Abstract
An obligate halophyte, Salicornia brachiata grows in salt marshes and is considered to be a potential resource of salt- and drought-responsive genes. It is important to develop an understanding of the mechanisms behind enhanced salt tolerance. To increase this understanding, a novel SbSRP gene was cloned, characterized, over-expressed, and functionally validated in the model plant Nicotiana tabacum. The genome of the halophyte S. brachiata contains two homologs of an intronless SbSRP gene of 1,262 bp in length that encodes for a stress-related protein. An in vivo localization study confirmed that SbSRP is localized on the plasma membrane. Transgenic tobacco plants (T1) that constitutively over-express the SbSRP gene showed improved salinity and osmotic stress tolerance. In comparison to Wild Type (WT) and Vector Control (VC) plants, transgenic lines showed elevated relative water and chlorophyll content, lower malondialdehyde content, lower electrolyte leakage and higher accumulation of proline, free amino acids, sugars, polyphenols, and starch under abiotic stress treatments. Furthermore, a lower build-up of H2O2 content and superoxide-radicals was found in transgenic lines compared to WT and VC plants under stress conditions. Transcript expression of Nt-APX (ascorbate peroxidase), Nt-CAT (catalase), Nt-SOD (superoxide dismutase), Nt-DREB (dehydration responsive element binding factor), and Nt-AP2 (apetala2) genes was higher in transgenic lines under stress compared to WT and VC plants. The results suggested that overexpression of membrane-localized SbSRP mitigates salt and osmotic stress in the transgenic tobacco plant. It was hypothesized that SbSRP can be a transporter protein to transmit the environmental stimuli downward through the plasma membrane. However, a detailed study is required to ascertain its exact role in the abiotic stress tolerance mechanism. Overall, SbSRP is a potential candidate to be used for engineering salt and osmotic tolerance in crops.
Keywords: abiotic stress tolerance, drought, halophytes, novel gene, salinity, transgenic
Introduction
About 450 million small-scale producers around the world are dependent on agriculture for their sustenance. The Food and Agriculture Organization of the United Nations (FAO, 2016) estimates that feeding the world's population will require a 60% expansion in overall agriculture production by 2050. Many resources are needed for sustainable food security as the challenges faced by industries are huge and there is an urgent need for crop improvements to mitigate crop failure during unfavorable conditions (FAO, 2016). Plants encounter a wide range of environmental stresses during their lifecycles and they have evolved different mechanisms to combat these stresses, for example through modulating their physiology and interactive molecular and cellular alterations (Knight and Knight, 2001). The Intergovernmental Panel on Climate Change (IPCC) reported that high temperatures, salinity, floods, drought, and deterioration of arable land severely affect the agricultural economy in the developing world (Annan et al., 2009).
According to a report, about 800 million ha of land are salt-affected worldwide and soil salinity is gradually increasing (Munns, 2005; Tuteja et al., 2011). Furthermore, it has also been predicted that drought stress will become more frequent because of the long-term effects of global warming. The impact of abiotic stresses differs regionally and depends on the adopted agricultural practices. An adverse impact is envisaged for the agricultural economy of developing countries, therefore, major precautionary steps are needed to develop adaptive strategies for sustainable agriculture according to the changing environment (Ashraf and Foolad, 2007; Tuteja et al., 2011). The majority of crops are glycophytes, which means they are not able to grow in soil that has a high salt concentration (Shabala, 2013). The extent to which plants can endure high salinity varies among species, and a way to differentiate these species is to establish whether they profit from a large amount (100 mM or more) of NaCl in the soil (Shabala and Mackay, 2011). Salt tolerance is usually measured as the percentage biomass gain by plants in the saline environment compared to control conditions over a definite time period (Munns, 2002). Most commonly, the ions that cause high salinity are Na+ and Cl− as they prevail in seawater. Halophytes utilize these ions for a large portion of their osmotic change (Flowers et al., 2010). Plants have evolutionarily conserved mechanisms to endure harsh conditions by expressing enzymes, transcription regulators and other factors that function in pathways directed by phytohormones, such as abscisic acid (ABA), and second messengers, such as Ca2+ (Mukhopadhyay and Tyagi, 2015).
The complete waterfront zone of Gujarat (India) is gradually becoming increasingly saline due to salt farming, poor agricultural practices, urbanization and poor environmental practices (Jha et al., 2011). Salicornia brachiata is an obligate halophyte that belongs to the Amaranthaceae family. It is a leafless annual succulent plant and is abundant in salt marshes on the Gujarat coast of India. S. brachiata has the ability to grow in a wide range of NaCl concentrations (0.1–2.0 M) and also requires NaCl for in vitro regeneration (Joshi et al., 2012). This unusual characteristics, alongside other factors such as its oligosaccharides, proteome and metabolites, provides an opportunity to investigate its salt tolerance system (Jha et al., 2012; Joshi et al., 2012; Mishra et al., 2013, 2015; Patel et al., 2016a). Different salt tolerance mechanisms have been reported from halophytes (Jha et al., 2011; Chaturvedi et al., 2014; Singh et al., 2014a; Udawat et al., 2016) and several EST databases have been created for numerous halophytes, including S. brachiata (Jha et al., 2009), Alfalfa (Jin et al., 2010), and Chenopodium album (Gu et al., 2011).
Molecular processes that control Na+ compartmentalization in vacuoles receive much attention, while other key procedures in the tissue tolerance of Na+ and Cl− and osmotic change are neglected (Munns and Tester, 2008). From a developmental perspective, all resistance systems are modified and genotype specific (Vinocur and Altman, 2005). Several candidate genes and promoters responsible for enhanced abiotic stress tolerance have been cloned from Salicornia and characterized in model organisms and crop plants such as jatropha, castor, cumin, and groundnut (Chaturvedi et al., 2012; Joshi et al., 2013; Pandey et al., 2013, 2016; Singh et al., 2014b; Tiwari et al., 2014, 2015a,b, 2016; Udawat et al., 2014; Patel et al., 2015). However, it is challenging to identify key genes in the stress tolerance mechanism. A number of novel genes have been characterized, such as SbUSP, SbSDR1, MsZEP, and IbZFP1, but other novel or uncharacterized genes may play a critical role in stress tolerance (Singh et al., 2016; Udawat et al., 2016; Wang et al., 2016; Zhang et al., 2016). The EST database contains about 30% novel or uncharacterized clone sequences for Salicornia. The gene clone Sal-C-64 (EB484712) exhibited high expression under salt stress but shows no significant homology with the existing database. It was speculated that this gene might be involved in the abiotic stress tolerance mechanism; therefore, it was selected for functional validation. The full-length gene encodes for a protein that is designated as Salicornia brachiata stress-related protein (SbSRP).
Despite these developments, numerous difficulties remain, not just in explaining the stress response mechanism in plants, but also in utilization genes from halophytes to increase the salt tolerance of crop plants (Qin et al., 2011). In order to better understand the mechanism of salt tolerance, the role of a number of genes involved in plant homeostasis was investigated. These genes modulate physiology by compartmentalizing ions for osmotic alteration, combining perfect solutes, gathering fundamental supplements (especially K+) when under high salinity conditions (increased concentration of Na+ ions), confining the passage of saline ions into the transpiration stream, and managing transpiration under saline condition (Flowers et al., 2010). To accomplish the genetic engineering of plants with improved abiotic stress tolerance, a continuous effort to find “potential genes” is crucial in order to add to stress endurance in transgenic plants (Lee et al., 2012). The current study will identify novel salt determinants or master switches involved in ion homeostasis and osmobalance of halophytic plants. A deeper understanding of the underlying mechanisms of enhanced salt tolerance is important for the development of transgenic plants. Therefore, to understand the mechanisms responsible for improved abiotic stress tolerance of S. brachiata, a novel SbSRP gene was cloned, characterized, over-expressed and functionally validated in the model plant N. tabacum.
Materials and methods
Cloning and in-silico analysis
A novel EST clone (Sal-C-64; EB484712) was used to design primers (Table S1). The gene was converted to full length using RACE (rapid amplification of cDNA ends) and was sequenced and analyzed. The NCBI (National Center for Biotechnology Information) database was used to search for nucleotide and protein homologs. The secondary structure was predicted using ExPASy tools. Amino acid sequences deduced from nucleotide sequences were imported to the ProtParam tool of the ExPASy server (Artimo et al., 2012) for primary analysis and the PSIPRED server (Buchan et al., 2010) for the prediction and analysis of the secondary structure. The functional activity of SbSRP was predicted using TrSSP (Mishra et al., 2014). The amino acid sequences were subjected to BLAST (Basic Local Alignment Search Tool) and compared with the Protein Data Bank (PDB) and Conserved Domain Data bank (CDD). The phylogram study was accomplished using the Maximum Likelihood (ML) statistical method based on the JTT matrix-based model using Molecular Evolutionary Genetics Analyses version 6 [MEGA6] (Tamura et al., 2013).
Transcript profiling
For transcript profiling, 1-month-old seedlings were transferred to a hydroponic culture medium containing ¼ strength MS basal medium and grown in vitro with 8/16 h dark/light cycle at 25°C for 15 days. Different NaCl (0.05, 0.10, 0.25, 0.50, and 1.00 M) stress treatments were given to plants for 24 h by transferring plants grown under equivalent condition to new hydroponic culture media (¼ MS containing different NaCl concentrations). In the second set of experiments, different abiotic stresses (salt, 250 mM; desiccation; heat, 45°C; cold, 4°C) were applied for different time periods (2, 6, 12, 24 h) to plants. Total RNA was isolated from control and stressed plant samples using the GITC method (Chomczynski and Sacchi, 1987) and quantified with a Nanodrop spectrophotometer (NanoDrop, USA). cDNA was prepared using a ImpromII reverse transcriptase first strand cDNA synthesis kit (Promega, USA). The quantitative real-time PCR (qRT-PCR) reactions were carried out with gene-specific primers and β-tubulin was used as the internal reference gene (Table S1). At the end of the PCR cycles, products were retained through a melt curve analysis to check the specificity of PCR amplification in qRT-PCR. The amplified products were checked on 1% agarose gel to confirm the expected size. The qRT-PCR data were analyzed using the comparative CT method, and the relative fold-gene expression (2−ΔΔCT) of the SbSRP gene in stressed plants in comparison to control plants (without treatment) was determined after normalizing with internal control β-tubulin CT-values (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008).
Subcellular localization
A translational fusion cassette of SbSRP along with RFP (red fluorescent protein) was made to study subcellular localization. The SbSRP open reading frame (ORF) was amplified, cloned into pENTER/D-TOPO (Invitrogen, USA) and sequenced (Table S1). An LR recombination reaction was performed between an attL-containing pENTER/D-TOPO-SbSRP recombinant vector and an attR-containing pSITE-4CA (RFP) destination vector using LR Clonase II enzyme mix (Invitrogen, USA). Positive clones were selected, confirmed by PCR and transferred to onion epidermal cells using a gene gun (PDS-1000/He Biolistic, Biorad, USA). Vector pSITE-4CA:RFP was also transformed and used as a control. Transformants were incubated in the dark for 12 h and the transient expression of RFP was observed using an epifluorescence microscope (Axio Imager, Carl Zeiss AG, Germany).
Genomic organization
The SbSRP was amplified and the gene copy number was determined using DNA gel blot analysis (Joshi et al., 2011; Table S1). Genomic DNA of Salicornia was extracted using a modified CTAB (N-cetyl-N, N, Ntrimethyl ammonium bromide) method (Bubner et al., 2004) and was quantified (ND-1000, Nanodrop Technology, USA) and digested with different restriction enzymes (EcoRI, SacI, SmaI, and HindIII). Digested DNA was transferred to a Hybond N+ membrane (Amersham Pharmacia, UK) using an alkaline transfer buffer (0.4 N NaOH along with 1 M NaCl). The blot was hybridized with PCR-generated DIG-11-dUTP labeled SbSRP probe overnight at 42°C (Table S1). Signals were obtained using CDP-Star as a chemiluminescent substrate and detected on X-ray film.
Genetic transformation of tobacco plants and molecular confirmation
The SbSRP ORF was amplified (Table S1) and cloned in pCAMBIA1301 under the control of a 35S promoter via an intermediate pRT101 plant expression vector and the recombinant pCAMBIA1301:35S:SbSRP vector was mobilized into Agrobacterium tumefaciens (strain LBA4404). Tobacco (Nicotiana tabacum cv. Petit Havana) plants were transformed by the leaf disc method (Horsch et al., 1985) and transgenic plants (T0) were obtained using standard tissue culture methods. The T1 transgenic lines, obtained from T0 plants, were subjected to PCR and southern analysis for the confirmation of trans-gene (SbSRP) integration.
Semi-quantitative RT-PCR
The total RNA was extracted using the GITC method from six transgenic lines (L2, L7, L10, L16, and L17), vector control (VC) and wild-type (WT) plants (abiotic stressed and unstressed) and quantified (ND-1000). The mRNA was converted to cDNA using Superscript II RT (Invitrogen, USA). The β-tubulin gene was used as an internal reference gene and resulting cDNA of both (SbSRP and β-tubulin) were amplified using PCR (Table S1). The semi-quantitative RT-PCR was performed three times independently and amplicons were observed through agarose gel electrophoresis. Three transgenic lines (L2, L7, and L16) showed better over-expression of the SbSRP gene and so were selected for further analysis.
Analyses of transgenic plants
The copy number of the SbSRP gene in transgenic lines was determined by DNA gel blotting (Joshi et al., 2011) as described above using gene-specific probes (Table S1). The T1 seeds of SbSRP over-expressing transgenic lines (L2, L7, and L16) along with VC and WT plants were germinated on MS media that contained NaCl (200 mM) or Polyethylene glycol (10% PEG) under controlled conditions. The seedling growth percentage was calculated using the following equation:-
T1 seedlings that were uniform in size and germinated on MSB containing hygromycin (20 mg L−1) were transferred to a hydroponic system with ½ strength MS media, incubated for 45 days, and then subjected to stress treatment (for 24 h under 200 mM NaCl or 10% PEG) for biochemical and physiological analysis.
Quantification of osmotic adjustment
Leaf segments of 45 day old treated and untreated plants were collected and incubated overnight at −20°C. The next day, plant samples were thawed and centrifuged at 13,000 rpm for 10 min at 4°C. The cell sap extract was used to determine the cellular osmotic potential of the sample using a vapor pressure osmometer (VAPRO, Wescor Inc., USA).
Relative water content (RWC)
Leaf fresh weight was measured from treated and untreated, control (WT and VC), and transgenic plants, following overnight incubation of leaf samples in deionized water. The next day, turgid weight, and dry weight were determined after drying at a constant temperature (40°C). Relative water content (RWC, %) was calculated using the following equation:
Leaf senescence assay and quantification of chlorophyll content
Similar sized leaf discs (n = 10) from 45 day old control and transgenic plants were floated in ½ strength MS (control) media supplemented with NaCl (100, 150, or 200 mM) or PEG (10 or 20%) and incubated for 8 days under controlled conditions. The effects of salt and osmotic stress were examined by visually observing phenotypic alteration amongst different leaf segments. In addition, total chlorophyll content was calculated for leaf discs per gram of tissue fresh weight (Inskeep and Bloom, 1985).
XTT and TTC assay
Leaf segments (of a uniform size and weight) of 45 day old plants (treated and untreated, control and transgenic) were used to quantify the accumulation of superoxide radicals (after stress) using a 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (Hema et al., 2014). Leaf segments were kept in a potassium phosphate buffer (20 mM, pH 6.0) containing 500 μM XTT for 5 h and the increase in absorbance was measured at 470 nm. Likewise, leaf segments (of equal weight and size) of 45 day old treated and untreated, control and transgenic, plants were used to evaluate cell viability using a 2,3,5-triphenyltetrazolium chloride (TTC) assay (Hema et al., 2014). Leaf fragments were carefully rinsed in sterile water and kept in TTC solution in the dark for 6 h at room temperature (RT). Leaf samples were then boiled in 5 ml of 2-methoxy ethanol until dry, to release bound formazan. Then, 5 ml of 2-methoxy ethanol was added for the second time, and the change in absorbance was measured at 485 nm.
Membrane stability index (MSI) and electrolyte leakage (EL)
Healthy fresh leaves were harvested (from treated and untreated; control and SbSRP transgenic plants) and MSI was determined by the process explained by Sairam (1994). Leaf segments were kept in sterile water, and 1st set (L1) was stored at 40°C for 30 min where 2nd set (L2) was stored at 100°C for 10 min. Electrical conductivity (EC) of samples from both sets was measured, and MSI was calculated as MSI = 100 × 1−L1/L2.
For analyzing the electrolyte leakage, leaf samples were thoroughly rinsed with sterile water to remove surface bound electrolytes. Fresh leaf segments were stored in the sterile water in sealed tubes and incubated, overnight, on a rotating shaker. The next day, EC was determined (Lt) by a conductivity meter (SevenEasy, Mettler Toledo AG 8603, Switzerland). Then, leaf samples from the each treatment were autoclaved at 120°C for 15 min, subsequently, they were chilled to RT and EC (L0) was determined again (Lutts et al., 1996). The percentage EL was determined as EL(%) = 100 × Lt/L0.
Quantification of proline, H2O2, and lipid peroxidation
The free proline and H2O2 content in the treated and untreated, control, and transgenic plants were calculated by the methods described by Bates et al. (1973) and He et al. (2005), respectively. A standard curve was generated with the known concentrations of proline and H2O2, an increase in absorbance was recorded at 520 and 560 nm, respectively. Lipid peroxidation was calculated by quantifying the level of MDA (malondialdehyde) produced by TBA (thiobarbituric acid) reaction (Draper and Hadley, 1990).
Estimation of total soluble sugar, reducing sugar, oligo sugar, free amino acid, and polyphenol content
The total quantities of oligo and reducing sugars were determined by reference to a standard curve that was generated using glucose (Sigma-Aldrich, USA). The treated and untreated, control and transgenic leaf segments were crushed in 85% ethanol, following incubation with an anthrone reagent. All samples were boiled then cooled to room temperature, and absorbance was recorded at 620 and 540 nm. The level of reducing sugar was computed using a colorimetric test and the DNS method (Yemm and Willis, 1954). Total amino acids and polyphenol content were calculated by reference to a standard curve, which was generated by glycine and catechol solution, respectively (Pandey et al., 2015; Patel et al., 2016b). Fresh leaf samples were crushed in 85% ethanol, evaporated to dryness following re-dissolution in ninhydrin or the Folin-Ciocalteu reagent. Variation in color intensity was then analyzed at 570 or 650 nm.
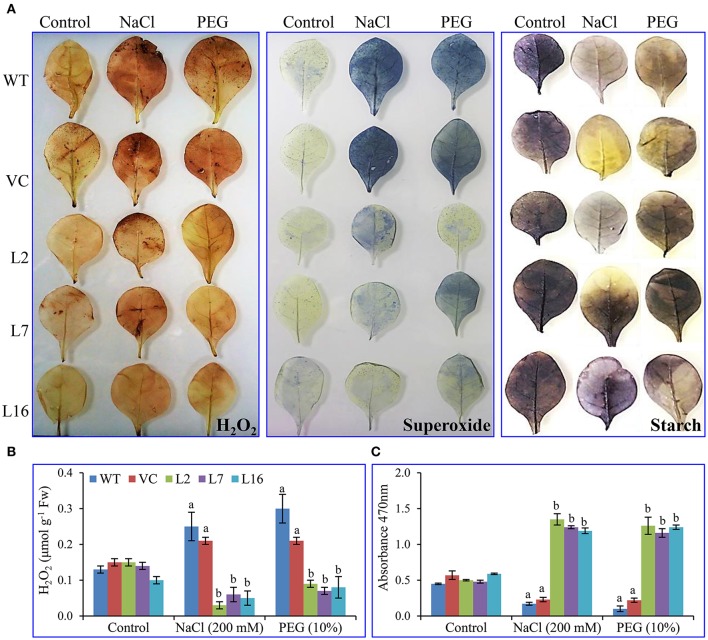
In vivo localization of H2O2, superoxide-radicals, and starch
The hydrogen peroxide, superoxide-radicals, and starch under salt (200 mM NaCl) or osmotic (10% PEG) stress were determined in vivo using a histochemical stain of 3,3-diaminobenzidine (DAB), nitro-blue tetrazolium (NBT), or potassium iodide (KI), respectively (Shi et al., 2010). DAB and NBT were prepared as 1 mg ml−1 solutions in 10 mM phosphate buffer (pH 7.8), whereas KI (1 mg ml−1) solution made in distilled water. Fresh leaf segments (of treated and untreated, control, and transgenic plants) were dipped in the freshly prepared stain solutions, kept in the dark for 2 h followed by exposure to white light (40 μmol m−2 s−1 spectral flux photon) for DAB (8 h) and NBT (1 h). For in vivo localization of starch, leaf segments were immersed in KI solution for 1 min. The blue or brown spots that appeared on the leaves indicated in vivo localization.
Analyses of ion content
The plant samples (treated and untreated, control, and transgenic plants) were dried at 65°C for 72 h and their dry weights were determined. Plant samples were acid digested overnight in perchloric acid-nitric acid solution (3:1), heated to dryness following re-dissolution in sterile water. Ion content was determined using an inductively coupled plasma optical emission spectrometer (Optima2000DV, PerkinElmer, Germany).
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from control and treated plant samples (WT, VC, and transgenic lines) and cDNA was synthesized using a Superscript RT III cDNA synthesis kit (Invitrogen, USA). A real-time qRT-PCR analysis was performed for antioxidant and transcription factor genes of the host plant (NtSOD, NtAPX, NtCAT, NtDREB, and NtAP2). A melt curve analysis was also implemented to validate the specificity of the qRT-PCR reaction (Table S1). The housekeeping gene β-tubulin was used as a reference and the relative expression change (compared to WT and control condition) was calculated using the CT method (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008).
Measuring photosynthesis and respiration
Photosynthesis was computed by determining the rate of change of CO2 over time in a leaf covered with a moderately big chamber, following the manufacturer's instructions (LI-6400XTv6, LI-COR Biosciences Inc., Nebraska). The treated (NaCl 200 mM and PEG 10%) and untreated plants of control and transgenic lines were kept under controlled conditions for 24 h and then chlorophyll fluorescence was computed. Photosynthesis, photochemical efficiency, and water use efficiency were investigated. All measurements were taken on four fully expanded leaves. The ratio of variable to maximum fluorescence was then determined as Fv/Fm = (Fm − F0/Fm). The computed light was switched on and actinic light was given for 30 s at an intensity of ~1,000 μmol photons m−2 s−1 PAR (photosynthetically active radiation) and Fm′ was calculated.
Statistical analysis
Data from 3 replicates, each containing 15 plants, were documented for each set of experiments. Results are expressed as mean ± standard error. Statistical significance was determined by ANOVA (analysis of variance) amongst the means of WT, VC, and transgenic plants. A Tukey HSD test was used for comparisons and the differences were considered to significant when p < 0.05 and these differences are designated by different letters.
Results
In-silico analysis predicts that the SbSRP may be a novel transporter
The full-length SbSRP gene (1262 bp; gene accession no. KX242114) contained a 5′ untranslated leader sequence (5′-UTR: 1–294 bases), an open reading frame (ORF: 753 bp; 295–1047), 3′-UTR (215 bp; 1048–1262) and a poly A tail of 19 base pairs (Figure S1). The SbSRP ORF (753 bp) encoded a protein of 250 amino acid residues (Figure S1) with an estimated molecular mass of 80–85 KDa and theoretical isoelectric point (pI) of 8.34. The total number of negatively charged residues (aspartate and glutamate) is 28 and there are 30 positively charged residues (arginine and lysine). The N-terminal of SbSRP is M (methionine) and its estimated half-life is 30 h for mammalian reticulocytes (in vitro), >20 h for yeast (in vivo), and >10 h for E. coli (in vivo). The instability index of the SbSRP protein was computed to be 34.52; this classifies the protein as stable according to ExPasy's ProtParam Tool (SIB, Swiss Institute of Bioinformatics). The TMpred program predicts membrane-spanning regions and their respective orientation (Figure S2). The prediction was made using an amalgamation of several weight matrices for the scoring of TMbase, a database of naturally occurring transmembrane proteins. The prediction of the topology of the membrane spanning domains revealed the presence of one transmembrane domain score from 25 to 44 at the N-terminal region (Figure S2). The predicted secondary structure of the deduced SbSRP protein contained topological arrangements of elements (coil, α-helices, and β-strand) with classical folding (Figure S2). The TrSSP program predicts that SbSRP is a transporter. The conserved domain database (CDD) categorized the deduced protein as part of the rubber elongation factor/stress related protein particle like superfamily (REF/SRPP). Phylogenetic analyses revealed that SbSRP showed proximity with the Beta vulgaris and Vitis vinifera hypothetical or predicted REF/SRPP like particle. The SbSRP gene sequence showed 78% identity with Beta vulgaris subsp. vulgaris predicted REF/SRPP like protein At3g05500 mRNA, 71% with Populus trichocarpa REF family protein, 71% with Populus trichocarpa unknown clone mRNA, 71% with Populus euphratica REF/SRPP like proteins At3g05500 mRNA, 70% with Ricinus communis REF like protein, 69% with Citrus sinensis predicted REF/SRPP like protein At3g05500 mRNA, 69% with Citrus clementina hypothetical protein mRNA, and 69% with Vitis vinifera predicted SRP like mRNA hence was considered uncharacterized.
The SbSRP is a plasma membrane localized protein
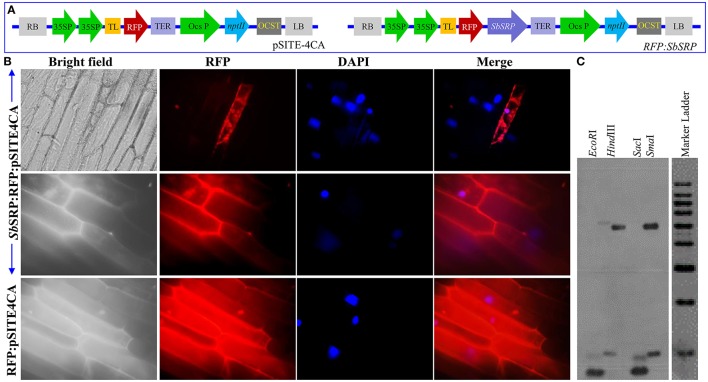
The transient expression of RFP only and SbSRP:RFP (Figure 1A) in onion epidermal cells revealed that SbSRP is expressed in the plasma membrane whereas RFP is expressed uniformly throughout the cells (Figure 1B). The sequence analysis of the cDNA clone compared to the genomic clone revealed a single exon structure of SbSRP gene, confirming the intronless genomic organization of the gene. In the DNA gel blot analysis, EcoRI, HindIII, SacI, and SmaI-digested genomic DNA produced two intense bands; hence, the existence of at least two homologs of the SbSRP gene was depicted (Figure 1C).
Figure 1.
Sub-cellular localization of SbSRP protein and gene copy number. (A) Vector pSITE-4CA(RFP) and SbSRP-1:RFP gene construct, (B) Transient expression of RFP alone and SbSRP:RFP translational fusion protein on transformed onion epidermal cells and (C) DNA hybridization of Salicornia brachiata genome to determine the copy number of SbSRP gene.
Transcript profiling confirms the differential expression of the SbSRP gene
The transcript profile of SbSRP was studied in Salicornia under salt, desiccation, heat, and cold stress using qRT-PCR (Figure S3). A 3.7-fold exponential increase was observed in the SbSRP transcript under 250 mM salt stress; when the salt concentrations was increased further to 500 and 1,000 mM, there was a reduction in the transcript level, and about 1.7 and 1.5-fold expression were detected respectively. Therefore, 250 mM NaCl was found to be the optimum concontration for SbSRP transcript expression. Consequently, plants were subjected to 250 mM salt stress for a range of durations from 2 to 24 h, and an exponential increase in the mRNA level of SbSRP from 2.5 to 6.5-fold was observed up to 12 h, thereafter expression decreaded and reached to 3.5-fold at 24 h compared to control condition. Under cold and desiccation stresses, relative expression of SbSRP transcript was maximum 8.6 and 3.8-fold, respectively at 12 h. Under heat, the SbSRP transcript reached a maximum of 25.63-fold at 24 h (Figure S3).
Analysis confirms overexpression of the SbSRP gene in transgenic tobacco lines
Putative transgenic lines (T1) were screened by germination on hygromycin (20 mg L−1) and the presence of transgenes was confirmed by PCR amplification of uidA and hptII genes (Figures S4A,B). Acclimatized putative SbSRP over-expressing transgenic lines were confirmed for the stable integration of the transgene into the tobacco genome by DNA hybridization (Figure S4C). Amplification of the uidA and hptII genes was observed in all the transgenic lines (L1–L17) and the positive control, whereas no amplification was observed in WT (wild type, non-transgenic) plants. DNA gel blot analysis revealed that transgenic lines L3, L6, and L12 had two copies of the transgene, whereas L2, L7, L10, L16, and L17 clearly showed a single transgenic event, validating the single-copy integration of T-DNA into the tobacco genome. All other lines showed faint or unclear transgenic events while WT and VC plants did not illustrate any detectable hybridization signal. Therefore, the comparative ectopic expression analysis of lines L2, L7, L10, L16, and L17 was carried out by semi-quantitative RT-PCR and real-time (qRT) PCR (Figures S5A,B). Transgenic lines L2, L7, and L16 showed 4.6, 5.9, and 5.6-fold increases whereas L10, L12, and L17 showed about 3–4-fold increases under salinity stress. The SbSRP over-expressing transgenic lines L2, L7, and L16 exhibited higher expression than the others lines, and no amplification was observed from the cDNA of WT and VC plants. The stringency of the PCR was checked by amplifying the β-tubulin as the internal reference gene using the same cDNA and PCR conditions. An amplicon of ~300 bp was obtained in all transgenic lines and the WT and VC plants. Semi-quantitative RT-PCR and qRT-PCR additionally confirmed the stable integration and expression of the transgene in putative transgenic lines. Transgenic lines (L2, L7, and L16) and control plants (WT and VC) were selected for further analysis. The activity of the reporter gene, β-glucuronidase, in the leaf segment of transgenic, VC and WT plants was visualized, and efficient GUS activity was observed (Figure S5C).
Overexpression of the SbSRP gene enhances plant growth
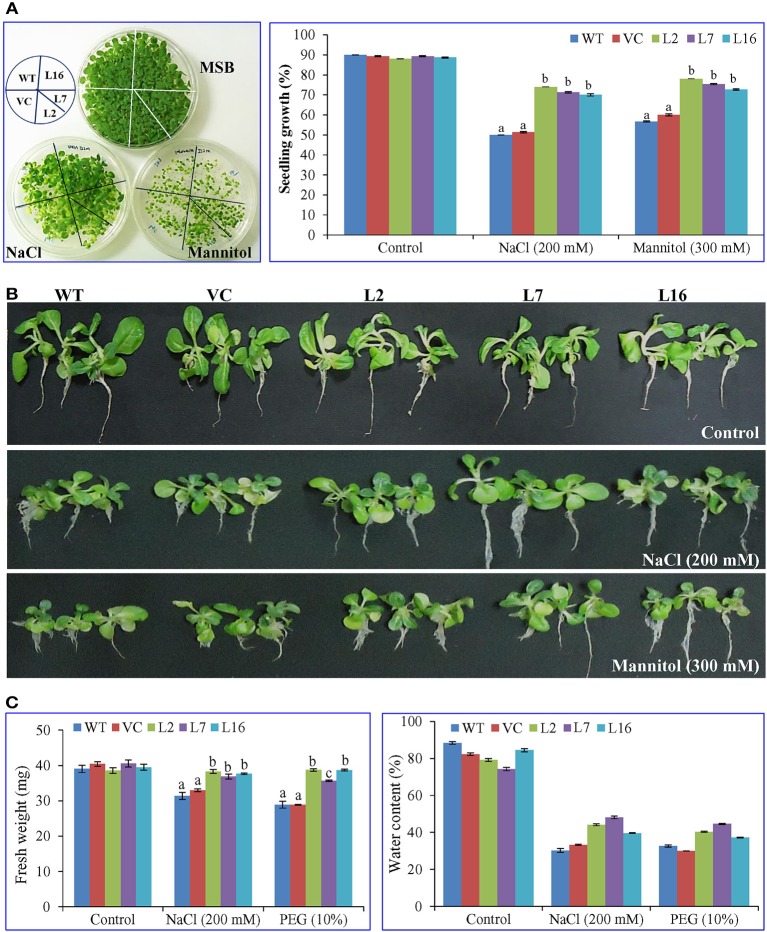
The seeds from WT, VC, and transgenic lines (L2, L7, and L16) were harvested from the greenhouse, surface-sterilized, and germinated under different conditions. The SbSRP over-expressing transgenic lines L2, L7, and L16 showed ~75% seedling growth under NaCl and osmotic stress conditions (Figure 2). This seedling growth percent was significantly different (p < 0.05) to that observed in WT and VC, which exhibited ~54% seedling growth. After 3 weeks of salinity (NaCl 200 mM) and osmotic stress (mannitol 300 mM) treatment, transgenic lines illustrated phenotypically better tolerance than WT and VC plants. Likewise, growth parameters were significantly (p < 0.05) enhanced in SbSRP over-expressing transgenic lines compared to WT and VC plants under stress conditions (Figure 2).
Figure 2.
Plant growth analyses of transgenic tobacco plants under abiotic stress. (A) Calculation of seedling growth percentage after 21 days. Comparative study of (B) morphology and (C) fresh weight and water content of transgenic lines (L2, L7, and L16) and control plants (WT and VC), grown for 21 days under salt and osmotic stress. Bars represent means ± SE and values with different letters are significant at P < 0.05.
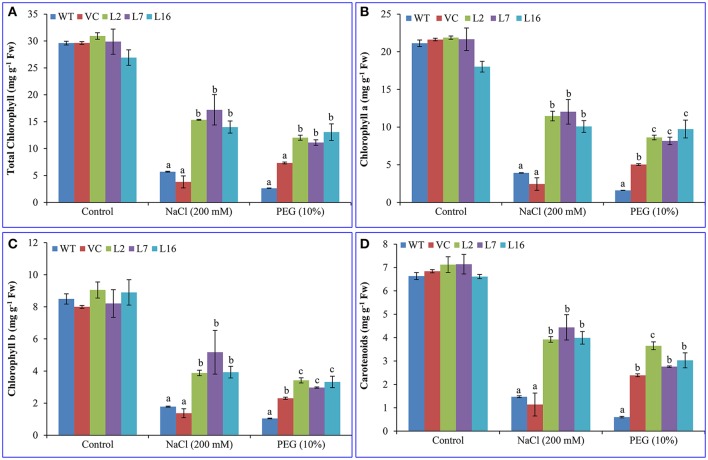
After 7 days of incubation in control conditions, NaCl (100, 150, 200 mM) or PEG (10 and 20%), leaf discs of SbSRP over-expressing transgenic lines showed lower degradation of chloroplasts and stayed greener than WT and VC leaf discs (Figure S6A). The total chlorophyll (Chl; about 29.5 mg g−1 Fw) and carotenoid (about 6.8 mg g−1 Fw) content were almost comparable between control (WT and VC) and transgenic plants under normal (unstressed) conditions (Figure 3). After NaCl and osmotic stress, the total Chl content was reduced ~80%. About 5.7 and 2.6 mg g−1 Fw Chl contents were observed in WT under NaCl and osmotic stress, respectively. Similarly, about 3.8 and 7.3 mg g−1 Fw Chl were detected in VC plant under NaCl and osmotic stress, respectively. The degree of reduction was less in transgenic lines and about 13.8 and 3.6 mg g−1 Fw total Chl and carotenoid contents were quantified, respectively. Transgenic lines showed better protection of Chl from salt and osmotic stress conditions than WT and VC plants. Individually, transgenic lines, L2, L7, and L16, showed about 50% (30.94–15.35 = 15.59 mg g−1 Fw), 42% (29.88–17.21 = 12.67 mg g−1 Fw) and 48% (26.92–14.01 = 12.91 mg g−1 Fw) reduction under salinity (NaCl) stress. Similarly, a 61% (30.94–12.03 = 18.91 mg g−1 Fw), 63% (29.88–11.12 = 18.76 mg g−1 Fw) and 52% (26.92–13.06 = 13.86 mg g−1 Fw) reduction was found in transgenic lines (L2, L7, and L16) under osmotic stress, respectively. Total carotenoid content also exhibited a similar pattern as chlorophyll content (Figure 3). About an average of 47% (6.8–3.6 = 3.2 mg g−1 Fw) decrease was observed in transgenic lines compared to 79.5% (6.8–1.4 = 5.4 mg g−1 Fw) reduction in control plants under stress conditions. Furthermore, transgenic lines showed significantly (p < 0.05) improved cell viability in contrast to WT and VC plants under salt and osmotic stress conditions (Figure S6B).
Figure 3.
Estimation of chlorophyll content and carotenoids. (A) Total chlorophyll, (B) chlorophyll a, (C) chlorophyll b, and (D) carotenoids contents of transgenic (L2, L7, and L16) and control plants (WT and VC) under salt and osmotic stress condition. Bars represent means ± SE and values with different letters are significant at P < 0.05.
Overexpression of the SbSRP gene improves physiology of the plant
Osmotic potential and water retention ability of the WT, VC, and transgenic plant leaves were evaluated under control, salinity, and osmotic stress conditions (Figure 4). All transgenic lines were better able to adjust osmotically (−1.7 to −1.9 MPa) under both salinity and osmotic stress conditions compared to WT and VC plant (−0.6 to −0.8 MPa) leaves. The relative water content (RWC) of all transgenic lines was significantly higher under stress conditions than that of WT and VC plants during salinity and osmotic stress. At control condition, an average of 55% RWC was observed in all plants. About 42–50% RWC was detected in transgenic lines under stress conditions compared to control plants (28–33% RWC in WT and VC). Lipid peroxidation of WT, VC, and transgenic lines was compared in leaf segments by estimating the MDA content (about 0.24 mmol g−1 Fw), which is generated after lipid peroxidation and is accumulated in leaves. The MDA content abruptly increased (about 6-fold) in the control (WT and VC) plants under stress, and 1.38 and 1.45 mmol g−1 Fw MDA contents were detected in WT and VC, respectively. The MDA content also increased in SbSRP over-expressing transgenic lines by 1.8–2.7-fold, and 0.43, 0.66, and 0.58 mmol g−1 Fw MDA contents were detected in transgenic lines, L2, L7, and L16, respectively, under salinity stress. Similarly, 2.2–4.7-fold increase was observed under osmotic stress, and 1.15, 0.89, and 0.54 mmol g−1 Fw MDA contents were measured. Electrolyte leakage (%) from the leaves of WT, VC, and transgenic lines were not significantly different and was approximately an average of 8% in control conditions. Under stress conditions, the leakage in WT and VC plants increased 2.9-fold, and approximately an average of 23% EL was observed in comparison to that of the control. The electrolyte leakage in SbSRP over-expressing transgenic lines (13.5%) increased about 1.7-fold upon stress treatment, but it was significantly lower (23.0–13.5 = 9.5%) than that of WT and VC plants (i.e., 23%). In control conditions, the membrane stability index (MSI) was comparable (about 0.92) in all plants (WT, VC, and transgenic lines). Transgenic lines showed a significantly (p < 0.05) higher MSI (about 0.83) under stress conditions compared to control plants (0.59; WT and VC). Results suggest that transgenic plants maintained membrane stability about 1.4-fold better under stress condition than that of WT and VC plants.
Figure 4.
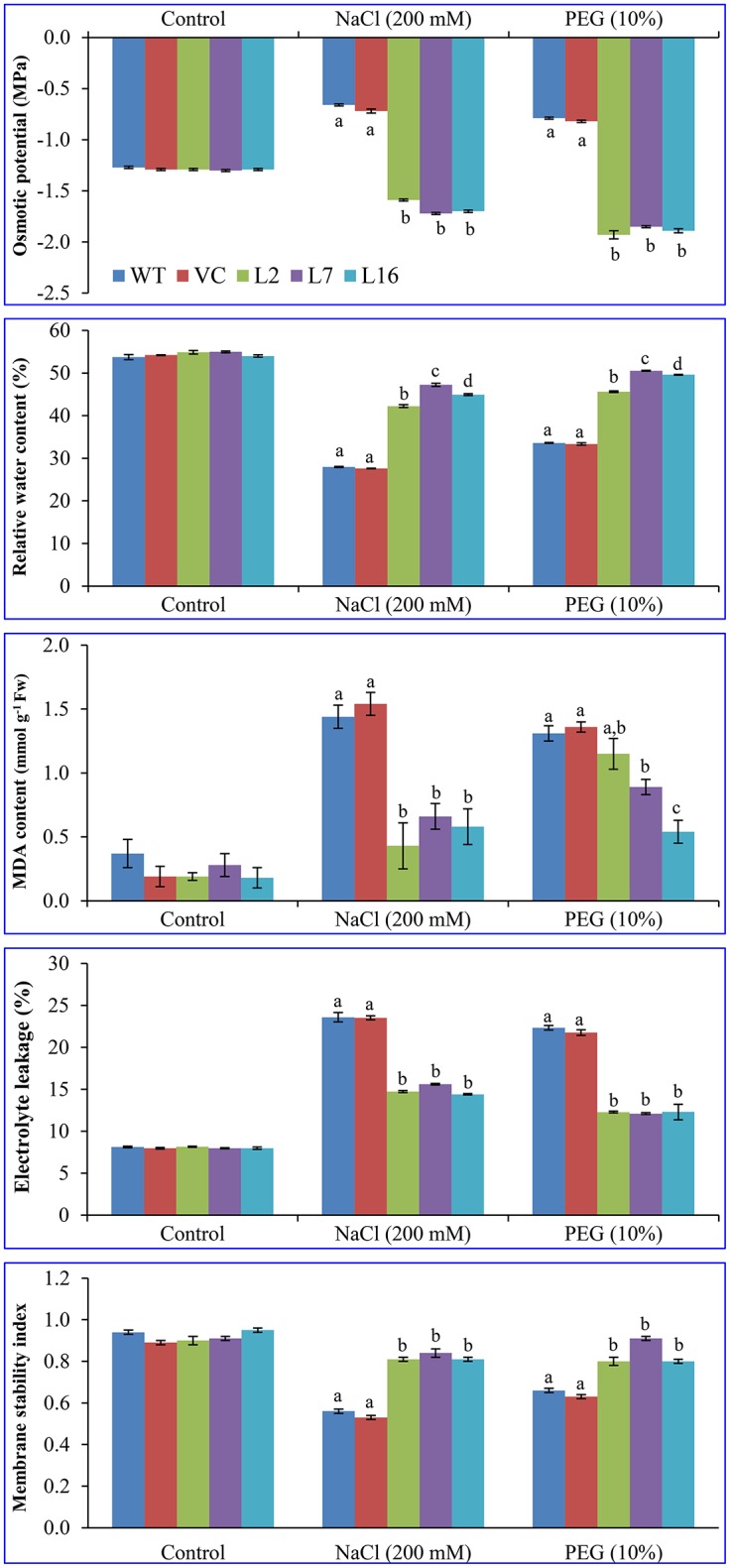
Physiological analyses of transgenic lines. Estimation of osmotic potential (OP), relative water content (RWC), lipid peroxidation (MDA content), electrolyte leakage (EL), and membrane stability index (MSI) from leaves of control plants (WT and VC) and transgenic lines (L2, L7, and L16) under control, salinity and osmotic stress conditions. Bars represent means ± SE and values with different letters are significant at P < 0.05.
The SbSRP gene involves in ion homeostasis
The Na+ content under control conditions was almost comparable (0.55 mg g−1 Dw) in WT, VC and transgenic lines but found lower under in transgenic lines (compared to WT and VC) under stress conditions. During salinity stress, Na+ content in WT and VC plants increased about 1.1-fold; transgenic lines also accumulated Na+, but not to the same extent as WT and VC plants (Figure S7). The K+ content under control conditions was similar (about 0.4 mg g−1 Dw) in all plant types, but WT and VC showed more than a 60% reduction in K+ content (0.4–0.15 = 0.25 mg g−1 Dw) under salinity stress, in comparison to transgenic lines (0.4–0.26 = 0.14 mg g−1 Dw) where the reduction was only 35%. Likewise, the K+/Na+ ratio was found to be significantly higher in transgenic lines over-expressing the SbSRP gene (Figure S7).
Overexpression of the SbSRP enhances biochemical status of the plant
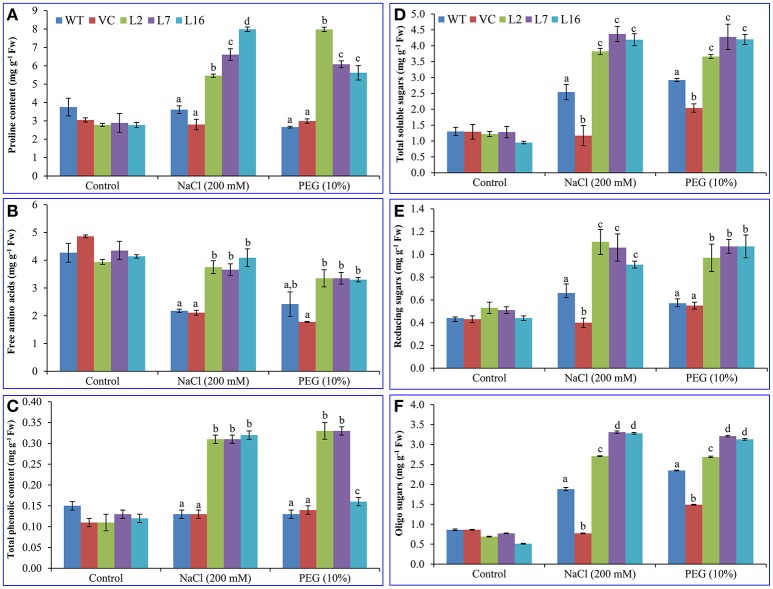
No significant difference was observed in the proline (~3.05 mg g−1 Fw), free amino acid (~4.32 mg g−1 Fw), and polyphenol (0.12 mg g−1 Fw) contents between control (WT and VC) and transgenic lines, over-expressing SbSRP gene, under control condition (Figure 5). Proline content increased about 1.5–2-fold under salinity stress (5.46, 6.61, and 7.99 mg g−1 Fw) and osmotic stress (5.2, 3.19, and 2.85 mg g−1 Fw) conditions in transgenic lines L2, L7, and L16, respectively, while WT and VC plants showed a non-significant increase (about 0.03 mg) in proline content (Figure 5A). Transgenic plants maintained the free amino acid content (about an average of 3.6 mg g−1 Fw) under stress conditions in contrast to WT and VC (2.1 mg g−1 Fw) plants (Figure 5B). The polyphenol content was increased about 2.6-fold (~ 0.32 mg g−1 Fw) in all transgenic lines under stresses, except L16, which showed a different result and accumulated only 0.04 mg (about 1.3 times) polyphenol than control plants (WT and VC) under osmotic stress (Figure 5C).
Figure 5.
Biochemical analyses of transgenic tobacco plants under abiotic stress. Estimation of (A) proline, (B) free amino acids (FAA), (C) total phenolic content (polyphenol; TPC), (D) total soluble sugars (TSS), (E) reducing sugars (RS), and (F) oligo sugars (OS) in control (WT and VC) and transgenic plants (L2, L7, and L16) under salinity and osmotic stress condition. Bars represent means ± SE and values with different letters are significant at P < 0.05.
Total soluble sugars (about an average of 1.2 mg g−1 Fw) increased about 3.5–4.5-fold under salinity and osmotic stress conditions in transgenic lines (about an average of 4.1 mg g−1 Fw) as compared to the control conditions, but only a slight change (1–2-fold) was observed in control (WT and VC; about an average of 2.2 mg g−1 Fw) plants (Figure 5D). The reducing sugar level (about an average of 0.47 mg g−1 Fw in control condition) in transgenic plants increased 2.2-fold (1.03 mg g−1 Fw) under salinity and drought stress conditions whereas a slight increase (about 1.2-fold) was observed in WT and VC (about an average of 0.55 mg g−1 Fw) plants (Figure 5E). The oligo-sugar content (about an average of 0.74 mg g−1 Fw) was comparable under control conditions, whereas transgenic lines L7 and L16 exhibited about 4.4-times higher oligo-sugar content (about an average of 3.23 mg g−1 Fw) under stress conditions (Figure 5F).
In-vivo localization and estimation of ROS
Fresh leaf segments (of treated and untreated, control and transgenic plants) were dipped in the freshly prepared DAB and NBT stain solutions, kept in the dark for 2 h followed by exposure to white light for in-vivo localization study. There was no variation in peroxide localization or free radicals among leaves of WT, VC, and SbSRP over-expressing transgenic lines under control conditions following staining with DAB and NBT (Figure 6A). In contrast, WT and VC plants leaves displayed elevated levels of brown and blue insoluble precipitate formation in comparison to transgenic lines under salt and osmotic stress. These results demonstrate that WT and VC leaves build up more superoxide-radicals and H2O2 than those of SbSRP over-expressing transgenic lines under stress, confirming that SbSRP mitigates abiotic oxidative stress in situ. Similarly, the cellular starch content was equivalent under control conditions, but transgenic lines showed a deep purple stain under salt and osmotic stress conditions, showing that SbSRP over-expressing transgenic lines maintained cellular starch content, even under stress conditions.
Figure 6.
In vivo localization and cell viability. (A) In vivo localization of H2O2, superoxide and starch, estimation of (B) H2O2 content and (C) superoxide radicals of transgenic (L2, L7, and L16) and control (WT and VC) leaves. Bars represent means ± SE and values with different letters are significant at P < 0.05.
Lower accumulation of ROS was observed in transgenic lines (an average of 0.063 μmol g−1 Fw), whereas WT and VC plants exhibited the higher accumulation of H2O2 (an average of 0.25 μmol g−1 Fw) under salt and osmotic stress conditions (Figure 6B). The superoxide content was measured by the reduction of XTT tetrazolium salt, where the transgenic lines showed a significant (p < 0.05) increase in absorbance (470 nm) in contrast to WT and VC plants under stress conditions (Figure 6C).
The SbSRP gene may regulate the transcript expression of antioxidative enzyme-encoding and transcription factor genes
The mRNA level of genes encoding antioxidative enzymes and transcription factors (ascorbate peroxidase [Nt-APX], catalase [Nt-CAT], superoxide dismutase [Nt-SOD], DREB [Nt-DREB], and AP2 [Nt-AP2]) were studied in WT, VC, and the transgenic lines (L2, L7, and L16) under control and stress conditions (salinity, osmotic, heat, cold, ABA, and SA; Figure 7). The expression of Nt-APX was upregulated by more than 8-fold under salt, drought, ABA, SA, heat, and cold stress in L2, whereas an ~5–7-fold increase was observed in L7 under stress compared to WT, VC plants, and control condition. However, L16 showed lower accumulation of the transcript of Nt-APX under stress conditions. The Nt-CAT gene transcript also showed a similar pattern, although L16 accumulated about 8 times more transcript under salt and drought stress than WT and VC plants. The transcript of Nt-SOD increased by 8-fold in L2, and by 7-fold in L7 and L16 under stress conditions. Likewise, the transcript of Nt-DREB and Nt-AP2 accumulated 4–6 times more in transgenic lines compared to WT and VC plants under stress conditions.
Figure 7.
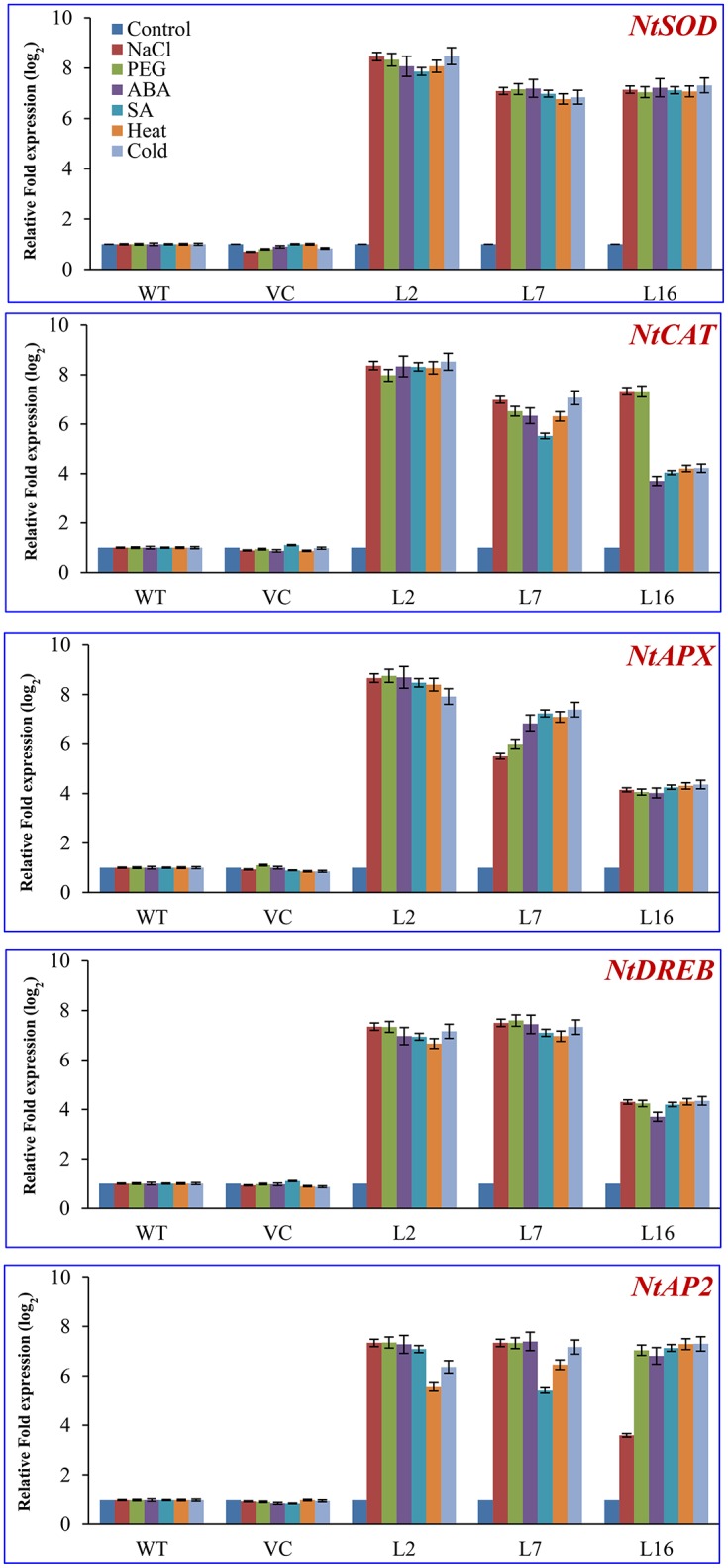
Transcript expression analysis of host stress responsive genes. Comparative transcript expression profile of antioxidant and transcription factor genes of the host plant (NtSOD, NtAPX, NtCAT, NtDREB, and NtAP2) under different stress conditions. The fold expression values obtained in WT (control or stress) were taken as one, relative expression change was calculated for VC and transgenic lines, and presented as relative fold expression (in log2). The housekeeping gene β-tubulin was used as a reference.
Overexpression of the SbSRP gene enhances photosynthesis of the plant
Net assimilation rate was ~12 μmol CO2 m−2 s−1 in WT, VC, and transgenic plants under control conditions. Under stress conditions, it reduced to 4 μmol CO2 m−2 s−1 in WT and VC plants, but transgenic plants (L2, L7, and L16) maintained values of 6.5, 8.0, and 5.0 μmol CO2 m−2 s−1, while transpiration rate was 2.0, 1.5, and 2.8 mmol H2O m−2 s−1. These results confirmed the role of SbSRP in providing abiotic stress tolerance to the transgenic tobacco plants by maintaining photosynthesis under stress condition (Figure 8).
Figure 8.
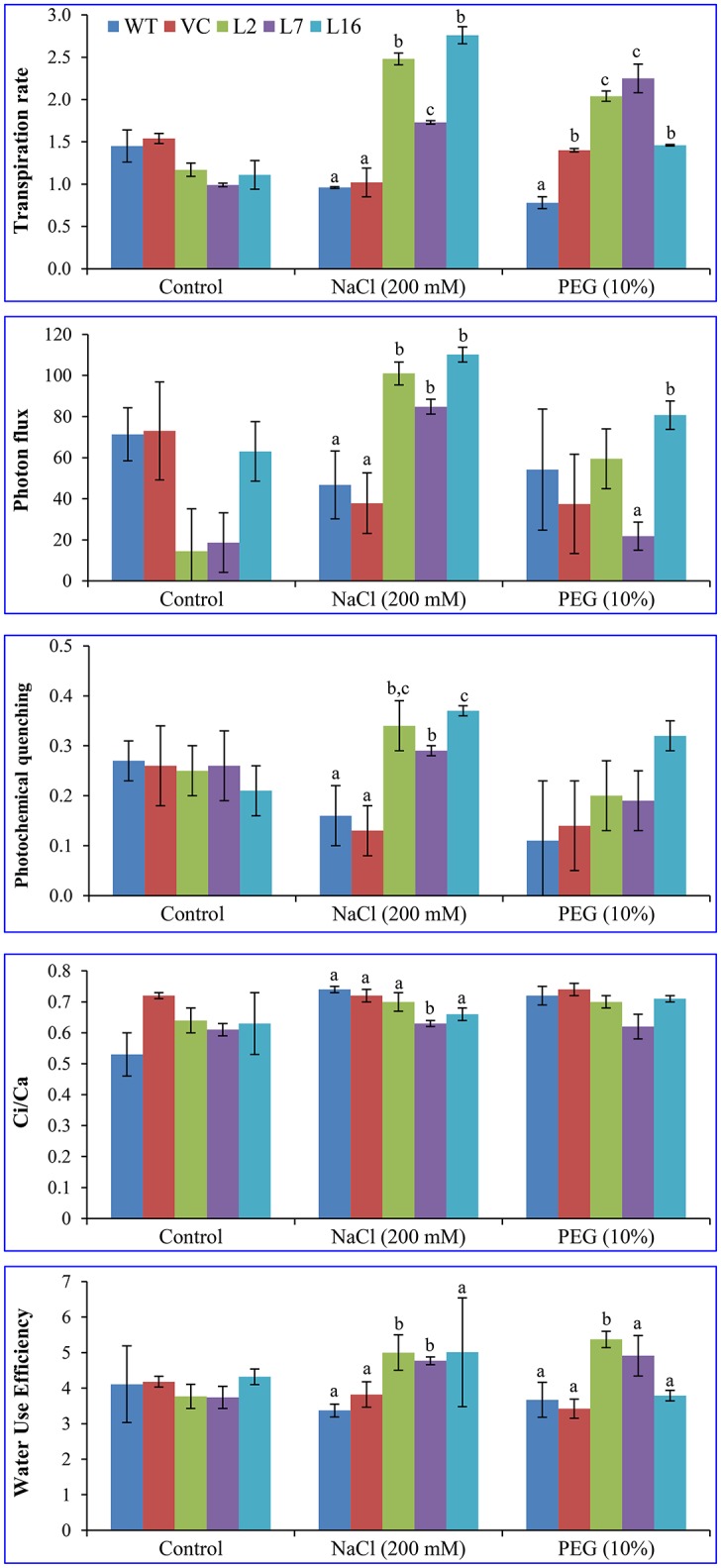
Quantitative analysis of the physiological status of transgenic lines. Transpiration rate, photon flux, photochemical quenching, Ci/Ca ratio, and water use efficiency of WT, VC, and transgenic lines (L2, L7, and L16) were measured under salinity and osmotic stress condition. Bars represent means ± SE and values with different letters are significant at P < 0.05.
Discussion
As plants are sessile in nature, they have to face numerous abiotic stresses such as salt, drought, extreme temperature, variation in light, and UV radiation. Salinity influences almost every aspect of plant physiology, commonly at the entire plant level, through osmotic stress in early stages, and ionic stress at later phases of development (Munns and Tester, 2008). Abiotic stresses, along with population increases and lack of food, create trouble for people globally, and cause losses worth hundreds of million dollars every year. Salinity is developing as a noteworthy issue, and halophytes give a remarkable opportunity to adjust crops to different saline biological niche (Mishra et al., 2015). Halophytes are well-adapted to high salinity due to their modified molecular, biochemical, morphological, anatomical, and physiological characteristics. Halophytes have therefore developed a cascade of genes in order to achieve tolerance, including ion compartmentalization, osmotic adjustment (osmolytes), succulence (ion sequestration in vacuoles), selective uptake and ion transport (SOS1, NHX1, HKT2, CLC), enzymatic (SOD, APX, GST, GR) and non-enzymatic (proline, glycine betaine, ascorbate, tocopherol, polyols, polyamines) antioxidant response, maintenance of redox/energetic status, salt inclusion/excretion, and genetic control (DREB, NAC, MYB). The present study reported the isolation, cloning and functional validation of a novel salt-responsive gene, SbSRP, from an extreme halophyte S. brachiata. The SbSRP gene was characterized at the genomic and transcriptomic levels and was genetically transformed into Nicotiana tabacum cv. Petit Havana. The role of the candidate SbSRP gene under salinity and osmotic stress tolerance was studied in the putative T1 generation of transgenic tobacco.
The full length SbSRP sequence was 1,262 bp and included a 5′ untranslated leader sequence (5′-UTR: 1–294 bp), an open reading frame (753 bp ORF that encoded a protein of 250 aa with an estimated molecular mass of 80–85 kDa), a 3′-UTR (215 bp; 1,047–1,262) and a poly A tail of 19 base pairs (Figure S1). The genomic organization of the SbSRP gene revealed that it is an intronless gene, consisting of a single exon. Jain et al. (2008) reported that the rice and Arabidopsis contain 19.9 and 21.7% intronless genes, respectively. These results may support future work on the role of intronless genes in plants and other higher organisms by comparative, evolutionary, and functional analysis. Yan et al. (2014) demonstrated that maize intronless genes (36.87%) play a crucial role in energy metabolism and translation. Intronless genes are widespread in prokaryotes and are also additionally seen as rapidly inducible eukaryotic genes (Giri et al., 2013). At least two homologs of the SbSRP gene are present in the S. brachiata genome, as shown by DNA gel blot analysis (Figure 1C). The DNA sample digested with HindIII, EcoRI, SmaI, and SacI showed only two bands, signifying the existence of two copies of the gene.
The SbSRP transcript showed higher expression under salt, desiccation, heat, and cold stress in S. brachiata (Figure S3). Zhang et al. (2014) stated that transcriptome data could provide insight into the molecular mechanisms behind the response to salt stress in the physic nut and might provide an asset for the genetic engineering of salt tolerance in crops. These results support the notion that Salicornia shows enhanced abiotic stress tolerance due to the accumulation of transcripts of several novel salt responsive genes, of which SbSRP is one (Jha et al., 2009).
The SbSRP gene sequence showed 80% identity with Beta vulgaris, 71% with Populus trichocarpa, 71% with Populus euphratica, 70% with Ricinus communis, and 69% with Citrus sinensis predicted REF/SRPP-like protein. Transient RFP: SbSRP expression analysis clearly indicated that SRP was preferentially compartmentalized in the plasma membrane (Figure 1). This was further confirmed by in silico TMpred analysis, which showed the presence of one transmembrane domain (Figure S2). To reveal the capacity of an unknown gene, subcellular localization studies are another way to identify the function of the relating protein. The area of the reporter gene in a subcellular compartment, as coordinated by the unknown intertwined protein, frequently provides support for identifying the function and confinement of the gene (Curtis and Grossniklaus, 2003). Crosstalk at the plasma membrane interface is imperative for sessile living beings, such as plants, which are required to keep up a various range of plasma membrane receptor and transport proteins so as to have the capacity to sense and react to changes in their surroundings (Luschnig and Vert, 2014). The present study confirms that SbSRP is a plasma membrane protein, which is in line with in silico studies (Figure 1 and Figure S2). Based on conserved motifs proposed for a special REF/SRPP-like family of uncharacterized proteins in plants, SbSRP is categorized as an SRP-like protein, which is a stress-related protein that may be released into the cytosol during osmotic lysis. The SbSRP gene was transformed and transgenic tobacco plants were developed. Successful integration of the transgene was validated by PCR and DNA gel blot analysis (Figure S4). The semi-quantitative RT-PCR and qRT-PCR showed higher expression of the transgene (Figure S5). Three transgenic lines (L2, L7, and L16) along with control plants (WT and VC) were selected for physio-morpho-biochemical and molecular analysis based on higher gene expression, histochemical GUS expression, and percent seedling growth. Yue et al. (2012) verified that transgenic tobacco plants illustrated higher rates of seed germination and chlorophyll content relative to WT in response to salt stress.
The WT and VC plants did not show any expression of the transgene while the transgenic lines L2, L7, and L16 showed a higher level of SbSRP transcript expression than that of other lines although a comparable amount of cDNA was used (Figure S5). Chlorophyll content was considered as one of the indicators of cellular stress, and it reduces in plants during abiotic stress. In the present study, Chl a, Chl b, total chlorophyll, and carotenoid content were considerably decreased in WT and VC plants under salinity or osmotic stress compared to transgenic lines (Figure 3). The differences between chlorophyll contents may vary while considering relative water contents. Similarly, transgenic plants (tobacco and groundnut) over-expressing SbpAPX and SbASR-1 showed elevated chlorophyll content under oxidative stress provoked by osmotic and salinity stress (Singh et al., 2014a,b; Tiwari et al., 2015b). Plant senescence is characterized by the degradation of cellular components, which leads to the loss of compartmentalization and tissue structure, resulting in plant death (Fan et al., 1997). Transgenic plants could withstand higher salt stress than the wild type in leaf disc assays and pots (Joshi et al., 2013; Singh et al., 2016). Based on this evidence, it is presumed that over-expressed SbSRP may have sheltered the chlorophylls from oxidative damage, and consequently, transgenic lines retain elevated chlorophyll, and carotenoid content. Ramel et al. (2012) reported that carotenoids play a key role in the protection of the photosynthetic machinery by stabilizing thylakoid phospholipids, hence reducing the excited triplet state of chlorophyll and singlet oxygen in salinity and osmotic stress conditions.
The RWC analysis of WT and VC plants and transgenic tobacco lines demonstrated higher relative water content in the transgenic lines than in WT and VC plants under salinity and osmotic stress conditions (Figure 4). Similar results were reported by Jha et al. (2011), Chaturvedi et al. (2014), Patel et al. (2015), Tiwari et al. (2015b), and Udawat et al. (2016), where SbASR-1, SbNHX-1, SbMT-2, and SbUSP over-expressing transgenic tobacco, castor, and groundnut lines showed a lower rate of water loss in salt and drought stress. MDA formation and electrolyte leakage are universal stress indicators that determine the degree of damage caused by stress in plants; stress-induced ROS generation is accountable for these leakages and the accumulation of MDA. MDA is the byproduct of lipid peroxidation due to the production of free radicals during stress, while electrolyte leakage is the release of K+ from ROS-activated cation channels against its counter ions Cl−, , , citrate and malate (Demidchik et al., 2014). The present study reported elevated MDA content and electrolyte leakage in WT and VC plants in comparison to SbSRP over-expressing transgenic lines under salinity and osmotic stress conditions (Figure 4). Conversely, under control conditions, all plant types showed a comparable level of MDA and electrolyte leakage.
The cellular stress marker proline was quantified, which accumulates during osmotic stress to balance the osmotic homeostasis across the membrane. In the present study, the SbSRP over-expressing putative transgenic lines exhibited higher accumulation of free proline than WT and VC plants under salinity and osmotic stress conditions (Figure 5). Analogous results were observed in transgenic tobacco lines over-expressing SbUSP grown under salinity and osmotic stress conditions (Udawat et al., 2016). The accumulation of this amino acid, primarily during salinity and drought stress, is considered to play an important role as an osmoregulatory osmolyte (Delauney and Verma, 1993) and also acts as a signaling molecule (Szabados and Savoure, 2010). Total soluble sugar, reducing sugars, oligo-sugars, and polyphenol content were found to be significantly higher in the transgenic tobacco lines over-expressing the SbSRP gene compared to that of WT and VC plants under salinity and osmotic stress conditions (Figure 5). Tobacco ASR-1 improved the transcript accumulation of sugar transporters, viz hexose transporter, sucrose transporter and vacuolar glucose transporter proteins (Dominguez et al., 2013). Glucose and sucrose are transported from the leaves to the phloem with the help of these transporters, in order to then mobilize the sugars to other plant organs. Based on our experimental results, we hypothesize that SbSRP may regulate several sugar transporter proteins and improve the abiotic stress tolerance in transgenic plants by the mobilization of plant photosynthate to the root or other non-photosynthetic organs.
Fresh leaf segments (of treated and untreated, control, and transgenic plants) were dipped in the freshly prepared DAB and NBT stain solutions, kept in the dark for 2 h followed by exposure to white light for in-vivo localization study. Peroxide and superoxide free radicals were in vivo localized in leaves of WT, VC, and transgenic plants (Figure 6). An almost negligible amount of free radicals (insoluble brown and blue-colored precipitate) was observed under control conditions, whereas under salt and osmotic stress, the leaves from the WT and VC plants exhibited higher accumulation of brown and blue-colored precipitate following DAB and NBT histochemical staining. This observation suggests that there is a greater accumulation of peroxide and superoxide free radicals in WT and VC plants under stress conditions. In agreement with our findings, transgenic tobacco and groundnut lines over-expressing SbpAPX and SbASR-1 also exhibited reduced accumulation of H2O2 content and superoxide-radicals free radicals in leaves grown under salt and drought stress (Singh et al., 2014a,b; Tiwari et al., 2015b). Likewise, leaves of the putative transgenic lines over-expressing SbSRP retained a higher amount of starch (observed as a purple precipitate), even under salinity and osmotic stress conditions, in contrast to WT and VC plant leaves. Parvaiz and Satyawati (2008) reviewed carbohydrates such as sugars (glucose, fructose, sucrose, fructans) and found that starch accumulation under salt stress may play a key role in osmoprotection, osmotic adjustment, carbon storage, and radical scavenging. Baena-Gonzalez et al. (2007) described the role of most the important KIN10 activated genes and their control over the majority of catabolic pathways, including cell wall, starch, sucrose, amino acid, lipid and protein degradation, that supply other sources of energy and metabolites during stress conditions.
To further validate the probable regulatory role of the SbSRP protein, transcript expression of antioxidant-encoding genes Nt-APX (cytosolic ascorbate peroxidase), Nt-CAT (catalase), Nt-SOD (mitochondrial superoxide dismutase), and transcription factor-encoding genes, Nt-DREB and Nt-AP2, were analyzed in WT, VC, and transgenic plants subjected to control conditions, salinity, drought, ABA, SA, heat, and cold stress (Figure 7). The relative increase in Nt-APX, Nt-CAT, Nt-SOD, Nt-DREB, and Nt-AP2 transcript expression was higher in transgenic lines under stress conditions. Liu et al. (2013) reported that Arabidopsis transgenic lines over-expressing LcASR-1 showed elevated transcript expression of CAT, SOD, APX, and glutathione reductase enzyme genes, thus contributing to improve ROS scavenging in transgenic plants.
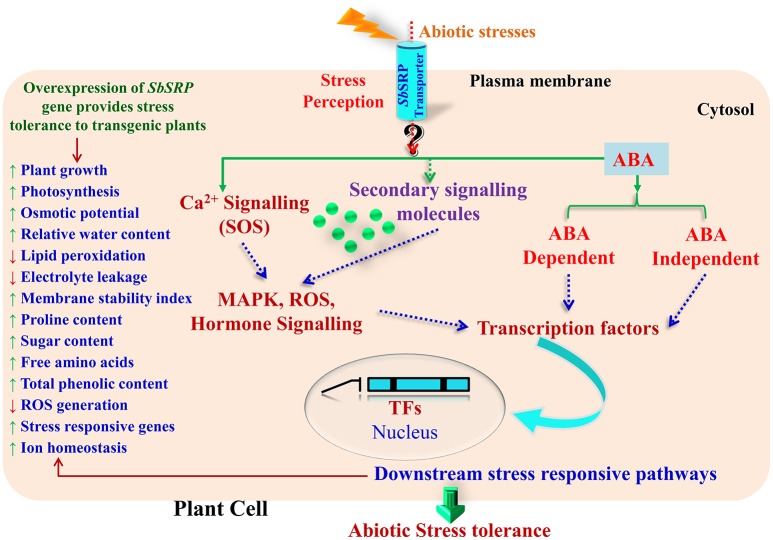
Based on transcript expression analysis, it was concluded that SbSDR-1 and SbUSP genes (cloned from S. brachiata) involve in the transcriptional regulation of antioxidant enzyme-encoding genes (Singh et al., 2016; Udawat et al., 2016). The present study is in agreement with these reports and suggests that SbSRP may be further implicated in ROS scavenging activity directly as a non-enzymatic antioxidant or indirectly by inducing the expression of several genes encoding antioxidative enzymes (Figure 7). Wang et al. (2011) demonstrated that the activity of ascorbate peroxidase (APX) and catalase (CAT), which are two key reactive oxygen species (ROS) detoxifying enzymes, were elevated in transgenic plants exposed to salt stress in comparison to WT plants. The primary job accomplished by APX and CAT is to detoxify the H2O2 into water molecules, which under normal conditions is kept under control. Similar results were found in transgenic tobacco and groundnut lines over-expressing SbASR-1 (Chaturvedi et al., 2014; Tiwari et al., 2015b). The main effect of abiotic stresses on plant growth/productivity is correlated with the communication occurs between organs and each cell types have less or more specific response to the stress. Based on results, it is speculated that membrane-localized SbSRP-like protein may regulate the expression of the antioxidative and transcription factor genes; it may sense environmental stimuli and transmits downward through the plasma membrane (Figure 9). The salt tolerance mechanism shown in this model is a schematic representation of a general salt tolerance mechanism (irrespective of organelle-specific) using previous studies.
Figure 9.
A hypothetical model to infer the putative role of membrane bound SbSRP-like protein in abiotic stress tolerance mechanism. The salt tolerance mechanism shown in this model is a schematic representation of a general salt tolerance mechanism (irrespective of organelle-specific) using previous studies.
Conclusion
In this study, the SbSRP gene was cloned from an extreme halophyte S. brachiata and functionally characterized using transgenic approach. The genome organization study revealed the two homologs of an intronless SbSRP gene. The in silico and in vivo localization study suggest that the SbSRP protein is confined to the plasma membrane. Morphological, biochemical, and physiological analyses of T1 transgenic tobacco lines showed that transgenic plants are more tolerant to salinity and osmotic stress than wild type plants. The higher transcript expression of APX, CAT, SOD, DREB, and AP2 genes were observed in transgenic lines under stress conditions compared to WT and VC plants. The results further support that the SbSRP gene could be used as a potential candidate gene to improve salt and osmotic stress tolerance of crop plants. It is also speculated that SbSRP may function as a transporter protein. However, a detailed study is needed to confirm the exact role of the SbSRP gene in the abiotic stress tolerance mechanism.
Author contributions
Conceived and designed the experiments: AM and BJ. Performed the experiments: PU and RJ. Analyzed the data and Wrote the paper: PU and AM.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
CSIR-CSMCRI Communication No.: PRIS-71/2016. This study was supported by the Council of Scientific and Industrial Research (CSIR; www.csir.res.in), Government of India, New Delhi [BSC0109-SIMPLE; Junior and Senior Research Fellowship to PU]. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. Authors are grateful to Arabidopsis Biological Resource Centre, Ohio State University, USA for providing the pSITE-4CA vector.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.00582/full#supplementary-material
References
- Annan K., Desai N., Egeland J., Huq S., Merkl A., Pachauri R., et al. (2009). Human Impact Report: Climate Change. The Anatomy of a Silent Crisis. Geneva: Global Humanitarian Forum; Available online at: http://www.ghf-ge.org/human-impact-report.pdf (Accessed May11, 2016). [Google Scholar]
- Artimo P., Jonnalagedda M., Arnold K., Baratin D., Csardi G., de Castro E., et al. (2012). ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 40, W597–W603. 10.1093/nar/gks400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Foolad M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59, 206–216. 10.1016/j.envexpbot.2005.12.006 [DOI] [Google Scholar]
- Baena-Gonzalez E., Rolland F., Thevelein J., Sheen J. (2007). A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–943. 10.1038/nature06069 [DOI] [PubMed] [Google Scholar]
- Bates L. S., Waldern R., Teare I. D. (1973). Rapid determination of free proline for water stress studies. Plant Soil 39, 205–207. 10.1007/BF00018060 [DOI] [Google Scholar]
- Bubner B., Gase K., Baldwin I. T. (2004). Twofold differences are the detection limit for determining transgene copy numbers in plants by real time PCR. BMC Biotechnol. 4:14 10.1186/1472-6750-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan D. W., Ward S. M., Lobley A. E., Nugent T. C., Bryson K., Jones D. T. (2010). Protein annotation and modeling servers at university college London. Nucleic Acids Res. 38, W563–W568. 10.1093/nar/gkq427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi A. K., Mishra A., Tiwari V., Jha B. (2012). Cloning and transcript analysis of type 2 metallothionein gene (SbMT-2) from extreme halophyte Salicornia brachiata and its heterologous expression in E. coli. Gene 499, 280–287. 10.1016/j.gene.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Chaturvedi A. K., Patel M. K., Mishra A., Tiwari V., Jha B. (2014). The SbMT-2 gene from a halophyte confers abiotic stress tolerance and modulates ROS scavenging in transgenic tobacco. PLoS ONE 9:e111379. 10.1371/journal.pone.0111379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. (1987). Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159. 10.1016/0003-2697(87)90021-2 [DOI] [PubMed] [Google Scholar]
- Curtis M., Grossniklaus U. (2003). A gateway cloning vector set for high throughput functional analyses of genes in planta. Plant Physiol. 133, 462–469. 10.1104/pp.103.027979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney A., Verma D. P. (1993). Proline biosynthesis and osmoregulation in plants. Plant J. 4, 215–223. 10.1046/j.1365-313X.1993.04020215.x [DOI] [Google Scholar]
- Demidchik V., Straltsova D., Medvedev S. S., Pozhvanov G. A., Sokolik A., Yurin V. (2014). Stress induced electrolyte leakage: the role of K+ permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 65, 1259–1270. 10.1093/jxb/eru004 [DOI] [PubMed] [Google Scholar]
- Dominguez P. G., Frankel N., Mazuch J., Balbo I., Iusem N., Fernie A. R. (2013). ASR1 mediates glucose hormone cross talk by affecting sugar trafficking in tobacco plants. Plant Physiol. 161, 1486–1500. 10.1104/pp.112.208199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper H. H., Hadley M. (1990). Malondialdehyde determination as index of lipid peroxidation. Meth. Enzymol. 186, 421–431. 10.1016/0076-6879(90)86135-I [DOI] [PubMed] [Google Scholar]
- Fan L., Zheng S., Wang X. (1997). Antisense suppression of Phospholipase D retards abscisic acid and ethylene promoted senescence of postharvest Arabidopsis leaves. Plant Cell 9, 2183–2196. 10.1105/tpc.9.12.2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2016). Food and Agriculture: Key to Achieving the 2030, Agenda for Sustainable Development, Job No. I5499. Food and Agriculture Organization of the United Nations, Rome, 23.
- Flowers T. J., Gala H. K., Bromham L. (2010). Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct. Plant Biol. 37, 604–612. 10.1071/FP09269 [DOI] [Google Scholar]
- Giri J., Dansana P., Kothari K., Sharma G., Vij S., Tyagi A. K. (2013). SAPs as novel regulators of abiotic stress response in plants. Bioessays 35, 639–648. 10.1002/bies.201200181 [DOI] [PubMed] [Google Scholar]
- Gu L., Xu D., You T., Li X., Yao S., Chen S., et al. (2011). Analyses of gene expression by ESTs from suppression subtractive hybridization library in Chenopodium album L. under salt stress. Mol. Biol. Rep. 38, 5285–5295. 10.1007/s11033-011-0678-5 [DOI] [PubMed] [Google Scholar]
- He J., Xu H., She X., Song X., Zhao W. (2005). The role and the interrelationship of hydrogen peroxide and nitric oxide in the UV-B-induced stomatal closure in broad bean. Funct. Plant Biol. 32, 237–247. 10.1071/FP04185 [DOI] [PubMed] [Google Scholar]
- Hema R., Vemanna R., Sreeramulu S., Reddy C., Senthil-Kumar M., Udayakumar M. (2014). Stable expression of mtlD gene imparts multiple stress tolerance in Finger millet. PLoS ONE 9:e99110. 10.1371/journal.pone.0099110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch R. B., Fry J. E., Hoffmann N. L., Eichholtz D., Rogers S. G., Farley R. T. (1985). A simple and general method for transferring genes into plants. Science 227, 1229–1231. 10.1126/science.227.4691.1229 [DOI] [PubMed] [Google Scholar]
- Inskeep W. P., Bloom P. R. (1985). Extinction coefficients of chlorophyll a and b in N, N-dimethyl formamide and 80% Acetone. Plant Physiol. 77, 483–485. 10.1104/pp.77.2.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M., Khurana P., Tyagi A. K., Khurana J. P. (2008). Genome-wide analysis of intronless genes in rice and Arabidopsis. Funct. Inegr. Genomics 8, 69–78. 10.1007/s10142-007-0052-9 [DOI] [PubMed] [Google Scholar]
- Jha B., Agarwal P. K., Reddy P. S., Lal S., Sopory S. K., Reddy M. K. (2009). Identification of salt induced genes from Salicornia brachiata, an extreme halophyte through expressed sequence tags analyses. Genes Genet. Syst. 84, 111–112. 10.1266/ggs.84.111 [DOI] [PubMed] [Google Scholar]
- Jha B., Sharma A., Mishra A. (2011). Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol. Biol. Rep. 38, 4823–4832. 10.1007/s11033-010-0625-x [DOI] [PubMed] [Google Scholar]
- Jha B., Singh N. P., Mishra A. (2012). Proteome profiling of seed storage proteins reveals the nutritional potential of Salicornia brachiata Roxb., an extreme halophyte. J. Agric. Food Chem. 60, 4320–4326. 10.1021/jf203632v [DOI] [PubMed] [Google Scholar]
- Jin H., Sun Y., Yang Q., Chao Y., Kang J., Jin H., et al. (2010). Screening of genes induced by salt stress from Alfalfa. Mol. Biol. Rep. 37, 745–753. 10.1007/s11033-009-9590-7 [DOI] [PubMed] [Google Scholar]
- Joshi M., Jha A., Mishra A., Jha B. (2013). Developing transgenic Jatropha using the SbNHX1 gene from an extreme halophyte for cultivation in saline waste land. PLoS ONE 8:e71136 10.1371/journal.pone.0071136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M., Mishra A., Jha B. (2011). Efficient genetic transformation of Jatropha curcas L. by microprojectile bombardment using embryo axes. Ind. Crops Prod. 33, 67–77. 10.1016/j.indcrop.2010.09.002 [DOI] [Google Scholar]
- Joshi M., Mishra A., Jha B. (2012). NaCl plays a key role for in vitro micropropagation of Salicornia brachiata, an extreme halophyte. Ind. Crops Prod. 35, 313–316. 10.1016/j.indcrop.2011.06.024 [DOI] [Google Scholar]
- Knight H., Knight M. R. (2001). Abiotic stress signaling pathways: specificity and cross talk. Trends Plant Sci. 6, 262–267. 10.1016/S1360-1385(01)01946-X [DOI] [PubMed] [Google Scholar]
- Lee K., Kim K., Kim Y., Lee B. H., Lee S. (2012). Identification of MsHsp23 gene using annealing control primer system. Acta Physiol. Plant. 34, 807–811. 10.1007/s11738-011-0853-2 [DOI] [Google Scholar]
- Liu J., Jia C., Dong F., Wang J., Zhang J., Xu Y. (2013). Isolation of an abscisic acid senescence and ripening inducible gene from litchi and functional characterization under water stress. Planta 237, 1025–1036. 10.1007/s00425-012-1820-x [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analyses of relative gene expression data using real time quantitative PCR and the 2(-Delta Delta C(T) method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Luschnig C., Vert G. (2014). The dynamics of plant plasma membrane proteins: PINs and beyond. Development 141, 2924–2938. 10.1242/dev.103424 [DOI] [PubMed] [Google Scholar]
- Lutts S., Kinet J. M., Bouharmont J. (1996). NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 78, 389–398. 10.1006/anbo.1996.0134 [DOI] [Google Scholar]
- Mishra A., Joshi M., Jha B. (2013). Oligosaccharide mass profiling of nutritionally important Salicornia brachiata, an extreme halophyte. Carbohyd. Polym. 92, 1942–1945. 10.1016/j.carbpol.2012.11.055 [DOI] [PubMed] [Google Scholar]
- Mishra A., Patel M. K., Jha B. (2015). Non targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 13, 21–31. 10.1016/j.jff.2014.12.027 [DOI] [Google Scholar]
- Mishra N. K., Chang J., Zhao P. X. (2014). Prediction of membrane transport proteins and their substrate specificities using primary sequence information. PLoS ONE 9:e100278. 10.1371/journal.pone.0100278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P., Tyagi A. K. (2015). OsTCP19 influences developmental and abiotic stress signaling by modulating ABI4-mediated pathways. Sci. Rep. 5:9998 10.1038/srep09998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25, 239–250. 10.1046/j.0016-8025.2001.00808.x [DOI] [PubMed] [Google Scholar]
- Munns R. (2005). Genes and salt tolerance: bringing them together. New Phytol. 167, 645–663. 10.1111/j.1469-8137.2005.01487.x [DOI] [PubMed] [Google Scholar]
- Munns R., Tester M. (2008). Mechanism of salinity tolerance. Ann. Rev. Plant Biol. 59, 651–681. 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- Pandey S., Mishra A., Patel M. K., Jha B. (2013). An efficient method for Agrobacterium-mediated genetic transformation and plant regeneration in cumin (Cuminum cyminum L.). Appl. Biochem. Biotechnol. 171, 1–9. 10.1007/s12010-013-0349-1 [DOI] [PubMed] [Google Scholar]
- Pandey S., Patel M. K., Mishra A., Jha B. (2015). Physio-biochemical composition and untargeted metabolomics of cumin (Cuminum cyminum L.) make it promising functional food and help in mitigating salinity stress. PLoS ONE 10:e0144469. 10.1371/journal.pone.0144469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S., Patel M. K., Mishra A., Jha B. (2016). In planta transformed cumin (Cuminum cyminum L.) plants, overexpressing the SbNHX1 gene showed enhanced salt endurance. PLoS ONE 11:e0159349. 10.1371/journal.pone.0159349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaiz A., Satyawati S. (2008). Salt stress and phyto-biochemical responses of plants- a review. Plant Soil Environ. 54, 89–99. Available online at: http://www.agriculturejournals.cz/web/pse.htm?volume=54&firstPage=89&type=publishedArticle [Google Scholar]
- Patel M. K., Joshi M., Mishra A., Jha B. (2015). Ectopic expression of SbNHX1 gene in transgenic castor (Ricinus communis L.) enhances salt stress by modulating physiological process. Plant Cell Tiss. Organ Cult. 122, 477–490. 10.1007/s11240-015-0785-4 [DOI] [Google Scholar]
- Patel M. K., Mishra A., Jha B. (2016a). Untargeted metabolomics of halophytes, in Marine Omics: Principles and Applications, ed Kim S. (Boca Raton, FL: CRC Press; ), 309–325. 10.1201/9781315372303-18 [DOI] [Google Scholar]
- Patel M. K., Mishra A., Jha B. (2016b). Non-targeted metabolite profiling and scavenging activity unveil the nutraceutical potential of psyllium (Plantago ovata Forsk). Front. Plant Sci. 7:431. 10.3389/fpls.2016.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F., Shinozaki K., Yamaguchi-Shinozaki K. (2011). Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 9, 1569–1582. 10.1093/pcp/pcr106 [DOI] [PubMed] [Google Scholar]
- Ramel F., Birtic S., Cuine S., Triantaphylides C., Ravanat J. L., Havaux M. (2012). Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 158, 1267–1278. 10.1104/pp.111.182394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam R. K. (1994). Effect of homobrassinolide application on metabolism and grain yield under irrigated and moisture stress conditions of two Wheat varieties. J. Plant Growth Reg. 14, 173–181. 10.1007/BF00025220 [DOI] [Google Scholar]
- Schmittgen T. D., Livak K. J. (2008). Analyzing real-time data by the comparative CT method. Nat. Prot. 3, 1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Shabala S. (2013). Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 112, 1209–1221. 10.1093/aob/mct205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S., Mackay A. (2011). Ion transport in halophytes. Adv. Bot. Res. 57, 151–187. 10.1016/B978-0-12-387692-8.00005-9 [DOI] [Google Scholar]
- Shi J., Fu X. Z., Peng T., Huang X. S., Fan Q. J., Liu J. H. (2010). Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 30, 914–922. 10.1093/treephys/tpq030 [DOI] [PubMed] [Google Scholar]
- Singh N., Mishra A., Jha B. (2014a). Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte Salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Mar. Biotechnol. 16, 321–332. 10.1007/s10126-013-9548-6 [DOI] [PubMed] [Google Scholar]
- Singh N., Mishra A., Jha B. (2014b). Ectopic over-expression of peroxisomal ascorbate peroxidase (SbpAPX) gene confers salt stress tolerance in transgenic peanut (Arachis hypogaea). Gene 547, 119–125. 10.1016/j.gene.2014.06.037 [DOI] [PubMed] [Google Scholar]
- Singh V. K., Mishra A., Haque I., Jha B. (2016). A novel transcription factor-like gene SbSDR1 acts as a molecular switch and confers salt and osmotic endurance to transgenic tobacco. Sci. Rep. 6:31686 10.1038/srep31686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados L., Savoure A. (2010). Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97. 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2013). MEGA6: molecular evolutionary genetics analyses using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 30, 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V., Chaturvedi A. K., Mishra A., Jha B. (2014). The transcriptional regulatory mechanism of the peroxisomal ascorbate peroxidase (pAPX) gene cloned from an extreme halophyte, Salicornia brachiata. Plant Cell Physiol. 55, 1774–1471. 10.1093/pcp/pct172 [DOI] [PubMed] [Google Scholar]
- Tiwari V., Chaturvedi A. K., Mishra A., Jha B. (2015a). An efficient method of Agrobacterium mediatedgenetic transformation and regeneration in local Indian cultivar of Groundnut (Arachis hypogaea) using grafting. App. Biochem. Biotechnol. 175, 436–453. 10.1007/s12010-014-1286-3 [DOI] [PubMed] [Google Scholar]
- Tiwari V., Chaturvedi A. K., Mishra A., Jha B. (2015b). Introgression of the SbASR-1 gene cloned from a halophyte Salicornia brachiata enhances salinity and drought endurance in transgenic groundnut (Arachis hypogaea) and acts as a transcription factor. PLoS ONE 10:e0135541 10.1371/journal.pone.0131567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V., Patel M. K., Chaturvedi A. K., Mishra A., Jha B. (2016). Functional characterization of the tau class Glutathione-S-Transferases gene (SbGSTU) promoter of Salicornia brachiata under salinity and osmotic Stress. PLoS ONE 11:e0148494. 10.1371/journal.pone.0148494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N., Gill S. S., Tuteja R. (2011). Plant responses to abiotic stresses: shedding light on salt, drought, cold and heavy metal stress, in Omics and Plant Abiotic Stress Tolerance, eds Tuteja N., Gill S. S., Tuteja R. (Sharjah: Bentham Science Publisher; ), 39–64. [Google Scholar]
- Udawat P., Jha R. K., Sinha D., Mishra A., Jha B. (2016). Overexpression of a cytosolic abiotic stress responsive universal stress protein (SbUSP) mitigates salt and osmotic stress in transgenic tobacco plants. Front. Plant Sci. 7:518. 10.3389/fpls.2016.00518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udawat P., Mishra A., Jha B. (2014). Heterologous expression of an uncharacterized universal stress protein gene (SbUSP) from the extreme halophyte, Salicornia brachiata, which confers salt and osmotic tolerance to E. coli. Gene 536, 163–170. 10.1016/j.gene.2013.11.020 [DOI] [PubMed] [Google Scholar]
- Vinocur B., Altman A. (2005). Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr. Opin. Biotechnol. 16, 123–132. 10.1016/j.copbio.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Wang B., Zhang J., Xia X., Zhang W. H. (2011). Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Reg. 65, 407–413. 10.1007/s10725-011-9595-9 [DOI] [Google Scholar]
- Wang F., Tong W., Zhu H., Kong W., Peng R., Liu Q., et al. (2016). A novel Cys2/His2 zinc finger protein gene from sweetpotato, IbZFP1, is involved in salt and drought tolerance in transgenic Arabidopsis. Planta 243, 783–797. 10.1007/s00425-015-2443-9 [DOI] [PubMed] [Google Scholar]
- Yan H., Zhang W., Lin Y., Dong Q., Peng X., Jiang H., et al. (2014). Different evolutionary patterns among intronless genes in maize genome. Biochem. Biophys. Res. Commun. 20, 146–150. 10.1016/j.bbrc.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Yemm E. W., Willis A. J. (1954). The estimation of carbohydrates in plant extracts by Anthrone. Biochem. J. 57, 508–514. 10.1042/bj0570508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Zhang M., Zhang J., Duan L., Li Z. (2012). SOS1 gene overexpression increased salt tolerance in transgenic tobacco by maintaining a higher K+/Na+ ratio. J. Plant Physiol. 169, 255–261. 10.1016/j.jplph.2011.10.007 [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang C., Wu P., Chen Y., Li M., Jiang H., et al. (2014). Global analyses of gene expression profiles in Physic nut (Jatropha curcas L.) seedlings exposed to salt stress. PLoS ONE 9:e97878. 10.1371/journal.pone.0097878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wang Y., Chang L., Zhang T., An J., Liu Y., et al. (2016). MsZEP, a novel zeaxanthin epoxidase gene from alfalfa (Medicago sativa), confers drought and salt tolerance in transgenic tobacco. Plant Cell Rep. 35, 439–453. 10.1007/s00299-015-1895-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.