Abstract
Background
Community-acquired pneumonia (CAP) is a severe infection, with high mortality. Antibiotic strategies for CAP differ across Europe.
The objective of the study was to describe the epidemiology of CAP in Denmark and evaluate the prognosis of patients empirically treated with penicillin-G/V monotherapy.
Methods
Retrospective cohort study including hospitalized patients with x-ray confirmed CAP. We calculated the population-based incidence, reviewed types of empiric antibiotics and duration of antibiotic treatment. We evaluated the association between mortality and treatment with empiric penicillin-G/V using logistic regression analysis.
Results
We included 1320 patients. The incidence of hospitalized CAP was 3.1/1000 inhabitants. Median age was 71 years (IQR; 58–81) and in-hospital mortality was 8%. Median duration of antibiotic treatment was 10 days (IQR; 8–12). In total 45% were treated with penicillin-G/V as empiric monotherapy and they did not have a higher mortality compared to patients treated with broader-spectrum antibiotics (OR 0.92, CI 95% 0.55–1.53).
Conclusion
The duration of treatment exceeded recommendations in European guidelines. Empiric monotherapy with penicillin-G/V was commonly used and not associated with increased mortality in patients with mild to moderate pneumonia. Our results are in agreement with current conservative antibiotic strategy as outlined in the Danish guidelines.
Keywords: Community-acquired pneumonia, Incidence, Penicillin, Prognosis
Background
Community-Acquired Pneumonia (CAP) is the most frequent lethal infection in Europe, and a substantial economic problem, mainly due to frequent hospital admission [1–3]. Reported incidence ranges from 1.1 to 8:1000 in different studies [4–6]. Lower respiratory tract infections (LRTI), including CAP, are the most frequent indications for prescribing antibiotics [7]. Due to the difficulties of obtaining a microbiological diagnosis, empirical treatment is far more common than pathogen specific treatment [1]. In Denmark, as in other Scandinavian countries and the Netherlands [5, 6], empirical treatment with penicillin-G/V alone is recommended for patients with a CURB-65 score < 3 and combination therapy for patients with CURB-65 ≥ 3 (http://www.infmed.dk/guidelines). Meanwhile, European and American guidelines recommend combination therapy for all hospitalized patients [7, 8]. To which extent monotherapy with penicillin G/V is used and whether this approach is associated with a poorer prognosis is sparsely documented. Moreover, there is a need to investigate the duration of treatment because of the risk of microbiological resistance, side-effects and higher costs that are associated with long treatment duration. Current European guidelines recommend 5–7 days in a responding patient [7], while Danish guidelines recommend 7 days antibiotic treatment for patients with CURB-65 < 3 and 10–14 days for patients with CURB-65 ≥ 3 (http://www.infmed.dk/guidelines).
Thus, in this study, we describe the epidemiology and characteristics of patients hospitalized with CAP in Denmark. Moreover, we investigate the duration of antibiotic treatment and the association between use of penicillin G/V and prognosis.
Methods
Design, setting and population
We conducted a retrospective cohort study including all patients admitted with CAP to one large regional and two smaller local hospitals in North Zealand, Denmark. The study period was January 1st 2011 until June 30th 2012. The number of cases hospitalized during the study period determined the sample size. The three hospitals supply acute care for 360,000 inhabitants in the region with urban and rural areas and a socio-demographically varied population. Denmark has universal health insurance that covers all acute care including hospital admissions.
Patients admitted with pneumonia were identified by ICD-10 codes registered at discharge. All patients who had one of the following ICD-10 codes either as primary or secondary diagnosis were considered for inclusion: pneumonia J10.0, J11.0, J12.X – J18.x and J69.X, mycoplasma B96.0, klebsiella B96.1, ornithosis A70.X and legionellosis A481.
We reviewed all patient files to assess whether inclusion and exclusion criteria were met. Each patient could only be included once in the study.
The inclusion criteria were: Adult patient’s ≥ 18 years admitted to hospital with CAP. CAP was defined as a new infiltrate on the chest X-ray assessed by the radiologist on-call and at least one of the following symptoms of LRTI: cough, purulent expectoration, fever (≥38.3 °C rectally or ≥37.8 °C auricular) or pathological lung auscultation. Only in-patients were included in the study.
The exclusion criteria were: hospital admission during the last 28 days, active tuberculosis or immunosuppression. Patients were classified as immunosuppressed if they had received treatment with corticosteroids (≥ 20 mg prednisolone-equivalent/day > 14 days), were HIV-positive, had received cancer-chemotherapy during the last 28 days and had neutropenia (neutrophil granulocytes < 1000/μl) or were immunosuppressed after an organ transplantation.
Patients admitted from nursing home and patients with frequent healthcare contacts were considered as having acquired pneumonia in the community [9, 10] and were not excluded from the cohort.
Data collection and variables
We registered data into the CAPNETZ database (www.capnetz.de) and into a local database in EpiData entry 3.1 (www.epidata.com). Data collection was performed from September 2014 until January 2015. All data stem from electronic patient files as well as laboratory, microbiological and radiological databases.
For each patient, we recorded demographics, comorbidities, symptoms, clinical values including CURB-65 [11], and biochemical test results on admission. Patients were divided in to risk groups according to CURB-65 score (score 0–1, 2 or 3–5). The number of co-morbidities was assessed by categorizing patients into three groups based on the sum of conditions (none, one, and more than one). In addition, we recorded microbiological test results, complications and the length of the hospital admission (LOS). We registered the total duration of antibiotic treatment (LOAB) and duration of intravenous antibiotic treatment (LOIVAB). Only the therapeutic agents given initially were recorded. We followed patients for 6 months after admission, to register deaths. Variables not mentioned in the patient file were noted as missing, except for co-morbidities which were recorded as absent if not mentioned.
Outcome measures, exposures and confounders
The main outcomes were in-hospital (short term) and 90-day (long term) mortality. Both outcomes were registered from electronic patient files, which also contain information on deaths outside of hospital. Patients were grouped according to empirical antibiotic treatment, which was the main exposure evaluated in this study. Patients who received empirical treatment with monotherapy penicillin-G/V were compared with patients receiving empirical treatment with all other antibiotics, as monotherapy or in combination. CURB-65 score, number of co-morbidities (0, 1 or >1), admission to the ICU, and age were viewed as potential confounders.
Confounding by indication could influence the results, which was addressed by adjusting for confounders, especially CURB-65 score. Further, we stratified patients by CURB-65 score and compared the two treatment groups within risk groups.
Statistics
We report descriptive statistics at baseline as counts (%) and as mean with standard deviation (SD) or medians with 25th to 75th interquartile range (IQR). When patients died in hospital, we recorded LOS as missing. For patients who died while on antibiotic treatment, we recorded LOIVAB and LOAB as missing values. Group comparisons between the two treatment groups and subgroup analysis were performed with chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables, which did not adhere to the normal distribution. The association between treatment with empiric penicillin-G/V monotherapy and mortality was evaluated with logistic regression analysis and adjusted for potential confounders (CURB65, number of comorbidities, admission to the ICU and age). Missing data are accounted for in Table 1 and only patients with complete data on outcome and potential confounders were included in the adjusted analysis.
Table 1.
Patient characteristics on admission
| Patient characteristics | Study population |
|---|---|
| N = 1320 | |
| Age, median (IQR) | 71 (58–81) |
| Gender, male | 47% (626) |
| Nursing home, Yes | 11% (145) |
| Co-morbidities | |
| COPD | 19% (243) |
| Asthma | 8% (105) |
| Other chronic respiratory diseasea | 4% (49) |
| Malignancy | 9% (119) |
| Chronic heart disease | 24% (320) |
| Chronic liver disease | 1% (12) |
| Chronic kidney failure | 3% (41) |
| Chronic neurological disease | 14% (188) |
| Diabetes | 12% (162) |
| Number of comorbidities | |
| One | 35% (466) |
| More than one | 25% (325) |
| Biochemistry, median (IQR) | |
| CRP, mg/l | 108 (47–214) |
| White blood cell count, x 109/l | 12.2 (9.2–15.7) |
| Urea, mmol/l | 6 (4–9) |
| Vital parameters on admission, median (IQR) | |
| Diastolic blood pressure, mmHg | 75 (65–85) |
| Systolic blood pressure, mmHg | 133 (119–149) |
| Pulse rate,/min | 94 (81–106) |
| Respiratory rate,/min | 20 (16–24) |
| Temperature, °C | 37.7 (37.0–38.5) |
| Oxygen therapy | 58% (744) |
| Findings on chest x-ray | |
| Multilobar infiltrate | 31% (403) |
| Risk assessment | |
| CURB-65: 0–1 | 53% (605) |
| CURB-65: 2 | 29% (330) |
| CURB-65: 3–5 | 18% (202) |
| Antibiotic treatmentb | |
| Penicillin-G/V monotherapyc | 45% (590) |
| Penicillin-G/V in combinationd | 6% (74) |
| Macrolide monotherapy | 3% (36) |
| Other beta-lactam monotherapye | 30% (396) |
| Other beta-lactam in combinationd | 10% (132) |
| Other | 7% (92) |
Data are presented as % (counts), unless otherwise indicated
COPD Chronic obstructive pulmonary disease, CRP C-reactive protein
abronchiectasis, pulmonary fibrosis, sarcoidosis, sleep apnoea and pulmonary cancer. All variables had less than 5% missing except smoking status (294 (22%)) and respiratory rate (144 (11%))
bEmpiric therapy
cRecommended dose: 2 mio units x 3 daily adjusted according to weight and renal function
dPreferably macrolide or quinolone
ePreferable Cefuroxime. Recommended dose: 1500 mg x 3 daily adjusted to weight and renal function
All p-values were two-sided and a p-value of <0.05 was regarded statistically significant.
We calculated the incidence of CAP only for 2011 by dividing the number of new cases by the number of persons at risk (inhabitants 18 years or older).
We used SAS for Windows statistical software, version 7.1 (SAS Institute, Inc., Carey, NC).
Results
Overall 3504 patients were hospitalized and diagnosed with pneumonia during the 18 months study-period (Fig. 1). The majority of patients were excluded because they did not have an infiltrate on the chest x-ray or they had a nosocomial infection. Ultimately 1320 patients were included in this study and constitute the CAP-North cohort.
Fig. 1.
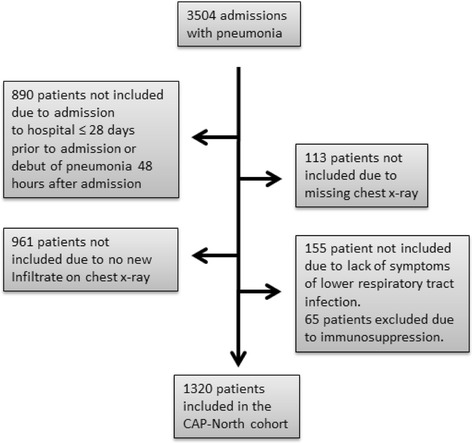
Screening of patients for inclusion
The incidence of hospitalized CAP, calculated on the basis of the population in the region, was 3.1:1000, increasing with age to 8.9:1000 in patients > 65 years and 14.7:1000 in patients >75 years (Fig. 2).
Fig. 2.
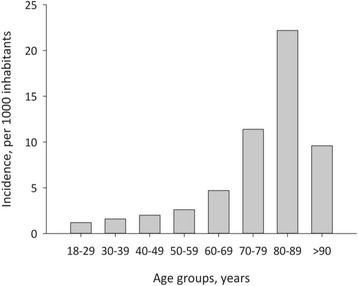
The incidence of community-acquired pneumonia
Baseline characteristics
Patient characteristics on admission are shown in Table 1. The median age was 71 years (IQR; 58–81), 11% were nursing-home residents and 60% had one or more co-morbidities. One or more chronic respiratory conditions were present in 363 (28%) patients. The majority of patients (53%) presented with mild (CURB-65: 0–1) and moderate (29%) (CURB-65: 2) CAP, and 31% had multi-lobular infiltrates on chest x –ray.
Microbiological findings
Overall, 19% (248/1320) of the patients and 23% (248/1083) of those who had a sample taken, had a pathogen detected. Streptococcus pneumoniae (n = 67) was the most common followed by Haemophilus influenza (n = 57), and Mycoplasma pneumoniae (n = 36) (Table 2).
Table 2.
Aetiology by full study population, by all microbiological tested and of pathogen specific testing
| Study population | Microbiologically tested | Pathogen specific tests | |
|---|---|---|---|
| N = 1320 | N = 1083 | ||
| Pathogen | |||
| Streptococcus pneumoniae | 67 (5.1%) | 67 (6.2%) | 67/1079 (6%)a |
| Haemophilus Influenzae | 57 (4.3%) | 57 (5.3%) | 57/1075 (5%)b |
| Mycoplasma pneumoniae | 36 (2.7%) | 36 (3.3%) | 36/213 (17%)c |
| Moraxella catarrhalis | 21 (1.6%) | 21 (2.0%) | 21/1075 (2%)b |
| Staphylococcus aureus | 12 (0.9%) | 12 (1.1%) | 12/1075 (1%)b |
| Pseudomonas aeruginosa | 11 (0.8%) | 11 (1.0%) | 11/1075 (1%)b |
| Escherichia coli | 11 (0.8%) | 11 (1.0%) | 11/1075 (1%)b |
| Legionella pneumophilia | 5 (0.4%) | 5 (0.5%) | 5/288 (1.7%)d |
| Others | 28 (2.1%) | 33 (3.0%) | 33/1083 (3%) |
Data are presented as counts (%)
aPathogen detected by microscopy, culture of sputum or blood or urinary test for Streptococcus pneumoniae
bPathogen detected by microscopy, culture of sputum or blood
cPathogen detected by PCR analysis
dPathogen detected by PCR analysis or urinary antigen test for Legionella pneumophilia
Blood cultures were performed in 74% of the patients and microscopy and culture of sputum samples in 38.5% (Table 3). 66 patients had bacteraemia. In patients diagnosed with Streptococcus pneumonia 60% (40/67) suffered from bacteraemia, while this only applied for 5% (3/57) of patients diagnosed with Haemophilus Influenzae. Microbiological testing was not performed in 237 (18%) patients.
Table 3.
Microbiological findings in different sample types
| Tested n(%) | Positive n (%a) | Three most common pathogens, n (%b) | |
|---|---|---|---|
| N = 1320 | |||
| Blood, (microscopy/culture) | 981 (74%) | 66 (7%) | Streptococcus pneumoniae, 40 (61%) |
| Staphylococcus areus, 4 (6%) | |||
| Escheria coli, 4 (6%) | |||
| Sputum/tracheobronchial secretion, (microscopy/culture) | 501 (38%) | 149 (30%) | Haemophilus influenza, 56 (36%) |
| Streptococcus pneumonia, 27 (18%) | |||
| Moraxella catarrhalis, 21 (14%) | |||
| Test for atypical bacteria, (PCR analysis) | 213 (16%) | 41 (19%) | Mycoplasma pneumoniae, 35 (85%) |
| Legionella pneumophilia, 3 (7%) | |||
| Chlamydia pneumoniae, 2 (5%) | |||
| Urine antigen test | 133 (10%) | 7 (3%) | Streptococcus pneumoniae, 4 (57%) |
| Legionella pneumophilia, 3 (43%) |
aPercentage of tested
bPercentage of positive tests
Empiric antibiotic treatment and clinical outcome
Penicillin-G/V was the most frequently used antibiotic, mainly as mono-therapy (44.7%) or in combination (5.6%), and 63 (4.8%) received the treatment orally. Cephalosporin’s were used as monotherapy in 25.5% of patients. In total, 77% of the patients initially received monotherapy (Table 1).
The median LOAB was 10 days (IQR 8–12) and 70% of the patients were treated for more than 8 days. The median LOIVAB was 3 days (IQR 2–6). Patients were admitted to the hospital for a median of 5 days (IQR 3–8) (Table 4).
Table 4.
The course of community-acquired pneumonia
| Outcome | Study population |
|---|---|
| N = 1320 | |
| Treatment duration, median days (IQR) | |
| IV antibiotica | 3 (2–6) |
| Total antibioticb | 10 (8–12) |
| Length of stay | 5 (3–8) |
| Complications | |
| Any complication | 21% (277) |
| Co-infection | 14% (182) |
| Pneumonia associatedc | 6% (79) |
| Renal failure | 1% (15) |
| Stroke | 1% (12) |
| Acute myocardial infarction | 3% (34) |
| ICU admission | 9.6% (127) |
| Mortality | |
| In-Hospital | 8% (111) |
| 30 days | 11% (149) |
| 90 days | 15% (203) |
| 180 days | 19% (246) |
Data are presented as % (counts), unless otherwise indicated
ICU Intensive care unit, IV Intravenous
All variables have less than 5% missing
aRange: 0–95 days
bRange: 0–97 days
cempyema, including pleural effusion treated as empyema, and lung-abscess
Complications, severe outcome, and mortality were assessed and we found that 270 (21%) had one or more complications, 127 (9.6%) patients were admitted to the ICU, and 111 (8%) died within the hospital. Mortality on days 30, 90 and 180 was 11, 15 and 19% respectively (Table 4).
Empiric treatment with small-spectrum penicillin
Patients treated with penicillin-G/V monotherapy had a lower CURB-65 score, less co-morbidities, fewer admissions to the ICU and a lower mortality in the unadjusted analysis (Table 5). We found no association between penicillin-G/V monotherapy and mortality after adjusting for confounders: CURB-65 score, number of co-morbidities (0, 1 or >1), admission to the ICU, and age (Table 6).
Table 5.
Comparison of patients by antibiotic treatment: penicillin-G/V monotherapy versus broad-spectrum antibiotics
| Monotherapy penicillin-G/V | Other than penicillin-G/V monotherapy | p-value | |
|---|---|---|---|
| n = 590 | n = 725 | ||
| Age, median (IQR) | 70.5 (57–81) | 72 (59–82) | 0.30b |
| CURB-65 | |||
| 0–1 | 58.8% (295/502) | 48.6% (306/630) | <0.0001a |
| 2 | 29.1% (146/502) | 29.1% (183/630) | |
| 3–5 | 12.1% (61/502) | 22.3% (141/630) | |
| Co-morbidities | |||
| 0 | 45.1% (264/585) | 35.1% (252/718) | 0.001a |
| 1 | 32.8% (192/585) | 37.7% (271/718) | |
| > 1 | 22.1% (129/585) | 27.2% (194/718) | |
| ICU admission | 6.1% (36/590) | 12.6% (91/725) | <0.0001a |
| In-hospital mortality rate | 6.4% (38/590) | 10.1% (73/725) | 0.02a |
| 90 day mortality rate | 11.7% (69/590) | 18.5% (134/720) | <0.0009a |
Data are presented as % (counts), unless otherwise indicated
aChi-square
bWilcoxon rank sum
Table 6.
Risk of death in patients treated with penicillin-G/V monotherapy
| Univariate | Multivariatea | |||
|---|---|---|---|---|
| OR (CI) | P-value | OR (CI) | P-value | |
| In hospital mortality | ||||
| Penicillin-G/V monotherapy | 0.62 (0.41–0.93) | 0.02 | 0.92 (0.55–1.53) | 0.74 |
| broad-spectrum (ref) | 1 | - | 1 | - |
| 90 day mortality | ||||
| Penicillin-G/V monotherapy | 0.58 (0.43–0.80) | 0.0008 | 0.77 (0.52–1.14) | 0.19 |
| broad spectrum (ref) | 1 | - | 1 | - |
aAdjusted for CURB-65, number of co-morbidities (0, 1 or >1), admission to an intensive care unit (Y/N) and age
OR (CI) odds ratio with 95% confidence interval. Cases used in the adjusted analysis: 1122
A subgroup analysis of the patients treated with penicillin-G/V monotherapy showed that patients who died in-hospital compared with survivors were older, 81 (IQR; 77–89) versus 70 (IQR; 56–80) years of age (p < 0.0001) and more often nursing home residents, 32% versus 6% (p < 0.0001). Further, they had more comorbidities, 76% versus 53% had ≥ 1 comorbidity (p = 0.002) and a higher CURB-65 score, 90% versus 38% had CURB-65 ≥ 2 (p < 0.0001). We found similar results when looking at 90-days mortality (data not shown). Of the 38 patients who received monotherapy with penicillin G/V and died in-hospital, 71% had an unknown aetiology. Of the remaining 11 patients, 3 were infected with Streptococcus spp. susceptible to penicillin G/V, 3 with Klebsiella pneumonia, 3 with Escherichia coli, 1 with Haemophilus influenza, and 1 with Moraxella catarrhalis. Of the 8 patients with pathogens non-susceptible to penicillin, 3 had a CURB65 score of more than 2 and monotherapy with penicillin was therefore an incorrect choice of empiric antibiotic according to guidelines. One had an unknown CURB-65 score due to missing data.
We addressed whether in-hospital mortality in the different CURB-65 categories was related to choice of empiric treatment and we found that the in-hospital mortality for patients treated with penicillin-G/V monotherapy with a CURB-65 score ≤ 2 was 4.3% and not different from 5.9% for patients treated with other than penicillin-G/V monotherapy (p = 0.26). Sixty-one patients received empiric treatment with monotherapy penicillin-G/V despite of having a CURB-65 score ≥ 3, and the mortality was 19.7% compared to 19.9% for patients treated with other than penicillin-G/V monotherapy (p = 0.98).
Discussion
The incidence of hospitalized CAP was 3.1:1000 adult inhabitants in the Danish region of North Zealand. The incidence increased substantially with age. Nearly half of the patients were treated empirically with penicillin-G/V and the median duration of antibiotic treatment was 10 days. Patients treated with penicillin-G/V had less severe pneumonia than patients treated with broad-spectrum antibiotics or combination therapy and penicillin monotherapy was not associated with increased mortality after adjusting for CURB-65, co-morbidities, admission the ICU and age.
The incidence is comparable to other studies from the UK, USA and Germany reporting incidences of hospitalized CAP of 1.1, 2.5 and 3.0 per 1000 respectively [12–14].
Microbiological sampling was not systematically performed; 18% did not have any microbiological samples taken and a pathogen was detected in only 19% of all the patients. This low yield does not differ from most other studies. Even in prospective studies systematically collecting samples, the diagnostic yield is low. In a recent study only 38% of the patient had a confirmed microbiological diagnosis [13]. In contrast to most other studies however, the empiric therapy was much more restrictive; monotherapy was administered to 77% of the patients and 45% received penicillin-G/V alone. Penicillin-G/V monotherapy is rarely used in southern European countries and the USA [15]. In a European observational multicentre study, 50% of adults hospitalized with CAP received monotherapy but none received penicillin-G/V [16]. Meanwhile, empiric treatment with β-lactam as monotherapy was given to 63% of the patients in a Dutch study [6].
The ERS guidelines for treatment of hospitalized CAP do not recommend penicillin as monotherapy [7] whereas, Danish guidelines during the current study recommended benzylpencillin as monotherapy for patients with a CURB-65/CRB-65 score < 3, and combination therapy with quinolones or macrolides for CURB-65 ≥ 3 (http://www.infmed.dk/guidelines). This is in line with guidelines from other Scandinavian countries and the Netherlands [5, 6]. The differences in recommendations are due to a low penicillin resistance of Streptococcus pneumonia in Denmark of < 1% whereas it is, e.g., in Spain > 10% (http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/database/Pages/map_reports) and a very conservative antibiotic policy encouraging small spectrum antibiotic.
In our study, patients treated with penicillin-G/V had less severe CAP. There were no differences in mortality associated with the use of penicillin-G/V or broader-spectrum antibiotics in the adjusted analysis, similarly to the findings in a Danish study from 2001 [17]. Because penicillin monotherapy was given to patients with mild disease (CURB-65 < 3), mortality is expected to be low independently of the treatment and we cannot conclude whether mortality would have been lower if broad spectrum antibiotic or combination therapy were given. Mortality in those with CURB-65 ≤ 2 was still substantial in both treatment groups (4.3–5.9% respectively) indicating that mortality is not solely related to initial severity assessment, but also to comorbidities and complications during admission [18, 19].
Patients who were treated with empiric penicillin-G/V monotherapy and died, were older, had more co-morbidities and a higher CURB-65 score than survivors. Penicillin-G/V monotherapy may have been insufficient in these patients and apparently guidelines were not followed in some cases. Pathogens non-susceptible to penicillin were found in 4 patients who were treated with penicillin-G/V monotherapy, had a CURB-65 score less than 3 and died. Although guidelines were followed for these patients, they received empirical treatment not matching their causative pathogen. However, due to the retrospective design, we cannot conclude if the poor outcome was due to monotherapy with penicillin and if outcome would have been better with another antibiotic agent.
Overall, penicillin-G/V appears safe in the correct patient group, namely those hospitalized with mild to moderate CAP (CURB-65 < 3) in a setting with a low degree of resistance of Streptococcus pneumonia against penicillin.
In our study, the median duration of treatment with antibiotics was 10 days and 70% of patients received more than the maximum 8 days which is recommended by European guidelines for responding patients [7]. Similarly, Reissig et al [20] and the REACH study [16] reported 11 and 10 days respectively. Duration of 10–11 days, however, exceeds recommendations in European and Danish guidelines ([7], http://www.infmed.dk/guidelines). Danish guidelines recommend duration of 10–14 days only for patients with CURB-65 ≥ 3, which can explain extended treatment duration for a smaller fraction of the study population. Due to the retrospective nature, the unsystematic microbiological sampling, and the low proportion of positive microbiological findings, we cannot draw firm conclusion on significant risk factors for prolonged treatment duration.
Recent studies have shown that antibiotic treatment for 3–5 days for mild to moderate CAP does not impair effectiveness or safety [21–23]. There is a need to focus on assessing alternative strategies to reduce antibiotic use in order to minimize the risk of adverse events and antibiotic resistance associated with extended duration of antibiotic therapy.
Comparing patients in the CAP-North cohort with other European CAP cohorts [14, 16, 24, 25] some differences were apparent. Compared with patients from the CAPNETZ cohort [25], our patients were older (mean age 72 versus 66), more frequently women (60% versus 47%), presented with more severe CAP (18% CURB-65 > 2 versus 4% CRB-65 > 2), and had a higher 30 days mortality (11% versus 4.3%). The differences could be due to our inclusion exclusively of hospitalized patients, as well as inclusion of all patients with severe CAP who are often challenging to include in a prospective study.
The major strength of this study is that we were able to identify all patients hospitalized and diagnosed with pneumonia in our region, due to our complete regional dataset. Our design did however not allow us to account for patients with CAP who were given an inaccurate ICD-10 code. We ensured that only patients with CAP were included by reviewing every patient-file for symptoms on admission, hospitalization within the last 28 days, and chest-x-ray for infiltrates. Moreover, the three hospitals cover both urban and rural areas of the capital region in Denmark and thus we believe our cohort to be representative of patients hospitalized with CAP in developed countries. Thus, our cohort represents a “real-life” population of patients hospitalized with CAP.
The main limitation of this study is the retrospective design, which made us dependent on the data recorded in the patient files and the results should be interpreted accordingly.
The cohort consists of a heterogeneous population, specifically patients who received treatment with monotherapy penicillin G/V were less sick, than patient who received broader spectrum antibiotics, according to the CURB65-score. We adjusted for this in the regression analysis, but we cannot exclude that residual confounding exists.
We were not able to account for changes in antibiotic therapy after initial assessment, i.e. due to complications or microbiological findings. Further, we could not account for the exact dosages prescribed but only recommended dosages. Furthermore, treatment duration varied among patients.
Approximately 50% of patients had a CURB-65 score of 0–1. It is suggested that CAP patients with a CURB-65 score of 0–1 can be treated as outpatients, albeit CURB-65 has several limitations and should not be the sole basis for deciding treatment allocation [26–28].
Conclusion
The incidence of patients hospitalized with CAP was 3.1:1000. Nearly half of the patients were treated with empiric penicillin-G/V monotherapy without an increase in mortality. Treatment duration exceeded recent guidelines. Our results are in agreement with the current conservative antibiotic strategy outlined in Danish guidelines, recommending penicillin treatment.
It is important to ensure compliance with guidelines concerning treatment duration and validation of strategies to reduce duration of treatment in order to minimize unnecessary side-effects. Finally prospective, randomized control trials would be necessary to confirm whether empiric monotherapy with penicillin-G/V is recommendable.
Acknowledgements
Acknowledgements go to our collaborators in The CAPNETZ stiftung, the CAPNETZ study group and the Pneumonia Research Group – Nordsjællands Hospital, including research nurse Gudrun Kaldan and medical student Sara Falk Jensen.
Funding
The study was supported by the Research council - Nordsjællands Hospital, Olga Brydes foundation, and Kaptajnløjtnant Jensen og Hustrus foundation. None of the funders had any influence on the study design, interpretation of data or content of the manuscript.
Availability of data and materials
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
GBE contributed to the study design, acquisition of data, performed the statistical analysis and interpretation of data and drafted the manuscript primarily. AVJ contributed to the study design, acquisition of data, interpretation of data, and critically revised the manuscript. SA contributed to acquisition of data and critically revised the manuscript. PTP contributed to acquisition of data and critically revised the manuscript. BL contributed to the study design, interpretation of data and critically revised the manuscript. CP contributed to the study design, interpretation of data, drafting of the manuscript, and critically revised the manuscript. GR contributed to the study design, interpretation of data, drafting of the manuscript, and critically revised the manuscript. PR contributed to the study design, interpretation of data, drafting of the manuscript, critically revised the manuscript and supervised the work. All authors have given final approval of the version to be published and agreed to be accountable for all aspects of the work.
Competing interests
GR (Dr. Rohde) reports personal fees from Pfizer, Boehringer Ingelheim, Solvay, GSK, Essex Pharma, MSD and Novartis for lectures including service on speaker bureaus outside the submitted work and personal fees from GSK for travel/accommodations/meeting expenses, outside the submitted work.
PR (Dr. Ravn) reports personal fees from MSD, Abb Vie and CSL Behring as invited speaker, outside the submitted work. Dr. Ravn reports personal fees from Statens Serum Institute as data safety monitoring board member, outside the submitted work. Dr. Ravn reports non-financial research collaboration with Astellas outside the submitted work.
The remaining authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the Danish Health and Medicines Authority (3-3013-340/1/) and by the Danish Data Protection Agency (HIH-2013-017). Danish legislation does not require informed consent for register-based studies.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CAP
Community-acquired pneumonia
- ICU
Intensive care unit
- LOAB
Length of antibiotic treatment
- LOIVAB
Length of intravenous antibiotic treatment
- LOS
Length of stay
- LRTI
Lower respiratory tract infection.
References
- 1.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 2.Blasi F, Mantero M, Santus P, Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect. 2012;18(Suppl 5):7–14. doi: 10.1111/j.1469-0691.2012.03937.x. [DOI] [PubMed] [Google Scholar]
- 3.Marti C, Garin N, Grosgurin O, Poncet A, Combescure C, Carballo S, Perrier A. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16(4):R141. doi: 10.1186/cc11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bewick T, Sheppard C, Greenwood S, Slack M, Trotter C, George R, Lim WS. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax. 2012;67(6):540–545. doi: 10.1136/thoraxjnl-2011-201092. [DOI] [PubMed] [Google Scholar]
- 5.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L, CDC EPIC Study Team Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewig S, Birkner N, Strauss R, Schaefer E, Pauletzki J, Bischoff H, Schraeder P, Welte T, Hoeffken G. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax. 2009;64(12):1062–1069. doi: 10.1136/thx.2008.109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarb P, Goossens H. European Surveillance of Antimicrobial Consumption (ESAC): value of a point-prevalence survey of antimicrobial use across Europe. Drugs. 2011;71(6):745–755. doi: 10.2165/11591180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Spindler C, Stralin K, Eriksson L, Hjerdt-Goscinski G, Holmberg H, Lidman C, Nilsson A, Ortqvist A, Hedlund J, Community Acquired Pneumonia Working Group of The Swedish Society of Infectious Diseases Swedish guidelines on the management of community-acquired pneumonia in immunocompetent adults--Swedish Society of Infectious Diseases 2012. Scand J Infect Dis. 2012;44(12):885–902. doi: 10.3109/00365548.2012.700120. [DOI] [PubMed] [Google Scholar]
- 9.Huijts SM, van Werkhoven CH, Boersma WG, Buijs J, Buunk G, Compaijen CJ, van Elde LJ, Gisolf JE, van der Kam R, Kluytmans JA, Kuipers BA, Mager JJ, Oppedijk B, Palmen F, Prins JM, van Reemst B, Silbermann MH, van Tiel FH, van der Wall E, van der Werf TS, Bonten MJ. Guideline adherence for empirical treatment of pneumonia and patient outcome. Treating pneumonia in the Netherlands. Neth J Med. 2013;71(10):502–7. [PubMed]
- 10.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, van der Heijden G, Read R, Verheij TJ, Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect. 2011;17(Suppl 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, Macfarlane JT, Read RC, Roberts HJ, Levy ML, Wani M, Woodhead MA, Pneumonia Guidelines Committee of the BTS Standards of Care Committee BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;Suppl 3:iii1–iii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 12.Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330–339. doi: 10.1093/cid/cit734. [DOI] [PubMed] [Google Scholar]
- 13.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratala J, El Solh AA, Ewig S, Fey PD, File TM, Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, Lewis SA, Macfarlane JT. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres A, Blasi F, Peetermans WE, Viegi G, Welte T. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis. 2014;33(7):1065–1079. doi: 10.1007/s10096-014-2067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasi F, Garau J, Medina J, Avila M, McBride K, Ostermann H, REACH study group Current management of patients hospitalized with community-acquired pneumonia across Europe: outcomes from REACH. Respir Res. 2013;14:44. doi: 10.1186/1465-9921-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirk O, Glenthoj J, Dragsted UB, Helweg-Larsen J, Aru TM, Benfield TL, Jensen K, Vestbo J, Lundgren JD. Penicillin as empirical therapy for patients hospitalised with community acquired pneumonia at a Danish hospital. Dan Med Bull. 2001;48(2):84–88. [PubMed] [Google Scholar]
- 18.Mortensen EM, Coley CM, Singer DE, Marrie TJ, Obrosky DS, Kapoor WN, Fine MJ. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162(9):1059–1064. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 19.Sintes H, Sibila O, Waterer G, Chalmers J. Severity assessment tools. In: Chalmers J, Pletz M, Aliberti S, editors. European respiratory monograph; community-acquired pneumonia. European Respiratory Society Publications; 2014:88.
- 20.Reissig A, Mempel C, Schumacher U, Copetti R, Gross F, Aliberti S. Microbiological diagnosis and antibiotic therapy in patients with community-acquired pneumonia and acute COPD exacerbation in daily clinical practice: comparison to current guidelines. Lung. 2013;191(3):239–246. doi: 10.1007/s00408-013-9460-x. [DOI] [PubMed] [Google Scholar]
- 21.Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, Grammatikos AP, Athanassa Z, Falagas ME. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs. 2008;68(13):1841–1854. doi: 10.2165/00003495-200868130-00004. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52(10):1232–1240. doi: 10.1093/cid/cir063. [DOI] [PubMed] [Google Scholar]
- 23.Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120(9):783–790. doi: 10.1016/j.amjmed.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Kothe H, Bauer T, Marre R, Suttorp N, Welte T, Dalhoff K, Competence Network for Community-Acquired Pneumonia study group Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J. 2008;32(1):139–146. doi: 10.1183/09031936.00092507. [DOI] [PubMed] [Google Scholar]
- 25.Bauer TT, Ewig S, Marre R, Suttorp N, Welte T, CAPNETZ Study Group CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260(1):93–101. doi: 10.1111/j.1365-2796.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 26.Aliberti S, Ramirez J, Cosentini R, Brambilla AM, Zanaboni AM, Rossetti V, Tarsia P, Peyrani P, Piffer F, Blasi F. Low CURB-65 is of limited value in deciding discharge of patients with community-acquired pneumonia. Respir Med. 2011;105(11):1732–1738. doi: 10.1016/j.rmed.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Chalmers JD, Mandal P, Singanayagam A, Akram AR, Choudhury G, Short PM, Hill AT. Severity assessment tools to guide ICU admission in community-acquired pneumonia: systematic review and meta-analysis. Intensive Care Med. 2011;37(9):1409–1420. doi: 10.1007/s00134-011-2261-x. [DOI] [PubMed] [Google Scholar]
- 28.Kolditz M, Ewig S, Schutte H, Suttorp N, Welte T, Rohde G, the CAPNETZ study group: Assessment of oxygenation and comorbidities improves outcome prediction in patients with community-acquired pneumonia with a low CRB-65 score. J Intern Med. 2015;278(2):193–202. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.


