Abstract
One of the most significant manifestations of environmental stress in plants is the increased production of Reactive Oxygen Species (ROS). These ROS, if allowed to accumulate unchecked, can lead to cellular toxicity. A battery of antioxidant molecules is present in plants for keeping ROS levels under check and to maintain the cellular homeostasis under stress. Ascorbate peroxidase (APX) is a key antioxidant enzyme of such scavenging systems. It catalyses the conversion of H2O2 into H2O, employing ascorbate as an electron donor. The expression of APX is differentially regulated in response to environmental stresses and during normal plant growth and development as well. Different isoforms of APX show differential response to environmental stresses, depending upon their sub-cellular localization, and the presence of specific regulatory elements in the upstream regions of the respective genes. The present review delineates role of APX isoforms with respect to different types of abiotic stresses and its importance as a key antioxidant enzyme in maintaining cellular homeostasis.
Keywords: APX, ROS, abiotic stress, antioxidant, ASC–GSH pathway
Introduction
Abiotic and biotic stresses are a regular feature in natural plant environment. Stress is quite unpredictable in its duration, occurrence, and intensity and thus maintaining the growth and survival is a herculean task in affected regions. Plants can perceive even a lowest environmental stress signal and reproductive stages are most sensitive to it. Reactive oxygen species or free radicals are produced as a by-product in various cellular compartments especially mitochondria and chloroplasts in association with different kinds of oxidases (Van Breusegem and Dat, 2006). These ROS are important for signaling in several growth and developmental processes and in comprehending biotic and abiotic stresses along with programmed cell death (Bailey-Serres and Mittler, 2006). But when ROS are present in excess amounts they bring about a severe damage to cellular structure and macromolecules. Scavenging systems comprising of many antioxidants and enzymes counter these ROS entities and convert them into less toxic products in the cell, sometimes even at the site of their generation. Under stress conditions, the redox homeostasis of the cell is rapidly disturbed accumulating abundant ROS (Halliwell, 2006). Antioxidant enzymes and their isoforms come into play to remove these free radicals and APX is one of the central enzymes in this system. Its role in maintaining redox balance has been seen both in normal plant life cycle and during various abiotic stress conditions, validated through various transgenic approaches. In this review, we describe the importance of APX as a key antioxidant enzyme and the myriad roles that its isoforms perform in mitigating various environmental stresses.
Plant stress and reactive oxygen species
Plant growth and development is squarely dependent on the availability of optimal environmental conditions and nutritional factors and any deviation from these conditions constitutes stress. It induces non-specific reversible changes and responses at various functional levels which may become permanent if allowed to persist for a longer duration. Plants, being sessile, have to confront these adverse conditions, and essentially require special adaptive mechanisms to combat them. The duration and magnitude of stress determines the severity of symptoms while the physiological manifestations involve an increase in respiration, alterations in electron transport system, inhibition of photosynthesis, and reduction in biomass. The cellular responses to stress include altered cell cycle, changes in the induction of vacuolization, and cell wall organization allowing to tolerate the stress (Cramer et al., 2007; dos Reis et al., 2012). There are alterations in the anatomy, physiology, and energy consumption which increases due to a shift in cellular metabolism to maintain cellular homeostasis. Plants react to stress by either acclimatization i.e., adjusting to the new conditions and reaching a state of homeostasis or adaptations which involve permanent alterations introduced to resist stress (Chinnusamy and Zhu, 2009).
Stress is generally categorized into two primary groups: biotic and abiotic. Biotic stress is perceived when other living organisms such as weeds, microbial pathogens, and insects induce damage to the plant while abiotic stress is caused by a physical or chemical entity in the immediate environment resulting in altered growth and productivity. The growth recovery is facilitated in case of the stress being short term, of low intensity, or the plant being tolerant. But when it cannot withstand this attack, its metabolic functions are severely affected, the phenological stages are hindered and it ultimately dies. The primary abiotic stresses include water-deficit, salinity, cold, heat, and oxidative stress.
ROS are inevitable components of aerobic metabolism. Sequential reduction of molecular oxygen produces 1O2 (singlet oxygen), H2O2 (hydrogen peroxide), O2.− (superoxide radical), and OH. (hydroxyl radical) by electron transport systems in different sub-cellular compartments like cytosol, chloroplasts, mitochondria, and microbodies (Dias et al., 2014). They have critical signaling roles at lower concentrations in processes like seed germination, pollen tube growth, leaf development and senescence, root hair growth, cell elongation, embryo formation, gravitropism, self-incompatibility, and many more (Gechev et al., 2006). This is achieved with the help of redox sensitive proteins (redox protein), mobilization of calcium, phosphorylation of protein, and gene expression.
These ROS are also the resultants of alterations in cellular metabolism which are induced in response to various environmental stresses culminating in oxidative stress (Shigeoka et al., 2002). ROS activate and/or regulate the primary and secondary signaling pathways during abiotic, oxidative, wounding, or pathogen stresses by their synthesis or detoxification (Duque et al., 2013). Temporary production of ROS, also termed as “respiratory burst,” is a common feature in biotic stress occurring during early wounding or plant-pathogen interactions. Various key players in ROS signaling pathways include zinc finger proteins (Zat 12, Zat 7) and WRKY TFs. ROS are secondary messengers in ABA transduction pathway in guard cells during abiotic stress. ABA induces H2O2 to reduce water loss (Baxter et al., 2014). Salicylic acid (SA) is also reported to be a regulator of ROS during wounding (Sharma et al., 2012).
Antioxidative defence system in plants
The level of ROS in the cell is determined from balance between their production and scavenging by antioxidants. When ROS levels exceed a threshold required for plant metabolic processes, they become able to damage the major macromolecules of the cell i.e., proteins, lipids, and nucleic acids (Das and Roychoudhury, 2014; Kapoor, 2015). Since abiotic stresses produce abundant ROS which cause detrimental effects, its detoxification is of paramount importance to protect cellular integrity. To maintain a redox homeostasis, antioxidant defense systems are continuously activated in the plant. They comprise of two components: enzymatic and non-enzymatic. The enzymatic components (Figure 1) include superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione-S-transferase (GST), guaiacol peroxidase (GP), glutathione peroxidase (GPX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR). The non-enzymatic components are compounds like ascorbic acid (ASC), carotenoids, flavanoids, phenolics, reduced glutathione (GSH), and α-tocopherol which act as antioxidant buffers removing higher levels of ROS and are found in all sub-cellular compartments (Miller et al., 2010).
Figure 1.
Localization of APX enzymes and detoxification of ROS in subcellular compartments.
The stress conditions always result in an excessive production of ROS which causes a major shift in redox environment. This interferes with signaling pathways, thus, leading to the scavenging and detoxification of free radicals and other intermediate compounds through antioxidant systems (Yun et al., 2010). Excess of activates SOD which converts to H2O2 and the latter is removed by APX. Thus, these antioxidant systems eliminate excess ROS not required for basic plant processes and stabilize the internal biochemical state of the cell during various abiotic stresses, leading to acclimatization as well as tolerance (Bowler and Fluhr, 2000; Scandalios, 2005).
Ascorbate peroxidase is reported to be an efficient regulator of ROS, as it contributes maximally to hydrogen peroxide detoxification. Being present in various sub-cellular organelles, APX is also one of the most regulated enzymes (Saxena et al., 2011).
Ascorbate peroxidase (EC 1.11.1.11)
APX belongs to the family of heme containing peroxidases that catalyse the H2O2-dependent oxidation of a wide range of organic molecules. It is present across a wide spectrum of plant kingdom and plays a crucial role in growth regulation. APX differs from other peroxidases in its dependency on ASC as the source of reducing power and becomes unstable in its absence (Shigeoka et al., 2002). There are multigenic families of APX in higher plants for e.g., Arabidopsis has 9 APX genes (AtAPX1-AtAPX6, sAPX, tAPX, lAPX) and Oryza sativa has 8 isozymes (OsAPX1-OsAPX8), having two APX each in cytosol, peroxisome, chloroplast and mitochondria (Table 1). Similarly, tomato has seven genes encoding different APX isozymes (Chew et al., 2003; Teixeira et al., 2004). Different isozymes of APX, which are classified on the basis of their sub-cellular localization, show different structural, and kinetic properties, including the presence of specific conserved domains and signal peptides. The thylakoidal isoform is reported to be the first one to intercept an H2O2 molecule as it is located adjacent to the acceptor of photosystem I (Huseynova et al., 2014). Broadly, on the basis of amino acid composition, five isoforms of APX have been classified in plants, viz cytosolic (cAPX: APX 1&2), mitochondrial (mitAPX), chloroplastic (chlAPX: stromal-APX and thylakoidal-APX), and peroxisomal/glyoxysomal (mAPX). It is interesting to note that all the isoforms of APX originate from alternative splicing, which contributes to the differential regulation of expression of various isoforms (Caverzan et al., 2012). All isoforms differ in their kinetic properties like molecular weight, optimal pH, stability, catalytic rate, and substrate affinity. As APXs are heme-dependent oxido-reductases, iron is critical for the catalytic activity and iron deficiency reduces its activity. Similar effect is observed when ascorbate concentrations are reduced, wherein the activity and stability of the enzymes is adversely affected. The cytosolic APX isoforms are more sensitive for reduction in ascorbate than chloroplastic, both stromal and thylakoid membrane-bound APX.
Table 1.
Localization of different APX isoforms in Oryza sativa.
| Gene | Cellular location | Locus I.D. | Chr. | Function | References |
|---|---|---|---|---|---|
| Oryza sativa | |||||
| OsAPx1 | Cytosolic | Os03g17690 | 3 | Cellular response to oxidative stress, ROS salinity stress tolerance, pathogen attack | Mittler and Zilinskas, 1991; Wang et al., 2005 |
| OsAPx2 | Os07g49400 | 7 | |||
| OsAPx3 | Peroxisomal | Os04g14680 | 4 | Salinity and drought tolerance | Teixeira et al., 2004 |
| OsAPx4 | Os08g43560 | 8 | |||
| OsAPx5 | Stromal | Os12g07830 | 12 | Response to salinity stress | Yoshimura et al., 2000; Hong et al., 2007 |
| OsAPx6 | Os12g07820 | 12 | |||
| OsAPx7 | Os04g35520 | 4 | |||
| OsAPx8 | Thylakoid | Os02g34810 | 2 | Involve in water- water cycle | Hong et al., 2007; Zhu et al., 2013 |
APXs have two important histidine residues, His42 and His163 and a K+ binding site which are required for APX activity. Ascorbate binds to the active site of the protein by four hydrogen bonds with lysine and arginine residues and the heme moiety, with the site of substrate binding (Cys32, Arg172, Lys30) being highly conserved (Chen and Asada, 1989). APX activity has been found to increase in presence of other antioxidant enzymes like superoxide dismutase and glutathione reductase, indicating a cross talk amongst various antioxidant enzymes. APX is unable to scavenge lipid hydroperoxides and is inhibited by cyanide, azide, and thiol-modifiers. The whole family of APX shows inducive responses to ABA treatment, with the cytosolic one being the most induced one (Zhang et al., 2014). The existence of multiple molecular forms of APX within the cells and organelles signifies the important role played by them in developmental processes and stress tolerance (Ishikawa et al., 1998; Shigeoka et al., 2002).
Cytosolic APX has been reported to be the most responsive isoform that is encoded by a single gene apx1 and is also the best characterized apx gene. It has a heat shock responsive element in the 5′ regulatory region and an anti-peroxidative element (ARE) specifically for H2O2 scavenging. APX and its various isoforms are actively expressed under biotic stresses (pathogen attack, herbivory, physical damage) as well as under abiotic stresses (salt, drought, heat, cold, UV radiations, oxidative stress etc.) (Table 2). The extent of expression of APX directly correlates with the duration and intensity of the imposed stress, as well as the multiplicity of stresses. Further, the same kind of stress can induce differential expression of isozymes in different sub-cellular sites (Shigeoka et al., 2002).
Table 2.
Role of different APX isoforms in plant abiotic stress tolerance.
| S. No | Gene name | Promoter | Source crop | Recipient crop | Stress | Outcome | References |
|---|---|---|---|---|---|---|---|
| SALT | |||||||
| 1 | CytAPX | CaMV35S | NA | Solanum lycopersicum | Salt/Chilling | APX activity was 10 fold higher during chilling and salt stress | Wang et al., 2005 |
| 2 | APX | CaMV 35S | Nicotiana tabacum | Nicotiana tabacum | Salt/Paraquat | APX was up-regulated in multiple stresses | Lee Y. P. et al., 2007 |
| 3 | CytAPX | CaMV35S | Oryza sativa | Arabidopsis thaliana | Salt | APX was up-regulated in salt stress | Lu et al., 2007 |
| 4 | PrAPX | CaMV35S / rd29 | Populus sps. | Nicotiana tabacum | Drought/Salt | Drought and salt tolerance during vegetative stage | Li et al., 2009 |
| 5 | StAPX | CaMV 35S | Suaeda salsa | Arabidopsis thaliana | Salt | Protection against salt stress | Li et al., 2012 |
| 6 | CytAPX | CaMV35S/Pcambia 3301 | Oryza sativa | Medicago sativa | Salt/Drought | APX activity was up-regulated in salt and drought | Qian et al., 2013 |
| 7 | CytAPX | CaMV35S | NA | Prunus domestica | Salt | Enhances the tolerance to salinity | Diaz-Vivancos et al., 2013 |
| 8 | TbAPX | CaMV 35S/Nos | Jatropha curcas | Nicotiana tabacum | Salt | Enhances the tolerance to salinity | Liu et al., 2013 |
| 9 | CytAPX | CaMV 35S | Jatropha curcas | Arabidopsis thaliana | Salt | APX activity up-regulated in salinity | Chen et al., 2015 |
| 10 | APX | CaMV 35S/SWPA2 | NA | Ipomoea batatas | Salt | APX up-regulated to 7.8 and 13.3 folds under salinity | Yan et al., 2016 |
| HEAT | |||||||
| 11 | PrAPX | SWPA2 | Hordeum vulgare L. | Arabidopsis thaliana | Heat stress | APX up-regulated in heat stress | Shi et al., 2001 |
| 12 | ChlAPX | pCAMBIA2300 | NA | Solanum tuberosum | Heat/M-V | Up-regulated in multiple environment stresses | Tang et al., 2006 |
| 13 | StAPX | CaMV35S | Cyanidioschyzon merolae | Arabidopsis thaliana | High temperature | Up-regulated during Heat stress | Hirooka et al., 2009 |
| 14 | CytAPX | Ubiquitin | Brassica campestris | Arabidopsis thaliana | Heat Stress | APX activity up-regulated in Heat stress | Chiang et al., 2015 |
| COLD | |||||||
| 15 | ChlAPX | NA | Gossypium hirsutum | Gossypium hirsutum | Cold | APX activity up-regulated during low temperature | Kornyeyev et al., 2003 |
| 16 | TbAPX | 35S-CaMV-LetAPX | LetAPX | Solanum lycopersicum | Chilling stress. | Tolerance against chilling stress. | Duan et al., 2012 |
| 17 | CytAPX | CaMV 35S | NA | Brazilian arrowroot | Cold | Improved tolerance against cold stress. | Xu et al., 2014 |
| DROUGHT | |||||||
| 18 | APX6 | Ubi/35S | Solanum melongena | Oryza sativa | Water stress | Stronger resistance to flood tolerance | Chiang et al., 2015 |
| OXIDATIVE | |||||||
| 19 | PrAPX | NA | Arabidopsis thaliana | Nicotiana tabacum | Oxidative stress | APX3 up-regulated in peroxisomes | Wang et al., 1999 |
| 20 | TbAPX | CaMV 35S | NA | Nicotiana tabacum | Photo-oxidative | Up-regulated TbAPX activity in chloroplast | Yabuta et al., 2002 |
| 21 | APX | CaMV 35S | Nicotiana tabacum | Nicotiana tabacum | M-V stress | APX activity highly up-regulated | Kwon et al., 2002 |
| 22 | TbAPX | NA | Triticum aestivum | Triticum aestivum | High light stress | Role in removal of H2O2 generated during photosynthesis | Danna et al., 2003 |
| 23 | TbAPX | CaMV35S | Arabidopsis thaliana | Arabidopsis thaliana | Photo-oxidation | Resistance to photo-oxidative and nitric oxide stresses | Murgia et al., 2004 |
| 24 | ChlAPX | SWPA2/ CaMV 35S | NA | Ipomoea batatas | M-V and Chilling | APX highly up-regulated in chloroplast | Lim et al., 2007 |
| 25 | CytAPX | NA | Theobroma cacao | Moniliophthora perniciosa | Oxidative stress | APX activity up-regulated in witches' broom disease | Camillo et al., 2013 |
| BIOTIC | |||||||
| 26 | OsAPX8 | CaMV 35S | NA | Oryza sativa | Biotic | Tolerance to bacterial blight | Jiang et al., 2016 |
| OTHERS | |||||||
| 27 | ChlAPX | CaMV35S | NA | Nicotiana tabacum | NA | Does not provide protection against ozone | Torsethaugen et al., 1997 |
| 28 | PrAPX | GFP–APX3 fus | NA | Arabidopsis thaliana | NA | Dispensable for Arabidopsis thaliana growth and development | Narendra et al., 2006 |
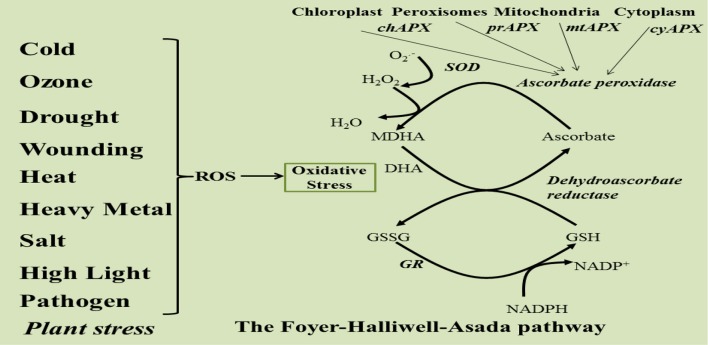
Ascorbate-glutathione (ASC-GSH) pathway
The enzymatic and non-enzymatic antioxidant molecules work in a close coordination to give a meaningful protection from oxidative stress. Two pathways are interconnected via APX enzyme which functions as a linking molecule for maintaining the redox balance under stress. Ascorbate and glutathione have long been known to have a close association. Since both of them were assumed to have a role in detoxification, they have been studied in chloroplast samples and found to be associated with NADPH (Foyer and Halliwell, 1976, 1977). Thus, the APX-ascorbate link also serves to regulate the NADPH/NAD ratio under stress. This central scheme in which ascorbate peroxidase-ascorbate and glutathione work in tandem is known as the Ascorbate-Glutathione (ASC-GSH) pathway or Foyer-Halliwell-Asada pathway (Figure 2) that functions in both plants and animals. The importance of this pathway can be gauged from the fact that it is present in cytosol, chloroplast (stromal and thylakoid bound), mitochondria, and peroxisomes (Mittler and Zilinskas, 1991; Jimenez and Hernandez, 1997). It is known as the heart of the redox homeostasis and performs the function of a unified scavenger of ROS, although other antioxidant enzymes and components are also present in plant cells. It has been reported that detoxification of ROS through ASC-GSH pathway causes transient adjustments in the levels of most of the intermediates of this pathway (Noctor et al., 2000; Mittler et al., 2004; Noctor, 2006; Foyer and Shigeoka, 2011).
Figure 2.
Schematic representation of Foyer-Halliwell-Asada Pathway.
The stress generated H2O2 inactivates photosynthetic mechanism and disturbs the electron transport system and cellular respiration (Kaiser, 1976; Charles and Halliwell, 1980). Accumulation of H2O2 is highly toxic for the cell as H2O2 is the only radical species that can pass through the biological membranes and invade other sub-cellular compartments. Further, H2O2 can also lead to the production of highly reactive hydroxyl radicals in the presence of divalent cations (Figure 2). Therefore, rapid scavenging of H2O2, via ascorbate peroxidase, is extremely important for a potent antioxidant system (Asada, 1999). The possibility that ASC and GSH might function independently has been studied. Andrea Polle developed a metabolic model in 2001 as a tool to analyse the network of redox reactions in Superoxide dismutase (SOD)-ascorbate (ASC)-glutathione (GSH) cycle. The computational simulation analysis was based on previously determined concentrations of all components of the cycle, kinetic properties of antioxidative enzymes and some more others crucial parameters. The simulation results concluded a higher production rate of H2O2 in the absence of APXs without significant effects on the redox balance of ASC/DHA/ or GSH/GSSG and the coupling between ASC and GSH-related redox systems was weak (Polle, 2001).
APX expression under various environmental conditions
APX gene expression has been reported to increase on exposure to drought, salt, cold, heat, pathogen infection, wound stress, and other biotic or abiotic stresses. However, the quantitative expression varies in different sub-cellular compartments and is also dependent on the developmental stages of the plant and the imposed stress conditions. Increase in APX activity is many times supplemented with the activity of other antioxidant enzymes that work in tandem with APX (Teixeira et al., 2006; Lee Y. P. et al., 2007).
Direct oxidative stress
There are many biotic and abiotic factors present in the environment that are responsible for the generation of oxidative stress/ROS in plants. These factors may directly contribute to ROS production via dissipation of excess energy, reducing power, or may indirectly cause ROS production by altering the cellular metabolism. In case of methyl viologen treatment stress, chlAPX (sAPX and tAPX) is the primary target for inactivation apart from cAPX and mAPXs which show lesser sensitivity toward the chemical (Mano et al., 2001). Different plant species have differential abilities to up-regulate APX activity under stress, e.g., the tAPX usually cannot scavenge excess amounts of ROS; but when over-expressed it reduces damage under pathogen attack by reducing NO (Nitric Oxide) symptoms. Over-expression of cAPX in tobacco chloroplasts leads to increased tolerance to oxidative stress induced by paraquat as well as salinity (Dąbrowska et al., 2007). APX activity is reported to increase in Arabidopsis during exposure to UV-B radiations (Rao et al., 1996). cAPX transcripts were increased in germinating rice embryos upon treatment with hydroxyl-urea or amino-triazole that resulted in increased cellular H2O2 levels. Similarly, APX was up-regulated in Arabidopsis bundle sheath cells, upon exposure to high light intensity, due to increase in H2O2 accumulation (Karpinski et al., 1999; Morita et al., 1999). Interestingly, double mutants for APX1 and CAT1 were found to be less sensitive to oxidative stress than individual single mutants, as the former probably activated a compensatory scavenging antioxidant and defense mechanism (Rizhsky et al., 2002).
Expression of APXs under saline conditions
Salinity stress creates ion imbalances and induces physiological drought like conditions by limiting the amount of water available to the plant. Under such conditions, APX provides salinity tolerance at different levels to the affected plants. Loss of function cAPX mutants show susceptibility to salinity induced oxidative stress, while constitutive over-expression lines show improved tolerance to 100 mM NaCl stress (Diaz-Vivancos et al., 2013). Tomato plants over expressing pea cAPX were reported to be tolerant to salinity stress (Wang et al., 2005). Over-expression of OsAPX2 shows better tolerance to salt stress as compared to OsAPX1 in transgenic Arabidopsis plants. However, the observed differential tolerance affect could be due to the positional effect of different transgenic lines. A cAPX from Arabidopsis in transgenic tobacco increased salt, drought, and PEG tolerance. Salt stress leads to lipid peroxidation and damaged membranes in sensitive plants which is accompanied by low levels of antioxidant enzymes. BY-2 cell lines of transgenic tobacco, having 50 and 75% lower cAPX activity, showed increased ROS accumulation. Ascorbate peroxidase gene expression during stress led to salt and heat tolerance with no significant changes in levels of other ROS scavenging enzymes (Gueta-Dahan et al., 1997; Badawi et al., 2004; Ishikawa et al., 2005; Lu et al., 2007). During saline conditions, pea chloroplast APXs behaved differently, with sAPX increasing and tAPX decreasing gradually while a tAPX from Solanum lycopersicum expressed in tobacco provided increased tolerance to salt and osmotic stress. An increased activity of chlAPX under salt stress also provides protection against ROS produced in mitochondria and/or peroxisomes. Salt stress tolerance was induced in transgenic tobacco accumulating increased ascorbate. In French bean seedlings, drought, and salt conditions up-regulated expression of gene encoding APX (Shalata et al., 2001; Mittova et al., 2003; Gómez et al., 2004; Eltelib et al., 2012; Nageshbabu and Jyothi, 2013). Over-expression of an APX from Puccinellia tenuiflora, a salinity tolerant wild grass in Arabidopsis increased its tolerance to 175mM NaCl in addition to protection from lipid peroxidation. Transcripts for a mAPX from Hordeum vulgare was increased under salinity conditions and another peroxisomal APX from Populus, transformed in tobacco, imparted salt, drought, and MV stress tolerance along with longer roots (Shi et al., 2001; Li et al., 2009). Additionally, APX deficient mutants are able to up-regulate other peroxidases to compensate for APX loss and to provide stress tolerance. This is validated by the expression of rice GPX in rice APX 1/2 mutants and other enzymes (CAT and GP) were upregulated in APX 3 knockout mutants of Arabidopsis showing no signs of stress. The APX expression is also affected by SA when externally applied by increasing APX and GR activity, which increases salt tolerance in mung bean (Narendra et al., 2006; Bonifacio et al., 2011; Nazar et al., 2011; Guan et al., 2015). Response of all antioxidant enzymes during salt stress in Brazilian indica rice was analyzed in two developmental stages and it was found that cAPX was up-regulated in 11 day old seedlings, while no significant APX expression was observed in 6-week old plants (Menezes-Benavente et al., 2004). This stress induces genesis of ROS, therefore, response of APX isozymes to this situation in plant developmental stages is regulated. The normal salinity tolerant rice leaf basal region showed an increase in CAT and APX transcripts under salinity stress and levels of APX8 were slightly reduced. OsAPX2 showed no alteration in expression under salinity (Yamane et al., 2010). The same reduction in response of APX8 was seen in another report where the other isoforms, APX2, and APX7 were highly expressed during salt stress. In another contrasting experiment, OsAPX8 showed high expression in a range of salt concentrations, viz., 150–300 mM in rice roots while there was a drastic decrease in transcripts of OsAPX7 at 300 mM. This variability in expression of different APX genes is due to differences in age, cultivar, plant parts, and physiological conditions of plant growth (Teixeira et al., 2004, 2006; Hong et al., 2007). Sweet potato plants differing in their sensitivity to salt stress showed differential accumulation of APX transcripts with the higher levels in tolerant genotypes. The expression of different isoforms was tissue and stress duration dependent (Lin and Pu, 2010). Salinity stress increased APX transcripts in soybean (Weisany et al., 2012). Therefore, it can be deduced that salt stress causes severe alterations in expression of antioxidant enzymes, it is spatially and temporally variable and different isoforms lead to a stable redox state in the cell.
Expression of APXs under high light or photo-oxidative damage
High light leads to reduced efficiency of the photosynthetic apparatus and rapid ROS generation. During high light conditions, APX2 is specifically reported to have high expression while other APXs like cAPX (APX1) and tAPX transcripts increase substantially to overcome damage (Karpinski et al., 1999; Yabuta et al., 2002; Fryer et al., 2003; Pignocchi et al., 2003). cAPX and chlAPX showed differential expression in spinach leaves during high light, while the former increased, the latter decreased. A wheat mutant with deficient tAPX had lower photosynthetic efficiency in high light implying importance of this isoform (Yoshimura et al., 2000; Danna et al., 2003). Compared to wild type plants, Arabidopsis mutants with deficiency in either tAPX or sAPX had damaged proteins in light or MV stress with a more pronounced effect with tAPX (Maruta et al., 2010). A simultaneous mutation in two APXs of Arabidopsis, viz., tAPX and cAPX led to mixed results like reduced protein damage, late flowering and high anthocyanin concentrations (Miller et al., 2007b). Mutants lacking either tAPX, sAPX or both in Arabidopsis showed symptoms of partial chlorophyll loss in tAPX mutants, and total bleaching in seedlings for the latter two plants. Mature leaves of the same mutants were susceptible for MV and light stress (Kangasjärvi et al., 2008). Arabidopsis plants deficient in APX1 showed suppression in high light of transcripts of several crucial genes involved in basic plant growth and development processes. In contrast to normal conditions, high light produced induction of enzymes (Pnueli et al., 2003).
APX1 also complements chloroplastic and mitochondrial APXs in tolerating excess light and its absence leads to protein oxidation and photosynthetic failure stating its role in protection of chloroplasts (Davletova et al., 2005). But the transcript levels of chloroplastic or mitochondrial APXs do not increase unlike cAPX to tolerate stress implying their capacity to neutralize ROS even during stress. A 10-fold increase in APX expression also does not contribute to tolerance against ozone stress in tobacco chloroplasts (Torsethaugen et al., 1997). High light intensity and Mg deficiency markedly increased the expression of APX and other antioxidant enzymes in Phaseolus vulgaris L, while the levels in Mg-sufficient plant parts remained constant. The increase in expression was directly proportional to the light intensity (Cakmak and Marschner, 1992).
Expression of APXs under temperature fluctuations
Extremely low or high temperature conditions negatively affect the plant physiology. Chilling stress leads to induced expression of APX in tolerant maize lines, as compared to sensitive lines (Pinhero et al., 1997). Low temperature induces higher cAPX expression in potato tubers as compared to heat stress implying its role in cold acclimation (Kawakami et al., 2002). A mAPX was induced under cold in Arabidopsis (Zhang et al., 1997). A tAPX over-expressed in tobacco improved tolerance to chilling and light stress while Arabidopsis lacking tAPX were tolerant to heat stress (Yabuta et al., 2002; Miller et al., 2007a). Homologous over-expression of a cAPX in rice was highly tolerant toward cold at booting stage due to increased activity of APX in spikelets than wild type plants (Sato et al., 2011). An inducible promoter SWPA2 working under oxidative stresses was used to induce SOD and APX gene expression in potato chloroplasts. The plants obtained were tolerant to high heat and MV stresses with a significant difference from control (Tang et al., 2006). A similar experiment in sweet potato resulted in tolerance against cold and MV stresses (Lim et al., 2007). The tomato tAPX expressed in tobacco led to tolerance against both temperature stresses and photosynthetic efficiency was maintained better in transgenic than non-transformed plants (Sun et al., 2010). In sweet potato, high heat induces cAPX in leaves while in cucumber, cAPX, mAPX, and sAPX were all up-regulated after an initial reduction (Park et al., 2004; Song et al., 2005). A cAPX has been reported to decrease immediately after heat shock treatment, negating its beneficial role in this stress (Vacca et al., 2004) but another report by Karpinski and colleague claims APX2 to be induced under heat conditions (Karpinski et al., 1999). APX1 is reported to be primarily active in case of heat and drought stresses in Arabidopsis cells (Koussevitzky et al., 2008). A mAPX from barley was over-expressed in Arabidopsis to reveal heat stress tolerance (Shi et al., 2001). Thus, different isoforms of APX and antioxidative systems in multiple sub-cellular locations can be exploited to raise environmental stress tolerant plants.
Expression of APXs under drought conditions
APX has an important role in drought stress tolerance and recovery of plants. APX transcripts are fairly increased under drought in transgenic soybean and tobacco which over-expressed P5CS gene. In case of woody plants, APX and other ASC-GSH pathway enzymes were up-regulated after drought in Prunus spp. and declined during recovery phase. Glycine betaine is also reported to increase APX during drought (Sofo et al., 2005; Kausar et al., 2012; Zarei et al., 2012; Cruz et al., 2013). The cAPX (APX1) over-expression also alleviates drought symptoms and transgenic tobacco fared better than non-transgenic plants. Loss of function APX2 mutants also revealed the importance of this isoform in plant growth and development and such mutants were over sensitive to drought as compared to over-expression lines. A mAPX from Salicornia brachiata in over-expression lines provided increased drought tolerance compared to control plants (Zhang et al., 2013; Singh et al., 2014). Faize and colleagues revealed the importance of cAPX in drought stress tolerance in tobacco where a major beneficial effect was on membrane protection (Faize et al., 2011). The APX activity is reported to be higher in tolerant cowpea plants even in non-stress conditions. In stress, the sensitive cultivar up-regulates cAPX and mAPX, while chlAPX was up-regulated in tolerant cultivar (D'Arcy-Lameta et al., 2006). Wheat genotypes show differential APX expression under water deficit. cAPX1 was up-regulated in both genotypes, sAPX2 only in sensitive, while tAPX and cAPX2 only in the tolerant type (Sečenji et al., 2009). The tAPX was down-regulated after 15 days of stress in rice, several other isoforms were up-regulated, still some microsomal isoforms were slightly or not affected at all (Rosa et al., 2010). This represents a differential expression of APXs in different species and various stresses.
Expression of APXs under metal toxicity
Soil contamination with heavy metal ions is a major issue hindering crop productivity. Induced expression of APXs have been observed under Cadmium and Arsenic stress in leaves of A. thaliana, Solanum nigrum, and Brassica juncea while it was reduced in B. napus (Smeets et al., 2008; Khan et al., 2009; Markovska et al., 2009; Nouairi et al., 2009; Pinto et al., 2009). In case of Copper stress in Elsholtzia splendens, there was increased expression of APX in leaves while in Withania somnifera it was variable according to concentration of metal ions (Peng et al., 2006; Khatun et al., 2008). In Nicotiana tabacum and Typha angustifolia, cadmium stress led to changed and unchanged expression of APX isoforms respectively while in Typha leaves, chromium and lead stresses did not induce any change in APX expression (Bah et al., 2011). Cadmium stress in Zea mays led to variable expression of APX (Ekmekçi et al., 2008). In coffee cells, lower concentrations of Cadmium induced activity of APX but higher concentrations did not cause any change after 24 h. While nickel increased APX activity with little difference between two extreme concentrations (Gomes-Junior et al., 2006a,b). Aluminum exposure also induces activity of almost all APX isoforms in rice. cAPX1/2 double mutants were normal and had increased tolerance to high concentrations (Sharma and Dubey, 2007; Rosa et al., 2010). This heavy metal increases activity of cAPX in pea, at higher concentrations and longer durations while it declines and becomes constant beyond it (Panda and Matsumoto, 2010). Iron induces activity of cAPX in de-rooted bean plants and tobacco plants with deficient cAPX were sensitive to iron (Pekker et al., 2002). Copper and cadmium increased APX activity in tall fescue plants over-expressing APX compared to control while arsenic decreased its activity in both transgenic and control (Lee S. H. et al., 2007). Lead increased APX activity in seedlings of Eichhornia crassipes (Malar et al., 2014). Cadmium chloride increased APX activity in both salt tolerant and sensitive rice varieties with a higher activity in the former (Roychoudhury et al., 2012; Malar et al., 2014). Similar increase in APX activity was seen in Vigna radiata under Cadmium Chloride stress (Roychoudhury and Ghosh, 2013). Doubled stress of salt and lead on V. radiata seedlings resulted in increased APX activity (Siddiqui, 2013). Therefore, we have seen an important role of APXs in protecting plants against heavy metal stress in soil from different scientific reports.
Summary and future perspectives
From the above literature, it is amply clear that ascorbate peroxidase constitutes one of the most important component of the cellular antioxidant defense, and plays a crucial role in regulating the levels ROS in plants when exposed to a variety of environmental stresses. The fact that APX constitutes the first line of defense against ROS is signified by the fact that H2O2 at low levels is beneficial to the plant system, as it acts as a secondary messenger in initiating cellular defense pathway. The same molecule can be a cause of oxidative injury to the plant if accumulated at higher concentrations. The role of APX in maintaining redox homeostasis is supported by the presence of specific isoforms of the enzyme at different sub-cellular locations. The quantitative expression of APX regulates how the plant system adapts to different types of environmental stresses, at the cellular and molecular level by interacting with various signaling molecules. The multitude of regulatory processes and molecules present in the cell helps ascorbate peroxidase enzyme to cross talk with various other antioxidant enzymes which is essentially required for a meaningful antioxidant defense system. Further, the mutant studies indicate that APX also has a complex compensatory relationship with other enzymes of the antioxidant pathway. However, the enigma created by the presence of different isoforms of APX needs to be resolved and further studies are required to delineate the genetic regulation of APX gene expression, in response to different types of stresses. This will help in designing better strategies for stress tolerance in plants.
Author contributions
SP prepared manuscript, formulated the idea, wrote the article, attached references, figures, table, and final proof reading. DF and AA formulated the idea, wrote the article, re-written and edited the manuscript, attached references, figures, table, and final proof reading. TS reproved and reorganized the manuscript. DJ helped in manuscript preparation. TK helped in guiding the article. YN helped in guiding the article. SA gave the idea of Ascorbate peroxidase (Plant APX) Review article; helped in guiding the article. MR gave the guidance for framework and final evaluation of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
SP thanks SERB for the PDF/2015/000230/LS grant. The authors also thank DST, CSIR, and DBT for INSPIRE fellowship to DF, SRF, to AA and SRF to DJ, respectively. The author SA is thankful to DBT for financial support in the form of PMS-Phase-II grant. The authors finally extend a big thanks to our reviewers for their critical comments and continuous support and patience.
References
- Asada K. (1999). The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639. 10.1146/annurev.arplant.50.1.601 [DOI] [PubMed] [Google Scholar]
- Badawi G. H., Yamauchi Y., Kawano N., Tanaka K., Tanaka K. (2004). Enhanced tolerance to water deficit and high salt stress by overexpressing superoxide dismutase and ascorbate peroxidase in tobacco chloroplasts. Plant Cell Physiol. 45, 806 10.1111/j.0031-9317.2004.00308.x [DOI] [PubMed] [Google Scholar]
- Bah A. M., Dai H., Zhao J., Sun H., Cao F., Zhang G., et al. (2011). Effects of cadmium, chromium and lead on growth, metal uptake and antioxidative capacity in Typha angustifolia. Biol. Trace Elem. Res. 142, 77–92. 10.1007/s12011-010-8746-6 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J., Mittler R. (2006). The roles of reactive oxygen species in plant cells. Plant Physiol. 141, 311–311. 10.1104/pp.104.900191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A., Mittler R., Suzuki N. (2014). ROS as key players in plant stress signalling. J. Exp. Bot. 65, 1229–1240. 10.1093/jxb/ert375 [DOI] [PubMed] [Google Scholar]
- Bonifacio A., Martins M. O., Ribeiro C. W., Fontenele A. V., Carvalho F. E., Margis-Pinheiro M., et al. (2011). Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ. 34, 1705–1722. 10.1111/j.1365-3040.2011.02366.x [DOI] [PubMed] [Google Scholar]
- Bowler C., Fluhr R. (2000). The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 5, 241–246. 10.1016/S1360-1385(00)01628-9 [DOI] [PubMed] [Google Scholar]
- Cakmak I., Marschner H. (1992). Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 98, 1222–1227. 10.1104/pp.98.4.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camillo L. R., Filadelfo C. R., Monzani P. S., Corrêa R. X., Gramacho K. P., Micheli F., et al. (2013). Tc-cAPX, a cytosolic ascorbate peroxidase of Theobroma cacao L. engaged in the interaction with Moniliophthora perniciosa, the causing agent of witches' broom disease. Plant Physiol. Biochem. 73, 254–265. 10.1016/j.plaphy.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Caverzan A., Passaia G., Rosa S. B., Ribeiro C. W., Lazzarotto F., Margis-Pinheiro M. (2012). Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 35, 1011–1019. 10.1590/S1415-47572012000600016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. (1980). Effect of hydrogen peroxide on spinach (Spinacia oleracea) chloroplast fructose bisphosphatase. Biochem. J. 189, 373–376. 10.1042/bj1890373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Asada K. (1989). Ascorbate Peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 30, 987–998. [Google Scholar]
- Chen Y., Cai J., Yang F. X., Zhou B., Zhou L. R. (2015). Ascorbate peroxidase from Jatropha curcas enhances salt tolerance in transgenic Arabidopsis. Genet. Mol. Res. 14, 4879–4889. 10.4238/2015.May.11.20 [DOI] [PubMed] [Google Scholar]
- Chew O., Whelan J., Millar A. H. (2003). Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 278, 46869–46877. 10.1074/jbc.M307525200 [DOI] [PubMed] [Google Scholar]
- Chiang C. M., Chen L. F. O., Shih S. W., Lin K. H. (2015). Expression of eggplant ascorbate peroxidase increases the tolerance of transgenic rice plants to flooding stress. J. Plant Biochem. Biotech. 24, 257–267. 10.1007/s13562-014-0265-7 [DOI] [Google Scholar]
- Chinnusamy V., Zhu J. K. (2009). Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 12, 133–139. 10.1016/j.pbi.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer G. R., Ergül A., Grimplet J., Tillett R. L., Tattersall E. A., Bohlman M. C., et al. (2007). Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct. Int. Genomics 7, 111–134. 10.1007/s10142-006-0039-y [DOI] [PubMed] [Google Scholar]
- Cruz F. J. R., Castro G. L. S., Júnior D. D. S., Pinheiro H. A. (2013). Exogenous glycine betaine modulates ascorbate peroxidase and catalase activities and prevent lipid peroxidation in mild water-stressed Carapa guianensis plants. Photosynthetica 51, 102–108. 10.1007/s11099-013-0004-7 [DOI] [Google Scholar]
- D'Arcy-Lameta A., Ferrari-Iliou R., Coutour-Ansel D., Pham-Thi A. T., Zuily-Fodil Y. (2006). Isolation and characterization of four ascorbate peroxidase cdnas responsive to water deficit in cowpea leaves. Ann. Bot. 97, 133–140. 10.1093/aob/mcj010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dąbrowska G., Kata A., Goc A., Szechyńska-Hebda M., Skrzypek E. (2007). Characteristics of the plant ascorbate peroxidase family. Acta Biol. Cracow. Ser. Bot. 49, 7–17. Available online at: http://www2.ib.uj.edu.pl/abc/pdf/49_1/01dabrow.pdf [Google Scholar]
- Danna C. H., Bartoli C. G., Sacco F., Ingala L. R., Santa-María G. E., Guiamet J. J., et al. (2003). Thylakoid-bound ascorbate peroxidase mutant exhibits impaired electron transport and photosynthetic activity. Plant Physiol. 132, 2116–2125. 10.1104/pp.103.021717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K., Roychoudhury A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2:53 10.3389/fenvs.2014.00053 [DOI] [Google Scholar]
- Davletova S., Rizhsky L., Liang H., Shengqiang Z., Oliver D. J., Coutu J., et al. (2005). Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17, 268–281. 10.1105/tpc.104.026971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias M. C., Azevedo C., Costa M., Pinto G., Santos C. (2014). Melia azedarach plants show tolerance properties to water shortage treatment: an ecophysiological study. Plant Physiol. Biochem. 75, 123–127. 10.1016/j.plaphy.2013.12.014 [DOI] [PubMed] [Google Scholar]
- Diaz-Vivancos P., Faize M., Barba-Espin G., Faize L., Petri C., Hernández J. A., et al. (2013). Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums. Plant Biotech. J. 11, 976–985. 10.1111/pbi.12090 [DOI] [PubMed] [Google Scholar]
- dos Reis S. P., Lima A. M., de Souza C. R. (2012). Recent molecular advances on downstream plant responses to abiotic stress. Int. J. Mol. Sci. 13, 8628–8647. 10.3390/ijms13078628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M., Feng H. L., Wang L. Y., Li D., Meng Q. W. (2012). Overexpression of thylakoidal ascorbate peroxidase shows enhanced resistance to chilling stress in tomato. J. Plant Physiol. 169, 867–877. 10.1016/j.jplph.2012.02.012 [DOI] [PubMed] [Google Scholar]
- Duque A. S., Farinha A. P., da Silva A. B., de Almeida A. M., Santos D., da Silva J. M., et al. (2013). Chapter 3 Abiotic stress responses in plants: unraveling the complexity of genes and networks to survive, in Abiotic Stress: Plant Responses and Applications in Agriculture, eds Vahdati K., Leslie C.(Croatia: INTECH Open; ), 49–102. 10.5772/45842 [DOI] [Google Scholar]
- Ekmekçi Y., Tanyolac D., Ayhan B. (2008). Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J. Plant Physiol. 165, 600–611. 10.1016/j.jplph.2007.01.017 [DOI] [PubMed] [Google Scholar]
- Eltelib H. A., Fujikawa Y., Esaka M. (2012). Overexpression of the acerola (Malpighia glabra) monodehydroascorbate reductase gene in transgenic tobacco plants results in increased ascorbate levels and enhanced tolerance to salt stress. S. Afr. J. Bot. 78, 295–301. 10.1016/j.sajb.2011.08.005 [DOI] [Google Scholar]
- Faize M., Burgos L., Faize L., Piqueras A., Nicolas E., Barba-Espin G., et al. (2011). Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J. Exp. Bot. 62, 2599–2613. 10.1093/jxb/erq432 [DOI] [PubMed] [Google Scholar]
- Foyer C. H., Halliwell B. (1976). The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133, 21–25. 10.1007/BF00386001 [DOI] [PubMed] [Google Scholar]
- Foyer C. H., Halliwell B. (1977). Purification and properties of dehydroascorbate reductase from spinach leaves. Phytochemistry 16, 1347–1350. 10.1016/S0031-9422(00)88779-8 [DOI] [Google Scholar]
- Foyer C. H., Shigeoka S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155, 93–100. 10.1104/pp.110.166181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer M. J., Ball L., Oxborough K., Karpinski S., Mullineaux P. M., Baker N. R. (2003). Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 33, 691–705. 10.1046/j.1365-313X.2003.01656.x [DOI] [PubMed] [Google Scholar]
- Gechev T. S., Van Breusegem F., Stone J. M., Denev I., Laloi C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28, 1091–1101. 10.1002/bies.20493 [DOI] [PubMed] [Google Scholar]
- Gomes-Junior R. A., Moldes C. A., Delite F. S., Gratão P. L., Mazzafera P. (2006a). Nickel elicits a fast antioxidant response in Coffea arabica cells. Plant Physiol. Biochem. 44, 420–429. 10.1016/j.plaphy.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Gomes-Junior R. A., Moldes C. A., Delite F. S., Pompeu G. B., Gratão P. L., Mazzafera P., et al. (2006b). Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere 65, 1330–1337. 10.1016/j.chemosphere.2006.04.056 [DOI] [PubMed] [Google Scholar]
- Gómez J. M., Jiménez A., Olmos E., Sevilla F. (2004). Location and effects of long-term NaCl stress on superoxide dismutase and ascorbate peroxidase isoenzymes of pea (Pisum sativum cv. Puget) chloroplasts. J. Exp. Bot. 55, 119–130. 10.1093/jxb/erh013 [DOI] [PubMed] [Google Scholar]
- Guan Q., Wang Z., Wang X., Takano T., Liu S. (2015). A peroxisomal APX from Puccinellia tenuiflora improves the abiotic stress tolerance of transgenic Arabidopsis thaliana through decreasing of H2O2 accumulation. J. Plant Physiol. 175, 183–191. 10.1016/j.jplph.2014.10.020 [DOI] [PubMed] [Google Scholar]
- Gueta-Dahan Y., Yaniv Z., Zilinskas B. A., Ben-Hayyim G. (1997). Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta 203, 460–469. 10.1007/s004250050215 [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2006). Oxidative stress and neurodegeneration: where are we now?. J. Neurochem. 97, 1634–1658. 10.1111/j.1471-4159.2006.03907.x [DOI] [PubMed] [Google Scholar]
- Hirooka S., Misumi O., Yoshida M., Mori T., Nishida K., Yagisawa F., et al. (2009). Expression of the Cyanidioschyzon merolae stromal ascorbate peroxidase in Arabidopsis thaliana enhances thermotolerance. Plant Cell Rep. 28, 1881–1893. 10.1007/s00299-009-0791-2 [DOI] [PubMed] [Google Scholar]
- Hong C.-Y., Hsu Y. T., Tsai Y.-C., Kao C. H. (2007). Expression of ascorbate peroxidase 8 in roots of rice (Oryza sativa L.) seedlings in response to NaCl. J. Exp. Bot. 58, 3273–3283. 10.1093/jxb/erm174 [DOI] [PubMed] [Google Scholar]
- Huseynova I. M., Aliyeva D. R., Aliyev J. A. (2014). Subcellular localization and responses of superoxide dismutase isoforms in local wheat varieties subjected to continuous soil drought. Plant Physiol. Biochem. 81, 54–60. 10.1016/j.plaphy.2014.01.018 [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Morimoto Y., Madhusudhan R., Sawa Y., Shibata H., Yabuta Y., et al. (2005). Acclimation to diverse environmental stresses caused by a suppression of cytosolic ascorbate peroxidase in tobacco BY-2 cells. Plant Cell Physiol. 46, 1264–1271. 10.1093/pcp/pci135 [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Yoshimura K., Sakai K., Tamoi M., Takeda T. (1998). Molecular characterization and physiological role of a glyoxysome-bound ascorbate peroxidase from spinach. Plant Cell Physiol. 39, 23–34. 10.1093/oxfordjournals.pcp.a029285 [DOI] [PubMed] [Google Scholar]
- Jiang G., Yin D., Zhao J., Chen H., Guo L., Zhu L., et al. (2016). The rice thylakoid membrane-bound ascorbate peroxidase OsAPX8 functions in tolerance to bacterial blight. Sci. Rep. 6:26104. 10.1038/srep26104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A., Hernandez J. (1997). Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 114, 275–284. 10.1104/pp.114.1.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. (1976). The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim. Biophys. Acta 440, 476–482. 10.1016/0005-2728(76)90035-9 [DOI] [PubMed] [Google Scholar]
- Kangasjärvi S., Lepistö A., Hännikäinen K., Piippo M., Luomala E. M., Aro E. M., et al. (2008). Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochem. J. 412, 275–285. 10.1042/BJ20080030 [DOI] [PubMed] [Google Scholar]
- Kapoor D. (2015). Redox homeostasis in plants under abiotic stress: role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front. Environ. Sci. 3:13 10.3389/fenvs.2015.00013 [DOI] [Google Scholar]
- Karpinski S., Reynolds H., Karpinska B., Wingsle G., Creissen G., Mullineaux P. (1999). Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284, 654–657. 10.1126/science.284.5414.654 [DOI] [PubMed] [Google Scholar]
- Kausar R., Hossain Z., Makino T. (2012). Characterization of ascorbate peroxidase in soybean under flooding and drought stresses. Mol. Biol. Rep. 39, 10573–10579. 10.1007/s11033-012-1945-9 [DOI] [PubMed] [Google Scholar]
- Kawakami S., Matsumoto Y., Matsunaga A., Mayama S., Mizuno M. (2002). Molecular cloning of ascorbate peroxidase in potato tubers and its response during storage at low temperature. Plant Sci. 163, 829–836. 10.1016/S0168-9452(02)00232-7 [DOI] [Google Scholar]
- Khan I., Ahmad A., Iqbal M. (2009). Modulation of antioxidant defense system for arsenic detoxification in Indian mustard. Ecotoxic. Environ. Saf. 72, 626–634. 10.1016/j.ecoenv.2007.11.016 [DOI] [PubMed] [Google Scholar]
- Khatun S., Babar M., Hahn E., Paek K. (2008). Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. J. Environ. Exp. Bot. 64, 279–285. 10.1016/j.envexpbot.2008.02.004 [DOI] [Google Scholar]
- Kornyeyev D., Logan B. A., Allen R. D., Holaday A. S. (2003). Effect of chloroplastic overproduction of ascorbate peroxidase on photosynthesis and photoprotection in cotton leaves subjected to low temperature photoinhibition. Plant Sci. 165, 1033–1041. 10.1016/S0168-9452(03)00294-2 [DOI] [Google Scholar]
- Koussevitzky S., Suzuki N., Huntington S., Armijo L., Sha W., Cortes D., et al. (2008). Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 283, 34197–34203. 10.1074/jbc.M806337200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S. Y., Jeong Y. J., Lee H. S., Kim J. S., Cho K. Y., Allen R. D., et al. (2002). Enhanced tolerances of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen-mediated oxidative stress. Plant Cell Environ. 25, 873–882. 10.1046/j.1365-3040.2002.00870.x [DOI] [PubMed] [Google Scholar]
- Lee S. H., Ahsan N., Lee K. W., Kim D. H., Lee D. G., Kwak S. S., et al. (2007). Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J. Plant Physiol. 164, 1626–1638. 10.1016/j.jplph.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Lee Y. P., Kim S. H., Bang J. W., Lee H. S., Kwak S. S., Kwon S. Y. (2007). Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Rep. 26, 591–598. 10.1007/s00299-006-0253-z [DOI] [PubMed] [Google Scholar]
- Li M., Ji L., Yang X., Meng Q., Guo S. (2012). The protective mechanisms of CaHSP26 in transgenic tobacco to alleviate photoinhibition of PSII during chilling stress. Plant Cell Rep. 31, 1969–1979. 10.1007/s00299-012-1309-x [DOI] [PubMed] [Google Scholar]
- Li Y. J., Hai R. L., Du X. H., Jiang X. N., Lu H. (2009). Over-expression of a Populus peroxisomal ascorbate peroxidase (PpAPX) gene in tobacco plants enhances stress tolerance. Plant Breed. 128, 404–410. 10.1111/j.1439-0523.2008.01593.x [DOI] [Google Scholar]
- Lim S., Kim Y. H., Kim S. H., Kwon S. Y., Lee H. S., Kim J. S., et al. (2007). Enhanced tolerance of transgenic sweetpotato plants that express both CuZnSOD and APX in chloroplasts to methyl viologen-mediated oxidative stress and chilling. Mol. Breed. 19, 227–239. 10.1007/s11032-006-9051-0 [DOI] [Google Scholar]
- Lin K. H., Pu S. F. (2010). Tissue- and genotype-specific ascorbate peroxidase expression in sweet potato in response to salt stress. Biol. Plant. 54, 664–670. 10.1007/s10535-010-0118-8 [DOI] [Google Scholar]
- Liu Z., Bao H., Cai J., Han J., Zhou L. (2013). A novel thylakoid ascorbate peroxidase from Jatropha curcas enhances salt tolerance in transgenic tobacco. Int. J. Mol. Sci. 15, 171–185. 10.3390/ijms15010171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Liu D., Liu S. (2007). Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 26, 1909–1917. 10.1007/s00299-007-0395-7 [DOI] [PubMed] [Google Scholar]
- Malar S., Vikram S. S., Favas P. J. C., Perumal V. (2014). Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot. Stud. 55, 54 10.1186/s40529-014-0054-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano J. I., Ohno C., Domae Y., Asada K. (2001). Chloroplastic ascorbate peroxidase is the primary target of methylviologen-induced photooxidative stress in spinach leaves: its relevance to monodehydroascorbate radical detected with in vivo ESR. Biochim. Biophys. Acta 1504, 275–287. 10.1016/S0005-2728(00)00256-5 [DOI] [PubMed] [Google Scholar]
- Markovska Y. K., Gorinova N. I., Nedkovska M. P., Miteva K. M. (2009). Cadmium-induced oxidative damage and antioxidant responses in Brassica juncea plants. Biol. Plant. 53, 151–154. 10.1007/s10535-009-0023-1 [DOI] [Google Scholar]
- Maruta T., Tanouchi A., Tamoi M., Yabuta Y., Yoshimura K., Ishikawa T., et al. (2010). Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 51, 190–200. 10.1093/pcp/pcp177 [DOI] [PubMed] [Google Scholar]
- Menezes-Benavente L., Teixeira F. K., Kamei C. L. A., Margis-Pinheiro M. (2004). Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza sativa L.). Plant Sci. 166, 323–331. 10.1016/j.plantsci.2003.10.001 [DOI] [Google Scholar]
- Miller G., Suzuki N., Ciftci-Yilmaz S., Mittler R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33, 453–467. 10.1111/j.1365-3040.2009.02041.x [DOI] [PubMed] [Google Scholar]
- Miller G., Suzuki N., Rizhsky L., Hegie A., Koussevitzky S., Mittler R. (2007a). Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 144, 1777–1785. 10.1104/pp.107.101436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Suzuki N., Rizhsky L., Hegie A., Koussevitzky S., Mittler R. (2007b). Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 144, 1777–1785. 10.1104/pp.107.101436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Gollery M., Breusegem F., Van Breusegem F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. 10.1016/j.tplants.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Mittler R., Zilinskas B. A. (1991). Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiol. 97, 962–968. 10.1104/pp.97.3.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittova V., Tal M., Volokita M., Guy M. (2003). Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 26, 845–856. 10.1046/j.1365-3040.2003.01016.x [DOI] [PubMed] [Google Scholar]
- Morita S., Kaminaka H., Masumura T., Tanaka K. (1999). Induction of rice cytosolic ascorbate peroxidase mRNA by oxidative stress; the involvement of hydrogen peroxide in oxidative stress signalling. Plant Cell Physiol. 40, 417–422. 10.1093/oxfordjournals.pcp.a029557 [DOI] [Google Scholar]
- Murgia I., Tarantino D., Vannini C., Bracale M., Carravieri S., Soave C. (2004). Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to Paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J. 38, 940–953. 10.1111/j.1365-313X.2004.02092.x [DOI] [PubMed] [Google Scholar]
- Nageshbabu R., Jyothi M. N. (2013). Profile of small interfering RNAs from french bean Phaseolus vulgaris under abiotic stress conditions. Int. J Pharm. Bio. Sci. 4, 176–185. Available online at: http://www.ijpbs.net/cms/php/upload/2243_pdf.pdf [Google Scholar]
- Narendra S., Venkataramani S., Shen G., Wang J., Pasapula V., Lin Y., et al. (2006). The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development. J. Exp. Bot. 57, 3033–3042. 10.1093/jxb/erl060 [DOI] [PubMed] [Google Scholar]
- Nazar R., Iqbal N., Syeed S., Khan N. A. (2011). Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 168, 807–815. 10.1016/j.jplph.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Noctor G. (2006). Metabolic signalling in defense and stress: the central roles of soluble redox couples. Plant Cell Environ. 29, 409–425. 10.1111/j.1365-3040.2005.01476.x [DOI] [PubMed] [Google Scholar]
- Noctor G., Veljovic-Jovanovic S., Foyer C. H. (2000). Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1465–1475. 10.1098/rstb.2000.0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouairi I., Ammar W. B., Youssef N. B., Miled D. D. B., Ghorbal M. H., Zarrouk M. (2009). Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol. Plant. 31, 237–247. 10.1007/s11738-008-0224-9 [DOI] [Google Scholar]
- Panda S. K., Matsumoto H. (2010). Changes in antioxidant gene expression and induction of oxidative stress in pea (Pisum sativum L.) under Al stress. Biometals 23, 753–762. 10.1007/s10534-010-9342-0 [DOI] [PubMed] [Google Scholar]
- Park S. Y., Ryu S. H., Jang I. C., Kwon S. Y., Kim J. G., Kwak S. S. (2004). Molecular cloning of a cytosolic ascorbate peroxidase cDNA from cell cultures of sweetpotato and its expression in response to stress. Mol. Genet. Genomics 271, 339–346. 10.1007/s00438-004-0986-8 [DOI] [PubMed] [Google Scholar]
- Pekker I., Tel-Or E., Mittler R. (2002). Reactive oxygen intermediates and glutathione regulate the expression of cytosolic ascorbate peroxidase during iron-mediated oxidative stress in bean. Plant Mol. Biol. 49, 429–438. 10.1023/A:1015554616358 [DOI] [PubMed] [Google Scholar]
- Peng H.-Y., Yang X.-E., Yang M.-J., Tian S.-K. (2006). Responses of antioxidant enzyme system to copper toxicity and copper detoxification in the leaves of Elsholtzia splendens. J. Plant Nutr. 29, 1619–1635. 10.1080/01904160600851478 [DOI] [Google Scholar]
- Pignocchi C., Fletcher J. M., Wilkinson J. E., Barnes J. D., Foyer C. H. (2003). The Function of ascorbate oxidase in tobacco. Plant Physiol. 132, 1631–1641. 10.1104/pp.103.022798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhero R. G., Rao M. V., Paliyath C., Murr P., Fletcher R. A. (1997). Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazolh induced chilling tolerance of maize seedlings. Plant Physiol. 114, 695–704. 10.1104/pp.114.2.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A. P., Alves A. S., Candeias A. J., Cardoso A. I., de Varennes A., Martins L. L., et al. (2009). Cadmium accumulation and antioxidative defenses in Brassica juncea L. Czern, Nicotiana tabacum L. and Solanum nigrum L. Int. J. Environ. Anal. Chem. 89, 661–676. 10.1080/03067310902962585 [DOI] [Google Scholar]
- Pnueli L., Liang H., Rozenberg M., Mittler R. (2003). Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J. 34, 187–203. 10.1046/j.1365-313X.2003.01715.x [DOI] [PubMed] [Google Scholar]
- Polle A. (2001). Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol. 126, 445–462. 10.1104/pp.126.1.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Peng X., Han X., Ren J., Sun L., Fu Z. (2013). Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 25, 1947–1956. 10.1016/S1001-0742(12)60301-5 [DOI] [PubMed] [Google Scholar]
- Rao M. V., Paliyath G., Ormrod D. P. (1996). Ultraviolet-B and Ozone-lnduced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 110, 125–136. 10.1104/pp.110.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L., Hallak-Herr E., Van Breusegem F., Rachmilevitch S., Barr J. E., Rodermel S., et al. (2002). Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J. 32, 329–342. 10.1046/j.1365-313X.2002.01427.x [DOI] [PubMed] [Google Scholar]
- Rosa S. B., Caverzan A., Teixeira F. K., Lazzarotto F., Silveira J. A., Ferreira-Silva S. L., et al. (2010). Cytosolic APX knockdown indicates an ambiguous redox responses in rice. Phytochemistry 71, 548–558. 10.1016/j.phytochem.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Roychoudhury A., Basu S., Sengupta D. N. (2012). Antioxidants and stress-related metabolites in the seedlings of two indica rice varieties exposed to cadmium chloride toxicity. Acta Physiol. Plant. 34, 835–847. 10.1007/s11738-011-0881-y [DOI] [Google Scholar]
- Roychoudhury A., Ghosh S. (2013). Physiological and biochemical responses of mungbean (Vigna radiata L. Wilczek) to varying concentrations of cadmium chloride or sodium chloride. Unique J. Pharm. Biol. Sci. 1, 11–21. Available online at: http://ujconline.net/wp-content/uploads/2013/09/2-UJPBS-1345-Rs.pdf [Google Scholar]
- Sato Y., Masuta Y., Saito K. (2011). Enhanced chilling tolerance at the booting stage in rice by transgenic overexpression of the ascorbate peroxidase gene, OsAPXa. Plant Cell Rep. 30, 399–406. 10.1007/s00299-010-0985-7 [DOI] [PubMed] [Google Scholar]
- Saxena S. C., Joshi P., Grimm B., Arora S. (2011). Alleviation of ultraviolet-C induced oxidative through overexpression of cytosolic ascorbate peroxidase. Biologia 66, 1052–1059. 10.2478/s11756-011-0120-4 [DOI] [Google Scholar]
- Scandalios J. G. (2005). Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 38, 995–1014. 10.1590/S0100-879X2005000700003 [DOI] [PubMed] [Google Scholar]
- Sečenji M., Hideg É., Bebes A., Györgyey J. (2009). Transcriptional differences in gene families of the ascorbate-glutathione cycle in wheat during mild water deficit. Plant Cell Rep. 29, 37–50. 10.1007/s00299-009-0796-x [DOI] [PubMed] [Google Scholar]
- Shalata A., Mittova V., Volokita M., Guy M., Tal M. (2001). Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol. Plant 112, 487–494. 10.1034/j.1399-3054.2001.1120405.x [DOI] [PubMed] [Google Scholar]
- Sharma P., Dubey R. S. (2007). Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep. 26, 2027–2038. 10.1007/s00299-007-0416-6 [DOI] [PubMed] [Google Scholar]
- Sharma P., Jha A. B., Dubey R. S., Pessarakli M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012, 1–26. 10.1155/2012/217037 [DOI] [Google Scholar]
- Shi W. M., Muramoto Y., Ueda A., Takabe T. (2001). Cloning of peroxisomal ascorbate peroxidase gene from barley and enhanced thermotolerance by overexpressing in Arabidopsis thaliana. Gene 273, 23–27. 10.1016/s0378-1119(01)00566-2 [DOI] [PubMed] [Google Scholar]
- Shigeoka S., Ishikawa T., Tamoi M., Miyagawa Y., Takeda T., Yabuta Y., et al. (2002). Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 53, 1305–1319. 10.1093/jxb/53.372.1305 [DOI] [PubMed] [Google Scholar]
- Siddiqui Z. S. (2013). Effects of double stress on antioxidant enzyme activity in Vigna radiata (L.) Wilczek. Acta Bot. Croatica 72, 145–156. 10.2478/v10184-012-0011-y [DOI] [Google Scholar]
- Singh N., Mishra A., Jha B. (2014). Ectopic over-expression of peroxisomal ascorbate peroxidase (SbpAPX) gene confers salt stress tolerance in transgenic peanut (Arachis hypogaea). Gene 547, 119–125. 10.1016/j.gene.2014.06.037 [DOI] [PubMed] [Google Scholar]
- Smeets K., Ruytinx J., Semane B., Van Belleghem F., Remans T., Van Sanden S., et al. (2008). Cadmium-induced transcriptional and enzymatic alterations related to oxidative stress. Environ. Exp. Bot. 63, 1–8. 10.1016/j.envexpbot.2007.10.028 [DOI] [Google Scholar]
- Sofo A., Tuzio A. C., Dichio B., Xiloyannis C. (2005). Influence of water deficit and rewatering on the components of the ascorbate – glutathione cycle in four interspecific Prunus hybrids. Plant Sci. 169, 403–412. 10.1016/j.plantsci.2005.04.004 [DOI] [Google Scholar]
- Song X. S., Hu W. H., Mao W. H., Ogweno J. O., Zhou Y. H., Yu J. Q. (2005). Response of ascorbate peroxidase isoenzymes and ascorbate regeneration system to abiotic stresses in Cucumis sativus L. Plant Physiol. Biochem. 43, 1082–1088. 10.1016/j.plaphy.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Sun W.-H., Duan M., Shu D.-F., Yang S., Meng Q.-W. (2010). Over-expression of StAPX in tobacco improves seed germination and increases early seedling tolerance to salinity and osmotic stresses. Plant Cell Rep. 29, 917–926. 10.1007/s00299-010-0878-9 [DOI] [PubMed] [Google Scholar]
- Tang L., Kwon S. Y., Kim S. H., Kim J. S., Choi J. S., Cho K. Y., et al. (2006). Enhanced tolerance of transgenic potato plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against oxidative stress and high temperature. Plant Cell Rep. 25, 1380–1386. 10.1007/s00299-006-0199-1 [DOI] [PubMed] [Google Scholar]
- Teixeira F. K., Menezes-Benavente L., Galvão V. C., Margis R., Margis-Pinheiro M. (2006). Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta 224, 300–314. 10.1007/s00425-005-0214-8 [DOI] [PubMed] [Google Scholar]
- Teixeira F. K., Menezes-benavente L., Margis R., Margis-Pinheiro M. (2004). Analysis of the molecular evolutionary history of the ascorbate peroxidase gene family: inferences from the rice genome. J. Mol. Evol. 59, 761–770. 10.1007/s00239-004-2666-z [DOI] [PubMed] [Google Scholar]
- Torsethaugen C., Pitcher L. H., Zilinskas B. A., Pell E. J. (1997). Overproduction of Ascorbate peroxidase in the tobacco chloroplast does not provide protection against ozone. Plant Physiol. 114, 529–537. 10.1104/pp.114.2.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca R. A., de Pinto M. C., Valenti D., Passarella S., Marra E., De Gara L. (2004). Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiol. 134, 1100–1112. 10.1104/pp.103.035956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F., Dat J. F. (2006). Reactive oxygen species in plant cell death. Plant Physiol. 141, 384–390. 10.1104/pp.106.078295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhang H., Allen R. D. (1999). Over-expression of an Arabidopsis peroxisomal ascorbate peroxidase gene in tobacco increases protection against oxidative stress. Plant Cell Physiol. 40, 725–732. 10.1093/oxfordjournals.pcp.a029599 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wisniewski M., Meilan R., Cui M., Webb R., Fuchigami L. (2005). Overexpression of cytosolic ascorbate peroxidase in tomato confers tolerance to chilling and salt stress. J. Am. Soc. Hortic. Sci. 130, 167–173. Available online at: http://journal.ashspublications.org/content/130/2/167.full.pdf+html [Google Scholar]
- Weisany W., Sohrabi Y., Heidari G., Siosemardeh A., Ghassemi-Golezani K. (2012). Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (‘Glycine max’ L.). Plant Omics 5, 60 Available online at: http://www.pomics.com/sohrabi_5_2_2012_60_67.pdf [Google Scholar]
- Xu J., Yang J., Duan X., Jiang Y., Zhang P. (2014). Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz). BMC Plant Biol. 14:208. 10.1186/s12870-014-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta Y., Motoki T., Yoshimura K., Takeda T., Ishikawa T., Shigeoka S. (2002). Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J. 32, 915–925. 10.1046/j.1365-313X.2002.01476.x [DOI] [PubMed] [Google Scholar]
- Yamane K., Mitsuya S., Taniguchi M., Miyake H. (2010). Transcription profiles of genes encoding catalase and ascorbate peroxidase in the rice leaf tissues under salinity. Plant Prod. Sci. 13, 164–168. 10.1626/pps.13.164 [DOI] [Google Scholar]
- Yan H., Li Q., Park S. C., Wang X., Liu Y. J., Zhang Y. G., et al. (2016). Overexpression of CuZnSOD and APX enhance salt stress tolerance in sweet potato. Plant Physiol. Biochem. 109, 20–27. 10.1016/j.plaphy.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Yabuta Y., Ishikawa T., Shigeoka S. (2000). Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol. 123, 223–234. 10.1104/pp.123.1.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K. Y., Park M. R., Mohanty B., Herath V., Xu F., Mauleon R., et al. (2010). Transcriptional regulatory network triggered by oxidative signals configures the early response mechanisms of japonica rice to chilling stress. BMC Plant Biol. 10:16. 10.1186/1471-2229-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei S., Ehsanpour A. A., Abbaspour J. (2012). The role of over-expression of P5CS gene on proline, catalase, ascorbate peroxidase activity and lipid peroxidation of transgenic tobacco (Nicotiana tabacum L.) plant under in vitro drought stress. J. Cell Mol. Res. 4, 43–49. 10.22067/jcmr.v4i1.18249 [DOI] [Google Scholar]
- Zhang H., Liu Y., Wen F., Yao D., Wang L., Guo J., et al. (2014). A novel rice C2H2-type zinc finger protein, ZFP36, is a key player involved in abscisic acid-induced antioxidant defense and oxidative stress tolerance in rice. J. Exp. Bot. 65, 5795–5809. 10.1093/jxb/eru313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang J., Nickel U., Allen R. D., Goodman H. M. (1997). Cloning and expression of an Arabidopsis gene encoding a putative peroxisomal ascorbate peroxidase. Plant Mol. Biol. 34, 967–971. 10.1023/A:1005814109732 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhang Q., Wu J., Zheng X., Zheng S., Sun X. (2013). Gene knockout study reveals that cytosolic ascorbate peroxidase 2 (osapx2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS ONE 8:e57472. 10.1371/journal.pone.0057472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Zuo M., Liang Y., Jiang M., Zhang J., Scheller H. V., et al. (2013). MAP65-1a positively regulates H2O2 amplification and enhances brassinosteroid-induced antioxidant defense in maize. J. Exp. Bot. 64, 3787–3802. 10.1093/jxb/ert215 [DOI] [PMC free article] [PubMed] [Google Scholar]