Summary
Branched-chain α-ketoacid (BCKA) dehydrogenase complex (BCKDC) regulates branched-chain amino acid (BCAA) metabolism at the level of BCKA catabolism. It has been demonstrated that the activity of hepatic BCKDC is markedly decreased in type 2 diabetic animal models. In this study, we examined the regulation of hepatic BCKDC in rats with diet-induced obesity (DIO). Rats were fed a control or a 60% of energy high-fat diet (HFD) for twelve weeks. Concentrations of blood components and the activities and protein amounts of hepatic BCKDC and its specific kinase (BDK) were measured. The concentrations of plasma glucose, insulin, and corticosterone were significantly elevated in DIO rats compared to those fed the control diet, suggestive of insulin resistance. Blood BCAA concentrations were not increased. The activity of hepatic BCKDC that was present in the active form in the liver was higher in DIO rats compared to controls, although the total activity and the enzyme amount were not different between two diet groups. The activity of hepatic BDK and the abundance of BDK bound to the BCKDC were decreased in DIO rats. The total amount of hepatic BDK was also significantly decreased in DIO rats. In rats made obese through HFD feeding, in contrast to prior studies in rat models of type 2 diabetes, hepatic BDK was down-regulated and thereby hepatic BCKDC was activated, suggesting that DIO promotes liver BCKA catabolism. In this model there was no evidence that increased blood BCAAs drive DIO-associated insulin resistance, since concentrations of BCAAs were not altered by DIO.
Keywords: BCAA catabolism, hepatic BCKDC, hepatic BDK, high-fat diet, diet-induced obesity
INTRODUCTION
Branched-chain amino acid (BCAA) catabolism is regulated by the mitochondrial branched-chain α-ketoacid dehydrogenase (BCKDH) complex (BCKDC), which catalyzes the irreversible oxidative decarboxylation of the three branched-chain α-ketoacids (BCKAs) generated by reversible transamination of BCAAs in the BCAA catabolic pathway [1]. The BCKDC is a multienzyme complex composed of E1 (consisting of α and β components), E2, and E3 [2], and its activity is subject to regulation through reversible phosphorylation (inactivation) and dephosphorylation (activation) of the E1 α component by a specific kinase (BCKDH kinase (BDK)) and a specific phosphatase, respectively [1]. The BDK bound to the complex (bound form) plays a particularly important role in the regulation of BCKDC activity, as the amount of bound BDK is inversely correlated with the complex activity [2].
The activity of the BCKDC is responsive to alterations in various nutritional and metabolic conditions [1]. Several hormones that control energy metabolism are reported as regulators of the expression of BCKDC subunits and BDK; insulin down-regulates expression of the BCKDC E1α subunit [3] and up-regulates BDK [4], and glucocorticoid up-regulates the BCKDC E2 subunit [5,6] and down-regulates the BDK expression [7]. Considering that metabolic and hormonal cues regulate BCKDC expression and activity, it is possible that BCKDC is an important site contributing to perturbations in systemic BCAA concentrations typically associated with obesity and insulin resistance [8,9]. We have reported that BCKDC activity was increased in the liver of streptozotocin-induced diabetic rats [10]. In contrast to the animal model of type 1 diabetes, hepatic BCAA catabolic enzymes have been shown to be down-regulated in animal models of type 2 diabetes, Otsuka Long-Evans Tokushima Fatty (OLETF) rats and Zucker diabetic fatty (ZDF) rats, in which blood insulin levels were high and the activity of hepatic BCKDC was decreased by high activity of the BDK [11,12]. Although high-fat diet (HFD) feeding is also known to elicit obesity and insulin resistance in rodents, there are few reports concerning the BCAA catabolic state in obesity and insulin resistance caused by diet-induced obesity (DIO).
In the present study, we sought to elucidate the regulation of BCKDC in obesity and insulin resistance induced by HFD feeding, through examination of enzyme activities and protein levels of the hepatic BCKDC and BDK in rats.
MATERIALS AND METHODS
Materials
Lambda protein phosphatase was obtained from New England BioLabs (Beverly, MA). Antiserum against the E2 subunit of the BCKDC and monoclonal antibody against the BDK were prepared as described previously [13]. Goat anti-rabbit and anti-mouse secondary antibodies used in the Western blotting analyses were purchased from Bio-Rad Laboratories (Hercules, CA), and protein A-agarose was from Upstate Biotechnology (Lake Placid, NY). All other reagents were of analytical grade and were purchased from Wako (Osaka, Japan), Oriental Yeast (Tokyo, Japan), Nacalai Tesque (Kyoto, Japan), or Sigma–Aldrich Japan (Tokyo, Japan).
Animals, Diets and Experimental Design
All procedures were approved by the Animal Care Committee of Nagoya University Graduate School of Bioagricultural Sciences. Fourteen male Sprague-Dawley rats aged 8 weeks were obtained from Japan SLC (Hamamatsu, Japan) and were singly-housed in a conventional animal room with controlled temperature (22 ± 1°C) and a 12-h light-dark cycle (lights on at 8:00 h). Rats were randomly allocated to two groups and fed either a control diet (D12450B [Research Diets, New Brunswick, NJ]: 10% of energy from fat, 20% of energy from protein, and 70% of energy from carbohydrate, n=7) or a 60% HFD (D12492 [Research Diets]: 60% of energy from fat, 20% of energy from protein, and 20% of energy from carbohydrate, n=7) for 12 weeks.
On the final day of the experiment, rats were deprived of food for ~8 h (from 08:00) and then sacrificed under anesthesia with sodium pentobarbital (50 mg/kg body weight) by exsanguination; blood samples were obtained from the inferior vena cava with syringe to prepare serum and post-heparin plasma. Subsequently, livers were rapidly removed, freeze clamped at a liquid nitrogen temperature, and stored at −80°C until analyses.
Analyses of blood Components
Concentrations of plasma glucose, and serum free fatty acids (FFAs) and triglyceride (TG) were measured using Wako kits (Wako Pure Chemical Co.; Osaka, Japan). Plasma insulin concentration was measured using a rat insulin ELISA kit (Shibayagi Co.; Gunma, Japan). Plasma BCAAs were analyzed using an amino acid auto-analyzer (Amino Tac, JLC-500/V; Jeol Ltd, Tokyo, Japan) with ninhydrin derivatisation. Plasma corticosterone concentration was measured using a rat corticosterone EIA kit (Cayman Chemical Co.; MI, USA).
Enzyme Activity Assays
The BCKDC associated with BDK was extracted from the liver by the method reported previously [14]. The activity of the hepatic BCKDC was measured by a spectrophotometric assay [14]. One unit of enzyme activity refers to the formation of 1 μmol of NADH/min. The actual activity (the activity of in vivo active (dephosphorylated) enzyme) and the total activity (the activity of the fully dephosphorylated enzyme) of the BCKDC were measured separately. Dephosphorylation of the complex was accomplished by incubating the enzyme extract with lambda protein phosphatase, as described previously [13]. Assay of the hepatic BDK was performed by measuring the ATP-dependent inactivation of the BCKDC [2]. Kinase activity is expressed as the first-order rate constant of the BCKDC inactivation over time.
Immunoprecipitation and Immunoblotting
Rat liver extracts were prepared as reported previously [2] and used for immunoprecipitation with polyclonal antibody against the E2 subunit of the BCKDC [2]. The liver extracts, precipitates, and supernatants were used in the immunoblotting analyses for the determination of the amounts of total, bound and free BDK, respectively [2,13]. Target immunoreactive proteins on the membranes were visualized using ECL Western blotting detection reagents (GE Healthcare UK Limited; Buckinghamshire, UK) and quantified using the AE6962 Light Capture system (ATTO; Tokyo, Japan). The intensities of the bands are expressed relative to the mean values of the control group of rats.
Statistical Analysis
All values are expressed as means ± standard error (SE). Statistical analysis was performed using the StatView (version 5.0) software (SAS Institute, Cary, NC). Data were analyzed by an unpaired two-group Student t-test and differences were considered significant at P < 0.05.
RESULTS
Body Weight, Food Intake and Concentrations of Blood Components
Consistent with prior reports [15–17], body weight and caloric intake were significantly higher in rats fed HFD than in those fed the control diet (Table 1). The concentrations of plasma glucose, insulin, and corticosterone were significantly higher in DIO rats compared to rats fed the control diet (Table 1). The concentrations of serum FFAs and TG, and plasma BCAAs were not significantly different between two diet groups (Table 1).
Table 1.
Body weight, food intake, and concentrations of blood components.
| Control diet group | High-fat diet group | |
|---|---|---|
| Body Weight (g) | 519 ± 15 | 625 ± 31# |
| Food intake (kcal/day) | 93.6 ± 3.1 | 112.3 ± 4.8# |
| Plasma glucose (mg/dL) | 170 ± 11 | 197 ± 9# |
| Plasma insulin (ng/mL) | 6.2 ± 0.6 | 12.3 ± 1.4# |
| Plasma corticosterone (ng/mL) | 220 ± 24 | 323 ± 27# |
| Serum FFAs (μM) | 443 ± 36 | 392 ± 30 |
| Serum TG (mg/dL) | 140 ± 27 | 72 ± 12 |
| Plasma BCAAs (μM) | 382 ± 24 | 401 ± 30 |
| Leucine (μM) | 127 ± 8 | 129 ± 11 |
| Isoleucine (μM) | 75 ± 5 | 78 ± 6 |
| Valine (μM) | 180 ± 11 | 193 ± 13 |
Rats were sacrificed ~8 h after food deprivation on the final day of the experiment. Values are means ± SE, n=7.
P<0.05 vs. control diet group.
Activities of Hepatic BCKDC and BDK
The total activity of hepatic BCKDC was not different between two diet groups (Fig. 1). However, the actual activity of the enzyme was higher in rats fed HFD than in those fed the control diet (Fig. 1). The activity of hepatic BDK, which is responsible for inactivation of BCKDC, was significantly lower in rats fed HFD (Fig. 2), being consistent with the higher actual activity of the BCKDC.
Fig. 1. Effect of HFD feeding on hepatic BCKDC complex activity in male rats fed a 60% of kcal HFD or control diet for 12 weeks.
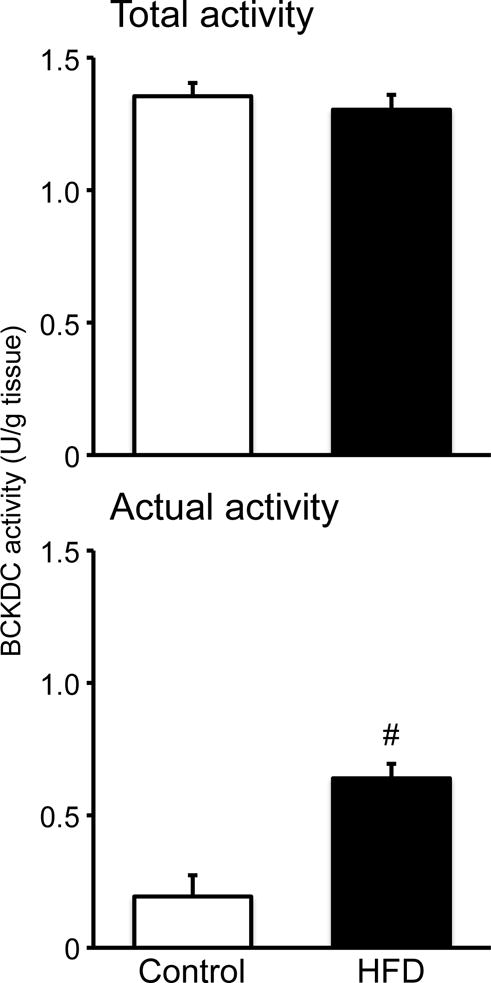
On the final day of experiment, rats were deprived of food for 8 h and sacrificed under anesthesia; liver measurements of actual (the active/dephosphorylated) and total (the fully activated/dephosphorylated) activities of the BCKDC were conducted separately. Values are means ± SE, n=7/treatment. #P<0.05 vs. control diet group.
Fig. 2. Effect of HFD feeding on hepatic BDK activity in male rats fed a 60% of kcal HFD or control diet for 12 weeks.
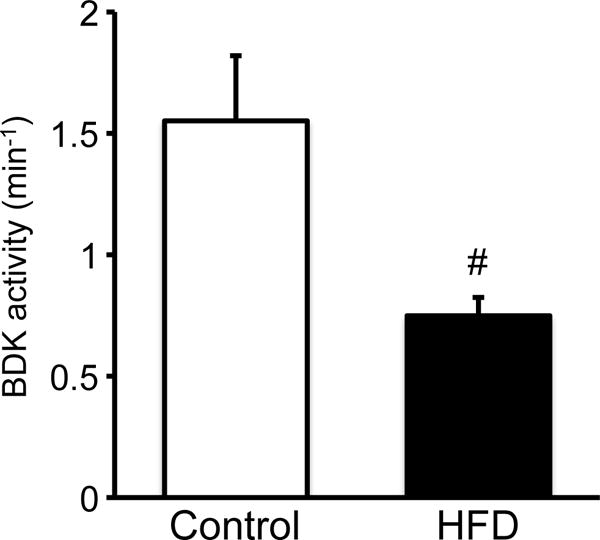
Rats were treated as described in the legend of Fig. 1. Assay of BDK was performed by measuring the ATP-dependent inactivation of the BCKDC [2]. Kinase activity is expressed as the first-order rate constant of BCKDC inactivation over time. Values are means ± SE, n=7/treatment. #P<0.05 vs. control diet group.
Abundance of the Subunits of BCKDC, and Total and Bound BDK
Protein amounts of E1α and E2 subunits of the BCKDC and BDK in the liver were analyzed by Western blot. The abundance of E1α and E2 subunits of the BCKDC was not different between two diet groups (Fig. 3), being consistent with the total activity of the BCKDC. On the other hand, the abundance of the total amount of BDK (Fig. 4A) and the bound form of BDK (Fig. 4B) were significantly lower in rats fed HFD than in animals fed the control diet. Interestingly, approximately 20% of the total BDK were found as free BDK in both groups (Fig. 4A). The amount of free BDK was much less than that reported previously [2].
Fig. 3. Amount of BCKDC subunits in liver extracts of male rats fed a 60% of kcal HFD or control diet for 12 weeks.
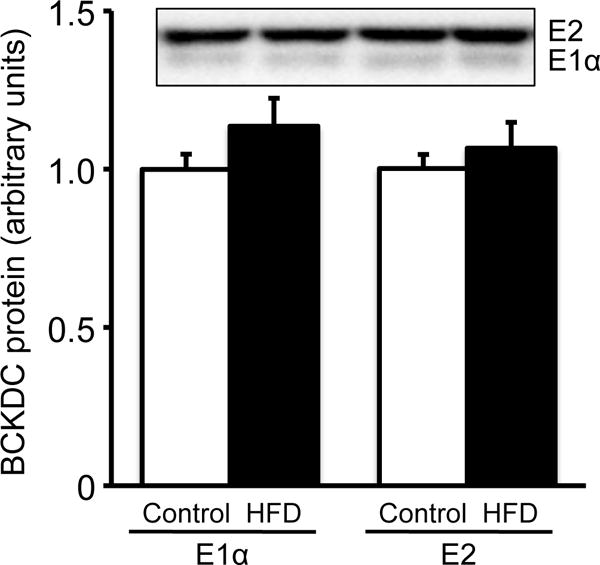
Abundance of BCKDC subunits (E1α and E2) was measured by Western blotting. The same amount of proteins (25 μg) of the liver extracts were applied on each lane of Western blotting. Band intensities are presented relative to the mean values of control diet group. Typical images of Western blots are shown above each bar. Values are means ± SE, n=7/treatment. #P<0.05 vs. control diet group.
Fig. 4. Amount of BDK in liver extracts of male rats fed a 60% of kcal HFD or control diet for 12 weeks.
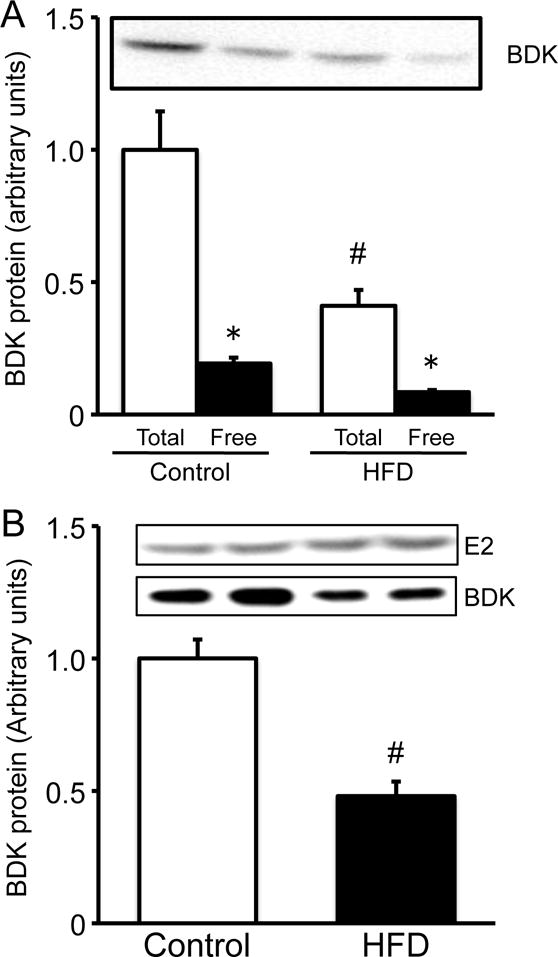
Abundance of total (A), free (A) and bound (B) BDK were measured by Western blotting. For analyzing the total and free BDK, the same amount of proteins (25 μg) of the liver extracts were applied on each lane of Western blotting. For analyzing the bound form of BDK, equivalent protein loading for immunoprecipitated proteins was verified by reprobing membranes with E2 antiserum [2]. Band intensities are presented relative to the mean values of control diet group. Typical images of Western blots are shown above each bar. Values are means ± SE, n=7/treatment. #P<0.05 vs. control diet group in the same form of BDK. *P<0.05 vs. Total BDK in the same diet group.
DISCUSSION
As reported the other studies [15–17], DIO in rats led to hyperinsulinemia, suggesting insulin resistance. Since the concentrations of plasma BCAAs were not altered by DIO in this model, there was no evidence that increased blood BCAAs drive DIO-associated insulin resistance. Hyperinsulinemia is also common in animal models of type 2 diabetes, and we have reported that hepatic BDK activity was significantly elevated in the type 2 diabetic rats, which was attributed to the increase in the BDK bound to the BCKDC [11,12]. The up-regulation of BDK is responsible for the reduced activity of the hepatic BCKDC, suggesting suppression of hepatic BCKA catabolism in the type 2 diabetic rats. From these findings, we hypothesized that insulin resistance induced by the diet-induced obesity would cause elevation of the hepatic BDK activity and thereby reduction of BCKDC activity. However, contrary to our hypothesis, the hepatic BDK activity was markedly reduced in rats fed HFD compared to rats fed the control diet. As described above, the BDK activity corresponds to the amount of the BDK bound to the BCKDC, suggesting that the bound form of the BDK is an active form of the enzyme. Therefore, we measured the amount of the bound BDK, and the results clearly indicated reduction of the bound BDK in rats fed HFD. These results suggest that the elevation of the BCKDC activity in rats fed HFD is attributed to the reduction of the bound BDK. Furthermore, in the present study, the ratio of bound to total BDK was not altered in both groups although the abundance of the total BDK was significantly reduced in rats fed HFD. These results suggest that reduction of the total BDK may contribute to the low activity of the BDK in the animals.
In the previous study, we found that the free BDK was fairly greater than the bound BDK in female rat livers [2]. However, this is not the case in the present study using male rats. This phenomenon is consistent with the gender difference of the regulation of BDK in rat liver [18].
It has been reported that the BDK expression is up-regulated by insulin [4] and down-regulated by corticosterone [7]. In the present study, the levels of both hormones were significantly increased in rats fed HFD. The effects of corticosterone may be dominant over insulin action to regulate expression of the BDK, because the total amount of the BDK was significantly decreased in rats fed HFD. In the ZDF rat model of type 2 diabetes, we observed that both plasma hormone levels were also significantly elevated, but the expression of hepatic BDK was significantly enhanced [12], being not consistent with the findings obtained in the present study. This discrepancy might be explained by the extremely high level (~10-fold) of plasma insulin concentration in the ZDF fatty rats compared to control rats. It is also notable that ZDF rats lack proper leptin signaling, which cannot be excluded as a potential confounding factor. A speculative, yet plausible teleological explanation is that the hyperphagia and hyperinsulinemia of the DIO condition mimics a fed state in which the liver is well-positioned for incoming BCKA combustion. In type 2 diabetes-like conditions coupled to hepatic insulin resistance on the other hand, liver biochemistry mimics the fasted state in which diminution of essential amino acid catabolism is adaptational. Under these conditions, it has been proposed that BCKDC activity would be reduced [8].
In summary, we demonstrated that hepatic BDK was down-regulated and thereby hepatic BCKDC was up-regulated in rats fed HFD, suggesting that events associated with HFD feeding promote BCKA catabolism. However, the concentrations of plasma BCAAs were not altered in this model. Further studies are required to clarify the mechanisms responsible for regulation of the liver BDK and BCKDC by HFD feeding.
Acknowledgments
This work was in part supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20300216 to YS). Amino acid research of Dr. Adams is supported in part by USDA-Agricultural Research Service Intramural Project 5306-51530-019-00, NIH-NIDDK R01DK078328, and the National Dairy Council (grant administered by the Dairy Research Institute). The USDA is an Equal Opportunity employer and provider.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Shimomura Y, Obayashi M, Murakami T, Harris RA. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care. 2001;4:419–23. doi: 10.1097/00075197-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Obayashi M, Sato Y, Harris RA, Shimomura Y. Regulation of the activity of branched-chain 2-oxo acid dehydrogenase (BCODH) complex by binding BCODH kinase. FEBS Lett. 2001;491:50–4. doi: 10.1016/s0014-5793(01)02149-4. [DOI] [PubMed] [Google Scholar]
- 3.Costeas PA, Chinsky JM. Effects of insulin on the regulation of branched-chain alpha-keto acid dehydrogenase E1 alpha subunit gene expression. Biochem J. 1996;318:85–92. doi: 10.1042/bj3180085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nellis MM, Doering CB, Kasinski A, Danner DJ. Insulin increases branched-chain alpha-ketoacid dehydrogenase kinase expression in Clone 9 rat cells. Am J Physiol Endocrinol Metab. 2002;283:E853–60. doi: 10.1152/ajpendo.00133.2002. [DOI] [PubMed] [Google Scholar]
- 5.Costeas PA, Chinsky JM. Glucocorticoid regulation of branched-chain alpha-ketoacid dehydrogenase E2 subunit gene expression. Biochem J. 2000;347:449–57. doi: 10.1042/0264-6021:3470449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Chinsky JM, Costeas PA, Price SR. Acidification and glucocorticoids independently regulate branched-chain alpha-ketoacid dehydrogenase subunit genes. Am J Physiol Cell Physiol. 2001;280:C1176–83. doi: 10.1152/ajpcell.2001.280.5.C1176. [DOI] [PubMed] [Google Scholar]
- 7.Huang YS, Chuang DT. Down-regulation of rat mitochondrial branched-chain 2-oxoacid dehydrogenase kinase gene expression by glucocorticoids. Biochem J. 1999;339:503–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr. 2011;2:445–56. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Murakami T, Nakai N, Nagasaki M, Obayashi M, et al. Modification by exercise training of activity and enzyme expression of hepatic branched-chain alpha-ketoacid dehydrogenase complex in streptozotocin-induced diabetic rats. J Nutr Sci Vitaminol. 2001;47:345–50. doi: 10.3177/jnsv.47.345. [DOI] [PubMed] [Google Scholar]
- 11.Kuzuya T, Katano Y, Nakano I, Hirooka Y, Itoh A, et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem Biophys Res Commun. 2008;373:94–8. doi: 10.1016/j.bbrc.2008.05.167. [DOI] [PubMed] [Google Scholar]
- 12.Doisaki M, Katano Y, Nakano I, Hirooka Y, Itoh A, et al. Regulation of Hepatic Branched-Chain Alpha-Keto Acid Dehydrogenase Kinase in A Rat Model for Type 2 Diabetes Mellitus at Different Stages of The Tisease. Biochem Biophys Res Commun. 2010;393:303–307. doi: 10.1016/j.bbrc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Honda T, Fukuda Y, Nakano I, Katano Y, Goto H, et al. Effects of liver failure on branched-chain α-keto acid dehydrogenase complex in rat liver and muscle: comparison between acute and chronic liver failure. J Hepatol. 2004;40:439–45. doi: 10.1016/j.jhep.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Nakai N, Kobayashi R, Popov KM, Harris RA, Shimomura Y. Determination of branched-chain α-keto acid dehydrogenase activity state and branchedchain α-keto acid dehydrogenase kinase activity and protein in mammalian tissues. Methods Enzymol. 2000;324:48–62. doi: 10.1016/s0076-6879(00)24218-3. [DOI] [PubMed] [Google Scholar]
- 15.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–8. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–12. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krawczewski Carhuatanta KA, Demuro G, Tschöp MH, Pfluger PT, Benoit SC, et al. Voluntary Exercise Improves High-Fat Diet-Induced Leptin Resistance Independent of Adiposity. Endocrinology. 2011;152:2655–64. doi: 10.1210/en.2010-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obayashi M, Shimomura Y, Nakai N, Jeoung NH, Nagasaki M, et al. Estrogen controls branched-chain amino acid catabolism in female rats. J Nutr. 2004;134:2628–33. doi: 10.1093/jn/134.10.2628. [DOI] [PubMed] [Google Scholar]


