Abstract
Although the adverse effects of neonatal hypoxia associated with premature birth on the central nervous system are well known, the contribution of hypoxic damage to the peripheral nervous system (PNS) has not been addressed. We demonstrate that neonatal hypoxia results in hypomyelination and delayed axonal sorting in mice leading to electrophysiological and motor deficits that persist into adulthood. These findings support a potential role for PNS hypoxic damage in the motor impairment that results from premature birth and suggest that therapies designed to protect the PNS may provide clinical benefit.
Improved care is increasing the number of neonates that survive prematurity, including those infants born at low birth weight (<1500 g).1 Unfortunately, neurodevelopmental disabilities caused by premature birth have not decreased, and more than half of preterm survivors experience motor, sensory, behavioral, and cognitive deficits that persist into adulthood.2, 3, 4 With the prevalence and persistence of these disabilities it is crucial to gain a better understanding of the pathophysiology of premature birth that results in neurologic deficits.
Most studies have focused on the central nervous system (CNS)-specific effects of neonatal hypoxic injury to premature infants resulting in diffuse white matter injury.5, 6 Nevertheless, little is known about the effects of neonatal hypoxia on the peripheral nervous system (PNS). This represents a gap in our understanding of damage caused by neonatal hypoxia, and a better understanding of these morbidities could provide novel opportunities for therapeutic intervention.
Here, we demonstrate that neonatal hypoxia results in PNS hypomyelination, characterized by thinner myelin sheets that persist into adulthood correlated with electrophysiological and motor behavior deficits. These results suggest that PNS myelin deficits may represent an underappreciated component of neurodevelopmental disabilities caused by neonatal hypoxia and that therapies designed to protect PNS myelin may improve clinical outcomes of these individuals.
Materials and Methods
Animals and Neonatal Hypoxia
All animals were housed under pathogen-free conditions, and all animal procedures were approved by the Institutional Animal Care and Use Committees of the University of Chicago. Neonatal hypoxia model was performed as previously described using both male and female mice.5 Briefly, male and female C57BL/6J mouse pups were fostered to lactating CD1 dams at postnatal day 2 (P2) and exposed to either neonatal hypoxia of 10% ± 0.5% oxygen or room air control (approximately 21% oxygen) from P3 to P11 after which time pups were returned to room air.
Electron Microscopy Analysis
Samples were prepared as previously described.7 G-ratio images were taken at ×1200 from four to six mice per group with >200 total axons counted per group and calculated according to the method previously described.8 Axon bundles images were taken at ×1200 or ×2900 from four to six mice with >20 bundles analyzed per group.
Total RNA Isolation and Real-Time Quantitative PCR
RNA was isolated from pooled sciatic nerves using the Bio-Rad Aurum Total RNA Fatty and Fibrous Tissue Kit (732-6830; Bio-Rad, Hercules, CA) and reverse transcribed using the Bio-Rad iScript cDNA Synthesis kit (1708891) according to manufacturer's instructions. Real-time quantitative PCR was run on a Bio-Rad CFX96 Real-Time PCR machine using SYBR Green detection. Results were analyzed using the ΔΔC(t) method with Rpl13A and Hprt1 used as reference genes. The following primer sets were used: Krox20 forward 5′-AATAGCTGGGCGAGGGG-3′, Krox20 reverse 5′-ATGTTGATTCATGCCATCTCCC-3′; P0 forward 5′-ACCTCTCAGGTCACGCTCTA-3′, P0 reverse 5′-CATGGCACTGAGCCTTCTCTG-3′; Mbp forward 5′-GCTCCCTGCCCCAGAAGT-3′, Mbp reverse 5′-TGTCACAATGTTCTTGAAGAAATGG-3′; Mag forward 5′-CTGCTCTGTGGGGCTGACAG-3′, Mag reverse 5′-AGGTACAGGCTCTTGGCAACTG-3′; Rpl13a forward 5′-TTCTCCTCCAGAGTGGCTGT-3′, Rpl13a reverse 5′-GGCTGAAGCCTACCAGAAAG-3′; Hprt1 forward 5′-TCAGACCGCTTTTTGCCGCGA-3′, and Hprt1 reverse 5′-ATCGCTAATCACGACGCTGGGAC-3′.
Immunohistochemistry
Mice were taken directly from hypoxia or room air and anesthetized by intraperitoneal injection with avertin (0.5 mg/g). Then the sciatic nerves were removed and embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA) and snap-frozen in isopentane with dry ice. Cross sections were cut from fresh frozen tissue, fixed for 10 minutes in 4% paraformaldehyde, washed in phosphate-buffered saline, and stained with 1:250 KROX20 (PRB-236P; Covance, Princeton, NJ) and 1:200 Oct-6 (sc-11661; Santa Cruz Biotechnology, Dallas, TX).
Motor Behavior Analysis
Motor coordination and balance of control and neonatal hypoxia-exposed mice were measured by the accelerating rotarod (Columbus Instruments, Columbus, OH) as previously described modified to accelerate from 5 to 45 rpm over a 300-second trial.9
Forelimb and hindlimb grip strength were measured as previously described using a computerized grip strength meter (0167-005L; Columbus Instruments).10, 11
Grid test measurements were performed by suspending mice inverted on a 1-inch mesh grid and measuring latency to fall during a 60-second trial. At 60 seconds the mouse was removed and given 5 minutes of rest. The average of four trials was calculated. All motor behavior analysis was performed by a blinded investigator (B.L.L.C).
Electrophysiology
Electrophysiology was performed in P60 mice with a Nicolet Viking Quest Laptop System (Natus Neurology, Middleton, WI) as previously described.10, 11
Statistical Analysis
Data are presented as means ± SEM. Multiple comparisons were made using analysis of variance with Tukey's post-test. Comparisons of two data points were made by a two-sided unpaired t-test. A P value of <0.05 was considered significant, and all statistical analyses were run with GraphPad Prism software version 7 (GraphPad Inc., San Diego, CA).
Results
Neonatal Hypoxia Leads to PNS Hypomyelination
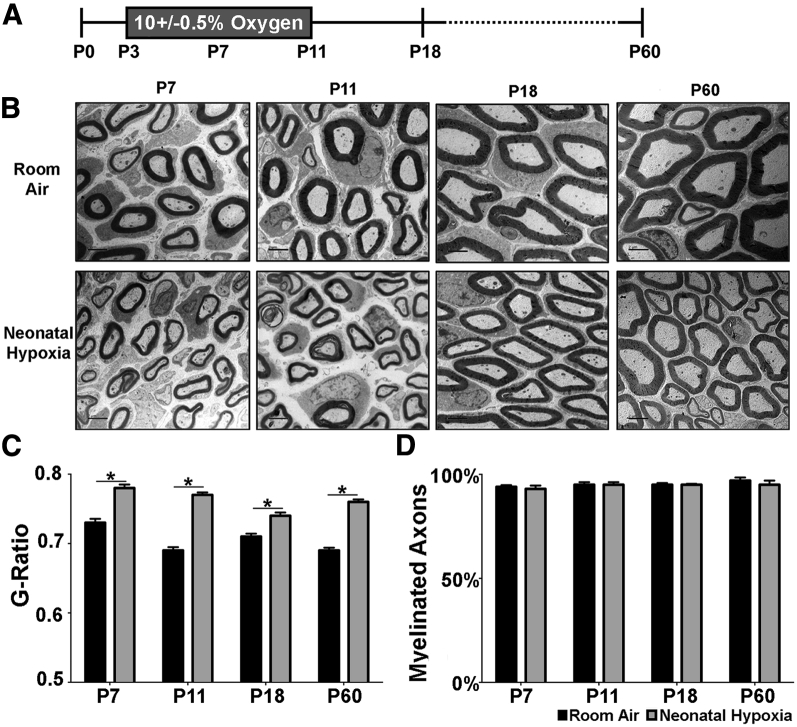
To examine the PNS effects of neonatal hypoxia we took advantage of a well-described model in which mice are exposed to 10% ± 0.5% oxygen from P3 to P11 (Figure 1A).5 Because the effect of chronic hypoxic rearing on PNS myelination has not been studied, we began by measuring myelin thickness of sciatic nerve axons in control and hypoxia-exposed mice. We found that g-ratios were increased in hypoxia-exposed pups at P7, P11, P18, and P60 (Figure 1, B and C). This chronic myelination deficit is similar to the effects of neonatal hypoxia on CNS myelin thickness.5 Nevertheless, the percentage of myelinated fibers did not differ at any age (Figure 1D). These results show that neonatal hypoxia leads to hypomyelination in the PNS that persists into adulthood.
Figure 1.
Neonatal hypoxia (NH) leads to thinner myelin in the peripheral nervous system. Myelination in the sciatic nerve of NH (gray bars) and room air (RA) (black bars) was analyzed using electron microscopy. A: For the NH model, mice were exposed to 10% ± 0.5% oxygen from P3 to P11, and tissue for electron microscopy analysis was collected at P7, P11, P18, and P60. B: Electron microscopy images from sciatic nerve of RA control and NH-exposed pups. C: Myelin thickness measured by g-ratio was significantly thinner in NH-exposed pups than in RA controls at all time points examined. D: NH had no effect on the percentage of myelinated axons in the sciatic nerve. Data are expressed as means ± SEM. n = 4 to 6 mice per group for g-ratio calculations >200 axons; n = 4 to 6 mice per group for percentage of myelination. ∗P < 0.05 by two-tailed t-test. Scale bar = 2 μm (B). P–, postnatal day–.
Neonatal Hypoxia Does Not Alter the Number of Schwann Cells
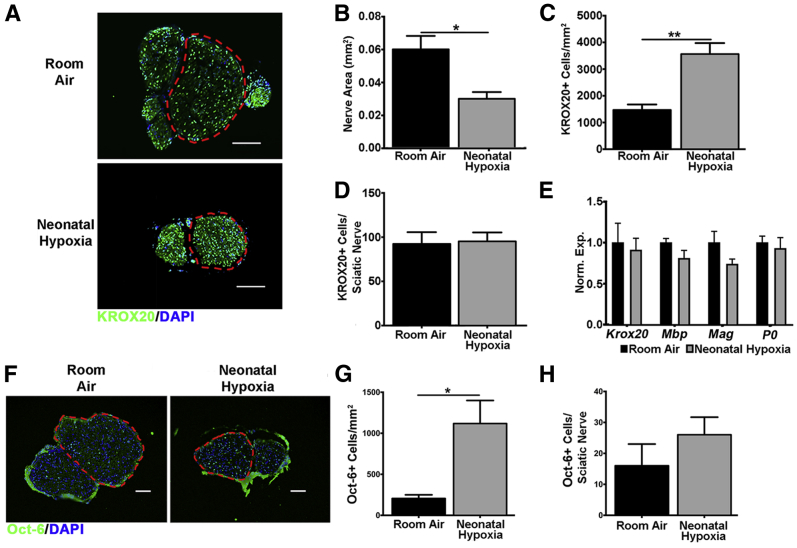
In the CNS neonatal hypoxia inhibits maturation of oligodendrocytes leading to hypomyelination.5 Therefore, we determined whether decreased numbers of mature Schwann cells contribute to PNS hypomyelination caused by neonatal hypoxia. Neonatal hypoxia increased the density of mature KROX20+ Schwann cells due to decreased sciatic nerve size (Figure 2, A–C). Nevertheless, neonatal hypoxia did not decrease the total number of mature Schwann cells in the sciatic nerve (Figure 2D), and there was no significant difference in the expression of mature Schwann cell genes (Figure 2E). These results show that neonatal hypoxia does not result in the loss of mature Schwann cells.
Figure 2.
Neonatal hypoxia does not decrease the number of immature or mature Schwann cells. Mature Schwann cell numbers in the sciatic nerve of neonatal hypoxia (gray bars) and room air (black bars) was analyzed by immunohistochemistry and qPCR. A: Neonatal hypoxia- and room air-exposed sciatic nerves from P11 mice stained with the mature Schwann cell marker KROX20 (green). Region of sciatic nerve used for cell counts is outlined by a red dashed line. B: P11 sciatic nerves from neonatal hypoxia-exposed mice were significantly smaller. C: Smaller sciatic nerves in P11 neonatal hypoxia-exposed mice led to a higher density of KROX20+ cells. D: However, there was no difference in the total number of KROX20+ cells per sciatic nerve between neonatal hypoxia- and room air-exposed mice at P11. E: There was no significant difference between expression of Krox20, Mbp, Mag, or P0 in neonatal hypoxia- and room air-exposed sciatic nerves. qPCR analysis was performed in sciatic nerves from P7 neonatal hypoxia- and room air-exposed mice. Normalized expression was calculated as expression relative to RPL13A and HPRT1 reference genes and normalized to controls. F: Neonatal hypoxia- and room air-exposed sciatic nerves from P11 mice stained with the promyelinating immature Schwann cell marker Oct-6 (green). Region of sciatic nerve used for cell counts is outlined by a red dashed line. G: Smaller sciatic nerves in P11 neonatal hypoxia-exposed mice led to a higher density of Oct-6+ cells. H: There was no significant difference in the total number of Oct-6+ cells per sciatic nerve between P11 neonatal hypoxia- and room air-exposed mice. Data are expressed as means ± SEM. n = 5 to 6 for KROX20+ cell counts; n = 3 for qPCR analysis of sciatic nerves; n = 3 Oct-6+ cell counts. ∗P < 0.05, ∗∗P < 0.01 by two-tailed t-test. Scale bars: 100 μm (A); 50 μm (F). Oct-6, octamer-binding protein 6; P–, postnatal day–; qPCR, real-time quantitative PCR.
It is also possible that neonatal hypoxia leads to an alteration in the number of immature Schwann cells in the PNS. Therefore, we examined these cells by staining for the transcription factor octamer-binding protein 6 (alias POU class 3 homeobox 1) that is present in immature Schwann cells and absent in myelinating Schwann cells.12 We found that at P11 octamer-binding protein 6–positive cells were significantly denser in neonatal hypoxia-exposed pups (Figure 2, F and G). Nevertheless, this difference in density was driven by decreased total nerve size and not by a decrease in the total number of octamer-binding protein 6–positive cells per nerve (Figure 2, F and H). These results indicate that neonatal hypoxia does not result in the loss of immature promyelinating Schwann cells.
Neonatal Hypoxia Increases the Size of PNS Nerve Bundles and the Number of Axons per Bundle
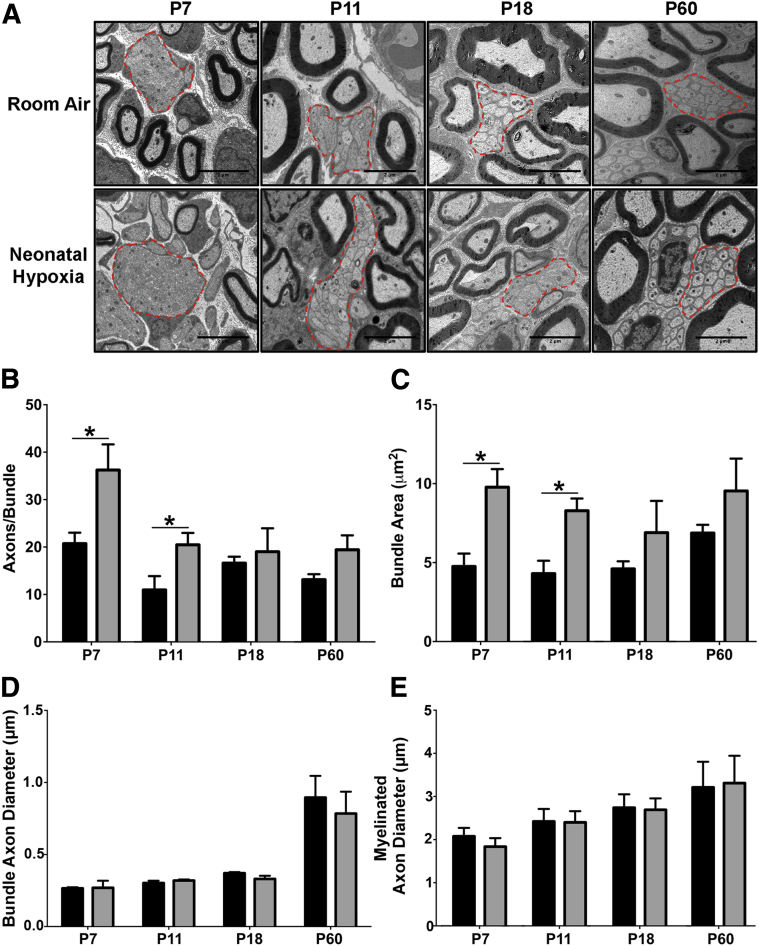
During PNS development axons are removed from nerve bundles and myelinated by Schwann cells in an event called axonal sorting.13 To determine whether neonatal hypoxia disrupts axonal sorting, we analyzed axon bundles in the developing sciatic nerve of control and neonatal hypoxia-exposed pups. We found that at P7 and P11 the size of axon bundles in hypoxia-exposed pups were significantly larger than controls due to increased axon numbers but not increased axon diameter (Figure 3, A–D). By P18 these differences were resolved. We also measured the size of myelinated axons to determine whether neonatal hypoxia may be delaying axonal growth and found no difference in the diameter of myelinated axons (Figure 3E). These data suggest that neonatal hypoxia transiently perturbs the early steps of the radial axonal-sorting process, which results in larger bundles containing increased numbers of axons.13
Figure 3.
Neonatal hypoxia (NH) leads to disrupted maturation of axon bundles in the peripheral nervous system (PNS). Axon bundles were examined in mice exposed to NH (gray bars) and room air (RA) (black bars) control mice using electron microscopy. A: Representative images of axonal bundles are outlined by red dashed lines. B: At P7 and P11, during hypoxic exposure, pups had significantly larger axon bundles in the PNS than RA controls. B: Bundle size recovered to control levels after 7 days of recovery at P18, and no long-term changes in axon bundle size were found at P60. C: Increased bundle size corresponded with increased number of axons per bundle in NH-exposed mice at P7 and P11, with no difference at P18 and P60. D and E: There was no significant difference in the size of either bundle axons (D) or myelinated axons (E) in the PNS of NH and RA control mice. Data are expressed as means ± SEM. n = 4 to 6 mice per group with >20 bundles analyzed. ∗P < 0.05 by two-tailed t-test. P–, postnatal day–.
Neonatal Hypoxia Results in PNS Electrophysiological and Motor Deficits in Adulthood
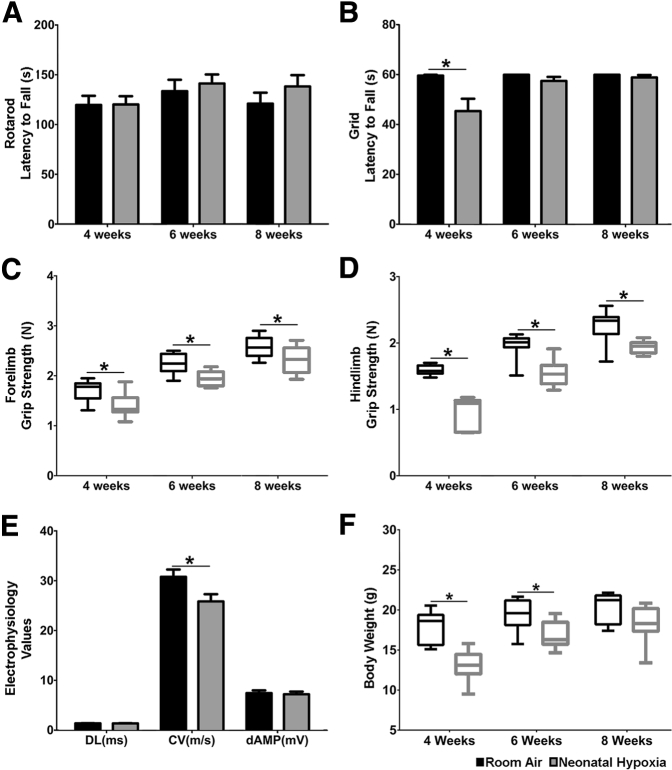
Myelin functions to increase the speed and fidelity of action potential propagation along the axon, and, given the decrease in PNS myelin thickness caused by neonatal hypoxia, we next examined whether this disturbance led to PNS motor deficits in older mice. We found that neonatal hypoxia had no effect on rotarod performance (Figure 4A) and resulted in a mild deficit at 4 weeks of age in performance on the inverted grid test (Figure 4B). Nevertheless, the rotarod and grid tests lack sensitivity. Therefore, we performed more-sensitive grip strength measures using a dynamometer. Both forelimb and hindlimbs showed that neonatal hypoxia-exposed pups had significantly weaker limbs at all time points examined (Figure 4, C and D). Importantly, decreased limb strength remained even after the reduced weight of neonatal hypoxia-exposed pups recovered (Figure 4F). Finally, we measured the latency, conduction velocity, and amplitude of motor responses recorded while stimulating the sciatic nerve. We found that sciatic motor responses from hypoxia-exposed mice had slower conduction velocity than those from control mice (Figure 4E), which correlates with the thinner myelin sheaths seen at P60 (Figure 1C). There was no difference in the amplitude or distal latency between the two groups (Figure 4E). These results show that the effects of neonatal hypoxia on PNS myelination correlate with sustained PNS motor and electrophysiological deficits in adult animals.
Figure 4.
Neonatal hypoxia causes long-term motor and electrophysiological deficits. Electrophysiological and motor deficits were examined in neonatal hypoxia (gray bars) and room air (black bars) control mice. A: Neonatal hypoxia had no effect on motor balance and coordination measured by rotarod. B: Compared with room air controls, neonatal hypoxia-exposed mice exhibited a decreased latency to fall in the grid test at 4 weeks of age that recovered by 6 weeks of age. C and D: Forelimb (C) and hindlimb (D) grip strength were significantly weaker in neonatal hypoxia-exposed mice at all time points measured. E: In agreement with peripheral nervous system myelin deficits, neonatal hypoxia-exposed pups had significantly slower conduction velocity (CV) at P60 with no distal latency (DL) or distal amplitude (dAMP). F: Neonatal hypoxia-exposed animals weighed less than room air-exposed animals at 4 and 6 weeks of age but recovered to control levels at 8 weeks. Data are expressed as means ± SEM. For box and whisker plots the box represents the 25th to 75th percentile with the median designated by a line and the whiskers representing the minimum and maximum values. n = 8 to 9 mice. ∗P < 0.05 by two-tailed t-test. P–, postnatal day–.
Discussion
In this study we demonstrate that neonatal hypoxia leads to PNS abnormalities, including delayed axonal sorting and hypomyelination that result in electrophysiological and motor deficits. These data indicate that neonatal hypoxia has similar adverse effects on the PNS as on the CNS. A large body of clinical research has established that preterm infants have significant motor impairments14, 15, 16 that are a risk factor for poor cognitive performance, behavioral problems, and learning disabilities.17, 18, 19 Our study suggests that hypoxia-induced PNS dysfunction likely contributes to the clinical manifestations that result from premature birth.
We found that neonatal hypoxia caused decreased myelin thickness in the PNS at all time points investigated, including in adult mice. This hypomyelination correlates with the decreased sciatic nerve conduction velocity and weaker limb strength observed in mice exposed to neonatal hypoxia. There was no decrease in the percentage of myelinated fibers nor was there a decrease in the number of mature Schwann cells. This suggests that the effect of neonatal hypoxia on the PNS involves a distinct mechanism from the arrested maturation of oligodendrocytes that leads to CNS hypomyelination after neonatal hypoxia. Neonatal hypoxia is known to effect maturation of neurons such that axonal damage cannot be excluded as a contributing factor to the observed PNS abnormalities.20 Further studies are needed to understand the mechanism behind neonatal hypoxia-induced deficits in PNS development.
In conclusion, our studies establish that neonatal hypoxia causes sustained hypomyelination in the PNS and leads to long-term electrophysiological and motor deficits. These results suggest that the effects of neonatal hypoxia on PNS myelination may be an unrecognized contributor to neurodevelopmental disabilities caused by premature birth and warrant greater attention.
Acknowledgments
B.L.L.C. and B.P. participated in the planning and design of the study; B.L.L.C., B.S., and B.P. participated in interpretation of the results; B.L.L.C., A.H., and D.D. participated in acquisition and analysis of the data; B.L.L.C., B.S., and B.P. wrote and edited the manuscript.
Footnotes
Supported by NIH grant NS34939 (B.P.).
Disclosures: None declared.
References
- 1.Salmaso N., Jablonska B., Scafidi J., Vaccarino F.M., Gallo V. Neurobiology of premature brain injury. Nat Neurosci. 2014;17:341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hack M., Flannery D.J., Schluchter M., Cartar L., Borawski E., Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 3.Marlow N., Wolke D., Bracewell M.A., Samara M., EPICure Study Group Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 4.Miller S.P., Ferriero D.M., Leonard C., Piecuch R., Glidden D.V., Partridge J.C., Perez M., Mukherjee P., Vigneron D.B., Barkovich A.J. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Scafidi J., Hammond T.R., Scafidi S., Ritter J., Jablonska B., Roncal M., Szigeti-Buck K., Coman D., Huang Y., McCarter R.J., Jr., Hyder F., Horvath T.L., Gallo V. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014;506:230–234. doi: 10.1038/nature12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuen T.J., Silbereis J.C., Griveau A., Chang S.M., Daneman R., Fancy S.P., Zahed H., Maltepe E., Rowitch D.H. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158:383–396. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussien Y., Podojil J.R., Robinson A.P., Lee A.S., Miller S.D., Popko B. ER chaperone BiP/GRP78 is required for myelinating cell survival and provides protection during experimental autoimmune encephalomyelitis. J Neurosci. 2015;35:15921–15933. doi: 10.1523/JNEUROSCI.0693-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auer R.N. Automated nerve fibre size and myelin sheath measurement using microcomputer-based digital image analysis: theory, method and results. J Neurosci Methods. 1994;51:229–238. doi: 10.1016/0165-0270(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 9.Traka M., Arasi K., Avila R.L., Podojil J.R., Christakos A., Miller S.D., Soliven B., Popko B. A genetic mouse model of adult-onset, pervasive central nervous system demyelination with robust remyelination. Brain. 2010;133:3017–3029. doi: 10.1093/brain/awq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbaz B., Traka M., Kunjamma R.B., Dukala D., Lutz A.B., Anton E.S., Barres B.A., Soliven B., Popko B. Adenomatous polyposis coli regulates radial axonal sorting and myelination in the PNS. Development. 2016;143:2356–2366. doi: 10.1242/dev.135913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levedakou E.N., Chen X.J., Soliven B., Popko B. Disruption of the mouse Large gene in the enr and myd mutants results in nerve, muscle, and neuromuscular junction defects. Mol Cell Neurosci. 2005;28:757–769. doi: 10.1016/j.mcn.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Zorick T.S., Syroid D.E., Arroyo E., Scherer S.S., Lemke G. The transcription factors SCIP and Krox-20 mark distinct stages and cell fates in Schwann cell differentiation. Mol Cell Neurosci. 1996;8:129–145. doi: 10.1006/mcne.1996.0052. [DOI] [PubMed] [Google Scholar]
- 13.Feltri M.L., Poitelon Y., Previtali S.C. How Schwann cells sort axons: new concepts. Neuroscientist. 2016;22:252–265. doi: 10.1177/1073858415572361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poole K.L., Schmidt L.A., Missiuna C., Saigal S., Boyle M.H., Van Lieshout R.J. Motor coordination and mental health in extremely low birth weight survivors during the first four decades of life. Res Dev Disabil. 2015;43-44:87–96. doi: 10.1016/j.ridd.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Cooke R.W., Foulder-Hughes L. Growth impairment in the very preterm and cognitive and motor performance at 7 years. Arch Dis Child. 2003;88:482–487. doi: 10.1136/adc.88.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salt A., Redshaw M. Neurodevelopmental follow-up after preterm birth: follow up after two years. Early Hum Dev. 2006;82:185–197. doi: 10.1016/j.earlhumdev.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Losch H., Dammann O. Impact of motor skills on cognitive test results in very-low-birthweight children. J Child Neurol. 2004;19:318–322. doi: 10.1177/088307380401900502. [DOI] [PubMed] [Google Scholar]
- 18.Piek J.P., Dawson L., Smith L.M., Gasson N. The role of early fine and gross motor development on later motor and cognitive ability. Hum Mov Sci. 2008;27:668–681. doi: 10.1016/j.humov.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y.D., Ou S., Cheng S.Y., Xiao Z., He W.J., Zhang J.H., Ruan H.Z. Dendritic development of hippocampal CA1 pyramidal cells in a neonatal hypoxia-ischemia injury model. J Neurosci Res. 2013;91:1165–1173. doi: 10.1002/jnr.23247. [DOI] [PubMed] [Google Scholar]