Highlights
-
•
DICER1 mutations play a significant role in gynecologic malignancy.
-
•
DICER1 may be involved in the sarcomagenesis of endometrial adenosarcoma.
-
•
The knowledge of a genetic mutation can help clarify a patient's medical history.
Keywords: Germline mutation, DICER1 gene, Mullerian adenosarcoma, Endometrial cancer
1. Background
Uterine adenosarcomas are composed of benign epithelium associated with a malignant stromal component. These tumors generally have low malignant potential resulting in a more favorable prognosis than other sarcomas (Kobayashi et al., 2013). Genetic associations or predispositions are largely unknown (Kobayashi et al., 2013).
The DICER1 gene product is an endoribonuclease responsible for processing microRNA (miRNA) (Chen et al., 2015). The majority of human genes are regulated by miRNAs. Given the key role of miRNA in gene expression, DICER1 is thought to play a large role in tumorigenesis and is oftentimes referred to as a tumor suppressor gene (Chen et al., 2015).
Germline mutations in the DICER1 gene have been associated with various lesions, specifically pleuropulmonary blastoma, cystic nephroma, multinodular goiter (MNG), Wilms tumor, ovarian Sertoli-Leydig cell tumor, and cervical embryonal rhabdomyosarcoma (Doros et al., 1993). Chen et al. recently demonstrated an association between somatic DICER1 mutations and endometrial carcinomas and carcinosarcomas (Chen et al., 2015). Similarly, Conlon et al. found that 2.4% of The Cancer Genome Atlas endometrial carcinoma genomes harbored DICER1 hotspot mutations (Conlon et al., 2016). Zigheilboim et al. demonstrated a correlation between decreased DICER1 expression and reduced disease-free survival in patients with endometrioid endometrial tumors (Zighelboim et al., 2011). Despite these associations between DICER1 and endometrial carcinomas, the association of DICER1 with uterine adenosarcomas has only recently been suggested (Piscuoglio et al., 2016).
We report on a 27-year-old woman who presented with a missed abortion. Pathologic examination of the products of conception revealed adenosarcoma and scant products of conception. Germline genetic testing revealed a DICER1 mutation.
2. Case
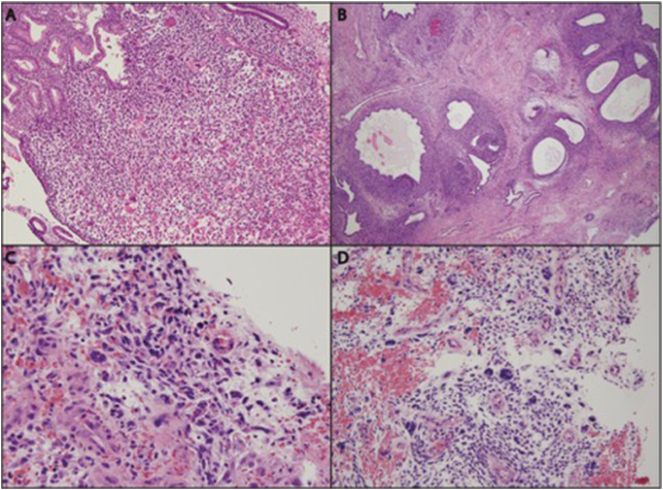
A 27-year-old woman, gravida 2, para 1 presented to her obstetrician for abnormal uterine bleeding. Her urine beta-human chorionic gonadotropin (HCG) was negative, but serum HCG was 17 IU/L. Ultrasound demonstrated a small gestational sac in the right cornu with a 6-week sized embryo with absent fetal heart motion. The patient underwent hysteroscopy, dilation, and curettage (D&C). The endometrial curettage specimen demonstrated fragments of endometrial tissue admixed with tissue that was highly atypical in appearance. Histologic sections showed abnormal endometrial tissue in polypoid leaf-like configurations and some glands with dilated, serrated profiles (Fig. 1A). The stroma was predominantly fibrotic, but in other areas it was cellular. The more cellular areas were concentrated around the serrated glands in the manner of a “cambium” layer (Fig. 1B). Stromal mitoses were identified in the periglandular cuffs (Fig. 1C). Clustered thick-walled blood vessels were seen elsewhere in the tissue fragments. Portions of tissue were largely infarcted. Within these infarcted areas were highly atypical stromal cells with pleomorphic nuclei and mitotic figures (Fig. 1D). These portions resembled a high-grade sarcoma. Another component represented normal background endometrium.
Fig. 1.
Histologic appearance of Mullerian adenosarcoma in endometrial curettings. (A) Abnormal endometrial tissue in a polypoid configuration, containing dilated and serrated glands. H&E, original magnification 100 ×. (B) Condensation of stroma around glands. H&E, 40 ×. (C) Abnormal stromal mitotic figures at center of panel, including one tripolar mitosis. H&E, 400 ×. (D) Vascular areas with bizarre stromal nuclei. H&E, 200 ×.
Immunostains were performed. Estrogen receptor was present in the endometrial-like areas, but absent in the high-grade sarcoma. The sarcomatoid areas were negative for p63, PLAP, and inhibin which would be positive in various forms of trophoblastic disease. Cytokeratins AE1/3 demonstrated a dot-like pattern of positivity in the sarcomatoid areas, which is characteristic of Mullerian adenosarcoma. Ki-67 showed a proliferation index approaching 80% in the most proliferative areas of the sarcoma and highlighted endometrial stromal cells within the cambium layer surrounding endometrial glands. An immunostain for beta-hCG highlighted one focus of tissue that on H&E appeared consistent with highly degenerated chorionic villi and trophoblast, confirming pregnancy. Myogenin and desmin stains were negative, excluding rhabdomyosarcoma. The final interpretation was Mullerian adenosarcoma with sarcomatous overgrowth. Repeat curettage of the endometrium demonstrated similar findings. An outside consultation was obtained, supporting the interpretation.
Computed tomography (CT) of the chest, abdomen, and pelvis was normal without evidence of metastatic disease. The patient underwent surgical staging with a DaVinci robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and sentinel lymph node dissection with ovarian preservation. She was referred to reproductive endocrinology prior to surgery for fertility preservation. On laparoscopy, the patient was noted to have a normal uterus, bilateral tubes, and ovaries with no evidence of metastatic disease. On bivalve of the uterus there was no obvious tumor. Pathology demonstrated no evidence of malignancy in the uterus, bilateral fallopian tubes, or sentinel lymph nodes. Given Stage I disease, the decision was made for no adjuvant therapy and follow-up with serial CT scans.
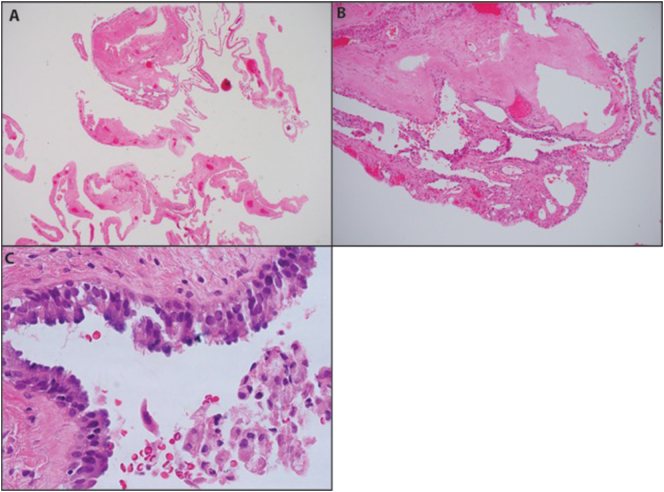
Of note, the patient had a history of an endometrial polyp in 2013, a lung bleb at age 16 years old, and a self-reported thyroid nodule in 2006 for which she underwent hemithyroidectomy. The polyp from 2013 was obtained for review. This showed an endocervical polyp that was predominantly necrotic. One of the rare areas of viable tissue showed stromal cells whose nuclei were enlarged. A few nuclei were hyperchromatic with coarse chromatin, and there was one apparent mitotic figure. Upon initial review these changes were attributed to vascular injury; however, the presence of atypical stromal cells in the 2013 material is noteworthy in retrospect. Also of note, at age 16 the patient presented with 6-month history of cough. Chest CT demonstrated a 20.3 × 10.4 cm, loculated emphysematous bleb. After resection the patient had no residual symptoms. The pathology was reported in 2005 as a cyst with focal fibrosis. However, upon reexamination of the specimen with knowledge of the patient's germline DICER1 mutation, pathology was felt to be consistent with a type I regressed (Ir) pleuropulmonary blastoma (PPB; Fig. 2). This was confirmed by expert consultation.
Fig. 2.
Histologic appearance of patient's type Ir PPB. (A) Thin-walled multilocular subpleural cysts. H&E, original magnification 20 ×. (B) Variably cellular cyst walls without obvious “cambium” layer. H&E, 40 ×. (C) Benign respiratory-type epithelium with focal cilia. H&E, 600 ×.
The patient was referred to a genetic counselor and underwent germline testing through a 42-gene Common Hereditary Cancers Panel (Invitae Genetics). Sequencing showed a heterozygous germline DICER1 mutation (c.3007C > T, p.Arg1003*). By creating a premature stop codon, this nonsense mutation is predicted to result in an absent or truncated protein with disrupted function. According to the COSMIC database, the mutation is present near the middle of the 1923-amino acid protein, in the PAZ domain, and is N-terminal to the two ribonuclease domains that are required for function. The mutation is C-terminal to the helicase and dimerization domains, suggesting a possible dominant-negative function of the mutant protein if one is produced. This variant is not listed as a polymorphism in the NHLBI Exome Variant Server or in the UCSC Genome Browser. However, this specific mutation has been reported previously in an individual with pleuropulmonary blastoma and the variant is therefore classified as pathogenic. We did not test tumor tissue to identify a possible somatic second-hit mutation.
3. Discussion
We report a case of adenosarcoma in a patient with a germline DICER1 mutation who initially presented for a missed abortion. Interestingly, the patient underwent polypectomy years prior, had a childhood pulmonary emphysematous bleb consistent with type Ir pleuropulmonary blastoma, and a thyroid nodule hypothetically representing multinodular goiter.
Adenosarcomas comprise ~ 8% of all uterine sarcomas (Kobayashi et al., 2013). The median age of presentation is 58 years, and the typical presentation is irregular bleeding secondary to a large polypoid mass in the endometrial cavity (Kobayashi et al., 2013). While this patient was younger than the median age of presentation, 13% of patients present prior to 30 years old and up to 38% prior to 50 years old (Clement and Scully, 1990). Hysteroscopy demonstrated a polypoid structure in the right cornual region characteristic of adenosarcoma's usual mass appearance. Her adenosarcoma was typical in that it was early-stage, but there was notable sarcomatous overgrowth which could represent a more aggressive variant (Kobayashi et al., 2013, Clement and Scully, 1990). The relationship between the adenosarcoma and our patient's pregnancy is difficult to ascertain. It is possible the missed abortion led to the diagnosis of the sarcoma. Additionally, the presence of the adenosarcoma may have contributed to pregnancy failure.
Adenosarcoma is often a difficult pathologic diagnosis and is frequently misinterpreted (Clement and Scully, 1990). In one series, 5% of patients with adenosarcoma were misdiagnosed with benign polyps on two or more occasions prior to their final diagnosis. Particularly in young women, recurrent endocervical and/or endometrial polyps are unusual and should raise a red flag (Clement and Scully, 1990). Of note, 22% of adenosarcomas contain heterologous sarcomatous stromal elements and samples often contain extensive fibrosis which can confer a misleading benign appearance (Clement and Scully, 1990).
Germline DICER1 mutations have never been described in endometrial adenosarcomas until this case. However, Piscuoglio et al. characterized somatic genetic mutations in 20 cases of uterine adenosarcomas and found DICER1 to be one of three genes recurrently mutated in the mesenchymal component of the uterine adenosarcomas occurring in 10.5% of the cases (Piscuoglio et al., 2016). Of note, one of the tumors with a hotspot DICER1 mutation had sarcomatous overgrowth similar to our patient. Interestingly, the pathology of uterine adenosarcoma shares similarities with PPB and Wilms tumor which are both commonly associated with germline DICER1 mutations. All three tumors demonstrate sarcomatous cells associated with an epithelial component (Chen et al., 2015, Priest et al., 2006). Therefore, it is not surprising that this tumor is associated with a similar genetic predisposition. Further research is necessary, but perhaps uterine adenosarcoma is the uterine dysontogenetic analogue to these tumors.
The patient's lung lesion was reinterpreted by a subspecialty pediatric pathologist as a type Ir PPB. Whereas the lung lesion was resected in 2005, the features of type I PPBs were not well described until 2006 (Seki et al., 2014), before which they were often diagnosed as benign blebs. Our patient has not experienced any sequelae from her lesion, which is not uncommon.
Lastly, while no data was available regarding the patient's thyroid nodule, the history might be considered suspicious for multinodular goiter (MNG) which has also been associated with germline DICER1 mutations (Doros et al., 1993).
This case demonstrates a patient with uterine adenosarcoma and a germline DICER1 mutation. Our observations provide insight into the genetic aberrations associated with adenosarcoma, and expand the spectrum of DICER1-related disease. Further research is necessary to determine the exact role, if any, that DICER1 plays in the pathogenesis of adenosarcoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Acknowledgments
I.S.H. is supported by NIH R25 CA190190. We thank Dr. Louis P. Dehner for reviewing the patient's lung specimen.
Footnotes
This research did not receive any specific funding. The authors have no conflicts of interest relevant to this article to disclose. The authors have no financial disclosures to report.
References
- Chen J., Wang Y., McMonechy M.K., Anglesio M.S., Yang W., Senz J. Recurrent DICER1 hotspot mutations in endometrial tumors and their impact on microRNA biogenesis. J. Pathol. 2015;237:215–225. doi: 10.1002/path.4569. [DOI] [PubMed] [Google Scholar]
- Clement P.B., Scully R.E. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum. Pathol. 1990;21(4):363–381. doi: 10.1016/0046-8177(90)90198-e. [DOI] [PubMed] [Google Scholar]
- Conlon N., Schultheis A.M., Piscuoglio S., Silva A., Guerra E., Tornos C., Reuter V.E. A survey of DICER1 hotspot mutations in ovarian and testicular sex cord-stromal tumors. Mod. Pathol. 2016;28(12):1603–1612. doi: 10.1038/modpathol.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doros L., Schultz K.A., Stewart D.R., Bauer A., Williams G., Rossi C.T. University of Washington, Seattle; Seattle (WA): 1993–2016. DICER1-related Disorders. GeneReviews [Internet]https://www.ncbi.nlm.nih.gov/books/NBK196157/ Available from: [Google Scholar]
- Kobayashi H., Uekuri C., Akasada J., Ito F., Shigemitsu A., Koike N. The biology of uterine sarcomas: a review and update. Mol. Clin. Oncol. 2013;1:599–609. doi: 10.3892/mco.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscuoglio S., Burke K.A., Ng C.K., Papanastasiou A.D., Geyer F.C. Uterine adenosarcomas are mesenchymal neoplasms. J. Pathol. 2016 Feb;238(3):381–388. doi: 10.1002/path.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest J.F., Hill D.A., Williams G.M., Moertel C.L., Messinger Y., Finkelstein M.J. Type I pleuropulmonary blastoma: a report from the international pleuropulmonary blastoma reigistry. J. Clin. Oncol. 2006 Sep 20;24(27):4492–4498. doi: 10.1200/JCO.2005.05.3595. [DOI] [PubMed] [Google Scholar]
- Seki M., Yoshida K., Shiraishi Y., Shimamura T., Sato Y., Nishimura R., Okuno Y., Chiba K., Tanaka H., Kato K., Kato M. Biallelic DICER1 mutations in sporadic pleuropulmonary blastoma. Cancer Res. 2014 May;74(10):2742–2749. doi: 10.1158/0008-5472.CAN-13-2470. [DOI] [PubMed] [Google Scholar]
- Zighelboim I., Reinhart A.J., Gao F., Schmidt A.P., Mutch D.G., Thaker P.H. DICER1 expression and outcomes in endometrioid endometrial adenocarcinoma. Cancer. 2011;117(7):1446–1453. doi: 10.1002/cncr.25665. [DOI] [PMC free article] [PubMed] [Google Scholar]