Abstract
Background
Both short and long-term exposure to traffic-related air pollutants have been associated with asthma and reduced lung function. We hypothesized that short-term indoor exposure to fine particulate matter <2.5 μm (PM2.5) and vanadium (V) would be associated with altered buccal cell DNA methylation of targeted asthma genes and decreased lung function among urban children in a nested subcohort of African American and Dominican children.
Methods
Six day integrated levels of air pollutants were measured from children’s homes (age 9–14; n = 163), repeated 6 months later (n = 98). Buccal samples were collected repeatedly during visits. CpG promoter loci of asthma genes (i.e., interleukin 4 (IL4), interferon gamma (IFNγ), inducible nitric oxide synthase (NOS2A), arginase 2 (ARG2)) were pyrosequenced and lung function was assessed.
Results
Exposure to V, but not PM2.5, was associated with lower DNA methylation of IL4 and IFNγ. In exploratory analyses, V levels were associated with lower methylation of the proinflammatory NOS2A-CpG+5099 among asthmatic overweight or obese children but not nonasthmatics. Short-term exposure to PM2.5, but not V, appeared associated with lower lung function (i.e., reduced z-scores for forced expiratory volume in one second (FEV1, FEV1/ forced vital capacity [FEV1/FVC] and forced expiratory flow at 25–75% of FVC [FEF25–75]).
Conclusions
Exposure to V was associated with altered DNA methylation of allergic and proinflammatory asthma genes implicated in air pollution related asthma. However, short-term exposure to PM2.5, but not V, appeared associated with decrements in lung function among urban children.
Electronic supplementary material
The online version of this article (doi:10.1186/s12931-017-0550-9) contains supplementary material, which is available to authorized users.
Keywords: Vanadium, PM2.5, Short-term exposure, DNA methylation, Overweight, Asthmatic children
Background
Both short-term and long-term exposure to fine particulate matter <2.5 μm (PM2.5) have been associated with reduced lung function [1–5]. However, pediatric cohort studies on short-term effects of PM2.5 on lung function are relatively scarce. Further, it is far from evident what components of PM2.5 cause these adverse health effects, and their underlying mechanisms. These components may include toxic agents like trace metals.
In the current study, we focused on vanadium (V) as the trace metal PM component because previous studies have shown that ambient levels of V, emitted from the burning of residual oil fuel mainly from residential heating and shipping ports [6] and traffic emissions [7], exhibited marked spatial variability in New York City (NYC) [8]. Measures of V have been associated with increased 1) cellular stress responses (i.e., Nuclear Factor kappa B) in human bronchial epithelial cells [9], 2) risk of PM2.5-related respiratory and cardiovascular hospitalizations [10], 3) mortality among elderly individuals [11], and 4) wheeze [12] and decreased lung function (i.e., forced vital capacity (FVC)) among children [13]. In the latter case, the V findings persisted after adjusting for co-pollutants (e.g., PM2.5 or elemental carbon (EC)), suggesting that V itself may be an important independent contributor of adverse respiratory effects of PM2.5. Moreover, susceptibility to these pollutants may vary by asthma phenotype, including obesity-related asthma [14, 15]. Overweight asthmatics exhibited more asthma-like symptoms in association with exposure to PM2.5, nitrogen dioxide (NO2) and polycyclic aromatic hydrocarbons (PAH) [16, 17], and greater declines in lung function in association with exposure to ozone [14].
Environmental epigenetic regulation also may underlie mechanisms of air pollution-associated asthma [18, 19]. As examples, recent ambient PM2.5 levels were associated with lower DNA methylation of the proinflammatory gene inducible nitric oxide synthase (iNOS encoded by NOS2A) [20], and chronic exposure to PAH was associated with methylation of the asthma regulatory gene Forkhead box transcription factor 3 (FOXP3) [21]. In a study of boilermakers, higher occupational levels of PM2.5, presumably representing high levels of metals [22], were associated positively with methylation of long interspersed nuclear element-1 (LINE-1) in peripheral blood leukocytes [23], indicating higher global methylation. However, pediatric cohorts have not yet investigated epigenetic changes of asthma genes in response to measures of air pollutants, and specifically for the V component.
Our objective was to delineate the association between residential exposure to air pollution, including PM2.5 and its metal component V, on epigenetic regulation and lung function in a nested cohort of asthmatics and healthy urban children. We specifically targeted epigenetic loci previously implicated in air pollution-related asthma [24–27]. We also quantified DNA methylation levels in buccal cells, aerodigestive track epithelium where air pollution-related molecular changes have been documented [28], via pyrosequencing technology to capture small differences [29–32]. We hypothesized that exposure to PM2.5 and V, assessed by residential measures integrated over 6 days, repeated 6 months later, would be associated with changes in buccal cell DNA methylation of targeted CpG loci in the promoter region of several asthma inflammatory genes (e.g., interleukin 4 (IL4), interferon gamma (IFNγ), NOS2A and arginase2 (ARG2)) among urban African American and Dominican children. Further, we explored whether such methylation would vary by obesity-asthma. We also hypothesized that short-term residential exposure to PM2.5 and V would be associated with decreased lung function.
Methods
Study population and residential air monitoring
Seven hundred twenty seven nonsmoking mothers of African-American and Dominican ethnicity living in Northern Manhattan and the South Bronx were recruited during pregnancy as part of the Columbia Center for Children’s Environmental Health cohort (CCCEH) birth cohort [33]. For this nested study, participants were recruited in order based on age criteria (9–14 years old still enrolled in CCCEH) and enriched for current asthma status (57% asthmatic vs 43% non-asthmatic). Children were classified as asthmatic if a specialized physician (allergist, pulmonologist) diagnosed them with asthma using study standardized and objective criteria, and if they had symptoms or used asthma medication in the 12 months prior to enrollment in the nested study [34]. Children without any asthma-related symptoms between age 5 and enrollment, or determined not to be asthmatic by our standardized criteria [34], in the nested study were classified as non-asthmatic. Height, weight, and body fat percentages (%BF) were measured at each visit (Time 1 and Time 2, 6 months later) using a portable stadiometer (SECA, Hamburg, Germany) and a segmental body composition monitor (Tanita Corporation, Tokyo, Japan). Children with body mass index (BMI) ≥ the age- and sex-specific 85th percentile of the year 2000 CDC growth charts for age and sex were classified as ‘overweight’ [35]. Waist circumference (WC) was measured twice at a level midway between the top of the hip bone and the lowest rib. High %BF and high WC, classified as the upper 33% of mean whole %BF and mean WC, respectively, were further investigated based on the strong correlations between BMI and %BF or WC in studies with young adolescents [36, 37].
Indoor air monitors collected six-day integrated PM2.5 filter samples at each of the 163 homes between March 2012 and August 2015 (Time 1; initial set-up) (Fig. 1). Samplings started on Wednesday or Thursday to minimize variation in air pollution exposure by day of the week [38]. The sampling was repeated approximately six months after the initial sampling period (Time 2; n = 98) to capture the seasonal variability in air pollution levels. Residential indoor monitors were placed in a room where the child spent most of his or her time. Data were analyzed for those children (n = 149) for whom measures of residential PM2.5 and V were available (Fig. 2). In order to perform a sensitivity analysis including chronic exposure to PM2.5, we obtained residential indoor PM2.5 data that were collected 4–8 years prior to Time 1, using the same sampling methodology. Of 163 children, 106 children had previously available PM2.5. The study was approved by the Columbia University Institutional Review Board and written informed consent and assent were obtained.
Fig. 1.
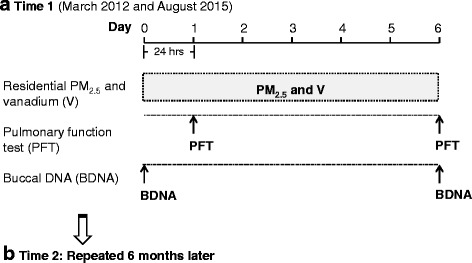
Study design. Residential indoor monitoring, pulmonary lung function test, and buccal sample collection over a 6 day sampling period, repeated 6 months later, are displayed. a Time 1 (March 2012 and August 2015). b Time 2: Repeated 6 months later
Fig. 2.
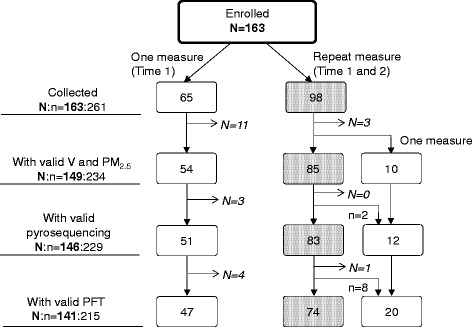
Schematic demonstration of study data. Numbers in the box represent the number of participants. N:n = number of repeat subjects: number of observations. Grey dotted box indicates two measures (both Time 1 and Time 2, 6 months apart) available and white box only one measure (Time 1) available. N = 14 participants dropped due to invalid residential air pollution data, resulting in n = 234 (54 + 85 × 2 + 10) data points available. N = 3 participants (equivalent to n = 5 observations as two participants with repeat measure had one invalid data) were excluded from the analysis of DNA methylation due to technical failures in the laboratory and N = 5 more participants (equivalent to n = 14 observations) were removed due to invalid pulmonary function tests
Residential indoor assessment
PM2.5 was analyzed by weighing (post-pre weight) Teflon filter samples collected from a cyclone with a 2.5 μm aerodynamic-diameter cut point (model SCC 1.062, BGI, Inc.) that operated at 1.5 L/min (±15% of standard deviation; SD) for six days. V levels on the same filters were analyzed using XEPOS Energy-Dispersive X-Ray Fluorescence Spectrometer (XEPOS 3, Spectro, Kleve, Germany) in specially-designed polytetrafluoroethylene (PTFE) holders to keep the filters in a fixed flat geometry [39]. The XEPOS had been calibrated for PM2.5 filters for V and other metals. Filter blanks and an internal standard consisting of a NYC PM2.5 filter were counted with each analysis batch of 10 filters.
Buccal sample collection, DNA extraction and methylation
Buccal samples were collected during in-home visits on Day 0 (set-up day) and Day 6 (take-down day; Fig. 1) to capture changes in DNA methylation over 6 days. The participants were asked to rinse out their mouths with water before buccal swabs sampling to avoid contamination from food and then brush the inside of each cheek for fifteen seconds with a CytoSoft cytology brush (Fischer Scientific, Pittsburgh, Pennsylvania). The brushes were placed immediately in cell lysis solution (Qiagen Sciences, Germantown, Maryland) and stored at room temperature until processing. To ensure a homogenous cell population, brush smears were generated from twenty-five participants selected at random and stained using hematoxylin and eosin. Eight fields were randomly selected and imaged at 10X using a Nikon Eclipse TS100 (Tokyo, Japan) brightfield microscope. Two individuals independently counted and determined that 94% percent of the total cells were squamous epithelial cells among the 24 participants (kappa agreement score 0.934).
DNA was extracted and isolated using the Gentra Puregene Buccal Cell kit (Qiagen), qualified and quantified using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA). Extracted DNA was bisulfite converted with the EZ-96 DNA Methylation-Lightning Kit (Zymo Research, Irvine, CA). Targeted promoter region CpG loci were selected based on their known importance to allergy and to NOS2A-related inflammation [25, 40], and published epigenetic links to asthma-related environmental exposures and/or asthma outcomes (Additional file 1: Figure S1 and Table S1). PCR primers were designed for the targeted regions using Pyromark Assay Design SW 2.0 Software (Qiagen) (Additional file 1: Table S2). PCR was performed using Qiagen HotStarTaq DNA Polymerase (Qiagen), and methylation levels for each of the targeted CpGs was measured using the Pyromark Q96 MD pyrosequencing instrument (Qiagen).
Quality assurance measures included: 1) methylated and unmethylated DNA (Qiagen) was added to each PCR plate as a control, and 2) the methylation level of a duplicate buccal sample collected at the same time was compared for 13% of the cohort (n = 23-29). The average absolute percent differences for the IL4, IFNγ, and NOS2A measures between the primary and duplicate buccal sample ranged between 2% and 7%, indicating a good agreement. The ARG2 loci exhibited greater average percent differences, ranging from 54% to 76% for CpG -30 and CpG−32, respectively. One additional data point was excluded due to PCR contamination.
Pulmonary function tests (PFTs)
PFTs were conducted during in-home visits on Day 1 and Day 6 using a portable spirometer (Koko, nSpire Health, Longmont, Colorado), in accordance with ATS and ERS guidelines [34] and repeated 6 months later (Fig. 1). Tests were considered acceptable if they met the following criteria: 1) rapid upstroke, 2) volume extrapolated <5% of FVC, 3) minimal premature termination of exhalation (premature termination = termination at >15% of peak flow), and 4) smooth exhalatory limb [41], as determined by two pulmonologists. PFTs that did not meet the acceptability criteria were excluded (n = 14 out of 229; Fig 2). Four spirometry outcome measures were included for analysis: FVC, forced expiratory volume in one second (FEV1), FEV1/FVC, and forced expiratory flow at 25–75% of forced vital capacity (FEF25–75).
Statistical analyses
Chi-square and Mann–Whitney tests were used to detect the difference in demographic characteristics between groups and air pollutant levels by heating season (i.e. October-April), respectively. Consideration of heating season was to assess meteorological conditions such as temperature, humidity, and cold/flu season which could confound measures of air pollution and respiratory morbidity [12, 42, 43]. A spearman correlation coefficient was computed for correlations between PM2.5, and V while the intraclass correlation coefficient (ICC) was calculated for correlations among repeated measures of percent methylations. Air pollutant concentrations were log-transformed to assume normal distribution for subsequent analyses.
Due to non-normal distribution of the log-transformed methylation data, percent methylation of IL4, IFNγ, and NOS2A were dichotomized at the upper tertile of each individual CpG site [44]. Percent methylation of ARG2 were averaged across 3 selected CpG sites then further dichotomized as ‘unmethylated (0)’ vs ‘methylated (1)’ if the average percent methylation was zero or > 0, as described [25]. The associations among residential levels of PM2.5, V, and changes in DNA methylation were analyzed using a modified Poisson regression in generalized estimating equations (GEE) models to estimate relative risks (RR) [45]. The analyses were conducted using PM2.5 and V (two-pollutant models), and DNA methylation on Day 6 with adjustment of Day 0 DNA methylation to assess the independent effects of each pollutant on changes in DNA methylation. Final models were further adjusted for common covariates, including race/ethnicity, sex, age, heating season, asthma diagnosis, and overweight. Exploratory analyses were conducted to examine differences in air pollution-related DNA methylation by overweight asthmatic phenotype, suggested in previous in cross-sectional studies [26, 46]. Adjusted models stratified by overweight and asthma (i.e., 4 groups: overweight asthmatics, non-overweight asthmatics, overweight non-asthmatics, and non-overweight non-asthmatics) were run. A similar analysis was performed after replacing overweight with obesity (BMI ≥ the age- and sex-specific 95th percentile), high BF%, or high WC.
Spirometric variables were converted to ethnic-specific z-scores, which were adjusted for sex, age, and height, according to the Global Lung Initiative (GLI-2012) equations for African American and Dominican (mixed ethic origin treated as ‘other’) children using the GLI-2012 software for subsequent analyses (http://www.ers-education.org/guidelines/global-lung-function-initiative/tools.aspx) [47]. Multivariable linear regression analyses in two-pollutant models were used to examine the observed effects of PM2.5 on each lung function outcome on Day 6, after controlling for V, Day 1 lung function outcome, heating season, asthma diagnosis, and overweight. Of the 149 children with valid air monitoring data, 3 and 8 children, respectively, were excluded due to invalid methylation (i.e. failed pyrosequencing run) and lung function data, resulting in a final sample of 146, and of 141 for the DNA methylation and lung function analysis, respectively (Fig. 2).
Sensitivity analyses were conducted as follow: (1) reanalysis after controlling for time-spent at home to address the impact of residential exposure vs other microenvironments (e.g., school and outdoors), (2) reanalysis after replacing concurrent PM2.5 levels with those measured 4–8 years prior to Time 1, as a surrogate for chronic exposure to PM2.5 in methylation analysis, (3) reanalysis after controlling for food intake, by asking the question “In the past two hours, have you had anything to eat or drink? (Yes/No)”, and (4) reanalysis after replacing heating season with four seasons (i.e., spring, summer, fall and winter), given the reported seasonal variation in lung function outcomes [48]. All analyses were performed using SPSS Statistic version 23.0 (SPSS Inc., Chicago, IL, USA) where p < 0.05 was considered statistically significant.
Results
Subject characteristics and residential exposure
There were no significant differences in demographic variables by enrollment into the nested cohort, except for a higher proportion of asthma and seroatopy, consistent with our recruitment strategy (Table 1). On daily average, children spent 68% (16.3 h) of their time at home. At Time 1, children were exposed to the median levels (Interquartile range IQR) of 11.9 (9.1) μg/m3 and 1.44 (1.34) ng/m3 of residential PM2.5 and V, respectively. A significant seasonal pattern was detected for V. Levels of V, but not PM2.5, were higher during the heating compared to the nonheating season (Additional file 1: Figure S2). While residential levels of PM2.5 at Time 1 moderately correlated with those at Time 2, 6 months later, V weakly correlated with repeated measures, possibly due to substantial seasonal variations (Additional file 1: Figure S3). PM2.5 levels weakly correlated with V levels (Fig. 3). Furthermore, indoor levels of PM2.5 at Time 1 weakly correlated with those measured 4–8 years prior to Time 1, on the same children (Fig. 3), suggesting common chronic sources of PM2.5 air pollution.
Table 1.
Cohort characteristics
| Characteristic | Participants includeda (n = 149) | CCCEH cohort not included (n = 578) | P-valueh |
|---|---|---|---|
| Maternal ethnicity | 0.57 | ||
| Dominican | 94/149 (63%) | 379/578 (66%) | |
| African American | 55/149 (37%) | 199/578 (34%) | |
| Age mean [min-max], yrs | 12.5 (9.2–14.3) | - | |
| Girls | 76/149 (51%) | 300/578 (52%) | 0.85 |
| ≥Maternal high school degree | 82/144 (57%) | 374/569 (66%) | 0.05 |
| Maternal asthma (+) | 42/149 (28%) | 121/578 (21%) | 0.06 |
| Prenatal ETS exposureb (+) | 48/147 (33%) | 198/570 (35%) | 0.64 |
| Current ETS exposurec (+) | 12/121 (10%) | - | |
| Daily time spent homed hrs, mean ± SD | 16.3 ± 4.8 | ||
| BMIe z score, mean ± SD | 0.86 ± 1.11 | - | |
| Underweight (<5th %ile) | 5/149 (3%) | - | |
| Normal weight (5th–85th %ile) | 66/149 (44%) | - | |
| Overweight (85th–95th %ile) | 40/149 (27%) | - | |
| Obesity (≥95th %ile) | 38/149 (26%) | - | |
| % Body fat, mean ± SD | 26.7 ± 8.4 | - | |
| Waist circumference cm, mean ± SD | 71.5 ± 11.5 | - | |
| Asthmaf | 87/149 (58%) | 83/360 (23%) | <0.001 |
| Seroatopyg | 74/134 (55%) | 113/269 (42%) | 0.01 |
CCCEH Columbia Center for Children’s Environmental Health, BMI Body mass index, ETS: Environmental tobacco smoke, IgE: Immunoglobulin E, SD: standard deviation
The total number of CCCEH cohort participants enrolled at birth = 727; aIncludes only the children in nested study that had complete data available for current analysis. Participants excluded if residential indoor sampling data not collected due to entry criteria (n = 564) and invalid air pollution data (n = 14)
bReport of any smoker in the house during pregnancy. All mothers were nonsmokers during pregnancy
cReport of any smoker during 1-week sampling period
dDetermined by 24-hour questionnaire by answering “Between the time we dropped off the monitor and when you went to bed, did you leave home (Yes/No); then “What time did you leave home?”; “What time did you get home?”
eWeight (kg)/height (m)2, SD, standard deviation
fDetermined by a specialist physician using standardized criteria at age 5–12 year [34]
gTotal IgE ≥ 80 IU/mL
hChi-tests performed
Fig. 3.
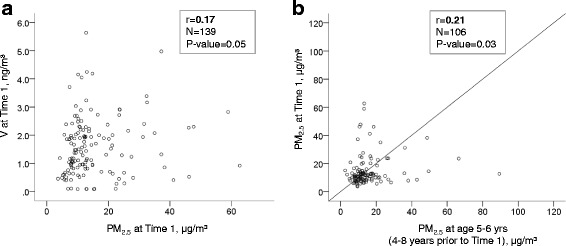
Correlations between (a) PM2.5 and V measured at Time 1, and (b) repeated residential indoor measures of PM2.5, 4–8 years apart Of 139 children who had valid air pollution data at Time 1, 106 had previous residential indoor measurements that were collected 4–8 years ago using the same sampling methodology
Buccal cell asthma gene methylation
In general, the targeted CpG sites in the IL4 promoter were heavily methylated, while the CpG sites in ARG2 were largely unmethylated; the latter is consistent with previous findings in buccal cells (Additional file 1: Figure S4) [25]. IFNγ methylation levels also were comparable to those reported in other urban cohorts [49]. Within each gene loci, two targeted CpG sites weakly correlated (Additional file 1: Figure S5). In general, repeated measures (Day 0 and Day 6) of percent methylation showed low values of ICCs (Additional file 1: Table S3), indicating substantial within-subject variability in methylation levels over the short-term, as previously described [50].
Associations among PM2.5, V exposures and DNA methylation
Overall, PM2.5 levels were not associated with DNA methylation at the CpG loci in IL4, IFNγ, NOS2A, and ARG2 gene in two-pollutant models (Table 2). In comparison, the relative risk of higher (i.e. upper tertile) IL4 CpG−326 and IFNγ CpG−54 methylation decreased with higher residential V exposure in adjusted models (Table 2). Associations between V exposure and DNA methylation in NOS2A and ARG2 loci were not significant.
Table 2.
Associations between residential measures of PM2.5, V and Day 6 DNA methylation in adjusted models (N:n = 146:229)
| RRadj a [95% CI] | |||
|---|---|---|---|
| Gene | CpG sites | PM2.5 | V |
| IL4 | −326 | 1.16 [0.84–1.61] | 0.80 [0.65–0.98]* |
| −48 | 1.04 [0.76–1.43] | 1.05 [0.83–1.34] | |
| IFNγ | −186 | 0.87 [0.64–1.19] | 1.23 [0.96–1.58] |
| −54 | 1.03 [0.79–1.35] | 0.81 [0.67–0.98]* | |
| NOS2A | +5099 | 1.07 [0.79–1.45] | 0.97 [0.80–1.19] |
| +5106 | 1.07 [0.74–1.54] | 0.98 [0.78–1.23] | |
| ARG2 | −32, −30, and −26b | 1.07 [0.97–1.17] | 0.96 [0.89–1.02] |
N: number of repeat subjects included for the analysis and n: number of observations from both Time 1 and Time 2
aAdjusted for race/ethnicity, sex, age, heating season, asthma diagnosis, overweight, and DNA methylation at Day 0 (Two-pollutant models)
bAverage of ARG2 CpG sites of −32, −30, and −26
* p-value < 0.05
In exploratory analyses that stratified by overweight and asthma (i.e., four groups), there were still no significant associations between PM2.5 and DNA methylations in the targeted asthma genes (Additional file 1: Table S4). In comparison, a distinct methylation pattern following V exposure was observed among the 4 groups. A significant association between V levels and lower methylation of NOS2A CpG+5099 was observed among overweight asthmatic children (Additional file 1: Table S5). Interestingly, the opposite pattern (higher methylation) was observed among the overweight non-asthmatic children at the IFNγ CpG−186 locus. Further, among non-overweight asthmatic children, V levels were associated with lower methylation of IFNγ CpG−54 (Additional file 1: Table S5). Methylation of ARG2 and IL4 was not associated with V levels among any of the phenotypes. Furthermore, when overweight was replaced by obesity, the observed associations of V with DNA methylation of NOS2A CpG+5099, IFNγ CpG−54, and IFNγ CpG−186 shown in Additional file 1: Table S5, remained strong (RR [95% CI]: 0.40 [0.26-0.63]; p = 0.03, 0.71 [0.54-0.92]; p = 0.001, 1.42 [1.02-1.97]; p = 0.039 for NOS2A CpG+5099, IFNγ CpG−54, and IFNγ CpG−186 respectively). Interestingly, a non-significant association, previously observed between V and lower methylation of IL4 CpG−326 among overweight asthmatic children, became significant when overweight was replaced by obesity (RR [95% CI]: 0.40 [0.24-0.68]; p = 0.001), high-BF (RR [95% CI]: 0.47 [0.27- 0.80]; p = 0.006), or high-WC in asthmatic children (RR [95% CI]: 0.21 [0.08- 0.53]; p = 0.001).
PM2.5 and V exposures and lung function
In multivariable linear regression models, residential PM2.5 levels, measured over 6 days, appeared associated with decreased z-scores for FEV1, FEV1/FVC and FEF25–75, including following adjustment for V levels (Table 3). In comparison, significant associations between V and lung function outcomes were not observed. Therefore, analysis for mediation by methylation of V on lung function was deferred.
Table 3.
Associations between residential levels of PM2.5 and V and Day 6 lung function z-scores
| Lung function | Beta coefficient a (95% CI) (N:n = 141:215) |
|
|---|---|---|
| z-scoreb | PM2.5 | V |
| FVC | −0.07 (−0.19, 0.05) | 0.07 (−0.02, 0.15) |
| FEV1 | −0.15 (−0.29, −0.01)* | 0.09 (−0.02, 0.19) |
| FEV1/FVC | −0.17 (−0.31, −0.03)* | 0.05 (−0.09, 0.19) |
| FEF25–75 | −0.18 (−0.32, −0.04)* | 0.03 (−0.12, 0.18) |
N: number of repeat subjects included for the analysis and n: number of observations from both Time 1 and Time 2
FVC Forced vital capacity, FEV 1 Forced expiratory volume in one second, FEF 25–75 Forced expiratory flow at 25–75% of Forced Vital Capacity
aTwo-pollutant models adjusted for heating season, asthma diagnosis, overweight, each lung function z- score measured on Day 1
bBased on reference equation from the Global Lung Initiative 2012 [47]
*p < 0.05
Sensitivity analyses
First, after controlling for time spent home, the main findings in Table 2 remained similar although the association between V and methylation of IL4 CpG−326 became borderline significant (RR [95% CI]: 0.81 [0.66-1.00]; p = 0.052 for IL4 CpG−326 and 0.81 [0.67-0.97]; p = 0.026 for IFNγ CpG−54). Second, with an adjustment of residential chronic exposure, assessed by previous PM2.5 levels measured 4–8 years prior to Time 1, significant association between V and lower methylation of IFNγ CpG−54 in Table 2 remained with a smaller RR (RR [95% CI]: 0.76 [0.63-0.92]; p = 0.005) while the association between V and methylation of IL4 CpG−326 lost statistical significance (p = 0.10), possibly due to a smaller sample (N[subjects] = 115 and n[obervations] = 172). Third, when we controlled for food intake, the main findings in Table 2, persisted (Data not shown). Lastly, when the two heating vs nonheating season was replaced with four seasons, the significant associations of PM2.5 and lung function outcomes were replicated (data not shown).
Discussion
In this nested cohort of African American and Dominican children living in NYC, we found that that 6 day-integrated residential V, but not PM2.5, was associated with lower buccal cell promoter DNA methylation of asthma T helper (Th) gene (i.e. IL4, IFNγ) loci, even after controlling for methylation levels 6 days previously. We also found that residential PM2.5 levels, but not V, were associated with lower lung function (i.e., z-scores for FEV1, FEV1/FVC and FEF25–75). To our knowledge, this is the first study to report altered asthma gene DNA methylation related to residential V exposure, and lung function decrements associated with short-term residential exposure to PM2.5 among urban children.
The strengths of this study include the 1) direct measurement of each child’s short-term (6 day) home exposure to air pollution, that may reduce misclassification of personal exposure and allow us to discern effects of individual key air pollutants (e.g., V and PM2.5), 2) use of repeat prospective measures of environmental air pollutants, DNA methylation, and lung function, which allow us to detect changes in each within the same children and consider seasonal effects (6 months apart), 3) targeted focus on the methylation of specific loci that previously were implicated in air pollution-related asthma. We did so by using pyrosequencing technology to capture small differences in their DNA methylation in order to validate the importance of these small differences to pediatric urban asthma, and 4) utilization of a well-phenotyped prospective birth cohort study with detailed data on children’s clinical status and past residential PM2.5 levels; the latter of which correlated over a 4–8 year time period (Fig. 3) and allowed us to use as a surrogate for residential chronic exposure to PM2.5 in sensitivity analyses.
We chose to focus on altered methylation of mechanistically relevant gene loci that were implicated previously in air pollution-related asthma, given the emerging environmental epigenetic literature (Additional file 1: Table S1). In cohort studies of electric furnace steel plant workers, boilermaker welders, chronic obstructive pulmonary disease (COPD) patients in China, and children in Southern California, exposure to PM2.5 was associated with altered NOS2A promoter region DNA methylation [20, 27, 51, 52], suggesting environmental epigenetic mechanisms. We found that residential V, but not PM2.5, was associated with lower methylation of several Th gene promoter loci, including IL4 CpG−326 and IFNγ CpG−54. The findings may be supported by observations in mouse CD4+ splenic T cells following concomitant exposure of Aspergillus fumigatus and diesel exhaust particles (DEP) [24], mouse CD4+ T cells from lung-draining lymph nodes following chronic ovalbumin challenge [53], and numerous human cohort experimental studies [54] (Additional file 1: Table S1). Because DNA methylation in promoter regions is usually critical to gene silencing in human cells [55], its decrease is consistent with upregulated expression of the proallergic immune response. In comparison, lower methylation of IFNγ CpG−54, while considered important to suppressing allergic immune responses in some mouse models [56], also has been observed paradoxically to enhance allergy or airway hyperresponsiveness [53, 57]. We did not identify associations between residential PM2.5 and altered DNA methylation. One explanation may be the lack of short-term variability in PM2.5 compared to V, given the moderate correlation over time (Additional file 1: Figure S3), thus making it hard to capture a methylation signal due to smaller variations in PM2.5 levels. Alternatively, our results suggest that instead of PM2.5, a complex particle mixture of various chemical constituents from multiple sources, a specific individual metal component, V, may be one of the important drivers of epigenetic changes despite V only contributes an average of less than 1% to total PM2.5 mass.
Further, in exploratory analyses, we observed the associations between V and select DNA methylation differed by overweight asthma stratum. In particular, V was associated with lower methylation (and presumed greater gene activation) of NOS2A CpG+5099 only among overweight or obese asthmatic children. Also, the association between V and IL4 CpG−326 among overweight asthmatic children gained statistical significance when restricted to obese, high BF or high WC in asthmatics. These findings appear consistent with one small cross-sectional study that showed dysregulated DNA methylation in peripheral blood mononuclear cells (PBMCs) that varied by the asthma and obesity phenotype [46]. This group found that obese asthmatics, when compared to obese non-asthmatics, exhibited altered methylation in many pathways related to IL4, IFNγ and NOS2A gene functions (as shown in Additional file 1: Table S5), including IgE signaling, and interferon and chemokine activity [58, 59]. Interestingly, in the absence of asthma, the overweight or obese children were instead susceptible to hypermethylation of IFNγ CpG−186 following V exposure. This may be consistent with enhanced inflammation and differential regulation of T helper responses previously observed in obesity [60]. Despite the smaller sample size in stratified analysis, our results were consistent across overweight, obese, high BF and high WC classification, suggesting that obesity may enhance susceptibility to the effect of V on epigenetic changes.
Another objective was to evaluate adverse effects of a specific metal component of V on lung function in relation to PM2.5. Our findings that PM2.5 seemed to drive decrements in lung function are novel because 1) to date, no studies have looked at lung function in relation to short-term exposure in children. We did this by controlling for previous lung function z-scores measured on Day 1 to assess changes in lung function over a short period, and 2) we used direct measurement of PM2.5 through residential indoor monitoring, rather than estimates from land-use regression models employed in most studies [3, 61]. In comparison, counter to our prediction based on recent reports of V effects on lung function in children [13, 61], we were not able to detect any associations between residential V levels and lung function z-scores. But our study differed in terms of the shorter timeline of exposure to V and by the differences detected in the obstructive airway physiology characteristic of asthma (i.e., reduced FEV1), and not FVC.
The focus on quantifying differences in pre-selected CpG specific targets also allows us to compare their potential differential impact across loci, across a short timeline of exposure, and in limited case, across studies. For example, we observed relatively weak correlations between neighboring CpG sites within the same gene at Time 1 (Additional file 1: Figure S5). This observation may challenge previous reports that DNA methylation at adjacent CpG sites tends to display similar amounts of methylation [62]. However, they are consistent with emerging evidence in humans that suggest individual CpG sites may methylate to a different extent [49]. Another group also reported, in a controlled exposure study of allergen and diesel exhaust, substantial differences in single CpG site methylation in human bronchial epithelial cells [32]. This suggests that neighboring CpG sites may respond to exposures independently.
We acknowledge several limitations. First, methylation was measured in buccal cells, previously shown to inform on airway molecular changes [20, 25], and did not compare to the target lung tissue. However, lung tissue cannot be accessed repeatedly in children, and previous studies have documented high correlations in gene expression between tissues obtained from the buccal mucosa and lung [63, 64]. The cells themselves represent a relatively homogenous population of epithelial cells, as we demonstrated. Second, we monitored residential indoor air for exposure assessment, but not outdoor or school environments that may have important confounding effects. However, urban children in this study spent the majority (68% on daily average) of their time home, and studies have shown that outdoor PM2.5 and V readily penetrate indoors [65, 66]. Further, sensitivity analysis with an adjustment of time-spent home showed that our findings persisted. Third, we recognize that other CpG loci upstream or downstream of targets, and certainly other genes, may contribute to the air pollution epigenetic effects, and that the presence of single nucleotide polymorphisms (SNPs) could impact methylation levels. However, the effect sizes of variants measured in genome-wide association studies also seem small, driving our approach to capture additional small epigenetic responses. Last, other metal components of PM2.5 such as nickel and iron, that may important along with V in respiratory health [9, 12, 13], were not available. Nonetheless, with targeted epigenetic loci previously implicated in asthma and the use of pyrosequencing technology, we demonstrated for the first time that short-term residential V exposure is associated with changes in the degree of methylation of important asthma genes in children. This furthers our understanding of epigenetic regulation and its susceptibility to exposure to a specific air pollutant. Further, we explored associations between V and methylation by asthma-obesity and observed differential DNA methylation patterns by asthma phenotype. While intriguing, the interpretation of these results should be considered with caution due to a relatively small sample size in stratified analyses.
Additional discussions of the ambient air pollution levels, DNA methylation values, and the further study limitations are presented in Additional file 1.
Conclusions
We found associations between short-term V exposure and DNA methylation of asthma gene loci. Short-term residential indoor measures of PM2.5, but not V, may have been associated with lower lung function among urban children. While previous studies have suggested possible links between metal exposure and particulate matter and differences in DNA methylation pattern relevant to asthma, none to our knowledge have specified the key component of these pollutants. Further, to the best of our knowledge, this is the first study to investigate the effect of short-term V exposure on altered DNA methylation of asthma genes, and suggests that V may be important to urban asthma via epigenetic regulation. Although requiring further investigation in a large cohort, our results suggest asthma phenotypes may need to be considered in environmental and epigenetic studies of asthma. In turn these findings ultimately may help guide policy-related or medical interventions against specific pollutants, and against obesity, as well as provide methods for early identification of at-risk children.
Acknowledgements
The authors thank Dr. Xinhua Liu for her review of the manuscript.
Funding
This work was supported by NIH (R01ES013163, P50ES015905, P01ES09600, 3R01ES013163-07S1, P30ES09089, S10OD016219-01), EPA (R827027, RD832141, RD834509), the Educational Foundation of America, the John & Wendy Neu Family Foundation, the New York Community Trust, and the Trustees of the Blanchette Hooker Rockefeller Fund. The funding bodies were not involved in the design of the study and collection, analysis, interpretation of data or in the writing of the manuscript.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
KHJ contributed to data analyses and interpretation of results, and co-wrote this manuscript with RLM. DT contributed to the collection of samples and analysis of DNA methylation. SLD contributed to the collection of samples, interpretation of results, and critical revision of the manuscript. MP contributed to the analytical approach and critical revision of the manuscript. JB contributed to sample collection. JRJ contributed to the analysis of DNA methylation. LH managed the database. JR contributed to the analysis of vanadium and particulate matter. FPP contributed to the study design and critical revision of the manuscript. SNC contributed to the study design, analysis of vanadium and particulate matter, and provided a critical revision of the manuscript. RLM conceived of the study, supervised all study elements, helped analyze the data, and co-wrote the manuscript. All authors reviewed and approved the final manuscript.
Competing interests
None of the authors have financial relationships with a commercial entity that has an interest in the subject of this manuscript. The authors declare that they have no competing interests.
Consent for publication
No data on an individual level is provided and thus this is not applicable.
Ethics approval and consent to participate
This study has been approved by Columbia University Human Subjects protocol AAAI0459. Written informed consent to participate has been obtained from study participants or their legal guardian.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- %BF
Body fat percentages
- ARG
Arginase
- BC
Black carbon
- BMI
Body mass index
- CCCEH
Columbia Center for Children’s Environmental Health
- COPD
Chronic obstructive pulmonary disease
- DEP
Diesel exhaust particle
- EC
Elemental carbon
- ETS
Environmental tobacco smoke
- FEF25–75
Forced Expiratory Flow at 25–75% of Forced Vital Capacity
- FeNO
Fractional exhaled nitric oxide
- FEV1
Forced expiratory volume in one second
- FOXP3
Forkhead box transcription factor 3
- FVC
Forced vital capacity
- GEE
Generalized estimating equation
- GLI
Global lung initiative
- ICC
Intraclass correlation coefficient
- IFNγ
Interferon gamma
- IL4
Interleukin 4
- IgE
Immunoglobulin E
- IQR
Interquartile range
- LINE-1
Long interspersed nuclear element-1
- NO2
Nitrogen dioxide
- NOS2A
Inducible nitric oxide synthase
- NYC
New York City
- PAH
Polycyclic aromatic hydrocarbon
- PBMCs
Peripheral blood mononuclear cells
- PEFR
Peak expiratory flow rate
- PFT
Pulmonary function test
- PM
Particulate matter
- PTFE
Polytetrafluoroethylene
- RR
Relative risk
- SNPs
Single nucleotide polymorphisms
- V
Vanadium
- WC
Waist circumference
Additional file
Promoter region CpG locations and rationale. Table S2. Primers for PCR and pyrosequencing. Table S3. Intraclass correlation coefficients (ICC) among repeated measures of buccal cell DNA methylation. Table S4. Associations between residential PM2.5 and Day 6 DNA methylation: by asthma and overweight. Table S5. Associations between residential V and Day 6 DNA methylation: by asthma and overweight. Figure S1. Targeted CpG sites in promoter region. Figure S2. Seasonal variations in (a) PM2.5 and (b) vanadium (V). Figure S3. Repeated residential indoor measures of (a) PM2.5 and (b) vanadium (V), 6 months later. Figure S4. Distribution of percent DNA methylation of IL4, IFNγ, NOS2A, and ARG2 at Day 6. Figure S5. Correlation matrix for Day 6-buccal cell DNA methylations of IL4, IFNγ, NOS2A, and averaged ARG2 at Time 1. (DOCX 286 kb)
Contributor Information
Kyung Hwa Jung, Phone: (212)-342-1671, Email: kj2237@cumc.columbia.edu.
David Torrone, Email: dt2339@cumc.columbia.edu.
Stephanie Lovinsky-Desir, Email: sl3230@cumc.columbia.edu.
Matthew Perzanowski, Email: mp2217@columbia.edu.
Joshua Bautista, Email: jbb2160@cumc.columbia.edu.
Jacqueline R. Jezioro, Email: js4641@cumc.columbia.edu
Lori Hoepner, Email: hoepner@nyspi.columbia.edu.
Jamie Ross, Email: jross@ldeo.columbia.edu.
Frederica P. Perera, Email: fpp1@columbia.edu
Steven N. Chillrud, Email: chilli@ldeo.columbia.edu
Rachel L. Miller, Email: rlm14@cumc.columbia.edu
References
- 1.Pope C, III, Dockery D. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 2.Jung KH, Hsu SI, Yan B, Moors K, Chillrud SN, Ross J, Wang S, Perzanowski MS, Kinney PL, Whyatt RM. Childhood exposure to fine particulate matter and black carbon and the development of new wheeze between ages 5 and 7 in an urban prospective cohort. Environ Int. 2012;45:44–50. doi: 10.1016/j.envint.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gehring U, Gruzieva O, Agius RM, Beelen R, Custovic A, Cyrys J, Eeftens M, Flexeder C, Fuertes E, Heinrich J. Air pollution exposure and lung function in children: the ESCAPE project. Environmental Health Perspectives (Online) 2013;121:1357. doi: 10.1289/ehp.1306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, Washko GR, O’Connor GT, Mittleman MA. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am J Respir Crit Care Med. 2013;188:1351–1357. doi: 10.1164/rccm.201308-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Götschi T, Heinrich J, Sunyer J, Künzli N. Long-term effects of ambient air pollution on lung function: a review. Epidemiology. 2008;19:690–701. doi: 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- 6.Peltier RE, Lippmann M. Residual oil combustion: 2. Distributions of airborne nickel and vanadium within New York City. J Expo Sci Environ Epidemiol. 2010;20:342–350. doi: 10.1038/jes.2009.28. [DOI] [PubMed] [Google Scholar]
- 7.Lough GC, Schauer JJ, Park J-S, Shafer MM, DeMinter JT, Weinstein JP. Emissions of metals associated with motor vehicle roadways. Environ Sci Technol. 2005;39:826–836. doi: 10.1021/es048715f. [DOI] [PubMed] [Google Scholar]
- 8.New York City Community Air Survey: Spatial Variation of Fine Particle Vandium and Ship Emissions in New York City. https://www1.nyc.gov/assets/doh/downloads/pdf/eode/nyccas-ship-report.pdf. Accessed 30 June 2016.
- 9.Maciejczyk P, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice: VIII. Source-related daily variations in in vitro responses to CAPs. Inhal Toxicol. 2005;17:243–253. doi: 10.1080/08958370590912914. [DOI] [PubMed] [Google Scholar]
- 10.Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am J Respir Crit Care Med. 2009;179:1115. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominici F, Peng RD, Ebisu K, Zeger SL, Samet JM, Bell ML. Does the effect of PM10 on mortality depend on PM nickel and vanadium content? A reanalysis of the NMMAPS data. Environ Health Perspect. 2007;1701–1703. [DOI] [PMC free article] [PubMed]
- 12.Patel M, Hoepner L, Garfinkel R, Chillrud S, Reyes A, Quinn J, Perera F, Miller R. Ambient Metals, Elemental Carbon, and Wheeze and Cough in New York City Children through Age 24 Months. Am J Respir Crit Care Med. 2009;180:1107–1113. doi: 10.1164/rccm.200901-0122OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habre R, Fruin S, Gauderman J, Lurmann F, Gilliland F, McConnell R, Urman R. Exposure To Transition Metals In Particulate Matter Air Pollution And Children’s Lung Function In The Southern California Children’s Health Study. In B15 HEALTH EFFECTS OF INDOOR/OUTDOOR POLLUTION IN CHILDHOOD. Am Thoracic Soc; 2016: A2873-A2873
- 14.Alexeeff SE, Litonjua AA, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure and lung function: effect modified by obesity and airways hyperresponsiveness in the VA normative aging study. CHEST Journal. 2007;132:1890–1897. doi: 10.1378/chest.07-1126. [DOI] [PubMed] [Google Scholar]
- 15.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;992–998. [DOI] [PMC free article] [PubMed]
- 16.Lu KD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, Williams D, Peng RD, McCormack MC, Matsui EC. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol. 2013;131:1017–1023. doi: 10.1016/j.jaci.2012.12.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung KH, Perzanowski M, Rundle A, Moors K, Yan B, Chillrud SN, Whyatt R, Camann D, Perera FP, Miller RL. Polycyclic aromatic hydrocarbon exposure, obesity and childhood asthma in an urban cohort. Environ Res. 2014;128:35–41. doi: 10.1016/j.envres.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller RL, Peden DB. Environmental impacts on immune responses in atopy and asthma. J Allergy Clin Immunol. 2014;134:1001–1008. doi: 10.1016/j.jaci.2014.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossnerova A, Tulupova E, Tabashidze N, Schmuczerova J, Dostal M, Rossner P, Jr, Gmuender H, Sram RJ. Factors affecting the 27 K DNA methylation pattern in asthmatic and healthy children from locations with various environments. Mutat Res Fundam Mol Mech Mutagen. 2013;741:18–26. doi: 10.1016/j.mrfmmm.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Salam MT, Byun H-M, Lurmann F, Breton CV, Wang X, Eckel SP, Gilliland FD. Genetic and epigenetic variations in inducible nitric oxide synthase promoter, particulate pollution, and exhaled nitric oxide levels in children. J Allergy Clin Immunol. 2012;129:232–239. doi: 10.1016/j.jaci.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, Tager I. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–852. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Magari SR, Schwartz J, Williams PL, Hauser R, Smith TJ, Christiani DC. The association of particulate air metal concentrations with heart rate variability. Environ Health Perspect. 2002;110:875. doi: 10.1289/ehp.02110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan T, Fang SC, Cavallari JM, Barnett IJ, Wang Z, Su L, Byun H-M, Lin X, Baccarelli AA, Christiani DC. Heart rate variability and DNA methylation levels are altered after short-term metal fume exposure among occupational welders: a repeated-measures panel study. BMC Public Health. 2014;14:1. doi: 10.1186/1471-2458-14-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Ballaney M, Al-alem U, Quan C, Jin X, Perera F, Chen L-C, Miller RL. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci. 2008;102:76–81. doi: 10.1093/toxsci/kfm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breton CV, Byun H-M, Wang X, Salam MT, Siegmund K, Gilliland FD. DNA methylation in the arginase–nitric oxide synthase pathway is associated with exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med. 2011;184:191–197. doi: 10.1164/rccm.201012-2029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang IV, Pedersen BS, Liu A, O’connor GT, Teach SJ, Kattan M, Misiak RT, Gruchalla R, Steinbach SF, Szefler SJ. DNA methylation and childhood asthma in the inner city. J Allergy Clin Immunol. 2015;136:69–80. doi: 10.1016/j.jaci.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarantini L, Bonzini M, Apostoli P, Pegoraro V, Bollati V, Marinelli B, Cantone L, Rizzo G, Hou L, Schwartz J. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ Health Perspect. 2009;117:217. doi: 10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhutani M, Pathak AK, Fan Y-H, Liu DD, Lee JJ, Tang H, Kurie JM, Morice RC, Kim ES, Hong WK. Oral epithelium as a surrogate tissue for assessing smoking-induced molecular alterations in the lungs. Cancer Prev Res. 2008;1:39–44. doi: 10.1158/1940-6207.CAPR-08-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richmond R, Simpkin A, Woodward G, Gaunt T, Lyttleton O, McArdle W, Ring S, Smith A, Timpson N, Tilling K, et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC) Hum Mol Genet. 2015;24:8. doi: 10.1093/hmg/ddu739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy S, Adigun A, Huang Z, Overcash F, Wang F, Jirtle R, Schildkraut J, Murtha A, Iversen E, Hoyo C. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene. 2012;494:36–43. doi: 10.1016/j.gene.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michel S, Busato F, Genuneit J, Pekkanen J, Dalphin J, Riedler J, Mazaleyrat N, Weber J, Karvonen A, Hirvonen M, et al. Farm exposure and time trends in early childhood may influence DNA methylation in genes related to asthma and allergy. Allergy. 2013;68:355–364. doi: 10.1111/all.12097. [DOI] [PubMed] [Google Scholar]
- 32.Clifford RL, Jones MJ, MacIsaac JL, McEwen LM, Goodman SJ, Mostafavi S, Kobor MS, Carlsten C. Inhalation of diesel exhaust and allergen alters human bronchial epithelium DNA methylation. J Allergy Clin Immunol. 2017;139:112–121. doi: 10.1016/j.jaci.2016.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Perera FP, Illman SM, Kinney PL, Whyatt RM, Kelvin EA, Shepard P, Evans D, Fullilove M, Ford J, Miller RL, et al. The challenge of preventing environmentally related disease in young children: community-based research in New York City. Environ Health Perspect. 2002;110:197–204. doi: 10.1289/ehp.02110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA, Arunajadai S, Canfield S, Resnick D, Calafat AM, Perera FP. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol. 2013;131:736–742. doi: 10.1016/j.jaci.2012.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CfDCa P. A SAS Program for the CDC Growth Charts. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 36.Jelena J, Baltic Z, Milica G, Jelena I, Marija B, Milka P, Mirjana L. Relationship between Body Mass Index and Body Fat Percentage among Adolescents from Serbian Republic. Journal of Childhood Obesity. 2016.
- 37.Spolidoro JV, Pitrez Filho ML, Vargas LT, Santana JC, Pitrez E, Hauschild JA, Bruscato NM, Moriguchi EH, Medeiros AK, Piva JP. Waist circumference in children and adolescents correlate with metabolic syndrome and fat deposits in young adults. Clin Nutr. 2013;32:93–97. doi: 10.1016/j.clnu.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Blanchard CL, Tanenbaum S, Lawson DR. Differences between weekday and weekend air pollutant levels in Atlanta; Baltimore; Chicago; Dallas–Fort Worth; Denver; Houston; New York; Phoenix; Washington, DC; and surrounding areas. J Air Waste Manage Assoc. 2008;58:1598–1615. doi: 10.3155/1047-3289.58.12.1598. [DOI] [PubMed] [Google Scholar]
- 39.Kinney PL, Chillrud SN, Ramstrom S, Ross J, Spengler JD. Exposures to multiple air toxics in New York City. Environ Health Perspect. 2002;110(Suppl 4):539–546. doi: 10.1289/ehp.02110s4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suresh V, Mih JD, George SC. Measurement of IL-13–Induced iNOS-Derived Gas Phase Nitric Oxide in Human Bronchial Epithelial Cells. Am J Respir Cell Mol Biol. 2007;37:97–104. doi: 10.1165/rcmb.2006-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cornell AG, Chillrud SN, Mellins RB, Acosta LM, Miller RL, Quinn JW, Yan B, Divjan A, Olmedo OE, Lopez-Pintado S. Domestic airborne black carbon and exhaled nitric oxide in children in NYC. J Expo Sci Environ Epidemiol. 2012;22:258–266. doi: 10.1038/jes.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stafoggia M, Schwartz J, Forastiere F, Perucci C. Does temperature modify the association between air pollution and mortality? A multicity case-crossover analysis in Italy. Am J Epidemiol. 2008;167:1476–1485. doi: 10.1093/aje/kwn074. [DOI] [PubMed] [Google Scholar]
- 43.Nawrot T, Torfs R, Fierens F, De Henauw S, Hoet P, Van Kersschaever G, De Backer G, Nemery B. Stronger associations between daily mortality and fine particulate air pollution in summer than in winter: evidence from a heavily polluted region in western Europe. J Epidemiol Community Health. 2007;61:146–149. doi: 10.1136/jech.2005.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidal AC, Semenova V, Darrah T, Vengosh A, Huang Z, King K, Nye MD, Fry R, Skaar D, Maguire R. Maternal cadmium, iron and zinc levels. DNA methylation and birth weight BMC Pharmacology and Toxicology. 2015;16:1. doi: 10.1186/s40360-015-0020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 46.Rastogi D, Suzuki M, Greally JM. Differential epigenome-wide DNA methylation patterns in childhood obesity-associated asthma. Sci Rep. 2013;3. [DOI] [PMC free article] [PubMed]
- 47.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;2012(40):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strachan P, Medarov BI. Seasonal variation in lung function. CHEST Journal. 2005;128:173S-a-173S. [Google Scholar]
- 49.Lovinsky-Desir S, Ridder R, Torrone D, Maher C, Narula S, Scheuerman M, Merle D, Kattan M, DiMango E, Miller RL. DNA methylation of the allergy regulatory gene interferon gamma varies by age, sex, and tissue type in asthmatics. Clin Epigenetics. 2014;6:1. doi: 10.1186/1868-7083-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torrone DZ, Kuriakose JS, Moors K, Jiang H, Niedzwiecki M, Perera F, Miller RL. Reproducibility and intraindividual variation over days in buccal cell DNA methylation of two asthma genes, interferon γ (IFNγ) and inducible nitric oxide synthase (iNOS) Clin Epigenetics. 2012;4:1. doi: 10.1186/1868-7083-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen R, Qiao L, Li H, Zhao Y, Zhang Y, Xu W, Wang C, Wang H, Zhao Z, Xu X. Fine particulate matter constituents, nitric oxide synthase DNA methylation and exhaled nitric oxide. Environ Sci Technol. 2015;49:11859–11865. doi: 10.1021/acs.est.5b02527. [DOI] [PubMed] [Google Scholar]
- 52.Kile ML, Fang S, Baccarelli AA, Tarantini L, Cavallari J, Christiani DC. A panel study of occupational exposure to fine particulate matter and changes in DNA methylation over a single workday and years worked in boilermaker welders. Environ Health. 2013;12:1. doi: 10.1186/1476-069X-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collison A, Siegle JS, Hansbro NG, Kwok C-T, Herbert C, Mattes J, Hitchins M, Foster PS, Kumar RK. Epigenetic changes associated with disease progression in a mouse model of childhood allergic asthma. Dis Model Mech. 2013;6:993–1000. doi: 10.1242/dmm.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhakta NR, Woodruff PG. Human asthma phenotypes: from the clinic, to cytokines, and back again. Immunol Rev. 2011;242:220–232. doi: 10.1111/j.1600-065X.2011.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Rohde C, Tierling S, Jurkowski TP, Bock C, Santacruz D, Ragozin S, Reinhardt R, Groth M, Walter J. DNA methylation analysis of chromosome 21 gene promoters at single base pair and single allele resolution. PLoS Genet. 2009;5:e1000438. doi: 10.1371/journal.pgen.1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wills-Karp M, Ewart SL. The genetics of allergen-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 1997;156:S89–S96. doi: 10.1164/ajrccm.156.4.12-tac-3. [DOI] [PubMed] [Google Scholar]
- 57.Cui J, Pazdziorko S, Miyashiro JS, Thakker P, Pelker JW, DeClercq C, Jiao A, Gunn J, Mason L, Leonard JP. T H 1-mediated airway hyperresponsiveness independent of neutrophilic inflammation. J Allergy Clin Immunol. 2005;115:309–315. doi: 10.1016/j.jaci.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 58.Losana G, Bovolenta C, Rigamonti L, Borghi I, Altare F, Jouanguy E, Forni G, Casanova J-L, Sherry B, Mengozzi M. IFN-γ and IL-12 differentially regulate CC-chemokine secretion and CCR5 expression in human T lymphocytes. J Leukoc Biol. 2002;72:735–742. [PubMed] [Google Scholar]
- 59.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 60.Eljaafari A, Robert M, Chehimi M, Chanon S, Durand C, Vial G, Bendridi N, Madec A-M, Disse E, Laville M. Adipose tissue–derived stem cells from obese subjects contribute to inflammation and reduced insulin response in adipocytes through differential regulation of the Th1/Th17 balance and monocyte activation. Diabetes. 2015;64:2477–2488. doi: 10.2337/db15-0162. [DOI] [PubMed] [Google Scholar]
- 61.Eeftens M, Hoek G, Gruzieva O, Mölter A, Agius R, Beelen R, Brunekreef B, Custovic A, Cyrys J, Fuertes E. Elemental composition of particulate matter and the association with lung function. Epidemiology. 2014;25:648–657. doi: 10.1097/EDE.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 62.Sormani G, Haerter JO, Lövkvist C, Sneppen K. Stabilization of epigenetic states of CpG islands by local cooperation. Mol Biosyst. 2016. [DOI] [PubMed]
- 63.Spivack SD, Hurteau GJ, Jain R, Kumar SV, Aldous KM, Gierthy JF, Kaminsky LS. Gene-environment interaction signatures by quantitative mRNA profiling in exfoliated buccal mucosal cells. Cancer Res. 2004;64:6805–6813. doi: 10.1158/0008-5472.CAN-04-1771. [DOI] [PubMed] [Google Scholar]
- 64.Kumar SV, Jain R, Mokhiber K, Venezia A, Sheehan A, Spivack SD. Exfoliated buccal and microdissected lung cell expression of antioxidant enzymes. Cancer Detect Prev. 2005;29:552–561. doi: 10.1016/j.cdp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Brody JG, Morello-Frosch R, Zota A, Brown P, Pérez C, Rudel RA. Linking exposure assessment science with policy objectives for environmental justice and breast cancer advocacy: The Northern California Household Exposure Study. Am J Public Health. 2009;99:S600–S609. doi: 10.2105/AJPH.2008.149088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung KH, Patel MM, Moors K, Kinney PL, Chillrud SN, Whyatt R, Hoepner L, Garfinkel R, Yan B. Effects of heating season on residential indoor and outdoor polycyclic aromatic hydrocarbons, black carbon, and particulate matter in an urban birth cohort. Atmos Environ. 2010;44:4545–4552. doi: 10.1016/j.atmosenv.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


