Abstract
Trypanosoma cruzi is a protozoan parasite known to cause Chagas disease (CD), a neglected sickness that affects around 6–8 million people worldwide. Originally, CD was mainly found in Latin America but more recently, it has been spread to countries in North America, Asia, and Europe due the international migration from endemic areas. Thus, at present CD represents an important concern of global public health. Most of individuals that are infected by T. cruzi may remain in asymptomatic form all lifelong, but up to 40% of them will develop cardiomyopathy, digestive mega syndromes, or both. The interaction between the T. cruzi infective forms and host-related immune factors represents a key point for a better understanding of the physiopathology of CD. In this context, the complement, as one of the first line of host defense against infection was shown to play an important role in recognizing T. cruzi metacyclic trypomastigotes and in controlling parasite invasion. The complement consists of at least 35 or more plasma proteins and cell surface receptors/regulators, which can be activated by three pathways: classical (CP), lectin (LP), and alternative (AP). The CP and LP are mainly initiated by immune complexes or pathogen-associated molecular patterns (PAMPs), respectively, whereas AP is spontaneously activated by hydrolysis of C3. Once activated, several relevant complement functions are generated which include opsonization and phagocytosis of particles or microorganisms and cell lysis. An important step during T. cruzi infection is when intracellular trypomastigotes are release to bloodstream where they may be target by complement. Nevertheless, the parasite uses a sequence of events in order to escape from complement-mediated lysis. In fact, several T. cruzi molecules are known to interfere in the initiation of all three pathways and in the assembly of C3 convertase, a key step in the activation of complement. Moreover, T. cruzi promotes secretion of plasma membrane-derived vesicles from host cells, which prevent the activity of C3 convertase C4b2a and thereby may hinder complement. In this review, we aim to present an overview on the strategies used by T. cruzi in order to circumvent the activation of complement and, consequently, its biological effects.
Keywords: Trypanosoma cruzi, complement system, complement regulatory proteins, evasion mechanism, innate immunity
Introduction
Trypanosoma cruzi is a hemoflagellate parasite of the order Kinetoplastida and Trypanosomatidae family (Levine et al., 1980) that causes CD. The parasite presents complex mechanisms of surveillance in the mammalian host and exerts direct influence on the course of CD (Watanabe Costa et al., 2016). CD is responsible for more expressive morbimortality than any other parasitic disease (World Health Organization [WHO], 2010; Bonney, 2014), resulting in a global annual burden of $627.5 million in health-care costs (Lee et al., 2013). It is estimated that 6–8 million people are infected with T. cruzi from 21 countries in Latin America (Stanaway and Roth, 2015), where 25 million people live at risk of acquiring the disease (Pereira and Navarro, 2013; World Health Organization [WHO], 2015). Furthermore, owing to the widespread human migration from CD-endemic areas, the disease has become an emerging global health concern, affecting several countries in Europe (World Health Organization [WHO], 2009; Navarro et al., 2012; European Centre for Disease Prevention and Control [ECDC], 2014), the United States (Bern and Montgomery, 2009), and Japan (Schmunis, 2007), where transmission occurs mainly through blood transfusions, organ transplants, or by congenital routes (Singh and Sehgal, 2010).
Although most of T. cruzi-infected people remain asymptomatic throughout their lives, the parasite-host interaction seems to be crucial for the development of the disease and the severity of the chronic symptomatic forms. In this context, the complement system plays an important role as a first line of host immune defense promoting the recognition, opsonization, and direct lysis of invading pathogens. However, during T. cruzi infection in humans, the activation of complement may present a dual role in both the acute and chronic phases of CD, initially being crucial in controlling the parasitemia, but later in the chronic phase contributing to the development or severity of the symptomatic forms because of its proinflammatory effect (Boldt et al., 2011; Weitzel et al., 2012; Luz et al., 2013, 2016). Considering that activation of complement by the lectin, classical, and APs leads to a proteolytic cascade and ultimately to a powerful lytic effect, this system is a special target for evasion strategies used by microbes in order to ensure infection success and possibly progression to chronic disease (Lambris et al., 2008). In fact, T. cruzi displays a range of different strategies to circumvent the harmful effects of the complement proteolytic cascade, which enables the parasite’s survival and development of CD. Therefore, we review here published information on T. cruzi-derived proteins that are involved in complement evasion which are critical to successful infection and disease progression.
Trypanosoma cruzi and Chagas Disease
T. cruzi Life Cycle
Trypanosoma cruzi infection occurs predominantly via vectorial transmission by triatomine bugs of the genera Triatoma, Rhodnius, and Panstrongylus. These bugs suck the blood of vertebrates infected with trypomastigotes, and this initiates the parasite life cycle. Once ingested by the insect vector, trypomastigotes are transformed in the anterior midgut either into spheromastigote or epimastigote forms. Epimastigotes multiply in the midgut and attach to the perimicrovillar membranes of the triatomine intestinal cells. At the most posterior region of the vector’s intestine and at the rectum, many epimastigotes detach from the intestinal surface and become infective metacyclic trypomastigotes, which are then released with feces and urine during blood meals. However, metacyclic trypomastigotes are not able to penetrate the intact host skin and enter through the rubbing or scratching of the bite wound, or through permissive mucosal or conjunctival surfaces at the inoculation site. Recruitment and fusion of the host lysosome with the parasite involves the formation of a parasitophorous endocytic vacuole, which is needed for parasite invasion of fibroblasts, macrophages, and epithelial cells (Souza et al., 2010; Nogueira et al., 2015). Subsequently, the parasite promotes enzymatic lysis of the vacuole membrane, differentiating into intracellular amastigotes, which after nine cycles of binary division become trypomastigotes that are eventually released into the circulation as bloodstream trypomastigotes (Dvorak and Hyde, 1973; Dvorak and Howe, 1976). These either infect new cells or are taken up by a new insect vector during a blood meal, repeating the parasite cycle.
Once in the bloodstream of a mammalian host, T. cruzi is able to infect several cell types from a variety of tissues thereby spreading to new infection sites where they differentiate into intracellular amastigotes (Watanabe Costa et al., 2016) (Figure 1). Besides vectorial transmission, T. cruzi can also be transmitted through blood transfusion, organ transplantation, transplacentally, the ingestion of contaminated food or drinks, or by accidental exposure; however, the physiopathological mechanisms of such transmissions are still unclear.
FIGURE 1.
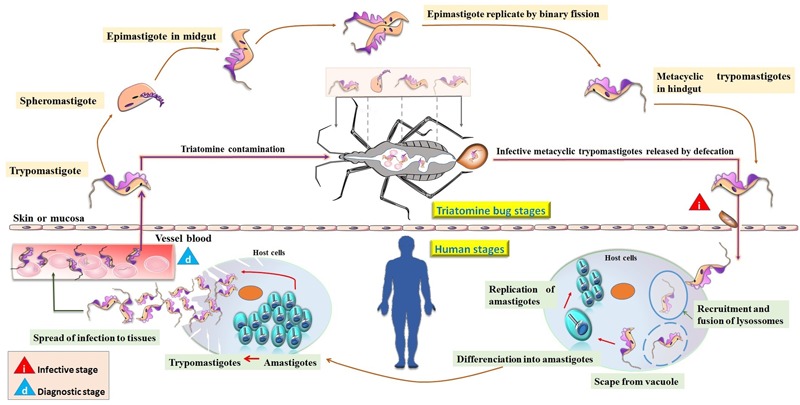
Life cycle of Trypanosoma cruzi. Transmission is initiated by insect vectors that defecate after a blood meal and release metacyclic trypomastigotes near the bite wound. This infective stage is characterized by the invasion of host cells by trypomastigotes forming the parasitophorous vacuole, from which they subsequently escape, differentiate into amastigotes, and replicate in the cytosol. The amastigotes then divide, differentiate into trypomastigotes, and upon rupture of the cell spread the infection to tissues. Trypomastigotes reach the bloodstream, where they are eventually taken up by the insect vector or infect new cells. In the triatomine bugs, trypomastigotes differentiate into spheromastigotes becoming initially short epimastigotes (mid-log). After migration to the bug’s hindgut, elongate epimastigotes (late-log) attach to the waxy gut cuticle and give rise to infectious metacyclic trypomastigotes, completing the parasite life cycle.
Chagas Disease
Several clinical manifestations may result in humans from T. cruzi infection. This initiates with an acute phase that last for about 2 months characterized by high parasitemia. In this stage, the diagnosis may be achieved by direct visualization of the parasite in blood and by the detection of IgG antibodies against T. cruzi antigens. Although most acute cases are oligosymptomatic or asymptomatic, initial dermatologic manifestations resulting in a skin lesion (chagoma), eyelid edema and conjunctivitis (Romaña sign), or generalized morbilliform eruption (schizotrypanides) may be present (Nunes et al., 2013). Other symptoms may include anorexia, fever, headache, dyspnea, abdominal pain, cough, hepatosplenomegaly, rash, painful nodules, generalized body swelling, and myocarditis (Hidron et al., 2010; Malik et al., 2015).
Following the acute phase, most infected people enter into a prolonged asymptomatic indeterminate form of the chronic disease and will never develop Chagas-related symptoms. However, after 10–30 years of infection approximately 30–40% of chronically infected people will present some clinical manifestations such as cardiomyopathy (20–30%), digestive commitment (10–15%) or both (1–5%) (Rassi and Marin-Neto, 2010). Chronic cardiomyopathy represents the most severe and life-threatening manifestation of human CD, ranging from asymptomatic electrocardiogram abnormalities to congestive heart failure, arrhythmias, and/or thromboembolic events (Biolo et al., 2010) that are associated with high morbidity and mortality. In fact, mortality related with CD is generally due to cardiovascular involvement, with approximately 12,000 deaths every year (Salvatella et al., 2013), with sudden death accounting for around 60%, heart failure 25–30% and stroke 10–15% (Rassi et al., 2001).
Standard approaches for CD diagnosis in the chronic phase require at least two different serological tests, generally using ELISA and IFA (Bern et al., 2007). Additionally, amplification of parasite DNA by PCR may be employed in some uncertain cases (Schijman et al., 2011) (Figure 2).
FIGURE 2.
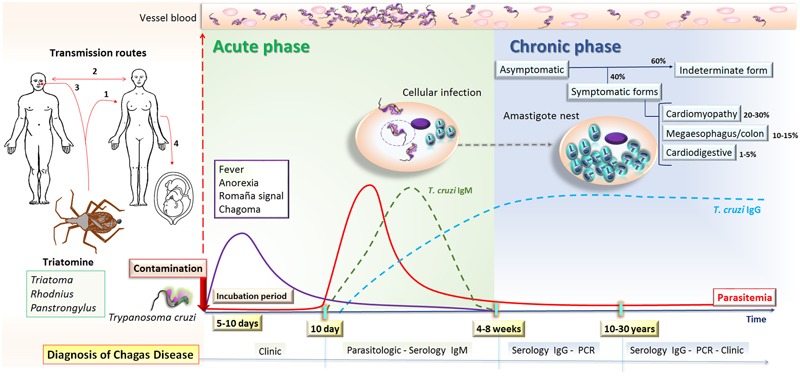
Natural course of human T. cruzi infection. T. cruzi transmission can occur by (1) vectorial (2) blood transfusion or organ transplantation, (3) oral, or (4) congenital routes. The incubation period lasts for 5–10 days after human contamination with T. cruzi from triatomines, which is followed by the acute phase of Chagas disease that lasts 4–8 weeks. This phase is characterized by circulating trypomastigotes, which can be visualized in the blood and IgM anti-T. cruzi antibodies can be detected after 10 days of infection. Most patients have non-specific symptoms, such as fever and anorexia, or are asymptomatic, and may develop inflammation and swelling at the site of inoculation in the skin or conjunctiva, characterizing chagoma and Romaña’s signal, respectively. The chronic phase begins once parasitemia falls below detectable levels by microscopy, usually 4 to 8 weeks after the onset of infection, and therefore diagnosis is based on the detection of anti-T. cruzi IgG antibodies or molecular tests. In this phase, most infected people enter a prolonged asymptomatic form known as the indeterminate form and will never develop Chagas-related symptoms. However, after 10–30 years around 30–40% of chronically infected people will present some clinical manifestations including cardiac, digestive, or cardiodigestive complaints.
The Complement System
The complement system constitutes a key component of innate immunity with a significant role in the first line of defense against invading microbes. It comprises more than 35 plasma and cell membrane receptor/regulator proteins that become activated mainly by three pathways: lectin (LP), classical (CP), and alternative (AP), resulting in important biological responses such as inflammation, phagocytosis, and lysis of pathogens (Ricklin et al., 2010). Activation of complement and its potent inflammatory response through the production of molecules with anaphylatoxin activity was first demonstrated by Dias da Silva and Lepow (1967). The functional role in vascular permeability, histamine release from mast cells and contraction of smooth muscle of the anaphylatoxins (C4a, C3a, and C5a) was subsequently reported (Dias da Silva and Lepow, 1967; Dias da Silva et al., 1967; Cochrane and Müller-Eberhard, 1968). Besides inflammatory response, the complement also plays an important role in the solubilization and removal of circulating immune complexes to avoid their deposition, which could result in tissue injury (Miller and Nussenzweig, 1974). In addition, the complement system links the innate and acquired responses through the activation of B lymphocytes and synthesis of immunoglobulins (Walport, 2001; Ricklin et al., 2010, 2016). Furthermore, complement is involved in the opsonization of apoptotic cells contributing to their phagocytosis and removal from circulation (Flierman and Daha, 2007).
Complement is activated mainly by three pathways, LP, CP, and AP, which lead to the generation of effector molecules, self-amplification, and the induction of immune signaling (Ricklin et al., 2010). The LP can be triggered through the binding of PRMs, such as MBL, ficolins (Ficolin-1 [or M-ficolin], Ficolin-2 [or L-ficolin], and Ficolin-3 [or H-ficolin]) and CL-K1, to PAMPs on the pathogen’s surface (Beltrame et al., 2015). Whereas carbohydrate-recognition domains in the MBL molecule bind to sugar moieties on the pathogen’s surface (such as D-mannose, glucose, L-fucose, and GlcNAc) (Weis et al., 1992), the three human ficolins bind to PAMPs by fibrinogen-like recognition domains and exhibit differences in their binding specificities (Gout et al., 2010). For instances, Ficolin-1 recognizes N-acetylated compounds (such as GlcNAc and N-acetylgalactosamine [GalNAc]) (Frederiksen et al., 2005), O-acetylated and glycan compounds containing sialic acid (Gout et al., 2010). Ficolin-2 also recognizes N-acetylated compounds and capsulated strains of bacteria (Frederiksen et al., 2005; Gout et al., 2010), while Ficolin-3 binds GalNAc, GlcNAc, D-fucose as mono/oligosaccharides and lipopolysaccharides (Sugimoto et al., 1998). In addition, CL-K1 senses mannose and fucose-containing microbial derived products (Keshi et al., 2006). Following the binding of MBL, ficolins, or CL-K1 to PAMPs, the LP is initiated by the activation of MASP-1 and MASP-2 resulting in active forms (Kjaer et al., 2013) that cleave C4 in C4a and C4b, and C2 in C2a and C2b, culminating with the formation of the LP C3 convertase (C4b2a) and C5 convertase (C4bC2aC3b) (Walport, 2001; Ricklin et al., 2010; Merle et al., 2015).
The activation of the CP depends mainly on the interaction of C1 with antigen-antibody complexes or alternatively, by PAMPs (lipopolysaccharides and porins from Gram-negative bacteria), phospholipids, apoptotic cells (phosphatidylserine) or pentraxins (C-reactive protein and pentraxin 3), among others (Alberti et al., 1993; Kishore et al., 2004). The C1 complex is formed of one C1q molecule and two molecules each of C1r and C1s (C1qC1r2C1s2). C1q initiates the activation of the CP by binding to CH3 or CH2 Fc domains of IgM and IgG, respectively, inducing a conformational change in C1q. This leads to the activation of C1r and C1s, and serine proteases that cleave C4 and C2 (Lörincz et al., 2000), forming the CP C3 and C5 convertases (C4b2a and C4bC2aC3b), similar to those generated in the LP (Ricklin et al., 2010).
The activation of the AP starts with spontaneous hydrolysis of the thiol-ester bond in C3 α-chain generating C3(H2O). This molecule exhibits a reactive site for the plasma protein FB, forming the complex C3(H2O)B. In this condition, FB can be cleaved by FD in Ba and Bb. The Bb fragment remains bound to C3(H2O), forming the first C3 convertase of AP (C3(H2O)Bb), which now exhibits serine protease activity cleaving further C3 molecules into C3a and C3b. Like C3(H2O), C3b exhibits reactive sites for FB binding allowing its cleavage by FD, resulting in the second C3 convertase of this pathway, C3bBb. Additionally, C3 binds to C3bBb forming C3bBbC3b, a complex with C5 convertase activity (Walport, 2001; Ricklin et al., 2010).
Once all three pathways are activated, the C5 convertases formed by both CP/LP (C4b2a3b) and AP (C3bBb3b) cleave C5 in C5a and C5b. The fragment C5b binds to C6, forming a stable complex C5b6, which recruits C7 resulting in a hydrophobic complex that targets cell membranes (mC5b-7). After C8 is incorporated to mC5b-7, the C5b678 complex becomes inserted in the cell membrane. Then, 12–18 copies of the C9 molecule polymerize forming the MAC. This pore-forming ring structure (C5b678(9)n) gets inserted into the cell as a transmembrane channel, favoring ionic imbalance and an increase in intracellular volume leading to membrane cell disruption (Kondos et al., 2010).
Since undesirable activation of complement may lead to inflammation and tissue damage, an effective and accurate system of regulatory molecules is required for the maintenance of its homeostasis. In this context, a variety of membrane-bound inhibitory proteins (such as CR1, CD59, CD46, and DAF or CD55) downregulate local complement activation, protecting host cells from unwanted complement lysis (Carroll and Sim, 2011; Noris and Remuzzi, 2013). In addition, several plasma regulator proteins (such as Factor H, Factor I, C1-INH, C4BP, and vitronectin) control soluble activated complement components and complexes (Carroll and Sim, 2011; Noris and Remuzzi, 2013). In general, either excessive or defective complement activation may be implicated in the pathogenesis of some conditions including autoimmune, chronic inflammatory, and infectious diseases (Ricklin and Lambris, 2013).
Complement Activation by T. cruzi
The interaction of complement with T. cruzi is a principal step in the immediate immune response of the host against the parasite. However, it is important to consider that this interaction is dependent on the evolutive forms of the parasite. Experimental studies showed that complement can be activated by amastigote (Iida et al., 1989), epimastigote (Nogueira et al., 1975), and trypomastigote forms (Kipnis et al., 1985), but only non-infective epimastigote forms are susceptible to complement lysis. However, some strains of metacyclic trypomastigotes have been shown to be susceptible to complement-mediated killing in vitro (Cestari and Ramirez, 2010). Epimastigotes can be recognize by MBL, ficolins, C3 and C1q, but C3b and C4b deposition assays revealed that complement activation occurred mainly by the LP and AP in non-immune sera (Cestari and Ramirez, 2010). CP can also be activated through the binding of specific antibodies to epimastigote’s surface (Nogueira et al., 1975). As previously shown, AP can be activated by the spontaneous hydrolysis of C3; C3b is deposited on the epimastigote’s surface supporting efficient formation of AP C3 convertase (Joiner et al., 1986). Since all pathways are activated and C3 convertases are formed, C3 is cleaved into C3a and C3b with additional C3b molecules binding to C3 convertases, forming C5 convertases, which in turn cleave C5 into C5a and C5b. Then C5b recruits C6, C7, and C8 forming a C5b-8 complex where C9 molecules polymerize forming the MAC that induces the lysis of epimastigotes (Figure 3). Abrahamsohn and Dias da Silva (1977) demonstrated that epimastigotes treated with specific immune serum in the presence of splenic lymphocytes may be also killed in vitro by antibody dependent cell-mediated cytotoxicity.
FIGURE 3.
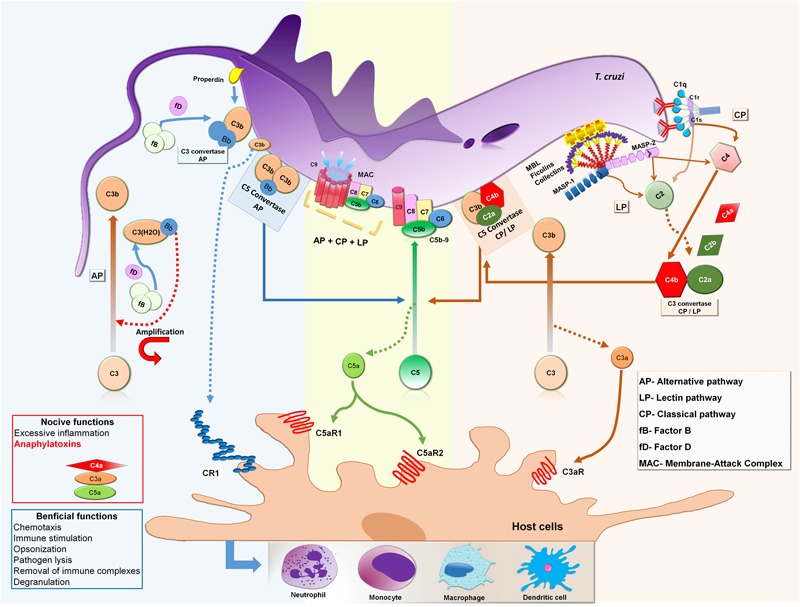
Activation of complement system by Trypanosoma cruzi epimastigote forms. MBL and ficolins recognize and bind to glycoproteins and carbohydrates present on the T. cruzi epimastigote surface initiating the LP. This interaction leads to conformational changes in MBL and ficolins and activation of MASP-1 followed by MASP-2 which cleavages C4 into C4a and C4b and C2 into C2a and C2b, generating C3 convertase (C4b2a). In presence of specific anti-T. cruzi antibodies, C1q molecules recognize and bind to IgM and IgG on the parasite’s surface initiating the CP. Subsequently, C1q suffers conformational changes and activates C1r, which cleaves and activates C1s that subsequently cleaves C4 and C2 generating C3 convertase (C4b2a), as in the LP convertase. When C3b binds to C4b2a the complex forms C4b2aC3b, with C5 convertase activity. The AP is initiated by the generation of C3(H2O) after spontaneous hydrolysis of C3, or as a consequence of loop amplification triggered during LP and CP activation,. Since FB binds to C3(H2O), FB can be cleaved by FD in Ba and Bb. The Bb fragment remains bound to C3(H2O) forming the first C3 convertase of AP (C3(H2O)Bb), which cleaves C3 into C3a and C3b. C3b also has a binding site for FB, allowing cleavage by FD, resulting in the second C3 convertase of AP, C3bBb. Additional C3b molecules are able to bind to the C3bBb complex to form C3bBb3bn, which exerts C5 convertase activity. Both C5 convertases, LP/CL and AP, cleave the C5 component into C5a and C5b. Newly formed C5b reacts with C6 to form the stable C5b6 complex that recruits C7 resulting in a hydrophobic complex that targets the membrane (mC5b-7). Membrane insertion is initiated upon binding of C8 (C5b-8) and 12–18 copies of C9 polymerize to form the membrane attack complex (MAC) that induces lysis of target membranes. In addition, as a product of all pathways being activated, the small fragments C4a, C3a, and C5a are formed, which are important anaphylatoxins, attracting and activating inflammatory cells to the activation site, such as neutrophils, monocytes, macrophages, and dendritic cells.
During the differentiation cycle from non-infective/complement susceptible to infective/complement resistance forms, trypomastigotes acquire the ability to circumvent complement lysis by TcCRT, TcCRP, TcCRIT, gp58/68, and T-DAF molecules. At the first stages of T. cruzi infection (seconds after infection), complement can be initially activated by the LP and AP since both pathways do not depend on a specific antibody response (Cestari et al., 2013). Thus, trypomastigotes can immediately be targeted by complement after accessing the host bloodstream. A wide range of carbohydrates (such as GalNAc and GlcNAc) anchored by glycosylphosphatidylinositol in the outer leaflet of T. cruzi plasma membrane (Lederkremer and Bertello, 2001; Buscaglia et al., 2006) can be recognized by PAMP sensor molecules, such as MBL and ficolins (Cestari et al., 2009; Cestari and Ramirez, 2010) leading to the activation of MASPs. Then, the serine protease MASP-2 cleaves C2 and C4 generating LP C3 convertase formation that activates C3 to form C3b, contributing to the AP amplification loop with simultaneous LP and AP activation (Ricklin et al., 2010; Cestari et al., 2013). In addition, the activation of the AP can also take place spontaneously by hydrolysis of C3, which leads to the generation of C3 convertase and C3 cleavage. However, deposition of C3b on trypomastigotes may contribute to T. cruzi internalization by CR1 (van Lookeren et al., 2007). Both LP and AP can be continually activated, not only at the first stages of T. cruzi infection, but also during the course of chronic CD (Lidani et al., 2015). At a later stage of T. cruzi infection (days post-infection), CP becomes activated when anti-trypomastigote-specific IgM and IgG antibodies are produced allowing C1q binding and the activation of C1r and C1s serine proteases that cleave C4 and C2 forming the CP C3 convertase. Although during T. cruzi infection complement is activated by these three pathways, the process is interrupted at the C3 convertase level by complement regulatory proteins derived from trypomastigotes that cause abrogation of the terminal pathway and MAC formation. This evasion from complement lysis allows trypomastigote cell invasion and tissue spreading driving the infection toward chronic disease.
Complement Evasion Strategies Used by T. cruzi
The success of T. cruzi infection depends on a series of complex mechanisms that enable the parasite to evade the host immune response. In fact, T. cruzi employs a range of strategies to escape the effects of both innate and adaptive immunity. A crucial step occurs during the first seconds of infection when trypomastigotes needs to circumvent the harmful lytic attack of complement (Sacks and Sher, 2002). During differentiation from epimastigote to metacyclic trypomastigote forms inside the insect vector, the parasite undergoes a series of morphological and physiological changes that confers the capacity to evade the lytic effect of complement. The mechanism controlling this resistance mainly involves the expression of complement binding molecules on the trypomastigote’s surface, such as T. cruzi calreticulin (TcCRT) (Ferreira et al., 2004a; Valck et al., 2010; Sosoniuk et al., 2014), T. cruzi complement regulatory protein (TcCRP) or Gp160 (Norris et al., 1989; Norris and Schrimpf, 1994), T. cruzi complement C2 receptor inhibitor trispanning (TcCRIT) (Cestari et al., 2008), gp58/68 (Fischer et al., 1988) and T-DAF (Tambourgi et al., 1993). In addition, it has been shown that T. cruzi metacyclic trypomastigote forms induce membrane-derived vesicles (microvesicles) from host cells, which interact with C3 convertase, resulting in inhibition of complement activation and increased parasite survival, as well as eukaryotic cell invasion (Cestari et al., 2012). Moreover, it has been reported that microvesicles, derived from the host cell membrane and also secreted by T. cruzi, can fuse thereby increasing host cell invasion (Ramirez et al., 2016) (Table 1). The molecules and microvesicles involved in T. cruzi evasion from the complement system will be covered in more detail (Figure 4).
Table 1.
Characteristics of the molecules involved in the evasion of Trypanosoma cruzi from the complement system.
| Parasite molecule | Complement component | Complement pathway affected | Reference |
|---|---|---|---|
| TcCRT | Binds to MBL collagen-like domain | Lectin pathway | Ferreira et al., 2004b |
| Binds to Ficolin-2 collagen-like domain | Lectin pathway | Sosoniuk et al., 2014 | |
| Interacts with C1q collagen-like domain | Classical pathway | Ferreira et al., 2004b; Valck et al., 2010 | |
| T-DAF | Binds to C3b and C4b accelerating the dissociation of C3 convertase | Alternative, classical, andlectin (probably) pathways | Joiner et al., 1988; Rimoldi et al., 1988; Tambourgi et al., 1993 |
| TcCRP/Gp160 | Binds to C3b and C4b preventing assembly of C3 convertase | Alternative and classical Lectin (probably) pathways | Norris et al., 1991, 1989; Norris and Schrimpf, 1994; Norris, 1998 |
| TcCRIT | Binds to C2 and prevent its cleavage by C1s and MASP2 | Classical and lectin pathways | Cestari et al., 2008, 2009 |
| gp58/68 | Interacts with Factor B blocking its binding to C3b | Alternative pathway | Fischer et al., 1988 |
| T. cruzi-induced membrane-derived vesicles from host cells or microvesicle (MV) | Binds to C3 convertase (C4b2a) on the T. cruzi surface, thereby inhibiting C3 cleavage | Classical and lectin pathway | Cestari et al., 2012; Ramirez et al., 2016 |
FIGURE 4.
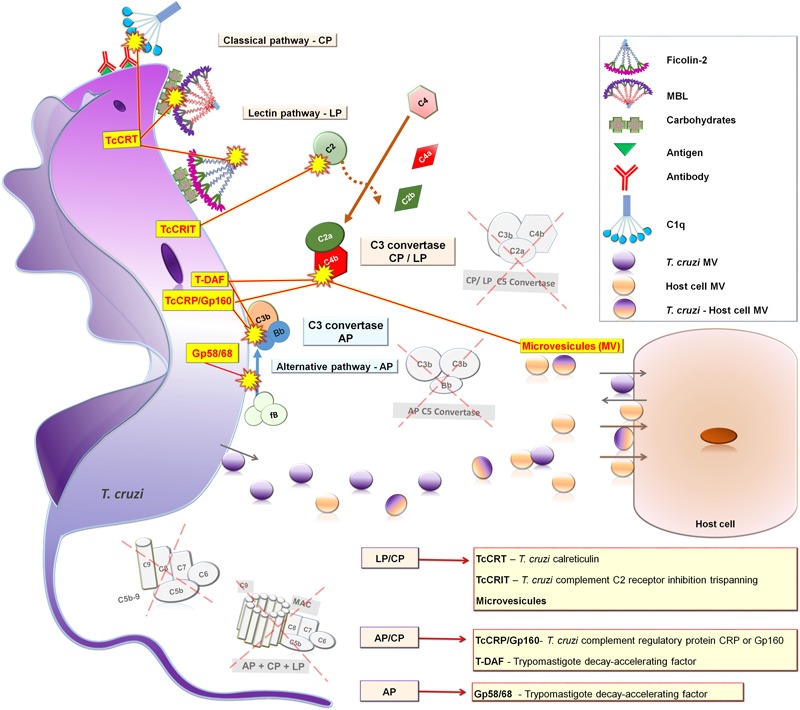
Complement evasion by strategies of T. cruzi trypomastigotes forms. TcCRT blocks CP and LP binding to C1q, MBL and Ficolin-2; T-DAF and Gp160/TcCRP block AP and CP C3 convertase assembly; TcCRIT blocks CP and LP binding to C2; gp58/68 blocks AP C3 convertase binding to factor B; and MV inhibit CP and LP C3 convertase assembly. AP, Alternative pathway; LP, Lectin pathway; and CP, Classical pathway.
T. cruzi Calreticulin (TcCRT)
Trypanosoma cruzi calreticulin (TcCRT) is a 45 kDa calcium-binding protein that is primarily expressed in the ER of infective trypomastigotes. Upon infection, TcCRT is translocated from the RE to the emerging area of the flagellum on the plasma membrane surface (Ferreira et al., 2004a,b; González et al., 2015). Interestingly, this area has been shown to be the initial contact with the host plasma membrane (González et al., 2015). TcCRT expressed on the T. cruzi trypomastigote’s surface is able to bind to host PRMs, similar to C1q, MBL (Ferreira et al., 2004b; Ramírez et al., 2011) and ficolins (Sosoniuk et al., 2014) interfering in the activation of the CP and LP. TcCRT also likely enhances the rate of the internalization of T. cruzi trypomastigotes by host cells (Ramírez et al., 2011, 2012).
Trypanosoma cruzi calreticulin was initially named Tc45 and described as a polypeptide present in lysates of epimastigotes and trypomastigotes. It was revealed to have an immunogenic role in the induction of specific antibodies in T. cruzi-infected mice (Ramos et al., 1991) and in human CD (Aguillón et al., 1997). Tc45 was subsequently characterized as T. cruzi calreticulin (TcCRT) as gene sequencing showed homology with calreticulin genes of other species (Aguillón et al., 2000). Later, TcCRT was found to exhibit a high homology with HuCRT, a multifunctional 46 kDa protein present predominantly in the ER and subcellular compartments, as well as in the plasma membrane (Ferreira et al., 2004a). Extracellular HuCRT has been reported to contribute to cell adhesion to the extracellular matrix and to facilitate the uptake of apoptotic and cancerous cells by phagocytes (Lu et al., 2015). The molecule may act as a receptor for the collagen-like domain of C1q and MBL, promoting phagocytosis through C1qR (Stuart et al., 1997). In fact, the role of C1q in the invasion of mononuclear phagocytes and fibroblasts by T. cruzi trypomastigotes has previously been demonstrated (Rimoldi et al., 1989). This parasite-derived molecule was later identified in infective trypomastigotes as TcCRT (Ferreira et al., 2004b) and its activity is considered one of the strategies that mediates T. cruzi uptake by mammalian phagocytic cells, mimicking the process of apoptotic cells ingestion (Ramírez et al., 2011).
In the early stages of infection, TcCRT is able to bind to the carbohydrate recognition domain of MBL resulting in abrogation of interaction with its natural ligands and the collagen domain of Ficolin-2, preventing C4 activation and impairing further activation of the LP (Ferreira et al., 2004b; Sosoniuk et al., 2014). This property however, is not shared by Ficolin-3 (Sosoniuk et al., 2014). During the late stages of T. cruzi infection the CP can be activated in the presence of specific T. cruzi antibodies, as mentioned earlier. Ferreira et al. (2004b) have demonstrated using hemolytic assays that recombinant TcCRT binds to C1q collagenous tails, impairing the activation of the CP. Subsequent studies showed that TcCRT competes with the (C1r-C1s)2 tetrameric complex for binding in the collagenous C1q tails and interferes in the capacity of C1s to activate C4 in a calcium-independent manner (Valck et al., 2010). In summary, TcCRT has been shown to be involved in the internalization of T. cruzi into mammalian cells, which increases infectivity. It is also known to be an important regulator of both the LP and CP.
Trypomastigote Decay-Accelerating Factor (T-DAF)
The T-DAF is an 87–93-kDa glycoprotein present on the surface of metacyclic and tissue-culture-derived trypomastigote forms of T. cruzi that mimics the activity of the complement regulatory protein DAF (Tambourgi et al., 1993). T-DAF regulates the activation of the AP, CP, and probably LP, by interfering in the assembly of C3 convertases.
During T. cruzi infection, the three complement pathways are activated culminating with the formation of C3 convertase that cleaves the central component C3. The presence of molecules modulating the C3 convertase of the CP in trypomastigote but not epimastigote forms of T. cruzi surface was first described by Kipnis et al. (1986). It was subsequently demonstrated in a supernatant culture of trypomastigotes that these modulator molecules ranged between 86 and 155 kDa in size, that were enriched for proteins of 86–98 kDa absent in epimastigotes. They were shown to accelerate the decay of both the AP and CP C3 convertases in vitro by interfering in the binding of factor B with C3b, or in the formation of C4b2a, respectively. Because of the similar behavior to human complement regulatory DAF the authors called these modulators as analogous to DAF (Rimoldi et al., 1988). In the same year Joiner et al. (1988) characterized biochemically an 87–93 kDa factor produced by T. cruzi trypomastigote forms responsible for accelerating the decay of C3 convertases, which appeared to be an immunogenic glycoprotein immunoprecipitated from sera of patients chronically infected with T. cruzi. Later, Tambourgi et al. (1993) called this molecule analogous to DAF as T-DAF owing to its ability in inhibiting complement activation in a manner functionally similar to mammalian DAF. In addition, polyclonal and monoclonal antibodies against T-DAF were shown to inhibit T-DAF activity in vitro, validating its functional role (Tambourgi et al., 1993). The authors also showed that T-DAF shared 40% similarity with a portion of the DNA coding region for human DAF, and that anti-T-DAF-specific antibodies were present in up to 96% of patients with both acute and chronic CD (Tambourgi et al., 1995). In summary, T-DAF either accelerates the dissociation or assembly efficiency of C3 convertases potentially affecting the AP, CP (Tambourgi et al., 1993), and probably LP. Both soluble and surface T-DAFs are essential for the escape of T. cruzi from complement activation and lytic effects as well as the development of infection.
Trypanosoma cruzi Complement Regulatory Protein (TcCRP)
Trypanosoma cruzi complement regulatory protein (TcCRP), also called Gp160, is a 160 kDa glycoprotein anchored into trypomastigote membranes (Norris et al., 1989) via glycosylphosphatidylinositol linkage (Norris and Schrimpf, 1994). It is also spontaneously shed in culture from trypomastigotes (Norris et al., 1991). Both membrane and soluble forms of TcCRP are able to bind to C3b and C4b inhibiting the formation of the AP and CP C3 convertases (Norris et al., 1991; Norris and Schrimpf, 1994), and probably LP C3 convertase as well.
Trypanosoma cruzi complement regulatory protein was initially purified and partially characterized by Norris et al. (1989) as Gp160 because of its molecular weight, and was found to be expressed in membrane extracts of both metacyclic and tissue-culture-derived T. cruzi trypomastigotes, but absent in insect epimastigotes or intracellular amastigotes. Norris and Schrimpf (1994) purified and characterized the membrane form of TcCRP having the glycolipid anchor attached which presented a molecular mass of 185 kDa. The conversion of the 185-kDa membrane form to the 160-kDa form was suggested to be the result of cleavage by endogenous phospholipase C. Both soluble (160 kDa) and membrane TcCRP forms (185 kDa) are able to bind C3b and C4b preventing the assembly of proteolytically active C3 convertase inhibiting both AP and CP activation (Norris et al., 1991; Norris and Schrimpf, 1994). It was then demonstrated that the TcCRP had similar activity to human CRP and DAF, being considered a member of the C3/C4 binding family of complement regulatory proteins, which provided infectious trypomastigotes another mean of evading the harmful effects of complement (Norris et al., 1991). Since TcCRP binds C4b, LP activation could be also affected at the same level. Moreover, anti-TcCRP lytic antibodies were present in the sera of T. cruzi-infected patients. Interesting, in the presence of anti-TcCRP antibodies the interaction between TcCRP and C3b was blocked allowing the AP C3 convertase assembly and parasite lysis (Norris et al., 1991).
Norris (1996) demonstrated that following the binding to C3b, TcCRP was released from the T. cruzi trypomastigote membrane by proteolytic cleavage, and these findings represented a new alternative mechanism of T. cruzi in evading complement activation. Interestingly, epimastigote forms transfected with a cDNA encoding full-length recombinant TcCRP were protected from complement-mediated lysis, confirming the role of TcCRP as a complement resistance factor of T. cruzi trypomastigotes (Norris et al., 1997; Norris, 1998). Recently, Henrique et al. (2016) demonstrated that surface expression of TcCRP differs among parasite strains, with a tendency of higher expression levels in the most virulent T. cruzi strains. In summary, TcCRP is directly involved in the evasion of T. cruzi from complement lysis by binding to C3b and C4b and consequently inhibiting both the AP, CP, and probably LP.
T. cruzi Complement C2 Receptor Inhibitor Trispanning (TcCRIT)
Trypanosoma cruzi complement C2 receptor inhibitor trispanning (TcCRIT) is a 32 kDa transmembrane protein that presents a sequence homology with the C4 beta-chain, the binding site of C2. Thus, TcCRIT inhibits the cleavage of C2 by C1s or MASP-2 and consequently prevents C3 convertase formation by competing with C4 (Inal, 1999; Inal and Schifferli, 2002; Cestari et al., 2012). TcCRIT is mainly expressed in complement resistant trypomastigote forms of T. cruzi regulating the activation of both the CP and LP (Inal, 1999; Inal et al., 2005).
The complement C2 receptor inhibitor trispanning (CRIT) was first described in the tegument of Schistosoma haematobium and subsequently S. mansoni (Inal, 1999; Inal and Sim, 2000) as a phosphorylated tyrosine molecule named TOR that inhibited C2 cleavage by C1s inhibiting the CP activation. It was then characterized as a novel complement regulator, and named CRIT (Inal and Sim, 2000). S. haematobium TOR (Sh-TOR) protein sequencing showed a long cytoplasmic tail with several consensus phosphorylation sites for enzymes, such as tyrosine kinases, characteristically associated with membrane receptors (Inal, 1999). Later, it was demonstrated that the Sh-TOR synthetic peptide pre-incubated with C2 inhibited the CP activation both in vitro (Inal and Schifferli, 2002) and in vivo (Inal et al., 2003) by competing with C4b (which presents some sequence identity to the first extracellular domain of Sh-TOR) (Inal and Sim, 2000). CRIT is highly conserved in Schistosoma species, T. cruzi strains, and mammals (Inal, 1999, 2005). Interestingly, both the Trypanosoma parasite and its human host share a receptor for C2, with a complement regulatory function (Inal et al., 2005).
Complement C2 receptor inhibitor trispanning protein was later shown to be expressed in the infective stage of T. cruzi preventing the lytic activity of NHS. In addition, overexpression of TcCRIT in transgenic epimastigotes increased the resistance to complement-mediated killing in the presence of non-immune NHS, blocking both the CP and LP. However, when epimastigotes were treated with exogenous C2 the complement activity was restored (Cestari et al., 2008). Although metacyclic trypomastigotes and epimastigotes differ regarding complement resistance, MBL, Ficolin-2 and Ficolin-3 are able to bind to glycosylated proteins on the surface of both parasites forms. However, only metacyclic trypomastigotes were shown to resist complement killing due to high expression levels of TcCRIT. TcCRIT circumvent LP activation through binding to C2, inhibiting its cleavage by MASP-2 and C3 convertase formation (Cestari et al., 2009). In summary, TcCRIT interferes in the activation of the LP and CP by binding to C2 and preventing its cleavage by the serine proteases C1s and MASP2, thereby evading both initial branches of complement activation. This leads to resistance against complement-mediated cell lysis, allowing T. cruzi survival and cell invasion.
T. cruzi Complement Regulatory gp58/68
The T. cruzi complement regulatory gp58/68 is a glycoprotein of an apparent molecular weight of 58 kDa (non-reduced) and 68 kDa (reduced) (Fischer et al., 1988) that is expressed on the parasite surface or can be released by trypomastigotes in culture (Ouaissi et al., 1988; Velge et al., 1988). It is part of the T. cruzi fibronectin/collagen receptor which consists of two molecules of 80–85 kDa and 58–68 kDa with an important role in the attachment of trypomastigote forms to mammalian cells (Ouaissi et al., 1984, 1986; Velge et al., 1988). Gp58/68 acts as a T. cruzi complement regulatory protein shown to inhibit the formation of cell-bound and fluid-phase AP C3 convertases (Fischer et al., 1988).
Gp58/68 was first identified in studies on the biological function of T. cruzi fibronectin receptors (TcFnR) by Ouaissi et al. (1984). Human fibronectin purified from blood were shown to bind specifically to T. cruzi trypomastigotes and to be involved in cell-parasite interaction. Anti-fibronectin antibodies were able to eradicate in vitro fibroblast infection by T. cruzi trypomastigotes (Ouaissi et al., 1984). The isolation and functional characterization of TcFnR were later achieved using immunoprecipitation assays, which identified the 85 kDa protein. Both affinity-purified TcFnR and anti-TcFnR antibodies exerted an inhibitory effect in the infection of fibroblasts by T. cruzi trypomastigotes in culture (Ouaissi et al., 1986). Subsequent studies characterized the other part of the fibronectin/collagen receptor, the collagen receptor, and showed that the same T. cruzi receptor binds to the host fibronectin and/or collagen, and that both the 80–85 kDa and 58/68 kDa glycoproteins are part of the same receptor (Velge et al., 1988). In the same year, the gp58/68 was purified by affinity chromatography from lysate of both culture and peripheral blood T. cruzi trypomastigotes and named according to its molecular weight (Fischer et al., 1988). This T. cruzi glycoprotein was able to inhibit the formation of cell-bound AP C3 convertase by preventing the initial association of FB with surface fixed C3b in a dose dependent manner. Moreover, gp58/68 was also shown to restrict the formation of fluid-phase AP C3 convertase by the consumption of fluid-phase FB (Fischer et al., 1988). In summary, gp58/68 is part of a fibronectin/collagen receptor of T. cruzi that has an important role in the interaction of T. cruzi with mammalian cells and confers the parasite’s ability to evade AP complement activation by inhibiting FB/C3b interaction.
Host and T. cruzi Microvesicles
Microvesicles (MVs) are 100–1000 nm vesicles originating from the plasma membrane and are released by a large number of cells from the blood, immune system, epithelial and endothelial tissues, among others (Evans-Osses et al., 2013). MVs are involved in intercellular communication owing to their capacity to transfer proteins, lipids, and nucleic acids, thereby influencing various physiological and pathological functions of both the recipient and parent cell (Yáñez-Mó et al., 2015). MVs are known to be released by different pathogens such as bacteria, fungus, and parasites, including T. cruzi (Silveira et al., 1979; Gonçalves et al., 1991; Geiger et al., 2010), which may carry virulence factors to host cells promoting the dissemination of the pathogen (Deatheragea and Cooksona, 2012; Barteneva et al., 2013). Host mammalian cells infected with T. cruzi release MVs that interfere in CP and LP C3 convertases assembly on the parasite’s surface, leading to the inhibition of its catalytic activity (Cestari et al., 2012) and consequently eliminating complement activation. Recent findings have demonstrated that interaction between T. cruzi trypomastigote and epimastigote forms with host cells also can induce MVs formation (Ramirez et al., 2016).
New insight into the mechanisms of complement immune evasion by T. cruzi were gain when Cestari et al. (2012) reported secretion of host MVs induced by parasitic infection. At the beginning of infection, metacyclic trypomastigotes induced the release of MVs from immune cells, such as lymphocytes, monocytes, and macrophages in a calcium-dependent process (Cestari et al., 2012). Under experimental conditions MVs were shown to strongly inhibit C3b deposition. However, C4b deposition was not significantly inhibited. These findings suggest that MVs interfered on complement at C3 level. In addition, MVs were shown to bind LP and CP C3 convertase complexes on the surface of T. cruzi inhibiting complement-mediated lysis and favoring the invasion of host cells. Moreover, C1q, Ficolin-2, and Ficolin-3 were also found to bind to MVs but did not impair parasite recognition by these PRMs. It has also been shown that MVs derived from lymphocytes and monocytes carry TGF-β, an important cytokine that enhances T. cruzi cell invasion and protects the parasite against complement-mediated lysis (Cestari et al., 2012). Recently, the release of MVs derived from infective (metacyclic trypomastigote and tissue-culture-derived trypomastigote) and non-infective (epimastigote) parasites, and their interaction with host cells was demonstrated. In addition, infective and non-infective T. cruzi forms were shown to induce different levels of MV release from host cells. Moreover, the fusion of MVs derived from both host cell and parasite was demonstrated, and this phenomenon appears to facilitate contact between T. cruzi and host cell plasma membranes, and probably membrane fusion. Thus, MVs released during interaction of the parasite with host cells were able to increase host cell invasion by metacyclic trypomastigotes (Ramirez et al., 2016). In summary, interaction between T. cruzi and host cells prompt the release of MVs from both parasite and host cells and this phenomenon may contribute to evasion of CP and LP complement activation and to increase host cell infection. Thus, both host and parasite MVs probably have a potential immunomodulatory effect in the pathogenesis of T. cruzi infection.
Immunomodulatory Perspectives
Although it has been suggested that the effectiveness of the etiological treatment for CD is inversely proportional to the duration of T. cruzi infection, the success of trypanosomatid drugs in preventing clinical progression to symptomatic forms is still controversial. Considering that T. cruzi makes use of several strategies to evade the host immune system in order to establish the infection, these molecules involved in evasion mechanisms could be interesting therapeutic targets to be explored in the context of prevention and treatment of CD. Immunomodulatory therapies could be based on the use of antibodies against overexpressed T. cruzi molecules, such as TcCRT, T-DAF, TcCRP, TcCRIT, and gp58/68. For instance, T. cruzi complement regulatory proteins, such as TcCRP, T-DAF, and gp58/68, may also be targets of anti-T. cruzi lytic antibodies (as reviewed by Krautz et al., 2000) and could be used as indicators of drug efficacy in T. cruzi infection and parasite clearance, as observed by anti-TcCRP and anti-T-DAF detected in sera of CD patients (Norris et al., 1994; Tambourgi et al., 1995). In addition, the association of MBL deficiency with protection against the development and progression of chronic CD cardiomyopathy (Luz et al., 2016) highlighted a potential marker for disease progression. Moreover, an interesting therapeutic approach to control the early stage of T. cruzi infection in patients with MBL deficiency and defects in LP activation may be the restitution of MBL or other PRMs involved in complement activation. Thus, new investigations are needed to explore which T. cruzi evasion molecules could be potential immunotherapeutic targets that could contribute to change the pathophysiological progression of this neglected disease.
Conclusion
The current knowledge on complement evasion strategies used by T. cruzi highlights the importance of the LP, AP, and CP as crucial components in the first line of defense against this parasitic infection. However, T. cruzi infective forms are able to regulate and inhibit the complement activation early in the proteolytic cascade by expressed and/or released regulator molecules thereby circumventing complement’s harmful effects. Thus, complement becomes prey of T. cruzi and has a bad day. Understanding these complement evasion strategies is crucial for the development of innovative strategies in the battle against T. cruzi infection and may pave the way for novel immunotherapies.
Author Contributions
KL, LB, and AA participated in the design and writing of the manuscript. IdM-R: participated in the design, coordination, and manuscript writing. KL developed the graphic design of all figures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer WDDS and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
This work was supported by research grants from Coordenação de Aperfeiçoamento de Pessoal Superior (CAPES Edital Parasitologia Básica 32/2010) and from Conselho Nacional de Desenvolvimento Científico e Tecnológico to IM-R (CNPq).
Abbreviations
- AP
alternative pathway
- C1-INH
C1 esterase inhibitor
- C1qR
C1q receptor
- C4BP
C4b-binding protein
- CD
chagas disease
- CD46
membrane cofactor protein
- CD59
inhibitor membrane attack complex-inhibitory protein
- CP
classical pathway
- CL-K1
collectin -11
- CRIT
complement C2 receptor inhibitor trispanning
- CR1
complement receptor 1
- DAF
decay accelerating factor or CD55
- ELISA
enzyme-linked immunosorbent assay
- ER
endoplasmic reticulum
- FB
factor B
- FD
factor D
- GlaNAc
N-acetylgalactosamine
- GlcNAc
N-acetyl-D-glucosamine
- HuCRT
human calreticulin
- IFA
immunofluorescence antibody test
- LP
lectin pathway
- MAC
membrane attack complex
- MASP-1
MBL-associated serine proteases - 1
- MASP-2
MBL-associated serine proteases - 2
- MBL
mannose-binding lectin
- MV
microvesicles
- NHS
normal human serum
- PAMPs
pathogen-associated molecular patterns
- PCR
polymerase chain reaction
- PRMs
pattern-recognition molecules
- Sh-TOR
Schistosoma haematobium
- T. cruzi
Trypanosoma cruzi
- TcCRT
Trypanosoma cruzi calreticulin
- TcCRIT
Trypanosoma cruzi complement C2 receptor inhibitor trispanning
- TcCRP
Trypanosoma cruzi complement regulatory protein or Gp160
- T-DAF
trypomastigote decay-accelerating factor
- TOR
trispanning orphan receptor
References
- Abrahamsohn I. A., Dias da Silva W. (1977). Antibody dependent cell-mediated cytotoxicity against Trypanosoma cruzi. Parasitology 75 317–323. 10.1017/S0031182000051866 [DOI] [PubMed] [Google Scholar]
- Aguillón J. C., Ferreira L., Pérez C., Colombo A., Molina M. C., Wallace A., et al. (2000). Tc45, a dimorphic Trypanosoma cruzi immunogen with variable chromosomal localization, is calreticulin. Am. J. Trop. Med. Hyg. 63 306–312. [PubMed] [Google Scholar]
- Aguillón J. C., Harris R., Molina M. C., Colombo A., Cortés C., Hermosilla T., et al. (1997). Recognition of an immunogenetically selected Trypanosoma cruzi antigen by seropositive chagasic human sera. Acta Trop. 63 159–166. 10.1016/S0001-706X(96)00619-5 [DOI] [PubMed] [Google Scholar]
- Alberti S., Marques G., Camprubi S., Merino S., Tomas J. M., Vivanco F., et al. (1993). Clq binding and activation of the complement classical pathway by Klebsiella pneumoniae outer membrane proteins. Infect. Immun. 61 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barteneva N. S., Maltsev N., Vorobjev I. (2013). Microvesicles and intercellular communication in the context of parasitism. Front. Cell. Infect. Microbiol. 3:49 10.3389/fcimb.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrame M. H., Catarino S. J., Goeldner I., Boldt A. B. W., Messias-Reason I. J. (2015). The lectin pathway of complement and rheumatic heart disease. Front. Pediatr. 2:148 10.3389/fped.2014.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C., Montgomery S. P. (2009). An estimate of the burden of chagas disease in the united states. Clin. Infect. Dis. 49 e52–4. 10.1086/605091 [DOI] [PubMed] [Google Scholar]
- Bern C., Montgomery S. P., Herwaldt B. L., Marin-neto J. A., Maguire J. H., Acquatella H., et al. (2007). Evaluation and treatment of chagas disease in the united states a systematic review. JAMA 298 2171–2181. 10.1001/jama.298.18.2171 [DOI] [PubMed] [Google Scholar]
- Biolo A., Ribeiro A. L., Clausell N. (2010). Chagas cardiomyopathy-where do we stand after a hundred years? Prog. Cardiovasc. Dis. 52 300–316. 10.1016/j.pcad.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Boldt A. B. W., Luz P. R., Messias-Reason I. J. T. (2011). MASP2 haplotypes are associated with high risk of cardiomyopathy in chronic chagas disease. Clin. Immunol. 140 63–70. 10.1016/j.clim.2011.03.008 [DOI] [PubMed] [Google Scholar]
- Bonney K. M. (2014). Chagas disease in the 21st century: a public health success or an emerging threat? Parasite 21 11 10.1051/parasite/2014012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaglia C. A., Campo V. A., Frasch A. C. C., Di Noia J. M. (2006). Trypanosoma cruzi surface mucins: host-dependent coat diversity. Nat. Rev. Microbiol. 4 229–236. 10.1038/nrmicro1351 [DOI] [PubMed] [Google Scholar]
- Carroll M. V., Sim R. B. (2011). Complement in health and disease. Adv. Drug Deliv. Rev. 63 965–975. 10.1016/j.addr.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Cestari I., Ansa-Addo E., Deolindo P., Inal J. M., Ramirez M. I. (2012). Trypanosoma cruzi immune evasion mediated by host cell-derived microvesicles. J. Immunol. 188 1942–1952. 10.4049/jimmunol.1102053 [DOI] [PubMed] [Google Scholar]
- Cestari I., Evans-Osses I., Schlapbach L. J., Messias-Reason I., Ramirez M. I. (2013). Mechanisms of complement lectin pathway activation and resistance by trypanosomatid parasites. Mol. Immunol. 53 328–334. 10.1016/j.molimm.2012.08.015 [DOI] [PubMed] [Google Scholar]
- Cestari I., Ramirez M. I. (2010). Inefficient complement system clearance of Trypanosoma cruzi metacyclic trypomastigotes enables resistant strains to invade eukaryotic cells. PLoS ONE 5:e9721 10.1371/journal.pone.0009721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestari I. S., Evans-Osses I., Freitas J. C., Inal J. M., Ramirez M. I. (2008). Complement C2 receptor inhibitor trispanning confers an increased ability to resist complement-mediated lysis in Trypanosoma cruzi. J. Infect. Dis. 198 1276–1283. 10.1086/592167 [DOI] [PubMed] [Google Scholar]
- Cestari I. S., Krarup A., Sim R. B., Inal J. M., Ramirez M. I. (2009). Role of early lectin pathway activation in the complement-mediated killing of Trypanosoma cruzi. Mol. Immunol. 47 426–437. 10.1016/j.molimm.2009.08.030 [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. (1968). The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J. Exp. Med. 127 371–386. 10.1084/jem.127.2.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatheragea B. L., Cooksona B. T. (2012). Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 80 1948–1957. 10.1128/IAI.06014-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias da Silva W., Eisele J. W., Lepow I. H. (1967). Complement as a mediator of inflammation. III. Purification of the activity with anaphylatoxin properties generated by interaction of the first four components of complement and its identification as a cleavage product of C’3. J. Exp. Med. 126 1027–1048. 10.1084/jem.126.6.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias da Silva W., Lepow I. H. (1967). Complement as a mediator of inflammation. II. Biological properties of anaphylatoxin prepared with purified components of human complement. J. Exp. Med. 125 921–946. 10.1084/jem.125.5.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak J. A., Howe C. L. (1976). The attraction of Trypanosoma cruzi to vertebrate cells In Vitro. J. Protozool. 23 534–537. 10.1126/science.294.5546.1483 [DOI] [PubMed] [Google Scholar]
- Dvorak J. A., Hyde T. P. (1973). Trypanosoma cruzi: interaction with vertebrate cells in vitro. Exp. Parasitol. 34 268–283. 10.1016/0014-4894(73)90087-8 [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control [ECDC] (2014). Assessing the Burden of Key Infectious Diseases Affecting Migrant Populations in the EU/EEA. Stockholm: European Centre for Disease Prevention and Control; 10.2900/28792 [DOI] [Google Scholar]
- Evans-Osses I., Messias-Reason I., Ramirez M. I. (2013). The emerging role of complement lectin pathway in trypanosomatids: molecular bases in activation, genetic deficiencies, susceptibility to infection, and complement system-based therapeutics. Sci. World J. 2013:675898 10.1155/2013/675898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira V., Molina M. C., Valck C., Rojas A., Aguilar L., Ramírez G., et al. (2004a). Role of calreticulin from parasites in its interaction with vertebrate hosts. Mol. Immunol. 40 1279–1291. 10.1016/j.molimm.2003.11.018 [DOI] [PubMed] [Google Scholar]
- Ferreira V., Valck C., Sánchez G., Gingras A., Tzima S., Molina M. C., et al. (2004b). The classical activation pathway of the human complement system is specifically inhibited by calreticulin from Trypanosoma cruzi. J. Immunol. 172 3042–3050. 10.4049/jimmunol.172.5.3042 [DOI] [PubMed] [Google Scholar]
- Fischer E., Ouaissi M. A., Velget P., Cornette J., Kazatchkine M. D. (1988). Gp 58/68, a prasite component that contributes to the escape of the trypomastigote form of T. cruzi from damage by the human alternative complement pathway. Imumunology 65 299–303. [PMC free article] [PubMed] [Google Scholar]
- Flierman R., Daha M. R. (2007). The clearance of apoptotic cells by complement. Immunobiology 212 363–370. 10.1016/j.imbio.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Frederiksen P. D., Thiel S., Larsen C. B., Jensenius J. C. (2005). M-Ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand. J. Immunol. 62 462–473. 10.1111/j.1365-3083.2005.01685.x [DOI] [PubMed] [Google Scholar]
- Geiger A., Hirtz C., Bécue T., Bellard E., Centeno D., Gargani D., et al. (2010). Exocytosis and protein secretion in Trypanosoma. BMC Microbiol. 10:20 10.1186/1471-2180-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves M. F., Umezawa E. S., Katzin A. M., Souza W., Alves M. J. M., Zingales B., et al. (1991). Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp. Parasitol. 72 43–53. 10.1016/0014-4894(91)90119-H [DOI] [PubMed] [Google Scholar]
- González A., Valck C., Sánchez G., Härtel S., Mansilla J., Ramírez G., et al. (2015). Trypanosoma cruzi calreticulin topographical variations in parasites infecting murine macrophages. Am. J. Trop. Med. Hyg. 92 887–897. 10.4269/ajtmh.14-0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout E., Garlatti V., Smith D. F., Lacroix M., Dumestre-Pérard C., Lunardi T., et al. (2010). Carbohydrate recognition properties of human ficolins: glycan array screening reveals the sialic acid binding specificity of M-Ficolin. J. Biol. Chem. 285 6612–6622. 10.1074/jbc.M109.065854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique P. M., Marques T., da Silva M. V., Nascentes G. A. N., de Oliveira C. F., Rodrigues V. I., et al. (2016). Correlation between the virulence of T. cruzi strains, complement regulatory protein expression levels, and the ability to elicit lytic antibody production. Exp. Parasitol. 170 66–72. 10.1016/j.exppara.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Hidron A., Vogenthaler N., Santos-Preciado J. I., Rodriguez-Morales A. J., Franco-Paredes C., Rassi A. (2010). Cardiac involvement with parasitic infections. Clin. Microbiol. Rev. 23 324–349. 10.1128/CMR.00054-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Whitlow M. B., Nussenzweig V. (1989). Amastigotes of Trypanosoma cruzi escape destruction by the terminal in the absence of antibody, the alternative pathway of the complement system can function as a first barrier in preventing infection. however, certain microorganisms have developed mec. Trop. Med. 169 881–891. [Google Scholar]
- Inal J. M. (1999). Schistosoma TOR (trispanning Orphan Receptor), a novel, antigenic surface receptor of the blood-dwelling, schistosoma parasite. Biochim. Biophys. Acta 1445 283–298. 10.1016/S0167-4781(99)00051-2 [DOI] [PubMed] [Google Scholar]
- Inal J. M. (2005). Complement C2 receptor inhibitor trispanning: from man to schistosome. Springer Semin. Immunopathol. 27 320–331. 10.1007/s00281-005-0009-9 [DOI] [PubMed] [Google Scholar]
- Inal J. M., Hui K. M., Miot C., Lange S., Ramirez M. I., Schneider B., et al. (2005). Complement C2 receptor inhibitor trispanning: a novel human complement inhibitory receptor. J. Immunol. 174 356–366. 10.4049/jimmunol.174.1.356 [DOI] [PubMed] [Google Scholar]
- Inal J. M., Schifferli J. A. (2002). Complement C2 receptor inhibitor trispanning and the Beta-chain of C4 share a binding site for complement C2. J. Immunol. 168 5213–5221. 10.4049/jimmunol.168.10.5213 [DOI] [PubMed] [Google Scholar]
- Inal J. M., Schneider B., Armanini M., Schifferli J. A. (2003). A peptide derived from the parasite receptor, complement C2 receptor inhibitor trispanning, suppresses immune complex-mediated inflammation in mice. J. Immunol. 170 4310–4317. 10.4049/jimmunol.170.8.4310 [DOI] [PubMed] [Google Scholar]
- Inal J. M., Sim R. B. (2000). A schistosoma protein, Sh-TOR, is a novel inhibitor of complement which binds human C2. FEBS Lett. 470 131–134. 10.1016/S0014-5793(00)01304-1 [DOI] [PubMed] [Google Scholar]
- Joiner K., Sher A., Gaither T., Hammer C. (1986). Evasion of alternative complement pathway by Trypanosoma cruzi results from inefficient binding of factor-B. Proc. Natl. Acad. Sci. U.S.A. 83 6593–6597. 10.1073/pnas.83.17.6593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., DaSilva W. D., Rimoldi M. T., Hammer C. H., Sher A., Kipnis T. L. (1988). Biochemical characterization of a factor produced by trypomastigotes of Trypanosoma cruzi that accelerates the decay of complement C3 convertases. J. Biol. Chem. 263 11327–11335. [PubMed] [Google Scholar]
- Keshi H., Sakamoto T., Kawai T., Ohtani K., Katoh T., Jang S., et al. (2006). Identification and characterization of a novel human collectin CL-K1. Microbiol. Immunol. 50 1001–1013. 10.1111/j.1348-0421.2006.tb03868.x [DOI] [PubMed] [Google Scholar]
- Kipnis T. L., Krettli A. U., Da Silva W. D. (1985). Transformation of trypomastigote forms of Trypanosoma cruzi into activators of alternative complement pathway by immune IgG fragments. Scand. J. Immunol. 22 217–226. 10.1111/j.1365-3083.1985.tb01874.x [DOI] [PubMed] [Google Scholar]
- Kipnis T. L., Tambourgi D. V., Sucupira M., da Silva W. D. (1986). Effect of Trypanosoma cruzi membrane components on the formation of the classical pathway C3 convertase. Braz. J. Med. Biol. 19 271–278. [PubMed] [Google Scholar]
- Kishore U., Ghai R., Greenhough T. J., Shrive A. K., Bonifati D. M., Gadjeva M. G., et al. (2004). Structural and functional anatomy of the globular domain of complement protein C1q. Immunol. Lett. 95 1–27. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer T. R., Thiel S., Andersen G. R. (2013). Toward a structure-based comprehension of the lectin pathway of complement. Mol. Immunol. 56 413–422. 10.1016/j.molimm.2013.05.007 [DOI] [PubMed] [Google Scholar]
- Kondos S. C., Hatfaludi T., Voskoboinik I., Trapani J. A., Law R. H. P., Whisstock J. C., et al. (2010). The structure and function of mammalian membrane-attack complex/perforin-like proteins. Tissue Antigens 76 341–351. 10.1111/j.1399-0039.2010.01566.x [DOI] [PubMed] [Google Scholar]
- Krautz G. M., Kissinger J. C., Krettli A. U. (2000). The targets of the lytic antibody response against Trypanosoma cruzi. Parasitol. Today 16 31–34. 10.1016/S0169-4758(99)01581-1 [DOI] [PubMed] [Google Scholar]
- Lambris J. D., Ricklin D., Geisbrecht B. V. (2008). Complement evasion by human pathogens. Nat. Rev. Microbiol. 6 132–142. 10.1038/nrmicro1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederkremer R., Bertello L. (2001). Glycoinositolphospholipids, free and as anchors of proteins, in Trypanosoma cruzi. Curr. Pharm. Des. 7 1165–1179. 10.2174/1381612013397519 [DOI] [PubMed] [Google Scholar]
- Lee B. Y., Bacon K. M., Bottazzi M. E., Hotez P. J. (2013). Global economic burden of chagas disease: a computational simulation model. Lancet Infect. Dis. 13 342–348. 10.1016/S1473-3099(13)70002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine N. D., Corliss J. O., Cox F. E. G., Deroux G., Grain J., Honigberg B. M., et al. (1980). A newly revised classification of the protozoa. J. Protozool. 27 37–58. 10.1111/j.1550-7408.1980.tb04228.x [DOI] [PubMed] [Google Scholar]
- Lidani K. C. F., Beltrame M. H., Luz P. R., Sandri T. L., Nisihara R. M., Messias-Reason I. J. (2015). Is pentraxin 3 a cardiovascular marker in patients with chronic chagas disease? Int. J. Cardiol. 190 233–235. 10.1016/j.ijcard.2015.04.106 [DOI] [PubMed] [Google Scholar]
- Lörincz Z., Gál P., Dobó J., Cseh S., Szilágyi K., Ambrus G., et al. (2000). The cleavage of two C1s subunits by a single active C1r reveals substantial flexibility of the C1s-C1r-C1r-C1s tetramer in the C1 complex. J. Immunol. 165 2048–2051. 10.4049/jimmunol.165.4.2048 [DOI] [PubMed] [Google Scholar]
- Lu Y. C., Weng W. C., Lee H. (2015). Functional roles of calreticulin in cancer biology. BioMed Res. Int. 2015 9 10.1155/2015/526524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz P. R., Miyazaki M. I., Neto N. C., Padeski M. C., Barros A. C. M., Boldt A. B. W., et al. (2016). Genetically determined MBL deficiency is associated with protection against chronic cardiomyopathy in chagas disease. PLoS Negl. Trop. Dis. 10:e0004257 10.1371/journal.pntd.0004257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz P. R., Velavan T. P., Kremsner P. G., Messias-Reason I. J. T. (2013). Association of IP-10 and PDGF-BB levels with clinical forms of chronic chagas disease. Int. J. Cardiol. 169 e53–e55. 10.1016/j.ijcard.2013.08.110 [DOI] [PubMed] [Google Scholar]
- Malik L. H., Singh G. D., Amsterdam E. A. (2015). The epidemiology, clinical manifestations, and management of chagas heart disease. Clin. Cardiol. 38 565–569. 10.1002/clc.22421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle N. S., Church S. E., Fremeaux-Bacchi V., Roumenina L. T. (2015). Complement system part I–molecular mechanisms of activation and regulation. Front. Immunol. 6:262 10.3389/fimmu.2015.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. W., Nussenzweig V. (1974). Complement as a regulator of interactions between immune complexes and cell membranes. J. Immunol. 113 464–469. [PubMed] [Google Scholar]
- Navarro M., Navaza B., Guionnet A., López-Vélez R. (2012). Chagas disease in spain: need for further public health measures. PLoS Negl. Trop. Dis. 6:e1962 10.1371/journal.pntd.0001962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Bianco C., Cohn Z. (1975). Studies on the selective lysis and purification of Trypanosoma cruzi. J. Exp. Med. 142 224–229. 10.1084/jem.142.1.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N. P., Saraiva F. M. S., Sultano P. E., Cunha P. R. B. B., Laranja G. A. T., Justo G. A., et al. (2015). Proliferation and differentiation of Trypanosoma cruzi inside its vector have a new trigger: redox status. PLoS ONE 10:e0116712 10.1371/journal.pone.0116712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M., Remuzzi G. (2013). Overview of complement activation and regulation. Semin. Nephrol. 33 479–492. 10.1016/j.semnephrol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K. A. (1996). Ligand-binding renders the 160 kDa Trypanosoma cruzi complement regulatory protein susceptible to proteolytic cleavage. Microb. Pathog. 21 235–248. 10.1006/mpat.1996.0058 [DOI] [PubMed] [Google Scholar]
- Norris K. A. (1998). Stable transfection of Trypanosoma cruzi epimastigotes with the trypomastigote-specific complement regulatory protein cDNA confers complement resistance. Infect. Immun. 66 2460–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K. A., Bradt B., Cooper N. R., So M. (1991). Characterization of a Trypanosoma cruzi C3 binding protein with functional and genetic similarities to the human complement regulatory protein, decay-accelerating factor. J. Immunol. 147 2240–2247. [PubMed] [Google Scholar]
- Norris K. A., Galvao L. M. C., Schrimpf J. E., Cancado J. R., Krettli A. U. (1994). Humoral immune response to the Trypanosoma cruzi complement regulatory protein as an indicator of parasitologic clearance in human chagas’. Dis. Infect. Immun. 62 4072–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K. A., Harth G., So M. (1989). Purification of a Trypanosoma cruzi membrane glycoprotein which elicits lytic antibodies. Infect. Immun. 57 2372–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K. A., Schrimpf J. E. (1994). Biochemical analysis of the membrane and soluble forms of the complement regulatory protein of Trypanosoma cruzi. Infect. Immun. 62 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K. A., Schrimpf J. E., Szabo M. J. (1997). Identification of the gene family encoding the 160-Kilodalton Trypanosoma cruzi complement regulatory protein. Infect. Immun. 65 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M. C. P., Dones W., Morillo C. A., Encina J. J., Ribeiro A. L. (2013). Chagas disease: an overview of clinical and epidemiological aspects. J. Am. Coll. Cardiol. 62 767–776. 10.1016/j.jacc.2013.05.046 [DOI] [PubMed] [Google Scholar]
- Ouaissi M. A., Afchain D., Capron A., Grimaud J. A. (1984). Fibronectin receptors on Trypanosoma cruzi trypomastigotes and their biological function. Nature 308 380–382. 10.1038/308380a0 [DOI] [PubMed] [Google Scholar]
- Ouaissi M. A., Cornette J., Capron A. (1986). Identification and isolation of Trypanosoma cruzi trypomastigote cell surface protein with properties expected of a fibronectin receptor. Mol. Biochem. Parasitol. 19 201–211. 10.1016/0166-6851(86)90002-2 [DOI] [PubMed] [Google Scholar]
- Ouaissi M. A., Cornette J., Taibi A., Velge P., Capron A. (1988). Major surface immunogens of Trypanosoma cruzi trypomastigotas. Mem. Inst. Oswaldo Cruz 83 502 10.1590/S0074-02761988000500058 [DOI] [PubMed] [Google Scholar]
- Pereira P. C. M., Navarro E. C. (2013). Challenges and perspectives of chagas disease: a review. J. Venom. Anim. Toxins Incl. Trop. Dis. 19:34 10.1186/1678-9199-19-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez G., Valck C., Aguilar L., Kemmerling U., López-Muñoz R., Cabrera G., et al. (2012). Roles of Trypanosoma cruzi calreticulin in parasite-host interactions and in tumor growth. Mol. Immunol. 52 133–140. 10.1016/j.molimm.2012.05.006 [DOI] [PubMed] [Google Scholar]
- Ramírez G., Valck C., Molina M. C., Ribeiro C. H., López N., Sánchez G., et al. (2011). Trypanosoma cruzi calreticulin: a novel virulence factor that binds complement C1 on the parasite surface and promotes infectivity. Immunobiology 216 265–273. 10.1016/j.imbio.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Ramirez M. I., Deolindo P., de Messias-Reason I. J., Arigi E. A., Choi H., Almeida I. C., et al. (2016). Dynamic flux of microvesicles modulate parasite-host cell interaction of Trypanosoma cruzi in Eukaryotic Cells. Cell. Microbiol. 19:e12672 10.1111/cmi.12672 [DOI] [PubMed] [Google Scholar]
- Ramos R., Juri M., Ramos A., Hoecker G., Lavandero S., Pena P., et al. (1991). An immunogenetically defined and immunodominant Trypanosoma cruzi Antigen. Am. J. Trop. Med. Hyg. 44 314–322. [DOI] [PubMed] [Google Scholar]
- Rassi A., Jr., Rassi S. G., Rassi A. (2001). Sudden death in Chagas’ disease. Arq. Bras. Cardiol. 76 86–96. 10.1590/S0066-782X2001000100008 [DOI] [PubMed] [Google Scholar]
- Rassi A., Marin-Neto J. A. (2010). Chagas Disease. Lancet 375 1388–1402. 10.1016/S0140-6736(10)60061-X [DOI] [PubMed] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J. D. (2010). Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11 785–797. 10.1038/ni.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Lambris J. D. (2013). Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 190 3831–3838. 10.4049/jimmunol.1203487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Reis E. S., Lambris J. D. (2016). Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 12 383–401. 10.1038/nrneph.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M. T., Sher A., Heiny S., Lituchy A., Hammer C. H., Joiner K. A. (1988). Developmentally regulated expression by Trypanosoma cruzi of molecules that accelerate the decay of complement C3 convertases. Proc. Natl. Acad. Sci. U.S.A. 85 193–197. 10.1073/pnas.85.1.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M. T., Tenner A. J., Bobak D. A., Joiner K. A. (1989). Complement component C1q enhances invasion of human mononuclear phagocytes and fibroblasts by Trypanosoma cruzi Trypomastigotes. J. Clin. Invest. 84 1982–1989. 10.1172/JCI114388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D., Sher A. (2002). Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 3 1041–1047. 10.1038/ni1102-1041 [DOI] [PubMed] [Google Scholar]
- Salvatella R., Irabedra P., Sánchez D., Castellanos L. G., Espinal M. (2013). South-south cooperation for chagas disease. Lancet 382 395–396. 10.1016/S0140-6736(13)61671-2 [DOI] [PubMed] [Google Scholar]
- Schijman A. G., Bisio M., Orellana L., Sued L., Duffy T., Jaramillo A. M. M., et al. (2011). International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from chagas disease patients. PLoS Negl. Trop. Dis. 5:e931 10.1371/journal.pntd.0000931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmunis G. A. (2007). Epidemiology of chagas disease in non-endemic countries: the role of international migration. Mem. Inst. Oswaldo Cruz 102(Suppl. 1) 75–85. 10.1590/S0074-02762007005000093 [DOI] [PubMed] [Google Scholar]
- Silveira J. F., da Abrahamsohn P. A., Colli W. (1979). Plasma membrane vesicles isolated from epimastigote forms of Trypanosoma cruzi. Biochim. Biophys. Acta 550 222–232. 10.1016/0005-2736(79)90209-8 [DOI] [PubMed] [Google Scholar]
- Singh G., Sehgal R. (2010). Transfusion-transmitted parasitic infections. Asian J. Transfus. Sci. 4 73 10.4103/0973-6247.67018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosoniuk E., Vallejos G., Kenawy H., Gaboriaud C., Thielens N., Fujita T., et al. (2014). Trypanosoma cruzi calreticulin inhibits the complement lectin pathway activation by direct interaction with L-ficolin. Mol. Immunol. 60 80–85. 10.1016/j.molimm.2014.03.014 [DOI] [PubMed] [Google Scholar]
- Souza W., Carvalho T. M. U., Barrias E. S. (2010). Review on Trypanosoma cruzi: host cell interaction. Int. J. Cell Biol. 2010:295394 10.1155/2010/295394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway J. D., Roth G. (2015). The burden of chagas disease estimates and challenges. Global Heart 10 139–144. 10.1016/j.gheart.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Stuart G. R., Lynch N. J., Day A. J., Schwaeble W. J., Sim R. B. (1997). The C1q and collectin binding site within C1q receptor (cell surface Calreticulin). Immunopharmacology 38 73–80. 10.1016/S0162-3109(97)00076-3 [DOI] [PubMed] [Google Scholar]
- Sugimoto R., Yae Y., Akaiwa M., Kitajima S., Shibata Y., Sato H., et al. (1998). Cloning and characterization of the hakata antigen, a member of the ficolin/opsonin p35 lectin family. J. Biol. Chem. 273 20721–20727. 10.1074/jbc.273.33.20721 [DOI] [PubMed] [Google Scholar]
- Tambourgi D. V., Cavinato R. A., De Abreu C. M., Peres B. A., Kipnis T. L. (1995). Detection of Trypanosoma-decay accelerating factor antibodies in mice and humans infected with Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 52 516–520. [DOI] [PubMed] [Google Scholar]
- Tambourgi D. V., Kipnis T. L., Da Silva W. D., Joiner K. A., Sher A., Heath S., et al. (1993). A partial cDNA Clone of Trypomastigote Decay-Accelerating Factor (T-DAF), a developmentally regulated complement inhibitor of Trypanosoma cruzi, has genetic and functional similarities to the human complement inhibitor DAF. Infect. Immun. 61 3656–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valck C., Ramírez G., López N., Ribeiro C. H., Maldonado I., Sánchez G., et al. (2010). Molecular mechanisms involved in the inactivation of the first component of human complement by Trypanosoma cruzi Calreticulin. Mol. Immunol. 47 1516–1521. 10.1016/j.molimm.2010.01.019 [DOI] [PubMed] [Google Scholar]
- van Lookeren C. M., Wiesmann C., Brown E. J. (2007). Macrophage complement receptors and pathogen clearance. Cell Microbiol. 9 2095–2102. 10.1111/j.1462-5822.2007.00981.x [DOI] [PubMed] [Google Scholar]
- Velge P., Ouaissi M. A., Cornette J., Afchain D., Capron A. (1988). Identification and isolation of Trypanosoma cruzi trypomastigote collagen-binding proteins: possible role in cell-parasite interaction. Parasitology 97(Pt 2) 255–268. 10.1017/S0031182000058467 [DOI] [PubMed] [Google Scholar]
- Walport M. J. (2001). Complement first of two parts. N. Engl. J. Med. 344 1058–1066. [DOI] [PubMed] [Google Scholar]
- Watanabe Costa R., Silveira J. F., Bahia D. (2016). Interactions between Trypanosoma cruzi secreted proteins and host cell signaling pathways. Front. Microbiol. 7:388 10.3389/fmicb.2016.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W. I., Drickamer K., Hendrickson W. (1992). Structure of a C-Type mannose-binding protein complexed with an oligosaccharide. Nature 360 127–134. 10.1038/360127a0 [DOI] [PubMed] [Google Scholar]
- Weitzel T., Zulantay I., Danquah I., Hamann L., Schumann R. R., Apt W., et al. (2012). Mannose-binding lectin and toll-like receptor polymorphisms and chagas disease in chile. Am. J. Trop. Med. Hyg. 86 229–232. 10.4269/ajtmh.2012.11-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization [WHO] (2009). Control and Prevention of Chagas Disease in Europe. Geneva: World Health Organization; 69. [Google Scholar]
- World Health Organization [WHO] (2010). Working to Overcome the Global Impact of Neglected Tropical Diseases First WHO Report on Neglected Tropical Diseases. Geneva: World Health Organization. [Google Scholar]
- World Health Organization [WHO] (2015). Chagas disease in latin america: an epidemiological update based on 2010 estimates. Wkly. Epidemiol. Rec. 6 33–44. 10.2147/IBPC.S70402 [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M., Siljander P. R. M., Andreu Z., Zavec A. B., Borràs F. E., Buzas E. I., et al. (2015). Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4:27066 10.3402/jev.v4.27066 [DOI] [PMC free article] [PubMed] [Google Scholar]


