Abstract
Background
Naturally-acquired immunity to Plasmodium falciparum malaria develops after several episodes of infection. Fc gamma receptors (FcγRs) bind to immunoglobulin G (IgG) antibodies and mediate phagocytosis of opsonized microbes, thereby, linking humoral and cellular immunity. FcγR polymorphisms influence binding affinity to IgGs and consequently, can influence clinical malaria outcomes. Specifically, variations in FcγRIIA -131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 modulate immune responses through altered binding preferences to IgGs and immune complexes. Differential binding, in turn, changes ability of immune cells to respond to infection through production of inflammatory mediators during P. falciparum infection.
Methods
We determined the association between haplotypes of FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 variants and severe malarial anemia (SMA; hemoglobin < 6.0 g/dL, any density parasitemia) in children (n = 274; aged 6–36 months) presenting for their first hospital visit with P. falciparum malaria in a holoendemic transmission region of western Kenya. FcγRIIA-131Arg/His and FcγRIIIA-176F/V genotypes were determined using TaqMan® SNP genotyping, while FcγRIIIBNA1/NA2 genotypes were determined using restriction fragment length polymorphism. Hematological and parasitological indices were measured in all study participants.
Results
Carriage of FcγRIIA-131Arg/FcγRIIIA-176F/FcγRIIIBNA2 haplotype was associated with susceptibility to SMA (OR = 1.70; 95% CI; 1.02–2.93; P = 0.036), while the FcγRIIA-131His/ FcγRIIIA-176F/ FcγRIIIB NA1 haplotype was marginally associated with enhanced susceptibility to SMA (OR: 1.80, 95% CI; 0.98–3.30, P = 0.057) and higher levels of parasitemia (P = 0.009). Individual genotypes of FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 were not associated with susceptibility to SMA.
Conclusion
The study revealed that haplotypes of FcγRs are important in conditioning susceptibility to SMA in immune-naive children from P. falciparum holoendemic region of western Kenya.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-017-2390-0) contains supplementary material, which is available to authorized users.
Keywords: FcγRs, Susceptibility, Polymorphisms, Malaria anemia
Background
In Plasmodium falciparum malaria holoendemic transmission regions, such as western Kenya, malaria manifests with a milieu of life-threatening conditions including severe malarial anemia (SMA), metabolic acidosis, high-density parasitemia (≥10,000 parasites/μL), respiratory distress, hypoglycaemia and other infrequent complications such as hypotension [1]. Even though not fully understood, severe clinical malaria is a multi-factorial process involving sequestration of infected red blood cells (iRBCs) in particular organs such as spleen [2], bone marrow suppression leading to dyserythropoiesis [3], and limited, malaria-specific antibody immunity and dysregulation in inflammatory responses [4]. Due to the gradual development of immunity against P. falciparum malaria in holoendemic areas, infants and young children suffer the greatest disease burden. The most common clinical manifestation of severe P. falciparum malaria infection in pediatric populations of western Kenya is SMA (hemoglobin, Hb < 6.0 g/dL, any density parasitemia) [5].
The binding of immunoglobulin domains to Fc receptors on target cells is important to initiate immunological defense against pathogens including antigen presentation, phagocytosis, cytotoxicity, induction of inflammatory processes and modulation of immune responses [6]. Therefore, Fc gamma receptors (FcγRs) are important in providing a significant link between the humoral and cellular immunity by bridging the interaction between specific antibodies and effector cells [7]. Previous studies demonstrate that polymorphic variability in these receptors is an important determinant of susceptibility to infections [8, 9].
Previous investigations have also shown that the efficacy of the cellular immune response is influenced by FcγR polymorphisms, and consequently, influence clinical outcomes for infectious diseases’ such as malaria [9, 10]. The human FcγRIIA mediates phagocytic function of monocytes, macrophages and neutrophils. The presence of FcγRIIA-131Arg/131His polymorphism affects the binding to the IgG1 and IgG3 [11]. As reviewed Grant and colleagues [12], FcγRIIA-131His/His homozygotes is associated with higher IgG2 levels and protection against high parasitemia and has been considered as protective against blood stage P. falciparum infection both in African and Asian populations [13].
FcγRIIIA is an activating receptor with two co-dominantly expressed alleles, the 176 V and the 176F that differ in an amino acid at position 176 in the extracellular domain (valine or phenylalanine, respectively) [14]. Dimorphisms in the amino acid at position 176F/V influences the binding of the immunoglobin G (IgG) subtype, with the 176 V variant having higher binding affinity for monomeric forms of IgG1 and IgG3, as compared to the 176F [15] which is potentially important in infectious disease immunity.
On the surface of polymorphonuclear leucocytes, the most abundantly expressed FcγRs is the FcγRIIIB. These receptors exhibits two allotypic forms i.e. neutrophil antigens (NAs) 1 and 2 which differ in minor amino acids at position 65 and 82 in two extra-glycosylation site in NA2 [16, 17] with different binding affinities. The NA2/NA2 allotype is associated with low immunoglobulin-mediated phagocytosis [18, 19]. The phagocytosis of IgG1-and IgG3-opsonized immune complexes is more efficient on neutrophils bearing FcγRIIIB-NA1 relative to FcγRIIIB-NA2 [18].
A number of genetic association studies have provided evidence that polymorphic variation in FcγRs have a strong effect on susceptibility to inflammatory mediated diseases [20–24]. Even though FcγRs are important in the immune response to infection, the effect of its haplotypes on susceptibility to SMA in immune-naїve children remain largely undetermined. In the present study, we determined the association between FcγRIIA, IIIA and IIIB haplotypes and SMA, and the influence of these haplotypes on peripheral parasite burden during acute falciparum infections in an extensively phenotyped cohort of children from a P. falciparum holoendemic transmission area western in Kenya.
Methods
Study site
The study was conducted at Siaya County Referral Hospital (SCRH), western Kenya, a P. falciparum holoendemic transmission region [25]. Over 98% of the inhabitants are from the Luo ethnic tribe, hence providing a homogenous population for immuno-genetic studies. Falciparum malaria prevalence is ~83% in children aged <4 years, with severe disease manifesting as SMA (Hb < 6.0 g/dL) with or without high-density parasitemia (HDP; ≥10,000 parasites/μL of blood) [5].
Study participants
Children [n = 274, aged 6–36 months] of both sexes were recruited at SCRH during their initial hospitalization for treatment of malaria. Recruitment followed a two-phase tier of screening and enrolment. The parent/legal guardian of the child received detailed explanation of the study. Enrollment decisions were made after initial HIV-1 screening of the child and a signed informed consent, which included authority to publish the findings. Questionnaires and written informed consent were administered in the language of choice (i.e. English, Kiswahili or Dholuo). Children with acute malaria were stratified into two categories: non-severe malarial anemia (non-SMA) group defined as a positive smear for asexual P. falciparum parasitemia (of any density) and Hb ≥ 6.0 g/dL; and SMA group defined by a positive smear for asexual P. falciparum parasitemia (of any density) and Hb < 6.0 g/dL [25]. Venous blood samples (<3.0 mL) were collected into EDTA-containing vacutainer tubes at the time of enrollment, prior to any treatment interventions or supportive care. Blood samples were used for malaria diagnosis, complete hematological profile measurements, HIV testing, bacterial culture and genetic analyses. Children were excluded from the study for any one of the following reasons; children with CM (a rare occurrence in this holoendemic area); clinical evidence of acute respiratory infection; and prior hospitalization. Participants were treated according to the Ministry of Health (MOH)-Kenya guidelines. This included the administration of oral artemether/lumefantrine (Coartem®) for uncomplicated malaria and intravenous quinine (and when indicted, blood transfusion) for severe malaria.
Laboratory procedures
Hemoglobin levels and complete blood counts were determined using the Beckman Coulter ACT diff2™ (Beckman-Counter Corporation, Miami, FL, USA). To determine parasitemia, 10% Giemsa-stained thick and thin blood smears were prepared and examined under a microscope on high power magnification. P. falciparum parasites per 300 white blood cells (WBCs) were determined, and parasitemia (per μL) was estimated using the total WBC count. In order to delineate severe anemia caused by malaria versus other anemia-promoting conditions, human immunodeficiency virus (HIV)-1, bacteremia and sickle-cell trait (HbAS) were determined in all study participants. The effect of these parameters on disease severity was controlled for during in all regression models. Pre- and post-test HIV counseling was provided for all participants. HIV-1 exposure and infection were determined serologically (i.e., Unigold™ and Determine™) and discordant results confirmed through HIV-1 proviral DNA PCR testing, according to previously published methods [26]. Bacteremia was determined using the Wampole Isostat Pediatric 1.5 system (Wamploe Laboratories, Town, Country). The presence of the sickle cell trait (HbAS) was determined by cellulose acetate electrophoresis (Helena Bio-Sciences, Oxford, United Kingdom) while G6PD deficiency was determined by fluorescent spot test using the manufacturer’s methods (Trinity Biotech Plc., Bray, Ireland).
Genotyping of FcγRs polymorphisms
Blood spots were made on FTA Classic® cards (Whatman Inc., Clifton, NJ, USA), air-dried, and stored at room temperature until used for DNA extraction. DNA was extracted using the Gentra System (Gentra System Inc., Minneapolis, MN, USA) based on the manufacturer’s instructions. The FcγRIIA-131Arg/His (rs1801274, assay ID: C__9077561_20) and FcγRIIIA -176F/V (rs396991, assay ID: C__25815666_10) polymorphisms were genotyped using the high-throughput TaqMan® 5′ allelic discrimination Assay-By-Design method, according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA), while the FcγRIIIB-NA1/NA2 genotyping for the rs448740 (N65S) and rs147574249 (N82D) was performed according to a previously described RFLP method [27].
Data analyses
SPSS® statistical software package version 20.0 (IBM SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Chi-square analysis was used to examine differences between proportions. Mann-Whitney U test was used for comparisons of demographic and clinical characteristics between the two clinical groups. The association between FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 genotypes, haplotypes and SMA was determined using bivariate logistic regression analysis controlling for confounding effects of age, gender, co-infections (bacteremia and HIV-1), G6PD deficiency, and sickle cell trait (HbAS). Student’s t-test was used to determine differences in the levels of parasitemia between the carriage and non-carriage of the haplotypes. Levels of parasitemia were log-transformed to normal distribution. FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 allele frequencies, consistency and/or deviations from Hardy-Weinberg Equilibrium (HWE) were determined using web-based site emerald.tufts.eduAQ3/~court01/Documents/Court%20lab%20-%20HW. Statistical significance was set at P ≤ 0.05.
Results
Demographic clinical and laboratory characteristics of study participants
We conducted a cross-sectional analysis of children (n = 274, aged 6–36 months) presenting with acute P. falciparum malaria (any density parasitemia) (See Additional file 1). Clinically, the study participants were classified into two categories based on a previous study in an age- and geographically-defined reference population from western Kenya [25], i.e., severe malaria anemia (SMA; Hb < 6.0 g/dL; n = 114) and non-SMA (Hb ≥ 6.0 g/dL, n = 160). There were more males in the non-SMA category compared to the SMA group (P = 0.039). Children with SMA were younger (age in months) [median (IQR); 8.0 (7.00)] than those in the non-SMA group [median (IQR); 13.5 (8.80)], P < 0.001. Parasitemia values (log10 of parasites/μL) was comparable between the study groups, SMA [mean (SEM); 4.09 (±0.07)] and non-SMA [mean (SEM); 4.24 (±0.06)], P = 0.088). The proportion of participants with high-density parasitemia (HDP) was also comparable between the clinical groups (62.3% in SMA and 71.9% in non-SMA, P = 0.094). Similarly, there was no difference in body temperature (°C) between the study groups, SMA [median (IQR); 38.0 (1.20)] and non-SMA [median, (IQR), [38.0; (1.40)], respectively, P = 0.430. Further analysis revealed that children with SMA had higher respiration rate (breaths/min), [median, (IQR); 32.0, (12.00)] than non-SMA, [median, (IQR); 26.0, (14.00)], P < 0.001. Analysis of hematological parameters revealed that red blood cells counts (RCBs × 1012/μL) were higher in children with non-SMA [median, (IQR); 3.72, (1.16)] than those with SMA, [median, (IQR); 2.20, (0.86)], P < 0.001. The SMA group were also characterized by elevated levels of white blood cells counts (WBC × 103/uL) [median (IQR); 13.50 (8.80)] relative to the non-SMA group [median (IQR); 10.95 (5.90)], P < 0.001. The platelet counts (platelets ×103/μL) were lower in children with SMA, [median, (IQR), 150.00 (93.00)] as compared to the non-SMA, [median, (IQR), 170.00 (13.10)], P = 0.025. The distribution of G6PD in SMA and non-SMA were comparable (7.00% in SMA and 7.50% in non-SMA, P = 0.880). Similarly, the distribution of those with sickle cell trait in SMA and non-SMA were comparable (SMA 19.30% while non-SMA 28.70% respectively, P = 0.074). These results are presented on Table 1.
Table 1.
Demographic clinical and laboratory characteristics of study participants
| Characteristics | Clinical groups | ||
|---|---|---|---|
| SMA | non-SMA | P-value | |
| (Hb < 6.0 g/dL) | (Hb ≥ 6.0 g/dL) | ||
| n = 114 | n = 160 | ||
| Sex, n (%) | |||
| Male | 49 (43.00) | 89 (55.40) | 0.039 a |
| Female | 65 (57.00) | 71 (44.60) | |
| Age, (months) | 8.0 (7.00) | 13.5 (8.80) | <0.001 b |
| Log10 of parasitemia | 4.09 (±0.07) | 4.24 (±0.06) | 0.088c |
| HDP (≥10, 000 parasites/μL) | 71/114 (62.3) | 115/160 (71.9) | 0.094a |
| Temperature, (°C) | 38.0 (1.20) | 38.0 (1.40) | 0.430b |
| Respiration rate, (breaths/min) | 32.0 (12.00) | 26.0 (14.00) | <0.001 b |
| Haematological indices | |||
| Hemoglobin, g/dL | 5.00 (1.00) | 7.95 (3.00) | <0.001 b |
| Hematocrit, % | 15.90 (4.30) | 25.00 (7.40) | <0.001 b |
| RBC, (× 1012/μL) | 2.20 (0.86) | 3.72 (1.16) | <0.001 b |
| RDW, (%) | 23.00 (5.20) | 20.45 (4.40) | <0.001 b |
| WBC (×103/uL) | 13.50 (8.80) | 10.95 (5.90) | <0.001 b |
| Platelet Counts (×103/μL) | 150.00 (93.00) | 170.00 (13.10) | 0.025 b |
| Genetic characteristics | |||
| G6PD n (%) | 8 (7.00) | 12 (7.50) | 0.880 |
| Sickle cell trait, n (%) | 22 (19.30) | 46 (28.70) | 0.074 |
Data are presented as the median (interquartile range) and n (%) of children unless stated otherwise. Parasitemic children (n = 274 were categorized as SMA (n = 114) and non-SMA (n = 160) according to modified definition of SMA (Hb < 6.0 g/dL, with any density parasitemia). aStatistical significance was determined by the Chi-square (χ2) analysis. bStatistical significance was determined using Mann-Whitney U test. cStatistical significance was determined using Student’s t-test. Abbreviations: G6PD Glucose-6-Phaspahte dehydrogenase, HDP high density parasitemia, RBC-Red blood cells, RDW - Red cell distribution width; WBC-White blood cells. Probability values were considered statistically significant at P ≤ 0.05
Values in bold are significant p-values at a cut-off of p≤0.05
Distribution of FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 genotypes in the clinical groups
Chi square (χ2) analysis showed that the distributions of the FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 genotypes were not significantly different between the clinical groups (P = 0.226, P = 0.162 and P = 0.632, respectively) (Table 2). FcγRIIA-131Arg/His genotypes within the SMA group were 30 (26.3%) Arg/Arg, 59 (51.8%) Arg/His and 25 (21.9%) His/His. Consistency with Hardy-Weinberg Equilibrium (HWE) in the SMA group for FcγRIIA-131Arg/His was observed (χ2 = 0.15, P = 0.692). FcγRIIA-131Arg/His genotypes distribution in non-SMA were 39 (24.3%) Arg/Arg, 71 (44.4.0%) Arg/Hist and 50 (31.1%) His/His. Frequencies of the genotypes in non-SMA showed deviation from HWE (χ2 = 4.92, P = 0.027). The overall genotype distribution for the FcγRIIA-131Arg/His did not deviate from HWE (χ2 = 0.703, P = 0.402) with an overall variant allele frequency of the FcγRIIA-131Arg/His at 0.49 (Arg). The genotypic distribution of the FcγRIIIA-176 F/V in SMA group was 61 (53.5%) FF, 45 (39.5%) FV and 8 (7.0%) VV. The distribution of these genotypes in SMA showed consistency with HWE (χ2 = 0.006, P = 0.939). Within the non-SMA group, the distributions was 77 (48.1%) FF, 60 (37.5%) FV and 23 (14.4%) for VV and the genotypes showed consistency with HWE (χ2 = 3.774, P = 0.052). The distribution of these genotypes in overall population showed consistency with HWE (χ2 = 2.510, P = 0.113) and had an overall mutant allele frequency of 0.30 (V). FcγRIIIB-NA1/NA2 genotypes distribution in the SMA group were 6 (5.3%) NA1, 73 (64.0%) NA1/NA2 and 35 (30.7%) NA2, while in non-SMA there was 8 (5.0%) NA1, 94 (58.8%) NA1/NA2 and 58 (36.2%) NA2. The distributions of the genotypes in both SMA and non-SMA revealed deviation from HWE normality (χ2 = 15.549, P < 0.001, and χ2 = 14.608, P < 0.001, respectively). In addition, HWE deviation was revealed by the FcγRIIIB-NA1/NA2 genotypes’ distribution considering the whole study group (χ2 = 29.47, P < 0.001) with variant allele frequency of 0.36 (NA1), Table 2.
Table 2.
Distribution of FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 genotypes within the study groups
| N (%) with genotype in groupa | HWE, P-value (SMA + non-SMA) c |
|||
|---|---|---|---|---|
| Genotypes | SMA (Hb < 6.0 g/dL) (n = 114) |
Non-SMA (Hb ≥ 6.0 g/dL) (n = 160) |
P-value b | |
| FcγRIIA-131Arg/His | ||||
| Arg/Arg, n (%) | 30 (26.3) | 39 (24.3) | ||
| Arg/His n (%) | 59 (51.8) | 71 (44.4) | 0.226b | 0.402b |
| His/His, n (%) | 25 (21.9) | 50 (31.3) | ||
| X(His) = 0.48 | ||||
| FcγRIIIA-176 F/V | ||||
| FF, n (%) | 61 (53.5) | 77 (48.1) | ||
| FV, n (%) | 45 (39.5) | 60 (37.5) | 0.162b | 0.113b |
| VV, n (%) | 8 (7.0) | 23 (14.4) | ||
| X(V) = 0.30 | ||||
| FcγRIIIB-NA1/NA2 | ||||
| NA1/NA1 | 6 (5.3) | 8 (5.0) | ||
| NA1/NA2 | 73 (64.0) | 94 (58.8) | 0.632b | <0.001 b |
| NA2/NA2 | 35 (30.7) | 58 (36.2) | ||
| X(NA1) = 0.36 | ||||
aData are presented as n (%) of children. Children with parasitemia were categorized on the basis of presence or absence of severe malarial anemia SMA based (defined as Hb < 6.0 g/dL, with any density parasitemia). bStatistical significance determined by χ2 analysis. X; the overall minor allele frequency in the study population. c HWE Hardy-Weinberg Equilibrium
Values in bold are significant p-values at a cut-off of p≤0.05
Association between FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 and severe malarial anemia (SMA, Hb < 6.0 g/dL)
We conducted genetic association analysis based on dominant, additive and recessive models of the FcγR polymorphisms. The FcγRIIA-131His/His dominant model did not reveal association with SMA susceptibility (OR = 0.59, 95% CI, 0.33–1.05, P = 0.077). Further analysis did not reveal association between SMA using the additive (OR = 1.52, 95% CI, 0.72–2.93, P = 0.298) or the recessive model (OR = 0.98, 95% CI, 0.56–1.75, P = 0.963). The dominant (OR = 1.27, 95% CI, 0.79–2.10, P = 0.343) and the additive (OR = 0.77, 95% CI, 0.63–1.83, P = 0.796) model of the FcγRIIIA-176 F/V dimorphism did not show associations with SMA. However, the recessive model of FcγRIIIA-176 F/V showed a trend towards protection against SMA, albeit with marginal significance (OR, 0.43, 95% CI, 0.18–1.02, P = 0.056). Analysis of all the genetic models of FcγRIIIB-NA1/NA2 variation did not reveal any association with SMA; dominant [OR = 0.76, 95% CI, 0.44–1.28, P = 0.786)], additive [OR = 1.34, 95% CI, 0.78–2.30, P = 0.288] and recessive [OR = 1.20, 95% CI 0.36–3.94, P = 0.767), Table 3.
Table 3.
Association between FcγRIIA-131Arg/His, FcγRIIIA-176F/V, FcγRIIIB-NA1/NA2 and severe malarial anemia (SMA, Hb < 6.0 g/dL)
| FcγR genotype models | SMA (Hb < 6.0 g/dL) | ||||
|---|---|---|---|---|---|
| SMA | Non-SMA | OR | 95% CI | P-value | |
| FcγRIIA-131Arg/His | |||||
| Dominant, (His/His, n = 75) | 25 | 50 | 0.59 | 0.33–1.05 | 0.077 |
| Additive, (Arg/His, n = 130) | 59 | 71 | 1.52 | 0.72–2.93 | 0.298 |
| Recessive, (Arg/Arg, n = 69) | 30 | 39 | 0.98 | 0.56–1.75 | 0.963 |
| FcγRIIIA-176 F/V | |||||
| Dominant, (F/F, n = 138) | 61 | 77 | 1.27 | 0.79–2.10 | 0.343 |
| Additive, (F/V, n = 105) | 45 | 60 | 0.77 | 0.63–1.83 | 0.796 |
| Recessive, (V/V, n = 31) | 8 | 23 | 0.43 | 0.18–1.02 | 0.056 |
| FcγRIIIB-NA1/NA2 | |||||
| Dominant, (NA2/NA2, n = 93) | 35 | 58 | 0.76 | 0.44–1.28 | 0.786 |
| Additive, (NA1/NA2, n = 167) | 73 | 94 | 1.34 | 0.78–2.30 | 0.288 |
| Recessive, (NA1/NA1, n = 14) | 6 | 8 | 1.20 | 0.36–3.94 | 0.767 |
Children with acute malaria (n = 274) were grouped based on SMA (defined as Hb < 6.0 g/dL, with any density parasitemia) [25]. Odds ratios (OR) and 95% confidence intervals (CI) were determined using bivariate logistic regression controlling for age, gender, co-infections (HIV-1 and bacteremia) sickle cell trait (HbAS) and G6PD deficiency. The reference groups in the logistic regression analysis were the absence of the respective models for each genotype. n = the number of participants with the respective genotype. P-values were considered significant at P ≤ 0.05
Values in bold are significant p-values at a cut-off of p≤0.05
FcγRIIA-131/FcγRIIIA-176/FcγRIIIB haplotypes distribution within the study groups and association with severe malarial anemia
Prior to performing regression analysis to determine the association between the FcγRIIA-131His/Arg, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 haplotypes and SMA, we compared the distribution of the carriage of the haplotypes within the study groups. In total, eight haplotypes were generated after haplotype construction. We selected four common haplotypes with an overall frequency > 8.0% in the whole population. The haplotypes were distributed as follows; FcγRIIA-131Arg/FcγRIIIA-176F/FcγRIIIBNA2, (0.33), FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA1 (0.12), FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA2 (0.17) and FcγRIIA-131His/FcγRIIIA176V/FcγRIIIBNA1 (0.15). Among these four common haplotypes, FcγRIIA-131Arg/FcγRIIIA-176F/FcγRIIIBNA2 haplotype was higher in children with SMA relative to non-SMA group (P = 0.044, Table 4). The distributions of the other three haplotypes were comparable between the SMA and non-SMA groups; FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA1 (P = 0.104), FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA2 (P = 0.269) and FcγRIIA-131His/FcγRIIIA-176 V/FcγRIIIBNA1 (P = 0.188, Table 4). Using bivariate logistic regression analysis controlling for age, sex, co-infection (HIV-1 status and bacteremia), sickle cell trait (HbAS) and G6PD deficiency [26, 28–30], we determined the association between carriages of the FcγRIIA-131/FcγRIIIA-176/FcγRIIIB haplotypic structures and severe malaria anemia (SMA; Hb < 6.0 g/dL and any density parasitemia). This analysis revealed that the carriage of the FcγRIIA-131Arg/FcγRIIIA-176F/FcγRIIIBNA2 haplotype was associated with increased risk of severe malaria anemia relative to none carriage (OR = 1.70, 95% CI, 1.02–2.93, P = 0.036). Further regression analysis did not show any association between carriage of FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA1 (OR = 1.80, 95% CI, 0.98–3.30, P = 0.057), FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA2 (OR = 0.76, 95% CI, 0.44–1.32, P = 0.334) and FcγRIIA-131His/FcγRIIIA-176 V/FcγRIIIBNA1 (OR = 0.71, 95% CI, 0.41–1.25, P = 0.234) haplotypes and SMA.
Table 4.
FcγRIIA-131/FcγRIIIA-176/FcγRIIIB haplotypes distribution within the study groups and association with severe malarial anemia
| FcγRIIA-131His/Arg, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 haplotypes | Study groups | P-value* | SMA (Hb < 6.0 g/dL | |||
|---|---|---|---|---|---|---|
| SMA n (%) |
non-SMA n (%) |
OR | 95% CI | P-value** | ||
| 131Arg/176F/NA2 (n = 171) | 79 (69.3) | 92 (57.5) | 0.044 | 1.70 | 1.02–2.93 | 0.036 |
| 131His/176F/NA1 (n = 59) | 30 (26.3) | 29 (18.1) | 0.104 | 1.80 | 0.98–3.30 | 0.057 |
| 131His/176F/NA2 (n = 87) | 32 (28.1) | 55 (34.4) | 0.269 | 0.76 | 0.44–1.32 | 0.334 |
| 131His/176 V/NA1 (n = 79) | 28 (24.6) | 51 (31.9) | 0.188 | 0.71 | 0.41–1.25 | 0.234 |
Children with acute malaria (n = 274) were grouped based on SMA (defined as Hb < 6.0 g/dL, with any density parasitemia) [25]. Odds ratios (OR) and 95% confidence intervals (CI) were determined using bivariate logistic regression controlling for age, gender, co-infections (HIV-1 and bacteremia) sickle cell trait (HbAS), alpha-thalassemia and G6PD deficiency. The reference groups in the regression analysis were the non-carriage of respective haplotypic structures. n; the number of participants with the respective haplotype. n (%); number (percentage) of participants with respective haplotype in each study group. *P-value determined using Chi-square (χ2). **P-values determined using logistics regression analysis. All P-values were considered statistically significant at P ≤ 0.05
Values in bold are significant p-values at a cut-off of p≤0.05
Association between FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIBNA1/NA2 haplotypes and parasitemia levels
Since the FcγRs are important determinants in phagocytosis of parasites, we determined if carriage of FcγRs haplotypes was associated with parasitemia levels. Results revealed that carriage of FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA1 haplotype [mean (SEM); 4.37 (± 0.079), n = 59] relative to non-carriage [mean (SEM); 4.12 (± 0.052), n = 215], P = 0.009), was associated with higher parasitemia. Additional analysis showed that the level of parasitemia was comparable between the carriage and non-carriage of FcγRIIA-131Arg/FcγRIIIA-176F/FcγRIIIBNA2 haplotype [mean (SEM); 4.18 (± 0.057), n = 171] versus non-carriage [mean (SEM); 4.17 (± 0.074), n = 103], P = 0.973) and FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA2 [mean (SEM); 4.23 (± 0.073), n = 87] versus non-carriage [mean (SEM); 4.16 (± 0.056), n = 187, P = 0.521]. Further analysis also showed that the level of parasitemia was also comparable between those with FcγRIIA-131His/FcγRIIIA-176 V/FcγRIIIBNA1 haplotype [mean (SEM); 4.21 (± 0.079), n = 79] versus those without the haplotype [mean (SEM); 4.16 (± 0.096), n = 195], P = 0.587), Fig. 1(a-d).
Fig. 1.
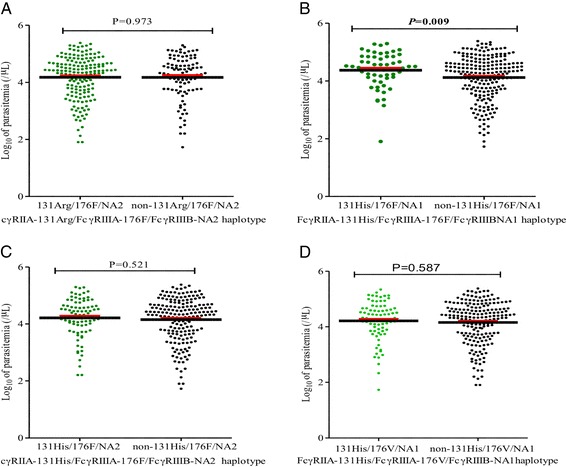
Data are presented as scatter plots for carriage and non-carriage of respective haplotype constructs. The thick black lines through the scatters represent mean, while the red lines above the mean line represent the standard error of the mean (SEM). The carriage of FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA1 haplotype which was marginally associated with susceptibility to SMA had higher levels of parasitemia (P = 0.009). Differences in parasitemia levels were determined using unpaired Student’s t-test with Welsch correction at 95% confidence interval
Discussion
Based on the observations that Fc gamma receptors (FcγRs) are important contributory factors for infectious disease immuno-pathogenesis, the association between the FcγRIIA-131Arg/His, FcγRIIIA-176F/V and FcγRIIIB-NA1/NA2 polymorphisms and pediatric severe malaria anemia (SMA; Hb < 6.0 g/dL, any density parasitemia) was determined. We further assessed whether the carriage of different haplotypes of FcγRs were associated with parasite levels in P. falciparum infections. The current study demonstrated that the FcγRIIA-131Arg/ FcγRIIIA-176F/FcγRIIIBNA2 haplotype was associated with an increased susceptibility to SMA, while the FcγRIIA-131Arg/ FcγRIIIA-176F/ FcγRIIIBNA1 haplotype was associated with increased levels of circulating parasites during infection. However, there was no association between the individual genotypes and SMA in this pediatric population from western Kenya.
The FcγRs constitute a crucial arm of host immune defense against extracellular challenges by infectious agents through engagement of IgGs to enable innate immune effectors cells carry out phagocytosis and other downstream processes leading to immunity [14, 31]. Some polymorphisms in the FcγRs have been identified as genetic determinants of susceptibility to infectious diseases [21, 32]. The FcγRIIA-131Arg/His polymorphism leads to Histidine to Arginine change at 131 located at its second extracellular immunoglobulin-like domain [8, 33]. The FcγRIIA-31His/His has efficient binding to IgG2 as opposed to FcγRIIA-131Arg/Arg. In addition, the IgG2 and IgG3 antibodies have been shown to confer resistance to malaria by some studies [34, 35]. In our current study, however, we did not find any association between FcγRIIA-131Arg/His polymorphism and SMA. An earlier study [23] in Ghanaian children demonstrated that FcγRIIA-131His/His was associated with an increased risk of severe malaria anemia, but not cerebral malaria or any other malarial complication. Of note is the fact that a number of studies have shown contradictory results on the actual role of this variant on malarial disease severity [36, 37]. These discrepancies may be attributed to clinical definitions of malaria, different genetic backgrounds from ethnic diversity and overall sample (population) size in previous studies.
The FcγRIIIA-176F/V gene displays a functional allelic polymorphism that generates allotypes exhibiting different receptor properties [38]. Our study revealed no association between the FcγRIIIA-176F/V polymorphism and susceptibility to SMA in this pediatric population. This may imply that this particular variant is not independently associated with susceptibility to SMA which is consistent with our previous study involving the combined effect of toll-like receptor 9 and FcγRIIIA polymorphisms [39]. The FcγRIIIB is a C-terminus linked glycosylphosphatidylinositol (GPI) moiety anchored receptor, exclusively expressed on neutrophils with three characterized allotypes i.e. human neutrophil antigen (HNA-1a or NA1, HNA-1b or NA2 and HNA-1c or SH) [27]. The NA variants, NA1 and NA2, are a product of five non-synonymous SNPs in the first Ig-like domain, with an asparagine to serine switch at amino acid position 65 resulting in glycosylation and reduced affinity in the NA2 allele [19, 38]. In the current study, we did not observe an association between either the NA1 or NA2 allotypes and susceptibility to SMA using common genetic models i.e., dominant, additive and recessive models. However, in Ghanaian children aged 1 to 12 years, the FcγRIIIB-NA2 was associated with susceptibility to clinical malaria [40]. In a different study of malaria patients in Thailand, the FcγRIIIB-NA2 allotype was associated with cerebral malaria, but not other forms of severe malaria [21]. Given the differences in findings from different populations and a diversity of clinical manifestations associated with malaria, the exact role of FcγRIIIB-NA2 in mediating outcome of malarial disease remains to be further explored.
It is important to note that FcγRs function synergistically, especially via crosslinking, resulting in phagocytosis of immunoglobulin-opsonized immune complexes or through stimulation of neutrophil granulation leading to production of reactive oxygen species (ROS) [41, 42]. Moreover, the additive and interaction effects of host genotype and infection affect malaria outcome [43] in malaria. In the current study, haplotypic analysis revealed that carriage of the FcγRIIA-131Arg/FcγRIIIA-176F/FcγRIIIBNA2 haplotype was associated with susceptibility to SMA. This is not surprising given that the haplotype had a higher frequency in the SMA group relative to the non-SMA group. Consistent with these observations, previous studies have demonstrated that the FcγR-131Arg/Arg is associated with low phagocytic activity and poor immune complex clearance [33], which may imply that its inheritance as a haplotype, together with FcγRIIIA-176F and the FcγRIIIBNA2 allotypes, impart decreased cellular responses to IgG-mediated stimulation [15, 18], and subsequently, susceptibility to SMA. Although the exact mechanisms through which the FcγRIIA-131Arg/FcγRIIIA-176F/FcγRIIIBNA2 haplotype result in severe malaria susceptibility were not evaluated in the current study, it is scientifically plausible to propose that carriage of the haplotype may lead to a reduced crosslinking in neutrophils, hence low phagocytic activity resulting in reduced antibody dependent respiratory burst (ADRB), a mechanism by which neutrophils provide protection against clinical malaria [44–46]. Moreover, the FcγRIIA-131Arg/Arg, FcγRIIIA-176F/F and FcγRIIIB-NA2 allotypes are associated with low binding to cytophilic antibodies, which have been shown to play major roles in ADRB [47, 48]. Taken together, the FcγRIIA-131Arg/FcγRIIIA-176F/FcγRIIIBNA2 haplotype may culminate in a reduced protective inflammatory response leading to enhanced susceptibility in children with SMA.
The finding that the FcγRIIA-131His/FcγRIIIA-176F/FcγRIIIBNA1 haplotype was associated with higher parasitemia levels is fascinating given the fact that the FcγRIIA-131His/His and FcγRIIIB-NA1 allotypes in this haplotype construct are associated with effective binding to cytophillic IgGs [49], leading to clearance of opsonized parasites as opposed to the FcγRIIIA-176 F/F. One possible explanation for this observation could be that high levels of parasitemia in the haplotype may be associated with the diluting effect of the FcγRIIA-176F allele, which has a low binding to cytophylic antibodies [15], and hence reduced clearance of parasites. However, it is worth noting that FcγRIIIA binding of IgG is important in induction of natural killer (NK) cells stimulatory properties which results in release of pro-inflammatory mediators, such as IL-1β, interferon-γ and tumor necrosis factor-α [50] whose imbalances have been implicated in pathogenesis of clinical malaria in children.
Differences in the allelic frequencies of the FcγRs SNPs observed in the current study likely suggest their indirect influence on malaria susceptibility and pathogenesis in the current population. The deviation from HWE of FcγRIIIB NA1/NA2 genotypes in the current study remains consistent with the results of FcγRIIIB genetic polymorphisms performed in our previous reporting in which we included 528 children [22]. It is likely that the observed NA1/NA2 genotype frequencies were in part due to consanguinity, however, this effect was not determined in the current study population. As much as HWE inconsistency may be due to genotyping errors [51], it is worth noting that the likelihood of this error was significantly reduced since in our previous population [22] we genotyped both FcγRIIA -131Arg/His and FcγRIIIB-NA1/NA2 using RFLP method in which the genotype frequencies were comparable to those in the current population in which TaqMan genotyping was used for FcγRIIA -131Arg/His. We thus hypothesize that the observed HWE deviation in FcγRIIIB could be due to unidentified mutation likely resulting from disease-related evolutionary selection pressure by P. falciparum (and potentially by other infectious disease in the population) that does not affect the neighboring FcγRIIA and FcγRIIIA genes. This, however, remains to be determined most preferably by whole genome sequencing so as to develop a conclusive explanation.
In summary, the current study demonstrates that FcγRs haplotypes, but not individual genotypes are associated with malarial disease severity, demonstrating the combinatorial effects of FcγRs on influencing clinical malaria outcomes. Future studies aimed at longitudinally measuring immune complexes over time will help to delineate the important role of FcγR haplotypes on susceptibility to severe malaria in pediatric populations.
Acknowledgements
We are grateful to the Siaya County Referral Hospital for clinical support. We are indebted to the parents/guardians of the study participants and children who took part in the study. This work is published with the approval of the Director, KEMRI.
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its Additional file 1).
Authors’ contributions
EOM, WAO, ER, SBA, TW, JMO, DJP and CO designed, carried out the survey studies in the rural population and participated in the drafting of the manuscript. EOM, ER and WAO performed the statistical analyses and participated in the drafting of the manuscript. All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the Scientific and Ethics Review Unit at the Kenya Medical Research Institute.
Consent
Informed written consent was obtained from the parent or legal guardian of all children participating in the study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ADRB
Antibody dependent respiratory burst
- CM
Cerebral malaria
- FcgR
Fc gamma receptor
- G6PD
Glucose-6-phosphate dehydrogenase
- Hb
Hemoglobin
- HbAS
Hemoglobin AS type
- HDP
High density parasitemia
- HWE
Hardy Weinberg equilibrium
- IgG
Immunoglobulin
- IQR
Interquartile range
- MOH
Ministry of Health
- NA
Neutrophil antigen
- NK
Natural killer cells
- SEM
Standard error of mean
- SMA
Severe malarial anemia
- SNP
Single nucleotide polymorphisms
- WBCs
White blood cells
Additional file
These are details of the raw data for the study participants (N=274) used in the analyses of results presented in the current paper. (XLS 222 kb)
Contributor Information
Elly O. Munde, Email: ellymunde@gmail.com
Winnie A. Okeyo, Email: okeyo.winnie@gmail.com
Evans Raballah, Email: eraballah@hotmail.com.
Samuel B. Anyona, Email: sbonuke@gmail.com
Tom Were, Email: mugogwe@yahoo.com.
John M. Ong’echa, Email: michaelongecha@yahoo.com
Douglas J. Perkins, Email: dperkins@salud.unm.edu
Collins Ouma, Email: collinouma@yahoo.com.
References
- 1.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332(21):1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 2.Dondorp AM, Angus BJ, Chotivanich K, Silamut K, Ruangveerayuth R, Hardeman MR, Kager PA, Vreeken J, White NJ. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. AmJTrop Med Hyg. 1999;60(5):733–737. doi: 10.4269/ajtmh.1999.60.733. [DOI] [PubMed] [Google Scholar]
- 3.Helleberg M, Goka BQ, Akanmori BD, Obeng-Adjei G, Rodriques O, Kurtzhals JA. Bone marrow suppression and severe anaemia associated with persistent Plasmodium falciparum infection in African children with microscopically undetectable parasitaemia. Malar J. 2005;4(1):56. doi: 10.1186/1475-2875-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415(6872):673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 5.Ong'echa JM, Keller CC, Were T, Ouma C, Otieno RO, Landis-Lewis Z, Ochiel D, Slingluff JL, Mogere S, Ogonji GA, et al. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. AmJTrop Med Hyg. 2006;74(3):376–385. [PubMed] [Google Scholar]
- 6.Pleass RJ, Woof JM. Fc receptors and immunity to parasites. Trends Parasitol. 2001;17(11):545–551. doi: 10.1016/S1471-4922(01)02086-4. [DOI] [PubMed] [Google Scholar]
- 7.Raghavan M, Bjorkman PJ. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 1996;12:181–220. doi: 10.1146/annurev.cellbio.12.1.181. [DOI] [PubMed] [Google Scholar]
- 8.van de Winkel JG, Capel PJ. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14(5):215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 9.van der Pol W, van de Winkel JG. IgG receptor polymorphisms: risk factors for disease. Immunogenetics. 1998;48(3):222–232. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]
- 10.Lehrnbecher TL, Foster CB, Zhu S, Venzon D, Steinberg SM, Wyvill K, Metcalf JA, Cohen SS, Kovacs J, Yarchoan R, et al. Variant genotypes of FcgammaRIIIA influence the development of Kaposi's sarcoma in HIV-infected men. Blood. 2000;95(7):2386–2390. [PubMed] [Google Scholar]
- 11.Warmerdam PA, van de Winkel JG, Gosselin EJ, Capel PJ. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32) J Exp Med. 1990;172(1):19–25. doi: 10.1084/jem.172.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant AV, Roussilhon C, Paul R, Sakuntabhai A. The genetic control of immunity to Plasmodium infection. BMC Immunol. 2015;16:14. [DOI] [PMC free article] [PubMed]
- 13.Zhao J, Ma L, Chen S, Xie Y, Xie L, Deng Y, He Y, Li T, Wang J, Li S, et al. Association between Fc-gamma receptor IIa (CD32) gene polymorphism and malaria susceptibility: a meta-analysis based on 6928 subjects. Infect Genet Evol. 2014;23:169–175. doi: 10.1016/j.meegid.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 15.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90(3):1109–1114. [PubMed] [Google Scholar]
- 16.Bux J, Kissel K, Hofmann C, Santoso S. The use of allele-specific recombinant Fc gamma receptor IIIb antigens for the detection of granulocyte antibodies. Blood. 1999;93(1):357–362. [PubMed] [Google Scholar]
- 17.Ory PA, Goldstein IM, Kwoh EE, Clarkson SB. Characterization of polymorphic forms of Fc receptor III on human neutrophils. J Clin Invest. 1989;83(5):1676–1681. doi: 10.1172/JCI114067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bredius RG, Fijen CA, De Haas M, Kuijper EJ, Weening RS, Van de Winkel JG, Out TA. Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes. Immunology. 1994;83(4):624–630. [PMC free article] [PubMed] [Google Scholar]
- 19.Salmon JE, Edberg JC, Kimberly RP. Fc gamma receptor III on human neutrophils. Allelic variants have functionally distinct capacities. J Clin Invest. 1990;85(4):1287–1295. doi: 10.1172/JCI114566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JY, Wang CM, Ma CC, Luo SF, Edberg JC, Kimberly RP, Wu J. Association of a transmembrane polymorphism of Fcgamma receptor IIb (FCGR2B) with systemic lupus erythematosus in Taiwanese patients. Arthritis Rheum. 2006;54(12):3908–3917. doi: 10.1002/art.22220. [DOI] [PubMed] [Google Scholar]
- 21.Omi K, Ohashi J, Patarapotikul J, Hananantachai H, Naka I, Looareesuwan S, Tokunaga K. Fcgamma receptor IIA and IIIB polymorphisms are associated with susceptibility to cerebral malaria. Parasitol Int. 2002;51(4):361–366. doi: 10.1016/S1383-5769(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 22.Ouma C, Davenport GC, Garcia S, Kempaiah P, Chaudhary A, Were T, Anyona SB, Raballah E, Konah SN, Hittner JB, et al. Functional haplotypes of Fc gamma (Fcgamma) receptor (FcgammaRIIA and FcgammaRIIIB) predict risk to repeated episodes of severe malarial anemia and mortality in Kenyan children. Hum Genet. 2012;131(2):289–299. doi: 10.1007/s00439-011-1076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuldt K, Esser C, Evans J, May J, Timmann C, Ehmen C, Loag W, Ansong D, Ziegler A, Agbenyega T, et al. FCGR2A functional genetic variant associated with susceptibility to severe malarial anaemia in Ghanaian children. J Med Genet. 2010;47(7):471–475. doi: 10.1136/jmg.2009.073643. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100(5):1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElroy PD, Lal AA, Hawley WA, Bloland PB, Kuile FO, Oloo AJ, Harlow SD, Lin X, Nahlen BL. Analysis of repeated hemoglobin measures in full-term, normal birth weight Kenyan children between birth and four years of age. III. The Asembo Bay cohort project. AmJTrop Med Hyg. 1999;61(6):932–940. doi: 10.4269/ajtmh.1999.61.932. [DOI] [PubMed] [Google Scholar]
- 26.Otieno RO, Ouma C, Ong'echa JM, Keller CC, Were T, Waindi EN, Michaels MG, Day RD, Vulule JM, Perkins DJ. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20(2):275–280. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 27.Bux J, Stein EL, Santoso S, Mueller-Eckhardt C. NA gene frequencies in the German population, determined by polymerase chain reaction with sequence-specific primers. Transfusion. 1995;35(1):54–57. doi: 10.1046/j.1537-2995.1995.35195090663.x. [DOI] [PubMed] [Google Scholar]
- 28.Aidoo M, Terlouw DJ, Kolczak MS, McElroy PD, ter Kuile FO, Kariuki S, Nahlen BL, Lal AA, Udhayakumar V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359(9314):1311–1312. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 29.Wambua S, Mwacharo J, Uyoga S, Macharia A, Williams TN. Co-inheritance of alpha+−thalassaemia and sickle trait results in specific effects on haematological parameters. Br J Haematol. 2006;133(2):206–209. doi: 10.1111/j.1365-2141.2006.06006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Were T, Davenport GC, Hittner JB, Ouma C, Vulule JM, Ong'echa JM, Perkins DJ. Bacteremia in Kenyan children presenting with malaria. J Clin Microbiol. 2011;49(2):671–676. doi: 10.1128/JCM.01864-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmon JE, Edberg JC, Brogle NL, Kimberly RP. Allelic polymorphisms of human Fc gamma receptor IIA and Fc gamma receptor IIIB. Independent mechanisms for differences in human phagocyte function. J Clin Invest. 1992;89(4):1274–1281. doi: 10.1172/JCI115712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouma C, Keller CC, Opondo DA, Were T, Otieno RO, Otieno MF, Orago AS, Ong'Echa JM, Vulule JM, Ferrell RE, et al. Association of FCgamma receptor IIA (CD32) polymorphism with malarial anemia and high-density parasitemia in infants and young children. AmJTrop Med Hyg. 2006;74(4):573–577. [PubMed] [Google Scholar]
- 33.Warmerdam PA, van de Winkel JG, Vlug A, Westerdaal NA, Capel PJ. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J Immunol. 1991;147(4):1338–1343. [PubMed] [Google Scholar]
- 34.Aucan C, Traore Y, Tall F, Nacro B, Traore-Leroux T, Fumoux F, Rihet P. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect Immun. 2000;68(3):1252–1258. doi: 10.1128/IAI.68.3.1252-1258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasr A, Elghazali G, Giha H, Troye-Blomberg M, Berzins K. Interethnic differences in carriage of haemoglobin AS and Fcgamma receptor IIa (CD32) genotypes in children living in eastern Sudan. Acta Trop. 2008;105(2):191–195. doi: 10.1016/j.actatropica.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Cooke GS, Aucan C, Walley AJ, Segal S, Greenwood BM, Kwiatkowski DP, Hill AV. Association of Fcgamma receptor IIa (CD32) polymorphism with severe malaria in West Africa. AmJTrop Med Hyg. 2003;69(6):565–568. [PubMed] [Google Scholar]
- 37.Nasr A, Iriemenam NC, Troye-Blomberg M, Giha HA, Balogun HA, Osman OF. Montgomery SM, ElGhazali G, Berzins K. Fc gamma receptor IIa (CD32) polymorphism and antibody responses to asexual blood-stage antigens of Plasmodium falciparum malaria in Sudanese patients. Scand J Immunol. 2007;66(1):87–96. doi: 10.1111/j.1365-3083.2007.01947.x. [DOI] [PubMed] [Google Scholar]
- 38.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170(2):481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munde EO, Okeyo W, Anyona SB, Raballah E, Konah S, Okumu W, Ogonda L, Vulule J, Ouma C. Polymorphisms in the Fc gamma receptor IIIA and toll-like receptor 9 are associated with protection against severe malarial anemia and changes in circulating gamma interferon levels. Infect Immun. 2012;80(12):4435–4443. doi: 10.1128/IAI.00945-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adu B, Dodoo D, Adukpo S, Hedley PL, Arthur FK, Gerds TA, Larsen SO, Christiansen M, Theisen M. Fc gamma receptor IIIB (FcgammaRIIIB) polymorphisms are associated with clinical malaria in Ghanaian children. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0046197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell MA, Huang MM, Chien P, Indik ZK, Pan XQ, Schreiber AD. Substitutions and deletions in the cytoplasmic domain of the phagocytic receptor Fc gamma RIIA: effect on receptor tyrosine phosphorylation and phagocytosis. Blood. 1994;84(6):1753–1759. [PubMed] [Google Scholar]
- 42.Salmon JE, Millard SS, Brogle NL, Kimberly RP. Fc gamma receptor IIIb enhances Fc gamma receptor IIa function in an oxidant-dependent and allele-sensitive manner. J Clin Invest. 1995;95(6):2877–2885. doi: 10.1172/JCI117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Idaghdour Y, Quinlan J, Goulet JP, Berghout J, Gbeha E, Bruat V, de Malliard T, Grenier JC, Gomez S, Gros P, et al. Evidence for additive and interaction effects of host genotype and infection in malaria. Proc Natl Acad Sci U S A. 2012;109(42):16786–16793. doi: 10.1073/pnas.1204945109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joos C, Marrama L, Polson HE, Corre S, Diatta AM, Diouf B, Trape JF, Tall A, Longacre S, Perraut R. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One. 2010;5(3) doi: 10.1371/journal.pone.0009871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapelski S, Klockenbring T, Fischer R, Barth S, Fendel R. Assessment of the neutrophilic antibody-dependent respiratory burst (ADRB) response to Plasmodium falciparum. J Leukoc Biol. 2014;96(6):1131–1142. doi: 10.1189/jlb.4A0614-283RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Investig. 2000;80(5):617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 47.Pleass RJ, Ogun SA, McGuinness DH, van de Winkel JG, Holder AA, Woof JM. Novel antimalarial antibodies highlight the importance of the antibody Fc region in mediating protection. Blood. 2003;102(13):4424–4430. doi: 10.1182/blood-2003-02-0583. [DOI] [PubMed] [Google Scholar]
- 48.Shi J, McIntosh RS, Adame-Gallegos J, Dehal PK, van Egmond M, van de Winkel J, Draper SJ, Forbes EK, Corran PH, Holder AA, et al. The generation and evaluation of recombinant human IgA specific for Plasmodium falciparum merozoite surface protein 1-19 (PfMSP1 19) BMC Biotechnol. 2011;11:77. doi: 10.1186/1472-6750-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garraud O, Mahanty S, Perraut R. Malaria-specific antibody subclasses in immune individuals: a key source of information for vaccine design. Trends Immunol. 2003;24(1):30–35. doi: 10.1016/S1471-4906(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 50.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72(10):5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox DG, Kraft P. Quantification of the power of Hardy-Weinberg equilibrium testing to detect genotyping error. Hum Hered. 2006;61:10–14. doi: 10.1159/000091787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Additional file 1).


