Abstract
Gliomas represent the most common primary intraparenchymal tumors of the central nervous system in adults and children and are a genetic and phenotypic heterogeneous group. Large multi-institutional studies and The Cancer Genome Atlas have provided firm insights into the basic genetic drivers in gliomas. The main molecular biomarkers routinely applied to evaluate diffuse gliomas include MGMT promoter methylation, EGFR alterations (eg, EGFRvIII), IDH1 or IDH2 mutations, and 1p19q co-deletion. Many of these markers have become standard of care for molecular testing and prerequisites for clinical trial enrollment. Other recent biomarkers include TERT promoter and ATRX mutations, alterations that identify specific molecular subgroups of diffuse gliomas with biological and clinical relevance. It has also become apparent that distinctive patterns of molecular genetic evolution develop in the context of current therapeutic regimens. Important insights have also been uncovered in the field of pediatric glioma, including the identification of recurrent mutation, fusion, and/or duplication events of the BRAF, FGFR1, MYB, and MYBL1 genes in pediatric low-grade gliomas, mutations affecting histone components (H3F3A p.K27M or p.G34) in pediatric high-grade gliomas, and aggressive subsets developing in midline central nervous system structures. Here, we summarize current concepts in molecular testing for glial tumors, including recent findings by large-scale discovery efforts and technologic advances that are affecting routine diagnostic work.
CME Accreditation Statement: This activity (“JMD 2016 CME Program in Molecular Diagnostics”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“JMD 2016 CME Program in Molecular Diagnostics”) for a maximum of 36 AMA PRA Category 1 Credit(s)™. Physicians should claim only credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Molecular Pathology of Glial Neoplasms: General Concepts
Glial neoplasms encompass a heterogeneous group characterized predominantly by an astrocytic or oligodendroglial morphology. The group of diffusely infiltrating astrocytomas is the most frequent and includes diffuse astrocytoma (World Health Organization grade II), recognized by cytologic atypia and low-moderate cellularity; anaplastic astrocytoma (World Health Organization grade III), characterized by moderate-high cellularity and obvious mitotic activity; and, at the end of the spectrum, glioblastoma (World Health Organization grade IV), containing necrosis or microvascular proliferation. Glioblastoma is also a morphologically heterogeneous neoplasm, with several variants and patterns.1 For example, the small cell astrocytoma pattern demonstrates minimal pleomorphism, but it tends to affect older age groups, has an aggressive course and frequent epidermal growth factor receptor (EGFR) (amplification in 70%), and phosphatase and tensin homolog (PTEN)/10q (approximately 100%) alterations. Conversely, giant cell glioblastoma is characterized by voluminous cell size and frequent TP53 mutations (83%) and aurora kinase B (AURKB) overexpression. Epithelioid glioblastoma may resemble a variety of non-central nervous system (CNS) tumor types, and of relevance has B-Raf proto-oncogene, serine/threonine kinase (BRAF) p.V600E mutations in approximately 50% of the cases. Gliosarcoma is characterized by neoplastic glial and mesenchymal components and a molecular profile similar to conventional glioblastoma but a lower frequency of EGFR amplification (<8%). Glioblastoma may be further subdivided on a clinical basis into primary and secondary subtypes, the latter evolving from documented or putative lower grade astrocytoma precursors and sharing with them early genetic driver events, for example, NADP+-dependent isocitrate dehydrogenase 1 or 2 (IDH1 or IDH2) gene mutations.2
Oligodendroglial tumors include low-grade oligodendroglioma (World Health Organization grade II) and anaplastic oligodendroglioma (World Health Organization grade III). The hallmark of oligodendroglial tumors is the presence of cellular monotony, including round nuclei with fine chromatin and a small nucleolus. Brisk mitotic activity, endothelial hypertrophy, and necrosis characterize the anaplastic oligodendrogliomas. The category of mixed glioma or oligoastrocytoma has increasingly fallen out of favor, given its low reproducibility and the lack of a distinguishing molecular signature from either astrocytic or oligodendroglial neoplasms in almost all instances.3
The circumscribed glioma group has a predilection for children and young adults and includes pilocytic astrocytoma (World Health Organization grade I), subependymal giant cell astrocytoma (World Health Organization grade I), and pleomorphic xanthoastrocytoma (World Health Organization grade II). At the molecular level, these neoplasms have frequent alterations in components of the mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) signaling pathways, often as the single genetic driver.
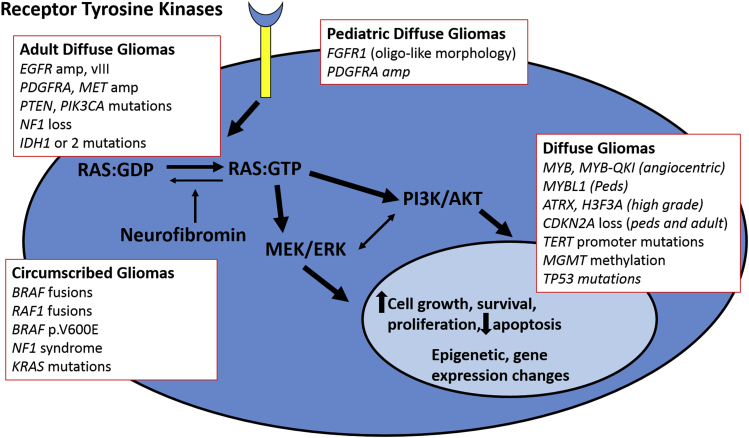
Although classic histology-based grading schemes have proven valuable in neuro-oncology practice for decades, it has been increasingly recognized that molecular genetics-based classification schemes provide robust prognostic information. The identification of key driver mutations in glial tumors, including activating mutations in oncogenes and inactivation of tumor suppressor genes, has been increasingly facilitated by the greater availability of high-throughput molecular assays and the development of immunohistochemical tests that more specifically identify key alterations in a practical manner.4 Updated diagnostic categories have been embodied in the recent International Society of Neuropathology-Haarlem consensus recommendations that advocate for an integrated histopathologic and molecular diagnosis approach in brain tumor classification schemes.5 An updated World Health Organization classification of brain tumors has also incorporated molecular genetic information into relevant entities and variants.1 In addition, it has been increasingly recognized that brain tumors that demonstrate similar morphologic features in children and adults represent distinct biological subgroups with varying proportions of alterations in genetic drivers and signaling pathways (Figure 1 and Tables 1 and 2). This is true regarding both low- and high-grade gliomas.
Figure 1.
Signaling pathways relevant to glial neoplasms in adults and children. A variety of signaling pathways are activated through mutations of oncogenes or tumor suppressor genes in diffuse gliomas in adults and children. MAPK pathway activation through receptor tyrosine kinase activation or downstream gene mutations and rearrangements (BRAF, NF1, RAS) is a universal feature of glial neoplasms. The PI3K/mTOR pathway is also activated through receptor tyrosine kinase activation and downstream gene mutations (PTEN, PI3KCA). Other relevant alterations include mutations affecting metabolic and epigenetic pathways (IDH1, IDH2, H3F3A) and telomere activity and/or maintenance (TERT, ATRX). Although there is some overlap regarding the pathways activated in adult and pediatric gliomas, the specific alterations and/or frequencies differ in these two broad subgroups. ERK, extracellular signal-regulated kinase; MEK, mitogen-activated protein kinase kinase; PI3K, phosphatidylinositol 3-kinase.
Table 1.
Selected Molecular Markers in Gliomas of Adults
| Marker | Full name | Gene location | Tumor type (% altered) | Function | Alteration type | Molecular Techniques | Biomarker Type | Therapeutic approaches |
|---|---|---|---|---|---|---|---|---|
| MGMT | O6-methylguanine-DNA methyltransferase | 10q26 | Glioblastoma (48.5%), lower grade glioma (85%–100% IDH mutants) | Removes alkyl adducts from the O6 position of guanine | DNA methylation (promoter) | MS-PCR (paraffin), bisulfite sequencing (gold standard), pyrosequencing | Predictive | Temozolomide |
| EGFR | Epidermal growth factor receptor | 7p12 | Glioblastoma (57%) | RAS/MAPK and PI3K pathway | Amplification, exome deletions (EGFRvIII), point mutations (rare) | FISH, aCGH, immunohistochemistry (EGFRvIII), PCR | Predictive (for anti-EGFRvIII immune therapies) | Tumor vaccines (eg, rindopepimut) |
| PDGFRA | Platelet-derived growth factor receptor | 4q12 | Glioblastoma (13%) | RAS/MAPK and PI3K pathway | Amplification, mutations, exome deletions | FISH, aCGH (amplifications), sequencing techniques (mutations) | Diagnostic | PDGFRA inhibitors (eg, Imatinib) |
| PTEN | Phosphatase and tensin homolog | 10q23.3 | Glioblastoma (25%–35%) | Phosphatase (preferentially dephosphorylates phosphoinositide substrates) | Mutations, deletions | Sequencing techniques (mutations), FISH, aCGH/SNP array (copy number), immunohistochemistry (protein loss) | Prognostic | mTOR inhibitors (eg, everolimus) |
| TP53 | Tumor protein p53 | 17p13.1 | Glioblastoma (25%–35%), lower grade adult glioma (>70%) | Regulates expression of target genes, inducing cell cycle arrest, apoptosis, DNA repair, or changes in metabolism | Mutations | Sequencing techniques, immunohistochemistry (strong positivity a surrogate for alterations in the pathway) | Diagnostic/prognostic | NA |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A | 9p21 | Glioblastoma (61%), lower grade glioma (11%) | Inhibitors of CDK4 kinase; ARF stabilizes p53 | Mutations, homozygous deletions | Sequencing techniques, FISH, aCGH/SNP array | Prognostic | NA |
| IDH1 and IDH2 | Isocitrate dehydrogenase (NADP(+)) | 2q33.3 (IDH1) and 15q26.1 (IDH2) | Primary glioblastoma (6%), lower grade glioma/secondary glioblastoma (>80%) | Affect citrate metabolism, leading to 2-hydrodxyglutarate metabolite | Mutations | Sequencing techniques, immunohistochemistry (R132H) | Prognostic, diagnostic | AG-120 (small molecule inhibitor) |
| 1p19q | Multiple genes | Near whole arm deletion | Oligodendrogliomas (>90%) | NA | Deletions | FISH, microsatellite PCR testing aCGH/SNP array (shows extent of deletion, more specific) |
Diagnostic, prognostic, predictive | PCV chemotherapy |
| CIC | Capicua transcriptional repressor | 19q13.2 | 1p19q codeleted oligodendroglioma (62%) | HMG-box transcriptional repressors | Deletion/mutation | Sequencing | Diagnostic, prognostic, predictive (but 1p19q more widely used) | PCV chemotherapy |
| FUBP1 | Far upstream element binding protein 1 | 1p31.1 | 1p19q codeleted oligodendroglioma (29%) | DNA-binding protein involved in MYC regulation | Deletion/mutation | Sequencing | Diagnostic, prognostic, predictive (but 1p19q more widely used) | PCV chemotherapy |
| ATRX | α-Thalassemia/mental retardation syndrome X-linked | Xq21.1 | Lower grade astrocytomas (86% of IDH mutant, 1p19q intact), pediatric glioblastoma (approximately 45%) | Chromatin remodeling, leads to alternative lengthening of telomeres phenotype | Mutation | Sequencing techniques, immunohistochemistry (protein loss) | Diagnostic, prognostic | DNA damaging agents |
| TERT | Telomerase reverse transcriptase | 5p15.33 | Primary glioblastomas (>80%), oligodendrogliomas (up to 96%) | Telomere maintenance | Promoter mutation | Sequencing techniques | Diagnostic, prognostic | NA |
Prevalence of specific alterations are obtained predominantly from The Cancer Genome Atlas data sets.
aCGH, array comparative genomic hybridization; ARF, alternative reading frame; CDK4, cyclin-dependent kinase 4; FISH, fluorescence in situ hybridization; HMG, high mobility group; MAPK, mitogen-activated protein kinase; MS-PCR, methylation-specific PCR; mTOR, mammalian target of rapamycin; NA, not applicable; PCV, procarbazine, CCNU, and vincristine; PDGFRA, platelet-derived growth factor receptor α polypeptide; PI3K, phosphatidylinositol 3-kinase; SNP, single nucleotide polymorphism.
Table 2.
Selected Molecular Markers in Gliomas of Children
| Marker | Full name | Gene location | Tumor type (% altered) | Function | Alteration type | Molecular techniques | Biomarker type | Therapeutic approaches |
|---|---|---|---|---|---|---|---|---|
| BRAF | B-Raf proto-oncogene, serine/threonine kinase | 7q34 | Pilocytic astrocytoma (85%), pleomorphic xanthoastrocytoma (50%–70%), pediatric diffuse astrocytoma (23%) | MAPK signaling | Fusions, point mutations, small deletions/insertions | FISH, aCGH, RT-PCR (fusion) Sequencing techniques (mutations) |
Diagnostic, prognostic, Predictive | BRAF inhibitors (dabrafenib, vemurafenib), MEK inhibitors (selumetinib and binimetinib) |
| H3F3A | H3 histone, family 3A | 1q42.12 | Diffuse midline gliomas (50%–80%) | Chromatin structure, gene transcription | Mutation | Sequencing techniques, immunohistochemistry (mutant protein) | Diagnostic, prognostic | NA |
| MYB | v-myb avian myeloblastosis viral oncogene homolog | 6q23.3 | Approximately 100% of angiocentric gliomas (predominantly through MYB-QKI fusions) | Transcriptional regulator | Rearrangements | Sequencing techniques, FISH, aCGH | Diagnostic | NA |
| MYBL1 | v-myb avian myeloblastosis viral oncogene homolog-like 1 | 8q13.1 | Diffuse pediatric astrocytoma (28%) | Transcriptional regulator | Rearrangements | Sequencing techniques, FISH, aCGH | Diagnostic | NA |
| FGFR1 | Fibroblast growth factor receptor 1 | 8p11.23-p11.22 | Low-grade neuroepithelial tumors with oligodendrocyte-like cells (40%–82%), pilocytic astrocytoma (6%) | MAPK, PI3K/mTOR signaling activation | Mutations, TK duplications | Sequencing techniques | Diagnostic, prognostic, predictive | FGFR inhibitors |
aCGH, array comparative genomic hybridization; FGFR, fibroblast growth factor receptor; FISH, fluorescence in situ hybridization; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; NA, not applicable; PI3K, phosphatidylinositol 3-kinase; TK, tyrosine kinase.
Adult Glioblastoma
Comprehensive molecular profiling of glioblastoma resulting in well-defined subclasses was facilitated by global gene expression array studies. Phillips et al6 proposed three different molecular subtypes, using a group of 76 high-grade diffuse gliomas (World Health Organization grade III and IV). These subgroups were labeled proneural, mesenchymal, and proliferative, and they formed the basis of subsequent, more comprehensive molecular classification efforts. These molecular subgroups were independently validated in a set of glioblastomas. The main outcome of these early gene expression profiling efforts was that molecular profiling was a more robust prognosticator than histopathologic classification alone and provide molecular classes with biological relevance.
One of the most important developments in the neuro-oncology community was the selection of glioblastoma as the model for The Cancer Genome Atlas (TCGA) first study. An early report was published in 2008,7 which included not only gene expression data as previous efforts but also DNA copy number and gene methylation in 206 glioblastomas. Standard gene sequencing was also performed, focusing on approximately 600 candidates in a representative group of tumors. This successful effort validated well-recognized glial oncogenes (EGFR, CDK4, PDGFRA, MDM4, MET) and tumor suppressor genes (CDKN2A/B, PTEN, RB1) that were altered at variable rates. In addition, a total of three molecular core pathways (ie, RB1, TP53, and receptor tyrosine kinase) were elucidated as aberrant in most tumors studied. PTEN encodes a phosphatidylinositol triphosphate phosphatase that negatively regulates AKT/protein kinase B signaling and is mutated in 25% to 35% of glioblastoma. TP53 encodes one of the most frequently mutated tumor suppressors in cancer, with important roles in the cellular response to DNA damage. The frequency of TP53 mutation in glioblastoma is similar to that of PTEN mutations (approximately 25% to 35%), but it is higher in secondary glioblastoma (approximately 70%), where it frequently co-occurs with IDH and ATRX mutations. The RB1 core pathway is also frequently activated in glioblastoma as confirmed in the TCGA data, where it frequently (approximately 60%) occurs through mutations in the closely placed cyclin-dependent kinase inhibitors 2A and B (CDKN2A/B) genes. An important novel finding in the tumor suppressor group was that the neurofibromin 1 (NF1) gene, a negative regulator of RAS signaling, was identified to be mutated in a subset of glioblastoma (14%), which demonstrated that NF1 was a key tumor suppressor, not only in neurofibromatosis type 1–associated gliomas but in sporadic glioblastomas as well. Concurrently, a major whole exome sequencing study of glioblastoma identified IDH1 and IDH2 mutations in another subset of glioblastomas, linking genetic alterations in metabolic enzymes to gliomagenesis.8
Subsequent multidimensional studies confirmed the power of the TCGA data in identifying robust molecular subgroups of glioblastomas defined not only by gene expression profiling but also by enrichment for specific gene alterations,9, 10, 11 including the classic (high-level EGFR amplification, chromosome 10 or PTEN loss), mesenchymal (NF1 gene alterations), and proneural (PDGFRA gains, IDH1 and TP53 mutations) subtypes. Follow-up analysis of 543 glioblastomas by the TCGA reinforced prior findings.12 This analysis also identified several novel mutated genes, including LZTR1, a putative transcriptional regulator, the telomerase reverse transcriptase (TERT) promoter, as well as complex rearrangement of receptor genes, including EGFR and PDGFRA. TERT promoter mutations have emerged as one of the more frequent somatic events in adult gliomas, even more frequent than IDH mutations, because they occur in most primary glioblastomas and oligodendroglial tumors.13
Historically, concurrent chromosome 7 gain and chromosome 10 loss (Chr 7+/Chr 10−) has been considered a cytogenetic hallmark of glioblastoma. In a comprehensive analysis of TCGA data of 1122 diffuse gliomas, an important finding by Ceccarelli et al14 was that almost all IDH wild-type gliomas with Chr 7+/Chr 10− had TERT promoter mutation or overexpression. Conversely, nearly one-half of IDH wild-type gliomas lacking Chr 7+/Chr 10− had TERT promoter mutation or overexpression, suggesting that TERT alterations may precede and even play a role in the development of these characteristic cytogenetic abnormalities.
The application of high-density methylation arrays has also proven a robust technique for molecular tumor subclassification of primary brain neoplasms in multiple studies.11, 15 This attests to the evolving evidence of how epigenetic states affect many if not all basic cell processes, including cell signaling, patterns of genetic alterations, and even the neoplastic microenvironment, which are reflected in epigenetic signatures and brain tumor cell identity.16 A popular platform in many molecular subclassification studies of brain tumors is the illumina Infinium HumanMethylation450 (450K) array, which also provides concurrent copy number analysis (an 850K array has been validated more recently).17 Methylation profiling using this platform was successfully applied to a large set of adult and pediatric glioblastoma, specifying six distinct subgroups enriched for specific DNA mutations (eg, codon 132 mutations of the IDH1 gene and codons 27 and 34 mutations of the H3F3A gene), as well as distinct clinical features with regard to age, anatomic location, and outcome.15 A key finding in the subsequent study by Ceccarelli et al14 of diffuse gliomas was also that epigenetic subgroups provide independent prognostic information from age and tumor grade. Global methylation profiling in brain tumor analysis also resulted in the identification of the CpG island methylator phenotype (CIMP) in glioblastoma by Noushmehr et al11 through analysis of TCGA data. The CIMP phenotype, defined by CpG island methylation in a subgroup of genes of a subset of tumors, was initially discovered in colon cancer, but it was later studied in a variety of tumor types. We now know that the clinical and biological relevance of CIMP is tumor specific. For example, CIMP in glioblastomas is associated with the proneural molecular subgroup, a more favorable molecular subgroup of glioblastoma, and some studies have concluded that it represents a direct consequence of mutations in IDH1 or IDH2 genes.18
Primary and Secondary Glioblastoma
From clinical presentation, glioblastomas have been broadly separated into primary and secondary types. Primary glioblastomas are by far the most common (>90%) and are characterized by a short clinical evolution (arising de novo), without evidence of a precursor lesion, and in older patients. Secondary glioblastomas represent a minority of the tumors (<10%), and by definition develop from a clinical or pathologically verified lower grade precursor. Relevant to molecular diagnostics, IDH mutations have emerged as robust molecular markers for secondary glioblastoma.2 Other known alterations in this group include TP53 (approximately 65%) and ATRX mutations (approximately 65%), as well as 19q loss (approximately 50%). Conversely, primary glioblastomas have a higher frequency of EGFR amplification (approximately 35%), PTEN mutation (approximately 25% to 35%), and whole chromosome 10 loss (>50%).
Post-Treatment Glioblastoma
Comprehensive molecular profiling has also provided new insights into molecular changes that develop after treatment in diffuse gliomas and the value of extending molecular diagnostic testing over time in specimens not only obtained at first diagnosis but also on progression. TCGA studies demonstrated that in the previously treated samples that were MGMT methylated, there was a predominance of transitions from G*C to A*T at non-CpG regions, particularly in mismatch repair genes.7 Subsequent studies using high-resolution genomic and whole exome sequencing have documented several important observations in post-treatment glioblastoma, involving linear or divergent/branched models of clonal evolution during progression and emergence of treatment resistance: the emergence of subclonal mutations associated with TP53 pathway deregulation,19 a hypermutation phenotype in secondary (but not primary) glioblastoma,20 and the development of post-treatment genetic drivers in the RB and AKT/mTOR pathways.21
Adult Lower Grade Glioma
The TCGA effort has also focused on the group of tumors labeled lower grade glioma. This heterogeneous group includes grade II and III astrocytomas, oligoastrocytomas, and oligodendrogliomas, that is, diffuse gliomas other than glioblastomas. The results on the first set of 293 tumors were recently published.22 The most consistent molecular finding in these tumors is the high frequency of TERT promoter and IDH mutations, as in other studies.13 Lower grade gliomas cluster in three main molecular subgroups, which are more strongly associated with prognosis than traditional histology. These subgroups include i) tumors with 1p19q codeletion and IDH mutations, a high frequency of CIC, FUBP1, NOTCH1, and TERT promoter mutations, associated with oligodendroglial histology and the best prognosis; ii) tumors with IDH mutations lacking 1p19q co-deletion, containing a high frequency of TP53 and ATRX mutations, and associated with astrocytic morphology and an intermediate prognosis; and iii) tumors with wild-type IDH, TERT promoter mutations, associated with astrocytic morphology and a poor prognosis similar to glioblastoma. It must be emphasized that these molecular groups have intrinsic importance, not only related to their robust prognostic power but also because they identify biologically separate disease entities, based on their distinct patterns of somatic alterations, epigenetic alterations (DNA methylation), and gene expression. Relevant biomarkers of adult glioblastoma and lower grade glioma and frequencies obtained from TCGA data are summarized in Table 1.
Specific Biomarkers and Targeted Therapeutics in Adult Diffuse Gliomas
MGMT Promoter Methylation
MGMT encodes an enzyme responsible for DNA repair, in particular for removing alkyl adducts from the O6 position of guanine, the mechanism through which alkylating chemotherapeutic agents work. In a study restricted to glioblastoma patients enrolled in a clinical trial of irradiation and temozolomide, MGMT gene promoter methylation emerged as a predictive marker of response.23 MGMT methylation may also be associated with the phenomenon of pseudoprogression, in which abnormal magnetic resonance imaging scans after chemoradiotherapy are secondary to treatment rather than progression or recurrence of the original tumor. Testing for methylation of the MGMT gene promoter has become routine in neuro-oncology practice. Currently, in many centers particularly in the United States, the presence or absence of MGMT methylation is not required for administration of temozolomide in the setting of standard care, because a subset of patients with unmethylated tumors may also benefit from temozolomide therapy, an observation of earlier clinical trials that is still being actively debated. However, testing for MGMT methylation may be required for clinical trial enrollment, and some investigators advocate for triaging patients based on MGMT methylation, including offering alternatives to temozolomide to patients with MGMT unmethylated tumors.24 Of additional interest, the recent TCGA study found O6-methylguanine-DNA methyltransferase (MGMT) to be predictive of treatment response only in the classic molecular subtype of glioblastoma.12
Because the development of the methylation-specific PCR assay, a variety of qualitative and quantitative molecular assays has been designed and validated to detect promoter methylation of the MGMT gene in brain tumors. The advantage and limitation of these molecular assays have been reviewed in detail elsewhere.25 Because the MGMT promoter contains large CpG islands, establishing reproducing cutoffs for methylation calls has been a challenge and variable in the literature, with approximately 48.5% of glioblastomas MGMT methylated in the recent TCGA study,12 a slightly higher prevalence than that in the study of Hegi et al23 (approximately 45%). The prevalence of MGMT methylation may be even higher in lower grade glioma, particularly those with IDH mutations, being present in approximately 85% IDH mutant, 1p19q intact diffuse gliomas and almost 100% of IDH mutant, 1p19q codeleted diffuse gliomas.14 Testing for the MGMT protein by immunohistochemistry (IHC), a widely available technique, has not proven of predictive value by many groups, which limits its widespread use.26
Activation of EGFR and Other Receptor Tyrosine Kinases
EGFR is almost always active or overexpressed in high-grade astrocytomas, particularly glioblastomas, most commonly through EGFR gene amplification and/or EGFR variant III deletion mutation (EGFRvIII). EGFRvIII occurs in approximately 20% of glioblastomas, leads to a truncated protein lacking the extracellular domain, and is frequently associated with amplification.12 Somatic EGFR alterations leads to constitutive activation of several signaling pathways critical for gliomagenesis, including MAPK and PI3K/AKT, ultimately promoting tumor growth. Recent studies have confirmed that EGFR is in fact one of the most frequently altered genes in glioblastoma (approximately 57% of tumors),12 with approximately 50% of tumors demonstrating amplification. In addition to EGFRvIII, a variety of other noncanonical recurrent EGFR mutations may be identified in glioblastoma, including C-terminal deletions and alternative intragenic alterations.
Regarding practical molecular diagnostics, EGFR amplification is frequently identified by fluorescence in situ hybridization (FISH) (Figure 2), whereas EGFRvIII expression may be tested by IHC or reverse-transcription PCR. It is important to note that there are some caveats in testing for EGFRvIII, because EGFRvIII is typically present in only a subpopulation of tumor cells, where it may drive tumorigenicity through paracrine effects on adjacent cells containing wild-type EGFR.27 Prior studies have specified at least focal moderate-to-strong staining with IHC for tumors to be considered positive for EGFRvIII, whereas a cutoff of at least 10% positive cells has been used in recent trials.28
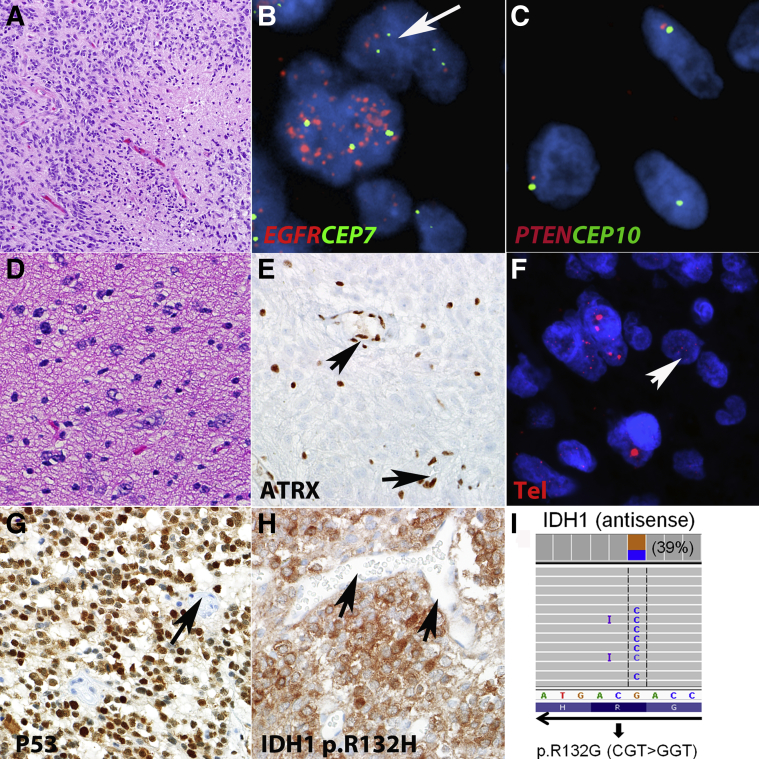
Figure 2.
Molecular pathology of adult diffuse astrocytomas. A: Histologically, glioblastoma is characterized by hypercellularity, mitotic activity, and necrosis. Amplification of receptor tyrosine kinases, particularly EGFR, is a frequent event in glioblastoma. B: FISH illustration of dual-color probe from Vysis, Abbott, targeting EGFR (red) and CEP7 (green). Other alterations frequently present in glioblastoma identifiable by FISH include monosomy 10/PTEN deletion. C: Dual-color probe targeting PTEN (red) and CEP10 (green), a single red and green signal on most cells is compatible with monosomy 10. D: Lower grade astrocytomas (diffuse and anaplastic astrocytomas) are characterized by infiltrating neoplastic astrocytes but lack the necrosis and microvascular proliferation typical of glioblastoma. E: These tumors have a high frequency of ATRX loss which is recognized by immunohistochemistry as negative staining in neoplastic cells but preserved expression in non-neoplastic elements. ATRX alterations lead to the ALT phenotype, which may be recognized by ultrabright signals in telomere-specific FISH in a subset of neoplastic cells. F and G: Increased TP53 levels by immunohistochemistry (F) are also frequent in these tumors and are consistent with mutation/pathway deregulation (G). H: A feature of most lower grade astrocytomas (grade II to III) and secondary glioblastomas (grade IV) is expression of mutant IDH1 protein which may be recognized through immunohistochemistry. I: Next-generation sequencing (AmpliSeq Cancer Hotspot Panel) is useful in identifying a variety of mutations in gliomas, including less common IDH1 R132 substitutions not recognizable by immunohistochemistry. Arrows demonstrate vessels as normal internal controls, including nonaltered cells in FISH and negative or positive vessels by immunohistochemistry. Telomere FISH image (F) published with permission of Dr. Christopher Heaphy. Original magnification: ×200 (A); ×1000 (B and C); ×600 (D–H). ALT, alternative lengthening of telomeres; ATRX, α thalassemia/mental retardation syndrome X-linked; FISH, fluorescence in situ hybridization; IDH1, isocitrate dehydrogenase 1.
Ongoing clinical trials should be able to assess the utility of EGFRvIII as a target more specifically. Encouraging recent results have demonstrated efficacy in preclinical animal models targeting EGFRvIII, as well as phase II trials of the rindopepimut vaccine (chemically conjugated to keyhole limpet hemocyanin).28 Therefore, increased testing of EGFRvIII in the clinical laboratory enables enrollment into trials using monoclonal antibodies and tumor vaccine strategies.
Amplification of other receptor tyrosine kinases is also relevant to glioblastoma biology, in addition to EGFR. For example, platelet-derived growth factor α (PDGFRA) is amplified in approximately 13% of glioblastomas, erb-b2 receptor tyrosine kinase 2 (ERBB2) in 8%, and MET proto-oncogene, receptor tyrosine kinase (MET) in 4%. Interestingly, multiple oncogenes (eg, EGFR, PDGFRA) can be amplified at the single-cell level within the same tumor in a mutually exclusive fashion, a reflection of intratumoral heterogeneity and an important current paradigm in cancer.29
IDH1 and IDH2 Mutations
Mutations in the IDH1 and IDH2 genes are uncommon in cancers in general but are restricted to specific relatively rare subtypes, including acute myeloid leukemia, cholangiocarcinoma, chondroid tumors, and particularly lower grade gliomas and the secondary glioblastomas (>80%) as described in the above section. IDH1 (residing in the cytosol) and IDH2 (residing in the mitochondria) are involved in the metabolism of citrate. The mutations are gain-of-function and essentially always involve the same codons, resulting in single amino acid substitutions in arginine at position 132 of IDH1 or the analogous hotspots R172 and R140 of IDH2. These mutations result in the production of increased levels of the 2-hydrodxyglutarate metabolite. Testing for these mutations is relatively straightforward at the present time. These mutations may be identified by various methods, including Sanger sequencing, melting-curve analysis, pyrosequencing, and next-generation sequencing platforms30, 31 (Figure 2). Of interest to routine pathologic diagnosis, IHC using a mutation-specific antibody against the predominant IDH1 p.R132H mutation works well in formalin-fixed, paraffin-embedded tissues and is widely available. Various algorithms using IDH1 and ATRX IHC may obviate the need for further molecular testing in a subset of cases (Figure 2). Of even greater relevance for therapeutic purposes is that IDH inhibitors and even vaccines have been developed, and molecular testing for these mutations is increasing because a prerequisite for specific clinical trials targeting these unique alterations. Specifically, a phase I clinical trial of AG-120, a small molecule inhibitor for IDH1 mutant solid tumors, including gliomas, is open (NCT02073994).
TERT Promoter Mutations, ATRX Mutations, and ALT Phenotype
Cell survival and proliferation requires activation of a mechanism that maintains telomeres which get shortened with each division cycle. This requirement is also relevant to cancer cells. In gliomas, there are two main mechanisms that mediate this process: first, an increase in telomerase expression, and, second, through the less common alternative lengthening of telomeres (ALT) phenotype.
In diffuse gliomas, the two mechanisms of telomere maintenance are facilitated at the genetic level by activating TERT promoter mutations,32 which lead to an increase in telomerase expression, and inactivating mutations in the α thalassemia/mental retardation syndrome X-linked (ATRX) gene, which are strongly associated with the ALT phenotype.33 With rare exceptions, these alterations are mutually exclusive,13 and are associated with different molecular tumor subclasses. These include the essentially 100% co-occurrence of TERT promoter and IDH mutations with 1p19q co-deletion in oligodendrogliomas; ATRX, IDH, and TP53 mutations in astrocytomas grades II to III and secondary glioblastomas; and TERT promoter mutations with wild-type IDH in primary glioblastomas.
Testing for TERT promoter mutations is relatively straightforward because they also occur at specific hotspots (p.C228T or p.C250T). Conversely, inactivating mutations in the large ATRX gene may occur at multiple sites. IHC has emerged as a more practical technique to identify loss of ATRX expression, which is limited to neoplastic cells and is relatively preserved in the non-neoplastic cells as a normal internal control. Loss of ATRX expression is strongly correlated with the ALT phenotype and may serve as a useful surrogate. The ALT phenotype may also be tested by using telomere-specific FISH, where ultrabright signals even in a small proportion of neoplastic cells is diagnostic (Figure 2).34
Adult Oligodendroglial Tumors, 1p19q Co-Deletions, and CIC/FUBP1 Mutations
Testing for 1p19q co-deletion is one of the most widely used tests available for prognostication in the molecular pathology of brain tumors. Initial conventional cytogenetic studies identified a high frequency of 1p and 19q co-deletion in diffuse gliomas with oligodendroglial features, which has become almost definitional of oligodendroglioma in the right context.1 This was further reinforced by the correlation of procarbazine, CCNU, and vincristine chemosensitivity of oligodendroglial tumors to be even more correlated with this alteration,35 findings confirmed prospectively by two large clinical trials (RTOG 9402 and EORTC 26951).36, 37 These codeletions almost always involve the whole 1p and 19q chromosomal arms in tumors satisfying morphologic criteria for oligodendroglioma, which is not unexpected given that this cytogenetic alteration is mediated by an unbalanced t(1;19) translocation.38, 39 Specific mutations involve the far upstream element binding protein 1 (FUBP1) gene (chromosome 1p), which encodes a DNA-binding protein involved in MYC regulation, and the capicua transcriptional repressor (CIC) gene (chromosome 19q), encoding a protein member of the high-mobility group-box transcriptional repressors, in 1p19q codeleted, IDH mutant tumors (29% and 62%, respectively).40, 41 As described earlier, 1p19q codeleted oligodendrogliomas almost universally have IDH and TERT promoter mutations.22
At the current time, 1p19q testing is recommended for all anaplastic oligodendroglial tumors and also low-grade oligodendrogliomas to predict chemosensitivity and prognosis. A variety of methods are available for 1p19q testing, which most frequently is performed by FISH. However, SNP/comparative genomic hybridization arrays and PCR-based microsatellite analysis are also performed in many laboratories (Figure 3). Regarding specific advantages and disadvantages, FISH has minimal tissue requirements and may identify the abnormality when present even in a small focus of a formalin-fixed, paraffin-embedded section. However, SNP/comparative genomic hybridization arrays may be preferable in other instances because they identify whole-arm deletions (in contrast to FISH), a more specific molecular property of oligodendrogliomas.42 The distinction of small deletions from whole-arm deletions may not be possible with other techniques (eg, FISH), which is relevant because presumably smaller deletions identifiable by FISH or microsatellite studies may occur in glioblastomas, for example, where they lack prognostic significance.43
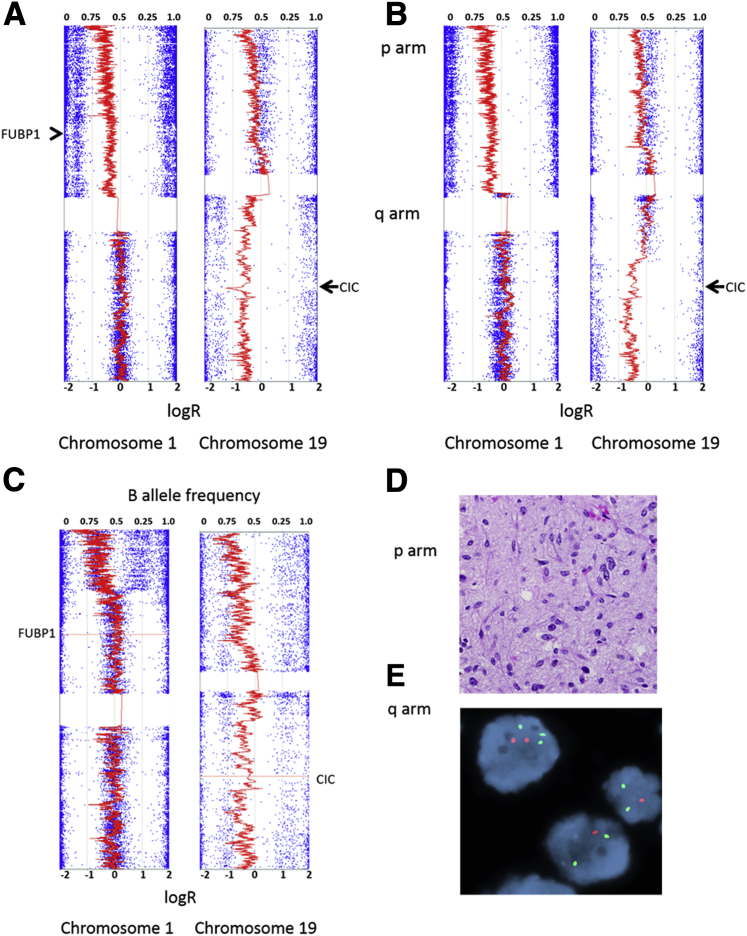
Figure 3.
Molecular testing for 1p19q alterations in oligodendroglial tumors. A: Classic oligodendrogliomas typically demonstrate whole arm 1p19q codeletions that are best identified by SNP arrays. Conversely, partial deletions are rare in oligodendrogliomas. B: In this case, partial deletions of 1p and 19q involving the FUBP1 and CIC genes were present in a neoplasm with features of classic oligodendroglioma. Partial deletions are more typical of astrocytic neoplasms. C–E: This example shows a distal partial deletion of 1p and monosomy 19 sparing the FUBP1 gene locus (C) in an astrocytoma (D) which predicted FISH patterns that may be interpreted as those for oligodendroglioma, particularly 1p loss (Vysis/Abbott probe set) (E). Original magnification: ×600 (D); ×1000 (E).
Pediatric High-Grade Glioma
High-grade gliomas in children are also classified histologically as high-grade gliomas of adults, including anaplastic astrocytomas (World Health Organization grade III) and glioblastomas (World Health Organization grade IV), and they are not necessarily separated in the World Health Organization classification.1 The number of mutations in coding genes is higher than lower grade examples, but interestingly much lower than high-grade adult counterparts in some data sets, with an average of 24 mutations per tumor in pediatric glioblastoma, and some tumors containing as few as four mutations, compared with an average of 47 mutations in adult glioblastoma.44 Integrated molecular profiling of pediatric glioblastomas have generated distinct subgroups with prognostic relevance, with tumors containing oncogene amplifications and/or H3F3A p.K27M mutations, in particular, having the worse outcome. Of interest, H3F3A p.K27M mutations have a predilection for diffuse astrocytomas involving midline structures, including diffuse intrinsic pontine gliomas (DIPGs) in children described in the section below, as well as high-grade astrocytomas occurring in the spinal cord in both children and adults.45 Other pediatric high-grade gliomas occurring outside of the pons/midline may contain alternative H3F3A mutations (p.G34R or p.G34V).15, 46, 47 Tumors with H3F3A p.G34 mutations appear to be histologically heterogeneous with some neoplasms corresponding to glioblastoma and others to embryonal neoplasms. They appear to represent a distinct entity, with uniform epigenetic signatures, frequent TP53 (88%) and ATRX (95%) alterations, as well as PDGFRA amplification (approximately 27%), 2q loss (67%), and 4q loss (70%).47 Other histone component mutations (eg, HIST1H3B, encoding for histone H3.1) occur at a lesser frequency. In the H3F3A p.K27M missense mutation, lysine at position 27 is changed to methionine in the N-terminal end of histone 3.3.46 Post-translational changes at this histone site alter a variety of cellular processes, including gene expression, DNA repair, and centromeres/telomere maintenance.48 This mutation was also associated with a variety of epigenetic changes, including a decrease in H3K27 trimethylation and global DNA hypomethylation.15, 48, 49 In addition to sequencing techniques, a mutation-specific antibody is applicable for routine IHC to detect the H3F3A p.K27M mutation (Figure 4).50
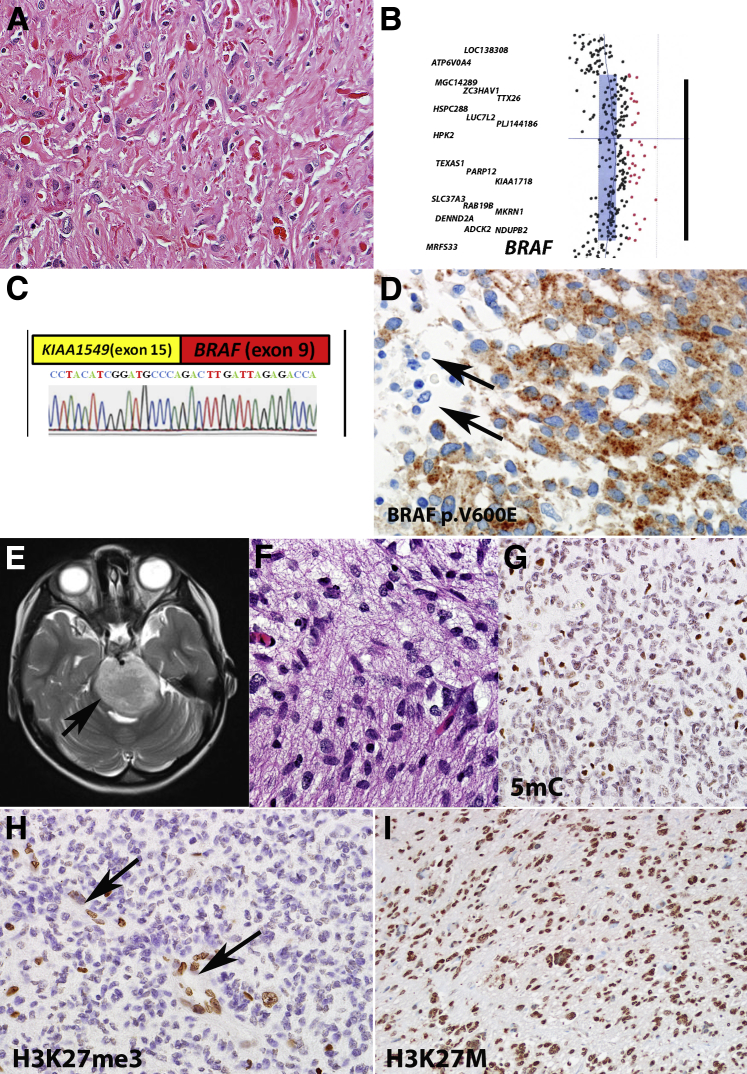
Figure 4.
Molecular alterations of pediatric gliomas. A: Pilocytic astrocytoma is the most frequent PLGA subtype. B and C: Most of these tumors are characterized by a duplication involving the BRAF kinase domain (array comparative genomic hybridization) (B), which leads most frequently to a novel KIAA1549-BRAF fusion (C).The black bar in B represents an approximately 2 megabase duplicated segment. D: Additional alterations that may be found in PLGAs include a BRAF p.V600E mutation, which may be detected by mutation-specific antibodies (negative non-neoplastic elements, arrows). E: A particular devastating subset of pediatric high-grade gliomas is DIPG, which diffusely expands the pons and may be diagnosed on clinical grounds (arrow). F: Histologically it overlaps with other diffuse gliomas (grades II to IV), but it is uniformly fatal. G and H: Epigenetic alterations typical of this tumor include global hypomethylation which may be tested with antibodies against 5mC (G) and loss of H3K27me3 (preserved labeling in vessels, arrows) (H). I: Most DIPGs have a H3F3A (H3K27M) mutation which is also associated with poor prognosis and may be detected by mutation-specific antibodies. Original magnification: ×400 (A and G–I); ×600 (D and F); DIPG, diffuse intrinsic pontine glioma; H3K27M, methionine 27 mutation in histone 3 variant; H3K27me3, histone 3 K27 trimethylation; PLGA, pediatric low-grade astrocytoma; 5mC, 5-methylcytosine.
DIPGs represent a distinct clinicopathologic variant of pediatric high-grade glioma, comprising approximately 10% of pediatric brain tumors, and are extremely aggressive. A variety of molecular studies have identified the genetic landscape of DIPGs, including oncogene amplifications (PDGFRA, MET) and PDGFRA mutations51 which they share in common with other pediatric glioblastomas. However, global molecular profiling studies have also separated distinct DIPG subgroups based predominantly on hedgehog (SHH) and MYCN pathway activation.52 Approximately 80% of DIPGs contain a p.K27M mutation of the H3F3A gene.53 More recently, ACVR1 mutations, encoding the transforming growth factor β superfamily member activin, have been reported in approximately 20% of DIPGs.54, 55
Pediatric Low-Grade Glioma
Major advances have been made recently in pediatric low-grade glioma (PLGA). BRAF kinase domain duplications frequently lead to a BRAF-KIAA1549 fusion, which is the most frequent recurrent alteration in pilocytic astrocytoma, the main PLGA subtype (Figure 4), occurring in >70% of tumors,56, 57 with the highest frequency occurring in the cerebellum. This and other genetic rearrangements and mutations lead to constitutive downstream oncogenic pathway activation, particularly the MAPK pathway.58 Comprehensive sequencing studies have uncovered genetic hits in MAPK components in essentially 100% of tumors.56 The relevance of this pathway to PLGA biology is also highlighted by the consistent inactivation of the NF1 gene in syndrome-associated cases. The mTOR pathway is also frequently active in these tumors,59, 60 and mTOR activation is the molecular hallmark of subependymal giant cell astrocytoma, a tumor frequently developing in the setting of tuberous sclerosis, and therefore containing alterations in the tumor sclerosis complex 1 or 2 (TSC1 or TSC2) tumor suppressor genes. A variety of other genetic alterations have been described in other PLGA subsets, including partial duplication of the transcription factor v-myb avian myeloblastosis viral oncogene homolog-like 1 (MYBL1) with truncated transcript as well as mutually exclusive intragenic duplication of the tyrosine kinase domain in the fibroblast growth factor receptor 1 (FGFR1) gene and rearrangement of the v-myb avian myeloblastosis viral oncogene homolog (MYB) gene in up to 68% diffuse PLGAs.57, 61 Of interest, MYB-QKI rearrangements are specific for angiocentric gliomas.62
On occasion, neoplasms resembling in all respects adult oligodendrogliomas affect pediatric patients. However, they frequently lack 1p19q codeletion and IDH1 or IDH2 mutations.63, 64 Interestingly, Zhang et al57 found FGFR1 tyrosine kinase domain duplications in three cases of pediatric oligodendrogliomas, and more recently rare subsets of pediatric low-grade tumors composed of oligodendrocyte-like cells had a high frequency of FGFR1 alterations, including 82% of dysembryoplastic neuroepithelial tumors and 40% pediatric oligodendrogliomas.44 Interestingly, a low-grade pediatric neoplasm with oligodendroglioma-like morphology but characterized at the clinical level by extensive superficial parenchymal and leptomeningeal dissemination has been increasingly characterized recently.65, 66 Several designations have been applied to these tumors, including disseminated oligodendroglioma-like leptomeningeal neoplasms and diffuse leptomeningeal glioneuronal tumor which has been incorporated in the recent World Health Organization classification update. Recent studies have demonstrated a high frequency of 1p deletion (59%) and BRAF:KIAA1549 fusion (72%) in67 a molecular signature distinct from adult and pediatric oligodendrogliomas.
BRAF Mutations and Targeted Therapeutics in Pediatric Glioma
BRAF is one of the three members of the RAF family proteins (ARAF, BRAF, and CRAF), all of them with serine-threonine kinase activity and directly regulated by RAS. The BRAF protein is an important element of the MAPK pathway, which in turn affects transcription factors involved in cell differentiation, proliferation, apoptosis, and senescence. More than 90% of all BRAF mutations in human cancers comprise a single amino acid substitution of valine by glutamic acid at codon 600 (p.V600E), as a result of c.1799T>A base variant. The BRAF p.V600E mutation was detected in 50% to 70% of pleomorphic xanthoastrocytomas (PXAs),57, 68 18% to 33% of gangliogliomas,68 and more recently in approximately one-third of non-pleomorphic xanthoastrocytomas, non-ganglioglioma PLGAs involving the diencephalon.69 It has also been described at lower frequencies in other low-grade glioma subtypes, such as pilocytic astrocytoma (9%), particularly noncerebellar pilocytic astrocytoma, and pediatric diffuse astrocytoma (23%),57, 68 as well as in variable subsets of high-grade astrocytomas, particularly the epithelioid glioblastoma subtype (50%).70 Furthermore, BRAF p.V600E combined with loss of CDKN2A leads to high-grade astrocytomas71 and may constitute a clinically distinct subtype of secondary high-grade gliomas arising from PLGAs.72 In contrast to BRAF p.V600E, a tandem duplication at 7q34 resulting in fusion of KIAA1549 and BRAF genes is the predominant oncogenic event in pilocytic astrocytoma.
Clinical detection of the BRAF p.V600E mutation has become the standard of care for patients with metastatic melanoma. BRAF inhibitors are also promising as targeted therapies for brain tumors with BRAF mutations. Early-phase clinical trials are testing the feasibility of BRAF inhibitors such as dabrafenib (clinicaltrials.gov; last accessed May 06, 2016; NCT01677741) and MAPK kinase inhibitors such as selumetinib and binimetinib (NCT01386450, NCT01089101, NCT02285439). A variety of molecular assays have been validated for clinical detection of BRAF mutations, including Sanger sequencing, pyrosequencing, allele-specific PCR, real-time PCR-based assays (such as the cobas 4800 BRAF V600 Mutation Test, the first companion BRAF test approved by the Food and Drug Administration), high-resolution melting analysis, primer extension-based assays, and next-generation sequencing assays.73 Next-generation sequencing assays provide not only a high analytic sensitivity but also a broad reportable range for clinical detection of BRAF p.V600E and non-p.V600E mutations. A p.V600E-specific mouse monoclonal antibody (VE1) has been applied to formalin-fixed, paraffin-embedded tissues, including brain tumors. Although VE1 IHC can only detect the p.V600E mutation, it may allow the identification of mutant tumors in small biopsy or fine-needle aspiration specimens or specimens with scattered tumor cells intermingled with abundant non-neoplastic cells.74
Conclusions
Our understanding of the biology of glial neoplasms has significantly increased in the past few years, with major scientific advances accomplished in both pediatric and adult glial tumors. The current status of this field is of particular relevance to molecular diagnostics, which will continue to evolve into a major player in the evaluation and treatment of adult and pediatric glial tumors.
Footnotes
Supported by Pilocytic/Pilomyxoid Fund, including Lauren's First and Goal (F.J.R.), and NIH grant P30 CA006973 to the Sidney Kimmel Comprehensive Cancer Center (PI: W. Nelson).
Disclosures: None declared.
This article is partly based on material presented at the Beaumont Health System 24th Annual Molecular Pathology Symposium on Clinical Applications of Genomic Medicine held September 16–17, 2015, Troy, MI.
References
- 1.Louis D.N., Ohgaki H., Wiestler B., Cavenee W., Ellison D.W., Figarella-Branger D., Perry A., Reifenberger G., Von Deimling A. ed 4. WHO/IARC; Lyon, France: 2016. WHO Classification of Tumours of the Central Nervous System. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H., Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 3.Sahm F., Reuss D., Koelsche C., Capper D., Schittenhelm J., Heim S., Jones D.T., Pfister S.M., Herold-Mende C., Wick W., Mueller W., Hartmann C., Paulus W., von Deimling A. Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. 2014;128:551–559. doi: 10.1007/s00401-014-1326-7. [DOI] [PubMed] [Google Scholar]
- 4.Tanboon J., Williams E.A., Louis D.N. The diagnostic use of immunohistochemical surrogates for signature molecular genetic alterations in gliomas. J Neuropathol Exp Neurol. 2016;75:4–18. doi: 10.1093/jnen/nlv009. [DOI] [PubMed] [Google Scholar]
- 5.Louis D.N., Perry A., Burger P., Ellison D.W., Reifenberger G., von Deimling A., Aldape K., Brat D., Collins V.P., Eberhart C., Figarella-Branger D., Fuller G.N., Giangaspero F., Giannini C., Hawkins C., Kleihues P., Korshunov A., Kros J.M., Beatriz Lopes M., Ng H.K., Ohgaki H., Paulus W., Pietsch T., Rosenblum M., Rushing E., Soylemezoglu F., Wiestler O., Wesseling P., International Society Of Neuropathology–Haarlem International Society Of Neuropathology–Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24:429–435. doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips H.S., Kharbanda S., Chen R., Forrest W.F., Soriano R.H., Wu T.D., Misra A., Nigro J.M., Colman H., Soroceanu L., Williams P.M., Modrusan Z., Feuerstein B.G., Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons D.W., Jones S., Zhang X., Lin J.C., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.M., Gallia G.L., Olivi A., McLendon R., Rasheed B.A., Keir S., Nikolskaya T., Nikolsky Y., Busam D.A., Tekleab H., Diaz L.A., Jr., Hartigan J., Smith D.R., Strausberg R.L., Marie S.K., Shinjo S.M., Yan H., Riggins G.J., Bigner D.D., Karchin R., Papadopoulos N., Parmigiani G., Vogelstein B., Velculescu V.E., Kinzler K.W. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhaak R.G., Hoadley K.A., Purdom E., Wang V., Qi Y., Wilkerson M.D., Miller C.R., Ding L., Golub T., Mesirov J.P., Alexe G., Lawrence M., O'Kelly M., Tamayo P., Weir B.A., Gabriel S., Winckler W., Gupta S., Jakkula L., Feiler H.S., Hodgson J.G., James C.D., Sarkaria J.N., Brennan C., Kahn A., Spellman P.T., Wilson R.K., Speed T.P., Gray J.W., Meyerson M., Getz G., Perou C.M., Hayes D.N., Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan X., Vengoechea J., Zheng S., Sloan A.E., Chen Y., Brat D.J., O'Neill B.P., de Groot J., Yust-Katz S., Yung W.K., Cohen M.L., Aldape K.D., Rosenfeld S., Verhaak R.G., Barnholtz-Sloan J.S. Molecular subtypes of glioblastoma are relevant to lower grade glioma. PLoS One. 2014;9:e91216. doi: 10.1371/journal.pone.0091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noushmehr H., Weisenberger D.J., Diefes K., Phillips H.S., Pujara K., Berman B.P., Pan F., Pelloski C.E., Sulman E.P., Bhat K.P., Verhaak R.G., Hoadley K.A., Hayes D.N., Perou C.M., Schmidt H.K., Ding L., Wilson R.K., Van Den Berg D., Shen H., Bengtsson H., Neuvial P., Cope L.M., Buckley J., Herman J.G., Baylin S.B., Laird P.W., Aldape K., Cancer Genome Atlas Research Network Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan C.W., Verhaak R.G., McKenna A., Campos B., Noushmehr H., Salama S.R. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckel-Passow J.E., Lachance D.H., Molinaro A.M., Walsh K.M., Decker P.A., Sicotte H. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceccarelli M., Barthel F.P., Malta T.M., Sabedot T.S., Salama S.R., Murray B.A. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturm D., Witt H., Hovestadt V., Khuong-Quang D.A., Jones D.T., Konermann C. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Mack S.C., Hubert C.G., Miller T.E., Taylor M.D., Rich J.N. An epigenetic gateway to brain tumor cell identity. Nat Neurosci. 2016;19:10–19. doi: 10.1038/nn.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran S., Arribas C., Esteller M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics. 2016;8:389–399. doi: 10.2217/epi.15.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turcan S., Rohle D., Goenka A., Walsh L.A., Fang F., Yilmaz E., Campos C., Fabius A.W., Lu C., Ward P.S., Thompson C.B., Kaufman A., Guryanova O., Levine R., Heguy A., Viale A., Morris L.G., Huse J.T., Mellinghoff I.K., Chan T.A. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H., Zheng S., Amini S.S., Virk S.M., Mikkelsen T., Brat D.J., Grimsby J., Sougnez C., Muller F., Hu J., Sloan A.E., Cohen M.L., Van Meir E.G., Scarpace L., Laird P.W., Weinstein J.N., Lander E.S., Gabriel S., Getz G., Meyerson M., Chin L., Barnholtz-Sloan J.S., Verhaak R.G. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015;25:316–327. doi: 10.1101/gr.180612.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J., Lee I.H., Cho H.J., Park C.K., Jung Y.S., Kim Y., Nam S.H., Kim B.S., Johnson M.D., Kong D.S., Seol H.J., Lee J.I., Joo K.M., Yoon Y., Park W.Y., Lee J., Park P.J., Nam D.H. Spatiotemporal evolution of the primary glioblastoma genome. Cancer Cell. 2015;28:318–328. doi: 10.1016/j.ccell.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Johnson B.E., Mazor T., Hong C., Barnes M., Aihara K., McLean C.Y., Fouse S.D., Yamamoto S., Ueda H., Tatsuno K., Asthana S., Jalbert L.E., Nelson S.J., Bollen A.W., Gustafson W.C., Charron E., Weiss W.A., Smirnov I.V., Song J.S., Olshen A.B., Cha S., Zhao Y., Moore R.A., Mungall A.J., Jones S.J., Hirst M., Marra M.A., Saito N., Aburatani H., Mukasa A., Berger M.S., Chang S.M., Taylor B.S., Costello J.F. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research Network. Brat D.J., Verhaak R.G., Aldape K.D., Yung W.K., Salama S.R., Cooper L.A. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegi M.E., Diserens A.C., Gorlia T., Hamou M.F., de Tribolet N., Weller M., Kros J.M., Hainfellner J.A., Mason W., Mariani L., Bromberg J.E., Hau P., Mirimanoff R.O., Cairncross J.G., Janzer R.C., Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 24.Hegi M.E., Stupp R. Withholding temozolomide in glioblastoma patients with unmethylated MGMT promoter--still a dilemma? Neuro Oncol. 2015;17:1425–1427. doi: 10.1093/neuonc/nov198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wick W., Weller M., van den Bent M., Sanson M., Weiler M., von Deimling A., Plass C., Hegi M., Platten M., Reifenberger G. MGMT testing–the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10:372–385. doi: 10.1038/nrneurol.2014.100. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez F.J., Thibodeau S.N., Jenkins R.B., Schowalter K.V., Caron B.L., O'Neill B.P., James C.D., Passe S., Slezak J., Giannini C. MGMT immunohistochemical expression and promoter methylation in human glioblastoma. Appl Immunohistochem Mol Morphol. 2008;16:59–65. doi: 10.1097/PAI.0b013e31802fac2f. [DOI] [PubMed] [Google Scholar]
- 27.Inda M.M., Bonavia R., Mukasa A., Narita Y., Sah D.W., Vandenberg S., Brennan C., Johns T.G., Bachoo R., Hadwiger P., Tan P., Depinho R.A., Cavenee W., Furnari F. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24:1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster J., Lai R.K., Recht L.D., Reardon D.A., Paleologos N.A., Groves M.D., Mrugala M.M., Jensen R., Baehring J.M., Sloan A., Archer G.E., Bigner D.D., Cruickshank S., Green J.A., Keler T., Davis T.A., Heimberger A.B., Sampson J.H. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17:854–861. doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snuderl M., Fazlollahi L., Le L.P., Nitta M., Zhelyazkova B.H., Davidson C.J., Akhavanfard S., Cahill D.P., Aldape K.D., Betensky R.A., Louis D.N., Iafrate A.J. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20:810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Catteau A., Girardi H., Monville F., Poggionovo C., Carpentier S., Frayssinet V., Voss J., Jenkins R., Boisselier B., Mokhtari K., Sanson M., Peyro-Saint-Paul H., Giannini C. A new sensitive PCR assay for one-step detection of 12 IDH1/2 mutations in glioma. Acta Neuropathol Commun. 2014;2:58. doi: 10.1186/2051-5960-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikiforova M.N., Wald A.I., Melan M.A., Roy S., Zhong S., Hamilton R.L., Lieberman F.S., Drappatz J., Amankulor N.M., Pollack I.F., Nikiforov Y.E., Horbinski C. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol. 2016;18:379–387. doi: 10.1093/neuonc/nov289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killela P.J., Reitman Z.J., Jiao Y., Bettegowda C., Agrawal N., Diaz L.A., Jr. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heaphy C.M., de Wilde R.F., Jiao Y., Klein A.P., Edil B.H., Shi C., Bettegowda C., Rodriguez F.J., Eberhart C.G., Hebbar S., Offerhaus G.J., McLendon R., Rasheed B.A., He Y., Yan H., Bigner D.D., Oba-Shinjo S.M., Marie S.K., Riggins G.J., Kinzler K.W., Vogelstein B., Hruban R.H., Maitra A., Papadopoulos N., Meeker A.K. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaphy C.M., Subhawong A.P., Hong S.M., Goggins M.G., Montgomery E.A., Gabrielson E., Netto G.J., Epstein J.I., Lotan T.L., Westra W.H., Shih Ie M., Iacobuzio-Donahue C.A., Maitra A., Li Q.K., Eberhart C.G., Taube J.M., Rakheja D., Kurman R.J., Wu T.C., Roden R.B., Argani P., De Marzo A.M., Terracciano L., Torbenson M., Meeker A.K. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179:1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cairncross J.G., Ueki K., Zlatescu M.C., Lisle D.K., Finkelstein D.M., Hammond R.R., Silver J.S., Stark P.C., Macdonald D.R., Ino Y., Ramsay D.A., Louis D.N. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 36.Cairncross G., Wang M., Shaw E., Jenkins R., Brachman D., Buckner J., Fink K., Souhami L., Laperriere N., Curran W., Mehta M. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31:337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Bent M.J., Brandes A.A., Taphoorn M.J., Kros J.M., Kouwenhoven M.C., Delattre J.Y., Bernsen H.J., Frenay M., Tijssen C.C., Grisold W., Sipos L., Enting R.H., French P.J., Dinjens W.N., Vecht C.J., Allgeier A., Lacombe D., Gorlia T., Hoang-Xuan K. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31:344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 38.Griffin C.A., Burger P., Morsberger L., Yonescu R., Swierczynski S., Weingart J.D., Murphy K.M. Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol. 2006;65:988–994. doi: 10.1097/01.jnen.0000235122.98052.8f. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins R.B., Blair H., Ballman K.V., Giannini C., Arusell R.M., Law M., Flynn H., Passe S., Felten S., Brown P.D., Shaw E.G., Buckner J.C. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 40.Bettegowda C., Agrawal N., Jiao Y., Sausen M., Wood L.D., Hruban R.H., Rodriguez F.J., Cahill D.P., McLendon R., Riggins G., Velculescu V.E., Oba-Shinjo S.M., Marie S.K., Vogelstein B., Bigner D., Yan H., Papadopoulos N., Kinzler K.W. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333:1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yip S., Butterfield Y.S., Morozova O., Chittaranjan S., Blough M.D., An J. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226:7–16. doi: 10.1002/path.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harada S., Henderson L.B., Eshleman J.R., Gocke C.D., Burger P., Griffin C.A., Batista D.A. Genomic changes in gliomas detected using single nucleotide polymorphism array in formalin-fixed, paraffin-embedded tissue: superior results compared with microsatellite analysis. J Mol Diagn. 2011;13:541–548. doi: 10.1016/j.jmoldx.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark K.H., Villano J.L., Nikiforova M.N., Hamilton R.L., Horbinski C. 1p/19q testing has no significance in the workup of glioblastomas. Neuropathol Appl Neurobiol. 2013;39:706–717. doi: 10.1111/nan.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettegowda C., Agrawal N., Jiao Y., Wang Y., Wood L.D., Rodriguez F.J., Hruban R.H., Gallia G.L., Binder Z.A., Riggins C.J., Salmasi V., Riggins G.J., Reitman Z.J., Rasheed A., Keir S., Shinjo S., Marie S., McLendon R., Jallo G., Vogelstein B., Bigner D., Yan H., Kinzler K.W., Papadopoulos N. Exomic sequencing of four rare central nervous system tumor types. Oncotarget. 2013;4:572–583. doi: 10.18632/oncotarget.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gessi M., Gielen G.H., Dreschmann V., Waha A., Pietsch T. High frequency of H3F3A (K27M) mutations characterizes pediatric and adult high-grade gliomas of the spinal cord. Acta Neuropathol. 2015;130:435–437. doi: 10.1007/s00401-015-1463-7. [DOI] [PubMed] [Google Scholar]
- 46.Bender S., Tang Y., Lindroth A.M., Hovestadt V., Jones D.T., Kool M. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell. 2013;24:660–672. doi: 10.1016/j.ccr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Schwartzentruber J., Korshunov A., Liu X.Y., Jones D.T., Pfaff E., Jacob K. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 48.Ahsan S., Raabe E.H., Haffner M.C., Vaghasia A., Warren K.E., Quezado M., Ballester L.Y., Nazarian J., Eberhart C.G., Rodriguez F.J. Increased 5-hydroxymethylcytosine and decreased 5-methylcytosine are indicators of global epigenetic dysregulation in diffuse intrinsic pontine glioma. Acta Neuropathol Commun. 2014;2:59. doi: 10.1186/2051-5960-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu G., Diaz A.K., Paugh B.S., Rankin S.L., Ju B., Li Y., St. Jude Children's Research Hospital-Washington University Pediatric Cancer Genome Project The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venneti S., Santi M., Felicella M.M., Yarilin D., Phillips J.J., Sullivan L.M., Martinez D., Perry A., Lewis P.W., Thompson C.B., Judkins A.R. A sensitive and specific histopathologic prognostic marker for H3F3A K27M mutant pediatric glioblastomas. Acta Neuropathol. 2014;128:743–753. doi: 10.1007/s00401-014-1338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paugh B.S., Zhu X., Qu C., Endersby R., Diaz A.K., Zhang J., Bax D.A., Carvalho D., Reis R.M., Onar-Thomas A., Broniscer A., Wetmore C., Zhang J., Jones C., Ellison D.W., Baker S.J. Novel oncogenic PDGFRA mutations in pediatric high-grade gliomas. Cancer Res. 2013;73:6219–6229. doi: 10.1158/0008-5472.CAN-13-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saratsis A.M., Kambhampati M., Snyder K., Yadavilli S., Devaney J.M., Harmon B., Hall J., Raabe E.H., An P., Weingart M., Rood B.R., Magge S.N., Macdonald T.J., Packer R.J., Nazarian J. Comparative multidimensional molecular analyses of pediatric diffuse intrinsic pontine glioma reveals distinct molecular subtypes. Acta Neuropathol. 2014;127:881–895. doi: 10.1007/s00401-013-1218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis P., Müller M., Koletsky M., Cordero F., Lin S., Banaszynski L., Garcia B., Muir T., Becher O., Allis C. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science (New York, NY) 2013;340:857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buczkowicz P., Hoeman C., Rakopoulos P., Pajovic S., Letourneau L., Dzamba M. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46:451–456. doi: 10.1038/ng.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor K.R., Mackay A., Truffaux N., Butterfield Y.S., Morozova O., Philippe C., Castel D., Grasso C.S., Vinci M., Carvalho D., Carcaboso A.M., de Torres C., Cruz O., Mora J., Entz-Werle N., Ingram W.J., Monje M., Hargrave D., Bullock A.N., Puget S., Yip S., Jones C., Grill J. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet. 2014;46:457–461. doi: 10.1038/ng.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones D.T., Hutter B., Jager N., Korshunov A., Kool M., Warnatz H.J., International Cancer Genome Consortium PedBrain Tumor Project Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45:927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J., Wu G., Miller C.P., Tatevossian R.G., Dalton J.D., Tang B., St. Jude Children's Research Hospital-Washington University Pediatric Cancer Genome Project Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forshew T., Tatevossian R.G., Lawson A.R., Ma J., Neale G., Ogunkolade B.W., Jones T.A., Aarum J., Dalton J., Bailey S., Chaplin T., Carter R.L., Gajjar A., Broniscer A., Young B.D., Ellison D.W., Sheer D. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 59.Hutt-Cabezas M., Karajannis M.A., Zagzag D., Shah S., Horkayne-Szakaly I., Rushing E.J., Cameron J.D., Jain D., Eberhart C.G., Raabe E.H., Rodriguez F.J. Activation of mTORC1/mTORC2 signaling in pediatric low-grade glioma and pilocytic astrocytoma reveals mTOR as a therapeutic target. Neuro Oncol. 2013;15:1604–1614. doi: 10.1093/neuonc/not132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaul A., Chen Y.H., Emnett R.J., Dahiya S., Gutmann D.H. Pediatric glioma-associated KIAA1549: BRAF expression regulates neuroglial cell growth in a cell type-specific and mTOR-dependent manner. Genes Dev. 2012;26:2561–2566. doi: 10.1101/gad.200907.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramkissoon L.A., Horowitz P.M., Craig J.M., Ramkissoon S.H., Rich B.E., Schumacher S.E. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A. 2013;110:8188–8193. doi: 10.1073/pnas.1300252110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bandopadhayay P., Ramkissoon L.A., Jain P., Bergthold G., Wala J., Zeid R. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016;48:273–282. doi: 10.1038/ng.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nauen D., Haley L., Lin M.T., Perry A., Giannini C., Burger P.C., Rodriguez F.J. Molecular analysis of pediatric oligodendrogliomas highlights genetic differences with adult counterparts and other pediatric gliomas. Brain Pathol. 2015;26:206–214. doi: 10.1111/bpa.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez F.J., Tihan T., Lin D., McDonald W., Nigro J., Feuerstein B., Jackson S., Cohen K., Burger P.C. Clinicopathologic features of pediatric oligodendrogliomas: a series of 50 patients. Am J Surg Pathol. 2014;38:1058–1070. doi: 10.1097/PAS.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez F.J., Perry A., Rosenblum M.K., Krawitz S., Cohen K.J., Lin D., Mosier S., Lin M.T., Eberhart C.G., Burger P.C. Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity. Acta Neuropathol. 2012;124:627–641. doi: 10.1007/s00401-012-1037-x. [DOI] [PubMed] [Google Scholar]
- 66.Schniederjan M.J., Alghamdi S., Castellano-Sanchez A., Mazewski C., Brahma B., Brat D.J., Brathwaite C.D., Janss A.J. Diffuse leptomeningeal neuroepithelial tumor: 9 pediatric cases with chromosome 1p/19q deletion status and IDH1 (R132H) immunohistochemistry. Am J Surg Pathol. 2013;37:763–771. doi: 10.1097/PAS.0b013e31827bf4cc. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez F.J., Schniederjan M.J., Nicolaides T., Tihan T., Burger P.C., Perry A. High rate of concurrent BRAF-KIAA1549 gene fusion and 1p deletion in disseminated oligodendroglioma-like leptomeningeal neoplasms (DOLN) Acta Neuropathol. 2015;129:609–610. doi: 10.1007/s00401-015-1400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schindler G., Capper D., Meyer J., Janzarik W., Omran H., Herold-Mende C., Schmieder K., Wesseling P., Mawrin C., Hasselblatt M., Louis D.N., Korshunov A., Pfister S., Hartmann C., Paulus W., Reifenberger G., von Deimling A. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 69.Ho C.Y., Mobley B.C., Gordish-Dressman H., VandenBussche C.J., Mason G.E., Bornhorst M., Esbenshade A.J., Tehrani M., Orr B.A., LaFrance D.R., Devaney J.M., Meltzer B.W., Hofherr S.E., Burger P.C., Packer R.J., Rodriguez F.J. A clinicopathologic study of diencephalic pediatric low-grade gliomas with BRAF V600 mutation. Acta Neuropathol. 2015;130:575–585. doi: 10.1007/s00401-015-1467-3. [DOI] [PubMed] [Google Scholar]
- 70.Kleinschmidt-DeMasters B.K., Aisner D.L., Birks D.K., Foreman N.K. Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol. 2013;37:685–698. doi: 10.1097/PAS.0b013e31827f9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huillard E., Hashizume R., Phillips J.J., Griveau A., Ihrie R.A., Aoki Y., Nicolaides T., Perry A., Waldman T., McMahon M., Weiss W.A., Petritsch C., James C.D., Rowitch D.H. Cooperative interactions of BRAFV600E kinase and CDKN2A locus deficiency in pediatric malignant astrocytoma as a basis for rational therapy. Proc Natl Acad Sci U S A. 2012;109:8710–8715. doi: 10.1073/pnas.1117255109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mistry M., Zhukova N., Merico D., Rakopoulos P., Krishnatry R., Shago M., Stavropoulos J., Alon N., Pole J.D., Ray P.N., Navickiene V., Mangerel J., Remke M., Buczkowicz P., Ramaswamy V., Guerreiro Stucklin A., Li M., Young E.J., Zhang C., Castelo-Branco P., Bakry D., Laughlin S., Shlien A., Chan J., Ligon K.L., Rutka J.T., Dirks P.B., Taylor M.D., Greenberg M., Malkin D., Huang A., Bouffet E., Hawkins C.E., Tabori U. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J Clin Oncol. 2015;33:1015–1022. doi: 10.1200/JCO.2014.58.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carter J., Tseng L.H., Zheng G., Dudley J., Illei P., Gocke C.D., Eshleman J.R., Lin M.T. Non-p.V600E BRAF mutations are common using a more sensitive and broad detection tool. Am J Clin Pathol. 2015;144:620–628. doi: 10.1309/AJCP85ATMJOZOUDJ. [DOI] [PubMed] [Google Scholar]
- 74.Koelsche C., Wohrer A., Jeibmann A., Schittenhelm J., Schindler G., Preusser M., Lasitschka F., von Deimling A., Capper D. Mutant BRAF V600E protein in ganglioglioma is predominantly expressed by neuronal tumor cells. Acta Neuropathol. 2013;125:891–900. doi: 10.1007/s00401-013-1100-2. [DOI] [PubMed] [Google Scholar]