Abstract
Rhagoletis pomonella is a model for sympatric speciation (divergence without geographic isolation) by means of host-plant shifts. Many Rhagoletis species are known to use fruit odor as a key olfactory cue to distinguish among their respective host plants. Because Rhagoletis rendezvous on or near the unabscised fruit of their hosts to mate, behavioral preferences for fruit odor translate directly into premating reproductive isolation among flies. Here, we report that reciprocal F1 hybrids between the apple and hawthorn host races of R. pomonella, as well as between the host races and an undescribed sibling species infesting Cornus florida (flowering dogwood) do not respond to host fruit volatiles in wind-tunnel assays at doses that elicit maximal directed flight in parental flies. The reduced ability of hybrids to orient to fruit volatiles could result from a conflict between neural pathways for preference and avoidance behaviors, and it suggests that hybrids might suffer a fitness disadvantage for finding fruit in nature. Therefore, host-specific mating may play a dual role as an important postzygotic as well as a premating reproductive barrier to isolate sympatric Rhagoletis flies.
Keywords: host-plant odors, postzygotic barrier, fruit maggots
True fruit flies belonging to the Rhagoletis pomonella sibling-species complex are host-specific frugivorous parasites specialized on unique, nonoverlapping sets of plants (1-3). Because these flies overlap broadly in their geographic distributions across North America and appear largely interfertile in crosses, Bush (3) hypothesized that the six or more members of the R. pomonella group radiated sympatrically in the absence of geographic isolation by means of host-plant shifts. In particular, the recent shift <150 years ago of the species R. pomonella from its native ancestral host hawthorn (Crataegus spp.) to introduced domesticated apple (Malus pumila) is often cited as an example of incipient sympatric speciation (4).
Habitat-specific mating is an important ecological adaptation, contributing to reproductive isolation and sympatric divergence for Rhagoletis flies (5). The trait is manifested through host fidelity, which is the tendency of adults to mate on and oviposit into the same species of unabscised host fruit that they fed within as larvae. Mark-recapture experiments have indicated that host fidelity reduces potential gene flow between apple and hawthorn flies to ≈4-6% per generation (5), and it has the capacity to completely isolate the host races from related sibling species, such as Rhagoletis mendax, the blueberry maggot (6).
R. pomonella flies use volatile compounds emitted from the surface of ripening fruit as important chemosensory cues to recognize and distinguish among their host plants (7, 8). In flight-tunnel assays and field tests, apple and hawthorn flies preferentially oriented to and were captured with chemical blends of their natal fruit volatiles (8). Flight-tunnel and field studies also have demonstrated that nonnatal volatiles can disrupt fly behavior (9). The same fruit volatile blend that acts as an agonist to attract natal apple or hawthorn flies also acts as an antagonist to cause the nonnatal race to tend to avoid an odor source (9). Because R. pomonella rendezvous exclusively on or near the unabscised fruit of their hosts to mate (10, 11), preference and avoidance behaviors for apple vs. hawthorn fruit odor translate directly into prezygotic reproductive isolation between the fly races.
Here, we investigate the genetics of fruit-odor discrimination between the apple and hawthorn host races of R. pomonella, as well as the “flowering dogwood fly,” which is an undescribed sibling species infesting Cornus florida that allozyme studies suggest is the sister taxon to the host races (12). We report an unexpected finding: F1 hybrids from pair-wise crosses of apple, hawthorn, and dogwood flies generally failed to respond to host fruit volatiles in wind-tunnel assays at concentrations that induce maximal oriented flight in parental flies. We discuss possible causes for the reduced chemosensory response of F1 hybrids and consider its implications for sympatric speciation.
Materials and Methods
Insects. Apple and hawthorn flies were collected as larvae from infested fruit at Grant, MI; Fennville, MI; and Urbana, IL, during the 1999-2002 field seasons, and they were reared to adulthood in the laboratory by using standard Rhagoletis protocols (13). Together, these three sites encompass the latitudinal range of overlap of the apple and hawthorn host races in the midwestern United States. Dogwood flies used in the study were collected from Granger, IN, and Raccoon Lake, IN, from 2000-2002, and they were treated in the same manner as the host races. After being overwintered as pupae in a refrigerator at 5°C for 4-7 months, eclosing adults were placed in holding cages in a controlled environmental chamber (24°C, 15 h light/9 h dark photoperiod, 60-70% relative humidity) and fed a diet containing sugar, vitamins, casein hydrolysate, and a salt mixture (14). A portion of these parental flies were tested for their behavioral responses to fruit-odor blends in the flight tunnel as odor-naïve, sexually mature adults of 10- to 21-days old. The remaining apple, hawthorn, and dogwood fly adults were mass crossed in 1 × 1 × 0.5-m Plexiglas cages in all possible reciprocal directions. Each mating cage contained a minimum of 20 females and 20 males, with flies being replenished as they died with virgin adults from holding cages. Each mating cage was supplied with water, food, and four Red Delicious apples for female oviposition. Apple and dogwood flies oviposit large numbers of eggs in apples if they are confined for several days without their natal fruit. Apples were replaced in the cages every 3 days. After removal from the cages, the apples were held on wire racks over plastic collecting trays in the constant-temperature chamber. The collecting trays were checked daily for puparia. Puparia were placed in small, clear, plastic Solo cups containing moist vermiculite. The Solo cups were held in the constant-temperature chamber to allow flies to directly develop into adults (Rhagoletis overwinters in a facultative pupal diapause that can be bypassed by continuously rearing flies at ambient room temperature and midsummer photoperiods). Sexually mature, odor-naïve F1 adults were tested in the flight tunnel in the same manner as parental flies. We also performed an additional set of reciprocal mass crosses between Fennville apple × hawthorn flies in which pupae were overwintered for 5 months in a refrigerator at 5°C. This second protocol provided a test for possible effects of direct development vs. diapause rearing on hybrid fly behavior.
Synthetic Blends. The following synthetic blends and sources of chemicals were the same as reported previously: apple, 10% butyl butanoate/4% propyl hexanoate/37% butyl hexanoate/44% hexyl butanoate/5% pentyl hexanoate (15); hawthorn, 94.3% ethyl acetate/4% 3-methylbutan-1-ol/1.5% isoamyl acetate/0.09% 4,8-dimethyl-1,3(E),7-nonatriene/0.01% butyl hexanoate/0.10% dihydro-β-ionone (16); and dogwood, 54.9% ethyl acetate/27.5% 3-methylbutan-1-ol/0.9% isoamyl acetate/1.9% dimethyl trisulfide/9% 1-octen-3-ol/5.8% β-caryophyllene (17).
Flight-Tunnel Assay. The behavioral responses of flies to synthetic apple, hawthorn, and dogwood fruit volatile blends were measured in a 183-cm-long, 61 × 61-cm square flight tunnel (see refs. 8 and 15-17 for details of the tunnel, flight conditions, and derivation of fruit-odor blends, as well as for an explanation of load doses). Solutions of the synthetic blends were prepared in methylene chloride and applied to acetone-washed, rubber septa at 60 min before flight testing (Thomas Scientific, Swedesboro, NJ). A septum was attached to a 7.5-cm-diameter red plastic sphere (Gempler's, Madison, WI) hung at the upwind end of the tunnel. Fresh sources and spheres were used for each trial run. Individual flies were transferred to a screen cage, which was then placed on a screen stand 1-m downwind of the odor-baited sphere, and fly behavior was recorded (see the legend to Fig. 1 for a description of behaviors).
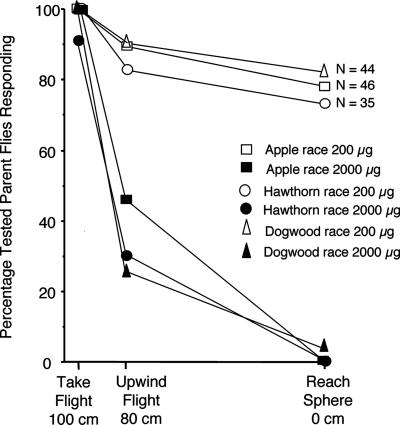
Fig. 1.
Percentages of tested parental flies collected from apple (rectangles; Grant), hawthorn (circles; Urbana), and dogwood (triangles; Granger) displaying the indicated behavioral acceptance of their natal volatile blend when tested at doses of 200 μg (open symbols) and 2,000 μg (filled symbols) in flight-tunnel assays. Behavioral responses in order of increasing blend acceptance are as follows: walk and groom (fly remaining in release cage), take flight (flight out of the release cage 100 cm from sphere), upwind flight (oriented flight to at least 80 cm from sphere), and reach sphere. The percentage of flies displaying walk and groom behavior was calculated as 100% minus the percentage that takes flight.
Flight-tunnel tests were conducted with parent and hybrid flies. Parental flies from three of the populations were tested successively to the 200- and 2,000-μg doses of their natal blend. For hybrid flies (six different crosses), six different odor treatments were tested, with each fly tested to the complete series in the following order: the parental female's natal fruit blend was assayed at doses of 200 and 2,000 μg; the parental male's natal blend was assayed at doses of 200 and 2,000 μg; and a 1:1 combination of both the parental female's and male's natal volatiles with each blend present was assayed at a dose of 200 μg or 2,000 μg (total combined blend doses of 400 and 4,000 μg). Also, a subset (n = 20) of parental (apple and hawthorn flies from the Fennville site, n = 20) and hybrid (apple × hawthorn from the Fennville site, n = 20) were tested only to a blank red sphere with a control-solvent-treated rubber septum.
It has been shown that blend doses of 200 μg elicit maximal responses from apple, hawthorn, and dogwood flies in the flight tunnel (8, 15-17). Therefore, we consider results from the 200-μg assays to reflect the normal behavior of parental fly taxa to fruit volatiles. The 2,000-μg dose was tested because preliminary experiments with F1 hybrids from the Grant apple and hawthorn populations indicated that levels of taking flight and oriented upwind flight to the 200-μg dose were reduced significantly compared with parent flies. Because of the low levels of taking flight, the earliest behavior in the response sequence that indicates recognition of a relevant odor, we hypothesized that hybrids might have a higher threshold for response to blends, and therefore, we also assayed flies to the higher order of magnitude (2,000-μg dose of volatiles). Combined blends were tested in this study because we recently found that at the standard 200-μg dose these mixtures can cause arrested upwind flight of apple, hawthorn, and dogwood flies due to certain nonnatal volatiles acting as behavioral antagonists (9). The earlier combined blend experiments did not test flies at the higher 2,000-μg dose.
Results
Odorless Control. No parental (apple and hawthorn flies from the Fennville site, n = 20), or hybrid (apple × hawthorn from the Fennville site, n = 20) fly tested flew upwind in the tunnel to reach a “blank” red sphere fitted with an odorless septum. This result agrees with previous flight-tunnel tests (16, 17) and shows that the sphere and septum used as a release point for the blends is not visually attractive from the 1-m distance at which flies were released.
Response of Parental Apple, Hawthorn, and Dogwood Flies to Natal Blends. Significant differences were observed in the levels of upwind flight of parent apple (Grant), hawthorn (Urbana), and dogwood (Granger) flies to the 200- and 2,000-μg doses of the natal blends (Fig. 1). At the standard 200-μg dose, most parent flies (> 74%) displayed upwind anemotactic flight in the tunnel to reach the source sphere containing their natal fruit blend. This result is consistent with previous studies for flies from the same, as well as different, geographic sites (8, 9, 16, 17) (C.E.L.J., unpublished results for dogwood flies from Raccoon Lake). We have also shown (8) that there was no difference between hawthorn flies from Urbana reared for two generations in the laboratory on apple and parental hawthorn flies reared directly from field-collected hawthorn fruit, discounting an effect of the larval host-fruit environment on adult fly behavior, at least for hawthorn flies.
When tested at the higher 2,000-μg dose, apple, hawthorn, and dogwood flies displayed dramatically reduced levels of completed flight to their natal fruit blend (Fig. 1). Although almost all of the flies that were tested took flight from the release stand, only 2 of 44 dogwood flies tested from Granger flew upwind and landed on the source sphere when it was baited with the dogwood blend at the higher dose. Moreover, no apple (n = 46) or hawthorn (n = 35) fly assayed from Grant flew upwind and landed on the 2,000-μg baited source sphere. The test results indicate that parental flies initially oriented to the 2,000-μg dose of their natal fruit blend, but their upwind flight was arrested by high amounts of volatiles in the odor plume.
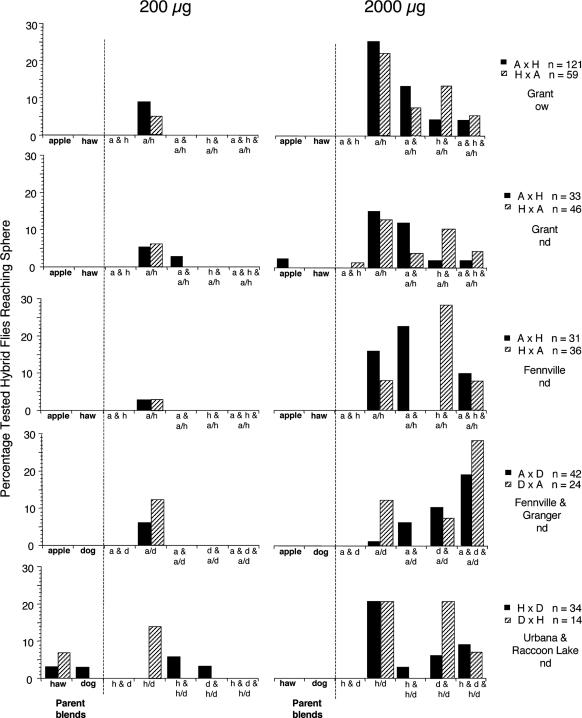
F1 Generation of Flies. The response of F1 flies differed in two major ways from that of their parents. First, hybrid flies displayed greatly reduced levels of response at all stages of the response sequence to 200-μg doses of volatile blends (200 μg, Fig. 2). Compared with 90-100% of apple, hawthorn, and dogwood flies tested to their natal blend (Fig. 1), <30% of hybrid flies took flight (the earliest behavior in the flight-tunnel sequence; data not shown) to any treatment. Low levels of taking flight resulted in significantly lower levels of upwind flight and landing on source spheres (signifying completion of the behavioral host odor acceptance sequence). Whereas ≈80% of parents reached the sphere (Fig. 1), almost no F1 hybrids did (Fig. 2). One apple × hawthorn hybrid [n = 313, including those reared directly to adulthood at 24°C (Grant, Fennville, and Urbana nondiapausing hybrid flies) or overwintered as pupae for 5 months (Grant, overwintering)], no apple × dogwood hybrids (n = 66), and only six hawthorn × dogwood hybrids (n = 48) reached the source sphere when it was baited with 200 μg of either parental fruit blend tested alone. The few F1 hybrids that flew upwind to spheres with 200-μg lures (3-14%) did so mostly with 1:1 combinations of each of their parent's natal blends (Fig. 2).
Fig. 2.
Percentages of F1 hybrid flies flying upwind and reaching spheres containing 200- or 2,000-μg doses of natal blends or combined blends. Crosses included the following: apple female × hawthorn male (A × H) and hawthorn female × apple male (H × A), including nondiapausing (nd) populations from Grant and Fennville and overwintering (ow) populations from Grant; apple female × dogwood male (A × D) and dogwood female × apple male (D × A) from Fennville (apple) and Granger (dogwood); and, female hawthorn × male dogwood (H × D) and female dogwood × male hawthorn (D × H) from Urbana (hawthorn) and Raccoon Lake (dogwood). Six hybrid H × A flies from Urbana (nd), all of which failed to reach source spheres in 200-μg-dose tests, are not shown. Individual flies were tested to each parental blend and the respective combined blend, at both doses, so that for each dose and set of populations there were seven response types. As an example, each fly from the female apple × male hawthorn crosses could have responded in one of the following ways: the maternal blend alone (a), paternal blend alone (h), both natal blends separately (a & h), the combined blends alone (a/h), the maternal blend and to the combined blend (a & a/h), the paternal blend and to the combined blend (h & a/h), or both blends separately and to the combined blend (a & h & a/h).
The second major difference between parental and F1 flies was that an increased proportion of hybrids responded in the higher 2,000-μg-dose assays. Increased response occurred not only with the combined blend alone but also to the higher dose of one or both of the natal blends and the combined blends (Fig. 2; e.g., apple and apple/hawthorn for flies responding to the parental apple blend and then to the apple/hawthorn combination). For each of the crosses, 55-63% of hybrids took flight (data not shown) and 30-50% flew upwind and landed only on spheres containing the combined blend or one (and sometimes both) of the natal blends and the combined blend. Parent flies were not tested to the 2,000-μg dose of the combined blends because a previous study showed that when a lower 200-μg dose of the combined blends was presented to flies, significant levels of arrested flight were observed because of antagonist effects of key nonhost volatiles on upwind oriented flight (9). Thus, the successful upwind flight of F1 flies to the 2,000-μg dose of the natal and combined blends represents a significant change in the threshold for upwind flight (recognition of the natal blend, a relevant odor mixture) and the antagonist properties of nonnatal compounds (upwind flight to combined blends).
Overall, the data for 2,000 μg also indicate considerable variability in response levels to different treatment combinations, both among the populations and between reciprocal crosses for each set of populations. For example, flies from the apple × hawthorn cross (Grant; overwintering) exhibited the greatest proportion of flights only to the sphere containing the combined blends (apple/hawthorn), compared with flies that responded to the natal blends as well as the combined blend (2,000 μg, Fig. 2). In contrast, flies from the apple × dogwood crosses (Fennville × Granger; nondiapausing) exhibited the greatest proportion of flights to both natal blends and the combined blend (apple, dogwood, and apple/dogwood), compared with the combined blends alone (apple/dogwood; 2,000 μg, Fig. 2). However, one significant trend for all three of the apple × hawthorn and hawthorn × apple crosses was a bias for preferring the maternal over paternal parent's natal volatiles (Fig. 2) [apple and apple/hawthorn and hawthorn and apple/hawthorn combinations; P < 0.0023 for combined apple female × hawthorn male crosses (n = 174), P < 0.0094 for hawthorn female × apple male crosses (n = 141), as determined by χ2 tests, 1 df; see Fennville crosses in Fig. 2 for the most dramatic maternal effect].
Discussion
We have shown that most F1 hybrids constructed between host races and sibling species of R. pomonella from a wide geographic sampling of flies fail to fly upwind to doses of synthetic fruit-odor blends that elicit maximal response in parental flies. In a study of R. pomonella (apple race) × R. mendax hybrids, Frey and Bush (18) found reduced electroantennogram responses of F1 hybrids to various chemical compounds compared with parental flies. The combined results of these studies suggest that impairment of F1 oriented flight response to fruit odors could be widespread in the R. pomonella species group.
Our results differ from other studies for phytophagous insects, which have mostly reported that F1 hybrids exhibit intermediate or dominant phenotypes compared with parental taxa (see Table 1, which is published as supporting information on the PNAS web site, for a list and description of several studies). However, most of these studies have focused on oviposition preference rather than long-range orientation. It is conceivable that part of the reason that our results differ from results for other phytophagous insects is that our flight-tunnel assay measured only one aspect of host discrimination: recognition of a specific blend of fruit odors that elicits upwind anemotactic flight from a distance, rather than the integrated constellation of chemical, visual, and tactile cues that are involved in within-tree close-range discrimination of host fruit/plants. Previous R. pomonella studies, using an experimental paradigm involving release of female flies into potted trees in field cage enclosures, have demonstrated that within-tree foraging behavior is a complex process, involving visual, chemical, and physical cues, as well as experience and learning (7). Flies rely strongly on visual cues at distances of <1 m to locate fruit in the tree canopy (7), and they also rely on chemical and physical cues from the fruit surface as soon as they have alighted on fruit. It is possible that if close-range oviposition choice tests were conducted, F1 flies might have shown intermediate or dominance preferences for apple, hawthorn, or dogwood trees.
However, other studies of host recognition in R. pomonella also support the conclusion that fruit odor is the most important long- to intermediate-range-cue used by flies to recognize and distinguish host from nonhost trees (7). Adult flies make long-distance dispersal flights in search of food sources and host fruit (5, 7, 10, 11), and thus, in habitats with mixed host types, chemically mediated oriented flight is an important component of host location. Therefore, it seems likely that even if additional cues were added to our upwind-flight choice tests, the altered chemosensory system of hybrids would be a significant factor impacting their host-finding ability and, consequently, their fitness. Thus, our finding that F1 apple × hawthorn, apple × dogwood, and hawthorn × dogwood hybrids appear to be very different from their parents with respect to their lack of response to host-fruit volatiles at normal dosages is important for a complete understanding of the genetic basis of host preference.
The decreased response of hybrid apple, hawthorn, and dog-wood flies to normal doses of fruit volatile blends can, in part, be explained as a shift in response thresholds. Hybrid flies, in contrast to parents, flew upwind in higher proportions to a 10-fold increase in concentration of natal blends (2,000 vs. 200 μg). However, our results show further that, for many hybrid individuals, there also was a change in the functionality of nonhost antagonist compounds, resulting in many more flies (30-50%) completing flights not only to their natal blend but also to spheres releasing a 1:1 combination of both parental blends. The results for 2,000 μg underscore that the olfactory systems (behavioral-response thresholds and agonist/antagonist properties) of R. pomonella hybrids are altered from that of their parents.
The genetics underlying reduced hybrid response to fruit volatiles could involve at least the following three types of factors, acting either alone or in concert. First, hybrids might suffer from general genomic incompatibilities that pleiotropically disrupt the development and functioning of key components of the chemosensory system. Second, the olfactory systems of F1 hybrids could be genetically conflicted; F1 flies might not be able to respond to normal doses of chemical cues because they possess alleles affecting both preference for and avoidance to alternative parental fruit blends. Third, a maternal effect could account for why a subset of apple × hawthorn F1 hybrids, although not responsive to low blend doses, orient to high doses of the natal blend of their mother.
If the altered behavior of F1 flies is a byproduct of a general developmental imbalance, then hybrids would be expected to exhibit various other phenotypic abnormalities with detrimental fitness consequences, in addition to their reduced olfactory response. Survivorship from pupal to adult life-history stages for apple and hawthorn hybrids is not qualitatively lower than that for parental flies (J.L.F., unpublished data). Also, there is no compelling evidence that hybrid males are more adversely affected than females (J.L.F., unpublished data), as might be expected for recently diverged taxa, such as the host races and dogwood fly, under Haldane's rule (19). Thus, if developmental abnormalities underlie the reduced behavioral response of hybrids to fruit volatiles, then they would appear to be fairly limited in their scope and probably due to a relatively small number of loci. Older, more divergent species, such as R. mendax, show more evidence of classic F1 inviability when hybridized to R. pomonella (20) than the recently diverged populations studied here.
A key question raised by our results is whether the reduced chemosensory ability of hybrids diminishes F1 fitness in the field. To address this issue, mark-release experiments need to be performed comparing hybrid and parental fly recapture on scented vs. odorless sphere traps positioned in host trees. The question of F1 fitness has important theoretical implications for sympatric host race formation and speciation. The adverse affects of chemosensory dysfunction for hybrids would likely extend beyond just mating success and affect oviposition efficiency as well, further compromising F1 fitness. Genetic constraints imposed on sympatric speciation by the selection-recombination antagonism (21) would be lessened because the same fruit-odor discrimination loci generating positive assortative mating in parental races would also have a negative impact on the mating and ovipositional success of hybrids. Thus, host-specific mating in parental races and hybrid performance would be multifarious traits that are pleiotropically linked, consistent with the single-variation model of Rice and Hostert (22). Therefore, evolving a new preference for a novel plant during a host shift would not only reduce the probability of a fly mating with individuals of the ancestral population but, in doing so, would also result in its offspring being less likely to find mates and oviposit into suitable host fruit. Another fitness-related possibility requiring future research is that avoidance of high volatile levels could be adaptive if high levels of volatiles are characteristic of overripe, nutritionally inferior fruit.
Further ramifications of impaired hybrid odor response in Rhagoletis are apparent when considered in the context of hybrid fitness in other organisms. Hybrid avoidance of the mating signal of parental species is rarely reported, perhaps because of the difficulties of demonstrating it, but it has been convincingly shown for mating song in the Drosophila biauraria-triauraria pair (23). However, hybrid impairment of mating behavior has been commonly observed in many organisms (e.g., refs. 24 and 25). Moreover, in several cases, ecological and mating impairment are linked, as they are in Rhagoletis. For example, in sticklebacks, hybrids between limnetic and benthic forms are ecologically inferior in both habitats and suffer impaired mating success as well (26). However, in the sticklebacks, there are two additional considerations that suggest important avenues for investigation in Rhagoletis. First, the reduced mating success of hybrid male fish seems to occur because of increased rejection by parental females (i.e., by sexual selection). Although hybrid males chose the same unvegetated habitats for breeding as do the limnetic fish, limnetic females preferentially choose limnetic males over hybrids as mates (26). Second, there is evidence in sticklebacks that such sexual selection could drive reinforcement of reproductive isolation between the limnetic and benthic forms (27). Therefore, analogous experiments examining female mate choice and differential hybrid and parental male success are required in Rhagoletis. In addition, comparative studies among the R. pomonella host races and closely related sibling species in areas of sympatry vs. allopatry are needed to test for possible reinforcement. The existence of reinforcement could help resolve a key issue in sympatric-speciation theory for Rhagoletis and other phytophagous insects; namely, how evolution proceeds from the host race to species stage (4).
Much further testing is required to confirm the ideas discussed above. Results from quantitative-trait loci mapping studies of later generation hybrids could help clarify the evolutionary dynamics of host-fruit-odor discrimination and support or refute these hypotheses. Regardless, the reduced ability of hybrids to orient to host-fruit odor opens lines of empirical enquiry and previously unrecognized theoretical dimensions to what appeared to be a straightforward and well understood relationship among habitat-specific mating, oviposition preference, reproductive isolation, and sympatric speciation in Rhagoletis.
Supplementary Material
Acknowledgments
We thank K. Catropia, K. Filchak, A. Forbes, R. Harrison, C. Musto, R. Oakleaf, K. Pelz, K. Poole, H. Reissig, J. Roethele, C. Smith, L. Stelinski, U. Stolz, F. Wang, F. Wang, Jr., B. Westrate, J. Wise, X. Xie, the Niles, MI, facility of the U.S. Department of Agriculture, Trevor Nichols Research Station (Fennville, MI), and the New York State Agricultural Experiment Station at Geneva. This article is dedicated to the work and memory of three tephritid workers: S. Polavarapu, who was an insightful ecologist, M. D. Huettel, who was a pioneer of behavioral genetics, and R. J. Prokopy, on whose broad shoulders all of us stand. This work was supported by grants from the National Science Foundation Integrated Research Challenges and the U.S. Department of Agriculture National Research Initiative to the authors, and the state of Indiana 21st Century Fund (to J.L.F).
Author contributions: C.E.L.J., J.L.F., S.H.B., S.N., and W.L.R. designed research; C.E.L.J., H.R.D., J.L.F., S.H.B., and S.N. performed research; C.E.L.J., H.R.D., J.L.F., S.H.B., S.N., and W.L.R. analyzed data; and C.E.L.J., H.R.D., J.L.F., S.H.B., and W.L.R. wrote the paper.
References
- 1.Bush, G. L. (1966) Bull. Mus. Comp. Zool. 134, 431-562. [Google Scholar]
- 2.Bush, G. L. (1969) Evolution (Lawrence, Kans.) 23, 237-251. [Google Scholar]
- 3.Bush, G.L. (1969) Am. Nat. 103, 669-672. [Google Scholar]
- 4.Berlocher, S. H. & Feder, J. L. (2002) Annu. Rev. Entomol. 47, 773-815. [DOI] [PubMed] [Google Scholar]
- 5.Feder, J. L., Opp, S., Wlazlo, B. Reynolds, K., Go, W. & Spisak, S. (1994) Proc. Natl. Acad. Sci. 91, 7990-7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feder, J. L. & Bush, G. L. (1989) Evolution (Lawrence, Kans.) 43, 1813-1819. [DOI] [PubMed] [Google Scholar]
- 7.Prokopy, R. J. & Papaj, D. R. (2000) in Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior, eds. Aluja, M. & Norbomm, A. L. (CRC, Boca Raton, FL), pp. 219-252.
- 8.Linn, C., Feder, J. L., Nojima, S., Dambroski, H., Berlocher, S. H. & Roelofs, W. (2003) Proc. Natl. Acad. Sci. USA 100, 11490-11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linn Jr., C., Nojima, S. & Roelofs, W. (2004) Entomol. Exp. Appl., in press.
- 10.Prokopy, R. J., Bennett, E. W. & Bush, G. L. (1971) Can. Entomol. 103, 1405-1409. [Google Scholar]
- 11.Prokopy, R. J., Bennett, E. W. & Bush, G. L. (1972) Can. Entomol. 104, 97-104. [Google Scholar]
- 12.Berlocher, S. H. (1999) Heredity 83, 652-662. [DOI] [PubMed] [Google Scholar]
- 13.Feder, J. L., Chilcote, C. A. & Bush, G. L. (1989) J. Hered. 80, 277-283. [Google Scholar]
- 14.Neilson, W. T. A. & McAllen, J. W. (1965) J. Econ. Entomol. 58, 542-543. [Google Scholar]
- 15.Zhang, A., Linn, C., Jr., Wright, S., Prokopy, R., Reissig, W. H. & Roelofs, W. L. (1999) J. Chem. Ecol. 25, 1221-1232. [Google Scholar]
- 16.Nojima, S., Linn, C., Jr., Morris, B., Zhang, A. & Roelofs, W. L. (2003) J. Chem. Ecol. 29, 319-334. [DOI] [PubMed] [Google Scholar]
- 17.Nojima, S., Linn, C., Jr., & Roelofs, W. (2003). J. Chem. Ecol. 29, 2347-2357. [DOI] [PubMed] [Google Scholar]
- 18.Frey, J. E. & Bush, G. L. (1996) Entomol. Exp. Appl. 80, 163-165. [Google Scholar]
- 19.Turelli, M. & Orr, H. A. (1995) Genetics 140, 389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bierbaum, T. J. & Bush, G. L. (1990) Entomol. Exp. Appl. 55, 105-118. [Google Scholar]
- 21.Felsenstein, J. (1981) Evolution (Lawrence, Kans.) 35, 124-138. [DOI] [PubMed] [Google Scholar]
- 22.Rice W. R. & Hostert, E. E. (1993) Evolution (Lawrence, Kans.) 47, 1637-1653. [DOI] [PubMed] [Google Scholar]
- 23.Tomaru, M. Matsubayshi, H. & Oguma, Y. (1995) Anim. Behav. 50, 905-914. [Google Scholar]
- 24.Noor, M. A. F., Grams, K. L., Bertucci, L. A., Almendarez, Y., Reiland, J. & Smith, K. R. (2001) Evolution (Lawrence, Kans.) 55, 512-521. [DOI] [PubMed] [Google Scholar]
- 25.Hobel, G. & Gerhardt, H. C. (2003) Evolution (Lawrence, Kans.) 57, 894-904. [DOI] [PubMed] [Google Scholar]
- 26.Vamosi, S. M. & Schluter, D. (1999) Evolution (Lawrence, Kans.) 53, 874-879. [DOI] [PubMed] [Google Scholar]
- 27.Rundle, H. D. & Schluter, D. (1998) Evolution (Lawrence, Kans.) 52, 200-208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.