Abstract
As part of a pharmacogenetic study, paired blood and oral fluid samples were tested for the IL28B polymorphism (rs12979860) before and after hematopoietic cell transplantation (HCT) to evaluate changes in the genotype and investigate the utility of genotyping in oral fluid in HCT recipients. In 54 patients with leukemia >18 years of age, samples were collected approximately 7 days before HCT and 60 days after HCT. IL28B polymorphism testing was performed using real-time PCR with allele-specific probes. Twenty-four patients had the same genotype as their donors. In 30 patients, the genotype was different from that of the donor. In the oral fluid samples, 4 retained the recipient's genotype, and 18 had a genotype that matched that of the donor. In the remaining 8 patients, the results could not be characterized and appeared to be a combination of both, suggesting mixed proportions of donor and recipient cells. The assumption was that the sloughed epithelial cells of the mouth are of recipient origin. However, oral fluid is a mixture that contains varying numbers of cells of the recipient and immunomodulatory cells from the donor. Therefore, the use of oral fluid after HCT for clinical pharmacogenetics purposes needs further investigation.
Pharmacogenetics are increasingly being studied in medicine. In particular, the effect of pharmacogenetics on hematopoietic cell transplantation (HCT) is being studied, especially with regard to dosing of medications.1, 2
IL28B is an innate cytokine in the interferon (IFN)-γ family that is expressed at low levels by a broad variety of cells in response to viral infections.3 A single-nucleotide polymorphism called rs12979860 CT, located 3 kb upstream from the IL28B gene locus on human chromosome 19q13 (Online Mendelian Inheritance in Man no. 607402), is associated with the response to pegylated interferon and ribavirin combination therapy in individuals with hepatitis C virus infection. Patients with the rs12979860CC genotype, compared with either the CT or TT genotypes, have approximately twofold to threefold greater rates of sustained viral response to combined pegylated interferon and ribavirin therapy.4 The CC genotype has also been associated with a threefold increase in rate of spontaneous clearance of hepatitis C virus.5 In addition, IFN-y is a common pathway for other viral infections, such as cytomegalovirus (CMV). Therefore, the rs12979860 polymorphism, which is presumed to affect the production of IFN-y, may also play an important role in CMV infection and disease. A recent study of 151 allogeneic HCT recipients found that the risk of CMV infection could be lower when donors have the rs12979860 TT genotype compared with donors with the CT and CC genotypes. In addition, recipients from donors with the TT genotype had a significantly shorter duration of first episodes of CMV compared with those with the CT and CC genotypes, thus indicating that the T allele (recessive genetic model) may be a protective factor against CMV infection in allogeneic HCT recipients.6
Blood is traditionally the specimen of choice for pharmacogenetic analysis; however, blood from HCT recipients would reflect the genotype of the donor not the recipient. Traditionally, oral fluid or buccal swabs, which contain the recipient's sloughed buccal cells, have been used to determine an HCT recipient's germline genotype. In this study, we used paired blood and oral fluid samples obtained from HCT recipients before and after HCT to describe changes in the IL28B genotype in these matrices.
Materials and Methods
Patient Selection and Sampling
We prospectively enrolled 63 adult (>18 years old) patients diagnosed as having leukemia in complete remission before they underwent allogeneic HCT at MD Anderson Cancer Center during 2014. Peripheral blood and oral fluid samples were collected from these patients approximately 7 days before HCT or conditioning regimen and approximately 60 days after the transplant, when most patients are fully engrafted, their clinical condition stabilized, and donor cells dominate in the blood. Patients in whom we did not obtain pre- or post-HCT genotypes (ie, those who died before day 60, dropped out, or refused to give a sample) were excluded. The final cohort included 54 patients. All samples were tested for the IL28B polymorphism (rs12979860) at the Mayo Clinic. Approval for this study was obtained from the institutional review boards where the patients were enrolled and where the genetic assays were performed. All patients provided written informed consent for participation in this study. Their demographic data are given in Table 1.
Table 1.
Characteristics of the 54 HCT Recipients
| Characteristic | Value |
|---|---|
| Age at time of HCT, years | |
| Median (range) | 56 (21–73) |
| Means ± SD | 51 ± 14 |
| Sex, No. (%) | |
| Male | 34 (63) |
| Female | 20 (37) |
| Time from HCT to engraftment, days | |
| Median (range) | 12 (10–33) |
| Means ± SD | 13 ± 4 |
| Race/ethnicity, No. (%) | |
| White | 41 (76) |
| Black | 6 (11) |
| Non-white Hispanic | 6 (11) |
| Asian | 1 (2) |
| Type of malignancy, No. (%) | |
| Acute myelogenous leukemia | 24 (44) |
| Myelodysplastic syndrome | 12 (22) |
| Acute lymphocytic leukemia | 11 (20) |
| Chronic myelogenous leukemia | 5 (9) |
| Chronic lymphocytic leukemia | 1 (2) |
| T-cell leukemia | 1 (2) |
| Type of transplant, No. (%) | |
| Matched related donor | 21 (39) |
| Matched unrelated donor | 28 (52) |
| Cord blood | 5 (9) |
| Type of conditioning regimen, No. (%) | |
| Myeloablative | 50 (93) |
| Nonmyeloablative | 4 (7) |
HCT, hematopoietic cell transplantation.
IL28B Determination
Oral samples were collected from patients in the following manner: patients were asked to refrain from drinking or eating for 3 hours and then to fill the Oragene DNA Self-Collection Kit with saliva. The kits were then shipped to the Mayo Clinic Referral Laboratory for processing and analysis. TaqMan real-time PCR analysis with allele-specific probes (Life Technologies, Carlsbad, CA) based on a method described by Cook et al7 was used to detect rs12979860 CT. Briefly, the assay consists of a primer pair that amplifies an upstream region in the IL28B gene. Two probes that are sequence specific for the 2 alleles, the C allele (wild type) and the T allele (mutant type), are labeled with 2 different reporter dyes, 1 for each allele. During PCR amplification, each probe annealed specifically to its complementary sequence between the forward and reverse primer sites. When the probe binds in the proximity of a quencher dye, the result is a quenching of the probe's fluorescence. Because the AmpliTaq-Gold DNA polymerase extends the primers, the polymerase cleaved probes hybridized to the target, separating the reporter dye from the quencher, resulting in increased fluorescence from the reporter. Thus, the fluorescence signal generated by PCR amplification indicates which alleles were present in the sample.
Results
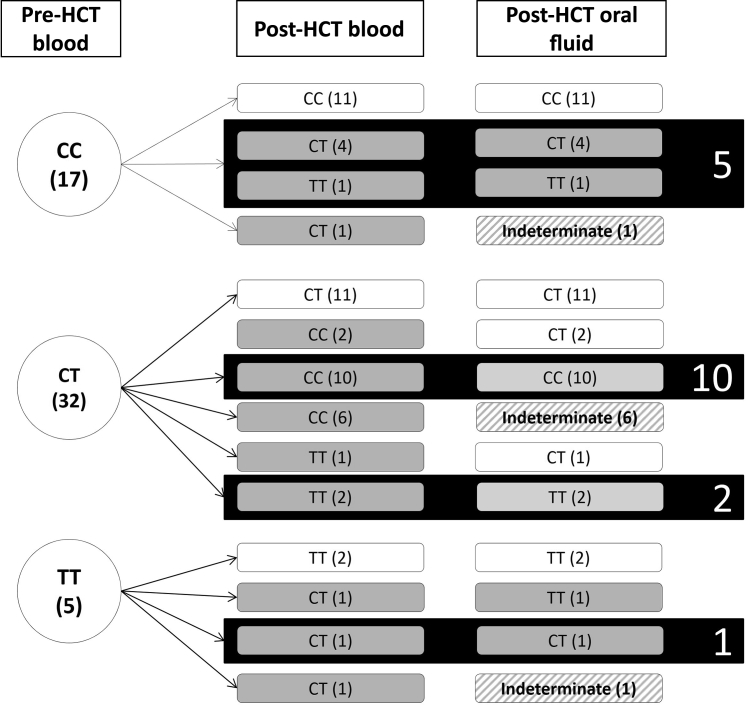
The frequency of changes in genotype from baseline to 60 days after HCT are shown in Figure 1. Among patients with an rs12979860 CC genotype before HCT (n = 17), 11 had the same genotype (CC) in blood samples after HCT, indicating that the donor had the same genotype as the recipient. For the remaining 6 patients, the genotype of the donor, as indicated by recipient post-HCT blood samples, was different from that of the recipient, with 5 patients receiving a CT genotype and 1 receiving a TT genotype. In patients with a CT genotype before HCT (n = 32), 11 had the same genotype after HCT, 18 received a CC genotype, and 3 received a TT genotype. Finally, in patients with a TT genotype before HCT (n = 5), 2 had the same genotype after HCT, and 3 received a CT genotype (Figure 1).
Figure 1.
Frequency and distribution of changes in genotype from baseline to 60 days after hematopoietic stem cell transplantation (HCT) comparing oral to blood testing.
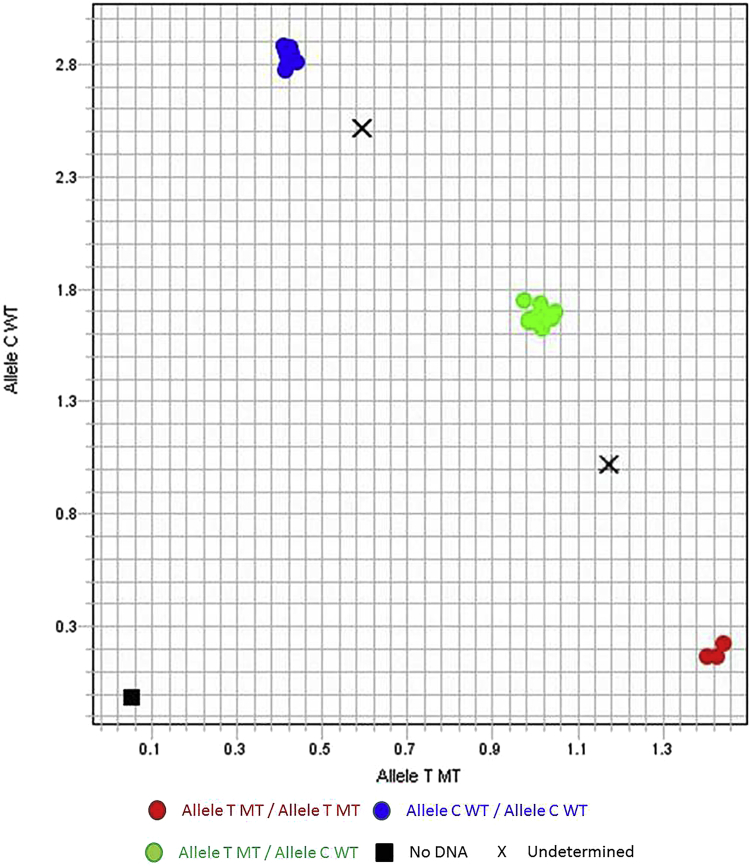
Overall, 24 HCT recipients had the same rs12979860 genotypes as their donors (11 sharing a CC genotype, 11 sharing a CT genotype, and 2 sharing a TT genotype), and in these cases, it would be anticipated that the oral fluid results would be the same, which was in fact the case. However, in 30 patients, the recipient and donor genotypes were an rs12979860 mismatch. Of these, 18 had an oral fluid genotype that matched the donor genotype, whereas 4 retained the recipient genotype in the oral fluid results. In the remaining 8 patients mismatched with their donors, an IL28B genotype was not identified in oral fluid because the fluorescent signals could not be characterized as either homozygous or heterozygous but rather appeared to be a combination of both. The allelic discrimination plots for the different genotype calls are shown in Figure 2. The samples represented by an X are examples of the oral fluid samples that could not be characterized. For those 2 samples, the genotype of the recipient and the donor were known, and the results fell between and could not be characterized as either genotype.
Figure 2.
Allelic discrimination plot showing the characterized genotypes: homozygous wild-type (WT) genotypes (upper left), homozygous mutant-type (MT) genotypes (bottom right), and heterozygous genotypes (middle center). The 2 samples represented by X are samples that are uncharacterized.
Discussion
After successful HCT, the recipient's blood should reflect the donor genotype, and the recipient genotype is expected to be reflected in the oral fluid because the oral fluid contains buccal cells from the recipient.8 In this study, we found that the oral fluid genotype frequently does not match the recipient's genotype and can be a mixture of both the donor and recipient or just that of the donor. The mechanism by which the IL28B genotype mediates response therapy is not yet understood and is the subject of intense ongoing research. In patients who have undergone bone marrow transplantation, the question of whether the recipient or donor genotype is more relevant to outcome is unknown.
Oral fluid is a complex mixture that contains secretions from the major salivary glands (parotid, submandibular, and sublingual), secretions from the minor glands (labial, buccal, and palatine), bacteria, sloughed epithelial cells, gingival fluid, food debris, and other particulate matter.8 In HCT recipients, the sloughed epithelial cells of the mouth are that of the recipient's germline genotype. However, oral fluid also contains varying numbers of immunomodulatory cells, such as lymphocytes, macrophages, and granulocytes. Few studies indicate that blood and oral rinses of patients who had underwent HCT were typically more similar to the genotype of the donor's marrow than to the pre-HCT genotype of the recipient.9, 10 Our study supports these findings; in 18 of 30 HCT recipients with a genotype mismatch to their donors, the oral fluid matched the genotype of the donor, suggesting that the donor cells, likely the immunomodulatory cells, predominated in the oral fluid. In contrast, in the 4 patients whose germline genotypes were reflected in the oral fluid but not in the blood, the recipient's likely buccal cells predominated in the oral fluid. Interestingly, in patients in whom the oral fluid genotype could not be determined, the genotyping data suggested a mixture of DNA, indicating the presence of cells from both the donor and recipient.
Our findings raise an important question about the appropriate sample to determine the recipient genotype after a successful HCT. Furthermore, the broader effect of recipient-donor genotype mismatch on the selection of appropriate sample types collected for determination of genotype is also brought into question. Does oral fluid reveal the recipient genotype, as expressed in the epithelial cells, or does oral fluid show the donor genotype, as expressed in the newly acquired immune system, or is it a combination of both? This is a complex issue that needs to be further investigated as we move further into the era of personalized and pharmacogenetic medicine.
In conclusion, oral fluid may be an inappropriate matrix to determine an HCT recipient's genotype after HCT. In the era of personalized medicine, the roles of the recipient and donor genotypes after HCT for clinical pharmacogenetic purposes need further investigation. Because of the potential presence of donor blood–derived cells, the utility of other sample sites (eg, biopsies or cultured fibroblasts) as alternatives for recipient genotype assessment after HCT needs to be determined in future studies.
Acknowledgments
L.J.L. and J.L.B. performed the testing, analyzed data, and reviewed the manuscript; R.F.C. designed the study, analyzed the data, and critically reviewed the manuscript; D.P.S. enrolled patients, collected and analyzed the data, and reviewed the manuscript; E.J.S. and K.R. reviewed the data and the manuscript; J.M.A. enrolled patients, collected data, and reviewed the manuscript; and L.N. designed the study, enrolled patients, analyzed data, and wrote the manuscript.
Footnotes
Supported in part by NIH Cancer Center Support grant P30CA016672 (University of Texas MD Anderson Cancer Center; D.P.S., J.M.A., E.J.S., K.R., and R.F.C.).
Disclosures: J.L.B. has licensed intellectual property to AssureX Health and OneOme.
References
- 1.Barreiro P., Fernandez-Montero J.V., de Mendoza C., Labarga P., Soriano V. Pharmacogenetics of antiretroviral therapy. Expert Opin Drug Metab Toxicol. 2014;10:1119–1130. doi: 10.1517/17425255.2014.930128. [DOI] [PubMed] [Google Scholar]
- 2.Hassan M., Andersson B.S. Role of pharmacogenetics in busulfan/cyclophosphamide conditioning therapy prior to hematopoietic stem cell transplantation. Pharmacogenomics. 2013;14:75–87. doi: 10.2217/pgs.12.185. [DOI] [PubMed] [Google Scholar]
- 3.Yin Z., Dai J., Deng J., Sheikh F., Natalia M., Shih T., Lewis-Antes A., Amrute S.B., Garrigues U., Doyle S., Donnelly R.P., Kotenko S.V., Fitzgerald-Bocarsly P. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J Immunol. 2012;189:2735–2745. doi: 10.4049/jimmunol.1102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge D., Fellay J., Thompson A.J., Simon J.S., Shianna K.V., Urban T.J., Heinzen E.L., Qiu P., Bertelsen A.H., Muir A.J., Sulkowski M., McHutchinson J.G., Goldstein D.B. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 5.Thomas D.L., Thio C.L., Martin M.P., Qi Y., Ge D., O'Huiqin C., Kidd J., Khakoo S.I., Alexander G., Goedert J.J., Kirk G.D., Donfield S.M., Rosen H.R., Tobler L.H., Busch M.P., McHutchinson J.G., Goldstein D.B., Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bravo D., Solano C., Gimenez E., Remigia J.M., Corrales I., Amat P., Navarro D. Effect of the IL28B Rs12979860 C/T polymorphism on the incidence and features of active cytomegalovirus infection in allogeneic stem cell transplant patients. J Med Virol. 2014;86:838–844. doi: 10.1002/jmv.23865. [DOI] [PubMed] [Google Scholar]
- 7.Cook L., Diem K., Kim W., Scott J.D., Jerome K.R. Allele-specific PCR for determination of IL28B genotype. J Clinical Microbiol. 2012;50:4144–4146. doi: 10.1128/JCM.02084-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hold K., de Boer D., Zuidema J., Maes R.A. Saliva as an analytical tool in toxicology. Int J Drug Test. 1995;1:1–36. [Google Scholar]
- 9.Endler G., Greinix H., Winkler K., Mitterbauer G., Mannhalter C. Genetic fingerprinting in mouthwashes of patients after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;24:95–98. doi: 10.1038/sj.bmt.1701815. [DOI] [PubMed] [Google Scholar]
- 10.Thiede C., Prange-Krex G., Freiberg-Richter J., Bornhauser M., Ehninger G. Buccal swabs but not mouthwash samples can be used to obtain pretransplant DNA fingerprints from recipients of allogeneic bone marrow transplants. Bone Marrow Transplant. 2000;25:575–577. doi: 10.1038/sj.bmt.1702170. [DOI] [PubMed] [Google Scholar]