Abstract
In the pursuit of sustainable agriculture through environment and human health friendly practices, we evaluated the potential of a novel gibberellins (GAs) producing basidiomycetous endophytic fungus Porostereum spadiceum AGH786, for alleviating salt stress and promoting health benefits of soybean. Soybean seedlings exposed to different levels of NaCl stress (70 and 140 mM) under greenhouse conditions, were inoculated with the AGH786 strain. Levels of phytohormones including GAs, JA and ABA, and isoflavones were compared in control and the inoculated seedlings to understand the mechanism through which the stress is alleviated. Gibberellins producing endophytic fungi have been vital for promoting plant growth under normal and stress conditions. We report P. spadiceum AGH786 as the ever first GAs producing basidiomycetous fungus capable of producing six types of GAs. In comparison to the so for most efficient GAs producing Gibberella fujikuroi, AGH786 produced significantly higher amount of the bioactive GA3. Salt-stressed phenotype of soybean seedlings was characterized by low content of GAs and high amount of ABA and JA with reduced shoot length, biomass, leaf area, chlorophyll contents, and rate of photosynthesis. Mitigation of salt stress by AGH786 was always accompanied by high GAs, and low ABA and JA, suggesting that this endophytic fungus reduces the effect of salinity by modulating endogenous phytohormones of the seedlings. Additionally, this strain also enhanced the endogenous level of two isoflavones including daidzen and genistein in soybean seedlings under normal as well as salt stress conditions as compared to their respective controls. P. spadiceum AGH786 boosted the NaCl stress tolerance and growth in soybean, by modulating seedlings endogenous phytohormones and isoflavones suggesting a valuable contribution of this potent fungal biofertilizer in sustainable agriculture in salt affected soils.
Keywords: endophytic fungi, Porostereum spadiceum, abscisic acid, gibberellins, isoflavonoids, soybean, salinity and biofertilization
Introduction
Plant–microbe interactions are among the vital processes that are not only essential for the survival of both the partners but also important in functioning of agricultural system. Among the plant interacting microbes, endophytic fungi have gained marvelous attention of plant biologists due to their mutualistic interactions with the host crops. Much of the attention has been focused on phytostimulation under environmental stress by these organisms in order to devise the most suitable biofertilizers (Baltruschat et al., 2008; Miransari, 2011). Plants hosting such beneficial fungi undergo significant changes in their key physiological aspects, including phytohormones balance, root exudation, composition of mineral nutrients, and physical modification in soil (Richardson, 2009; Kusari et al., 2013). These changes enable host plant to withstand different abiotic and biotic stresses which ultimately enhance their overall fitness (Chandanie et al., 2006; Khan et al., 2009).
An important stress among abiotic stresses is salinity, as it is responsible for diverting plant metabolism to deposit organic solutes so as to maintain turgor pressure. Such osmolytes including proline have been shown to function as osmoprotectants (Munns and Tester, 2008). Plants exposed to salinity have to cope with osmotic and ionic stresses to which they respond by induction of a defense response. Salt stressed plants are characterized by aggregation of compatible osmolytes, which induce additional water uptake in plant root from surrounding, to buffer the immediate effect within plant cell (Bartels, 2005; Misra and Gupta, 2005; Verbruggen and Hermans, 2008). In leguminous plants including soybean, salinity is correlated with poor yield and reduction in plant growth (Dinler et al., 2014). The severity of the situation is enhanced when salt stress interferes with the symbiotic association of rhizobia and roots of legumes (Dardanelli et al., 2010; Mhadhbi et al., 2014). Fungal endophytes may be of special significance because of their ability to colonize legumes and help their partner to survive under extreme environmental conditions by secreting beneficial secondary metabolites.
Soybean [Glycine max (L.) Merril] is an important legume cultivated worldwide for its oil and protein rich grains. The crop has been known as a rich source of beneficial endophytic fungi improving its fitness to withstand environmental stresses including salinity (Pimentel et al., 2006; Khan et al., 2011). About 187 species of fungal endophytes have been reported from different parts of soybean (Fernandes et al., 2015) which belong to different taxonomic groups including Ascomycota and Basidiomycota (de Souza Leite et al., 2013). Soybean leaves were comparatively richer in fungal endophytes than its roots.
Isoflavones are important class of compounds having twofold importance, acting as signals thereby mediating root interaction with fungi. Additionally, isoflavones may improve survival of soybean by acting as antimicrobial agents (Dakora and Phillips, 1996; Messina, 2010). Isoflavones have useful implications in human health as they decrease risks of cancers (breast, colon, and prostate), premature menopause and cardiovascular disorders (Anderson and Keifer, 1997; Allred et al., 2004; Phommalth et al., 2008). Information on the modulation of soybean endogenous isoflavone and proline content by phytostimulant fungi has been scarce. We preferred isoflavones determination in salt affected soybean because they act as important regulators of root nodulation, symbiotic interactions and plant defense thereby favoring plant to withstand environmental pressure. Silencing of soybean biosynthesis gene has been shown to increase susceptibility to Phytophthora sojae (Subramanian et al., 2005). Additionally, isoflavones are greatly valued in human health. Because of these properties, selection or development of soybean cultivars with higher isoflavone content has been focused in the past (Phommalth et al., 2008). Similarly, in planta GAs enhancement by fungal endophytes may promote the activity of phenyl alanine ammonia lyase, one of the key enzyme in isoflavone biosynthesis pathway, thereby enhancing synthesis of isoflavones by plant itself (Barnes and Jones, 1984).
Likewise, in stressed plants, endogenous level of phytohormones has been widely investigated, even then the role of endophytic fungi in alleviation of biotic and abiotic stresses by modulating phytohormones has been least explored. Similarly, the ability of endophytic fungi to produce GAs in pure culture and promote plant growth has widely been reported (Hamayun et al., 2009; Khan et al., 2009, 2011). However, use of endophytic fungi to recover plants from the damaging effects of salinity is limited to a few works (Waller et al., 2005; Khan et al., 2012). Most of the plant growth promoting endophytic fungi belong to the group of sac fungi known as Ascomycota (Khan et al., 2009; Zhou et al., 2015). However, members of club fungi (Basidiomycota) has also been shown to exist as endophytes in plant tissues and promote growth by different mechanisms (Waller et al., 2005). Among several mechanisms of phytostimulation is direct growth promotion by the secretion of phytohormones including gibberellins (Hamayun et al., 2009; Khan et al., 2009). However, GAs and other phytohormones may also modulate plant physiology making them resistant to different environmental stresses (Waqas et al., 2012, 2015). Several members of Ascomycota has been known to live as plant endophytes and promote plant growth by producing GAs (Ansari et al., 2015; Leitão and Enguita, 2016). However, to date none of the basidiomycetous fungal endophyte has been reported to produce GAs. Current study was proposed to evaluate the phytostimulatory potential of a novel GAs producing endophyte member of Basidiomycota, Porostereum spadiceum AGH786, and its ability to modulate endogenous contents of GAs, ABA, JA, and isoflavones.
Materials and Methods
Isolation of Endophytic Fungi from Soybean Roots
For isolation of endophytes, 27 days old soybean (cv. Hwangkeumkong), grown in 5.5 L plastic pots under greenhouse conditions were collected. The plants were grown in horticultural soil composed of peat moss (13–18%), perlite (7–11%), coco-peat (63–68%) and zeolite (6–8%), while the macro-nutrients were present as follows: NH4+∼90 mg/L; NO3-∼205 mg/L; P2O5∼350 mg/L, and K2O∼100 mg/L. The pH was neutral (pH 7).
To isolate endophytic fungi, roots of the selected plants were washed with running tap water and treated with Tween 80 solution. Thoroughly, washed roots of 10 soybean plants were cut to obtained 10 segments (0.5 cm) per plant making a total of 100 root segments which were then surface sterilized in perchloric acid (Khan et al., 2008). After surface sterilization, the sections were imprinted on Hagam medium plates to check their sterility (Berg et al., 2005). After incubation of 7 days, the sterile root segments were subjected to endophytes isolation procedure. The sterilized root sections were then injured by pressing against glass slide to expose internal tissues and inoculated on agar plates containing Hagem medium (5 pieces/plate) supplemented with streptomycin (for avoiding bacterial contamination). The plates were then incubated at 25°C till fungal colonies emerged from the plant sections (Khan et al., 2009). After the emergence of fungal spots, subculturing was done by shifting distinctly different fungal spots on to Hagem and PDA medium plates and allowing them to grow for 7 days at the above mentioned temperature. Subculturing was repeated until the establishment of pure cultures which were stored on PDA slants under mineral oil at -20°C.
Screening the Endophytic Fungi for Beneficial Traits of Plant Growth Promotion
Low GAs dwarf cultivar of rice (Waito-c rice) was used to screen the isolated fungal strains for GAs production and Phytostimulation. Rural Development Administration (RDA) Korea kindly provided seeds of Waito-c. Fungal cultures were grown in broth culture containing Czapek medium for 7 days at 30°C and 120 rpm. Fungal biomass was separated from culture medium by centrifugation (10000 rpm) at 4°C for 15 min. Culture supernatant and fungal biomass were separately lyophilized. Pellet containing fungal biomass was used to identify fungal isolates and the lyophilized supernatant was resuspended in sterilized distilled water making its volume to 1 mL. Surface sterilized healthy seeds of Gas deficient rice were dipped in 20 ppm uniconazol solution for 24 h. The seedlings were grown in 0.8% water-agar medium under axenic conditions in growth chamber (photoperiod of 14 h light and 10 h dark; light intensity 1000 μmm-2s-2 Natrium lamps and relative humidity 60–70%). Fungal culture supernatant (10 μL) was applied on the apices of seedlings at 2-leaf stage. Seedlings treated with distilled water and supernatant of Gibberella fujikuroi culture were used as negative and positive controls respectively. Seedlings were harvested 1 week post-supernatant treatment and different growth parameters were noted.
Fungal Identification and Phylogenetic Analysis
Molecular approach was adopted to identify the selected fungal strain AGH786. Protocol of Khan et al. (2008) was followed to isolate fungal genomic DNA and carry out PCR for the amplification of internally transcribed region of 18S rRNA gene using primers ITS-1 and ITS-4 (Khan et al., 2008). The ITS regions was sequenced and the obtained sequence was subjected to BLASTn1 for sequence homology estimation. The sequences obtained as a result of homology search, transformed to maximum parsimony tree through MEGA 4 (Tamura et al., 2007).
Screening Isolates for Halotolerance
Porostereum spadiceum AGH786 was grown in Czapek broth supplemented with different concentrations (50–500 mM) of salt (NaCl). After incubation of 7 days in shaking flasks, fungal mycelia were filtered out and subjected to fresh and dry weight determination. Czapek medium composed of standard recipe (Krzyśko-Lupicka et al., 1997) was used as control.
Soybean–endophytes Interaction
Surface sterilized seeds of soybean (Glycine max L. Hwangkeum) were grown in sterilized pots (15 L) containing autoclaved (once) horticultural soil (composition described above) and maintained axenically (Misra and Dwivedi, 2004). Pots were kept for 3 weeks under conditions mentioned above in randomized complete block design. The seedlings received two treatments with each treatment having two levels constituting four different sets of seedlings, e.g., seedlings receiving neither salt nor endophytic association (NEA), seedlings receiving both endophytic association and salt stresses (EAS), and seedlings with either endophytic association (EA) and or salt stress (NEAS) only.
To prepare inoculum of fungal strains, mycelium was harvested by centrifugation at 5000 × g and 4°C for 15 min from 7 days old fungal culture grown in Czapek broth (250 mL) at 30°C. To each pot, soil mixed with 50 mg of crushed fungal mycelium was added and the pots were kept in controlled environment for 3 days under conditions mentioned elsewhere to acclimatize fungal isolate.
Twenty one days old soybean seedlings were exposed to moderate (70 mM) and high (140 mM) NaCl stress. Seedlings were watered with 1400 mL of salt solution or distilled water (control) for 7 days.
Analysis of Plant Growth Attributes
Soybean seedlings were harvested to investigate shoot length and biomass. Chlorophyll was estimated through chlorophyll meter (SPAD-502 Minolta, Japan) and rate of photosynthesis of the selected leaves was also measured (ADC Bioscientific LCi analyzer; Model31655 UK). Area of mature leaves were measured with Laser Leaf Area meter (CI-203 model, CID, Inc., USA).
Determination of Phytohormones
Phytohormones including GAs were determined in fungal culture filtrate collected after growing AGH786 for 7 days in Czapek broth culture under conditions mentioned above. To know the endogenous status of phytohormones in seedlings, endogenous GAs, ABA, and JA were determined in the seedlings exposed to different treatments.
Determination of GAs
To determine fungal GAs, 30 mL of culture filtrate was used to extract GAs according to Khan et al. (2009). For determining endogenous GAs of soybean, seedlings were crushed under liquid nitrogen to fine powder. Gibberellins were extracted and quantified by using 0.5 g fine powder. Instrument used for the determination of GA was gas chromatograph (Hewlett-Packard 6890, 5973N mass selective detector) with HA-1 capillary column (30 m × 0.25 mm i.d. 0.25 μm film thickness) and oven with a range of temperature, i.e., initial 60°C for 1 min, elevated to 200°C at the rate of 15°C min-1, then to 285°C at the rate of 5°C min-1). The carrier gas (helium) was maintained under a pressure of 30 kPa. Gas chromatography and mass selective detector were directly connected with 280°C temperature of furnace, with ionizing voltage and dwell time of 70 eV and 100 ms respectively. Full scan mode (the first trial) and three major ions of supplemented [2H2], GAs internal standards (the second trial) and the endogenous gibberellins were monitored simultaneously. The amount of endogenous GA4, GA9, and GA12 (ng/g of dry weight) were calculated from the peak area ratios of 284/286, 298/300, and 300/360 respectively. The analysis was repeated two times.
Determination of ABA
The level of endogenous free ABA was determined in soybean shoots as described (Yoon et al., 2009). Soybean shoots (0.5 g) were ground to powder and then extracted with mixture (30 mL) composed of isopropanol and glacial acetic acid in 19:1 and 100 ng of [2H6]-ABA as internal standard. After filtration, the solvent was evaporated from the filtrate at reduced pressure using rotary evaporator. Further purification was exactly similar as described previously (Yoon et al., 2009). The pure sample was derivitized by treatment with diazomethane and analyzed by GC–MS SIM (6890N network GC system, equipped with 5973 network mass selective detector; Agilent Technologies, Palo Alto, CA, USA). The Lab-Base (ThermoQuset, Manchester, UK) data system software was employed to monitor responses to ions of m/e 162 and 190 for Me-ABA and 166 and 194 for Me-[2H6]-ABA.
Determination of JA
To determine the contents of JA in soybean shoots, 0.1 g of fine powder was extracted with a mixture of 50 mM citric acid and acetone (30:70, v/v) containing 20 ng internal standard, [9, 10-2H2]Dihydro-JA. To get rid of the volatile fatty acids, the extracts were left overnight for evaporation at room temperature. The aqueous phase obtained was passed through filter and extracted thrice with 10 mL aliquots of diethyl ether. The three aliquots of diethyl ether were pooled and subjected to a solid phase extraction column composed of 500 mg of sorbent, aminopropyl. The column was then washed with a 7 mL mixture containing 2-propanol and trichloromethane in 2:1, v/v. The column was eluted with 10 mL mixture of acetic acid and diethyl ether in 98:2, v/v. From the eluate containing JA, the solvent was evaporated and to the residue excess of diazomethane was added to derivatize JA for analysis by GC/MS (6890N network GC system, and 5973 network mass selective detector; Agilent Technologies, Palo Alto, CA, USA). Sensitivity of the protocol was maximized by recording the spectra in the selected ion mode. Peak area produced by JA in the sample was compared with the standard to estimate its amounts (ng/g dry weight). Three different samples were used to repeat the analysis.
Isoflavone Content Analysis
Previously established protocols were followed for the isolation and determination of isoflavones in soybean seedlings (Rostagno et al., 2003; Phommalth et al., 2008). Briefly, 10 mL of 80% EtOH was mixed with 0.2 g of dry powder and kept at 50°C for 1 h in ultrasonic bath (Kodo, Co., Korea). Afterward, the samples were shaken at 50°C and 150 rpm for 15 h. Syringe filteration through 0.45 μm filters was done to make the sample debris free and the filtrate (10 μL) was then eluted from HPLC (PerkinElmer series 200, USA) column (C18 column 4.6 mm × 150 mm; 5 μm) using gradient solutions viz. 0.1% of acetic acid and acetonitrile in deionized water keeping flow rate at 1.0 mL min-1 The UV detector was set to 260 nm for the detection of isoflavones in the eluant. Peak areas of the standards isoflavones genistein and daidzein (Sigma Chemical, Co, USA) were compared with that of the samples to estimate the quantity of the isoflavones.
Statistical Analysis
Experiments were repeated three times with exception of GA determination which was performed twice. Means were compared by performing one way analysis of variance (ANOVA) and Duncan multiple range test (DMRT) at p = 0.05 using SPSS for windows.
Results
Screening Bioassay for Isolated Strains
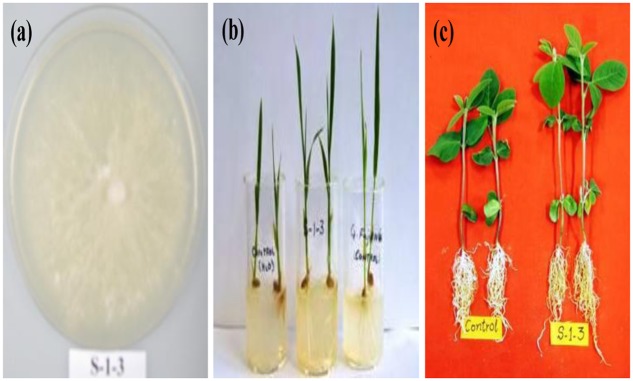
Isolated endophytes were screened for GAs production by applying their culture supernatant on Waito-c rice seedlings and their performance was compared with that of wild strain of G. fujikuroi. For this purpose, 10 μL of resuspended lyophilized supernatant from individual strains were applied on shoot apex of 10 days old seedlings. Growth attributes of Waito-c rice seedlings were analyzed 1 week after supernatants application. A total of 42 strains were initially isolated and tested for plant growth promotion. Assay on Waito-c rice showed that 34 strains were able to promote the growth attributes of soybean and eight strains were inhibitors (data not shown). Based upon its excellent performance in terms of growth promotion of Waito-c rice, S-1-3 was selected for further study (Figure 1). During further study, name of the strain was changed to AGH786.
FIGURE 1.
Seven days old colony of the endophytic fungus Porostereum spadiceum AGH786 (labeled as S-1-3) isolated from soybean plants on Czapek agar (a), which restored growth of wito-c rice (b middle flask) like Gibberella fujikuroi (b right flask) and enhanced growth of soybean seedlings (c) grown under axenic conditions for 3 weeks.
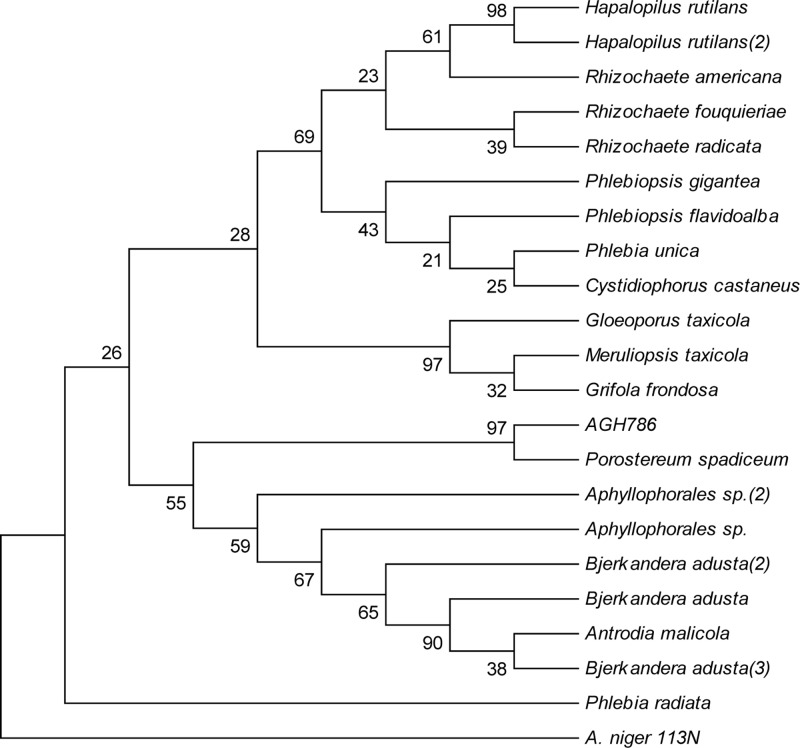
Identification of AGH786
Based upon BLAST results, sequence of 28S rDNA of AGH786 showed maximum homology (99%) with P. spadiceum. To confirm identity of the strain, its sequence was subjected to phylogenetic analysis (Khan et al., 2008). Phylogenetic consensus tree was constructed from 22 (21 reference and 1 clone) by using neighbor joining (NJ) method in MEGA 6 package (Figure 2). Our isolate AGH786 formed a clad with P. spadiceum supported by 99% boostrap value in the consensus tree. Combine results of sequence homology and phylogenetic analysis suggested that the strain AGH786 was P. spadiceum. Sequence of its 28S rDNA was submitted to GenBank under accession No. FJ808680.
FIGURE 2.
Phylogenetic tree constructed by Neighbor-Joining method showing the evolutionary history of AGH786. Ascomycetous Aspergillus niger 113N was used as outgroup.
Characterization of Endophyte AGH786
Halotolerance of AGH786
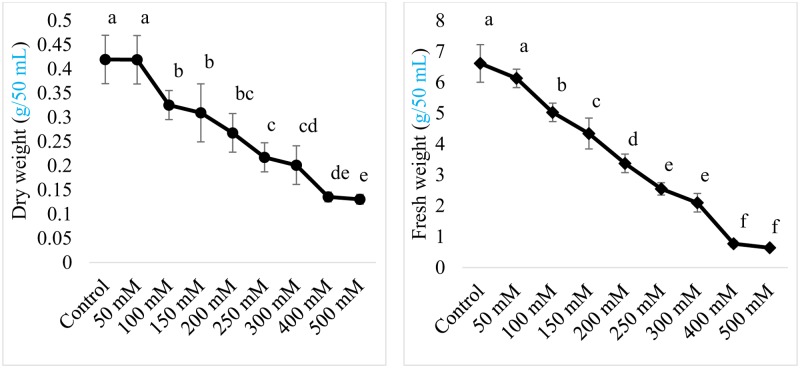
Halotolerance of the isolated endophyte AGH786 was tested by exposing the strain to different concentrations of NaCl. Current results showed that both fresh and dry weight of AGH786 was not affected by elevated NaCl stress (50 mM) showing its ability to tolerate salinity stress (Figure 3). However, further increase in salt concentration imposed significant reduction in growth of AGH786 when its fresh and dry biomass in salt supplemented and control media were compared (Figure 3). A strong negative correlation was recorded between salt and fresh weight (r = -0.979; p < 0.01) and also between salt concentration and dry weight (r = -0.969; p < 0.01). Increasing concentration of salt to 150 mM reduced fresh and dry fungal biomass by 29 and 26% respectively as compared to the fresh and dry weight of fungi grown in control media. Minimal inhibitory concentration of NaCl was around 250 mM, reducing fresh and dry weight of this fungus by 50%. Further increase was detrimental to fungus growth, reducing fresh biomass from 6.6 mg (in control media) to 0.64 mg (in media containing 500 mM NaCl). Decrease in dry biomass was less sharp as 500 mM NaCl reduced it by only threefold as compared to control (Figure 3).
FIGURE 3.
Effect of salt (NaCl) concentration on the fresh and dry weight of endophytic strain AGH786 of P. spadiceum. The strain was grown for 7 days in shaking flasks containing 50 mL Czapek culture broth provided different concentrations of NaCl. Bars show standard error of mean and data points labeled with different letters are significantly different (p < 0.05).
Free Amino Acids in Culture Filtrate of AGH786
Free amino acids in the rhizosphere are important source of nitrogen available to plants and they compete with microbes for obtaining free amino acids (Owen and Jones, 2001; Jones et al., 2005). Being important in plant growth, we observed whether this isolate can release free amino acids in the culture filtrate. Twenty seven different free amino acids were detected in the culture filtrate of this fungus (Table 1). Among different amino acids citrullin and sarcosine were the most abundant types. Hydroxylysine, tyrosin, cystein, glutamic acid, and aspartic acid were also released in handsome amount to the culture media.
Table 1.
Free amino acids determined in the culture filtrate of Porostereum spadiceum AGH786 grown in Czapek media for 7 days.
| S. no | Amino acid | Amount (μg mL-1) | S. no | Amino acid | Amount (μg mL-1) |
|---|---|---|---|---|---|
| 1 | Phosphoserine | 119.14 | 15 | Leucine | 253.48 |
| 2 | Urease | 7.8 | 16 | Tyrosine | 466.07 |
| 3 | Aspartic acid | 473.42 | 17 | Phenylalanine | 12.06 |
| 4 | Threonine | 283.46 | 18 | β-Amino isobutyric acid | 20.91 |
| 5 | Serine | 311.85 | 19 | γ-Amino-n-butyric acid | 21.06 |
| 6 | Glutamic acid | 402.63 | 20 | Ethanol amine | 3.94 |
| 7 | Sarcosine | 628.34 | 21 | Ammonia | 1.63 |
| 8 | α-Aminoadipic acid | 21.74 | 22 | Hydroxylysine | 588.22 |
| 9 | Glycine | 12.67 | 23 | 1-Methylhistidine | 38.36 |
| 10 | Alanine | 263.48 | 24 | Histidine | 160.42 |
| 11 | Citrulline | 664.83 | 25 | Anserine | 131.88 |
| 12 | α-Amino-n-butyric acid | 16.89 | 26 | Arginine | 8.44 |
| 13 | Cystine | 545.52 | 27 | Proline | 183.14 |
| 14 | Isoleucine | 8.51 |
Determination of GAs in the Culture Filtrate of AGH786
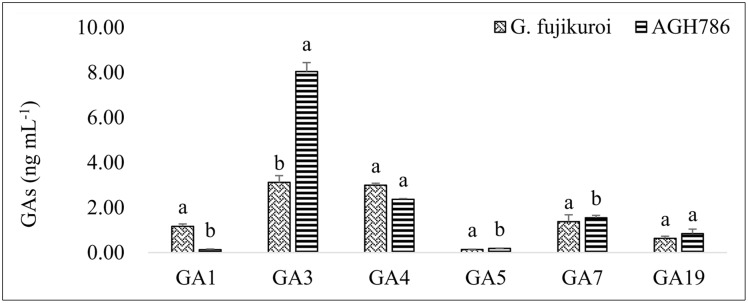
Production of bioactive GAs in the culture filtrate of fungal isolate AGH786 was determined qualitatively and quantitatively. We could detect six different types of bioactive GAs, including GA1, GA3, GA4, GA5, GA7, and GA19 (Figure 4). GA3 was the most abundant type of the GAs and its production by AGH786 was more than twofold greater than its amount produced by wild type G. fujikori. So far, this is the first fungal endophyte producing GA3 in greater amount than G. fujikori. However, the quantity of other GAs was either similar in both strains or greater in the filtrate of G. fujikuri than its amount in the culture filtrate of AGH786 (Figure 4). Bioactive GA3 and GA4 have been known to play exponential role in promoting plant growth (Eriksson et al., 2006) and higher growth of Waito-c rice seedlings by AGH786 supports our findings.
FIGURE 4.
Production of Gibbrellins (GAs) by P. spadiceum AGH786 and wild strain of G. fujikuroi. After 7 days of growth in Czapek broth, GAs were extracted from fungus culture filtrate and quantified through GC–MS/MS. Bars show standard error of mean and data points labeled with different letters are significantly different (p < 0.05).
Soybean–endophyte Interaction
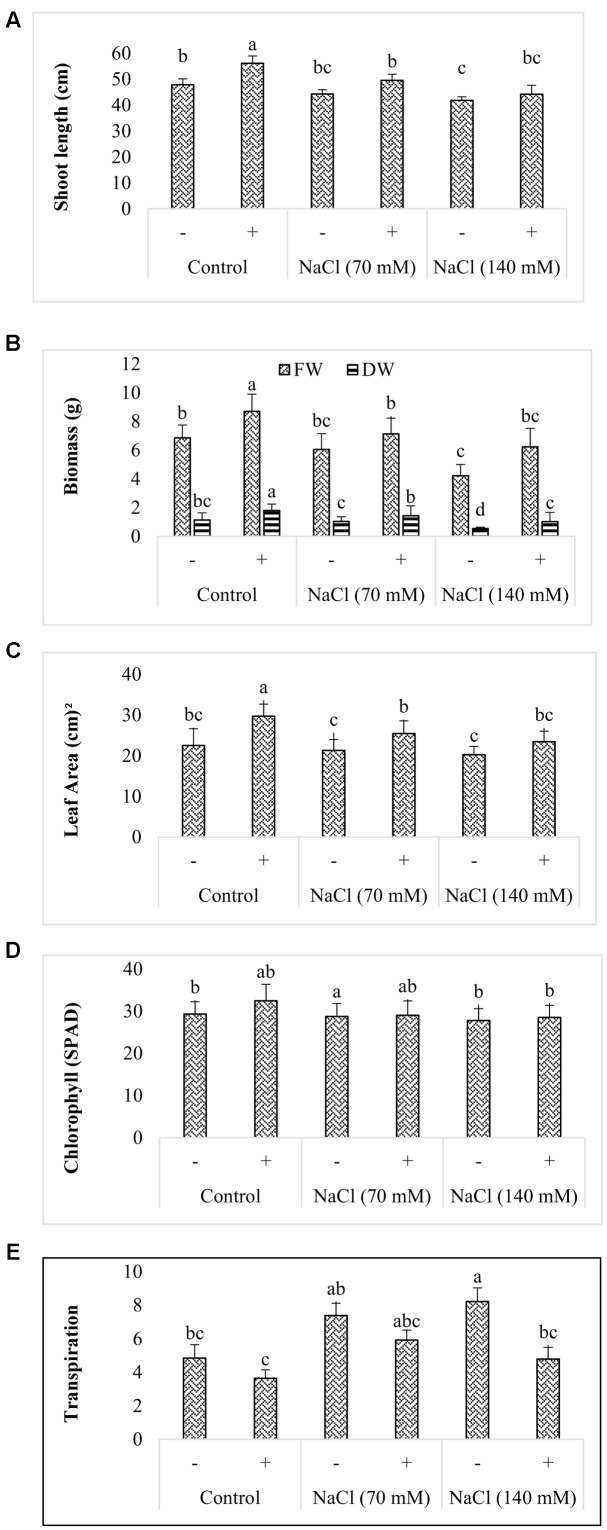
When co-cultured with AGH786, significant improvement was recorded in the growth of soybean seedlings. Soybean seedlings grown on soil with mixed AGH786 mycelium, showed higher shoot growth as compared to the plants grown without fungus (Figure 5A). However, shoot dry weight was not affected by the fungus indicating that AGH786 improved water absorption or its retention by the seedlings.
FIGURE 5.
Effect of P. spadiceum AGH786 on growth of soybean seedlings grown for 3 weeks under axenic conditions. Growth parameters include (A) shoot length, (B) biomass, (C) leaf area, (D) chlorophyll, and (E) transpiration. Data are means of three replicates along with standard error bars. Mean bars labeled with different letters are significantly different (p < 0.05).
The isolate significantly enhanced fresh and dry biomass of seedlings in the absence of salt stress (Figure 5B). In comparison to control, fresh and dry biomass was increased by 26.8 and 57.8% respectively. Salt stress (70 mM NaCl) reduced fresh and dry biomass by 11.8 and 8.7% respectively when compared with that of control. Further decrease of 38.6 and 52.6% respectively in these parameters was observed by elevating salt concentration to 140 mM. Inoculation of salt stressed seedlings with AGH786 alliviated the stress shown by restoration of fresh and dry biomass to the level of control in such plants (Figure 5B). In comparison to control, the strain caused an improvement of more than 31% in leaf area of seedlings grown under normal conditions (Figure 5C). The same behavior of the strain was observed in salt stress seedlings. However, exposure to the stress did not affect the parameter significantly (Figure 5C). Increase in the chlorophyll content in the inoculated seedlings compared to the non-inoculated plants was statistically non-significant (Figure 5D). By contrast, transpiration rate was significantly reduced in seedlings cocultivated with the strains under normal as well as saline conditions (Figure 5E).
Modulation of Endogenous Phytohormones in Soybean under Stress
GAs Analysis
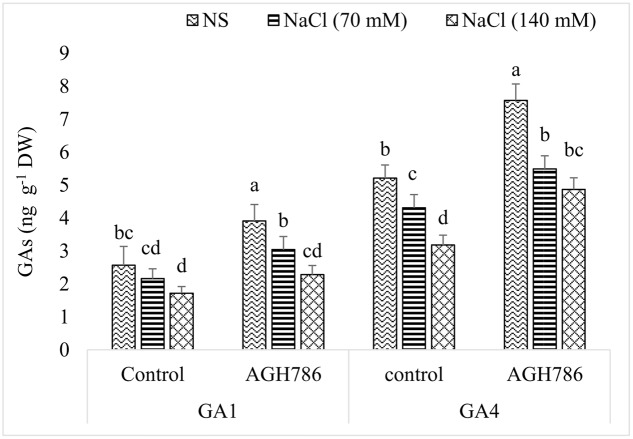
Bioactive GAs, i.e., GA1 and GA4 were determined because of their importance during salt stress. It was obvious that salt stress negatively regulated the synthesis of GA1 and GA4 in a dose dependent manner in seedlings grown with or without endophyte. However, in seedlings colonized by AGH786, production of GAs was always greater than that in their respective controls (Figure 6). For example, production of GA1 was 52% higher in control seedlings colonized by AGH786 than that of uncolonized seedlings. Similarly, in salt affected seedlings, 41 and 33% greater contents of endogenous GA1 was recorded in AGH786 treated seedlings exposed to 70 and 140 mM NaCl respectively when compared with their respective controls. In a similar fashion, endogenous level of GA4 was increased by 45% in seedlings grown in association with AGH786. In salt affected seedlings, synthesis of GA4 was enhanced by 27 and 53% in AGH786 treated seedlings exposed to 70 and 140 mM NaCl respectively when compared with their respective controls.
FIGURE 6.
Effect of endophytic fungus P. spadiceum AGH786 and different salt concentrations (70 and 140 mM) on the endogenous level of GAs in soybean seedlings axenically grown for 3 weeks. Concentrations of GAs were determined by GC–MS/MS. Data are means of three replicates along with standard error bars. Mean bars labeled with different letters are significantly different (p < 0.05).
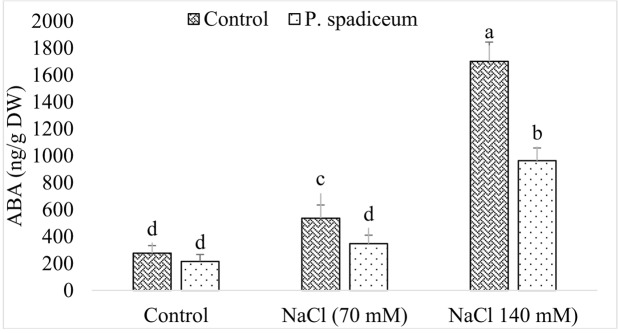
ABA Analysis
In the absence of NaCl, ABA contents in seedlings treated with fungal endophyte and untreated seedlings were almost similar. However, a sharp and dose dependent increase in the production of ABA was observed in seedlings exposed to salt stress (Figure 7). In comparison to control (not treated with AGH786), increase in ABA production was significantly less steep in seedlings colonized by AGH786 showing alleviation of the stress. Nevertheless, doubling the dose of salt from 70 nM to 140 mM enhanced ABA production in both endophyte associated and non-associated seedlings.
FIGURE 7.
Effect of endophytic fungus P. spadiceum AGH786 and different salt concentrations (70 and 140 mM) on the endogenous level of ABA in soybean seedlings. Data are means of three replicates along with standard deviation bars. Mean labeled with different letters are significantly different (p < 0.05).
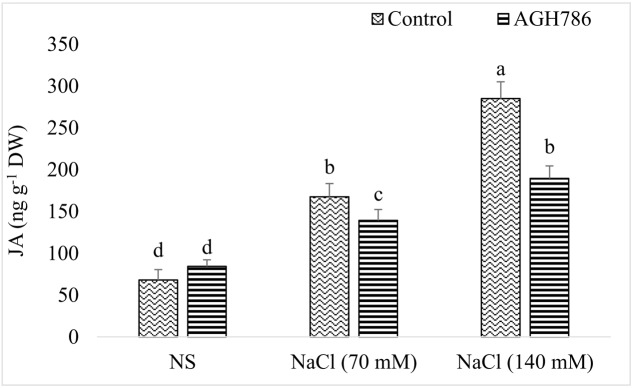
JA Analysis
In the absence of salt stress, JA content of soybean seedlings colonized by AGH786 was comparable to that of seedlings not associated with the endophyte (Figure 8). However, exposure to salt stress increased JA production by the seedlings in a dose dependent manner. More than twofold increase was recorded in the endogenous JA levels of seedlings treated with 70 mM NaCl as compared to that of the control which was reduced by 41% in seedlings colonized by AGH786. Similarly, doubling salt concentration in the media enhanced JA production by more than threefold when compared with control (seedlings grown in the absence of salt stress). However, colonization of AGH786 on the roots of the seedlings reduced JA contents down to half of the seedlings treated with this double dose of NaCl alone. Though endophyte treatment reduced JA production in salt affected seedlings, its amount was still higher than that of the control seedlings (seedlings grown in the absence of salt and endophyte).
FIGURE 8.
Effect of endophytic fungus P. spadiceum AGH786 and different salt concentrations (70 and 140 mM) on the endogenous level of JA in soybean seedlings. Data are means of three replicates along with standard error bars. Mean bars labeled with different letters are significantly different (p < 0.05).
Isoflavone Content Analysis
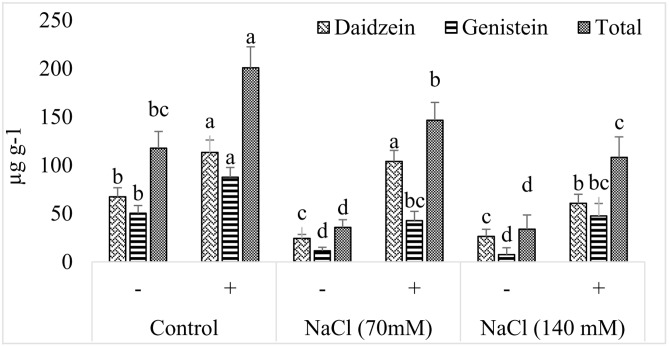
Soybean plants are famous for the production of isoflavones, daidzein, and genistein which are used as markers of food quality. It was noted that production of isoflavones was greatly reduced in soybean upon exposure to salt stress (Figure 8). In case of daidzein, more than twofold reduction was recorded in salt seedlings exposed to the selected concentration of NaCl. However, increaing concentration of NaCl was not linked with any further decrease in the production of daidzein. Contrary to this, effect of salt on the production of genistein was dose dependent and more drastic (Figure 9). In the presence of 70 mM NaCl, genistein production was reduced by more than fourfold and further decrease was noted by doubling the amount of NaCl. In the absence of NaCl, AGH786 associated seedlings produced isoflavones in significantly greater amount as compared to that of control seedlings. Interestingly, AGH786 restored the production of isoflavones in salt affected seedlings too (Figure 9).
FIGURE 9.
Effect of halotolerant fungal endophyte (P. spadiceum AGH786) on the endogenous isoflavone (daidzein and genistein) contents of soybean grown under elevated salt stress. Error bars represent ± standard error (of three replications). Mean bars labeled with different letters are significantly different at p < 0.05 analyzed by Duncan multiple range test.
Discussion
Soybean is an important legume cultivated worldwide for its oil and protein rich grains. However, its production is under threat due to ever increasing environmental stresses including salinity (Pimentel et al., 2006). Among the various strategies, use of endophytic fungi as biofertilizers are emerging as the most efficient tools for sustainable agricultural practices on salt affected areas (Radhakrishnan et al., 2014). Soybean has been known as a rich source of beneficial endophytic fungi improving its fitness to survive under stress (Khan et al., 2011). Soybean endophytes belong to diverse taxonomic groups such as Ascomycota and Basidiomycota (de Souza Leite et al., 2013). For soybean cultivation on salt affected soils, we attempted to isolate halotolerant endophytic fungus from Soybean itself having the potential to readily colonize its host and alleviate salt stress. One of the endophyte P. spadiceum strain AGH786, residing soybean root proved to be plant growth promoting, GAs producing (assayed on Waito-c rice) and halotolerant to NaCl. The GAs mutant Waito-c rice is an excellent system used to screen culture filtrate of endophytes for GAs and plant growth promotion (Potshangbam et al., 2017). Production of GAs is an important trait enabling endophytes to promote plant growth and mitigate salt stress (Hamayun et al., 2015). In addition to GAs, Several amino acids were also detected in the culture of this strain. Similarly, this strain not only enhanced soybean growth under control environment but also under salt stress.
Several endophytic fungi including Aspergillus niger, A. flavus, Fusarium oxysporum, Penicillium funiculosum, P. corylophilum, Rhizopus stolonifer, and Paecilomyces formosus have been reported to produce phytohormones including GAs and IAA (Khan et al., 2015; Deng and Cao, 2017). However, all the fungal species capable of GA production belong to ascomycetes, a group of ascus forming fungi. P. spadiceum strain AGH786 has become the first GAs producing fungal endophyte that belongs to the group Basidiomycota. Plants treated with endophytes are often healthier than those lacking such interaction (Khan et al., 2008; Larriba et al., 2015), which in most cases is attributed to the endophyte secretion of phytohormones such as GAs (Waqas et al., 2012). In endophyte–host symbioses, secondary metabolites may be a contribution of the endophytic partner for such mutualistic relationship (Shi et al., 2009; Kusari et al., 2013).
Phytohormones signaling and crosstalk is of great significance in plant growth and development under normal and stress conditions. Under salinity stress, plant protects itself by producing extra amount of ABA which mediate stomatal closure to minimize water loss and then mediates stress damage (Chaves et al., 2009). According to one theory, CKs: ABA ratios in xylem sap controls stress signaling (Tran et al., 2010; Nishiyama et al., 2011). During salt stress, ABA production shoots up which is capable of repressing the expression of isopentenyl transferase gene encoding a rate limiting enzyme in the biosynthesis of CKs, suppressing amount and signaling of CKs (Nishiyama et al., 2011). In line with this, we observed significantly lower ABA level in endophyte-associated soybean seedlings as compared to endophyte-free plants. Our findings confirmed previously recorded lower amount of ABA in salt stressed endophytes associated plants (Khan et al., 2011). Still other studies suggested enhanced ABA production in plants inoculated with endophytic fungi (Hao et al., 2010). Hence, different groups of endophytic fungi may cause different effects on the endogenous ABA levels of plants as revealed by some earlier reports. Lower ABA in fungal associated plants suggests the involvement of GAs, as exogenous application of GA3 improved soybean salinity stress tolerance accompanied by low level of ABA (Hamayun et al., 2010). In salt affected plants, higher ABA is correlated with inhibition of leaf expansion and shoots development (Cramer and Quarrie, 2002). Next to ABA, GAs biosynthesis was significantly lower in salt affect seedlings. Recently, antagonism in the signaling of ABA and GA has been suggested (Cutler et al., 2010). GA-deficient plants are more susceptible to stress than those with higher levels of this hormone (Achard et al., 2006). Also, upregulation of gibberellin-deactivating gene, GA2ox7 in salt stressed Arabidopsis has been reported which represses growth for stress adaptation (Magome et al., 2008). The higher amount of GA12 in endophyte-treated plants under salinity stress elucidates the activation of GAs biosynthesis pathway, while higher production of GA1 and GA4 confirms plant growth maintenance during stress condition. Thus, by maintaining GAs and, therefore, growth under stress conditions, the endophyte is having a beneficial effect on the plant long-term survival. There are many previous reports showing the ameliorative effects of exogenous application of GAs (GA1/GA4) and IAA on plant growth under abiotic stress (Siddiqui et al., 2008; Arteca, 2013; Kaya et al., 2013), while information on the regulation of plant endogenous hormones in association with phytohormones producing endophytic fungi under abiotic stress conditions are scarce. Some physiological evidences demonstrate that plants infected with endophytic fungi often have a distinct advantage against biotic and abiotic stress over their endophyte-free counterparts (Rodriguez and Redman, 2008). Foliar application of GAs has been known for its role in plant stem elongation and mitigation of abiotic stress (Yang et al., 1996; Kaur et al., 1998), while the same was observed in current study that endophytes producing GAs rescued plants from the adverse effects of salinity stress.
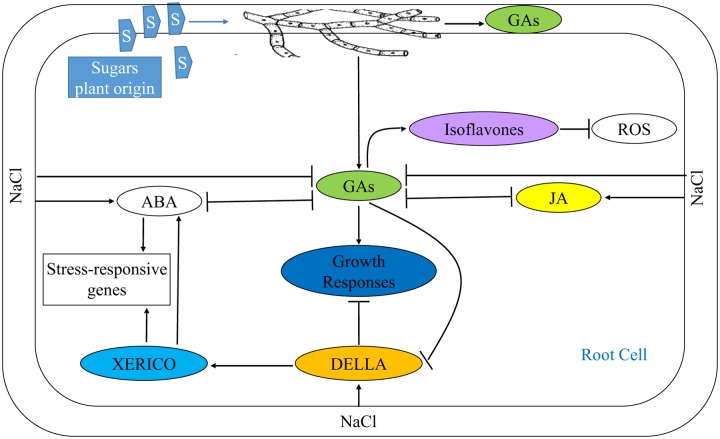
Restoration of ABA and GAs to their normal levels in salt affected plants associated with fungal endophytes demonstrates the mitigation of adverse effects of salt stress, probably by promoting plant growth and subsequently hindering build-up of DELLA proteins (Figure 10). ABA pathway is involved in plant responses to diverse abiotic and biotic inputs, and it is more likely that DELLA restraint provides a general mechanism for integration of plant growth responses to the environment. Salt stress also enhanced JA which shows the possible involvement of JA in the stress signal perception (Pedranzani et al., 2003). Inoculation of salt affected plants with fungal endophyte reduced endogenous JA to a level still higher than that of control plants advocating that alleviation of salt stress by fungal enodphyte is independent of JA signaling. JA signaling helps plants to combat against salt stress by enhancing the production of arginine decarboxylase, ribulose 1⋅5-bisphosphate carboxylase/oxygenase (Rubisco) activase and apoplastic invertase genes (Walia et al., 2007), that rationalizes the high level of JA in uninoculated salt stressed plants. High GAs in endophytes associated plants may act synergistically with SA in reducing JA signaling thereby making plant insensitive to JA. Additionally, enhanced GAs also promote SA signaling by degrading DELLA proteins (Navarro et al., 2008). Besides, GAs improve nodulation in soybean which may lead to stress alleviation (Hayashi et al., 2016).
FIGURE 10.
Mitigation of NaCl stress in soybean seedlings by modulating phytohomones through application of an endophytic fungus P. spadiceum S-1-3. NaCl stress suppresses GAs biosynthesis by enhanced biosynthesis of its antagonists, ABA and JA (or other independent mode), leading to retarded growth. Additionally, under NaCl stress, the stress responsive genes are induced either by enhanced biosynthesis of ABA or by DELLA protein in a XERICO dependent pathway. But in S-1-3 treated seedlings, the NaCl stress is alleviated by the boosted production of GAs that antagonize the biosynthesis of JA and ABA, and promotes biosynthesis isoflavones resulting in amelioration of different physiological and growth parameters of the stressed seedlings.
Isoflavones are highly valued for human health, and soybean cultivars with higher isoflavones content have been developed over the past years (Phommalth et al., 2008). In addition, isoflavones are important regulators of root nodulation and plant defense in soybean and therefore, induction of isoflavones would be desirable for better field performance under stress (Algar et al., 2014). Our isolate AGH786 significantly enhanced isoflavone content of soybean under normal and salt stress conditions, thereby enhancing quality of soybean. Fungi are known to synthesized and release flavonoids which may be absorbed by plant, improving the endogenous pool of plant isoflavones (Qiu et al., 2010). Alternatively, plant enhanced isoflavones production might be due to increased activity of a key enzyme in isoflavone biosynthesis pathway (phenyl alanine ammonia lyase), under the influence of endophyte produced GAs (Barnes and Jones, 1984).
Conclusion
Abiotic stress conditions prevailing in the environment are among the key constraints to agricultural production. This work reported a halophilic and halotolerant fungal strain, P. spadiceum AGH786 that not only boosted growth in soybean, but also helped it to resist salt stress. The fungal strain secreted bioactive GAs and free amino acids in the culture, modulated the endogenous phytohormones (GAs, ABA, JA, and SA) and enhanced isoflavones in inoculated seedlings intimating the ameliorative mechanism by which the fungal endophyte improved seedlings growth and their tolerance to salt stress. The reported strain is not only the ever first GAs producing endophytic basidiomycete, but is superior in terms of GA3 production to the so far reported most efficient GAs producing fungus G. fujikori. However, G. fujikori is still top GA1 producing fungus. This study opens an entry to unwrap this potential of basidiomycetes and urges for the use of such species as biofertilizers for enhancing productivity and food quality through real sustainable agriculture in saline soils.
Author Contributions
MH: performed plant growth experiments and phytohormones determination. AH: isolated fungal endophytes and analyzed data statistically. SK: screened salt tolerance of the endophytes. AK: sequenced the isolates and identified the strains. MW: performed isoflavones determination. MI, AI, SJ, and GR: wrote draft manuscript. I-JL: supervised the study and finalized the draft manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the Agenda Program (Project No. PJ01099002), Rural Development Administration, South Korea.
Footnotes
References
- Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., et al. (2006). Integration of plant responses to environmentally activated phytohormonal signals. Science 311 91–94. 10.1126/science.1118642 [DOI] [PubMed] [Google Scholar]
- Algar E., Gutierrez-Mañero F. J., Garcia-Villaraco A., García-Seco D., Lucas J. A., Ramos-Solano B. (2014). The role of isoflavone metabolism in plant protection depends on the rhizobacterial MAMP that triggers systemic resistance against Xanthomonas axonopodis pv. glycines in Glycine max (L.) Merr. cv. Osumi. Plant Physiol. Biochem. 82 9–16. 10.1016/j.plaphy.2014.05.001 [DOI] [PubMed] [Google Scholar]
- Allred C. D., Allred K. F., Ju Y. H., Goeppinger T. S., Doerge D. R., Helferich W. G. (2004). Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis 25 1649–1657. 10.1093/carcin/bgh178 [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Keifer J. (1997). The cerebellum and red nucleus are not required for in vitro classical conditioning of the turtle abducens nerve response. J. Neurosci. 17 9736–9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. W., Trivedi D. K., Sahoo R. K., Gill S. S., Tuteja N. (2015). A critical review on fungi mediated plant responses with special emphasis to Piriformospora indica on improved production and protection of crops. Plant Physiol. Biochem. 70 403–410. 10.1016/j.plaphy.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Arteca R. N. (2013). Plant Growth Substances: Principles and Applications. Berlin: Springer Science & Business Media. [Google Scholar]
- Baltruschat H., Fodor J. Z., Harrach B. L. D., Niemczyk E. B., Barna B. Z., Gullner G. B., et al. (2008). Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 180 501–510. 10.1111/j.1469-8137.2008.02583.x [DOI] [PubMed] [Google Scholar]
- Barnes L., Jones R. L. (1984). Regulation of phenylalanine ammonia lyase activity and growth in lettuce by light and gibberellic acid. Plant Cell Environ. 7 89–95. 10.1111/j.1365-3040.1984.tb01561.x [DOI] [Google Scholar]
- Bartels E. (2005). Evaluation of arteriovenous malformations (AVMs) with transcranial color-coded duplex sonography does the location of an AVM influence its sonographic detection? J. Ultrasound Med. 24 1511–1517. 10.7863/jum.2005.24.11.1511 [DOI] [PubMed] [Google Scholar]
- Berg G., Krechel A., Ditz M., Sikora R. A., Ulrich A., Hallmann J. (2005). Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol. Ecol. 51 215–229. 10.1016/j.femsec.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Chandanie W. A., Kubota M., Hyakumachi M. (2006). Interactions between plant growth promoting fungi and arbuscular mycorrhizal fungus Glomus mosseae and induction of systemic resistance to anthracnose disease in cucumber. Plant Soil 286 209–217. 10.1007/s11104-006-9038-y [DOI] [Google Scholar]
- Chaves M. M., Flexas J., Pinheiro C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103 551–560. 10.1093/aob/mcn125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer G. R., Quarrie S. A. (2002). Abscisic acid is correlated with the leaf growth inhibition of four genotypes of maize differing in their response to salinity. Funct. Plant Biol. 29 111–115. 10.1071/PP01131 [DOI] [PubMed] [Google Scholar]
- Cutler S. R., Rodriguez P. L., Finkelstein R. R., Abrams S. R. (2010). Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61 651–679. 10.1146/annurev-arplant-042809-112122 [DOI] [PubMed] [Google Scholar]
- Dakora F. D., Phillips D. A. (1996). Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Mol. Plant Physiol. 49 1–20. 10.1006/pmpp.1996.0035 [DOI] [Google Scholar]
- Dardanelli M. S., Manyani H., Barroso S., Carvajal M. A., Gil-Serrano A. M., Espuny M. R., et al. (2010). Effect of the presence of the plant growth promoting rhizobacterium (PGPR) Chryseobacterium balustinum Aur9 and salt stress in the pattern of flavonoids exuded by soybean roots. Plant Soil 328 483–493. 10.1007/s11104-009-0127-6 [DOI] [Google Scholar]
- de Souza Leite T., Cnossen-Fassoni A., Pereira O. L., Mizubuti E. S. G., De Araújo E. F., De Queiroz M. V. (2013). Novel and highly diverse fungal endophytes in soybean revealed by the consortium of two different techniques. J. Microbiol. 51 56–69. 10.1007/s12275-013-2356-x [DOI] [PubMed] [Google Scholar]
- Deng Z., Cao L. (2017). Fungal endophytes and their interactions with plants in phytoremediation: a review. Chemosphere 168 1100–1106. 10.1016/j.chemosphere.2016.10.097 [DOI] [PubMed] [Google Scholar]
- Dinler B. S., Antoniou C., Fotopoulos V. (2014). Interplay between GST and nitric oxide in the early response of soybean (Glycine max L.) plants to salinity stress. J. Plant Physiol. 171 1740–1747. 10.1016/j.jplph.2014.07.026 [DOI] [PubMed] [Google Scholar]
- Eriksson S., Böhlenius H., Moritz T., Nilsson O. (2006). GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 18 2172–2181. 10.1105/tpc.106.042317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes E. G., Pereira O. L., Da Silva C. C., Bento C. B. P., De Queiroz M. V. (2015). Diversity of endophytic fungi in Glycine max. Microbiol. Res. 181 84–92. 10.1016/j.micres.2015.05.010 [DOI] [PubMed] [Google Scholar]
- Hamayun M., Hussain A., Khan S. A., Irshad M., Khan A. L., Waqas M., et al. (2015). Kinetin modulates physio-hormonal attributes and isoflavone contents of Soybean grown under salinity stress. Front. Plant Sci. 6:377 10.3389/fpls.2015.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamayun M., Khan S. A., Iqbal I., Na C.-I., Khan A. L., Hwang Y.-H., et al. (2009). Chrysosporium pseudomerdarium produces gibberellins and promotes plant growth. J. Microbiol. 47 425–430. 10.1007/s12275-009-0268-6 [DOI] [PubMed] [Google Scholar]
- Hamayun M., Khan S. A., Khan A. L., Shin J.-H., Ahmad B., Shin D.-H., et al. (2010). Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem. 58 7226–7232. 10.1021/jf101221t [DOI] [PubMed] [Google Scholar]
- Hao G., Du X., Zhao F., Ji H. (2010). Fungal endophytes-induced abscisic acid is required for flavonoid accumulation in suspension cells of Ginkgo biloba. Biotechnol. Lett. 32 305–314. 10.1007/s10529-009-0139-6 [DOI] [PubMed] [Google Scholar]
- Hayashi S., Gresshoff P. M., Ferguson B. J. (2016). Mechanistic action of gibberellins in legume nodulation. J. Integr. Plant Biol. 56 971–978. 10.1111/jipb.12201 [DOI] [PubMed] [Google Scholar]
- Jones D. L., Shannon D., Junvee-Fortune T., Farrar J. F. (2005). Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol. Biochem. 37 179–181. 10.1016/j.soilbio.2004.07.021 [DOI] [Google Scholar]
- Kaur J., Ledward D. A., Park R. W., Robson R. L. (1998). Factors affecting the heat resistance of Escherichia coli O157:H7. Lett. Appl. Microbiol. 26 325–330. 10.1046/j.1472-765X.1998.00339.x [DOI] [PubMed] [Google Scholar]
- Kaya C., Ashraf M., Dikilitas M., Tuna A. L. (2013). Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indoleacetic acid (IAA) and inorganic nutrients-A field trial. Aust. J. Crop Sci. 7 249. [Google Scholar]
- Khan A. L., Hamayun M., Kang S.-M., Kim Y.-H., Jung H.-Y., Lee J.-H., et al. (2012). Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol. 12:1 10.1186/1471-2180-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. L., Hamayun M., Kim Y.-H., Kang S.-M., Lee I.-J. (2011). Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol. Biochem. 49 852–861. 10.1016/j.plaphy.2011.03.005 [DOI] [PubMed] [Google Scholar]
- Khan A. L., Hussain J., Al-Harrasi A., Al-Rawahi A., Lee I.-J. (2015). Endophytic fungi: resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 35 62–74. 10.3109/07388551.2013.800018 [DOI] [PubMed] [Google Scholar]
- Khan S. A., Hamayun M., Kim H.-Y., Yoon H.-J., Seo J.-C., Choo Y.-S., et al. (2009). A new strain of Arthrinium phaeospermum isolated from Carex kobomugi Ohwi is capable of gibberellin production. Biotechnol. Lett. 31 283–287. 10.1007/s10529-008-9862-7 [DOI] [PubMed] [Google Scholar]
- Khan S. A., Hamayun M., Yoon H., Kim H.-Y., Suh S.-J., Hwang S.-K., et al. (2008). Plant growth promotion and Penicillium citrinum. BMC Microbiol. 8:231 10.1186/1471-2180-8-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyśko-Lupicka T., Strof W., Kubś K., Skorupa M., Wieczorek P., Lejczak B., et al. (1997). The ability of soil-borne fungi to degrade organophosphonate carbon-to-phosphorus bonds. Appl. Microbiol. Biotechnol. 48 549–552. 10.1007/s002530051095 [DOI] [PubMed] [Google Scholar]
- Kusari S., Pandey S. P., Spiteller M. (2013). Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry 91 81–87. 10.1016/j.phytochem.2012.07.021 [DOI] [PubMed] [Google Scholar]
- Larriba E., Jaime M. D. L. A., Nislow C., Martín-Nieto J., Lopez-Llorca L. V. (2015). Endophytic colonization of barley (Hordeum vulgare) roots by the nematophagous fungus Pochonia chlamydosporia reveals plant growth promotion and a general defense and stress transcriptomic response. J. Plant Res. 128 665–678. 10.1007/s10265-015-0731-x [DOI] [PubMed] [Google Scholar]
- Leitão A. L., Enguita F. J. (2016). Gibberellins in Penicillium strains: challenges for endophyte-plant host interactions under salinity stress. Microbiol. Res. 183 8–18. 10.1016/j.micres.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Magome H., Yamaguchi S., Hanada A., Kamiya Y., Oda K. (2008). The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J. 56 613–626. 10.1111/j.1365-313X.2008.03627.x [DOI] [PubMed] [Google Scholar]
- Messina M. (2010). A brief historical overview of the past two decades of soy and isoflavone research. J. Nutr. 140 1350S–1354S. 10.3945/jn.109.118315 [DOI] [PubMed] [Google Scholar]
- Mhadhbi H., Mylona P. V., Polidoros A. N. (2014). “Legume-rhizobia symbiotic performance under abiotic stresses: factors influencing tolerance behaviour,” in Legumes Under Environmental Stress: Yield, Improvement and Adaptations, 1st Edn, eds Azooz M. M., Ahmad P. (Hoboken, NJ: John Wiley & Sons, Ltd; ). [Google Scholar]
- Miransari M. (2011). Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol. Adv. 29 645–653. 10.1016/j.biotechadv.2011.04.006 [DOI] [PubMed] [Google Scholar]
- Misra N., Dwivedi U. N. (2004). Genotypic difference in salinity tolerance of green gram cultivars. Plant Sci. 166 1135–1142. 10.1016/j.plantsci.2003.11.028 [DOI] [Google Scholar]
- Misra N., Gupta A. K. (2005). Effect of salt stress on proline metabolism in two high yielding genotypes of green gram. Plant Sci. 169 331–339. 10.1016/j.plantsci.2005.02.013 [DOI] [Google Scholar]
- Munns R., Tester M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59 651–681. 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- Navarro L., Bari R., Achard P., Lisón P., Nemri A., Harberd N. P., et al. (2008). DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 18 650–655. 10.1016/j.cub.2008.03.060 [DOI] [PubMed] [Google Scholar]
- Nishiyama R., Watanabe Y., Fujita Y., Le D. T., Kojima M., Werner T., et al. (2011). Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23 2169–2183. 10.1105/tpc.111.087395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A. G., Jones D. L. (2001). Competition for amino acids between wheat roots and rhizosphere microorganisms and the role of amino acids in plant N acquisition. Soil Biol. Biochem. 33 651–657. 10.1016/S0038-0717(00)00209-1 [DOI] [Google Scholar]
- Pedranzani H., Racagni G., Alemano S., Miersch O., Taleisnik E., Machado-Domenech E., et al. (2003). Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul. 41 149–158. 10.1023/A:1027311319940 [DOI] [Google Scholar]
- Phommalth S., Jeong Y.-S., Kim Y.-H., Dhakal K. H., Hwang Y.-H. (2008). Effects of light treatment on isoflavone content of germinated soybean seeds. J. Agric. Food Chem. 56 10123–10128. 10.1021/jf802118g [DOI] [PubMed] [Google Scholar]
- Pimentel I. C., Glienke-Blanco C., Gabardo J., Stuart R. M., Azevedo J. O. L. C. (2006). Identification and colonization of endophytic fungi from soybean (Glycine max (L.) Merril) under different environmental conditions. Braz. Arch. Biol. Technol. 49 705–711. 10.1590/S1516-89132006000600003 [DOI] [Google Scholar]
- Potshangbam M., Devi S. I., Sahoo D., Strobel G. A. (2017). Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 8:325 10.3389/fmicb.2017.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L.-M., Ma J., Wang J., Zhang F.-C., Wang Y. (2010). Thermal stability properties of an antifreeze protein from the desert beetle Microdera punctipennis. Cryobiology 60 192–197. 10.1016/j.cryobiol.2009.10.014 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R., Kang S.-M., Baek I.-Y., Lee I.-J. (2014). Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J. Plant Interact. 9 754–762. 10.1080/17429145.2014.930524 [DOI] [Google Scholar]
- Richardson M. (2009). The ecology of the Zygomycetes and its impact on environmental exposure. Clin. Microbiol. Infect. 15(Suppl. 5), 2–9. 10.1111/j.1469-0691.2009.02972.x [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Redman R. (2008). More than 400 million years of evolution and some plants still can’t make it on their own: plant stress tolerance via fungal symbiosis. J. Exp. Bot. 59 1109–1114. 10.1093/jxb/erm342 [DOI] [PubMed] [Google Scholar]
- Rostagno M. A., Palma M., Barroso C. G. (2003). Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 1012 119–128. 10.1016/S0021-9673(03)01184-1 [DOI] [PubMed] [Google Scholar]
- Shi Y., Lou K., Li C. (2009). Promotion of plant growth by phytohormone-producing endophytic microbes of sugar beet. Biol. Fertil. Soils 45 645–653. 10.1007/s00374-009-0376-9 [DOI] [Google Scholar]
- Siddiqui M. H., Khan M. N., Mohammad F., Khan M. M. A. (2008). Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 194 214–224. 10.1111/j.1439-037X.2008.00308.x [DOI] [Google Scholar]
- Subramanian S., Graham M. Y., Yu O., Graham T. L. (2005). RNA interference of soybean isoflavone synthase genes leads to silencing in tissues distal to the transformation site and to enhanced susceptibility to Phytophthora sojae. Plant Physiol. 137 1345–1353. 10.1104/pp.104.057257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). MEGA molecular evolutionary genetics analysis (MEGA) software version 4. Mol. Biol. Evol. 24 1596–1599. 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- Tran L.-S. P., Shinozaki K., Yamaguchi-Shinozaki K. (2010). Role of cytokinin responsive two-component system in ABA and osmotic stress signalings. Plant Signal. Behav. 5 148–150. 10.4161/psb.5.2.10411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N., Hermans C. (2008). Proline accumulation in plants: a review. Amino Acids 35 753–759. 10.1007/s00726-008-0061-6 [DOI] [PubMed] [Google Scholar]
- Walia H., Wilson C., Condamine P., Liu X., Ismail A. M., Close T. J. (2007). Large-scale expression profiling and physiological characterization of jasmonic acid-mediated adaptation of barley to salinity stress. Plant Cell Environ. 30 410–421. 10.1111/j.1365-3040.2006.01628.x [DOI] [PubMed] [Google Scholar]
- Waller F., Achatz B., Baltruschat H., Fodor J., Becker K., Fischer M., et al. (2005). The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. U.S.A. 102 13386–13391. 10.1073/pnas.0504423102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waqas M., Khan A. L., Hamayun M., Shahzad R., Kang S., Kim J., et al. (2015). Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot an example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 10 280–287. 10.1080/17429145.2015.1079743 [DOI] [Google Scholar]
- Waqas M., Khan A. L., Kamran M., Hamayun M., Kang S.-M., Kim Y.-H., et al. (2012). Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17 10754–10773. 10.3390/molecules170910754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Davies P. J., Reid J. B. (1996). Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light-grown peas. Plant Physiol. 110 1029–1034. 10.1104/pp.110.3.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. Y., Hamayun M., Lee S., Lee I. J. (2009). Methyl jasmonate alleviated salinity stress in soybean. J. Crop Sci. Biotechnol. 12 63–68. 10.1007/s12892-009-0060-5 [DOI] [Google Scholar]
- Zhou Z., Zhang C., Zhou W., Li W., Chu L., Yan J., et al. (2015). Diversity and plant growth-promoting ability of endophytic fungi from the five flower plant species collected from Yunnan, Southwest China. J. Plant Interact. 9 585–591. 10.1080/17429145.2013.873959 [DOI] [Google Scholar]