Abstract
Recent paleoanthropological discoveries reveal a diverse, potentially speciose human fossil record. Such extensive morphological diversity results from the action of divergent evolutionary forces on an evolving lineage. Here, we apply quantitative evolutionary theory to test whether random evolutionary processes alone can explain the morphological diversity seen among fossil australopith and early Homo crania from the Plio–Pleistocene. We show that although selection may have played an important role in diversifying hominin facial morphology in the late Pliocene, this is not the case during the early evolution of the genus Homo, where genetic drift was probably the primary force responsible for facial diversification.
Keywords: craniofacial biology, early hominins, evolutionary processes, Hominoidea, morphological diversification
Although there is much disagreement about the taxonomic status and phylogenetic relationships among early fossil hominins, the profusion of recent paleoanthropological discoveries (1–7) has led to a growing consensus that the record of human evolution is considerably more diverse than previously recognized. Such extensive phenotypic diversity suggests a high magnitude and rate of morphological change within the hominin clade, which results from complex genetic and developmental underpinnings (8, 9). Clearly, evolutionary processes have shaped this rich phenotypic diversity, although exactly which forces are involved is an unresolved question. Because substantial environmental changes associated with increasing aridity occurred in Africa toward the end of the Pliocene (10, 11), most explanations for diversity during this time, and especially those associated with the emergence of Homo and the demise of the robust australopiths, are mired in adaptationist perspectives, explaining much, if not most, morphological variation as adaptation to changing climate and environment (12), and hence primarily the result of diversifying selection. Here, we test hypotheses of evolutionary diversification in the human fossil record: specifically, whether the facial diversity seen among late Pliocene and early Pleistocene hominins could be explained by genetic drift alone, or whether nonrandom forces like selection played an important role (see ref. 13).
We compared hominoid within-group facial variation with hominin between-group patterns of variation across seven fossil taxa, to test whether these between-group and within-group patterns are proportional. Our quantitative approach is grounded in principles of evolutionary theory developed by Lande (14). Evolutionary forces depend on intraspecific variation as fuel for population diversification, and therefore morphological change through time is rooted in an understanding of population level variation and covariation (14). If populations have diversified through random evolutionary processes such as genetic drift, evolutionary theory predicts a proportional relationship between within-group and between-group phenotypic variation (14–16); a nonproportional relationship indicates the action of nonrandom processes such as differential selection. Although this methodological approach has been used successfully to look at drift versus selection in living New World monkeys (13, 17, 18), it has never been applied directly to the fossil record of human evolution. Such questions have, however, been addressed in other fossil primates (19) and modern humans (20, 21). An important way in which this approach differs from other methods is that it focuses on the relative amounts of variance (V) across traits (i.e., the pattern of variation) rather than the total V, allowing us to evaluate populations with substantial time depth; in drifting populations sampled at different points in time the magnitude of variation would be expected to differ among the populations but the pattern would remain consistent.
Materials and Methods
This analysis takes place at various levels in the hominin hierarchy to pinpoint where in the lineage random versus nonrandom processes were acting (see Fig. 1). Each analyzed hierarchical level contains at least three populations. Because of the inherently small sample sizes, individual fossil specimens are taken to represent population means; whether these populations represent different species or are time-successive taxa should not affect the analysis, as this approach has been shown to work at different levels in a phylogeny (13, 17, 18). As the fossil record does not allow for a direct estimate of fossil intraspecific variation, extant variation in humans and our two closest living relatives (chimps and gorillas) is substituted for within-population variation of fossil species (see below). It is important to emphasize that it is the pattern of variation (not the magnitude) that is used in this analysis, mitigating problems caused by differences in population structure, relic status, etc.
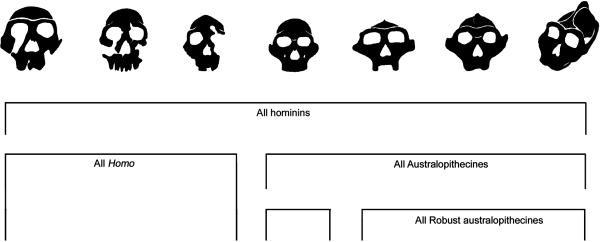
Fig. 1.
Analyzed hierarchical groups shown are all hominins, all Homo, all australopiths, and all robust australopiths. Fossil specimens depicted from left to right are African H. erectus (KNM-ER 3733), H. habilis (or H. rudolfensis: KNM-ER 1470), H. habilis (KNM-ER 1813), A. africanus (Sts 5), A (P.) robustus (SK 48), A. (P.) boisei (KNM-ER 406), and A. (P.) aethiopicus (KNM-WT 17000).
Sample Structure. The fossil specimens KNM-ER 1470, KNM-ER 1813, and KNM-ER 3733 represent adult Homo habilis (sometimes designated Homo rudolfensis), Homo habilis sensu stricto, and African Homo erectus, respectively, all from Koobi Fora, Kenya. Fossils KNM-ER 406, KNM-WT 17000, and SK 48 all are colloquially called “robust australopiths” and represent adult members of the species Australopithecus (Paranthropus) boisei (Kenya), A. (P.) aethiopicus (Kenya), and A. (P.) robustus (South Africa), respectively. Sts 5 is an adult gracile australopith, A. africanus, from Sterkfontein, South Africa. For all of these analyses, it is important to note that although individual fossils are taken here as representative of their species, the large amount of interspecific variation relative to the level of intraspecific variation makes the analyses robust, despite the paucity of fossils. Extant cranial material consists of cross-sectional samples of adult Homo sapiens (n = 141), Gorilla gorilla (n = 115), and Pan troglodytes (n = 65). Adult sample sizes consist of roughly equal numbers of male and female individuals.
Data Acquisition. The data set comprises eight Euclidean distances, derived from 3D coordinates of seven unilateral and midline facial landmarks that were reliably locatable on all seven fossil specimens (Table 1). All landmarks are based on sutural morphology, representing homologous structures across species. The choice of variables was dictated by the shared preservation of the fossil specimens, which restricted analysis to the face, and limited the number of landmarks. The number and distribution of landmarks are sufficient for identifying significant differences among the adult extant individuals. All analyses are done with raw data to evaluate both size and shape change.
Table 1. Landmarks recorded from crania by using a 3D digitizer.
| Landmark | Name | Description |
|---|---|---|
| NA | Nasion | Point where nasal-frontal suture intersects with suture dividing left and right nasal bones |
| NSL | Nasale | Most inferior point of suture dividing left and right nasal bones |
| ANS | Anterior nasal spine | Point marking the intersection of the nasal margin with the maxillary suture |
| IS | Intradentale superior | Most inferior and anterior point of maxillary suture lying between upper medial incisors |
| FMN | Frontal-maxillary-nasal suture | Point where nasal, frontal, and maxillary bones meet |
| ZS | Zygomaxillare superior | Intersection of zygo-maxillary suture and orbital border |
| FM | Fronto-malare | Intersection of frontal-zygomatic suture and orbital border |
Interlandmark distances were drawn from these landmarks as follows: NA-NSL, NA-FM, NSL-ANS, NSL-ZS, ANS-IS, ANS-ZS, FMN-ZS, ZS-FM.
Estimating Population Variation. Using patterns of variation in living populations to interpret past ones is a common approach to assessing fossil diversity. However, the assumption of constancy in covariation patterning is problematic. Although a number of studies have shown that there are many common aspects to morphological variation and integration in the primate cranium, and especially in the face (13, 22–27), it is also true that there are differences. To conservatively account for the possible effects of small differences in covariance (CV) structure (22, 28), we use three extant models rather than just one, as we realize that patterns of variation are not homogenous (although they are similar; see ref. 22) across these extant groups and assume that they would not be homogenous across the fossil populations either. Here, we do assume that the range of fossil variation patterns is encompassed within the range of the modern hominoids.
Human, chimp, and gorilla intraspecific patterns of variation serve as models for hominin within-population variability. Phenotypic within-population V/CV matrices for the facial variables from all three living primate populations were obtained by using the residual CV matrix from a multiple ANOVA with the eight traits as the dependent variables and subspecies as the independent variable, thus pooling the CV across subspecies, and were then simplified to their principal components (PCs). The PCs of the within-population V/CV matrix are ordered by their level of V (eigenvalues) and are uncorrelated with one another so that on the scale of the PCs, the within-population V/CV matrix is a simple diagonal matrix with no CVs among components. PC scores are calculated for each fossil population by multiplying trait means by the standardized within-population PC loadings. The between-population V for each PC can then be calculated as the V among these population mean PC scores; these values are given in Table 2 along with the within-population Vs (eigenvalues) for each extant model.
Table 2. Within- and between-population variances.
| Population | Extant | All | Homo | Australopiths | Robusts |
|---|---|---|---|---|---|
| Chimpanzee V/CV | 0.351 | 0.633 | 1.295 | 0.401 | 0.133 |
| 0.163 | 0.348 | 0.230 | 0.155 | 0.231 | |
| 0.145 | 0.212 | 0.396 | 0.084 | 0.033 | |
| 0.080 | 0.138 | 0.023 | 0.252 | 0.239 | |
| 0.055 | 0.156 | 0.060 | 0.221 | 0.048 | |
| 0.043 | 0.524 | 1.027 | 0.241 | 0.323 | |
| 0.022 | 0.209 | 0.028 | 0.284 | 0.426 | |
| 0.009 | 0.031 | 0.020 | 0.042 | 0.035 | |
| Gorilla V/CV | 1.349 | 0.824 | 1.568 | 0.603 | 0.130 |
| 0.333 | 0.233 | 0.001 | 0.180 | 0.244 | |
| 0.234 | 0.015 | 0.012 | 0.010 | 0.007 | |
| 0.126 | 0.154 | 0.235 | 0.087 | 0.071 | |
| 0.101 | 0.540 | 0.938 | 0.275 | 0.394 | |
| 0.068 | 0.287 | 0.087 | 0.373 | 0.559 | |
| 0.053 | 0.137 | 0.203 | 0.111 | 0.022 | |
| 0.008 | 0.062 | 0.036 | 0.042 | 0.042 | |
| Human V/CV | 0.258 | 1.013 | 1.861 | 0.665 | 0.356 |
| 0.131 | 0.253 | 0.243 | 0.133 | 0.167 | |
| 0.118 | 0.204 | 0.093 | 0.157 | 0.221 | |
| 0.085 | 0.096 | 0.066 | 0.072 | 0.008 | |
| 0.046 | 0.243 | 0.497 | 0.098 | 0.006 | |
| 0.037 | 0.217 | 0.268 | 0.254 | 0.314 | |
| 0.019 | 0.025 | 0.032 | 0.027 | 0.018 | |
| 0.008 | 0.202 | 0.019 | 0.274 | 0.380 |
The within-population variance (eigenvalues) for each of the three extant model populations is shown in the first column. The following columns give the between-population variances for each analysis, calculated under each extant model. Before performing regression analysis, these values were multiplied by 107 and then logged to the base 10.
Testing Hypothesis of Genetic Drift. Lande's (15) quantitative model for understanding the relationship between morphological change and variation/covariation under genetic drift is shown by the equation
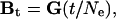 |
where Bt is the between-population V/CV matrix (dispersion matrix), in generation t, G is the additive genetic V/CV matrix of the founding population from which the group of species is derived, and Ne is the effective population size of the individual taxa (14–16). Because the phenotypic within-group V/CV matrix (W) has been shown to be proportional to the additive genetic V/CV matrix for morphological traits (29–32), it may be substituted for it, resulting in
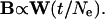 |
Ne and t are constants for any particular comparison, and therefore the expected pattern of between-group phenotypic variation should be proportional to the within-group phenotypic variation (B ∝ W), if the populations have diversified by random evolutionary processes. Similarly, if these patterns of variation are not proportional, other modes of evolutionary phenotypic divergence, such as differential selection, may have been at work. On a logarithmic scale, this equation can be written as a linear regression with
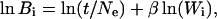 |
where Bi is the between-population V and Wi is the within-population V for the ith eigenvector. If differentiation was produced by genetic drift, we expect a regression slope (β) of 1.0 for the regression of between- on within-population V. A significant deviation from a slope of 1.0 indicates a pattern not likely to have been produced by genetic drift; nonsignificant or no deviation from 1.0 means we have failed to reject the null hypothesis of drift, leaving other nonrandom evolutionary processes such as selection as an alternative. Under a strictly neutral evolutionary model, increasing divergence time also will increase the dispersion among groups and consequently the regression constant (t/Ne), but this does not alter the expectation of 1.0 for the regression slope. Regression slopes >1.0 indicate that one or more of the first PCs are more variable, relative to other PCs, than expected under a model of drift, whereas slopes <1.0 indicate that populations are relatively highly divergent among minor PCs. For example, a positive deviation of the PC1 V would indicate that for PC1 the variation between populations is more than expected, given the variation within populations. Such deviations can occur via diversifying selection of the relatively variable PCs (in this example PC1) or stabilizing selection on the relatively invariable PCs. The very presence of even a single substantial outlier is evidence that the distribution of variation among taxa is not consistent with drift as the diversifying source. By design, the fewer taxa compared, the harder it is to reject drift (i.e., to detect deviations from a slope of 1.0). Because of this factor, plus the small sample sizes and the uncertainty surrounding which extant model V/CV would best represent variation in the fossil populations being compared, it is likely that any sign of significant selection under any single model will indicate selection.
Reconstructing Selection. If selection has acted to differentiate two populations, evolutionary theory provides an approach for reconstructing the selection necessary to produce the differences in observed population means by using the following relationship:
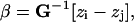 |
where β is the differential selection gradient summed over the generations (33), G–1 is the inverse of the pooled within-species genetic V/CV matrix, and [zi – zj] is the difference in means between species i and j, here, the fossil hominin individuals (14, 16). Again, the phenotypic within-group V/CV matrix is substituted for the genetic V/CV matrix. Because we know that V/CV structures are not strictly constant through time this is again done by using all three models of extant V. For this particular calculation, it is important to note that if there are differences between the fossil CV structure and that of the extant models these values may be inaccurate. Therefore, these vectors are considered accurate for each analysis only if all three models are in general agreement and should be interpreted only as a guide to the general pattern of selection and not its precise magnitude.
Results and Discussion
Results of all four analyses indicate that in many instances these early hominins are too variable in some features of the face (and not variable enough in others) for divergence to have occurred through random drift alone; however, some of the phenotypic diversity is consistent with random evolutionary processes. The regressions of logged between-group variation against logged within-group variation are shown in Table 3; of the four levels analyzed, one is consistent with drift, one is borderline, and two require selection.
Table 3. Results of regression of between-group on within-group variance as a test for genetic drift.
| Group | Extant model | Consistent with drift? | Slope | R2 | P value |
|---|---|---|---|---|---|
| All hominins | Chimpanzee | Yes | 0.59 | 0.54 | 0.11 |
| Gorilla | Maybe | 0.31 | 0.14 | 0.07 | |
| Human | Yes | 0.51 | 0.31 | 0.18 | |
| Genus Homo | Chimpanzee | Yes | 1.00 | 0.46 | 1.00 |
| Gorilla | Yes | 0.14 | 0.01 | 0.25 | |
| Human | Yes | 0.99 | 0.54 | 0.98 | |
| All australopiths | Chimpanzee | No | 0.30 | 0.22 | 0.02 |
| Gorilla | Maybe | 0.30 | 0.12 | 0.09 | |
| Human | Maybe | 0.28 | 0.10 | 0.07 | |
| Robust australopiths | Chimpanzee | No | 0.07 | 0.01 | 0.04 |
| Gorilla | Maybe | 0.14 | 0.02 | 0.08 | |
| Human | Yes | 0.14 | 0.01 | 0.23 |
In the first analysis, with all seven hominin specimens considered together, the results are somewhat ambiguous; while drift cannot be rejected outright as a cause of fossil variation the borderline significance indicates some effect of selection. This result suggests that both random and to a lesser extent nonrandom processes played an important role in the diversification of this morphologically diverse group; it does not necessarily mean that both played a role across all parts of the group. By inspecting the residuals, we can examine the reasons for this pattern, as negative or positive deviations of PCs from a slope of 1.0 indicate more or less between-group variation, respectively, than expected (see Materials and Methods). For all seven specimens together, the slope is <1.0, indicating that the first few eigen-vectors may have relatively too little between-fossil (species) variation compared with the smaller eigenvectors for divergence to be caused by drift alone.
Next, we look at subdivisions of these seven fossils. First, among the three Homo specimens, the variation is generally consistent with expectations caused by drift regardless of which extant V/CV matrix is used. That the support of a drift model is particularly strong when a human V/CV matrix is used further corroborates this hypothesis, as both fossils and the extant group come from the same genus. It is also strong when a chimpanzee model is used, and less so with the gorilla model. Next, looking at the four australopith individuals, we find that the first few eigenvectors have too little between-fossil variation, whereas the lesser components have too much, indicating differences that do not fit a drift model, and suggesting diversifying selection among the australopiths. Although this model is only strongly supported when V/CV matrices of chimpanzees are used, its acceptance seems reasonable because australopiths are generally considered to follow an ape-like morphological pattern and because the other analyses are borderline significant. When the one gracile australopith is removed from the analysis, the variation pattern among the robust australopiths remains generally consistent with selection, as supported most strongly by the analyses using ape (and especially the chimpanzee) V/CV matrices.
To further examine the nature of the selection that may have acted to diversify Homo from the australopiths, robust from gracile australopiths, and robust australopiths from each other, we reconstructed the selection necessary to produce the observed differences in fossil morphology (see Materials and Methods and Table 4). Mean vectors of all variables were calculated for the following five groups (where appropriate): Homo, gracile australopiths (i.e., Sts 5), robust australopiths, early robust australopiths (A. aethiopicus), and later robust australopiths. Assuming that early Homo and robust australopiths are more derived hominins than the gracile australopiths (this assumption is consistent with our understanding of the phylogenetic directionality among these groups), and that later robust australopiths are more derived than early ones, we estimate the selection required to produce: (i) Homo from a gracile australopith, (ii) a robust australopith from a gracile one, and (iii) a later robust australopith from an earlier one. For each of the three comparisons the vectors are similar regardless of which living V/CV estimate is used. Yet there are some important differences between the selection vectors among the three comparisons. The selection required to produce Homo from a gracile australopith is relatively strong to moderately positive in the upper face and orbit, moderately positive to null in the midface/nasal region, and relatively weakly negative along the lower orbits and zygomatics (see Fig. 2 and Table 4). The selection required to produce a later robust australopith from an earlier one is similar, albeit more strongly negative along the lower orbits and zygomatics. However, that required to produce a robust australopith from a gracile one differs considerably, being relatively strongly positive in the lateral orbital/zygomatic region, and weakly negative to null in the rest of the face.
Table 4. Standardized reconstructed differential selection vectors describing the selection needed to produce the Homo face from the gracile australopith face, the robust australopith face from the gracile australopith face, and a later robust australopith face from an earlier one.
| Nasal NA—NSL | Orbit NA—FM | Nasal NSL—ANS | Nasal NSL—ZS | Oral ANS—IS | Nasal ANS—ZS | Orbit FMN—ZS | Orbit/Zyg ZS—FM | |
|---|---|---|---|---|---|---|---|---|
| Homo-Gracile | ||||||||
| Difference vector | -0.37 | 1.30 | 0.82 | 0.77 | 0.35 | 0.35 | 0.37 | 0.28 |
| vc | -1.36 | 20.53 | 28.94 | 20.70 | -2.76 | -21.40 | -4.75 | 5.55 |
| vh | -0.30 | 29.38 | 9.69 | 2.77 | -2.18 | -13.47 | -2.86 | -13.37 |
| vg | -2.77 | 5.57 | 35.54 | 38.54 | -4.13 | -37.43 | -2.81 | 3.97 |
| Robust-Gracile | ||||||||
| Difference vector | 0.37 | 0.94 | 0.43 | 0.74 | 0.41 | 0.62 | -0.12 | 0.68 |
| vc | -0.65 | 6.93 | 16.43 | 22.85 | -1.79 | -11.05 | -8.92 | 15.30 |
| vh | -6.20 | -1.37 | -7.27 | 20.10 | 0.86 | 9.51 | -16.51 | 30.14 |
| vg | -1.79 | 3.53 | 14.52 | 20.70 | -3.06 | -17.67 | -4.14 | 7.77 |
| Boisei/Robustus-Aethiopicus | ||||||||
| Difference vector | 0.37 | 0.46 | -0.56 | -0.05 | -0.46 | -0.33 | -0.73 | -1.24 |
| vc | 10.52 | 30.22 | -10.51 | -17.54 | 0.56 | 3.47 | -11.22 | -49.88 |
| vh | 27.29 | 62.82 | 24.26 | -16.32 | -4.63 | -42.76 | -5.72 | -100.93 |
| vg | 4.96 | 10.94 | -11.93 | -7.92 | -2.74 | 5.75 | -4.65 | -17.26 |
Selection is relative, strongly negative and strongly positive selection are shown in bold and underlined, respectively. For each comparison, the difference vector between the two groups is given, as is the selection vector required to produce that difference, based on chimpanzee (vc), human (vh), and gorilla (vg) V/CV matrices.
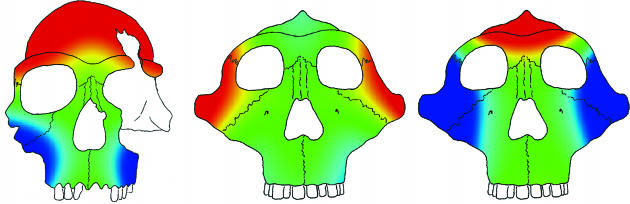
Fig. 2.
A visual representation of the selection vectors necessary to produce observed differences in facial morphology is shown. (Left) Selection required to produce a Homo from a gracile australopith. (Center) Selection required to produce a robust australopith from a gracile one. (Right) Selection required to produce a later robust australopith from an earlier one. Images shown are based on a chimp V/CV model. Selection is relative, with red indicating strongly positive selection, green no selection, and blue strongly negative selection.
The vast majority of explanations for facial diversity in the hominin fossil record are adaptive (34–36), despite wide acceptance that morphological change can be nonadaptive (37) and that other evolutionary processes or underlying developmental or functional differences can generate morphological diversity among populations providing a mechanism for evolutionary change. The results of these analyses suggest a more complex evolutionary scenario (Fig. 3). Certainly the unique, derived facial morphology of robust australopiths may have been selected for early in the evolutionary history of the lineage, perhaps driving the differentiation between them and the other gracile australopiths such as A. africanus, and selection continued to shape the differentiation within this lineage after the divergence of the robust clade from other australopiths. However, although the initial divergence of Homo from the australopiths may have involved selection, divergence after this time (at least in the facial characters analyzed) could have occurred through random processes alone. In other words, much of the facial diversity seen in the Homo lineage from ≈2.5 million to 1 million years may result from random evolutionary processes, rather than adaptive evolution. Other studies have shown that craniofacial diversity in most populations of modern humans can be explained by random processes (20, 21). Lynch (20) suggests that the development of cultural inheritance could have released many of the morphological traits of humans from the pressures of stabilizing selection. This study supports this idea and supplies it with temporal context, potentially providing direct biological evidence of a shift early on in this lineage toward nonbiological adaptation (i.e., culture) as early hominins increasingly relied on technology. Because drift tends to play a larger role in shaping diversity when populations are finite, these results also may reflect a demographic revolution toward increasingly isolated and widespread populations. Although this hypothesis would be consistent with the fossil record, which indicates widespread geographic dispersal of the genus Homo during the late Pliocene or early Pleistocene (4, 38–40), it remains to be tested with larger samples drawn from more disparate regions.
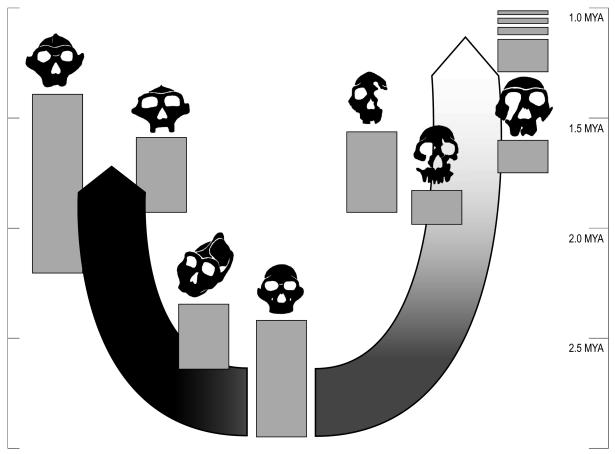
Fig. 3.
Evolutionary forces and diversity in early human evolution are shown in a temporal context. The arrows represent the action of selection and genetic drift, shown in black and white, respectively.
When selection has shaped diversity, it acts in a manner that is consistent with many current explanations of craniofacial diversity in the hominin lineage during this time period. For example, the relatively positive selection in the orbits and upper face necessary to produce Homo from a gracile australopith may be correlated with increasing endocranial volume, tied to increasing brain size and related shape changes. Similarly, the relatively positive selection in the lateral face and zygomatic region necessary to produce a robust australopith from a gracile one may be correlated to size and shape changes associated with the unique masticatory adaptation of robusts. It is also interesting that the selection necessary to produce Homo from a gracile australopith and later robust australopiths from earlier ones is similar. This congruence may offer one explanation for the morphological similarities between robust australopith and Homo faces (12), that these similarities result from similar response to a similar evolutionary pressure and are therefore homoplastic. Only further analyses with more fossils can finally answer the question of whether drift alone explains the variation we see throughout the Homo lineage; this is a promising avenue for future research.
Acknowledgments
Many thanks to those individuals and institutions who provided access to skeletal material, both extant and extinct: M. Leakey (National Museums of Kenya), F. Thackeray (Transvaal Museum, Northern Flagship Institution), P. V. Tobias and K. Kuykendall (University of the Witwatersrand), D. R. Hunt, D. Owsley, and R. Thorington (National Museum of Natural History), B. Latimer and L. Jellema (Cleveland Museum of Natural History), J. Haas (Field Museum of Natural History), and W. van Neer (Musée Royal de l'Afrique Centrale). We especially single out the National Museums of Kenya and the Transvaal Museum (Northern Flagship Institution) for their generosity in allowing access to fossil hominins. The manuscript was improved by comments from S. Athreya, G. Conroy, D. T. Rasmussen, R. Smith, E. Trinkaus, and two anonymous reviewers. Different aspects of this research were supported by the National Research Foundation of South Africa (R.R.A.), the National Science Foundation (R.R.A.), and the L. S. B. Leakey Foundation (R.R.A.).
Author contributions: R.R.A. and J.M.C. designed research; R.R.A. performed research; R.R.A. and J.M.C. analyzed data; and R.R.A. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: V, variance; CV, covariance; PC, principal component.
References
- 1.Asfaw, B., White, T., Lovejoy, O., Latimer, B., Simpson, S. & Suwa, G. (1999) Science 284, 629–635. [DOI] [PubMed] [Google Scholar]
- 2.White, T. D., Asfaw, B., DeGusta, D., Gilbert, H., Richards, G. D., Suwa, G. & Howell, F. C. (2003) Nature 423, 742–747. [DOI] [PubMed] [Google Scholar]
- 3.Haile-Selassie, Y. (2001) Nature 412, 178–181. [DOI] [PubMed] [Google Scholar]
- 4.Vekua, A., Lordkipanidze, D., Rightmire, G. P., Agusti, J., Ferring, R., Maisuradze, G., Mouskhelishvili, A., Nioradze, M., Ponce de Leon, M., Tappen, M., et al. (2002) Science 297, 85–89. [DOI] [PubMed] [Google Scholar]
- 5.Leakey, M. G., Spoor, F., Brown, F. H., Gathogo, P. N., Kiarie, C., Leakey, L. N. & McDougall, I. (2001) Nature 410, 433–440. [DOI] [PubMed] [Google Scholar]
- 6.Brunet, M., Guy, F., Pilbeam, D., Mackaye, H. T., Likius, A., Ahounta, D., Beauvilain, A., Blondel, C., Bocherens, H., Boisserie, J., et al. (2002) Nature 418, 145–151. [DOI] [PubMed] [Google Scholar]
- 7.Senut, B., Pickford, M., Gommery, D., Mein, P., Cheboi, K. & Coppens, Y. (2001) Earth Planetary Sci. 332, 137–144. [Google Scholar]
- 8.Rogers, J., Mahaney, M. C., Almasy, L., Comuzzie, A. G. & Blangero, J. (1999) Yearbook Phys. Anthropol. 42, 127–152. [DOI] [PubMed] [Google Scholar]
- 9.Carroll, S. B. (2003) Nature 422, 849–857. [DOI] [PubMed] [Google Scholar]
- 10.deMenocal, P. B. & Bloemendal, J. (1995) in Paleoclimate and Evolution with Emphasis on Human Origins, eds. Vrba, E. S., Denton, G. H., Partridge, T. C. & Burckle, L. H. (Yale Univ. Press, New Haven, CT), pp. 262–288.
- 11.Shackleton, N. J. (1995) in Paleoclimate and Evolution with Emphasis on Human Origins, eds. Vrba, E. S., Denton, G. H., Partridge, T. C. & Burckle, L. H. (Yale Univ. Press, New Haven, CT), pp. 242–248.
- 12.Klein, R. G. (1999) The Human Career (Univ. of Chicago Press, Chicago).
- 13.Ackermann, R. R. & Cheverud, J. M. (2002) Am. J. Phys. Anthropol. 117, 260–271. [DOI] [PubMed] [Google Scholar]
- 14.Lande, R. (1979) Evolution (Lawrence, Kans.) 33, 402–416. [Google Scholar]
- 15.Lande, R. (1980) Am. Nat. 116, 463–479. [Google Scholar]
- 16.Lofsvold, D. (1988) Evolution (Lawrence, Kans.) 42, 54–67. [DOI] [PubMed] [Google Scholar]
- 17.Marriog, G. & Cheverud, J. M. (2004) Am. Nat. 163, 417–428. [DOI] [PubMed] [Google Scholar]
- 18.Marriog, G., De Vivo, M. & Cheverud, J. M. (2004) J. Evol. Biol. 17, 144–155. [DOI] [PubMed] [Google Scholar]
- 19.Clyde, W. C. & Gingerich, P. D. (1994) Paleobiology 20, 506–522. [Google Scholar]
- 20.Lynch, M. (1990) Am. Nat. 136, 727–741. [Google Scholar]
- 21.Roseman, C. C. (2004) Proc. Natl. Acad. Sci. USA 101, 12824–12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackermann, R. R. (2002) J. Hum. Evol. 43, 167–187. [DOI] [PubMed] [Google Scholar]
- 23.Ackermann, R. R. & Cheverud, J. M. (2000) Am. J. Phys. Anthropol. 111, 489–501. [DOI] [PubMed] [Google Scholar]
- 24.Ackermann, R. R. & Cheverud, J. M. (2004) in Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes, eds. Pigliucci, M. & Preston, K. (Oxford Univ. Press, Oxford), pp. 302–319.
- 25.Cheverud, J. M. (1995) Am. Nat. 145, 63–89. [Google Scholar]
- 26.Cheverud, J. M. (1996) J. Evol. Biol. 9, 5–42. [Google Scholar]
- 27.Marroig, G. & Cheverud, J. M. (2001) Evolution (Lawrence, Kans.) 55, 2576–6000. [DOI] [PubMed] [Google Scholar]
- 28.Ackermann, R. R. (2003) S. Afr. J. Sci. 99, 255–258. [Google Scholar]
- 29.Cheverud, J. M. (1988) Evolution (Lawrence, Kans.) 42, 958–968. [DOI] [PubMed] [Google Scholar]
- 30.Roff, D. A. (1995) Heredity 74, 481–490. [Google Scholar]
- 31.Roff, D. A. (1996) Evolution (Lawrence, Kans.) 50, 1392–1403. [DOI] [PubMed] [Google Scholar]
- 32.Koots, K. R. & Gibson, J. P. (1996) Genetics 143, 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lande, R. & Arnold, S. J. (1983) Evolution (Lawrence, Kans.) 37, 1210–1226. [DOI] [PubMed] [Google Scholar]
- 34.Rak, Y. (1983) The Australopithecine Face (Academic, New York).
- 35.Grine, F. E. (1986) J. Hum. Evol. 15, 783–822. [Google Scholar]
- 36.Kay, R. F. & Grine, F. E. (1988) in Evolutionary History of the Robust Australopithecines, ed. Grine, F. E. (de Gruyter, New York), pp. 427–448.
- 37.Gould, S. J. & Lewontin, R. C. (1979) Proc. R. Soc. London 205, 581–598. [DOI] [PubMed] [Google Scholar]
- 38.Gabunia, L., Vekua, A., Lordkipanidze, D., Swisher, C. C., III, Ferring, R., Justus, A., Nioradze, M., Tvalchrelidze, M., Antón, S. C., Bosinski, G., et al. (2000) Science 288, 1019–1025. [DOI] [PubMed] [Google Scholar]
- 39.Antón, S. C. (2003) Yrbk. Phys. Anthropol. 46, 126–170. [DOI] [PubMed] [Google Scholar]
- 40.Antón, S. C. & Swisher, C. C. I. (2004) Annu. Rev. Anthropol. 33, 271–296. [Google Scholar]