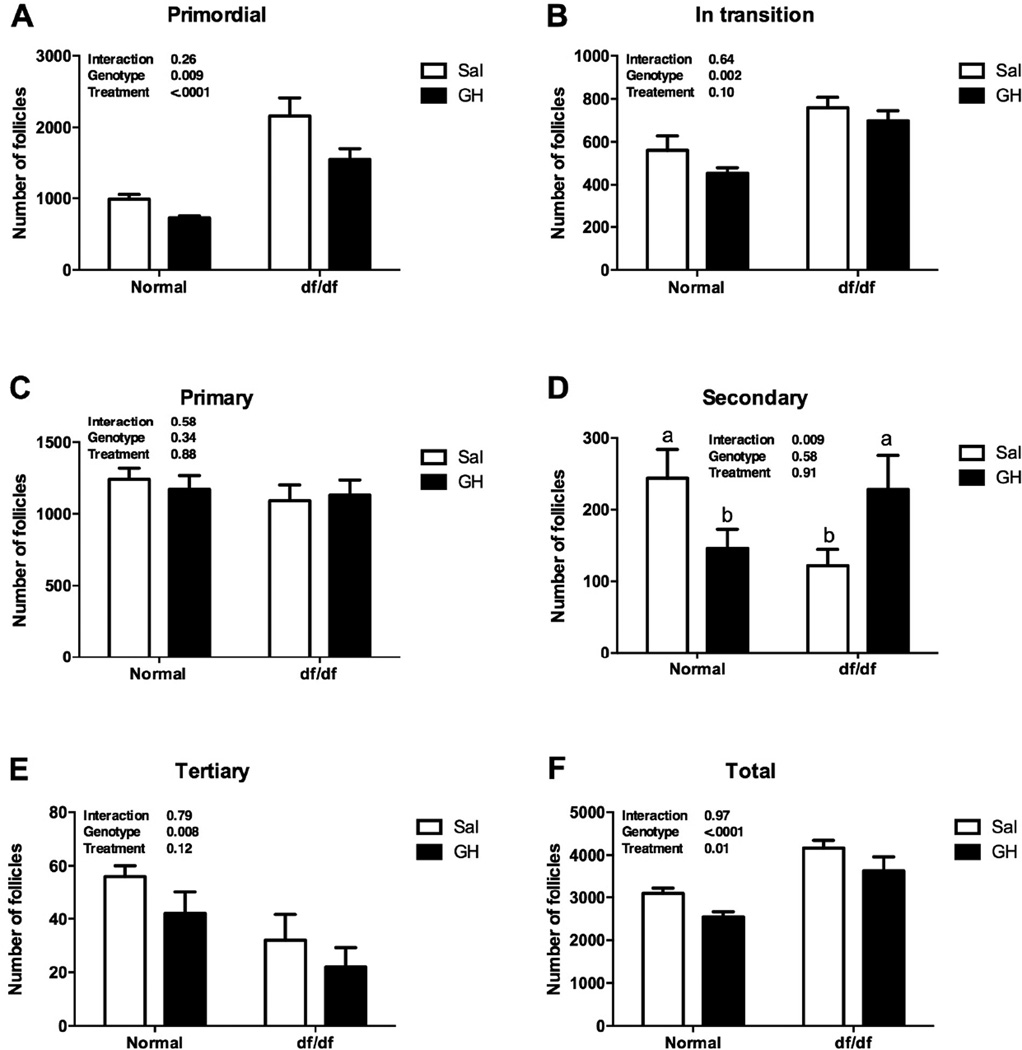
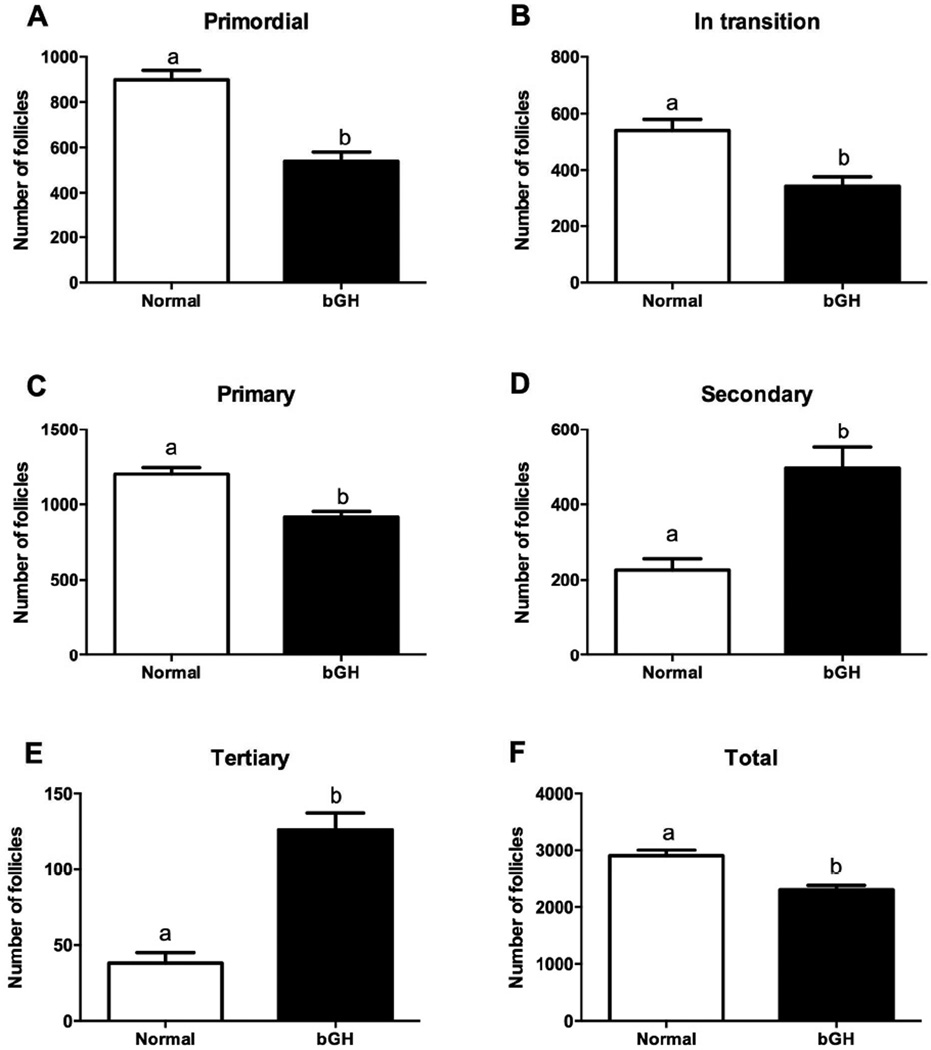
The number of primordial, in transition and total follicles was higher in df/df than N/df mice (P<0.01, Figures 1A, 1B and 1F). In addition, GH treatment decreased the number of primordial and total follicles in both df/df and N/df mice (P<0.02, Figures 1A and 1F). The number of primary follicles was not different between genotypes or treatments (P>0.10, Figure 1C). There was a genotype by treatment interaction for the number of secondary follicles, since GH treatment increased (P<0.01, Figure 1D) the number of follicles in df/df mice, while decreased it in N/df mice (P<0.01, Figure 1D). The number of tertiary follicles was lower in df/df than N/df mice (P<0.01, Figure 1E). The number of primordial, in transition, primary and total follicles was decreased in bGH compared to normal mice (P<0.01, Figures 2A, 2B, 2C and 2F), while the number of secondary and tertiary follicles was higher in bGH than in normal mice (P<0.01, Figures 2D and 2F).
Figure 1.
Number of primordial (A), transitional (B), primary (C), secondary (D), tertiary (E) and total follicles (F) in Ames dwarf (df/df) and Normal (N/df) mice receiving GH or saline treatment. Different letters indicate significant differences when the interaction was significant (P<0.05).
Figure 2.
Number of primordial (A), transitional (B), primary (C), secondary (D), tertiary (E) and total follicles (F) in normal and transgenic mice overexpressing GH (bGH). Different letters indicate significant differences (P<0.01).
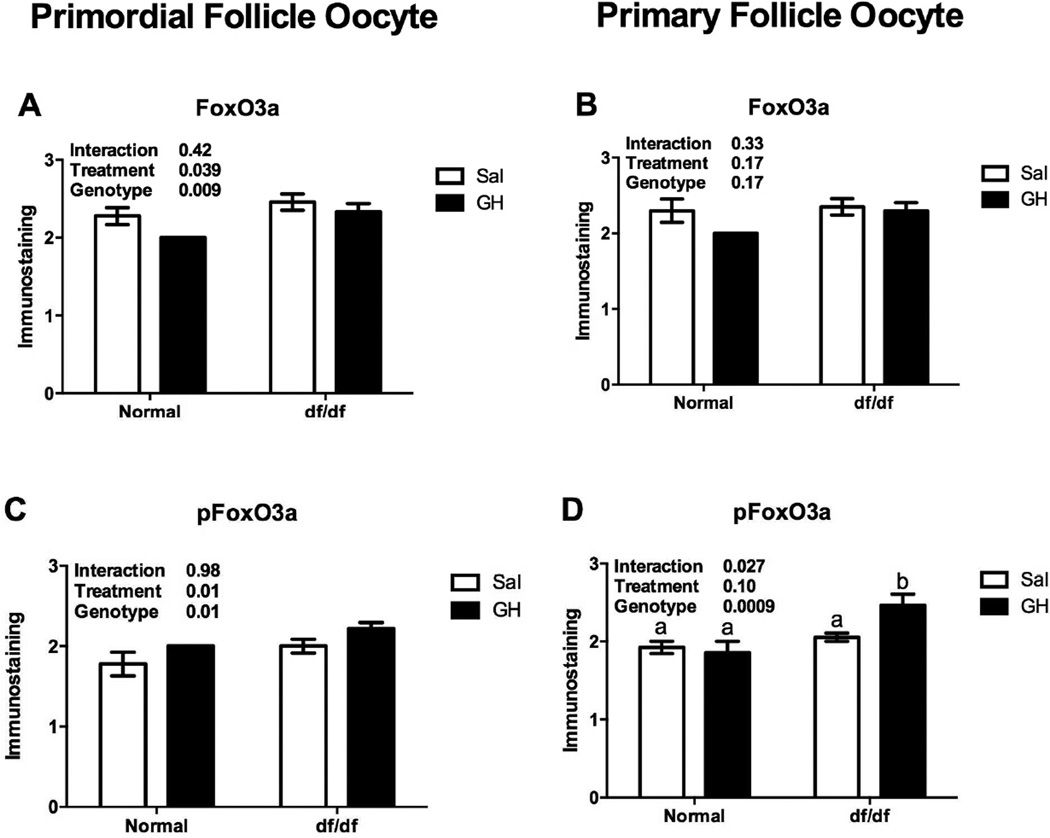
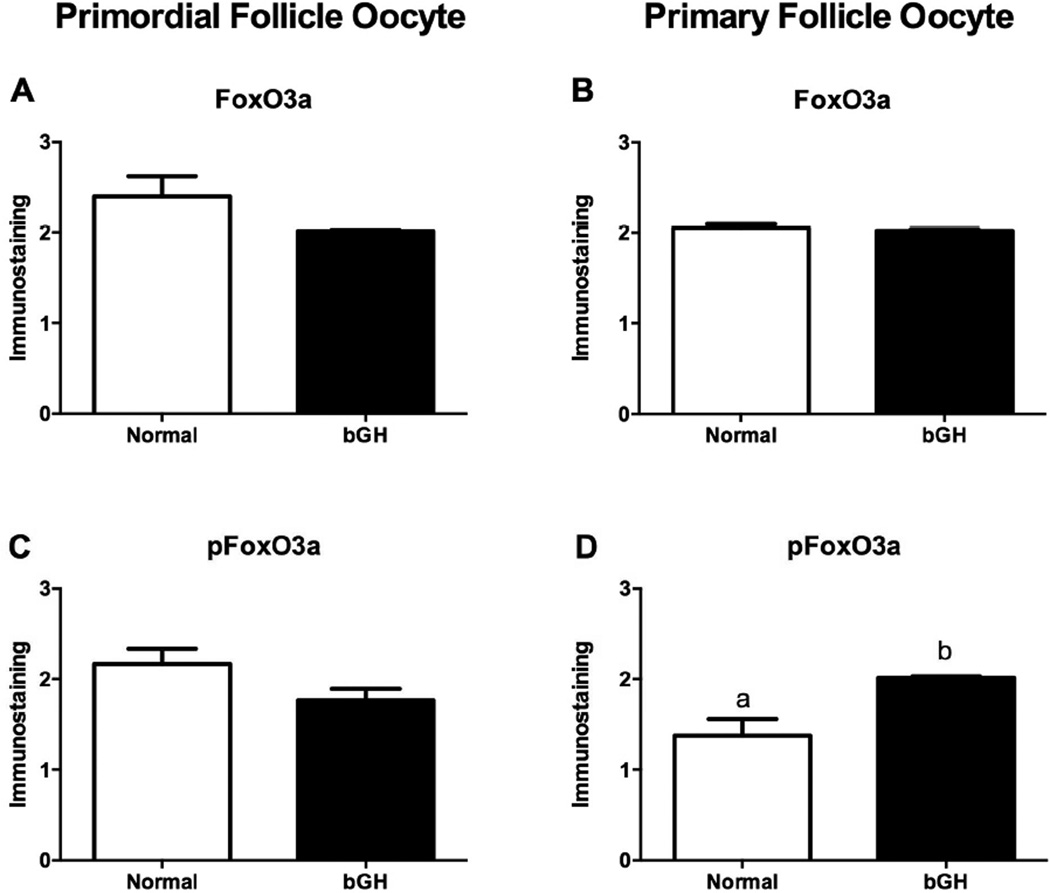
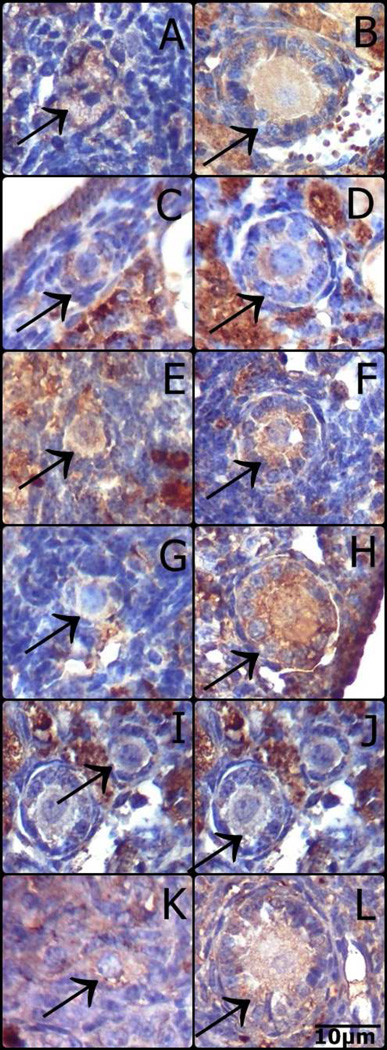
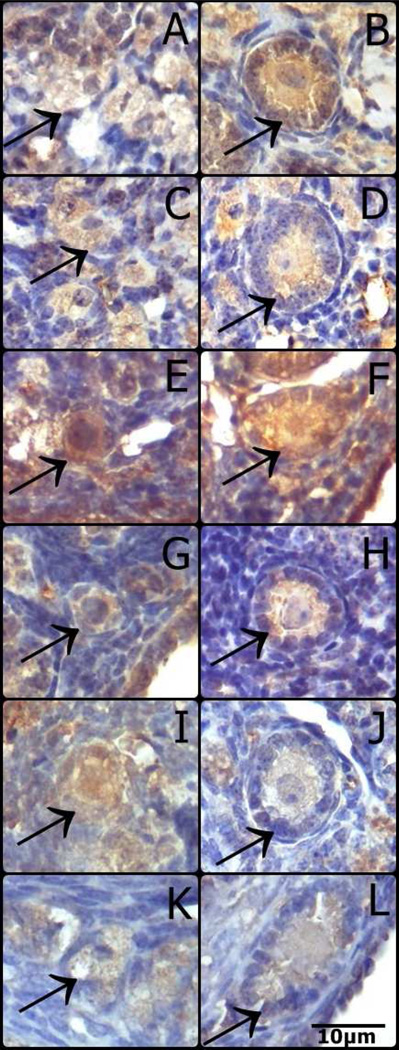
FoxO3a immunostaining in primordial follicles oocytes was higher in df/df mice (P=0.009, Figure 3A), however it was reduced in df/df and N/df mice treated with GH (P=0.03, Figure 3A). pFoxO3a immunostaining in primordial follicles oocytes was also higher in df/df mice (P=0.01, Figure 3C), and it was increased by GH treatment in both df/df and N/df mice (P=0.01, Figure 3C). FoxO3a immunostaining in primary follicles oocytes was not different between genotypes or treatments (P>0.10, Figure 3B). However, there was a genotype by treatment interaction for pFoxO3a immunostaining in primary follicles oocytes, which was higher for df/df treated with GH (P<0.05, Figure 3D). There was no difference in FoxO3a immunostaining in primordial and primary follicles oocytes of bGH compared to normal mice (P>0.05, Figures 4A and 4B). However, pFoxO3a immunostaining in primary follicles oocytes was higher in bGH compared to normal mice (P=0.006, Figure 4D). FoxO3a and pFoxO3a immunostaining images for the oocytes in the different groups are presented in Figures 5 and 6, respectively.
Figure 3.
Immunostaining for FoxO3a (A and B) and pFoxO3a (C and D) in oocytes from primordial/transitional and primary follicles in Ames dwarf (df/df) and Normal (N/df) mice receiving GH or saline treatment (n=18 oocytes/category/group). Different letters indicate significant differences when the interaction was significant (P<0.05). The initial values were converted to a scale from 0 (no staining) to 4 (intense staining).
Figure 4.
Immunostaining for FoxO3a (A and B) and pFoxO3a (C and D) in oocytes from primordial/transitional and primary follicles in normal mice and transgenic mice overexpressing GH (bGH) (n=18 oocytes/category/genotype). Different letters indicate significant differences (P<0.05). The initial values were converted to a scale from 0 (no staining) to 4 (intense staining).
Figure 5.
Representative images of immunostaining for FoxO3a in oocytes from primordial and primary follicles of N/df mice receiving saline (A, B) or GH (C and D), df/df mice receiving saline (E and F) or GH (G and H), bGH mice (I and J) and normal mice (K and L). (Primordial follicles: A, C, E, G, I, K; Primary follicles: B, D, F, H, J, L).
Figure 6.
Representative images of immunostaining for pFoxO3a in oocytes from primordial and primary follicles of N/df mice receiving saline (A, B) or GH (C and D), df/df mice receiving saline (E and F) or GH (G and H), bGH mice (I and J) and normal mice (K and L). (Primordial follicles: A, C, E, G, I, K; Primary follicles: B, D, F, H, J, L).
Data for nuclei, oocyte and follicle diameters are shown in Table 1 for df/df and N/df mice and in Table 2 for bGH and normal mice. GH treatment decreased oocyte and follicle diameters in primordial follicles of df/df and N/df mice (P<0.05, Table 1). In addition, oocyte diameter of secondary follicles was reduced in df/df mice (P=0.03, Table 1). Tertiary follicle size was reduced in df/df compared to N/df mice (P=0.03, Table 1) and oocyte nucleus diameter was increased in mice treated with GH (P=0.0001, Table 1). Nuclei diameters from oocytes of primordial, in transition and primary follicles were increased in bGH compared to normal mice (P<0.05, Table 2). Secondary and tertiary follicles oocyte diameter was higher in bGH than normal mice (P<0.05, Table 2).
Table 1.
Nuclei, oocyte and follicle diameters of primordial (n=18/group), in transition (n=18/group), primary (n=18/group), secondary (n=18/group) and tertiary (n=18/group) follicles in 16 months old Ames Dwarf and Normal mice treated with GH or saline solution for 6 weeks. Data is presented as average ± standard error of the mean.
| Normal | Ames dwarf | P value | |||||
|---|---|---|---|---|---|---|---|
| Diameter (µm) | Saline | GH | Saline | GH | Genotype | Treatment | Genot*Treat |
| Primordial follicle | |||||||
| Nucleus | 3.2 (±0.2) | 2.8 (±0.2) | 2.9 (±0.1) | 3.0 (±0.1) | 0.77 | 0.44 | 0.15 |
| Oocyte | 6.4 (±0.4) | 5.2 (±0.2) | 5.7 (±0.3) | 5.6 (±0.3) | 0.54 | 0.04* | 0.07 |
| Follicle | 9.6 (±0.8) | 7.8 (±0.3) | 8.3 (±0.3) | 8.0 (±0.4) | 0.25 | 0.03* | 0.14 |
| In transition follicle | |||||||
| Nucleus | 3.1 (±0.1) | 3.2 (±0.2) | 3.1 (±0.1) | 2.8 (±0.1) | 0.17 | 0.29 | 0.10 |
| Oocyte | 6.5 (±0.3) | 6.3 (±0.3) | 6.3 (±0.3) | 6.4 (±0.3) | 0.71 | 0.83 | 0.59 |
| Follicle | 10.4 (±0.4) | 10.0 (±0.4) | 9.7 (±0.4) | 9.9 (±0.4) | 0.29 | 0.79 | 0.44 |
| Primary follicle | |||||||
| Nucleus | 3.4 (±0.2) | 3.8 (±0.3) | 3.2 (±0.2) | 3.6 (±0.3) | 0.36 | 0.08 | 0.77 |
| Oocyte | 10.6 (±1.0) | 10.8 (±0.8) | 9.6 (±0.7) | 10.2 (±0.9) | 0.34 | 0.63 | 0.76 |
| Follicle | 17.3 (±1.5) | 16.3 (±1.1) | 15.7 (±0.9) | 15.6 (±1.2) | 0.32 | 0.65 | 0.70 |
| Secondary follicle | |||||||
| Nucleus | 6.1 (±0.5) | 5.9 (±0.4) | 4.8 (±0.4) | 6.0 (±0.3) | 0.12 | 0.19 | 0.10 |
| Oocyte | 25.7 (±0.9) | 24.4 (±1.0) | 23.3 (±0.9) | 22.9 (±0.7) | 0.03* | 0.33 | 0.62 |
| Follicle | 50.2 (±3.2) | 52.8 (±3.6) | 56.8 (±4.9) | 55.9 (±5.7) | 0.29 | 0.88 | 0.72 |
| Tertiary follicle | |||||||
| Nucleus | 5.4 (±0.3) | 6.3 (±0.3) | 4.1 (±0.5) | 6.6 (±0.5) | 0.21 | 0.0001* | 0.21 |
| Oocyte | 45.6 (±15.2) | 30.2 (±0.9) | 29.8 (±1.1) | 31.0 (±1.5) | 0.40 | 0.42 | 0.35 |
| Follicle | 150.8 (±9.0) | 170.7 (±7.3) | 129.1 (±6.7) | 132.1 (±10.2) | 0.001* | 0.19 | 0.33 |
Significant P value – P<0.05
Table 2.
Nuclei, oocyte and follicle diameters of primordial (n=18/group), in transition (n=18/group), primary (n=18/group), secondary (n=18/group) and tertiary (n=18/group) follicles in 12 months old bovine growth hormone transgenic (bGH) and normal mice. Data is presented as average ± standard error of the mean.
| Diameter (µm) | Normal | bGH | P value |
|---|---|---|---|
| Primordial follicle | |||
| Nucleus | 2.6 (±0.1) | 3.1 (±0.1) | 0.03* |
| Oocyte | 5.7 (±0.3) | 5.7 (±0.2) | 0.89 |
| Follicle | 8.6 (±0.3) | 11.4 (±3.3) | 0.40 |
| In Transition follicle | |||
| Nucleus | 2.9 (±0.1) | 3.4 (±0.1) | 0.01* |
| Oocyte | 6.2 (±0.2) | 6.3 (±0.3) | 0.69 |
| Follicle | 8.9 (±0.2) | 9.4 (±0.3) | 0.21 |
| Primary follicle | |||
| Nucleus | 3.3 (±0.1) | 4.1 (±0.2) | 0.009* |
| Oocyte | 8.5 (±0.4) | 10.6 (±0.6) | 0.007* |
| Follicle | 14.6 (±0.5) | 16.4 (±0.7) | 0.03* |
| Secondary follicle | |||
| Nucleus | 5.8 (±0.4) | 5.5 (±0.3) | 0.53 |
| Oocyte | 25.2 (±0.8) | 22.1 (±0.9) | 0.01* |
| Follicle | 54.8 (±3.7) | 53.8 (±4.1) | 0.85 |
| Tertiary follicle | |||
| Nucleus | 6.8 (±0.3) | 6.2 (±0.3) | 0.17 |
| Oocyte | 31.3 (±1.1) | 32.6 (±1.0) | 0.35 |
| Follicle | 172.6 (±4.3) | 164.7 (±5.7) | 0.28 |
Significant P value – P<0.05
4. Discussion
The current study points to the GH/IGF-I axis as a central player in regulating the female reproductive lifespan. The results show that df/df mice have a greater number of primordial follicles than N/df mice, while bGH mice have a reduced number of primordial follicles in comparison to normal mice. Additionally, the treatment of both df/df and N/df mice with exogenous GH enhanced the depletion of the primordial follicle reserve. Therefore, these results demonstrate that the GH/IGF-I axis via its downstream effectors can regulate activation of the ovarian primordial follicle reserve, confirming the close association between somatic aging and reproductive lifespan.
The number of primordial and in transition follicles was higher, while the number of secondary follicles was lower, in df/df compared to N/df mice. In contrast, bGH mice had a lower number of primordial and in transition follicles, with a higher number of secondary and tertiary follicles, compared to N mice. Collectively these data indicate that follicles are trapped in the primordial stage, not being activated and therefore not progressing to the secondary stage due to GH/IGF-I deficiency. No previous record of ovarian follicular quantification in df/df and bGH mice was found in the literature, showing that the data from the present study are unique and provide novel evidence for these mice as models for the study of ovarian aging. This is important, since other mouse models for ovarian aging (like the knockouts of PTEN and FoxO3) have complete ovarian failure early after puberty (Castrillon et al., 2003; John et al., 2008; Reddy et al., 2009) and do not allow for comparative studies at adult life as the df/df and bGH mice. It was previously demonstrated that young female GHRKO mice have an increased primordial follicle reserve (Slot et al., 2006). The difference observed in the GH deficient mice in the present study was even bigger than observed for GHRKO before and this can be due the fact we used much older mice. Still, the comparison is important since previous studies have shown that not all characteristics are shared between df/df and GHRKO mice (Al-Regaiey et al., 2005). In addition, rats subjected to caloric restriction also have increased primordial follicle reserve compared to ad libitum fed rats, which is associated with reduced levels of circulating insulin and IGF-I (Li et al., 2011). Therefore, our findings are in agreement with these previous models, indicating that alterations in the GH/IGF-I axis that increase or decrease longevity also affect the reproductive lifespan.
The treatment of adult df/df and N/df mice with GH was able to activate primordial follicle growth, since we observed a decrease in the number of primordial follicles in both genotypes. Interestingly, the reduction in the number of primordial follicles was followed by increased number of secondary follicles in df/df mice, but not in N/df mice. This can indicate that while GH is stimulating follicle progression and continuous growth in df/df mice, it may be stimulating higher follicular atresia in N/df mice, since they do not continue to grow to further stages. Previous studies found that the number of primordial follicles was reduced in younger GHRKO mice treated with IGF-I for two weeks (Slot et al., 2006). Together, this evidence suggest that low levels of GH/IGF-I may cause the accumulation of primordial follicles, extending the longevity of the ovarian reserve, but as its levels are increased even at older ages, the activation of primordial follies is restored. It should be noted that both df/df and bGH mice have reduced pituitary secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH) (Chandrashekar and Bartke, 1996; Tang et al., 1993). The transition of follicles from the primordial to primary stages is gonadotropin independent (Scaramuzzi et al., 2011), however prolactin deficiency can reduce primordial follicle activation (Ormandy et al., 1997). Therefore, our results should be interpreted carefully as in rodents porcine GH can bind to both GH and prolactin receptors (Amit et al., 1992) and have lactotropic and somatotropic effects in these GH/prolactin deficient df/df mice. On the other hand, bGH has purely somatogenic effects (Amit et al., 1992; Chandrashekar and Bartke, 2003), but the sustained increase of GH in the transgenic bGH mice results in higher serum levels of prolactin (Chandrashekar and Bartke, 1996), which can also affect the rate of primordial follicle activation.
The presence of FoxO3a in its non-phosphorylated form is crucial to maintaining the primordial follicles in their quiescent state (John et al., 2008). Primordial follicles begin to grow irreversibly when oocyte FoxO3a is phosphorylated by stimulus from the surrounding granulosa cells (John et al., 2007; John et al., 2008; Zhang et al., 2014). Therefore, the higher level of non-phosphorylated FoxO3a observed in primordial follicles oocytes may be an explaination why follicles remain for longer periods in the primordial stage in df/df mice. In addition, GH treatment reduced FoxO3a protein level, while it increased its phosphorylated form in primordial follicles oocytes. Again, this evidence suggests that GH treatment is activating the primordial follicle pool by promoting increased phosphorylation of the FoxO3a transcription factor. We also observed that df/df mice had increased pFoxO3a level in primordial follicle oocytes, but this increase was proportional to the increase in FoxO3a total protein. In a previous study we observed that pFoxO3a protein levels in primordial/primary follicles were lower in 12 mo old df/df compared to N/df mice, despite no difference in total FoxO3a protein levels (Schneider et al., 2014), which can suggest an age-dependent FoxO3a regulation in the ovary. FoxO3a and pFoxO3a were not different in primordial follicles of bGH and normal mice. However, the level of pFoxO3a was higher in primary follicles oocytes of bGH than normal mice, which could be related to the higher rate of primordial follicle activation and depletion observed in bGH mice.
Bowen and Atwood (2004) postulated the Reproductive-Cell Cycle Theory of Aging, which states that the hormones that regulate reproduction act in an antagonistic pleiotrophic manner to control aging via cell cycle signaling, with many suggested pathways that can be implicated is this trade-off (Atwood and Bowen, 2011). Additionally, it has been shown before that lower GH/IGF levels correlate with increased lifespan and delayed sexual maturation in different strains of mice, which also indicates the existence of a tradeoff between growth rate, sexual maturation and longevity (Yuan et al., 2012; Yuan et al., 2015). The FoxO transcription factor can be a central player in this regulation, since its increased activation is linked to accelerated aging in several model organisms (Kenyon, 2010). The nuclear presence of the non-phosphorylated FoxO is associated with activation of pathways related to cell self-preservation, maintenance of the quiescent state, stress resistance, maintenance of the stem cell pool and tumor suppression (Salih and Brunet, 2008). Based on this previous evidence and our current results, we can suggest that the modulation of the GH/IGF-I axis and its downstream effector FoxO3a can have a central role in the regulation of the trade-off between the somatic and reproductive aging in mice. Therefore, accelerated aging is linked to higher GH/IGF-I levels, increased primordial oocyte FoxO3a activation and faster depletion of follicular reserves. The opposite situation is observed in mice with reduced levels of circulating GH/IGF-I.
It is well established that the growth of primordial, in transition and secondary follicles is directly linked to the growth of the oocyte and its nucleus (Lintern-Moore and Moore, 1979), with an intense cross-talk between the oocyte and its surrounding granulosa cells (Sobinoff et al., 2013; Zhang and Liu, 2015). Granulosa cells secreted factors can activate the Pi3k/FoxO3a pathway in the primordial follicle oocyte and initiate follicle growth (Zhang et al., 2014; Zhang and Liu, 2015). Our results show that oocytes from primordial, in transition and primary follicles of bGH mice had increased nuclei diameter. Growth of oocyte nuclei is associated with increased gene expression activity (Moore and Lintern-Moore, 1974), thus indicating that the oocyte is awakening the machinery necessary for its growth and differentiation. In addition, primary and secondary follicles oocyte diameter was increased in bGH mice. On the other hand, df/df mice tended to have smaller secondary follicle oocyte diameter, although GH treatment decreased primordial follicles oocyte size in both df/df and N/df mice. Overall this may be an indication that higher GH levels are associated with increased oocyte transcriptional activity, which would lead to early activation of oocyte and follicular growth, as was observed for bGH mice. It is possible that FoxO3a activation is an important regulator of oocyte growth, since the FoxO3a knockout mice also have a rapid increase in primordial follicle oocyte diameter, which is followed by premature ovarian failure due to exhaustion of the follicular reserve (Castrillon et al., 2003). The same association between increased primordial follicle oocyte size and early activation of the ovarian reserve was observed for the Pten knockout mice (Reddy et al., 2008).Thus, we can hypothesize that there may be a relationship between the activation of oocyte FoxO3a signaling, leading to oocyte growth and awakening of quiescent follicles in the reserve.
5. Conclusion
GH-deficient df/df female mice had an increased ovarian primordial follicle reserve in comparison to N/df mice, and GH treatment in adulthood was able to activate primordial follicle growth and reduce the size of the reserve in both df/df and N/df mice. The opposite was observed in transgenic mice overexpressing bGH, which had accelerated ovarian aging and a decreased ovarian primordial follicle reserve. Overall, this indicates that the size of the ovarian primordial follicle reserve at older ages can be a direct reflection of the circulating levels of GH/IGF-I, and that even transient changes in GH/IGF-I levels after maturation can alter the rate of follicle depletion and ovarian aging. Our study also has shown that FoxO3a phosphorylation is increased by GH and is associated with decreased primordial follicle reserve, possibly linking changes in the GH/IGF-I axis to the rate of ovarian aging.
Supplementary Material
Body weight of Ames dwarf (df/df) and Normal (N) mice before the beginning of growth hormone (GH) or saline (Sal) treatment and after six weeks of treatment. Mice were 14 months-old at the beginning of treatment.
Highlights.
GH deficient df/df mice had increased, while transgenic mice overexpressing GH had decreased ovarian primordial follicle reserve;
GH treatment at adult age can reduce the primordial follicle reserve in both df/df and N/df mice;
Reduced ovarian reserve is associated to increased presence of pFoxO3a in oocytes enclosed in primordial follicles.
Acknowledgments
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This work was also supported by the National Institute on Aging (NIA) (grant numbers AG031736, AG032290, AG19899).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors disclose no competing interests.
References
- Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- Amit T, Hochberg Z, Waters MJ, Barkey RJ. Growth hormone- and prolactin-binding proteins in mammalian serum. Endocrinology. 1992;131:1793–1803. doi: 10.1210/endo.131.4.1396325. [DOI] [PubMed] [Google Scholar]
- Andersen B, Pearse RV, 2nd, Jenne K, Sornson M, Lin SC, Bartke A, Rosenfeld MG. The Ames dwarf gene is required for Pit-1 gene activation. Dev Biol. 1995;172:495–503. doi: 10.1006/dbio.1995.8040. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Bowen RL. The reproductive-cell cycle theory of aging: an update. Exp Gerontol. 2011;46:100–107. doi: 10.1016/j.exger.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Bachelot A, Monget P, Imbert-Bollore P, Coshigano K, Kopchick JJ, Kelly PA, Binart N. Growth hormone is required for ovarian follicular growth. Endocrinology. 2002;143:4104–4112. doi: 10.1210/en.2002-220087. [DOI] [PubMed] [Google Scholar]
- Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7:285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- Blackburn A, Dressendorfer RA, Blum WF, Erhard M, Brem G, Strasburger CJ, Wolf E. Interactions of insulin-like growth factor (IGF)-II and growth hormone in vivo: circulating levels of IGF-I and IGF-binding proteins in transgenic mice. Eur J Endocrinol. 1997;137:701–708. doi: 10.1530/eje.0.1370701. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bowen RL, Atwood CS. Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology. 2004;50:265–290. doi: 10.1159/000079125. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Bartke A. Induction of endogenous insulin-like growth factor-I secretion alters the hypothalamic-pituitary-testicular function in growth hormone-deficient adult dwarf mice. Biol Reprod. 1993;48:544–551. doi: 10.1095/biolreprod48.3.544. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Bartke A. Influence of hypothalamus and ovary on pituitary function in transgenic mice expressing the bovine growth hormone gene and in growth hormone-deficient Ames dwarf mice. Biol Reprod. 1996;54:1002–1008. doi: 10.1095/biolreprod54.5.1002. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Bartke A. The role of insulin-like growth factor-I in neuroendocrine function and the consequent effects on sexual maturation: inferences from animal models. Reprod Biol. 2003;3:7–28. [PubMed] [Google Scholar]
- Chandrashekar V, Zaczek D, Bartke A. The consequences of altered somatotropic system on reproduction. Biol Reprod. 2004;71:17–27. doi: 10.1095/biolreprod.103.027060. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- Gesing A, Al-Regaiey KA, Bartke A, Masternak MM. Growth hormone abolishes beneficial effects of calorie restriction in long-lived Ames dwarf mice. Exp Gerontol. 2014;58:219–229. doi: 10.1016/j.exger.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Relationship between the supply of primordial follicles and the onset of follicular growth in rats. Biol Reprod. 1994;50:421–428. doi: 10.1095/biolreprod50.2.421. [DOI] [PubMed] [Google Scholar]
- John GB, Shirley LJ, Gallardo TD, Castrillon DH. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction. 2007;133:855–863. doi: 10.1530/REP-06-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147:3228–3234. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- Li L, Fu YC, Xu JJ, Chen XC, Lin XH, Luo LL. Caloric restriction promotes the reproductive capacity of female rats via modulating the level of insulin-like growth factor-1 (IGF-1) Gen Comp Endocrinol. 2011;174:232–237. doi: 10.1016/j.ygcen.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Li L, Fu YC, Xu JJ, Lin XH, Chen XC, Zhang XM, Luo LL. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of Mammalian target of rapamycin signaling. Reprod Sci. 2015;22:60–67. doi: 10.1177/1933719114542016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintern-Moore S, Moore GP. The initiation of follicle and oocyte growth in the mouse ovary. Biol Reprod. 1979;20:773–778. doi: 10.1095/biolreprod20.4.773. [DOI] [PubMed] [Google Scholar]
- Louis A, Bartke A, Masternak MM. Effects of growth hormone and thyroxine replacement therapy on insulin signaling in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65:344–352. doi: 10.1093/gerona/glq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Panici JA, Wang F, Wang Z, Spong A. The effects of growth hormone (GH) treatment on GH and insulin/IGF-1 signaling in long-lived Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65:24–30. doi: 10.1093/gerona/glp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquet JG, Sotelo AI, Dominici FP, Bonkowski MS, Bartke A, Turyn D. Increased sensitivity to GH in liver of Ames dwarf (Prop1df/Prop1df) mice related to diminished CIS abundance. J Endocrinol. 2005;187:387–397. doi: 10.1677/joe.1.06001. [DOI] [PubMed] [Google Scholar]
- Moore GP, Lintern-Moore S. A correlation between growth and RNA synthesis in the mouse oocyte. J Reprod Fertil. 1974;39:163–166. doi: 10.1530/jrf.0.0390163. [DOI] [PubMed] [Google Scholar]
- Moreira F, Corcini CD, Mondadori RG, Gevehr-Fernandes C, Mendes FF, Araujo EG, Lucia T., Jr Leptin and mitogen-activated protein kinase (MAPK) in oocytes of sows and gilts. Anim Reprod Sci. 2013;139:89–94. doi: 10.1016/j.anireprosci.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramuzzi RJ, Baird DT, Campbell BK, Driancourt MA, Dupont J, Fortune JE, Gilchrist RB, Martin GB, McNatty KP, McNeilly AS, Monget P, Monniaux D, Vinoles C, Webb R. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev. 2011;23:444–467. doi: 10.1071/RD09161. [DOI] [PubMed] [Google Scholar]
- Schneider A, Zhi X, Moreira F, Lucia T, Jr, Mondadori RG, Masternak MM. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res. 2014;7:120. doi: 10.1186/s13048-014-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot KA, Kastelijn J, Bachelot A, Kelly PA, Binart N, Teerds KJ. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction. 2006;131:525–532. doi: 10.1530/rep.1.00946. [DOI] [PubMed] [Google Scholar]
- Sluczanowska-Glabowska S, Laszczynska M, Piotrowska K, Glabowski W, Kopchick JJ, Bartke A, Kucia M, Ratajczak MZ. Morphology of ovaries in laron dwarf mice, with low circulating plasma levels of insulin-like growth factor-1 (IGF-1), and in bovine GH-transgenic mice, with high circulating plasma levels of IGF-1. J Ovarian Res. 2012;5:18. doi: 10.1186/1757-2215-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluczanowska-Glabowska S, Laszczyn Ska M, Piotrowska K, W GB, Rumianowski B, Masternak M, Arum O, Kucia M, Kopchick JJ, Bartke A, Ratajczak MZ. The effect of calorie restriction on the presence of apoptotic ovarian cells in normal wild type mice and low-plasma-IGF-1 Laron dwarf mice. J Ovarian Res. 2013;6:67. doi: 10.1186/1757-2215-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobinoff AP, Sutherland JM, McLaughlin EA. Intracellular signalling during female gametogenesis. Mol Hum Reprod. 2013;19:265–278. doi: 10.1093/molehr/gas065. [DOI] [PubMed] [Google Scholar]
- Tang K, Bartke A, Gardiner CS, Wagner TE, Yun JS. Gonadotropin secretion, synthesis, and gene expression in human growth hormone transgenic mice and in Ames dwarf mice. Endocrinology. 1993;132:2518–2524. doi: 10.1210/endo.132.6.8504754. [DOI] [PubMed] [Google Scholar]
- te Velde ER, Scheffer GJ, Dorland M, Broekmans FJ, Fauser BC. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol. 1998;145:67–73. doi: 10.1016/s0303-7207(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Wolf E, Kahnt E, Ehrlein J, Hermanns W, Brem G, Wanke R. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev. 1993;68:71–87. doi: 10.1016/0047-6374(93)90141-d. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Xu J, Li L, Lin X, Chen X, Zhang X, Fu Y, Luo L. Calorie restriction increases primordial follicle reserve in mature female chemotherapy-treated rats. Gene. 2012;493:77–82. doi: 10.1016/j.gene.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Yuan R, Meng Q, Nautiyal J, Flurkey K, Tsaih SW, Krier R, Parker MG, Harrison DE, Paigen B. Genetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strains. Proc Natl Acad Sci U S A. 2012;109:8224–8229. doi: 10.1073/pnas.1121113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Gatti DM, Krier R, Malay E, Schultz D, Peters LL, Churchill GA, Harrison DE, Paigen B. Genetic Regulation of Female Sexual Maturation and Longevity Through Circulating IGF1. J Gerontol A Biol Sci Med Sci. 2015;70:817–826. doi: 10.1093/gerona/glu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaczek D, Hammond J, Suen L, Wandji S, Service D, Bartke A, Chandrashekar V, Coschigano K, Kopchick J. Impact of growth hormone resistance on female reproductive function: new insights from growth hormone receptor knockout mice. Biol Reprod. 2002;67:1115–1124. doi: 10.1095/biolreprod67.4.1115. [DOI] [PubMed] [Google Scholar]
- Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, Bosbach B, Brannstrom M, Liu K. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–2508. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Human Reproduction Update. 2015 doi: 10.1093/humupd/dmv037. dmv037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Body weight of Ames dwarf (df/df) and Normal (N) mice before the beginning of growth hormone (GH) or saline (Sal) treatment and after six weeks of treatment. Mice were 14 months-old at the beginning of treatment.