Abstract
Few studies have explored the neurobiological basis of insight level in obsessive-compulsive disorder (OCD), though the salience network (SN) has been implicated in insight deficits in schizophrenia. This study was then designed to investigate whether resting-state (rs) functional connectivity (FC) of SN was associated with insight level in OCD patients. We analyzed rs-functional magnetic resonance imaging (fMRI) data from 21 OCD patients with good insight (OCD-GI), 19 OCD patients with poor insight (OCD-PI), and 24 healthy controls (HCs). Seed-based whole-brain FC and ROI (region of interest)-wise connectivity analyses were performed with seeds/ROIs in the bilateral anterior insula (AI) and dorsal anterior cingulate cortex (dACC). The right AI-right medial orbital frontal cortex (mOFC) connectivity was found to be uniquely decreased in the OCD-PI group, and the value of this aberrant connectivity correlated with insight level in OCD patients. In addition, we found that the OCD-GI group had significantly increased right AI-left dACC connectivity within the SN, relative to HCs (overall trend for groups: OCD-GI > OCD-PI > HC). Our findings suggest that abnormal right AI-right mOFC FC may mediate insight deficits in OCD, perhaps due to impaired encoding and integration of self-evaluative information about OCD-related beliefs and behaviors. Our findings indicate a SN connectivity dissociation between OCD-GI and OCD-PI patients and support the notion of considering OCD-GI and OCD-PI as two distinct disorder subtypes.
Keywords: Obsessive-compulsive disorder, Insight, Salience network, Resting-state functional connectivity, Insula
Highlights
-
•
We examined the functional connectivity of SN in OCD-GI and OCD-PI.
-
•
OCD-PI patients had decreased right AI-right mOFC connectivity.
-
•
Right AI- right mOFC connectivity correlated with insight level in OCD.
-
•
OCD-GI patients had elevated right AI-left dACC connectivity within SN.
-
•
These results are helpful toward elucidating insight presentation in OCD.
1. Introduction
Obsessive-compulsive disorder (OCD) is a relatively common mental disorder with a lifetime prevalence of 1–3%; it is characterized by the presence of unwanted, intrusive thoughts termed obsessions and/or repetitive, ritualistic behaviors known as compulsions (DSM-V; APA, 2013). Previously, patients with OCD who did not have the ability to recognize the excessiveness or unreasonableness of their OCD behaviors were classified as having poor insight (DSM-IV; APA, 1994). Because, OCD patients have been described historically as having preserved insight into their symptoms, the notion of poor insight in OCD is a relatively new feature in OCD research relative to, for instance, schizophrenia, wherein poor insight has long been regarded as a hallmark of psychosis.
The portion of patients with OCD who are classified as having poor insight has been estimated to be in the range of 15–36% of the OCD population (Matsunaga et al., 2002, Kishore et al., 2004, Catapano et al., 2010). Poor insight in OCD has been associated with early age of onset (Catapano et al., 2010), intense symptom severity (Storch et al., 2008, Catapano et al., 2010), co-morbidity with schizotypal personality disorder and body dysmorphic disorder (Catapano et al., 2010, Costa et al., 2012), unfavorable responses to behavioral and pharmacological interventions (Erzegovesi et al., 2001, Himle et al., 2006), and poor prognosis (Matsunaga et al., 2002, Catapano et al., 2010). Furthermore, relative to their counterparts with good insight, OCD patients with poor insight have been reported to have more severe neuropsychological deficits in conflict resolution/response inhibition and verbal memory (Tumkaya et al., 2009, Kashyap et al., 2012). These results suggest that poor insight in OCD may represent an important neuropsychological characteristic. If so, understanding insight may be helpful for OCD management.
The neural underpinnings of insight in OCD have not been well elucidated. The only two neuroimaging studies examining this characteristic explicitly pointed to different specific brain regions. Aigner et al. (2005) found evidence for the involvement of the basal ganglia and parietal lobe, whereas Fan et al. (2017) found evidence for involvement of temporal regions. Thus, clarification of the neurobiological basis of insight in OCD is desperately needed.
In recent years, psychiatric disorders have come to be examined extensively on the level of brain-network dysfunction. In particular, analyses of functional connectivity (FC) during a resting-state (rs) within brain networks (intra-rsFC) or between different networks (inter-rsFC) have gained favor as methods for clarifying the phenomenology of mental illness (Shin et al., 2014). Notably, emerging recent evidence has linked intra- and inter-rsFC of the salience network (SN) to insight in psychosis and schizophrenia (Lena Palaniyappan et al., 2012, Raij et al., 2016).
The SN, a stable core brain network, is a large-scale paralimbic-limbic system anchored to the anterior insula (AI) and dorsal anterior cingulated cortex (dACC). It has been shown to be involved in detecting, processing, and integrating internal and external salient information (Sridharan et al., 2008, Menon, 2011). Besides the intra network function, SN was also found to play a crucial role in controlling interactions between task-negative (usually known as default mode network [DMN]) and task-positive (usually known as central executive network [CEN]) networks, which was achieved by initiating transient control signals that engage the CEN to mediate cognitive control processes while disengaging the DMN (Menon and Uddin, 2010, Menon, 2011). SN-related intra- and inter-rsFC alternations have been associated with psychosis in several psychiatric disorders, including schizophrenia, depression, and bipolar disorder (Skaf et al., 2002, Ellison-Wright and Bullmore, 2010, Manoliu et al., 2013, Wotruba et al., 2013).
To examine psychopathology insight directly, Raij et al. (2016) designed a clinical insight task wherein participants evaluate statements about insight content, such as “whether an individual's psychosis-related experiences should be described as a mental illness”. They found that SN activation was related to evaluation of insight-related questions in first-episode psychosis patients. van der Meer et al. (2010) also found that AI activation was associated with clinical insight in schizophrenic patients. Though not found in OCD cohorts, these results suggest that SN connectivity data could be helpful for understanding the neural underpinnings of insight in OCD.
To the best of our knowledge, no prior study has investigated the relationship between SN connectivity and insight in OCD directly. The a few studies that have explored FC of the SN in OCD patients have yielded inconsistent results (Stern et al., 2011, Stern et al., 2012, Weber et al., 2014, Posner et al., 2016, Matsumoto et al., 2010, Song et al., 2011a, Tan et al., 2013, de Wit et al., 2014). These inconsistencies may be related, perhaps in part, to methodological differences. Additionally, given the evidence implicating SN connectivity in psychopathology insight, we supposed that the lack of consideration of the participants' insight presentation may help explain the aforementioned discrepant findings. Thus, we hypothesized that patients with OCD with differing insight levels might differ with respect to the involvement of intra- and inter-rsFCs of the SN in OCD.
The aim of the present study was to investigate whether intra- and/or the inter-rsFC of the SN may underlie insight presence in OCD. To explore specific alterations in intra- and inter-rsFC of the SN that may distinguish between OCD patients with poor insight (OCD-PI) versus those with good insight (OCD-GI), we compared seed-based whole-brain FC of the SN—using the regions of interest (ROIs) of the AI and dACC as seeds—and ROI-wise connectivity within the SN among OCD-GI, OCD-PI, and HC groups. The bilateral AI and bilateral dACC were selected as ROIs because they exhibit stable, reliable SN properties (Pannekoek et al., 2014, Posner et al., 2016). Subsequently, to identify core connectivity changes that could account for degree of insight in OCD, we examined whether the insight levels of patients with OCD correlated with altered FCs involving the SN. Given the exploratory nature of this preliminary study, we chose an unbiased approach with no a priori hypothesis regarding the specific FC pathways that may be associated with insight in OCD.
2. Materials and methods
2.1. Participants
Forty-four patients fulfilled the DSM-IV criteria for OCD participated in the current study. Twenty-two of them were OCD-GI, and the other 22 were OCD-PI.
All the patients were recruited from the psychological clinic at Second Xiangya Hospital of Central South University. The diagnoses of OCD and comorbidity of axis I psychiatric disorder were established by an experienced psychiatrist according to the Structured Clinical Interview for the DSM-IV (SCID), wherein insight quality was also rated and according to which the patients were dichotomized into OCD-GI and OCD-PI groups. The exclusion criteria were: (1) any axis I psychiatric disorder comorbidity, such as schizophrenia, schizoaffective disorder, major depression disorder, bipolar disorder, autism spectrum disorder, drug dependence and eating disorders; (2) a history of major medical or neurological problems (e.g., hypothyroidism, seizure disorder, or brain injury). To control for potential medication effects on our results, only patients who were drug-naïve (19 patients) or did not take psychotropic medications for a minimum of 3 months at the time of enrollment were recruited.
Twenty-five age- and gender-matched students or staff members at Central South University were recruited to form the HC group. The exclusion criteria for HCs were: (1) a history of any psychiatric illnesses; and (2) any major medical or neurological problems.
All participants were right-handed, 16–35 years of age, with ≥ 9 years of formal education. This study was approved by Ethics Committee of the Second Xiangya Hospital of Central South University and all the subjects signed written consent forms before they participated in the study.
2.2. Clinical assessments and verification of OCD-GI vs. OCD-PI classification
After being diagnosed, each participant then underwent a semi-structured interview conducted by an experienced research psychiatrist. During the interview, demographic data and information related to clinical variables were recorded; handedness was classified using the Edinburgh Handedness Inventory (EHI; Oldfield, 1971); and general intelligence was evaluated with the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). All of the participants completed the Beck Depression inventory (BDI; Beck et al., 1961) and the State Trait Anxiety Inventory (STAI; Spielberger et al., 1983) to determine their depression and anxiety levels. OCD severity was assessed with the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Goodman et al., 1989).
Verification of OCD-GI vs. OCD-PI classification was based on the Brown Assessment of Beliefs Scale (BABS; Eisen et al., 1998). The BABS is a clinician-administrated 7-item scale that was developed to assess insight across a variety of psychiatric disorders. The Chinese version of the BABS has been confirmed to have good reliability and validity (Niu et al., 2016). Specific probes of this scale included conviction, perception of other's views or beliefs, explanation of differing views, fixity of ideas, attempts to disprove beliefs, insight, and ideas/delusions of reference. In the BABS, each item is rated on a scale ranging from 0 (non-delusional or least pathological) to 4 (delusional or most pathological), and scores for the first six items are summed to create a total score (range: 0 to 24). Previous research has demonstrated the utility of the BABS in classifying individuals into OCD-GI vs. OCD-PI subgroups (Eisen et al., 2001, Kishore et al., 2004, Catapano et al., 2010) wherein poor insight can be indicated by a total BABS score ≥ 12 and a score ≥ 3 for the conviction item (fairly or completely convinced that belief/worry is true). The research psychiatrist, blinded to the patients' prior classification, rated the BABS for each patient. Notably, our BABS ratings were fully consistent with all 44 of the patients' prior classifications (kappa = 1.00).
2.3. Scan acquisition
Imaging data were acquired on a Siemens Skyra 3-T magnetic resonance scanner at the Second Xiangya Hospital of Central South University. All participants were instructed to lie supine with their closed eyes, to remain still, and to think of nothing in particular but to avoid falling asleep. Their heads were fixed snugly with foam pads and straps to minimize head movement. We collected resting-state fMRI series using an echoplanar imaging sequence with the following parameters: 39 axial slices, 3.5-mm slice thickness, no gap, 2500-ms repetition time (TR), 25-ms echo time (TE), 3.8 × 3.8 × 3.5-mm voxel size, 90° flip angle, 240-mm field of view, 64 × 64 data matrix, and 200 volumes. In addition, three-dimensional T1-weighted, magnetization-prepared rapid gradient echo (MPRAGE) sagittal images were acquired with the follow parameters: 176 slices, 1900-ms TR, 2.01-ms TE, 1.00-mm slice thickness, 1.0 × 1.0 × 1.0-mm voxel size, 9° flip angle, 900-ms inversion time, 256-mm field of view, and 256 × 256 matrix.
2.4. Image preprocessing
Images were processed in Data Processing Assistant for Resting-State fMRI software (DPARSF V2.3; Yan and Zang, 2010, http://www.restfmri.net). After discarding of the first 10 volumes of each functional time series, slice timing correction, and realignment of head motion, five subjects (1 OCD-GI, 3 OCD-PI, and 1 HC) were excluded from the data analysis for translation > 1 mm in any direction or rotation > 1° around any axis in six head motion parameters. Then, linear spatial normalization to the Montreal Neurological Institute (MNI) atlas space was conducted for each participant. Each participant's T1-weighted MPRAGE images were co-registered to the mean functional image after head motion correction, and then segmented into gray matter, white matter, and cerebrospinal fluid according to a unified segmentation algorithm (Ashburner and Friston, 2005). Parameters estimated in segmentation were used to normalize motion-corrected functional volumes onto MNI space, with resampling to a voxel size of 3 × 3 × 3 mm. After normalization, images were smoothed using a Gaussian kernel of 8 × 8 × 8 mm full-width at half maximum and then processed by linear detrending. Nuisance covariates, including six head motion parameters, white matter signal, and cerebrospinal fluid signal, were regressed out. Finally, temporal band-pass filtering (0.01–0.08 Hz) was performed.
2.5. Seed selection and functional connectivity analysis
Based on previous research, the classical four SN ROIs (bilateral AI and bilateral dACC) were selected (Pannekoek et al., 2014, Posner et al., 2016). ROIs were defined as spheres with 6-mm radius centered on the following coordinates: bilateral AI (− 45, 5, 9; 45, 3, 15; MNI) and bilateral dACC (± 6, 18, 28; MNI) (Pannekoek et al., 2014, Posner et al., 2016). To better elaborate the intra- and inter-rsFCs of the SN, we calculated both ROI-based whole-brain voxel-wise connectivity and the ROI-wise connectivity (Jung et al., 2013). Whole-brain rsFC maps were obtained for all four seed regions by DPARSF. ROI-wise connectivity was measured by extracting average time courses for each ROI, calculating the correlation coefficients (CCs) between each ROI pair, and then applying the Fisher z transformation.
2.6. Statistical analysis
Differences of demographic and clinical variables were evaluated with chi-square tests, two-sample t-tests, and one-way analyses of variance (ANOVAs). Pearson correlations were used to evaluate relationships between insight level (BABS score) and other clinical variables (e.g., YBOCS score, BDI score, STAI score, illness duration, and age of onset).
For the FC maps (seed-based whole brain voxel-wise connectivity), a one-way ANOVA was firstly used to compare rsFC differences across the three groups for each seed/ROI. Subsequently, post hoc t-tests were performed to examine specific differences between each group pair by applying the ANOVA results as a mask. Significance thresholds for ANOVAs and post hoc t-tests were set at p < 0.05 with AlphaSim corrected. Age, gender, educational level, and general intelligence were controlled as covariates for all the ANOVAs and post hoc analyses, while for the post hoc t-test between OCD-GI and OCD-PI, an additional covariate of illness severity (YBOCS scores) was controlled since a significant correlation between BABS scores and YBOCS scores was detected (see details in Results section). These statistical analyses were carried out with the Resting-State fMRI Data Analysis Toolkit (REST V1.8; Song et al., 2011b, http://www.restfmri.net). Notably, for each AlphaSim correction, the individual voxel threshold was set at p < 0.005, and before computing each corresponding threshold of cluster size, the amount of Gaussian smoothing was re-estimated (see Table S1).
Analyses of ROI-wise connectivity were performed in SPSS 17.0. All Z values indicating ROI-pair correlations were each submitted to a one-way ANOVA. If a main group effect was detected (p < 0.05), post hoc t-tests were conducted to identify the specific group differences and Jonckheere-Terpstra tests were used, if necessary, to explore whether there were trends across the three groups; both these two types of tests were conducted with a Bonferroni adjustment for p values (< 0.05).
After identifying FCs that differed between the two OCD groups via statistical analysis of FC maps and ROI-wise connectivity, correlation analysis were carried out to examine whether these abnormal FCs were related to insight level as indicated by BABS score in OCD patients.
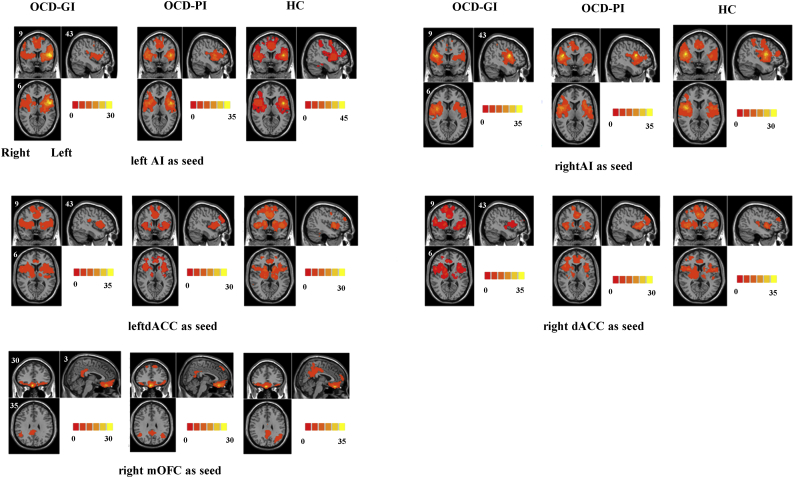
In addition, to verify that the 4 ROIs selected (bilateral AI and bilateral dACC) can reliably identify the SN, we also analyzed the FC spatial pattern starting from each ROI for each group (see details in Supplementary material).
3. Results
3.1. Demographic and clinical variables
The demographic and clinical characteristics of the participants are summarized in Table 1. The three groups were similar in terms of age, gender, education, and general intelligence. STAI-T, STAI-S, and BDI scores differed among the three groups, with both patient groups demonstrating higher scores than HCs on all three measures, but similar scores to each other. The OCD-GI and OCD-PI groups were also similar to each other in terms of age of onset, illness duration, and OCD severity; as expected, BABS scores were higher (indicating poorer insight) in the OCD-PI group than in the OCD-GI group. Among all of the clinical variables examined, only insight level correlated with OCD symptom severity (Table 2).
Table 1.
Demographic and clinical characteristics of OCD-GI, OCD-PI and HC groups.
| Characteristics | OCD-GI (N = 21) | OCD-PI (N = 19) | HC (N = 24) | F/t/χ2 | p |
|---|---|---|---|---|---|
| Age (years) | 22.19 (5.64) | 23.58 (5.65) | 21.92 (2.21) | 0.74 | 0.479 |
| Gender (male, %) | 14 (66.7) | 12 (63.2) | 9 (37.5) | 4.627 | 0.099 |
| General intelligence | 117.38(13.96) | 113.16(13.22) | 119.71(9.58) | 1.53 | 0.224 |
| Education (years) | 13.52 (2.29) | 14.55 (3.47) | 15.25 (1.39) | 2.79 | 0.069 |
| Age onset | 18.74 (4.06) | 18.95(4.03) | – | − 0.16 | 0.871 |
| Duration(months) | 45.00 (61.09) | 53.95 (60.39) | – | − 0.47 | 0.644 |
| STAI-T | 55.86 (8.52) | 56.79 (7.95) | 40.67 (6.59) | 31.23 | < 0.001 |
| STAI-S | 50.67 (10.73) | 54.16 (11.92) | 41.00 (6.95) | 10.10 | < 0.001 |
| BDI | 16.86 (9.12) | 18.79 (9.24) | 5.91 (4.93) | 16.80 | < 0.001 |
| Y-BOCS | 30.71 (5.19) | 32.21 (6.11) | – | − 0.84 | 0.407 |
| BABS | 8.24 (3.86) | 15.32 (2.77) | – | − 6.62 | < 0.001 |
Means with standard deviations in parentheses.
OCD-GI, OCD with good insight; OCD-PI, OCD with poor insight; HC, healthy control; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale; STAI-T, Spielberger State-Trait Anxiety Inventory-Trait Form; STAI-S, Spielberger State-Trait Anxiety Inventory-State Form; BDI, Beck Depression Inventory; BABS, Brown Assessment of Beliefs Scale.
F/t/χ2: variables of age, education, STAI-T, STAI-S, and BDI were tested by one-way ANOVAs (results were indicated by F); Categorical data such as gender was tested using chi-squared tests (results were indicated by χ2), and variables such as age onset, duration, YBOCS and BABS were statistically tested by two-sample t-test (results were indicated by t); p, statistical significance, significant at p < 0.05.
Significant post hoc tests: STAI-T: OCD-GI > HC (p < 0.001), OCD-PI > HC (p < 0.001); STAI-S: OCD-GI > HC (p < 0.01), OCD-PI > HC (p < 0.001); BDI: OCD-GI > HC (p < 0.001), OCD-PI > HC (p < 0.001).
Table 2.
Correlations between insight level and other clinical variables in OCD patients.
| YBOCS |
STAI-T |
STAI-S |
BDI |
Age onset |
Duration |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| BABS | 0.30 | 0.031 | 0.01 | 0.923 | 0.21 | 0.136 | 0.17 | 0.226 | 0.004 | 0.976 | 0.04 | 0.782 |
Y-BOCS, Yale-Brown Obsessive-Compulsive Scale; STAI-T, Spielberger State-Trait Anxiety Inventory-Trait Form; STAI-S, Spielberger State-Trait Anxiety Inventory-State Form; BDI, Beck Depression Inventory; BABS, Brown Assessment of Beliefs Scale.
r, Pearson's correlation coefficient; p, statistical significance, significant at p < 0.05.
3.2. Seed-based whole-brain FC
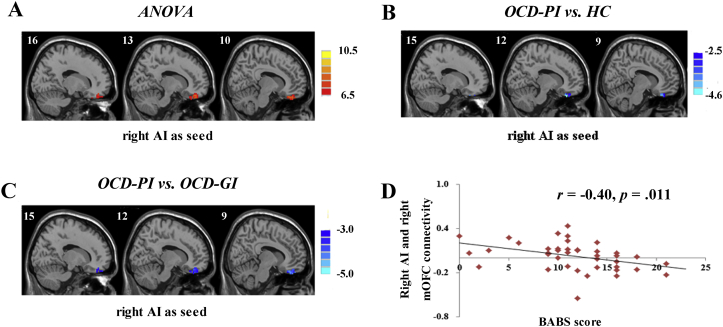
For the right AI seed, a one-way ANOVA revealed group differences in FC with the right medial OFC (mOFC), which were driven by reduced connectivity in the OCD-PI group compared with that in the OCD-GI and HC groups (p < 0.05, AlphaSim correction; see details in Table 3, Fig. 1A, B, C). No significant group differences in whole-brain FC were detected with the left AI, right dACC, and left dACC as seeds.
Table 3.
Brain regions showing functional connectivity differences based on salience network seeds among OCD-GI, OCD-PI and HC.
| Brain regions | Voxel | Peak coordinates (x/y/z; MNI) | Peak F/T values | Effect sizea | ||
|---|---|---|---|---|---|---|
| Right AI as seed | ||||||
| ANOVA | ||||||
| Right Frontal_Sup_Orb (BA11) | 59 | 9 | 36 | − 27 | 9.220 | 0.23 |
| Post hoc t-tests | ||||||
| OCD-PI < HC | ||||||
| Right Frontal_Sup_Orb (BA11) | 23 | 12 | 33 | − 30 | − 4.5436 | − 1.48 |
| OCD-PI < OCD-GI | ||||||
| Right Frontal_Sup_Orb (BA11) | 51 | 6 | 36 | − 27 | − 4.7404 | − 1.54 |
OCD-GI, patients with obsessive-compulsive disorder with good insight; OCD-PI, patients with obsessive-compulsive disorder with poor insight; HC: healthy control; BA, Broadmann area; x, y, z, coordinates of peak locations in the Montreal Neurological Institute space (MNI). p < 0.05, Alphasim correction.
Results of ANOVA were indicated by peak F values and effect size of which was indexed byηp2; results of post hoc t-tests were indicated by peak T values and effect size of which was indexed by Cohen's d.
Fig. 1.
(A) Statistic maps showing ANOVA results of functional connectivity differences among OCD patients with good insight (OCD-GI), OCD patients with poor insight (OCD-PI) and healthy controls (HC) with seed in right anterior insula (AI). (B) Post hoc t-test revealed decreased right AI and right medial orbital frontal cortex (mOFC) connectivity in OCD-PI as compared with HC. (C) Post-hoc t-test revealed decreased right AI and right mOFC connectivity in OCD-PI as compared with OCD-GI. (D) Scatter plots demonstrating significant negative correlations between BABS scores and functional connectivity of right AI and right mOFC in OCD patients (p < 0.05). Age, gender, educational level, and general intelligence were controlled as covariates for the ANOVA and post hoc t-tests. For the post hoc t-test between OCD-GI and OCD-PI, an additional covariate of illness severity (YBOCS scores) was controlled. Two-sample t-test results are expressed within a mask showing significant group differences from the ANOVA. Red and blue denote connectivity increases and decreases, respectively, and color bars indicate F/t values. p < 0.05, AlphaSim corrected. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. ROI-wise FC within the SN
In terms of the ROI-wise connectivity, a main effect of group was found only for the right AI-left dACC region pair (F2, 61 = 3.641, p < 0.05, uncorrected, ηp2 = 0.11). Specifically, the OCD-GI group had greater rsFC (0.49 ± 0.24) of the right AI and left dACC than the HC group (0.29 ± 0.26, p < 0.05, Cohen's d = 0.80, Bonferroni corrected; see details in Table 4). Although significant differences between the OCD-PI and HC groups and between the OCD-GI and OCD-PI groups were not detected with post hoc t-tests, the Jonckheere-Terpstra test indicated that there was a significant OCD-GI > OCD-PI > HC trend for the FC of the right AI and left dACC (Std. J-T Statistic = − 2.52, p = 0.012).
Table 4.
ROI-wise functional connectivity differences within salience network among OCD-GI, OCD-PI and HC groups.
| ROI wise FC | OCD-GI (N = 21) | OCD-PI (N = 19) | HC (N = 24) | F | p | ηp2 |
|---|---|---|---|---|---|---|
| Left AI - right AI | 0.58 (0.29) | 0.59 (0.15) | 0.56 (0.22) | 0.07 | 0.929 | – |
| Left AI - left dACC | 0.60 (0.25) | 0.53 (0.21) | 0.47 (0.15) | 2.46 | 0.094 | – |
| Left AI - right dACC | 0.58(0.26) | 0.50(0.22) | 0.44(0.13) | 2.61 | 0.081 | – |
| Right AI - left dACC | 0.49 (0.24) | 0.42 (0.25) | 0.29 (0.26) | 3.64 | 0.032 | 0.11 |
| Right AI - right dACC | 0.47 (0.23) | 0.40(0.24) | 0.33(0.19) | 2.24 | 0.115 | – |
| Left dACC - right dACC | 1.33 (0.35) | 1.39 (0.30) | 1.26(0.29) | 0.95 | 0.391 | – |
Means with standard deviations in parentheses.
F: variables of ROI wise FCs were tested by one-way ANOVAs (results were indicated by F); p, statistical significance.
Significant post hoc tests: right AI-left dACC: OCD-GI > HC (p < 0.05; Bonferroni correction, Cohen's d = 0.80).
3.4. Brain-behavior associations
As shown in Fig. 1D, the extracted FC values of clusters with significant differences between the OCD-GI and OCD-PI groups (here, right AI seed to right mOFC) were significantly correlated with insight level in OCD patients (r = − 0.40, p = 0.011).
4. Discussion
This study explored the neural correlates of insight in OCD by using both the seed-based whole-brain FC analysis and the ROI-wise connectivity. We found that FC of the right AI to the right mOFC was decreased in the OCD-PI group, relative to both HCs and OCD-GI. The FC value for this aberrant connectivity correlated with insight level in OCD patients. In addition, ROI-wise FC analysis revealed that the OCD-GI group had significantly increased FC of the right AI and left dACC than HCs, with a OCD-GI > OCD-PI > HC trend also stood. These data suggest that connectivity between the right AI and right mOFC may be an important neural correlate of insight in OCD. Furthermore, these findings provide evidence of a dissociation of impaired intra- versus inter-FC of the SN in OCD-GI and OCD-PI patients and support the notion that insight presentation should be considered in the delineation of the clinical heterogeneity of OCD.
The most outstanding findings of the current study were our identification of right AI-right mOFC connectivity as being abnormal specifically in OCD-PI patients, but not OCD-GI patients, and our finding of a correlation between this connectivity and insight level in OCD patients. Although the SN participates in many diverse functions (e.g., salience detection, attention control, and switching between large-scale networks), the two core hubs of SN, that is, the AI and dACC, appear to have quite different focuses. Unlike dACC, which focuses more on modulating responses in sensory, motor, and association cortices, the AI has been suggested to play a prominent role in information integration—receiving and integrating information about both internal and external sensations, representations of goals and plans, and stimulus-independent thoughts, to update expectations or enable action to be initiated or modified—as well as a role in salience detection (Averbeck and Seo, 2008, Craig, 2009, Menon and Uddin, 2010, Lena Palaniyappan et al., 2012). Interestingly, the mOFC (part of the vmPFC) has consistently been thought to play an important role in emotional valuation (Grabenhorst and Rolls, 2011), assigning subjective value not only to explicit rewards (e.g. food, money), but also to visual objects, faces, personal goals, and belongings, as well as to aspects of self-reflection (judgments of one's own traits) (Murray et al., 2012, van der Meer et al., 2010, Kim et al., 2007, Hare et al., 2009, Lebreton et al., 2009, D'Argembeau, 2013). By assigning, evaluating, and representing these values, the mOFC can then play an important role in forming personal preferences, self-image, and guiding one's choices and decisions. Altered AI-mOFC connectivity in the present study could impair encoding and integration of such value information; thus, our results suggest that patients with OCD-PI may have difficulty with integrating value information regarding their OCD behaviors, which may prevent them from realizing that their feared outcomes are unrealistic or that their compulsions are unlikely to produce outcomes of true value, thereby producing the characteristic of poor insight.
It should be noted that structural abnormality of the right mOFC (Shad et al., 2006) and impaired insula-vmPFC rsFC (Gerretsen et al., 2014) were implicated previously in insight level in schizophrenic patients. Additionally, Ćurčić-Blake et al. (2015) found that altered insula-vmPFC connectivity during a self-reflection task was associated with insight level in schizophrenic patients, though the correlation did not survive FDR correction (p = 0.055). Our findings may be consistent with these results to some extent, at least in terms of supporting the importance of AI-mOFC connectivity in insight capacity. In addition, although the OFC has been recognized as a consistently altered brain region in the pathophysiology of OCD, a meta-analysis demonstrated a high level of heterogeneity for the right OFC in OCD (Rotge et al., 2009). Interestingly, this heterogeneity of the right OFC could be associated with OCD refractoriness (Atmaca et al., 2006). The previously established association of the OCD-PI designation with a less favorable responsivity to behavioral and pharmacological interventions (Erzegovesi et al., 2001, Himle et al., 2006) raises the possibility of an overlap between OCD-PI and so-called refractory OCD. The persistent OFC alteration in refractory patients might be in line with our present finding of specific abnormal right AI-right mOFC connectivity impairment in OCD-PI to some extent.
Further, the connectivity between right AI and right mOFC may have implication from a network level. Specifically, the OFC is a complex and heterogeneous brain region that encompasses subregions with differing functional domains and involvement in different brain networks. For example, the mOFC, related to OCD insight in the present study, is part of the ventral medial prefrontal cortex (vmPFC). The vmPFC, which is part of the DMN, is distinct from the lateral OFC, which is considered to be involved with the CEN (Andrews-Hanna et al., 2010, Stern et al., 2011, Göttlich et al., 2014). It's also important to note that our results of FC spatial patterns starting from the mOFC cluster were largely in line with the previously established templates of DMN (see details in Supplementary material). Thus, these may suggest that our AI-mOFC connectivity results reflect a potential role of impaired SN-DMN interactions in insight level in OCD. Several prior studies have demonstrated altered SN-DMN connectivity in OCD patients. However, the specific connections found to be abnormal and the direction of the abnormality (increased vs. decreased) differed across those studies (Posner et al., 2016, Beucke et al., 2014, Stern et al., 2012). Our finding of an SN-DMN connectivity abnormality in only OCD-PI patients may be consistent with the possibility that the failure to account for insight could help explain, at least in part, the previous discrepancies.
In addition, our ROI-wise FC analysis also revealed aberrant connectivity within the SN in OCD patients. Specifically, we found that right AI-left dACC connectivity within the SN was elevated in OCD-GI patients, relative to that in HCs, together with a OCD-GI > OCD-PI > HC trend. The AI and ACC both contain an unusual specialized class of neurons, known as von Economo neurons, which have distinctive anatomical and functional features that enable them to facilitate rapid signaling between these two brain regions and with other areas (Allman et al., 2010). The AI-ACC connectivity makes bottom-up attention and salience detection (which were mainly sub-served by AI), and subsequent top-down cognitive control and motor responses (which were mainly sub-served by ACC) coherent and possible (Menon and Uddin, 2010). Thus, increased AI-dACC connectivity may explain, at least in part, why certain triggers can initiate particularly more frequent/severe response consequences in OCD. Also, from a clinical aspect, with more uncertainty and doubts about their OC beliefs, the OCD-GI may have more struggles in whether performing obsessions and especially compulsions, which may be in line with their more enhanced AI and ACC connectivity. However, given that the ANOVA p value related to this finding did not survive a Bonferroni correction, this result should be treated with caution and should be re-examined.
Several limitations should be considered when interpreting the results of the present study. First, rsFC analysis cannot detect causal interactions between brain regions. Previous structural neuroimaging studies revealed reciprocal insula-OFC projections (Augustine, 1985). Thus, though we hypothesized that reduced AI-mOFC connectivity may underlie poor insight due to the AI having an impaired ability to integrate subjective value information from the mOFC, we cannot rule out the possibility that this abnormal connectivity might prevent signaling of the mOFC to generate value information after saliency has been detected. Second, our explanations of the results were limited to being speculative to some extent. We cannot specify the behavioral significance of the aberrant rsFC with any certainty. Thus, though rsFC analysis is a promising, relatively simple tool, more thoroughly developed behavioral indexes and fMRI paradigms with task activation (e.g., a valuation task) should be employed to examine our hypothesis. Third, the relatively small sample size may limit the interpretation of our findings. Studies with larger sample sizes and more detailed neurocognitive domain assessments are needed to examine these results and to clarify the specific mechanisms mediating insight in OCD.
5. Conclusion
In conclusion, we found that the impaired right AI-right mOFC connectivity may be involved in insight level in OCD patients. It is possible that altered AI-mOFC connectivity affects valuation in a manner that results in patients with OCD-PI failing to integrate subjective value information regarding their OCD-related beliefs and symptoms. Moreover, this aberrant connectivity may implicate altered communication between SN and DMN.
The following are the supplementary data related to this article.
Fig. S1.
The respective spatial pattern for each group starting from each ROI revealed by the one-sample t-test. p < 0.05, family-wise error corrected. OCD-GI, OCD with good insight; OCD-PI, OCD with poor insight; HC, healthy control; AI, anterior insula; dACC, dorsal anterior cingulate cortex; mOFC, medial orbital frontal cortex.
Supplementary material
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was supported by National Natural Science Foundation of China (Mingtian Zhong, grant number 81371487).
Acknowledgement
We are grateful for the generosity of time and effort by all the participants, and all researchers who make this project possible.
Contributor Information
Jinyao Yi, Email: jinyaoyi2001@163.com.
Changlian Tan, Email: tanchanglianxy@aliyun.com.
References
- Aigner M., Zitterl W., Prayer D., Demal U., Bach M., Prayer L., Stompe T., Lenz G. Magnetic resonance imaging in patients with obsessive–compulsive disorder with good versus poor insight. Psychiatry Res. 2005;140(2):173–179. doi: 10.1016/j.pscychresns.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Allman J.M., Tetreault N.A., Hakeem A.Y., Manaye K.F., Semendeferi K., Erwin J.M., Park S., Goubert V., Hof P.R. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain. Struct. Func. 2010;214(5–6):495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Fouth ed. American Psychiatric Association; Washington, DC (Text Revision): 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. (Text Revision) [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Atmaca M., Yildirimb H., Ozdemirb H., Aydinb A., Tezcana E., Ozlera S. Volumetric MRI assessment of brain regions in patients with refractory obsessive–compulsive disorder. Progress. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(6):1051–1057. doi: 10.1016/j.pnpbp.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Augustine J.R. The insular lobe in primates including humans. Neurol. Res. 1985;7(1):2–10. doi: 10.1080/01616412.1985.11739692. [DOI] [PubMed] [Google Scholar]
- Averbeck B.B., Seo M. The statistical neuroanatomy of frontal networks in the macaque. PLoS Comput. Biol. 2008;4(4) doi: 10.1371/journal.pcbi.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beucke J.C., Sepulcre J., Eldaief M.C., Sebold M., Kathmann N., Kaufmann C. Default mode network subsystem alterations in obsessive-compulsive disorder. Br. J. Psychiatry. 2014;205(5):376–382. doi: 10.1192/bjp.bp.113.137380. [DOI] [PubMed] [Google Scholar]
- Catapano F., Perris F., Fabrazzo M., Cioffi V., Giacco D., De Santis V., Maj M. Obsessive–compulsive disorder with poor insight: a three-year prospective study. Progress. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(2):323–330. doi: 10.1016/j.pnpbp.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Costa C., Lucas D., Chagas Assunção M., Arzeno Ferrão Y., Archetti Conrado L., Hajaj Gonzalez C., Fontenelle F.L., Fossaluza V., Constantino Miguel E., Rodrigues Torres A., Gedanke Shavitt R. Body dysmorphic disorder in patients with obsessive–compulsive disorder: prevalence and clinical correlates. Depress. Anxiety. 2012;29(11):966–975. doi: 10.1002/da.21980. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Ćurčić-Blake B., van der Meer L., Pijnenborg G.H., David A.S., Aleman A. Insight and psychosis: functional and anatomical brain connectivity and self-reflection in schizophrenia. Hum. Brain Mapp. 2015;36(12):4859–4868. doi: 10.1002/hbm.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front. Hum. Neurosci. 2013;10:372. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen J.L., Phillips K.A., Baer L., Beer D.A., Atala K.D., Rasmussen S.A. The brown assessment of beliefs scale: reliability and validity. Am. J. Psychiatry. 1998;155(1):102–108. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- Eisen J.L., Rasmussen S.A., Phillips K.A., Price L.H., Davidson J., Lydiard R.B., Ninan P., Piggott T. Insight and treatment outcome in obsessive-compulsive disorder. Compr. Psychiatry. 2001;42(6):494–497. doi: 10.1053/comp.2001.27898. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I., Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr. Res. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Erzegovesi S., Cavallini M.C., Cavedini P., Diaferia G., Locatelli M., Bellodi L. Clinical predictors of drug response in obsessive-compulsive disorder. J. Clin. Psychopharmacol. 2001;21(5):488–492. doi: 10.1097/00004714-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Fan J., Zhong M., Gan J., Liu W., Niu C., Liao H., Zhang H., Tan C.L., Yi J., Zhu X. Spontaneous neural activity in the right superior temporal gyrus and left middle temporal gyrus is associated with insight level in obsessive-compulsive disorder. J. Affect. Disord. 2017;207:203–211. doi: 10.1016/j.jad.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Gerretsen P., Menon M., Mamo D.C., Fervaha G., Remington G., Pollock B.G., Graff-Guerrero A. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: resting state functional connectivity. Schizophr. Res. 2014;160(1):43–50. doi: 10.1016/j.schres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Heninger G.R., Charney D.S. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch. Gen. Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Göttlich M., Krämer U.M., Kordon A., Hohagen F., Zurowski B. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum. Brain Mapp. 2014;35(11):5617–5632. doi: 10.1002/hbm.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn. Sci. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Himle J.A., Van Etten M.L., Janeck A.S., Fischer D.J. Insight as a predictor of treatment outcome in behavioral group treatment for obsessive–compulsive disorder. Cogn. Thera. Res. 2006;30(5):661–666. [Google Scholar]
- Jung W.H., Kang D.H., Kim E., Shin K.S., Jang J.H., Kwon J.S. Abnormal corticostriatal-limbic functional connectivity in obsessive–compulsive disorder during reward processing and resting-state. NeuroImage. Clin. 2013;3:27–38. doi: 10.1016/j.nicl.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap H., Kumar J.K., Kandavel T., Reddy Y.J. Neuropsychological correlates of insight in obsessive–compulsive disorder. Acta Psychiatr. Scand. 2012;126(2):106–114. doi: 10.1111/j.1600-0447.2012.01845.x. [DOI] [PubMed] [Google Scholar]
- Kim H., Adolphs R., O'Doherty J.P., Shimojo S. Temporal isolation of neural processes underlying face preference decisions. Proceedings. Nat. Aca. Sci. U S A. 2007;104(46):18253–18258. doi: 10.1073/pnas.0703101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore V.R., Samar R., Reddy Y.J., Chandrasekhar C.R., Thennarasu K. Clinical characteristics and treatment response in poor and good insight obsessive–compulsive disorder. Eur. Psychiatry. 2004;19(4):202–208. doi: 10.1016/j.eurpsy.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Lebreton M., Jorge S., Michel V., Thirion B., Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;64(3):431–439. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Lena Palaniyappan M.B.B.S., M. B.M.B.C., L. P.F. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 2012;37(1):17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Riedl V., Zherdin A., Mühlau M., Schwerthöffer D., Scherr M., Peters H.M., Zimmer C., Förstl H., Bäuml J., Wohlschläger A.M., Sorg C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 2013:sbt037. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R., Ito H., Takahashi H., Ando T., Fujimura Y., Nakayama K., Okubo Y., Obata T., Fukui K., Suhara T. Reduced gray matter volume of dorsal cingulate cortex in patients with obsessive–compulsive disorder: a voxel-based morphometric study. Psychiatry Clin. Neurosci. 2010;64(5):541–547. doi: 10.1111/j.1440-1819.2010.02125.x. [DOI] [PubMed] [Google Scholar]
- Matsunaga H., Kiriike N., Matsui T., Oya K., Iwasaki Y., Koshimune K., Miyata A., Stein D.J. Obsessive-compulsive disorder with poor insight. Compr. Psychiatry. 2002;43(2):150–157. doi: 10.1053/comp.2002.30798. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.J., Schaer M., Debbané M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self-and other-reflection. Neurosci. Biobehav. Rev. 2012;36(3):1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Niu C., Liu W., Lei H., Gan J., Fan J., Wang X., Zhu X. The Chinese version of the Brown Assessment of Beliefs Scale: psychometric properties and utility in obsessive compulsive disorder. Journal of Obsessive-Compulsive and Related Disorders. 2016;11:39–42. [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. (9) [DOI] [PubMed] [Google Scholar]
- Pannekoek J.N., Werff S.J.A., Meens P.H., Bulk B.G., Jolles D.D., Veer I.M., Jolles D.D., van Lang N.D., Rombouts S.A., van der Wee N.J., Vermeiren R.R. Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naive clinically depressed adolescents. J. Child Psychol. Psychiatry. 2014;55(12):1317–1327. doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- Posner J., Song I., Lee S., Rodriguez C.I., Moore H., Marsh R., Blair Simpson H. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum. Brain Mapp. 2016;23 doi: 10.1002/hbm.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij T.T., Mäntylä T., Mantere O., Kieseppä T., Suvisaari J. Cortical salience network activation precedes the development of delusion severity. Psychol. Med. 2016;46(13):2741. doi: 10.1017/S0033291716001057. [DOI] [PubMed] [Google Scholar]
- Rotge J.Y., Guehl D., Dilharreguy B., Tignol J., Bioulac B., Allard M., Burbaud P., Aouizerate B. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol. Psychiatry. 2009;65(1):75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Shad M.U., Muddasani S., Keshavan M.S. Prefrontal subregions and dimensions of insight in first-episode schizophrenia—a pilot study. Psychiatry Res. 2006;146(1):35–42. doi: 10.1016/j.pscychresns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Shin D.J., Jung W.H., He Y., Wang J., Shim G., Byun M.S., Jang J.H., Kim S.N., Lee T.Y., Park H.Y., Kwon J.S. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol. Psychiatry. 2014;75(8):606–614. doi: 10.1016/j.biopsych.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Skaf C.R., Yamada A., Garrido G.E., Buchpiguel C.A., Akamine S., Castro C.C., Busatto G.F. Psychotic symptoms in major depressive disorder are associated with reduced regional cerebral blood flow in the subgenual anterior cingulate cortex: a voxel-based single photon emission computed tomography (SPECT) study. J. Affect. Disord. 2002;68(2):295–305. doi: 10.1016/s0165-0327(00)00365-7. [DOI] [PubMed] [Google Scholar]
- Song A., Jung W.H., Jang J.H., Kim E., Shim G., Park H.Y., Choi C.H., Kwon J.S. Disproportionate alterations in the anterior and posterior insular cortices in obsessive–compulsive disorder. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., Li S.F., Zuo X.N., Zhu C.Z., He Y., Yan C.G., Zang Y.F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger D.C., Gorsuch R.L., Lushene R. Consulting Psychologists Press, Inc.; Palo Alto: 1983. Manual for the State-Trait Anxiety Inventory (Form Y) [M] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E.R., Welsh R.C., Fitzgerald K.D., Gehring W.J., Lister J.J., Himle J.A., Abelson J.L., Taylor S.F. Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biol. Psychiatry. 2011;69(6):583–591. doi: 10.1016/j.biopsych.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E.R., Fitzgerald K.D., Welsh R.C., Abelson J.L., Taylor S.F. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch E.A., Milsom V.A., Merlo L.J., Larson M., Geffken G.R., Jacob M.L., Murphy T.K., Goodman W.K. Insight in pediatric obsessive-compulsive disorder: associations with clinical presentation. Psychiatry Res. 2008;160(2):212–220. doi: 10.1016/j.psychres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Tan L., Fan Q., You C., Wang J., Dong Z., Wang X., Chen K., Xiao Z., Jiang K. Structural changes in the gray matter of unmedicated patients with obsessive-compulsive disorder: a voxel-based morphometric study. Neurosci. Bull. 2013;29(5):642–648. doi: 10.1007/s12264-013-1370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumkaya S., Karadag F., Oguzhanoglu N.K., Tekkanat C., Varma G., Ozdel O., Ateşçi F. Schizophrenia with obsessive-compulsive disorder and obsessive-compulsive disorder with poor insight: a neuropsychological comparison. Psychiatry Res. 2009;165(1):38–46. doi: 10.1016/j.psychres.2007.07.031. [DOI] [PubMed] [Google Scholar]
- van der Meer L., Costafreda S., Aleman A., David A.S. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34(6):935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Weber A.M., Soreni N., Noseworthy M.D. A preliminary study of functional connectivity of medication naive children with obsessive–compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;53:129–136. doi: 10.1016/j.pnpbp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation/Harcourt Brace; New York: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- de Wit S.J., Alonso P., Schweren L., Mataix-Cols D., Lochner C., Menchón J.M.…Hoexter M.Q. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am. J. Psychiatry. 2014;171(3):340–349. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- Wotruba D., Michels L., Buechler R., Metzler S., Theodoridou A., Gerstenberg M., Walitza S., Kollias S., Rössler W., Heekeren K. Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophr. Bull. 2013:sbt161. doi: 10.1093/schbul/sbt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Zang Y.F. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material