Abstract
Background
Use of antigen-detecting malaria rapid diagnostic tests (RDTs) has increased exponentially over the last decade. WHO’s Global Malaria Programme, FIND, and other collaborators have established a quality assurance scheme to guide product selection, lot verification, transport, storage, and training procedures. Recent concerns over the quality of buffer packaging and test accessories suggest a need to include these items in product assessments. This paper describes quality problems with buffer and accessories encountered in a project promoting private sector RDT use in five African countries and suggests steps to avoid or more rapidly identify and resolve such problems.
Methods
Private provider complaints about RDT buffer vials and kit accessories were collected during supervisory visits, and a standard assessment process was developed. Using 100 tests drawn from six different lots produced by two manufacturers, lab technicians visually assessed alcohol swab packaging, blood transfer device (BTD) usability, and buffer appearance, then calculated mean blood volume from 10 BTD transfers and mean buffer volume from 10 individual buffer vials. WHO guided complaint reporting and follow-up with manufacturers.
Results
Supervisory visits confirmed user reports of dry alcohol swabs, poorly functioning BTDs, and non-uniform volumes of buffer. Lot testing revealed further evidence of quality problems, leading one manufacturer to replace buffer vials and accessories for 40,000 RDTs. In December 2014, WHO issued an Information Notice for Users regarding variable buffer volumes in single-use vials and recommended against procurement of these products until defects were addressed.
Discussion
Though not necessarily comprehensive or generalizable, the findings presented here highlight the need for extending quality assessment to all malaria RDT test kit contents. Defects such as those described in this paper could reduce test accuracy and increase probability of invalid, false positive, or false negative results. Such deficiencies could undermine provider confidence in RDTs, prompting a return to presumptive treatment or reliance on poor quality microscopy. In partial response to this experience, WHO, FIND, and other project partners have developed guidance on documenting, troubleshooting, reporting, and resolving such problems when they occur.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1820-1) contains supplementary material, which is available to authorized users.
Background
With the adoption of artemisinin-based combination therapy (ACT) as the first-line treatment for falciparum malaria in the early 2000s, the World Health Organization (WHO) recommended parasite-based diagnosis first for older children and adults, then for all suspected cases of malaria regardless of patient age [1]. This recommendation led to greatly expanded use of antigen-detecting malaria rapid diagnostic tests (RDTs) worldwide. Total annual RDT sales grew from 46 million in 2008 to 314 million in 2014 [2]. To guide selection of quality RDTs in the face of multiple products on the market, variable reports of test performance, and weak national regulatory systems, WHO, the Foundation for Innovative New Diagnostics (FIND), and other collaborators established the WHO Malaria RDT Product Testing Programme in 2008 at the US Centers for Disease Control and Prevention (CDC) [3]. By December 2015, the programme had completed six rounds of assessment and evaluated 171 products. The programme constitutes the laboratory evaluation component of WHO’s prequalification process for in vitro diagnostics (IVDs) and forms the basis for WHO’s malaria RDT procurement recommendations. Results inform procurement decisions by multilateral and donor agencies, non-governmental organizations (NGOs), ministries of health, and others across the malaria-endemic world [2].
Procuring good quality RDTs, however, does not necessarily guarantee good field performance. Shipping, handling, and storage can affect RDT accuracy as can the training and supervision of health workers in both test preparation and interpretation [4–9]. All have received attention in the literature.
One area that has received less attention is the quality of the buffer and accessories required to conduct RDT testing. The term “accessories” here refers to alcohol swabs, lancets, and blood transfer devices (BTDs). Buffer is referred to as a component. “Accessories” are so described because one brand or style can be substituted for another without compromising test performance. For instance, if tests come packaged with dry alcohol swabs, users can substitute alcohol and cotton or a different alcohol swab. Buffer, in contrast, is manufactured for a specific RDT brand and lot. Substituting buffer from a different brand, lot, or type of test, using water, or using any solution other than the one specifically designed for that lot and test, is contraindicated and could produce incorrect results. For this reason, WHO classifies buffer as a “component”: an integral part of the test. Manufacturers package alcohol swabs, lancets, BTDs, and buffer solution into boxes of 25, 30 or 50 test cassettes, commonly known as ‘hospital packs.’ Alternatively, RDT ‘single packs,’ include a test cassette, accessories and a single-use buffer vial in one envelope. In either case, defective accessories can contribute to anomalous test results. For example, some substandard BTDs can result in collection of too much blood; this can obscure results if the test strip background remains red and test lines illegible. Others can result in collection of too little blood; this can generate false negative results if the blood volume contains insufficient antigen to generate a visible test line. With RDT single packs, single-use vials that contain too little buffer or allow leakage or evaporation can also produce invalid or false negative results. In hospital packs, bottles with insufficient buffer volume may run out before all tests are used. This can lead to tests being wasted or providers substituting water or some other inappropriate solution for buffer. Until recently, however, the WHO-FIND Lot Testing Programme included only limited assessment of buffer and accessories [10].
This paper reports on problems with leakage and stability of single-use buffer vials and quality concerns with accessories encountered in a project promoting RDT use among private sector health care providers in Kenya, Madagascar, Nigeria, Tanzania and Uganda. The paper also describes resulting actions taken by WHO and project partners. The project, “Creating a Private Sector Market for Quality-Assured RDTs in Malaria Endemic Countries,” took place between April 2013 and April 2016. Project details and outcomes will be described elsewhere. Briefly, private providers in the five countries were recruited, trained in malaria diagnosis using RDTs, supplied with an initial stock of test kits, linked with local supply chain partners, and supported by the project’s two implementing partners: Population Services International (PSI) in Kenya, Madagascar, and Tanzania, and Malaria Consortium (MC) in Nigeria and Uganda. WHO, FIND, and the Johns Hopkins Bloomberg School of Public Health (JHSPH) provided support in health worker training, product quality assurance activities, public-sector engagement, and development of relevant guidelines and standard operating procedures (SOPs).
The project’s first year involved working with national regulatory authorities, ministries of health, national malaria control programmes and others to develop or revise guidelines and policies on private sector RDT use. Project partners developed procurement protocols, carried out lot testing on samples drawn from among 500,000 RDTs, recruited and trained an initial cadre of private providers, and engaged with manufacturers, importers, and distributors [11].
Methods
Context
Results reported here are based on data from two sources: (1) supervisory and M&E visits to project-based private sector outlets and (2) more extensive assessments carried out off site by FIND and WHO. During 2014, the project trained 801 providers at 682 outlets in the five project countries. Trained providers began offering RDT-based diagnosis in April (Kenya and Madagascar), May (Tanzania), August (Uganda) and September (Nigeria) [12].
Pre- and post-shipment lot testing
All project-procured RDTs underwent lot testing as per standard protocol prior to shipment from the manufacturer and again after arrival and customs clearance in each project country [13]. Lot testing was carried out at the Research Institute for Tropical Medicine in Manila, Philippines and the Institut Pasteur of Cambodia in Phnom Penh, both WHO-FIND lot testing laboratories [10]. The African Network for Drugs and Diagnostics Innovation (ANDI) Centre of Excellence for Malaria Diagnosis, College of Medicine, University of Lagos, Nigeria, also carried out lot-testing on RDTs used in Nigeria. As part of routine lot testing procedures, major observations—such as partially or completely empty buffer bottles or vials, missing accessories, damaged desiccant sachets, or incorrect package insert sheets—were noted in the comments section of lot testing reports [14].
Supervisory and M&E visits
Project staff and distributors conducted 478 supervisory visits across the five project countries from June–December 2014. Over the same period, the project M&E team, including representatives from all project partners, visited providers in all five countries. Many providers mentioned problems with buffer vials and test accessories. During routine supervisory visits, Ugandan providers complained of what they perceived to be an unusually large number of negative test results and of vials with insufficient buffer. In response, representatives from the Uganda National Drug Authority (NDA), Malaria Consortium, and FIND visited 65 (31.7%) of the 205 outlets registered with the project in October 2014. To rule out user error, the team observed each provider preparing a test, checking to ensure that the provider (1) followed the preparation instructions correctly, (2) added the correct volume of blood and buffer to the correct test wells, (3) waited the requisite time before interpreting the test results, and (4) correctly interpreted the test outcome. The team then queried each provider about problems with buffer and accessories and recorded any complaints in a log. In a follow-up visit to 95 (46.3%) randomly selected outlets, the team retrieved and evaluated 170 single packs, including test cassettes, single-use buffer vials, BTDs, alcohol swabs, lancets and test instructions [15].
Qualitative assessment
In January and February 2015, PSI conducted a qualitative study of private provider and client perceptions of RDTs in Tamatave, Madagascar. Study team members interviewed 22 participating providers and 12 adult patients or caregivers of child patients about their experiences using RDTs. While test-kit accessories were not a specific study focus, many providers spontaneously reported problems with lancets, BTDs, alcohol swabs and desiccant packets.
Accessory assessment during RDT lot testing
RDT instructions for use, buffer and accessory review procedures
One partner (MC) requested more extensive assessment of RDT buffer and accessories at the beginning of the project. In response, a standard operating procedure (SOP) was developed and applied to RDTs shipped to Nigeria and Uganda, the two MC project countries (Additional file 1). Qualitative assessments included a review of test instructions, visual inspection of accessories, and observations during lot testing to confirm whether:
the instructions clearly described (a) target malaria species, (b) target antigens, (c) correct blood and buffer volumes, (d) proper BTD use, (e) illustrations or text identifying the cassette’s blood and buffer wells, (f) minimum and maximum reading time, and (g) an illustration and text explaining how to interpret test results;
BTDs were (a) easy to use (pick-up, transfer and deposit of required blood volume could be done in no more than two attempts) and (b) did not result in blood spillage during transfer;
buffer vials appeared intact, buffer colour consistent, and buffer easy to deliver into the test well (dispensing the required volume could be accomplished in a single attempt);
alcohol swabs were in sealed undamaged envelopes;
desiccant colour indicated that the test kit had not been exposed to excess humidity.
Both buffer vials and BTDs were also assessed quantitatively:
Following manufacturer’s instructions, a laboratory technician used a BTD from one RDT test kit to make 10 blood transfers from a lot testing quality control sample. The technician deposited each sample on Whatman 3 M filter paper on a precision balance pre-set to zero and calculated the mean weight of the ten samples. For comparison, ten transfers were then made using a calibrated micropipette set at the manufacturer-specified volume to determine the mean reference weight. The mean blood volume of the 10 BTD transfers was calculated using the reference weight divided by the reference volume as a conversion factor. This was compared with the allowable volume variation when available from the manufacturer.
Ten buffer vials selected from individual test kits were used to deposit the manufacturer’s indicated buffer volume in a container on a precision balance pre-set to zero. Mean weight was calculated. The same process was repeated using a calibrated micropipette set at the buffer volume specified by the manufacturer, and calculating the mean reference weight. The buffer volume transferred with vials was calculated using reference weight divided by reference volume, and compared with the allowed volume variation when available from the RDT manufacturer.
Samples from the RDT lots undergoing accessories assessment were provided for either pre-shipment or post-shipment lot testing, with samples drawn by either the RDT manufacturer from the production lot or the project implementer from the shipment pallet in the country port of entry. RDTs were sent to WHO-FIND lot testing laboratories. A total of 100 RDTs were sampled for Plasmodium falciparum-only tests, and 150 RDTs for P. falciparum plus other species (‘combo’) tests. Shipment and storage were at room temperature throughout the process, and RDTs were assessed within 5 days of receipt at the lot testing laboratories.
Results
Supervisory and M&E visits
In Uganda, 13 of 65 outlets visited (20%) reported dry alcohol swabs or single-use buffer vials that were empty, had inconsistent volumes, or were difficult to open or use, resulting in incomplete dispensing of buffer into the cassette. The initial NDA assessment identified five out of 65 providers (7.7%) who reported empty buffer vials. However, the follow-up assessment of 170 kits from 95 providers found that variation in buffer volume fell within acceptable limits based on manufacturer specifications.
In Kenya, where accessories assessment was not part of the lot verification done prior to field deployment, quality assurance team visits identified test kits with no BTDs, dry alcohol swabs, and stained packaging indicating buffer vial leakage (Fig. 1). In Madagascar, M&E team members examined accessories collected from a convenience sample of about 20 providers during a September 2014 visit. Almost all alcohol swabs evaluated were completely dry. Test kits contained one-piece eye dropper-like plastic pipettes designed to collect blood by squeezing a bulb at the top (Fig. 2). Stiff plastic made the bulb difficult to compress and blood volume difficult to control. Stiffness also made it difficult to avoid suctioning the blood beyond the pipette tube into the bulb itself from where it could not easily be dispensed into the test cassette. Even when the blood remained in the pipette tube, bulb stiffness hindered delivery into the cassette. Pinholes or cracks in some pipettes also inhibited suction. Plastic flashing at some pipette tips interfered with collecting blood and depositing it into the cassette. Finally, the blood volume indicator line was often not visible. Buffer volume appeared to vary significantly from vial to vial. Some vials appeared nearly or completely empty (Figs. 3, 4).
Fig. 1.
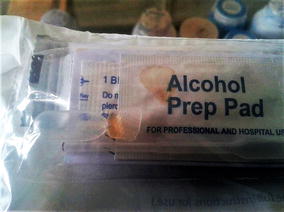
Leakage from buffer vials evidenced by stained packaging (Kenya)
Fig. 2.

Plastic pipettes designed to collect blood by squeezing a bulb at the top (Madagascar)
Fig. 3.
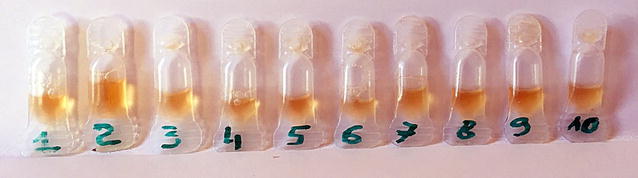
Buffer vials showing different volumes of buffer (Madagascar)
Fig. 4.
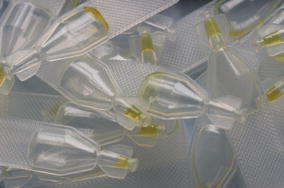
Buffer vials nearly empty with buffer stuck at tip
Half the 22 providers in the early 2015 Madagascar qualitative study mentioned similar findings. Along with dry alcohol swabs, evaporated buffer and defective pipettes, participants reported lancet tips that had detached from their plastic housings or were bent or that broke upon use, and desiccant that had changed colour indicating test exposure to excess humidity.
Accessory assessment during RDT lot testing
Qualitative results
Lots assessed from Uganda (6) and Nigeria (2) complied with most qualitative criteria:
Instruction sheets described target malaria species and antigens, blood and buffer volume required, proper BTD and buffer vial use, and minimum and maximum reading times. Sheets included pictures or text indicating correct blood and buffer wells and illustrating how to read and interpret test results.
The inverted cup BTD (Fig. 5), was rated easy to use and caused no blood spills.
Buffer vials and alcohol swab envelopes appeared sealed and showed no external evidence of leaking.
Desiccant packets showed no indication of exposure to humidity.
Fig. 5.

Inverted cup blood collection device (FIND Geneva)
However, for one lot assessed twice during post-shipment testing, once each in Nigeria and Uganda, visual inspection revealed variation in buffer volume and an unusual yellowish colour, possibly indicating evaporation during transport and storage. In both cases, one vial out of 34 randomly opened was completely dry; in others, buffer remained trapped at the tip of the vial and could not be dispensed (Figs. 3, 4).
Quantitative results
Table 1 summarizes quantitative BTD results. Variation in mean transferred blood volume ranged from 45.8% below the target 5 µl volume to 9.2% above. However, no manufacturer specified the allowable variation from the 5 µl standard, so it was not possible to determine whether deviations fell within acceptable range.
Table 1.
Volume of blood collected using pre-packaged blood-collection devices (inverted cup—10 devices per lot tested)
| Test lot # | Mean | SD | Min | Max | % variation |
|---|---|---|---|---|---|
| 1 (Uganda) | 2.71 | 0.56 | 1.75 | 3.50 | −46 |
| 1 (Nigeria) | 3.28 | 0.63 | 2.27 | 4.20 | −34 |
| 2 | 3.98 | 0.61 | 3.07 | 5.11 | −20 |
| 3 (Uganda) | 3.93 | 0.49 | 3.33 | 4.70 | −21 |
| 3 (Nigeria) | 4.76 | 0.33 | 4.39 | 5.31 | −5 |
| 4 | 5.33 | 0.49 | 4.29 | 6.02 | 7 |
| 5 | 5.46 | 0.53 | 4.69 | 6.12 | 9 |
| 6 | 5.43 | 0.62 | 4.80 | 6.43 | 9 |
All manufacturers specify a 5 μl fingerstick blood sample. % variation is based on the difference between this 5 μl standard and the mean volume in column 2. Lots 1 and 3 were sampled from both Uganda and Nigeria; all other lots were sampled from Nigeria only
Table 2 presents quantitative buffer volume results based on post-shipment lot assessments. While the volume in individual buffer vials sometimes fell outside the manufacturer’s specified allowable variation, the mean volume from five of the seven lot assessments fell within it. Mean volume from the sixth lot, assessed using samples from both Nigeria and Uganda, fell outside acceptable range.
Table 2.
Volume of buffer (μl) contained in pre-packaged vials (10 vials per lot tested)
| Test lot # | Specified volume | Acceptable deviation (μl) | Mean volume | SD | Min–max | Mean volume falls within acceptable deviation? | % variation |
|---|---|---|---|---|---|---|---|
| 1 (Uganda) | 70 | ±10 | 53.17 | 18.07 | 17.78–77.44 | No | −24.0% |
| 1 (Nigeria | 70 | ±10 | 43.32 | 15.77 | 21.15–66.88 | No | −38.1% |
| 2 | 130 | ±39 | 110.02 | 9.48 | 93.48–120.44 | Yes | −15.4% |
| 3 (Uganda) | 130 | ±39 | 109.08 | 5.48 | 101.18–118.35 | Yes | −16.1 |
| 3 (Nigeria) | 130 | ±39 | 113.06 | 4.25 | 107.91–121.07 | Yes | −13.0% |
| 4 | 130 | ±39 | 112.12 | 8.05 | 95.91–123.11 | Yes | −13.8% |
| 5 | 130 | ±39 | 111.34 | 5.55 | 103.67–121.53 | Yes | −14.4% |
| 6 | 130 | ±39 | 116.13 | 4.65 | 108.91–123.98 | Yes | −10.7% |
Lots 1 and 3 sampled from Uganda and Nigeria; all other lots sampled from Nigeria only. % variation is based on the difference between the manufacturer’s specified volume in column 2 and the mean volume in column 4
Discussion
This paper’s results reveal several quality issues related to malaria RDT buffer and accessories heretofore not described in the literature. Previous studies have focused primarily on clarity of instructions, package labelling, or desiccant status [16–19]. Some of the issues described here raise more serious concerns than others. Dry alcohol swabs, for instance, may represent only a minor additional cost and inconvenience to providers who must purchase their own alcohol and cotton. Without access to these supplies, however, a provider might wipe the patient’s finger with the dry swab or water or leave it uncleaned, augmenting the risk of infection. Bent, broken or detached lancet tips could pose a risk to both health worker and patient. Some users might procure their own lancets—another additional cost—others might be tempted to substitute a non-sterile or non-disposable device. Poorly functioning BTDs represent an even greater concern. A stiff, difficult to use plastic pipette, manufacturing defects such as pinholes that interfere with suction, or absence of a mark indicating correct volume could result in transferring the wrong blood quantity to the RDT. Excess blood could leave a dark red background in the results window, obscuring test lines. This could cause a health worker to misread a positive result as negative, especially with a low parasite density infection where test lines tend to be faint. Too little blood could also produce false negative results if antigen quantity is insufficient to generate a visible test line. In either case, a malaria-infected patient could go untreated.
Assessment during lot testing showed blood volume variations using the inverted cup, a BTD previously reported as easier to use and more accurate than others [20, 21]. Variations exceeded those in past reports, perhaps because different BTD manufacturers use different designs or plastics. (Note that the Hopkins et al. study was based on 3 transfers from each of 227 health workers; data here are based on 10 transfers with a single BTD by a single technician [21]). The fact that some BTDs show up to 46% volume variation suggests that not all RDT manufacturers perform an adequate quality control of BTDs. Most RDT manufacturers source accessories such as BTDs from other suppliers; under ISO 13485, manufacturers are responsible for verifying the quality of any materials they include in their test kits.
Problems with single-use buffer vials represent another significant concern. An empty vial might tempt an RDT user to substitute water or some other liquid. At least one study has shown that substitution of tap water, distilled water, or saline solution for buffer can all produce false positive results in both P. falciparum-only and multi-species RDTs [22]. For vials in which evaporation has left insufficient buffer volume, reagent concentration poses a similar risk. Beyond lots procured for this project, the FIND-WHO RDT lot-testing programme documented buffer evaporation in 145 lots of single-use RDT kits representing 11 different products from three manufacturers between 2014 and 2015 [23]. Anecdotal reports from various countries were also discussed during a meeting of the malaria RDT procurement task force in October 2014. As a result of these various findings, the WHO IVD Prequalification Programme (WHO PQ) issued a notice of concern recommending a halt to procurement and delisting of WHO-prequalified affected products [24]. In parallel, WHO PQ also asked relevant manufacturers to notify those who procured the affected products, investigate the problem, and develop or source alternative, stable, single-use buffer vials. Two affected manufacturers accomplished this using higher density plastic, but sourcing the material and conducting the required stability studies resulted in the product being unavailable for 20 months.
Deficiencies with buffer and accessories could undermine both provider and client confidence in RDTs. It has been widely reported that RDT users doubt negative test results and prescribe antimalarials to RDT-negative patients for fear of leaving an infected but incorrectly diagnosed client untreated [25–28]. Research also shows that both health care providers and patients are reluctant to end a clinical visit without prescribing or receiving some sort of treatment [25–29]. Quality problems with buffer and accessories could reinforce this type of incorrect practice, ultimately leading providers to substitute non-quality assured RDTs or return to poor-quality microscopy or presumptive treatment.
Putting more emphasis on quality assessment of buffer and accessories in WHO laboratory evaluations and WHO PQ manufacturer dossier and site assessments could diminish the likelihood of such problems. This might spur manufacturers to more closely monitor the quality of accessories and RDT components which are often supplied by third parties. FIND, WHO and the other private sector project partners have developed a troubleshooting guide and problems protocol to assist supervisors and end-users in investigating frequently observed anomalous results and errors [30, 31]. The publications offer guidance documenting problems with RDTs, buffer, and accessories and reporting them to NMCPs, national regulatory authorities, and WHO PQ. Timely reporting can help trigger corrective actions such as the previously described WHO PQ notice of concern. Reporting should include investigation to verify that anomalies are due to quality problems rather than end-user errors. The WHO PQ website also accepts problem reports related to malaria and other IVDs [32].
FIND’s expanded assessment has now been updated to include verifying the volume of buffer, the number of accessories and the moisture of alcohol swabs. Because this expanded assessment is highly labour intensive, however, WHO-FIND laboratories can only conduct it by special request on a limited number of lots. A shortened assessment is now included in routine WHO-FIND RDT lot testing, the main difference being that buffer volume uniformity is checked visually rather than measured. Lot testing reports now summarize results and include photos of the accessories. A lot with insufficient buffer for preparing the test will fail the assessment.
The aforementioned interventions would likely reduce buffer- and accessory-related anomalies and provide a mechanism for addressing them at a system level when they occur. They would not, however, respond to the immediate needs of front-line private sector providers who encounter problems with buffer or accessories and need defective items replaced rapidly. Many private providers operate on very narrow financial margins and cannot wait the several months required to resolve an issue through international channels. Similarly, many lack capital to purchase replacement supplies while waiting. During the private sector project, project partners or their distributors could resolve problems more rapidly by intervening directly with international entities and manufacturers. Manufacturers supplying the project generally responded quickly and positively. One manufacturer replaced 40,000 alcohol swabs and BTDs while confirming that individual buffer vial volume, though varied, fell within acceptable limits. Two others also replaced buffer vials after receiving complaints. PSI, MC, and their distributors delivered replacements directly to affected providers.
Outside a project setting, an individual provider facing such problems may lack the wherewithal to contact a manufacturer or regulator directly. An individual provider might likewise have difficulty determining whether a problem was limited or widespread or even whom to contact locally to report one. Guidelines such as those in the aforementioned RDT problems protocol should make it easier for a local or regional distributor to compile provider complaints, pass them on to regulators and manufacturers, and serve as a go-between to ensure that provider concerns are addressed. This could protect private providers from unsustainable losses and reduce provider attrition.
The data reported here have many limitations. Information compiled from the private sector project’s M&E and supervisory visits is based on small non-random samples of providers. Visits included direct qualitative examination of test accessories but did not follow a structured protocol or attempt quantitative measurements (e.g., of blood or buffer volume). The systematic laboratory-based assessment of buffer vials, instructions for use, and accessories, though based on random sampling within lots, included only six lots and two test brands procured for project use in Nigeria and Uganda. Lots for other project countries were procured before the accessories assessment was in place. Further, while the systematic assessment made it possible to quantify the volume of blood transferred to each test, all tests evaluated used the inverted cup BTD, widely considered the most accurate and easiest to use device currently available [21]. Testing with other types of BTD would have provided a more representative picture of the accuracy of devices currently in use. It should also be noted that the assessment is not fully representative of real life since the blood used in testing the BTDs came from a frozen quality control sample, typically less viscous than fresh blood, and was transferred to Whatman filter paper which has a different structure and density than the RDT sample pad. Nevertheless, the procedure allows for a standardized approach to assessing BTDs. Systematic assessment enables quantitative measurement of buffer volume, but leaves open the question of whether the lower-than-specified volume observed is due to inadequate quality control in manufacturing, evaporation during storage, or some other cause. To resolve this, manufacturers would need to carry out root cause analysis. During initial accessory assessments, alcohol swabs were not checked for moisture, lancets were not examined, and accessories were not counted to ensure that their numbers were sufficient. Subsequently, assessment procedures have been updated to address some of these shortcomings, and a shortened procedure for accessories assessment is now incorporated into routine RDT lot testing [13].
Taken together, these limitations preclude generalizable conclusions about the extent of quality issues related to buffer and accessories or the effect of such issues on test performance and acceptability. However, quality issues clearly exist and manufacturers must apply good quality control standards to buffer vials and test accessories as well as to the test devices themselves. Both private and public entities that procure RDTs should be aware of these issues and have a system in place to document, troubleshoot, and rapidly resolve them. This should include a mechanism for replacing defective products on a timeline acceptable to front-line providers. Programmes that evaluate rapid test performance should include assessment of buffer and accessories as an integral part of routine quality control procedures.
Additional note
A key objective of publishing this paper was to highlight the need for more effective quality control of buffer and accessories for all malaria RDTs, something the authors believe merits greater attention than it has received to date. As a result, the authors elected not to name specific brands or products. Manufacturers of all the products described here have either discontinued sale of these products or remedied the problem by sourcing new materials that meet higher quality control standards. Further, since the sample described here was small and non-representative, it is possible that the buffer or accessories packaged with other brands of RDTs suffer from the same or similar defects that have not yet come to light. Singling out one or two manufacturers for specific mention might lead some readers to conclude that both the problem and the recommendations for improved quality control relate to a small number of specific brands or products rather than to an industry-wide issue.
Authors’ contributions
SAH conceived of the topic of the paper, helped assemble and interpret the data on which this paper was based and took principal responsibility for preparing and revised the manuscript, incorporating input from all other authors. SI helped assemble and interpret the data on which this paper was based, helped develop the methods for assessing rapid diagnostic test quality described in the paper, and contributed to writing and revision of the manuscript. NM helped analyse the data and contributed to the manuscript. CL helped assemble and interpret the data and contributed to the manuscript. ES directed project implementation in Uganda and Nigeria, made the initial request for assessment of RDT accessories and buffer that led to the findings reported here, and contributed to the writing and revision of the manuscript. SD served as deputy director of the 5-country project, managed day-to-day project operations including the monitoring and evaluation that contributed to the findings reported here, and contributed to the writing and revision of the manuscript. NC supervised and helped carry out the evaluations of RDT accessories and buffer reported here. She also contributed to the writing and revision of the manuscript. DK supervised and contributed to accessory and buffer assessment in Uganda and Nigeria, contributed to developing the SOPs for accessory and buffer assessment in the project as a whole, and contributed to the writing and revision of the manuscript. RM was the Uganda country lead for project implementation, contributed to assessment of accessories of buffer in Uganda, and contributed to the writing and revision of the manuscript. GN was the Nigeria country lead for project implementation, contributed to assessment of accessories of buffer in Nigeria, and contributed to the writing and revision of the manuscript. NN was the Kenya country lead for project implementation, contributed to assessment of accessories of buffer in Kenya, and contributed to the writing and revision of the manuscript. RR was the Madagascar country lead for project implementation, contributed to assessment of accessories of buffer in Madagascar, and contributed to the writing and revision of the manuscript. JC helped develop the standard operating procedures for testing many aspects of RDT quality including quality of assessments, participating in field visits to project sites to gather data on problems with accessories and buffer and made substantial contributions to drafting and revising the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to thank the private medical providers whose participation in the Private Sector RDT Marketing Project led to the findings from which this paper emerged. The authors would also like to thank the anonymous reviewer for his/her helpful comments.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data on which this paper is based contain proprietary information which the authors are not at liberty to share. To protect the confidentiality of rapid diagnostic test manufacturers and maintain a focus on the problems with RDT accessories discussed in the paper rather than on specific manufacturers, the authors have elected to exclude manufacturers’ names from the manuscript. Sharing the original data would risk a breach of this confidentiality.
Funding
The project under which the data reported here was gathered, “Creating a Private Sector Market for Quality-Assured RDTs in Malaria Endemic Countries,” was funded by UNITAID. UNITAID participated in the design of both the project and the project evaluation. However, UNITAID had no participation in the collection or analysis of the data reported here and no role in preparing or editing the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACT
artemisinin-based combination therapy
- ANDI
African Network for Drugs and Diagnostics Innovation
- BTD
blood transfer device
- CDC
US Centers for Disease Control and Prevention
- FIND
Foundation for Innovative New Diagnostics
- IVDs
in-vitro diagnostics
- JHSPH
Johns Hopkins Bloomberg School of Public Health
- MC
Malaria Consortium
- NDA
Uganda National Drug Authority
- NGO
non-governmental organization
- PSI
Population Services International
- RDT
rapid diagnostic test
- SOP
standard operating procedure
- TDR
WHO Special Programme for Research and Training in Tropical Diseases
- WHO
World Health Organization
- WHO-PQ
WHO IVD Prequalification Programme
Additional file
Additional file 1. Standard Operation Procedures (SOP) 04.00: Assessment of accessories of individually packaged malaria RDT kits.
Contributor Information
Steven A. Harvey, Email: Steven.Harvey@jhu.edu
Sandra Incardona, Email: Sandra.Incardona@finddx.org.
Nina Martin, Email: nina.martin@jhu.edu.
Cristina Lussiana, Email: clussiana@psi.org.
Elizabeth Streat, Email: e.streat@malariaconsortium.org.
Stephanie Dolan, Email: sdolan@psi.org.
Nora Champouillon, Email: nora.champouillon@finddx.org.
Daniel J. Kyabayinze, Email: Daniel.kyabayinzed@finddx.org
Robert Mugerwa, Email: r.mugerwa@malariaconsortium.org.
Grace Nakanwagi, Email: g.nakanwagi@malariaconsortium.org.
Nancy Njoki, Email: nnjoki@pskenya.org.
Ratsimandisa Rova, Email: rovar@psi-southsudan.org.
Jane Cunningham, Email: cunninghamj@who.int.
References
- 1.World Health Organization . Guidelines for the treatment of malaria. 2. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 2.World Health Organization, Foundation for Innovative New Diagnostics, Centers for Disease Control. Malaria rapid diagnostic test performance: summary results of WHO product testing of malaria RDTs: rounds 1–6 (2008–2015). Geneva: World Health Organization; 2015.
- 3.World Health Organization, Foundation for Innovative New Diagnostics, Centers for Disease Control and Prevention, Special Programme for Research and Training in Tropical Diseases (TDR). Malaria rapid diagnostic test performance: Results of WHO product testing of malaria RDTs: Round 1 (2008). Geneva: WHO; 2009.
- 4.Albertini A, Lee E, Coulibaly SO, Sleshi M, Faye B, Mationg ML, et al. Malaria rapid diagnostic test transport and storage conditions in Burkina Faso, Senegal, Ethiopia and the Philippines. Malar J. 2012;11(1):1–9. doi: 10.1186/1475-2875-11-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiodini PL, Bowers K, Jorgensen P, Barnwell JW, Grady KK, Luchavez J, et al. The heat stability of Plasmodium lactate dehydrogenase-based and histidine-rich protein 2-based malaria rapid diagnostic tests. Trans R Soc Trop Med Hyg. 2007;101(4):331–337. doi: 10.1016/j.trstmh.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen P, Chanthap L, Rebueno A, Tsuyuoka R, Bell D. Malaria rapid diagnostic tests in tropical climates: the need for a cool chain. Am J Trop Med Hyg. 2006;74(5):750–754. [PubMed] [Google Scholar]
- 7.Kyabayinze DJ, Asiimwe C, Nakanjako D, Nabakooza J, Bajabaite M, Strachan C, et al. Programme level implementation of malaria rapid diagnostic tests (RDTs) use: outcomes and cost of training health workers at lower level health care facilities in Uganda. BMC Public Health. 2012;12(1):1–8. doi: 10.1186/1471-2458-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey SA, Jennings L, Chinyama M, Masaninga F, Mulholland K, Bell DR. Improving community health worker use of malaria rapid diagnostic tests in Zambia: package instructions, job aid and job aid-plus-training. Malar J. 2008;7(1):160. doi: 10.1186/1475-2875-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rennie W, Phetsouvanh R, Lupisan S, Vanisaveth V, Hongvanthong B, Phompida S, et al. Minimising human error in malaria rapid diagnosis: clarity of written instructions and health worker performance. Trans R Soc Trop Med Hyg. 2007;101(1):9–18. doi: 10.1016/j.trstmh.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Lot testing: pre and post-purchase—WHO-FIND RDT evaluation programme: World Health Organization. 2016. http://www.who.int/malaria/areas/diagnosis/rapid-diagnostic-tests/evaluation-lot-testing/en/.
- 11.FIND, Johns Hopkins Bloomberg School of Public Health, Malaria Consortium, PSI, WHO. UNITAID Private Sector RDT Project Annual Report 2013. Nairobi: UNITAID Private sector malaria RDT project partners; 2013.
- 12.Population Services International, Malaria Consortium, Foundation for Innovative New Diagnostics, World Health Organization, Johns Hopkins Bloomberg School of Public Health. Private Sector RDT Project 2014 Annual Report. Nairobi: Population Services International; 2014.
- 13.Foundation for Innovative New Diagnostics (FIND), WHO Global Malaria Programme (GMP). Methods Manual for Laboratory Quality Control Testing of Malaria Rapid Diagnostic Tests Geneva: Foundation for Innovative New Diagnostics (FIND) and WHO Global Malaria Programme (GMP); 2016 Version 8: manual of standard operating procedures for: Laboratory—based quality control testing of malaria rapid diagnostic tests using stored dilutions of malaria parasites and Preparation of quality control samples from malaria parasite field collections. http://www.who.int/malaria/publications/methods-manual-laboratory-quality-control-testing-malaria-rdt.pdf.
- 14.FIND. Guide for the interpretation of observations noted during lot testing of malaria RDTs 2013 (Standard Operating Procedure). http://www.finddx.org/wp-content/uploads/2016/02/malaria_rdt_guide_for_observations_30jul13.pdf.
- 15.Private Sector RDT Project_2014 Annual Report FINAL.pdf p 10.
- 16.Barbe B, Gillet P, Beelaert G, Fransen K, Jacobs J. Assessment of desiccants and their instructions for use in rapid diagnostic tests. Malar J. 2012;11:326. doi: 10.1186/1475-2875-11-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillet P, Maltha J, Hermans V, Ravinetto R, Bruggeman C, Jacobs J. Malaria rapid diagnostic kits: quality of packaging, design and labelling of boxes and components and readability and accuracy of information inserts. Malar J. 2011;10:39. doi: 10.1186/1475-2875-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillet P, Mukadi P, Vernelen K, Van Esbroeck M, Muyembe JJ, Bruggeman C, et al. External quality assessment on the use of malaria rapid diagnostic tests in a non-endemic setting. Malar J. 2010;9:359. doi: 10.1186/1475-2875-9-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs J, Barbé B, Gillet P, Aidoo M, Serra-Casas E, Van Erps J, et al. Harmonization of malaria rapid diagnostic tests: best practices in labelling including instructions for use. Malar J. 2014;13(1):1–10. doi: 10.1186/1475-2875-13-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luchavez J, Lintag ME, Coll-Black M, Baik F, Bell D. An assessment of various blood collection and transfer methods used for malaria rapid diagnostic tests. Malar J. 2007;6:149. doi: 10.1186/1475-2875-6-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins H, Oyibo W, Luchavez J, Mationg ML, Asiimwe C, Albertini A, et al. Blood transfer devices for malaria rapid diagnostic tests: evaluation of accuracy, safety and ease of use. Malar J. 2011;10(1):30. doi: 10.1186/1475-2875-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillet P, Mori M, Vanden Ende J, Jacobs J. Buffer substitution in malaria rapid diagnostic tests causes false-positive results. Malar J. 2010;9:215. doi: 10.1186/1475-2875-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Results of the FIND-WHO Lot Testing Programme for Malaria Rapid Diagnostic Tests (RDTs): 2007–2015. Geneva: FIND; 2016.
- 24.WHO Information Notice for Users. WHO-identifier: 2014/01, version 4. http://www.who.int/diagnostics_laboratory/procurement/150116_single_buffer_ampulla_information_notice_for_users_v4.pdf. Accessed 22 July 2016.
- 25.Altaras R, Nuwa A, Agaba B, Streat E, Tibenderana JK, Strachan CE. Why do health workers give anti-malarials to patients with negative rapid test results? A qualitative study at rural health facilities in western Uganda. Malar J. 2016;15(1):1–14. doi: 10.1186/s12936-015-1020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudhinaraset M, Briegleb C, Aung M, Khin H, Aung T. Motivation and challenges for use of malaria rapid diagnostic tests among informal providers in Myanmar: a qualitative study. Malar J. 2015;14(1):61. doi: 10.1186/s12936-015-0585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mubi M, Kakoko D, Ngasala B, Premji Z, Peterson S, Bjorkman A, et al. Malaria diagnosis and treatment practices following introduction of rapid diagnostic tests in Kibaha District, Coast Region, Tanzania. Malar J. 2013;12(1):293. doi: 10.1186/1475-2875-12-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asiimwe C, Kyabayinze D, Kyalisiima Z, Nabakooza J, Bajabaite M, Counihan H, et al. Early experiences on the feasibility, acceptability, and use of malaria rapid diagnostic tests at peripheral health centres in Uganda-insights into some barriers and facilitators. Implement Sci. 2012;7(1):5. doi: 10.1186/1748-5908-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams HA, Causer L, Metta E, Malila A, O’Reilly T, Abdulla S, et al. Dispensary level pilot implementation of rapid diagnostic tests: an evaluation of RDT acceptance and usage by providers and patients—Tanzania, 2005. Malar J. 2008;7:239. doi: 10.1186/1475-2875-7-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FIND, JHSPH, MC, PSI, WHO. Troubleshooting guide for supervisors overseeing users of malaria RDTs. Geneva: FIND, JHSPH, MC, PSI and WHO; 2015.
- 31.FIND. Protocol on responding to problems with malaria RDTs Geneva: FIND; 2016.
- 32.World Health Organization—in vitro diagnostics and laboratory technology: Complaints and product alerts. http://www.who.int/diagnostics_laboratory/procurement/complaints/en/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data on which this paper is based contain proprietary information which the authors are not at liberty to share. To protect the confidentiality of rapid diagnostic test manufacturers and maintain a focus on the problems with RDT accessories discussed in the paper rather than on specific manufacturers, the authors have elected to exclude manufacturers’ names from the manuscript. Sharing the original data would risk a breach of this confidentiality.


