Abstract
Background and objectives
We retrospectively evaluated the value of PET/CT in predicting survival and histopathological tumour-response in patients with distal oesophageal and gastric adenocarcinoma following neoadjuvant treatment.
Methods
Twenty-one patients with resectable distal oesophageal adenocarcinoma and 14 with gastric adenocarcinoma between January 2002 and December 2011, who had undergone serial PET before and after neoadjuvant therapy followed by surgery, were enrolled. Maximum standard uptake value (SUVmax) and metabolic tumour volume were measured and correlated with tumour regression grade and survival.
Results
Histopathological tumour response (PR) is a stronger predictor of overall and disease-free survival compared to metabolic response. ∆%SUVmax ≥70% was the only PET metric that predicted PR (82.4% sensitivity, 61.5% specificity, p = 0.047). Histopathological non-responders had a higher risk of death (HR 8.461, p = 0.001) and recurrence (HR 6.385, p = 0.002) and similarly in metabolic non-responders for death (HR 2.956, p = 0.063) and recurrence (HR 3.614, p = 0.028). Ordinalised ∆%SUVmax showed a predictive trend for OS and DFS, but failed to achieve statistical significance.
Conclusions
PR was a stronger predictor of survival than metabolic response. ∆%SUVmax ≥70% was the best biomarker on PET that predicted PR and survival in oesophageal and gastric adenocarcinoma. Ordinalisation of ∆%SUVmax was not helpful in predicting primary outcomes.
Keywords: Oesophageal cancer, Gastric cancer, Treatment response, FDG, PET, PET-CT
Introduction
Despite the increase in the incidence of oesophageal adenocarcinoma since 1988 and the steady decline in the incidence of gastric adenocarcinoma since 1982, oesophageal and gastric malignancies collectively accounted for 2317 deaths in Australia in 2010 [1]. While surgery remains the mainstay of curative treatment, the administration of neoadjuvant multimodality therapy has been shown to increase rates of histopathological complete response and entail modest survival benefit over surgery alone [2]. Positron emission tomography (PET) using 18F-2-fluoro-2-deoxyglucose (18F-FDG) permits in vivo characterisation of pathological 18F-FDG retention and PET has gained acceptance for initial staging of oesophageal malignancy by improving the detection of occult distant metastases [3]. Recently, several authors have investigated the predictive value of PET metrics for early response to therapy in oesophageal and gastric neoplasms, given that PET has been shown to be useful in other cancers [4–7].
Tumour regression grades (TRG), a measure of histopathological tumour response (PR) based on an estimation of the percentage of residual tumour tissue in relation to the macroscopically identifiable tumour bed at the primary site, was adopted from studies conducted on gastric cancers [8] and oesophageal adenocarcinomas [9]. Lordick, et al. [10] validated the use of therapy-induced changes in PET metrics to predict PR and to stratify distal oesophageal and gastric cardia adenocarcinoma patients into different prognostic groups. They concluded that 35% regression of maximum standardised uptake value (SUVmax), a semi-quantitative measure of 18F-FDG retention in the primary tumour bed was the optimal cut-off to identify histopathological responders with 100% sensitivity and 58% specificity. However, the evidence regarding the predictive value of PET-based biomarkers in gastric cancers is limited and inconsistent. One prospective study on advanced gastric cancer patients confirmed the highly predictive value of 35% reduction in SUVmax on PR with 77% sensitivity and 86% specificity [11], while one small study comprising locally advanced gastric cancers showed a 45% decrease in SUV from baseline and day 35 significantly predicted PR whereas the change at day 15 did not [12].
There is increasing interest in investigating the prognostic value of PET volumetric parameters such as metabolic tumour volume (MTV), but the utility of this more novel imaging biomarker is experimental. One study reported that a 63% reduction in MTV was the optimal cut-off to identify histopathologically responding distal oesophageal adenocarcinoma with 91% sensitivity and 90% specificity [13]. The evidence on the predictive value of MTV in gastric cancer is scarce.
Several authors have validated that metabolic responders (MR+) identified on PET had better prognosis [10, 11, 14]. Some studies have shown variable thresholds [15] in predicting PR and survival on PET, while others failed to validate the predictive power of PET metrics [16, 17]. Lee did not find a correlation between the change in SUVmax of the primary tumour with PR after neoadjuvant chemotherapy and surgery in gastric carcinoma [18]. Vallbohmer also did not find a correlation between a change in SUVmax (baseline and post-treatment PET at 2 weeks) and PR (<10% viable cells) or overall survival in oesophageal carcinoma [19]. This study aims to retrospectively evaluate the performance of PET in predicting survival and PR to neoadjuvant therapy in patients with surgically resected distal oesophageal and gastric adenocarcinoma.
Method
Patient population
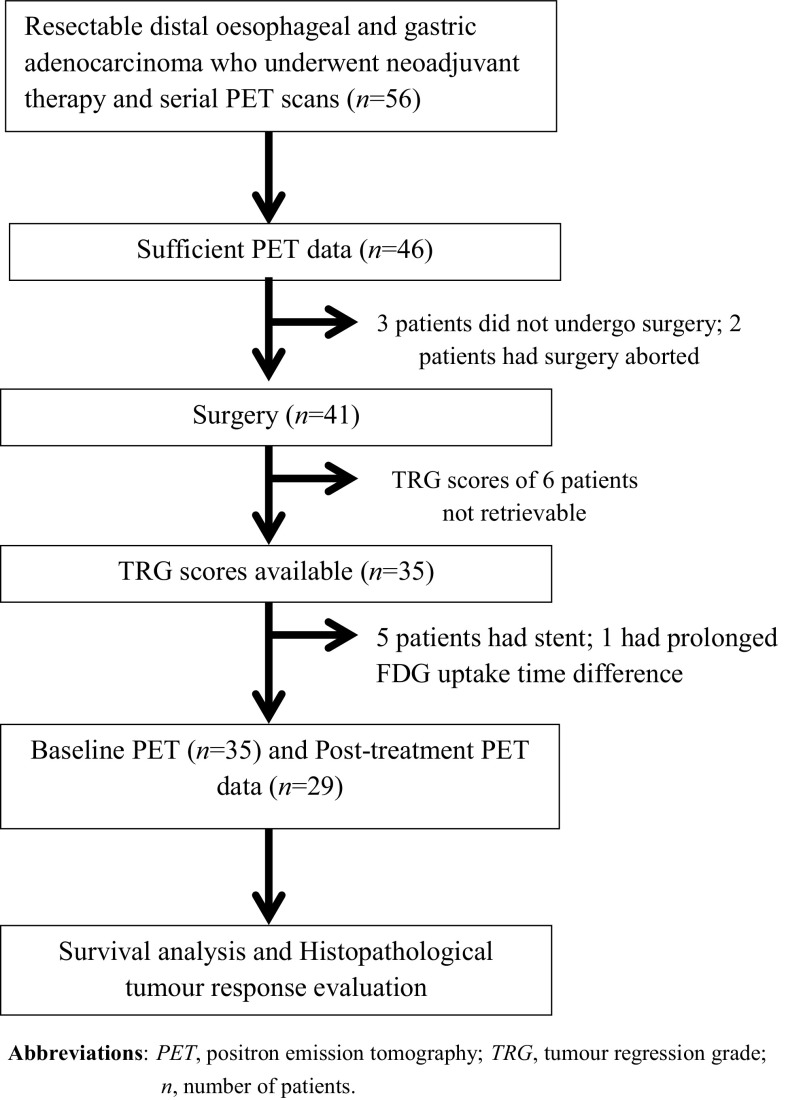
Fifty-six patients with newly diagnosed resectable distal oesophageal and gastric adenocarcinoma who underwent neoadjuvant therapy and serial 18F-FDG PET scans at Liverpool Hospital (Sydney, Australia) between January 2002 and December 2011 were included. 35 patients (21 patients with distal oesophageal adenocarcinoma and 14 patients with gastric adenocarcinoma) with sufficient PET data and TRG scores were analysed (Fig. 1). Patients with non-resectable disease at diagnosis, non-FDG-avid primary tumour on initial PET scan (PET-1), insufficient PET data, active concurrent cancer unrelated to oesophageal and gastric cancer, previous neoadjuvant therapy prior to initial PET and those who did not undergo surgery were excluded. Post-treatment PET (PET-2) parameters were not evaluated in one patient with a significant difference in uptake time between the two PET scans (64 min) and in five patients who had oesophageal stents implanted after PET-1. Most patients underwent PET/CT scans apart from two patients where PET-alone imaging was performed.
Fig. 1.
Patient selection. PET positron emission tomography, TRG tumour regression grade, n number of patients
Tumour staging was based on the sixth edition of the American Joint Committee of Cancer (AJCC) Staging Manual [20], but classification of tumour location was based on the seventh edition [21].
Patient information
Demographic, clinical and follow-up data were gathered through various databases including the South Western Sydney Area Health Service’s (SWSAHS) online patient information system, Cancer Therapy Centre (MOSAIQ), Departments of Surgery and Nuclear Medicine and PET.
PET imaging
All patients underwent a baseline PET scan for staging (PET-1) and a post-treatment PET scan (PET-2). PET-alone scans operating in three-dimensional mode (Allegro, Philips Medical Systems, Milpitas, CA, US) with germanium source attenuation were performed prior to February 2006, and PET/CT scans (Gemini GXL-6, Philips Medical Systems, Miltipas, CA, US) using low-dose CT without contrast enhancement for attenuation correction.
A standardised protocol comprised a minimum 4-h fasting period and blood glucose levels <10 mmol/L prior to 18F-FDG (5.14 MBq/kg), administration intravenously. Patients were scanned after an uptake period of approximately 60 min.
PET data analysis
PET scans were analysed by two accredited Nuclear Medicine physicians in consensus (M.L. and J.Y.) according to a standardised protocol where the SUVmax was measured using a 15 mm wide region of interest around the primary tumour. MTV was measured using vendor’s software with a SUVmax threshold that best delineated the tumour. Scans (PET-1 and PET-2) were analysed blinded from all clinical, pathological and imaging data apart from the knowledge that all patients had oesophageal and gastric malignancy and had completed neoadjuvant therapy. PET-1 and PET-2 measurements and any absolute and relative differences in 18F-FDG uptake were correlated with TRG and survival. If no residual tumour was visible and uptake was indistinguishable from background oesophageal or gastric activity on post-treatment scan, no volumetric measurement was attempted and the percentage reduction in abnormal tracer uptake is assigned 100%. Metabolic responders (MR+) are patients with ∆%SUVmax ≥70%.
Histopathological response evaluation
Surgical specimens were retrospectively examined by a single pathologist (S.L.). TRG score was assessed semi-quantitatively into either complete (TRG 1a: no residual tumour), subtotal (TRG 1b: <10% of residual tumour), partial (TRG 2: 10–50% of residual tumour) and minimal response (TRG 3: >50% residual tumour) based on Becker et al. [8]. The pathologist was blinded from all clinical, pathological and imaging data. Patients with complete or subtotal tumour regression were classified as histopathological responders (PR+). All other patients were classified as non-responders (PR−).
Follow-up
Disease-status and survival status at the time of census were recorded. Overall survival (OS) was calculated from the date of PET-1 to date of death or date of most recent follow-up. Disease-free survival (DFS) was calculated from date of surgery to the date of confirmed recurrence. If death was a direct consequence of surgery within 2 weeks of surgery, then the patient was excluded from survival analyses.
Statistical analysis
Absolute numbers and percentages were computed to describe the patient population, and quantitative values are expressed as median and range. Chi-square test was used to examine associations between categorical variables. Receiver operator characteristics (ROC) curve was performed to find the optimal cut-offs of the PET parameters. Survival curves were generated using Kaplan–Meier estimates and significance of difference between curves was tested with log-rank tests. Univariate analysis of survival was performed using Cox regression analysis and the estimated hazard ratio (HR) and 95% confidence interval (CI) were reported. All statistical analyses were performed using IBM SPSS Statistics 21 and p < 0.05 was considered statistically significant.
Results
Patient characteristics
There was a preponderance of male subjects and neoadjuvant chemotherapy-alone treatment in the cohort (Table 1). Most patients were node negative on staging PET.
Table 1.
Patient characteristics and clinicopathological parameters
| Parameters | n (%) |
|---|---|
| Gender | |
| Males | 30 (85.7) |
| Females | 5 (14.3) |
| Median age at diagnosis | 61.7 years (40.6–74.5) |
| Histology | |
| Signet ring cell | 4 (11.4) |
| Non-Signet ring cell | 31 (88.6) |
| Tumour Location (AJCC 7th ed.) | |
| Distal oesophagus | 18 (51.4) |
| Stomach | 17 (48.6) |
| Cardia of stomach | 9 |
| Fundus | 1 |
| Body of stomach | 4 |
| Antrum | 3 |
| Pylorus | 0 |
| Tumour Grade | |
| Moderate | 17 (48.6) |
| Poor | 18 (51.4) |
| Clinical T stage at diagnosis (AJCC 6th ed.) | |
| T2 | 3 (8.6) |
| T3 | 23 (65.7) |
| T4 | 6 (17.1) |
| Missing | 3 (8.6) |
| Nodal involvement on staging PET | |
| N− | 26 (74.3) |
| N+ | 9 (25.7) |
| Overall clinical stage at diagnosis (AJCC 6th ed.) | |
| I | 2 (5.7) |
| II | 12 (48.6) |
| III | 20 (55.1) |
| Missing | 1 (2.9) |
| Type of neoadjuvant therapy | |
| CT alone | 20 (57.1) |
| CRT | 9 (25.7) |
| RT alone | 3 (8.6) |
| Not specified | 3 (8.6) |
| TRG | |
| 1a | 3 (8.6) |
| 1b | 13 (37.1) |
| 2 | 4 (11.4) |
| 3 | 15 (42.9) |
| Recurrence | |
| No | 18 (51.4) |
| Yes | 17 (48.6) |
| Median disease-free survival | 13.8 months (2.1-122.4) |
| Dead | |
| No | 15 (42.9) |
| Yes | 20 (57.1) |
| Median overall survival | 22.3 months (9.6-122.4) |
| Median follow-up period | 22.7 months (10.8-122.4) |
| Two-year overall survival rate | 40.0% |
T stage, depth of invasion, N stage nodal involvement, PET positron emission tomography, AJCC American Joint Committee of Cancer Staging Manual, N − negative nodal involvement, N + positive nodal involvement; CT chemotherapy, CRT chemoradiotherapy, RT radiotherapy, TRG tumour regression grade
There was a significant difference (p = 0.002) in the median patient weight at PET-1 (79 kg, 48–115) compared to PET-2 of (76 kg, 55–118). Median uptake time at PET-1 was 63 min and for PET-2 was 64 min (p = 0.878) with a median difference in uptake times of 6 min (0–22). The median interval between the two PET scans was 106 days (55–153).
PET and histopathological tumour response evaluation
The relative reduction in SUVmax (∆%SUVmax) ≥70% was the only optimum cut-off to predict PR (p = 0.047) on ROC analysis with 82.4% sensitivity and 61.5% specificity. There was a trend for a greater proportion of PR+ having an absolute reduction in SUVmax (∆SUVmax) ≥5.75 (p = 0.071). Other PET metrics did not retain statistical significance and were dichotomised at the respective median values (Table 2). By ordinalising ∆%SUVmax into a schema similar to that of TRG with complete response (∆%SUVmax = 100%), subtotal response (∆%SUVmax ≥70%), partial response (∆%SUVmax ≥35%) and minimal response (∆%SUVmax <35%), no significant result was attained.
Table 2.
Predictive value of various PET parameters of tumour regression grade (TRG) score
| n (%) | Chi-square | p | ||
|---|---|---|---|---|
| TRG1a–1b | TRG2–3 | |||
| Baseline SUVmax | ||||
| SUV1max ≥9.70 | 10 (28.6) | 10 (28.6) | 0.345 | 0.557 |
| SUV1max <9.70 | 6 (17.1) | 9 (25.7) | ||
| Post-treatment SUVmax | ||||
| SUV2max ≥3.75 | 5 (17.2) | 8 (27.6) | 0.386 | 0.534 |
| SUV2max <3.75 | 8 (27.6) | 8 (27.6) | ||
| Metabolic response based on ∆SUVmax | ||||
| ∆SUVmax ≥5.75 | 10 (34.5) | 7 (24.2) | 3.254 | 0.071 |
| ∆SUVmax <5.75 | 3 (10.3) | 9 (31.0) | ||
| Metabolic response based on ROC analysis of ∆%SUVmax | ||||
| ∆%SUVmax ≥70% | 8 (27.6) | 4 (13.8) | 3.948 | 0.047 |
| ∆%SUVmax <70% | 5 (17.2) | 12 (41.4) | ||
| Baseline MTV (cm3) | ||||
| MTV1 ≥47.30 | 8 (27.6) | 10 (34.5) | 0.024 | 1.000 |
| MTV1 <47.30 | 8 (27.6) | 9 (31.0) | ||
| Post-treatment MTV (cm3) | ||||
| MTV2 ≥12.00 | 3 (11.0) | 7 (22.8) | 1.008 | 0.315 |
| MTV2 <12.00 | 8 (27.6) | 8 (27.6) | ||
| Missing | 3 (11.0) | |||
| Metabolic response based on ∆MTV | ||||
| ∆MTV ≥39.40 | 7 (22.8) | 9 (31.0) | 0.035 | 0.851 |
| ∆MTV <39.40 | 4 (13.2) | 6 (22.0) | ||
| Missing | 3 (11.0) | |||
| Metabolic response based on ∆% MTV | ||||
| ∆%MTV ≥80% | 5 (14.0) | 6 (22.0) | 0.077 | 0.781 |
| ∆%MTV <80% | 6 (22.0) | 9 (31.0) | ||
| Missing | 3 (11.0) | |||
| Metabolic response based on various cut-offs of ∆%SUVmax | ||||
| ∆%SUVmax = 100% | 2 (6.9) | 2 (6.9) | 3.883 | 0.274 |
| ∆%SUVmax ≥70–99% | 6 (20.7) | 2 (6.9) | ||
| ∆%SUVmax ≥35–69% | 5 (17.2) | 9 (31.1) | ||
| ∆%SUVmax <35% | 0 (0.0) | 3 (10.3) | ||
Statistical significant result is in bold
Post-treatment scans in 5 patients were excluded from analysis due to oesophageal stent insertion. One patient was excluded due to significant difference in uptake times between the two scans
PET positron emission tomography, TRG tumour regression grade, SUV1 max baseline SUVmax, SUV2 max post-treatment SUVmax, ∆SUV max absolute reduction in SUVmax, ∆%SUV max relative reduction in SUVmax, MTV metabolic tumour volume, MTV1 baseline MTV, MTV2 post-treatment MTV, ∆MTV absolute reduction in MTV, ∆%MTV relative reduction in MTV
PET, TRG and survival analysis
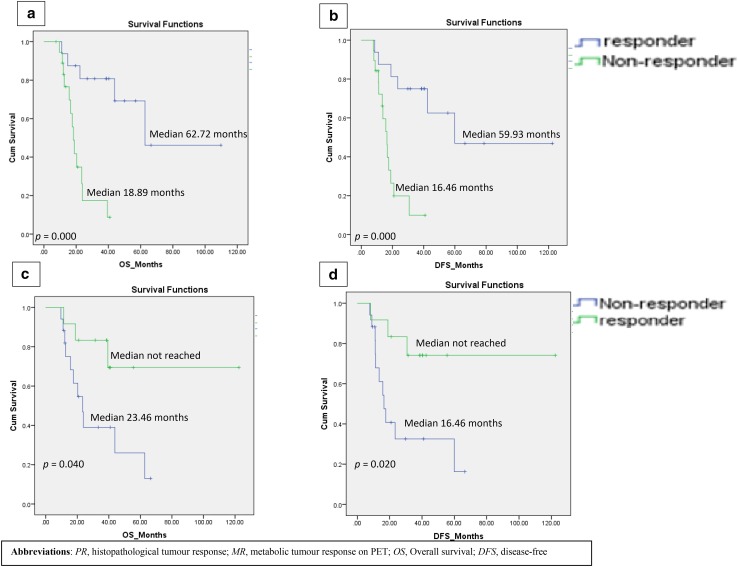
MR+ and PR+ had a significantly longer OS and DFS than their non-responding counterparts (Fig. 2). Median OS and DFS were not reached in MR+.
Fig. 2.
Kaplan–Meier Analysis. PR and OS (a), PR and DFS (b), MR and OS (c), MR and DFS (d). PR histopathological tumour response, MR metabolic tumour response on PET, OS overall survival, DFS disease-free
PR− had a significantly greater risk of death (HR 8.461; p = 0.001) and recurrence (HR 6.385; p = 0.002) (Table 3). Similarly, metabolic non-responders (MR−) with ∆%SUVmax <70% entailed a significantly greater risk of death (HR 2.956, p = 0.063) and recurrence (HR 3.614; p = 0.028) (Table 3). PR was a stronger predictor of OS (HR 8.461 vs 2.956) and DFS (HR 6.385 vs 3.614) compared to ∆%SUVmax≥70%.
Table 3.
Prognostic value of metabolic parameters on PET and histopathological tumour response for survival
| n (%) | Univariate Cox regression test | Univariate Cox regression test | |||||
|---|---|---|---|---|---|---|---|
| OS HR | 95% CI | p | DFS HR | 95% CI | p | ||
| Baseline SUVmax | |||||||
| SUV1max ≥9.70 | 20 (57.1) | 1.000 | 0.044 | 1.000 | 0.030 | ||
| SUV1max < 9.70 | 15 (42.9) | 2.589 | 1.025–6.535 | 2.797 | 1.107–7.071 | ||
| Post-treatment SUVmax | |||||||
| SUV2max ≥3.75 | 13 (44.8) | 1.000 | 0.376 | 1.000 | 0.352 | ||
| SUV2max < 3.75 | 16 (55.2) | 1.627 | 0.554–4.780 | 1.658 | 0.572–4.812 | ||
| Metabolic response based on ∆SUVmax | |||||||
| ∆SUVmax ≥ 5.75 | 17 (58.6) | 1.000 | 0.112 | 1.000 | 0.066 | ||
| ∆SUVmax < 5.75 | 12 (41.2) | 2.223 | 0.829–6.013 | 2.563 | 0.941–6.981 | ||
| Metabolic response based on ROC analysis of ∆%SUVmax | |||||||
| ∆%SUVmax ≥70% | 12 (41.2) | 1.000 | 0.063 | 1.000 | 0.028 | ||
| ∆%SUVmax <70% | 17 (58.6) | 2.956 | 0.945–9.247 | 3.614 | 1.150–11.533 | ||
| Baseline MTV (cm3) | |||||||
| MTV1 ≥47.30 | 18 (51.4) | 1.000 | 0.141 | 1.000 | 0.133 | ||
| MTV1 < 47.30 | 17 (48.6) | 1.996 | 0.795–5.013 | 2.031 | 0.806–5.118 | ||
| Post-treatment MTV (cm3) | |||||||
| MTV2 ≥12.00 | 10 (34.5) | 1.000 | 0.817 | 1.000 | 0.795 | ||
| MTV2 < 12.00 | 16 (55.2) | 0.860 | 0.241–3.074 | 0.845 | 0.238–3.003 | ||
| Missing | 3 (10.3) | ||||||
| Metabolic response based on ∆MTV | |||||||
| ∆MTV ≥39.40 | 16 (55.2) | 1.000 | 0.305 | 1.000 | 0. 362 | ||
| ∆MTV < 39.40 | 10 (34.5) | 1.182 | 0.583–5.639 | 1.698 | 0.544–5.296 | ||
| Missing | 3 (10.3) | ||||||
| Metabolic response based on ∆% MTV | |||||||
| ∆%MTV ≥80% | 10 (34.5) | 1.000 | 0.707 | 1.000 | 0. 681 | ||
| ∆%MTV < 80% | 16 (55.2) | 0.792 | 0.235–2.672 | 0.775 | 0.231–2.605 | ||
| Missing | 3 (10.3) | ||||||
| Metabolic response based on various cut-offs of ∆%SUVmax | |||||||
| ∆%SUVmax = 100% | 4 (13.8) | 1.000 | 1.000 | ||||
| ∆%SUVmax ≥70–99% | 8 (27.6) | 0.402 | 0.056–2.898 | 0.366 | 0.454 | 0.063–3.247 | 0.431 |
| ∆%SUVmax ≥35–69% | 14 (48.3) | 1.552 | 0.331–7.727 | 0.577 | 2.137 | 0.460–9.922 | 0.332 |
| ∆%SUVmax <35% | 3 (10.3) | 3.241 | 0.429–24.495 | 0.254 | 3.718 | 0.491–28.183 | 0.204 |
| Histopathological tumour response | |||||||
| TRG1a–1b | 16 (45.7) | 1.000 | 1.000 | ||||
| TRG2–3 | 19 (54.3) | 8.461 | 2.355–30.396 | 0.001 | 6.385 | 2.019–20.195 | 0.002 |
Statistical significant result is in bold
Post-treatment scans in five patients were excluded from analysis due to oesophageal stent insertion. One patient was excluded due to significant difference in uptake times between the two scans
PET positron emission tomography, OS overall survival, DFS Disease-free survival, HR Hazard ratio, CI confidence interval, n number of patients, SUV1 max baseline SUVmax, SUV2 max post-treatment SUVmax, ∆SUV max absolute reduction in SUVmax, ∆%SUV max relative reduction in SUVmax, MTV metabolic tumour volume, MTV1 Baseline MTV, MTV2 post-treatment MTV, ∆MTV absolute reduction in MTV, ∆%MTV relative reduction in MTV, TRG tumour regression grade
For PET-1, baseline SUVmax <9.70 was the only statistically significant predictor of poor OS (HR 2.589, p = 0.044). The ∆%SUVmax <70% cut-off showed a trend (p = 0.063) towards poorer OS. Both groups reported a higher risk of recurrence (p = 0.030; p = 0.028) (Table 3). All other PET metrics were not statistically significant.
When we analysed TRG as a binary parameter-complete or subtotal (TRG 1a and TRG 1b), and partial or minimal response (TRG 2 and 3), TRG was significantly associated with survival status (p = 0.002) on Chi-square analysis.
Discussion
The correlations between TRG, survival and PET-metrics have not been widely reported in oesophageal and gastric adenocarcinomas. This study comprised a heterogeneous group with resectable adenocarcinoma of the distal oesophagus and stomach. Although surgical treatment of oesophageal and gastric cancers occurs in relatively low volume centres in Australia, we have equivalent short and long term outcomes compared to Asian and European institutions [22, 23]. Therefore, our findings on the predictive performance of PET for survival and PR in this group are applicable to other populations. The study is also strengthened with centralized PET scan interpretation in consensus.
We found that a favourable metabolic response is associated with favourable PR. A reduction of SUVmax of the primary ≥70% following neoadjuvant treatment was associated with a TRG1a–1b. Our study showed that ∆%SUVmax ≥70% (p = 0.047) was the optimal cut-off that characterised PR with 82.4% sensitivity and 61.5% specificity. To our knowledge, the association between PR and PET metrics have not been studied extensively in gastric cancers. This is in line with a small study of 35 patients using PET-alone system which demonstrated ∆%SUVmax ≥35% predicted survival in locally advanced gastric cancer [11]. Our data included only two patients who underwent PET-alone imaging and while we acknowledge PET-alone and PET-CT can produce differences in SUV due to different methods of attenuation correction, the difference is reported to be relatively small in the clinical setting for non-osseous lesions of a sufficient size (our cohort comprised mostly T3 or T4 tumours) [24, 25].
Although there was a trend for an absolute therapy-induced change in SUVmax (p = 0.07) in predicting PR, we showed relative reduction as a stronger predictor. This may be due to the variable physiologic background FDG uptake in the oesophagus and stomach and relative reduction appears to be a better metric to gauge response. There is currently no consensus on the optimal thresholds in determining metabolic response on PET and various investigators have found different cut-offs [10, 17, 26, 27]. This most likely reflects different camera specifications and methodology and a standardised protocol in future prospective trials is mandatory [28].
There are conflicting results regarding the use of PET metrics in predicting survival in gastric cancer [29–31]. Our results demonstrated both MR+ and PR+ had significantly longer OS and DFS than non-responders (Fig. 2). Becker et al. [8] showed that PR+ (TRG 1a/1b) in 480 gastric adenocarcinoma patients had a significantly longer OS (128.6 months) compared to partial (61.9 months) and minimal (40.1 months) responders and similar conclusions were drawn from studies on distal oesophageal adenocarcinoma [9]. In our study, MR+ had a significantly longer OS (median NR vs 23.5 months) and longer DFS (median NR vs 16.5 months). These findings are similar to those from the MUNICON I trial [10]. There was a trend for MR− to have an almost threefold increase in risk of death (p = 0.06) and a significantly shorter DFS (p = 0.028) (Table 3). Although high SUVmax in many tumours have been shown to be poor prognosticators [32], in our study a high baseline SUVmax (>9.7) did not predict poor OS. This observation has also been shown in one study where patients with metastatic disease had lower SUVmax and SUVmean compared to M0 patients [33] and in another, no significant difference was found between limited and disseminated gastric cancers [34]. This may be due to several poor prognostic histological sub-types having low glucose metabolism, e.g. signet-ring cell or mucinous adenocarcinoma. Recent evidence also suggests a relationship between FDG avidity and HER2 expression and PET may have the potential to predict tumour phenotype [35].
PR+ in our study had a greater reduction in tumour 18F-FDG metabolism, highlighting the potential to formulate PET-guided treatment algorithm, which has been validated in the MUNICON II trial [36]. The greater histopathological remission rate among MR− of the MUNICON II trial compared to those in the MUNICON I trial was attributed to the PET-based early metabolic assessment and subsequent escalation of therapy in MR− from chemotherapy-alone to chemoradiotherapy [10, 36]. MR− in the MUNICON I trial had their chemotherapy stopped after 2 weeks and went directly to surgical resection potentially avoiding toxicity from futile chemotherapy. Despite the addition of radiotherapy to cisplatin or 5-fluorouracil based chemotherapy in MUNICON II trial, MR− still had a poor prognosis. A recent trial comprising a small number of patients suggested that changing chemotherapy regimens (to taxane-based) in PET non-responding patients may improve outcomes [37].
To our knowledge, this is the first study to ordinalise ∆%SUVmax similar to TRG scores using ∆%SUVmax ≥70% and ∆%SUVmax ≥35% cut-offs. Although it failed to achieve statistical significance, our analysis showed a predictive trend for DFS and OS (Table 3). This warrants further investigation in a larger cohort.
Volumetric measurements on PET is emerging as an important novel imaging biomarker in predicting prognosis in non-small cell lung (NSCLC) [7] and oesophageal cancers [13, 38], but there is limited evidence in gastric malignancies. In our study, neither the median MTV nor the MTV determined by ROC analysis (data not shown) predicted PR and survival, perhaps due to the difficulty and inaccuracy in delineating MTV using different SUV thresholds. This may partly be due to the variable physiological 18F-FDG uptake in gastric mucosa which attenuates the tumour to background ratio. Hence, MTV may not have the same prognostic value in gastric cancer compared with NSCLC or oesophageal cancer.
This retrospective study comprised only FDG-avid gastric tumours. It is well known that a significant proportion of gastric cancers can be falsely negative on FDG PET in particular in tumours rich in mucin and our data is not applicable to all gastric cancers [39–41]. We combined patients with distal oesophageal and gastric cancers due to small sample size and a larger population could have allowed subgroup analyses.
In conclusion, PR was a stronger prognostic indicator than metabolic response, and ∆%SUVmax was the best PET-based biomarker that predicted PR and survival in oesophageal and gastric adenocarcinoma. This study highlighted the potential role of PET in optimising treatment protocols and allows non-responders to be detected early to have escalation of treatment in this poor prognostic group.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflict of interests.
Ethical standard
Ethics approval was obtained from the Research and Ethics Office of the South Western Sydney Local Health District.
References
- 1.Australian. Institute of Health and Welfare [AIHW] Cancer in Australia: an overview 2012. Canberra: Australian Institute of Health and Welfare; 2010. [Google Scholar]
- 2.Merett ND. Multimodality treatment of potentially curative gastric cancer: Geographical variations and future prospects. World J Gastroenterol. 2014;20(36):12892–12899. doi: 10.3748/wjg.v20.i36.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai T, Popa E, Shah MA. The role of 18F-FDG PET imaging in upper gastrointestinal malignancies. Curr Treat Options Oncol. 2014;15:351–364. doi: 10.1007/s11864-014-0301-9. [DOI] [PubMed] [Google Scholar]
- 4.Kostakoglu L, Coleman M, Leonard JP, Kuji I, Zoe H, Goldsmith SJ. Positron emission tomography predicts prognosis after one cycle of chemotherapy in aggressive lymphoma and Hodgkin’s disease. J Nucl Med. 2002;43:1018–1027. [PubMed] [Google Scholar]
- 5.Schwarz-Dose J, Untch M, Tiling R, Sassen S, Mahner S, Kahlert S, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27(4):535–541. doi: 10.1200/JCO.2008.17.2650. [DOI] [PubMed] [Google Scholar]
- 6.Avril N, Sassen S, Schmalfeldt B, Naehrig J, Rutke S, Weber WA, et al. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J Clin Oncol. 2005;23(30):7445–7453. doi: 10.1200/JCO.2005.06.965. [DOI] [PubMed] [Google Scholar]
- 7.Yoo SW, Kim J, Chong A, Kwon SY, Min JJ, Song HC, et al. Metabolic tumor volume measured by F-18 FDG PET/CT can further stratify the prognosis of patients with Stage IV non-small cell lung cancer. Nucl Med. Mol Imaging. 2012;46:286–296. doi: 10.1007/s13139-012-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker K, Langer R, Reim D, Novotny A, zum Buschenfelde CM, Engel J, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253(5):934–939. doi: 10.1097/SLA.0b013e318216f449. [DOI] [PubMed] [Google Scholar]
- 9.Langer R, Ott K, Feith M, Lordick F, Siewert JR, Becker K. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol. 2009;22(12):1555–1563. doi: 10.1038/modpathol.2009.123. [DOI] [PubMed] [Google Scholar]
- 10.Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8(9):797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 11.Ott K, Fink U, Becker K, Stahl A, Dittler HJ, Busch R, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21(24):4604–4610. doi: 10.1200/JCO.2003.06.574. [DOI] [PubMed] [Google Scholar]
- 12.Shah MA, Yeung H, Coit D, Trocola R, Ilson D, Randazzo J, et al. A phase II study of preoperative chemotherapy with irinotecan(CPT) and cisplatin(CIS) for gastric cancer(NCI 5917): FDG-PET/CT predicts patient outcome. J Clin Oncol. 2007;25:4502. doi: 10.1200/JCO.2007.12.5062. [DOI] [Google Scholar]
- 13.Roedl JB, Colen RR, Holalkere NS, Fischman AJ, Choi NC, Blake MA. Adenocarcinomas of the esophagus: Response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET–CT. Comparison to histopathologic and clinical response evaluation. Radiother Oncol. 2008;89:278–286. doi: 10.1016/j.radonc.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Wieder HA, Brucher BL, Zimmermann F, Becker K, Lordick F, Beer A, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22(5):900–908. doi: 10.1200/JCO.2004.07.122. [DOI] [PubMed] [Google Scholar]
- 15.Kwee RM. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F FDG PET: a systematic review. Radiology. 2010;254(3):707–717. doi: 10.1148/radiol.09091324. [DOI] [PubMed] [Google Scholar]
- 16.Konski AA, Cheng JD, Goldberg M, Li T, Maurer A, Yu JQ, et al. Correlation of molecular response as measured by 18-FDG positron emission tomography with outcome after chemoradiotherapy in patients with esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2007;69(2):358–363. doi: 10.1016/j.ijrobp.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song SY, Kim JH, Ryu JS, Lee GH, Kim SB, Park SI, et al. FDG-PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. Int J Radiat Oncol Biol Phys. 2005;63(4):1053–1059. doi: 10.1016/j.ijrobp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Lee SM, Kim SH, Lee JM, Im SA, Bang YJ, Kim WH, et al. Usefulness of CT volumetry for primary gastric lesions in predicting pathologic response to neoadjuvant chemotherapy in advanced gastric cancer. Abdom Imaging. 2009;34:430–440. doi: 10.1007/s00261-008-9420-8. [DOI] [PubMed] [Google Scholar]
- 19.Vallbohmer D, Holscher AH, Dietlein M, Bollschweiler E, Baldus SE, Monig SP, et al. [18F]-Fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg. 2009;250(6):888–894. doi: 10.1097/SLA.0b013e3181bc9c0d. [DOI] [PubMed] [Google Scholar]
- 20.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 21.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Awan N, Haveman JW, Apostolou C, Chang DK, Merrett N. Gastric cancer: Australian outcomes of multi-modality treatment with curative intent. ANZ J Surg. 2016;86(5):386–390. doi: 10.1111/ans.12693. [DOI] [PubMed] [Google Scholar]
- 23.Thomson IG, Gotley DC, Barbour AP, Martin I, Jayasuria N, Thomas J, et al. Treatment results of curative gastric resection from a specialist Australian unit: low volume with satisfactory outcomes. Gastric Cancer. 2014;17(1):152–160. doi: 10.1007/s10120-013-0240-3. [DOI] [PubMed] [Google Scholar]
- 24.Kamel E, Hany TF, Burger C, Treyer V, Lonn AHR, von Schulthess GK, et al. CT vs 68Ge attenuation correction in a combined PET/CT system: evaluation of the effect of lowering the CT tube current. Eur J Nucl Med Mol Imaging. 2002;29:346–350. doi: 10.1007/s00259-001-0698-9. [DOI] [PubMed] [Google Scholar]
- 25.Nakamoto Y, Osman M, Cohade C, Marshall LT, Links JM, Kohlmyer S, et al. PET/CT: comparison of quantitative tracer uptake between germanium and CT transmission attenuation corrected images. J Nucl Med. 2002;43:1137–1143. [PubMed] [Google Scholar]
- 26.Miyata H, Yamasaki M, Takahashi T, Murakami K, Tanaka K, Yukinori K, et al. Determinants of response to neoadjuvant chemotherapy for esophageal cancer using 18F-fluorodeoxiglucose positron emission tomography (18F-FDG-PET) Ann Surg Oncol. 2014;21:575–582. doi: 10.1245/s10434-013-3343-5. [DOI] [PubMed] [Google Scholar]
- 27.Port JL, Lee PC, Korst RJ, Liss Y, Meherally D, Christos P, et al. Positron emission tomographic scanning predicts survival after induction chemotherapy for esophageal carcinoma. Ann Thorac Surg. 2007;84(2):393–400. doi: 10.1016/j.athoracsur.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 28.Boellaard R. Need for standardization of 18F-FDG PET/CT for treatment response assessment. Eur J Nucl Med. 2011;52(12):93S-100 S. doi: 10.2967/jnumed.110.085662. [DOI] [PubMed] [Google Scholar]
- 29.Dassen AE, Lips DJ, Hoekstra CJ, Pruijt JFM, Bosscha K. FDG-PET has no definite role in preoperative imaging in gastric cancer. Eur J Surg Oncol. 2009;35(5):449–455. doi: 10.1016/j.ejso.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Vallbohmer D, Holscher AH, Schneider PM, Schmidt M, Dietlein M, Bollschweiler E, et al. [18F]-fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemotherapy in gastric cancer. J Surg Oncol. 2010;102(2):135–140. doi: 10.1002/jso.21592. [DOI] [PubMed] [Google Scholar]
- 31.Chung HW, Lee EJ, Cho YH, Yoon SY, So Y, Kim SY, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol. 2010;136(12):1929–1935. doi: 10.1007/s00432-010-0852-5. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, et al. [18F] Fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non–small-cell lung cancer. J Clin Oncol. 2005;23:1136–1143. doi: 10.1200/JCO.2005.06.129. [DOI] [PubMed] [Google Scholar]
- 33.Tamandl D, Ta J, Schmid R, Preusser M, Paireder M, Schoppmann SF, et al. Prognostic value of volumetric PET parameters in unresectable and metastatic esophageal cancer. Eur J Radiol. 2016;85:540–545. doi: 10.1016/j.ejrad.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Grabinska K, Pelak M, Wydmanski J, Tukiendorf A, d’Amico A. Prognostic value and clinical correlations of 18-Fluorodeoxyglucose metabolism quantifiers in gastric cancer. World J Gastroenterol. 2015;21(19):5901–5909. doi: 10.3748/wjg.v21.i19.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen R, Zhou X, Liu J, Huang G. Relationship between 18F-FDG PET/CT findings and HER2 expression in gastric cancer. J Nucl Med. 2016;57:1040–1044. doi: 10.2967/jnumed.115.171165. [DOI] [PubMed] [Google Scholar]
- 36.zum Buschenfelde CM, Herrmann K, Schuster T, Geinitz H, Langer R, Becker K, et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med. 2011;52(8):1189–1196. doi: 10.2967/jnumed.110.085803. [DOI] [PubMed] [Google Scholar]
- 37.Won E, Shah MA, Schöder H, Strong VE, Coit DG, Brennan MF, et al. Use of positron emission tomography scan response to guide treatment change for locally advanced gastric cancer: the Memorial Sloan Kettering Cancer Center experience. J Gastrointest Oncol 2016;7(4):506–14. [DOI] [PMC free article] [PubMed]
- 38.Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-Fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17(1):115–122. doi: 10.1245/s10434-009-0719-7. [DOI] [PubMed] [Google Scholar]
- 39.Coupe NA, Karikios D, Chong S, Yap J, Ng W, Merrett N, et al. Metabolic information on staging FDG-PET–CT as a prognostic tool in the evaluation of 97 patients with gastric cancer. Ann Nucl Med. 2014;28:128–135. doi: 10.1007/s12149-013-0791-8. [DOI] [PubMed] [Google Scholar]
- 40.Kato H, Takita J, Miyazaki T, Nakajima M, Fukai Y, Masuda N, et al. Correlation of 18-F-fluorodeoxyglucose (FDG) accumulation with glucose transporter (Glut-1) expression in esophageal squamous cell carcinoma. Anticancer Res. 2003;23(4):3263–3272. [PubMed] [Google Scholar]
- 41.Schreurs LMA, Smit JK, Pavlov K, Pultrum BB, Pruim J, Groen H, et al. Prognostic impact of clinicopathological features and expression of biomarkers related to 18F-FDG uptake in esophageal cancer. Ann Surg Oncol. 2014;21(12):3751–3757. doi: 10.1245/s10434-014-3848-6. [DOI] [PubMed] [Google Scholar]