Abstract
Helicobacter pylori infects over half the world's population and is a leading cause of peptic ulcer and gastric cancer. H. pylori infection results in chronic inflammation of the gastric mucosa, and progression of chronic inflammation leads to glandular atrophy and intestinal metaplasia. However, how this chronic inflammation is induced or maintained is not well known. Here, we show that chronic inflammation caused by H. pylori infection is highly correlated with de novo synthesis of peripheral lymph node addressin (PNAd) presented on high-endothelial venule (HEV)-like vessels. The number of HEV-like vessels dramatically increases as chronic inflammation progresses. We found that the PNAd is bound by L-selectin·IgM chimeric protein, and decorated by NCC-ST-439 antibody, which is suggested to recognize both nonsulfated and 6-sulfated sialyl Lewis X on core 2 branched O-glycans, and MECA-79 antibody, which reacts with 6-sulfo N-acetyllactosamine on extended core 1 O-glycans. These results indicate that PNAd on HEV-like vessels present in the gastric mucosa subsequent to H. pylori infection is similar to those on HEVs present in the secondary lymphoid organs, which are essential for lymphocyte circulation. Moreover, eradication of H. pylori is associated with the disappearance of HEV-like vessels in the gastric mucosa. By contrast, very few PNAd were found in the gastric mucosa of patients with chemical gastritis caused by nonsteroidal antiinflammatory drugs. These results strongly suggest that PNAd in HEV-like vessels plays a critical role in lymphocyte recruitment during chronic inflammation induced by H. pylori infection.
Keywords: inflammation, peptic ulcers, gastric carcinoma
Helicobacter pylori is a Gram-negative microaerophilic bacterium that infects >50% of the world's population (1). The infection of H. pylori is usually confined to the surface mucous cell-derived mucin (2). If untreated, this infection leads to chronic active gastritis and develops pyloric gland atrophy and intestinal metaplasia expressing intestine-specific genes, including MUC2, sucrase/isomaltase, and carbonic anhydrase 1 (3-7). This second advanced stage of gastritis is closely associated with the pathogenesis of peptic ulcers.
The host responds to H. pylori infection primarily by mounting a strong neutrophilic response. Such a response contributes to gastric epithelial damage and is followed by chronic inflammatory infiltrates composed of lymphocytes and plasma cells, forming mucosa-associated lymphoid tissue (8). Although it has not been formally proven, it is suggested that this mucosal inflammation in response to H. pylori infection might lead to gastric carcinoma and malignant lymphoma (4, 5, 9-11). It is thus important to understand how lymphocytes are recruited to the gastric mucosa during the progression of chronic inflammation. However, such mechanisms are not fully understood.
In chronic inflammatory states of other systems, L-selectin and its ligands are implicated in lymphocyte recruitment in those diseases for which peripheral lymph node addressin (PNAd) is induced on high-endothelial venule (HEV)-like vessels (12, 13). Such HEV-like vessels have been observed in rheumatoid arthritis, lymphocytic thyroiditis, and inflammatory bowel diseases (14-17). In these studies, the induction of PNAd is detected by MECA-79 antibody (18), which decorates PNAd on HEV-like vessels. Indeed, BCA-1, a homing chemokine in the lymphoid tissue, and MECA-79-positive vessels were detected in mucosa-associated lymphoid tissue associated with H. pylori infection (19, 20).
MECA-79+ HEVs in the secondary lymphoid organs play a major role in lymphocyte circulation (12). The MECA-79 epitope has been shown to be 6-sulfo N-acetyllactosamine attached to extended core 1 O-glycans, Galβ1→4(SO3→6) GlcNAcβ1→3Galβ1→3GalNAcα1→Ser/Thr (21). Moreover, MECA-79 antibody can also bind to its sialylated and fucosylated form that constitutes PNAd (21). Structural studies also showed that 6-sulfo sialyl Lewis X on core 2 branched O-glycans, sialic acid-α2 → 3Galβ1 → 4[Fucα1 → 3(SO3 → 6)]GlcNAcβ1 → 6 (Galβ1→3) GalNAcα1→Ser/Thr is present as a major L-selectin ligand on HEVs (21, 22).
In the present study, we found that inflammatory response to H. pylori infection is associated with the formation of HEV-like vessels in the gastric mucosa. HEV-like vessels express PNAd, characterized by binding to MECA-79, HECA-452 (23), and NCC-ST-439 (24) antibodies, and L-selectin·IgM chimeric protein. The expression of HEV-like vessels, assessed by MECA-79 and HECA-452 antibody staining, was highly correlated with the degree of lymphocyte infiltration. Moreover, we show that HEV-like vessels disappear once H. pylori is eradicated. These results indicate that inflammatory response to H. pylori infection is, at least in part, facilitated by induction of PNAd, thereby recruiting lymphocytes to the gastric mucosa.
Materials and Methods
Histological Specimens. We retrieved 143 formalin-fixed, paraffin-embedded blocks of histological specimens with various degrees of chronic gastritis from the archives of the Department of Laboratory Medicine, Shinshu University Hospital. Tissue sections 3 μm thick were stained with hematoxylin and eosin. Based on the updated Sydney system (25), gastritis was evaluated by using a visual analogue scale to evaluate five factors, including (i) H. pylori density, (ii) polymorphonuclear neutrophil activity, (iii) chronic infiltration of lymphocytes, (iv) glandular atrophy, and (v) intestinal metaplasia. Each factor was categorized into four grades: normal, mild, moderate, and marked. This grading is illustrated in Fig. 6, which is published as supporting information on the PNAS web site.
Biopsy specimens obtained from 20 rheumatoid arthritis patients who had been taking nonsteroidal anti-inflammatory drugs (NSAIDs) were evaluated according to a system (26) modified from Dixon et al. (27) for grading of chemical gastritis. Five histological features, which are composed of (i) foveolar hyperplasia, (ii) edema and prominence of muscle fibers in the lamina propria, (iii) vasodilatation and congestion, (iv) inverse scale for polymorphonuclear neutrophil activity, and (v) inverse scale for chronic lymphocyte infiltration, were graded from 0 (normal or absent) to 3 (marked). Each grade of five categories was added to provide a total chemical score that could range from 0 to 15. Scores from 11 to 15 were considered diagnostic of chemical gastritis. The Ethical Committee of Shinshu University School of Medicine approved these study plans.
Antibodies and Immunohistochemistry. The antibodies used in this study were CSLEX-1 (mouse IgM, Pharmingen), HECA-452 (rat IgM, Pharmingen), MECA-79 (rat IgM, Pharmingen), NCC-ST-439 (mouse IgM, Nippon Kayaku, Tokyo), mouse IgG anti-human CD31 (DAKO), QBEND10 (mouse IgG directed to human CD34, Immunotech, Luminy, France), and rabbit anti-H. pylori polyclonal (DAKO). Immunostaining was performed by using the EnVision+ system (DAKO). Briefly, serial sections were deparaffinized and rehydrated, and endogenous peroxidase activity was blocked by soaking in 0.3% H2O2 methanol solution. Before immunostaining, the antigens were retrieved by incubating sections in a microwave in 10 mM Tris·HCl buffer (pH 8.0) containing 1 mM EDTA for CD31 and CD34 or by 5 min of treatment with 20 μg/ml proteinase K for H. pylori. The tissue sections were blocked with 3% FBS in PBS and incubated with primary antibody. After being washed in PBS, the slides were incubated with horseradish peroxidase- and secondary antibody-conjugated polymer, EnVision+ (DAKO). The color reaction was developed with 3,3′-diaminobenzidine containing 0.02% H2O2 (Vector). Sections were briefly counterstained with hematoxylin. Negative controls were obtained by omitting the primary antibodies. Tonsil tissue was used as a positive control (Fig. 7, which is published as supporting information on the PNAS web site).
The numbers of CD34+, MECA-79+, and HECA-452+ vessels in 10 high-power fields of view with ×400 magnification were obtained. The numbers of MECA-79+ and HECA-452+ vessels each were divided by the number of CD34+ vessels, yielding the percentages of MECA-79+ and HECA-452+ vessels, respectively, as described in ref. 28.
Construction of L- and E-Selectin·IgM Chimeras. The Fc region of human IgM was amplified with the oligonucleotides 5′-CGGGATCCTGTGATTGCTGAGCTGCCTCCCA-3′ and 5′-GCTCTAGATCAGTAGCAGGTGCCAGCTGTGT-3′ by using pcDNA1-mouse P-selectin·IgM (29) as a template and subcloned into the BamHI/XbaI site of pcDNA1.1 (pcDNA1.1-IgM). For construction of pcDNA1.1-human L-selectin·IgM, the 5′ end of L-selectin was excised from pCDM8-human L-selectin·IgG by EcoRI digestion and blunted with Klenow fragment (Roche). After digestion of the 3′ end with BamHI, the excised cDNA was subcloned into the blunted HindIII site and BamHI site of pcDNA1.1-IgM, to form pcDNA1.1-human L-selectin·IgM. Similarly, the PCR product obtained by using pcDNA1-human E-selectin·IgG (30) as a template, was subcloned into pcDNA1.1-IgM, forming pcDNA1.1-human E-selectin·IgM.
Selectin·IgM Chimeric Protein-Binding Assay. Human L-selectin·IgM and E-selectin·IgM chimeric proteins were obtained from the cultured medium of COS-1 cells after transient transfection of pcDNA1.1-L-selectin·IgM and pcDNA1.1-E-selectin·IgM as described in ref. 31.
After blocking endogenous peroxidase activity as described above, we incubated tissue sections with selectin·IgM chimeric protein and rinsed them with PBS containing Ca2+. The sections were then incubated with horseradish peroxidase-conjugated goat anti-human IgM antibody (Pierce). After being rinsed in PBS containing Ca2+, the color reaction was developed with 3,3′-diaminobenzidine as described above. The sections were briefly counterstained with hematoxylin. A control experiment was done with PBS containing 1 mM EDTA.
Statistical Analysis. The statistical difference in the percentages of positive vessels in different chronic infiltrate grades was analyzed by the Kruskal-Wallis test followed by Dunn's post hoc test. The difference of proportions (percentage of patients) was analyzed by Fisher's exact test. In both tests, P values at <0.05 are considered significant.
Stable Expression of P-Selectin Glycoprotein Ligand 1 (PSGL-1), Fucosyltransferase VII (FucT-VII), Core 1 Extension β1,3-N-acetylglucosaminyltransferase (Core1-β3GlcNAcT), Core 2 β1,6-N-acetylglucosaminyltransferase I (Core2GlcNAcT-I), and L-Selectin Ligand Sulfotransferase (LSST) in CHO Cells. CHO cells stably expressing PSGL-1 and Core1-β3GlcNAcT, Core2GlcNAcT-I, or FucT-VII were established as described in ref. 32. CHO cells stably expressing PSGL-1, Core1-β3GlcNAcT, and FucT-VII, CHO-PSGL-1·C1·F7 (32) were further stably transfected with pcDNA1.1-LSST (30) and pCMV/Bsd (Invitrogen) and selected with Blasticidin S (Invitrogen). The cells were sorted and cloned for MECA-79+ staining to obtain CHO-PSGL-1·C1·F7·LSST.
CHO-PSGL-1·C2·F7 cells (32) were transfected with pcDNA1.1-LSST. Cloned cells positive for HECA-452 and negative for CSLEX-1 were chosen as CHO-PSGL-1·C2·F7·LSST. Previously, it was shown that 6-sulfo sialyl Lewis X is recognized by HECA-452 antibody but not by CSLEX-1 antibody (33). Similarly, CHO-PSGL-1·C2·F7·LSST cells were stably transfected with Core1-β3GlcNAcT, yielding MECA-79+ CHO-PSGL-1·C1·C2·F7·LSST.
Results
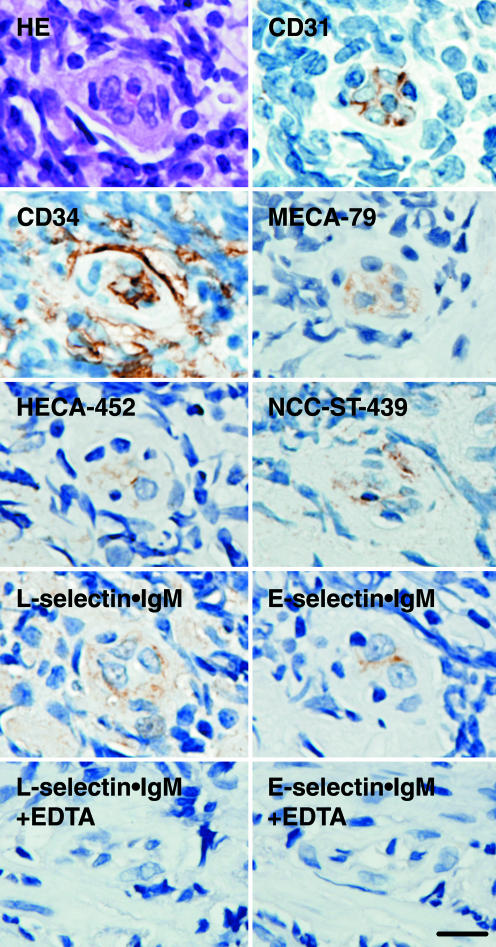
HEV-Like Vessels Are Induced in H. pylori-Induced Inflammation. Because it has been reported that de novo formation of HEV-like vessels, which express PNAd, is associated with various chronic inflammatory diseases, we determined whether chronic inflammation caused by H. pylori infection is also associated with the formation of HEV-like vessels. To do so, gastric mucosa from patients infected with H. pylori was stained with MECA-79 antibody, which reacts with 6-sulfo sialyl Lewis X on extended core 1, and HECA-452 antibody, which reacts equally well with sialyl Lewis X and 6-sulfo sialyl Lewis X-capping structure on extended core 1 and core 2 branches (Fig. 1; see also Fig. 8, which is published as supporting information on the PNAS web site). As shown in Fig. 2, gastric mucosa derived from H. pylori-infected patients displayed HEV-like vessels expressing MECA-79 and HECA-452 antigens, as well as CD31 and CD34, which are markers for vascular endothelial cells. Moreover, these HEV-like vessels can potentially recruit L-selectin-expressing lymphocytes, because L-selectin·IgM chimeric protein bound the same vessels in a Ca2+-dependent manner. Indeed, a large number of B and T lymphocytes were recruited in the gastric mucosa infected with H. pylori (Fig. 9, which is published as supporting information on the PNAS web site). These results indicate that H. pylori-induced inflammation is associated with the formation of PNAd present on HEV-like vessels.
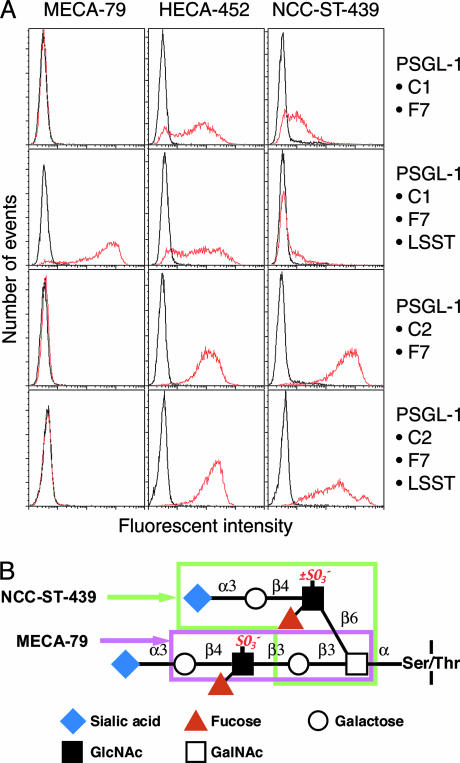
Fig. 1.
FACS analysis of transfected CHO cells expressing sialyl Lewis X or 6-sulfo sialyl Lewis X on extended core 1 or core 2 branched O-glycans (32) and structures of MECA-79 and NCC-ST-439 epitopes are shown. (A) MECA-79 binds CHO cells expressing 6-sulfo sialyl Lewis X on extended core 1 (CHO-PSGL-1·C1·F7·LSST) but not sialyl Lewis X (CHO-PSGL-1·C1·F7). NCC-ST-439 binds CHO cells expressing sialyl Lewis X (CHO-PSGL-1·C2·F7) and 6-sulfo sialyl Lewis X (CHO-PSGL-1·C2·F7·LSST) on core 2 branched O-glycans but barely binds those expressing sialyl Lewis X or 6-sulfo sialyl Lewis X on extended core 1 O-glycans. HECA-452 binds all cells tested, but its reactivity apparently depends on the expression level of extended core 1 O-glycans. (B) L-selectin ligand containing 6-sulfo sialyl Lewis X on core 2 branch and extended core 1 structure is shown. The epitopes for NCC-ST-439 and MECA-79 are shown in boxes.
Fig. 2.
MECA-79, HECA-452, and NCC-ST-439 antigens in a HEV-like vessel in the gastric mucosa with H. pylori-associated chronic active gastritis. Serial sections were subjected to immunostaining with anti-CD31, anti-CD34, MECA-79, HECA-452, and NCC-ST-439 antibodies and to a binding assay with L-selectin·IgM and E-selectin·IgM chimeric proteins in the absence or presence of 1 mM EDTA. HE, hematoxylin and eosin staining. (Bar, 10 μm.)
The above results demonstrated that 6-sulfo sialyl Lewis X on extended core 1 O-glycans is present based on positive staining by MECA-79 and HECA-452 antibodies. To elaborate further the chemical nature of L-selectin ligands on these HEV-like vessels, the NCC-ST-439 monoclonal antibody was used. NCC-ST-439 antibody binding has been tested for sialyl Lewis X-capping structure on Galβ1→4GlcNAcβ1→6GalNAcα1→R but not on natural core 2 branched O-glycan Galβ1→ 4GlcNAcβ1→6(Galβ1→3) GalNAcα1→R (missing Galβ1→3 shown in bold) (24). Moreover, it has not been determined whether 6-sulfo sialyl Lewis X is also recognized by this antibody. To test these possibilities, we made CHO cells expressing various types of O-glycans and stained the cells with NCC-ST-439 antibody. Fig. 1 illustrates that NCC-ST-439 antibody binds to CHO cells expressing nonsulfated and 6-sulfo sialyl Lewis X on core 2 branched O-glycans but barely to CHO cells expressing those capping structures on extended core 1 O-glycans. Fig. 2 shows that NCC-ST-439 antibody can also stain HEV-like vessels formed in the gastric mucosa. These results combined suggest that PNAd induced by H. pylori infection expresses 6-sulfo sialyl Lewis on both extended core 1 and core 2 branched structures in the same manner as PNAd expressed in the secondary lymphoid organs (21).
Increased Formation of HEV-Like Vessels Is Correlated with Progression of Inflammation. Based on morphological examination, progression of inflammation initiated by H. pylori infection can be roughly divided into four stages i.e., normal, mild, moderate, and marked (25). In the moderate and marked stages, intestinal metaplasia frequently takes place, which indicates an advanced stage of the disease.
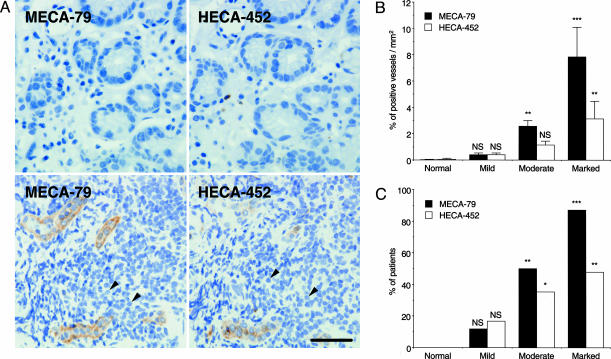
Fig. 3A Lower indicates a marked stage of inflammation in which recruitment of mononuclear cells obscured proper glands in gastric mucosa compared with glands shown in mucosa at mild stage (Fig. 3A Upper). These observations demonstrate that lymphocyte infiltration is more prominent when HEV-like vessels are more abundant.
Fig. 3.
Gastric mucosa of different degrees of chronic inflammation and association of HEV-like vessels with progression of inflammation. (A) (Upper) Gastric mucosa at a mild stage barely expresses HEV-like vessels with minimum recruitment of lymphocytes. (Lower) Gastric mucosa at a marked stage express a significant number of recruited lymphocytes (arrowheads) around HEV-like vessels. (B) The number of MECA-79+ or HECA-452+ vessels is positively correlated with the progression of chronic inflammation. Each group consists of 11 (normal), 42 (mild), 67 (moderate), and 23 (marked) patients. (C) The number of patients exhibiting ≥1% MECA-79+ or HECA-452+ vessels is highly correlated with the progression of chronic inflammation. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant. (Bar, 50 μm.)
After examining >140 human specimens, we found that the number of HEV-like vessels, as detected by MECA-79 or HECA-452 antibody, is positively correlated with the progression of inflammation (Fig. 3B and Table 1, which is published as supporting information on the PNAS web site). Fig. 3C illustrates that more patients display HEV-like vessels as the inflammation progresses. H. pylori was detected in 0%, 21%, 82%, and 87% of patients in normal, mild, moderate, and marked stages of the inflammation, respectively. Overall, HEV-like vessels were found in 79.2% of H. pylori-infected patients. These results combined indicate that progression of inflammation, initiated by H. pylori infection, is highly correlated with formation of HEV-like vessels at the gastric mucosa.
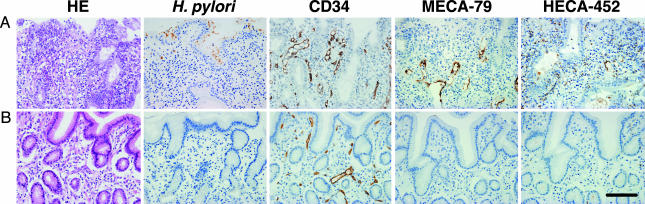
Formation of HEV-Like Vessels Depends on Continuous H. pylori Infection. To determine whether the formation of HEV-like vessels is correlated with the presence of H. pylori infection, gastric biopsies were obtained from 17 patients with chronic active gastritis before and after eradication of H. pylori by treatment with antibiotics and a proton pump inhibitor. Patients with a moderate inflammation stage displayed both H. pylori and HEV-like vessels detected by MECA-79 and HECA-452 antibodies (Fig. 4A). After eradication of H. pylori, the gastric mucosa of all of the patients examined no longer displayed HEV-like vessels as assessed by MECA-79 and HECA-452 staining and showed minimum lymphocyte infiltration (Fig. 4B). These results indicate that continuous infection of H. pylori is necessary for formation and maintenance of HEV-like vessels expressing PNAd.
Fig. 4.
Disappearance of HEV-like vessels in the gastric mucosa after eradication of H. pylori. Gastric mucosa infected by H. pylori was examined before and 2 months after a treatment to eradicate H. pylori. (A) Before the treatment, HEV-like vessels detected by MECA-79 and HECA-452 antibodies were abundant, and large numbers of mononuclear cells (lymphocytes) were present around these vessels. (B) After eradication of H. pylori, HEV-like vessels were no longer present and few mononuclear cells were present. (Bar, 100 μm.)
HEV-Like Vessels in NSAIDs-Induced Gastritis. To determine whether HEV-like vessels are induced in chronic inflammatory response by causes other than H. pylori infection, gastric mucosa obtained from long-standing rheumatoid arthritis patients taking NSAIDs were examined, because it is known that continuous use of NSAIDs results in chemical gastritis. The majority of 20 patients examined exhibited chemical gastritis phenotype and were devoid of HEV-like vessels (Fig. 5A; see also Fig. 10A, which is published as supporting information on the PNAS web site). HEV-like vessels were found in the specimens from 6 of 20 patients, but 3 of these patients were also infected with H. pylori. All of those three patients display lower scores for chemical gastritis, and HEV-like vessels were likely formed by inflammation caused by H. pylori infection. In three H. pylori-free patients, HEV-like vessels were found in only 0.68%, 0.67%, and 0.21% of CD34+ vessels, and two of them displayed intestinal meta-plasia, suggesting a possible prior infection of H. pylori. Fig. 5B illustrates that the remaining patient displayed both chemical gastritis phenotype and lymphocyte recruitment. Interestingly, the intensity of MECA-79 staining was much less for this patient than for those infected with H. pylori (compare Fig. 5B with Figs. 2, 3, 4). These results indicate that chemical gastritis negligibly induces PNAd in the gastric mucosa.
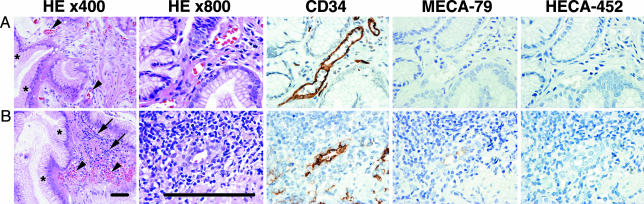
Fig. 5.
Few HEV-like vessels are associated with chemical gastritis caused by NSAIDs. (A and B) Gastric mucosa obtained from two rheumatoid arthritis patients taking NSAIDs. In both patients, typical chemical gastritis phenotype, such as foveolar hyperplasia (asterisks) and vasodilatation and congestion (arrowheads), is evident. (B) MECA-79+ HEV-like vessels are associated only with substantial lymphocyte recruitment (arrows), which is rather atypical for chemical gastritis. Images for HE ×800, CD34, MECA-79, and HECA-452 are further enlarged in the same scale to show details. (Bar, 50 μm.)
Discussion
The present studies demonstrate that H. pylori-induced inflammation is associated with the recruitment of lymphocytes through de novo formation of PNAd on HEV-like vessels. HEV-like vessels are absent in human gastric mucosa under normal conditions. Once chronic inflammation occurs, these vessels, which bind L-selectin·IgM chimeric protein in a Ca2+-dependent manner, appear. Our observations of de novo formation of HEV-like vessels are similar to those described previously in various human chronic inflammatory diseases, such as rheumatoid arthritis, lymphocytic thyroiditis, inflammatory bowel diseases, and in acute heart allograft rejection (14-17, 34). However, none of the previous studies showed disappearance of HEV-like vessels by removing causes that led to their formation. We have established here that the abundance of HEV-like vessels is highly correlated with the progression of H. pylori-associated chronic active gastritis, and that HEV-like vessels are no longer expressed after H. pylori is eradicated. By contrast, NSAIDs-induced gastritis patients display only very few HEV-like vessels, if any.
In the present study, we showed that HEV-like vessels are positive for MECA-79 and HECA-452 staining, suggesting that 6-sulfo sialyl Lewis X on the extended core 1 structure is present on PNAd. We also found that HEV-like vessels were stained by NCC-ST-439, the intensity of which was similar to that on neutrophils expressing sialyl Lewis X on core 2 branched O-glycans (35, 36). These results suggest that HEV-like vessels in the gastric mucosa should contain 6-sulfo sialyl Lewis X and sialyl Lewis X on extended core 1 and core 2 branched O-glycans. In previous studies, MECA-79 has been frequently used to characterize PNAd on HEV-like vessels. Although HECA-452 antibody was also used in one study, no structural basis for differential staining was inferred (28). By using HECA-452 and NCC-ST-439 antibodies in addition to MECA-79 antibody, we have obtained more detailed knowledge of the chemical nature of L-selectin ligands on HEV-like vessels induced by H. pylori infection. Because it is difficult to isolate a sufficient number of cells expressing PNAd from many pathological specimens, a combination of MECA-79, HECA-452, and NCC-ST-439 staining would be useful to determine the chemical nature of HEV-like vessels formed in these chronic inflammatory diseases.
It has been shown that the H. pylori adhesin Sab A binds sialyl dimeric Lewis X-bearing glycosphingolipids in the surface mucous cells (37). The expression of L-selectin ligands including 6-sulfo sialyl Lewis X may thus enhance adhesion of H. pylori to mucosa expressing HEV-like vessels. At the same time, colonization of H. pylori on the gastric mucosa may contribute to gastritis by producing autoantibodies, because both H. pylori and acid-secreting parietal cells share Lewis X antigen (38). Core 2 branched O-glycans are capped by α1,4-linked N-acetylglucosamine in the gastric mucosa, and those O-glycans are secreted from gland mucous cells (39). Strikingly, our recent studies demonstrate that this glandular mucin containing α1,4-GlcNAc-capped O-glycans inhibits H. pylori growth and thus acts as a natural antibiotic against H. pylori infection, whereas MUC5AC secreted from surface mucous cells stimulates H. pylori growth (39). The results are consistent with the observation that H. pylori rarely colonizes the layer where gland mucous cell-derived mucins are present (2). These combined results suggest that the expression of sialyl Lewis X may facilitate H. pylori infection and resultant inf lammation, whereas α1,4-GlcNAc-capped O-glycans in the gland mucous cells inhibits this process.
The present study also demonstrated that HEV-like vessels disappear once H. pylori is eradicated by antibiotic treatment. The results indicate that the formation of HEV-like vessels and thus recruitment of lymphocytes to chronic inflammatory sites in the gastric mucosa can be reversed once H. pylori infection is abolished. It is tempting to speculate that lipopolysaccharides and/or other components from the organism, acting through Toll-like receptor-dependent pathways in the gastric epithelium, exerts the elaboration of cytokines, i.e., lymphotoxin α. This effect might in turn modulate gene expression in postcapillary venules in ways that cause their biochemical, functional, and morphological transformation by up-regulating chemokines, such as CCL19 and CCL21, that act on CCR7 receptors.
Several enzymes play major roles in the biosynthesis of PNAd at the secondary lymphoid organs, including LSST (also called GlcNAc6ST-2 or HEC-GlcNAc6ST) (30, 40), Core2GlcNAcT-I (21, 31), and Core1-β3GlcNAcT (21, 32). Mice that are mutant in any of these enzymes exhibit reduced lymphocyte homing activity compared with wild-type mice (31, 32, 40). Future studies that determine whether inhibition of L-selectin ligand synthesis can inhibit the chronic inflammation induced by H. pylori infection and, hence, the formation of gastric carcinoma would be of great interest. Mice with targeted mutations in various glycosyltransferases and sulfotransferases responsible for L-selectin ligand synthesis will likely provide critical tools for those studies.
Supplementary Material
Acknowledgments
We thank Yasuyo Shimojo and Akiko Ishida for preparation of tissue sections, Dr. John Lowe (University of Michigan Medical School, Ann Arbor) for providing pcDNA1-P-selectin·IgM, Dr. Elise Lamar for critical reading of the manuscript, Yoav Altman for assisting in FACS analysis, and Aleli Morse for organizing the manuscript. This work was supported by the National Institutes of Health Grant R37 CA33000 (to M.F.) and by Ministry of Education, Culture, Sports, Science, and Technology of Japan Priority Area 14082201 and the Ministry of Health, Labor, and Welfare of Japan (Third Term Comprehensive Control Research for Cancer) (to J.N.).
Author contributions: M.K., T.K., J.N., and M.F. designed research; M.K. performed research; M.K., J.N., and M.F. analyzed data; J.M. and N.N. contributed new reagents/analytical tools; and M.K., J.M., J.N., and M.F. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HEV, high-endothelial venule; PNAd, peripheral lymph node addressin; NSAID, nonsteroidal antiinflammatory drug; LSST, L-selectin ligand sulfotransferase; FucT-VII, fucosyltransferase VII; Core1-β3GlcNAcT, core 1 extension β1,3-N-acetylglucosaminyltransferase; Core2GlcNAcT-I, core 2 β1,6-N-acetylglucosaminyltransferase I; PSGL-1, P-selectin glycoprotein ligand 1.
References
- 1.Marshall, B. J. & Warren, J. R. (1984) Lancet i, 1311-1315. [DOI] [PubMed] [Google Scholar]
- 2.Hidaka, E., Ota, H., Hidaka, H., Hayama, M., Matsuzawa, K., Akamatsu, T., Nakayama, J. & Katsuyama, T. (2001) Gut 49, 474-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sipponen, P. & Hyvarinen, H. (1993) Scand. J. Gastroentrol., Suppl., 196, 3-6. [DOI] [PubMed] [Google Scholar]
- 4.Peek, R. M., Jr. & Blaser, M. J. (2002) Nat. Rev. Cancer 2, 28-37. [DOI] [PubMed] [Google Scholar]
- 5.Yuasa, Y. (2003) Nat. Rev. Cancer 3, 592-600. [DOI] [PubMed] [Google Scholar]
- 6.Freund, J. N., Domon-Dell, C., Kedinger, M. & Duluc, I. (1998) Biochem. Cell. Biol. 76, 957-969. [DOI] [PubMed] [Google Scholar]
- 7.Hinoi, T., Lucas, P. C., Kuick, R., Hanash, S., Cho, K. R. & Fearon, E. R. (2002) Gastroenterology 123, 1565-1577. [DOI] [PubMed] [Google Scholar]
- 8.Bayerdorffer, E., Neubauer, A., Rudolph, B., Thiede, C., Lehn, N., Eidt, S. & Stolte, M. (1995) Lancet 345, 1591-1594. [DOI] [PubMed] [Google Scholar]
- 9.Nomura, A., Stemmermann, G. N., Chyou, P. H., Kato, I., Perez-Perez, G. I. & Blaser, M. J. (1991) N. Engl. J. Med. 325, 1132-1136. [DOI] [PubMed] [Google Scholar]
- 10.Parsonnet, J., Freidman, G. D., Vandersteen, D. P., Chang, Y., Vogelman, J. H., Orentreich, N. & Sibley, R. K. (1991) N. Engl. J. Med. 325, 1127-1131. [DOI] [PubMed] [Google Scholar]
- 11.Higashi, H., Tsutsumi, R., Muto, S., Sugiyama, T., Azuma, T., Asaka, M. & Hatakeyama, M. (2002) Science 295, 683-686. [DOI] [PubMed] [Google Scholar]
- 12.Rosen, S. D. (2004) Annu. Rev. Immunol. 22, 129-156. [DOI] [PubMed] [Google Scholar]
- 13.von Andrian, U. H. & Mackay, C. R. (2000) N. Engl. J. Med. 343, 1020-1034. [DOI] [PubMed] [Google Scholar]
- 14.Duijvestijn, A. M., Kerkhove, M., Bargatze, R. F. & Butcher, E. C. (1987) J. Immunol. 138, 713-719. [PubMed] [Google Scholar]
- 15.Kabel, P. J., Voorbij, H. A., de Haan-Meulman, M., Pals, S. T. & Drexhage, H. A. (1989) J. Clin. Endocrinol. Metab. 68, 744-751. [DOI] [PubMed] [Google Scholar]
- 16.van Dinther-Janssen, A. C., Pals, S. T., Scheper, R., Breedveld, F. & Meijer, C. J. (1990) J. Rheumatol. 17, 11-17. [PubMed] [Google Scholar]
- 17.Salmi, M., Granfors, K., MacDermott, R. & Jalkanen, S. (1994) Gastroenterology 106, 596-605. [DOI] [PubMed] [Google Scholar]
- 18.Streeter, P. R., Rouse, B. T. & Butcher, E. C. (1988) J. Cell. Biol. 107, 1853-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzucchelli, L., Blaser, A., Kappeler, A., Schärli, P., Laissue, J. A., Baggiolini, M. & Uguccioni, M. (1999) J. Clin. Invest. 104, R29-R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dogan, A., Du, M., Koulis, A., Briskin, M. J. & Isaacson, P. G. (1997) Am. J. Pathol. 151, 1361-1369. [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh, J. C., Hiraoka, N., Petryniak, B., Nakayama J., Ellies, L. G., Rabuka, D., Hindsgaul, O., Marth, J. D., Lowe, J. B. & Fukuda, M. (2001) Cell 105, 957-969. [DOI] [PubMed] [Google Scholar]
- 22.Hemmerich, S., Leffler, H. & Rosen, S. D. (1995) J. Biol. Chem. 270, 12035-12047. [DOI] [PubMed] [Google Scholar]
- 23.Duijvestijn, A. M., Horst, E., Pals, S. T., Rouse, B. N., Steere, A. C., Picker, L. J., Meijer, C. J. & Butcher, E. C. (1988) Am. J. Pathol. 130, 147-155. [PMC free article] [PubMed] [Google Scholar]
- 24.Kumamoto, K., Mitsuoka, C., Izawa, M., Kimura, N., Otsubo, N., Ishida, H., Kiso, M., Yamada, T., Hirohashi, S. & Kannagi, R. (1998) Biochem. Biophys. Res. Commun. 247, 514-517. [DOI] [PubMed] [Google Scholar]
- 25.Dixon, M. F., Genta, R. M., Yardley, J. H. & Correa, P. (1996) Am. J. Surg. Pathol. 20, 1161-1181. [DOI] [PubMed] [Google Scholar]
- 26.Taha, A. S., Nakshabendi, I., Lee, F. D., Sturrock, R. D. & Russel, R. I. (1992) J. Clin. Pathol. 45, 135-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon, M. F., O'Connor, H. J., Axon, A. T., King, R. F. & Johnston, D. (1986) J. Clin. Pathol. 39, 524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renkonen, J., Tynninen, O., Hayry, P., Paavonen, T. & Renkonen, R. (2002) Am. J. Pathol. 161, 543-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maly, P., Thall, A., Petryniak, B., Rogers, C. E., Smith, P. L., Marks, R. M., Kelly, R. J., Gersten, K. M., Cheng, G., Saunders, T. L., et al. (1996) Cell 86, 643-653. [DOI] [PubMed] [Google Scholar]
- 30.Hiraoka, N., Petryniak, B., Nakayama, J., Tsuboi, S., Suzuki, M., Yeh, J. C., Izawa, D., Tanaka, T., Miyasaka, M., Lowe, J. B. & Fukuda, M. (1999) Immunity 11, 79-89. [DOI] [PubMed] [Google Scholar]
- 31.Hiraoka, N., Kawashima, H., Petryniak, B., Nakayama, J., Mitoma, J., Marth, J. D., Lowe, J. B. & Fukuda, M. (2004) J. Biol. Chem. 279, 3058-3067. [DOI] [PubMed] [Google Scholar]
- 32.Mitoma, J., Petryniak, B., Hiraoka, N., Yeh, J. C., Lowe, J. B. & Fukuda, M. (2003) J. Biol. Chem. 278, 9953-9961. [DOI] [PubMed] [Google Scholar]
- 33.Mitsuoka, C., Kawakami-Kimura, N., Kasugai-Sawada, M., Hiraiwa, N., Toda, K., Ishida, H., Kiso, M., Hasegawa, A. & Kannagi, R. (1997) Biochem. Biophys. Res. Commun. 230, 546-551. [DOI] [PubMed] [Google Scholar]
- 34.Toppila, S., Paavonen, T., Nieminen, M. S., Häyry, P. & Renkonen, R. (1999) Am. J. Pathol. 155, 1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda, M., Carlsson, S. R., Klock, J. C. & Dell, A. (1986) J. Biol. Chem. 261, 12796-12806. [PubMed] [Google Scholar]
- 36.Wilkins, P. P., McEver, R. P. & Cummings, R. D. (1996) J. Biol. Chem. 271, 18732-18742. [DOI] [PubMed] [Google Scholar]
- 37.Mahdavi, J., Sonden, B., Hurtig, M., Olfat, F. O., Forsberg, L., Roche, N., Angstrom, J., Larsson, T., Teneberg, S., Karlsson, K. A., et al. (2002) Science 297, 573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guruge, J. L., Falk, P. G., Lorenz, R. G., Dans, M., Wirth, H. P., Blaser, M. J., Berg, D. E. & Gordon, J. I. (1998) Proc. Natl. Acad. Sci. USA 95, 3925-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawakubo, M., Ito, Y., Okimura, Y., Kobayashi, M., Sakura, K., Kasama, S., Fukuda, M. N., Fukuda, M., Katsuyama, T. & Nakayama, J. (2004) Science 305, 1003-1006. [DOI] [PubMed] [Google Scholar]
- 40.Hemmerich, S., Bistrup, A., Singer, M. S., van Zante, A., Lee, J. K., Tsay, D., Peters, M., Carminati, J. L., Brennan, T. J., Carver-Moore, K., et al. (2001) Immunity 15, 237-247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.