Abstract
The sweetpotato whitefly Bemisia tabaci (Gennadius) was challenged with different combinations of matrine (insecticide) and Lecanicillium muscarium (entomopathogenic fungus). Our results revealed a synergistic relationship between matrine and L. muscarium on mortality and enzyme activities of B. tabaci. To illustrate the biochemical mechanisms involved in detoxification and immune responses of B. tabaci against both control agents, activities of different detoxifying and antioxidant enzymes were quantified. After combined application of matrine and L. muscarium, activities of carboxylestrease (CarE), glutathione-s-transferase (GSTs) and chitinase (CHI) decreased during the initial infection period. Acetylcholinestrase (AChE) activities increased during the entire experimental period, whereas those of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) decreased during the later infection period. The increased mortality and suppression of enzymatic response of B. tabaci following matrine and L. muscarium application suggests a strong synergistic effect between both agents. The strong synergistic effect is possibly related to the disturbance of acetylcholine balance and changes in AchE activities of the whitefly as both matrine and L. muscarium target insect acetylcholine (Ach) receptors which in turn effects AchE production. Therefore, our results have revealed the complex biochemical processes involved in the synergistic action of matrine and L. muscarium against B. tabaci.
The sweetpotato whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is a serious pest of agricultural crops in different regions of the world1,2. From the 1980s, B. tabaci Middle East-Asia Minor 1 (MEAM1) cryptic species (previously known as ‘B biotype’) has drastically increased in distribution. This has been attributed to the development and increase in global trade. Direct damage by B. tabaci occurs as a result of sucking plant sap from the phloem and secretion of honey dew which serves as a substrate for growth of sooty moulds3,4. In addition, adults can transmit more than 150 plant viruses to commercial crops5. Management of B. tabaci has been dominated by the frequent use of broad spectrum conventional chemical pesticides6,7. The consistent use of synthetic chemicals for B. tabaci management has resulted in environmental pollution and adverse effects on humans, mammals and other non-target organisms8. This injudicious use of chemicals leads to the intermission of natural biological control systems and outbreaks of B. tabaci8. All these factors have necessitated research and development of environmentally secure, biodegradable and indigenous methods for insect pest management9. Hence, the search for effective chemical constituents of naturally occurring entomopathogenic fungi based biopesticides or the combined use of entomopathogenic fungi along with synthetic chemicals to provide potential methods to overcome environmental pollution and resistance problems.
More than 750 fungi from over 90 species have been described as entomopathogenic. Lecanicillium muscarium10 is a well-known entomopathogenic fungus which has been commercialized for aphid and whitefly control11. Strains of L. muscarium have been isolated from aphids, scales, whiteflies and other insects in various regions of the world and have also been shown to be pathogenic against various other insects12. The host range of this species is quite broad and includes hemipteran insects as well as other arthropod orders. Wang et al.13 studied the virulence of six strains of L. muscarium against sweetpotato whitefly. Their results indicated that strain V16063, V3450 and Vp28 were virulent against B. tabaci having LC50 values of 2.57 × 105, 6.03 × 105 and 6.05 × 105 conidia/ml respectively. Commercial preparations of L. muscarium have been on the market since 1980 and have been used successfully against whiteflies infesting different crops13.
Matrine is a quinolizidine alkaloid derived from the roots of Sophora flavescens and S. alopecuroides14. Matrine has been used as a traditional Chinese medicine and pesticide15. Matrine exhibits a variety of pharmacological and cytotoxic activities16. Recently, matrine has been used alone or in combination with other chemicals to control insect pests of different vegetables, fruits, flowers and tea crops in China17,18. In addition to the contact toxicity, matrine has shown antifeedant activities against Formosan subterranean termites (Coptotermes formosanus Shiraki) and two spotted spider mite (Tetranychus urticae Koch)14,19. Hwang et al.20 described the efficacy of a chemical formulation (KNI3126) based on a mixture of matrine and neem oil against different sucking insect pests and phytophagous mites confirming the toxic as well as biological action of matrine against phytophagous arthropods with different feeding habits.
Insects have a well developed defence mechanism against insecticides and natural pathogens consisting of multiple enzyme systems21. Cuticle degrading enzymes, multifunction oxidases (MFO), glutathione-S-transferase (GST), esterases (EST), acetylcholinestrase (AChE) and antioxidant enzymes (SOD, CAT, POD) are enzyme systems commonly involved in insect defence against toxic chemicals22. These enzymes play a pivotal role in detoxification and cellular antioxidant defences against the oxidative stress caused by chemicals or an invading pathogen23. It is well documented that insecticide poisoning and fungal infection can cause significant changes in enzymes24,25. A few studies have reported the complex changes in enzyme profiles in response to the joint application of insecticide and pathogens against insects21,23. However, detailed investigation on the effects of the synergistic action between L. muscarium and insecticides on insect enzyme systems has not been reported.
In this study, we aim to explain the possibility of synergistic effect between matrine and L. muscarium against B. tabaci as both of these agents affects their host through partial disruption of acetylcholine receptors. Matrine is known to target insect acetylcholine (Ach) receptors which in turn effects AchE production16. Lecanicillium muscarium can produce a secondary metabolite named bassianolide (a cyclooligomer depsipeptide) which can affect acetylcholine receptors of insect muscles reducing the production of AchE26,27. In addition, changes in activity profiles of different antioxidant and detoxifying enzymes of B. tabaci were also quantified as a function of the possible synergism between the entomopathogenic fungus L. muscarium and the insecticide matrine. Initially, the co-toxicity of matrine and L. muscarium infection was evaluated. Many previous synergistic studies have used only one dose level of each component. However, in the current study multiple dose levels of matrine, L. muscarium and their combinations were utilized for investigation. Their effects on different detoxifying as well as antioxidant enzyme activities in B. tabaci were also studied. The results will provide basic information on the interactions between L. muscarium and the insecticide. These findings will improve our knowledge concerning the mechanism by which L. muscarium overcomes the immune system of an insect host. The information obtained from this study will help in designing better control strategies involving insecticides and insect pathogens against B. tabaci.
Results
Virulence of matrine and L. muscarium alone or in combinations against B. tabaci
Mortality (%) of 2nd instar B. tabaci nymphs differed significantly among different concentrations of matrine and the control after 8 days of application (F5,12 = 23.72; P < 0.001). The mortality data were used for probit analysis to calculate medial lethal concentrations (LC50) as shown in Table 1. The medial lethal concentration (LC50) of matrine was 0.83 ± 0.09 mg/L.
Table 1. Virulence of Lecanicillium muscarium, matrine, and combined Lecanicillium muscarium + matrine treatments against Bemisia tabaci.
| Treatments | Mortality | LC50 | 95% C.I. | Slope | Chi-square | CTC* | Type of action | |
|---|---|---|---|---|---|---|---|---|
| Matrine (mg/L) | 0.005 | 19.07 ± 0.87 e | 0.83 ± 0.09 a | 0.54–1.13 | 0.09 | 16.65 | — | — |
| 0.05 | 31.45 ± 1.35 d | |||||||
| 0.5 | 48.29 ± 1.97 c | |||||||
| 5 | 67.74 ± 2.68 b | |||||||
| 15 | 84.36 ± 4.65 a | |||||||
| Control | 4.49 ± 0.36 f | |||||||
| L. muscarium (Conidia/mL) | 1 × 104 | 28.97 ± 1.04 e | 0.11 ± 0.02 b | 0.08–0.20 | 0.08 | 12.37 | — | — |
| 1 × 105 | 45.14 ± 2.65 d | |||||||
| 1 × 106 | 65.32 ± 3.47 c | |||||||
| 1 × 107 | 74.21 ± 3.78 b | |||||||
| 1 × 108 | 83.36 ± 5.09 a | |||||||
| Control | 4.49 ± 0.37 f | |||||||
| CT1 | 0.001 + 0.8 × 105 | 61.23 ± 2.98 d | 0.034 ± 0.01 d | 0.02–0.04 | 0.47 | 18.97 | 125.99 | Synergistic |
| 0.01 + 0.8 × 106 | 74.63 ± 3.71 c | |||||||
| 0.1 + 0.8 × 107 | 82.54 ± 4.09 b | |||||||
| 0.75 + 0.8 × 108 | 98.31 ± 4.56 a | |||||||
| Control | 4.45 ± 0.36 e | |||||||
| CT2 | 0.0025 + 0.5 × 105 | 54.13 ± 2.89 d | 0.063 ± 0.01 c | 0.05–0.08 | 0.34 | 15.74 | 200 | Synergistic |
| 0.025 + 0.5 × 106 | 61.89 ± 3.65 c | |||||||
| 0.25 + 0.5 × 107 | 79.76 ± 3.97 b | |||||||
| 1.88 + 0.5 × 108 | 95.06 ± 5.23 a | |||||||
| Control | 4.45 ± 0.36 e | |||||||
| CT3 | 0.004 + 0.2 × 105 | 46.38 ± 3.21 d | 0.21 ± 0.01 b | 0.13–0.36 | 0.1 | 19.82 | 165.75 | Synergistic |
| 0.04 + 0.2 × 106 | 55.19 ± 2.85 c | |||||||
| 0.4 + 0.2 × 107 | 72.21 ± 3.65 b | |||||||
| 4.5 + 0.2 × 108 | 93.72 ± 4.56 a | |||||||
| Control | 4.45 ± 0.36 e | |||||||
CT1, CT2 and CT3 are combined treatments of Lecanicillium muscarium, matrine mixed at different ratios; CTC stands for co-toxicity coefficients; Mortalities (% ± S.E.) mentioned for each treatment followed by different letters are significantly different (Tukey’s test, P < 0.01).
The percentage mortality of 2nd instar B. tabaci nymphs differed significantly among different conidial concentrations of L. muscarium following 8 days application (F5,12 = 19.73; P < 0.001). The medial lethal concentration (LC50) of L. muscarium alone against B. tabaci nymphs was 0.16 mg/L (~5.5 × 105 conidia/ml) following 8 days application (Table 1).
Combining matrine with L. muscarium in different ratios (CT1, CT2 and CT3) during bioassay-III resulted in higher mortality rates with LC50 values of 0.034, 0.063 and 0.21 mg/L for CT1, CT2 and CT3, respectively (Table 1). The co-toxicity coefficients based on initial toxicity data of matrine and L. muscarium were 125.99, 200 and 165.75 for CT1, CT2 and CT3 respectively, thus showing a synergistic interaction between matrine and L. muscarium.
In bioassay IV (Table 2), B. tabaci nymphs were first treated with different concentrations of L. muscarium. Following 24 h post fungal application different concentrations of matrine were then applied. The combined application resulted in a LC50 value of 0.02 mg/L and a co-toxicity coefficient of 223; again showing a synergistic interaction between matrine and L. muscarium (Table 2).
Table 2. Susceptibility of Bemisia tabaci to matrine after 24-h exposure to Lecanicillium muscarium.
| Treatments | Mortality | LC50 | 95% C.I. | Slope | Chi-square | CTC | Type of action | |
|---|---|---|---|---|---|---|---|---|
| Matrine (mg/L) | 0.005 | 19.07 ± 0.87 e | 0.97 ± 0.06 a | 0.63–1.28 | 0.06 | 19.56 | — | — |
| 0.05 | 27.41 ± 1.23 d | |||||||
| 0.5 | 42.36 ± 1.89 c | |||||||
| 5 | 63.49 ± 3.32 b | |||||||
| 15 | 82.18 ± 4.16 a | |||||||
| Control | 5.2 ± 0.37 f | |||||||
| L. muscarium (Conidia/mL) | 1 × 104 | 28.97 ± 1.04 d | 0.13 ± 0.001 b | 0.10–0.20 | 0.07 | 11.23 | — | — |
| 1 × 105 | 42.8 ± 2.31 c | |||||||
| 1 × 106 | 61.03 ± 3.35 b | |||||||
| 1 × 107 | 72.36 ± 4.56 a | |||||||
| 1 × 108 | 79.81 ± 5.13 a | |||||||
| Control | 5.3 ± 0.36 e | |||||||
| CT4 | 0.005 + 1 × 105 | 57.19 ± 3.81 d | 0.02 ± 0.001 c | 0.01–0.03 | 0.04 | 14.36 | 223 | Synergistic |
| 0.05 + 1 × 106 | 69.87 ± 3.56 c | |||||||
| 0.5 + 1 × 107 | 82.34 ± 4.23 b | |||||||
| 5 + 1 × 108 | 96.78 ± 6.31 a | |||||||
| Control | 5.3 ± 0.36 e | |||||||
CT4 is combined treatment of Lecanicillium muscarium and, matrine mixed at 5:5 ratio; CTC stands for co-toxicity coefficients; Mortalities (% ± S.E.) mentioned for each treatment followed by different letters are significantly different (Tukey’s test, P < 0.01).
Effect of matrine and L. muscarium on enzyme activities in B. tabaci
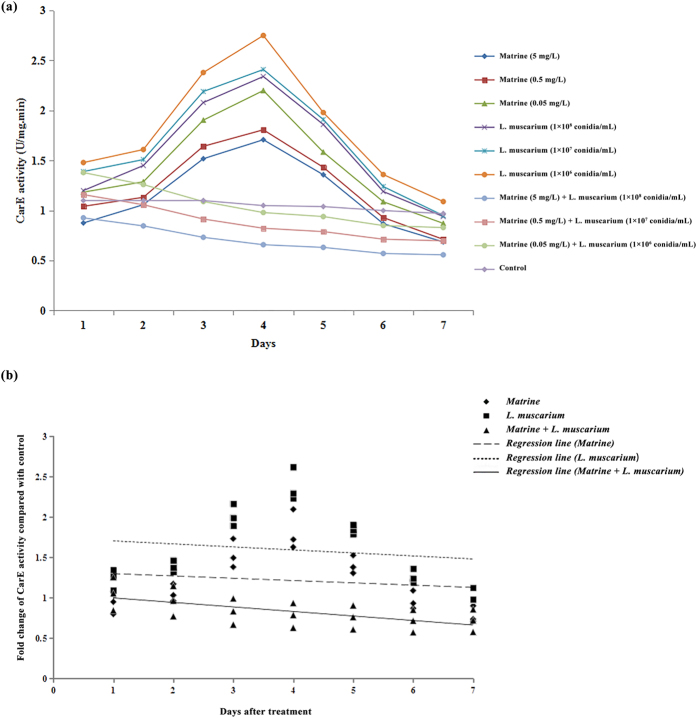
The Carboxylestrease (CarE) activities of B. tabaci in response to different concentrations of matrine and L. muscarium alone or in combinations are shown in Fig. 1a. An increase in CarE activity was observed during the initial four days post matrine treatment with a sharp decline in enzyme activity being observed afterwards. Lecanicillium muscarium application also increased the activities of CarE in B. tabaci during the initial 4 days post treatment. A significant reduction in CarE activity was observed in response to the joint application of matrine and L. muscarium up to 7 days post treatment. The fold changes in CarE activities over control in response to different treatments and their fitted regression lines are shown in Fig. 1b. The results showed that the regression line for matrine + L. muscarium treatment was more sensitive than those for matrine or L. muscarium alone. The fitted regression lines yielded significantly different slope (F2,6 = 10.31; P < 0.01) and Y-intercept (F2,6 = 22.45; P < 0.01) values for different treatments.
Figure 1.
(a) Carboxylestrase activities of Bemisia tabaci at different time intervals following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment; (b) Linear regression analysis between fold changes in carboxylestrase activities and different days following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment.
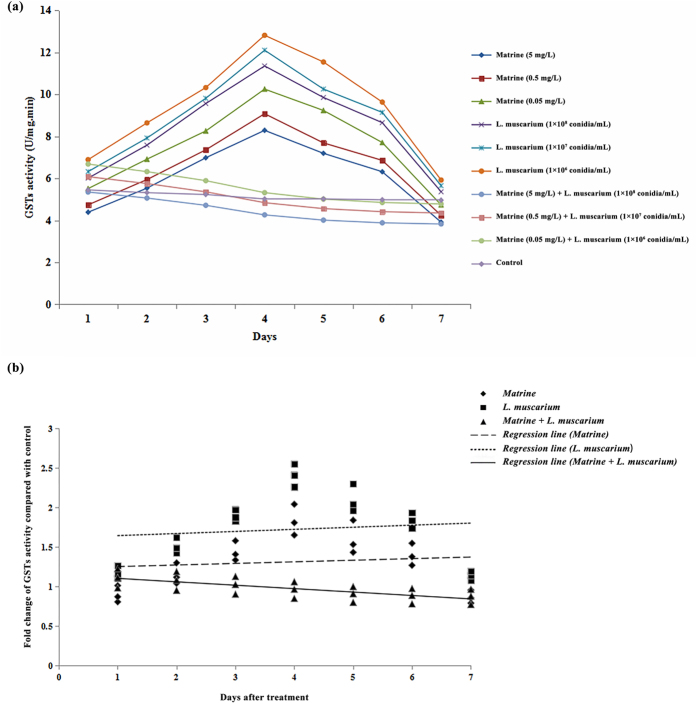
The activities of GSTs in B. tabaci when treated with matrine increased up to 4 days post treatment followed by a decrease in enzyme activity during the later periods. GSTs activity also increased during the initial 4 days following L. muscarium treatment. The GSTs activity in B. tabaci decreased significantly throughout the experimental period in response to the joint application of matrine and L. muscarium (Fig. 2a). The fold changes in GSTs activities over the control in response to different treatments and their fitted regression lines are shown in Fig. 2b. The results showed that the regression line for matrine + L. muscarium treatment was more sensitive than those for matrine or L. muscarium alone. The fitted regression lines yielded significantly different slope (F2,6 = 16.42; P < 0.01) and Y-intercept (F2,6 = 19.91; P < 0.01) values for different treatments.
Figure 2.
(a) Glutathione-s-transferase activities of Bemisia tabaci at different time intervals following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment; (b) Linear regression analysis between fold changes in glutathione-s-transferase activities and different days following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment.
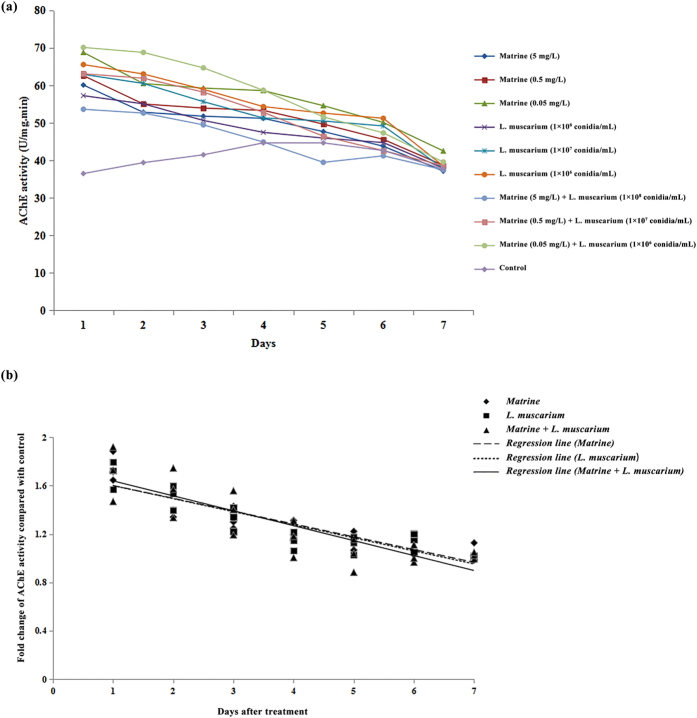
Acetylcholinestrase (AChE) activity decreased in B. tabaci during the entire experimental period following treatment with matrine alone. When treated with L. muscarium alone, AChE activity in B. tabaci decreased throughout the experimental period. AChE activity also decreased during the whole experimental period after B. tabaci was treated with a mixture of matrine and L. muscarium (Fig. 3a). The fold changes in AchE activities over the control in response to different treatments and their fitted regression lines are shown in Fig. 3b. The results showed that the regression line for different treatments were similar to each other. The fitted regression lines yielded significantly similar slope (F2,6 = 15.36; P = 0. 54) and Y-intercept (F2,6 = 14.39; P = 0.61) values for different treatments.
Figure 3.
(a) Acetylcholinestrase activities of Bemisia tabaci at different time intervals following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment; (b) Linear regression analysis between fold changes in acetylcholinestrase activities and different days following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment.
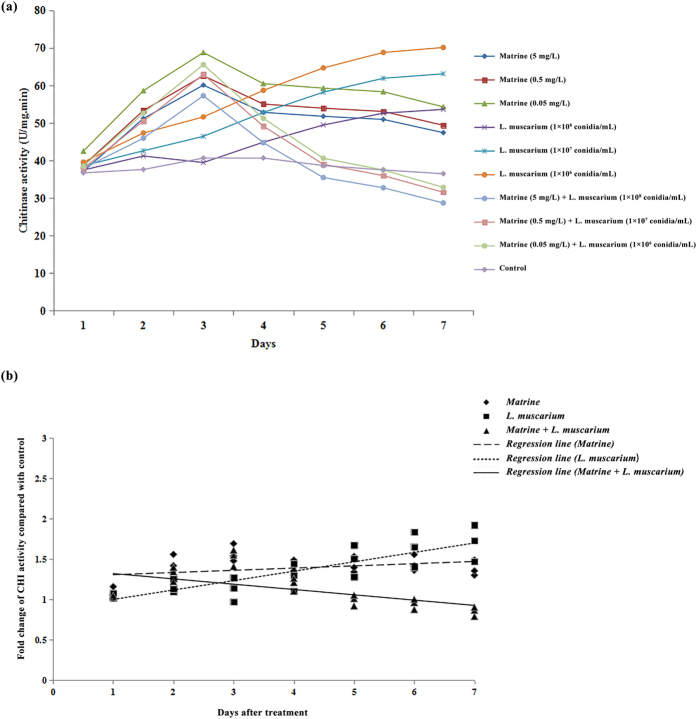
The activities of chitinase (CHI) in B. tabaci increased during the initial 3 days of the experiment when whiteflies were treated with matrine alone. When whiteflies were treated with L. muscarium alone, the enzyme activities increased throughout the experimental period. When whiteflies were treated with a combination of matrine and L. muscarium, enzyme activities increased during the initial 3 days and then decreased during the later time period (Fig. 4a). The fold changes in CHI activities over the control in response to different treatments and their fitted regression lines are shown in Fig. 4b. The results showed that the regression line for matrine + L. muscarium treatment was more sensitive than those for matrine or L. muscarium alone. The fitted regression lines yielded significantly different slope (F2,6 = 25.49; P < 0.001) and Y-intercept (F2,6 = 17.56; P < 0.01) values for different treatments.
Figure 4.
(a) Chitinase activities of Bemisia tabaci at different time intervals following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment; (b) Linear regression analysis between fold changes in chitinase activities and different days following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment.
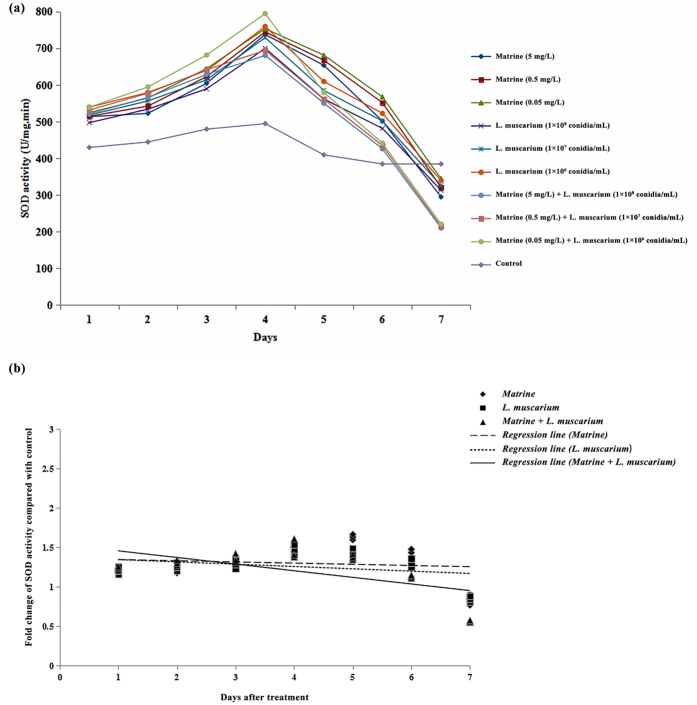
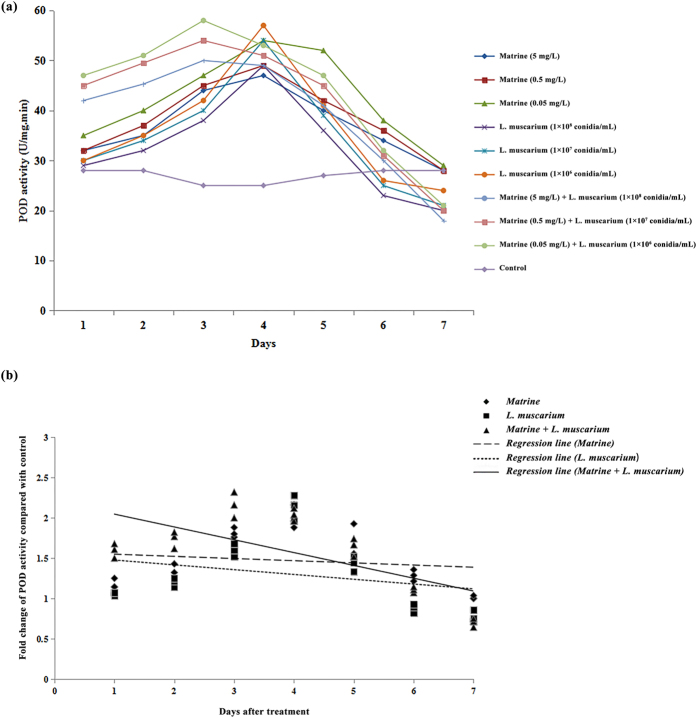
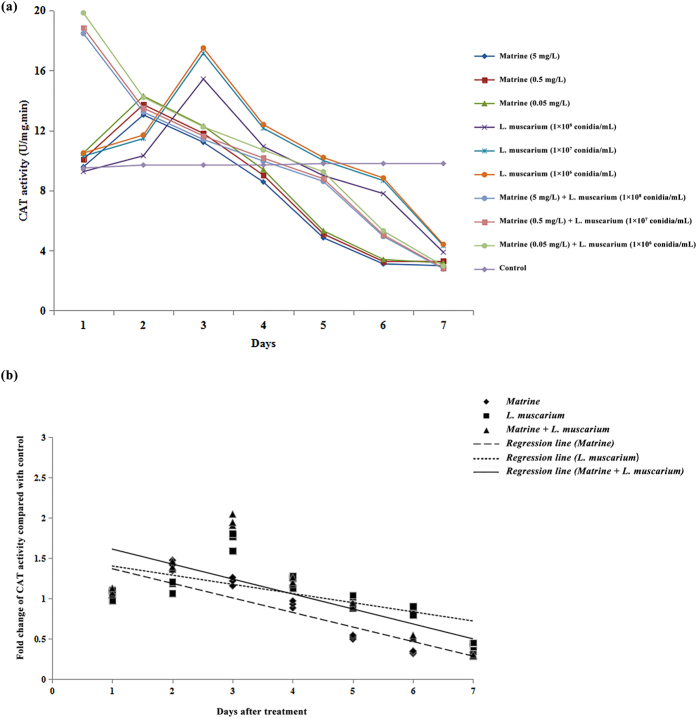
The activities of different antioxidant enzymes (SOD, POD and CAT) in B. tabaci increased during the initial 4 days after treatment with matrine or L. muscarium alone. When whiteflies were treated with a mixture of matrine and L. muscarium, activities of the antioxidant enzymes (SOD, POD and CAT) increased for up to 3 days. At the end of the experimental period the enzyme activities observed were significantly lower than the control (Figs 5a–7a). The fold changes in activities of the different antioxidant enzymes (SOD, POD and CAT) over the control in response to different treatments and their fitted regression lines are shown in Figs 5b–7b. The results showed that the regression lines for matrine + L. muscarium treatment were more sensitive than those for matrine or L. muscarium alone for SOD and POD while in case of CAT the fitted regression line of matrine was more sensitive when compared with the other treatments. The fitted regression lines yielded significantly different slope (SOD: F2,6 = 23.65, P < 0.001; POD: F2,6 = 14.27, P < 0.01 and CAT: F2,6 = 15.19, P < 0.01) and Y-intercept (SOD: F2,6 = 19.63, P < 0.01; POD: F2,6 = 13.22, P < 0.01 and CAT: F2,6 = 25.78, P < 0.01) values for different treatments.
Figure 5.
(a) Superoxide dismutase activities of Bemisia tabaci at different time intervals following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment; (b) Linear regression analysis between fold changes in superoxide dismutase activities and different days following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment.
Figure 6.
(a) Peroxidase activities of Bemisia tabaci at different time intervals following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment; (b) Linear regression analysis between fold changes in peroxidase activities and different days following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment.
Figure 7.
(a) Catalase activities of Bemisia tabaci at different time intervals following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatment; (b) Linear regression analysis between fold changes in catalase activities and different days following matrine, Lecanicillium muscarium, and matrine + Lecanicillium muscarium treatments.
Discussion
Development of integrated control programs against B. tabaci involving L. muscarium and chemical pesticides requires a clear understanding concerning the effects of chemicals on the physiology and pathogenicity of entomopathogenic fungi28. There have been a few in vivo studies which have indicated that combinations of L. muscarium and insecticides can have synergistic, antagonistic or additive mortality effects against B. tabaci29,30. However, most of the studies reporting on L. muscarium-insecticide interactions have shown the insect mortality or LC50 values by combining a single dose of fungi or chemical. We believe this is not an appropriate approach to determine the most effective synergistic formulation23,31. In this study Sun and Johnson’s co-toxicity coefficient was used to estimate the efficacy of different matrine and L. muscarium combinations (prepared at different ratios) against B. tabaci. We observed synergistic interactions when 2nd instar nymphs of B. tabaci were treated with different doses of matrine and L. muscarium. All the combined treatments of matrine and L. muscarium (CT1, CT2, CT3 in bioassay III and CT4 in bioassay IV) had higher rates of B. tabaci mortality (>93%) when compared with the mortality of B. tabaci in response to matrine or L. muscarium applied alone. The combined treatments CT1, CT2, CT3 (in bioassay III) and CT4 (in bioassay IV) had synergistic interactions with co-toxicity coefficients of 125.99, 200, 165.75 and 223 respectively. These findings illustrate the possible effectiveness of dual attack strategy for whitefly management. Our results showed that matrine can improve the efficacy of L. muscarium against B. tabaci during the infection period. The observed synergistic effect can be related to the target site of action of matrine and the toxin produced by L. muscarium. Both agents attack acetylcholine production by the insect which makes their interaction strongly synergistic, although the strength of this synergistic interaction can vary with the proportion of both agents applied, as well as, the timing of their application. This study has also shown possible physiological or biochemical consequences of the joint matrine and L. muscarium application against B. tabaci. The enzymatic response of B. tabaci observed was of a complex nature as the immune system of the whitefly was simultaneously trying to detoxify the insecticide as well as defend against fungal attack. The enzyme quantified during this study showed moderate to high variation in response to matrine and L. muscarium application.
In insects, detoxification of insecticides or pathogens is mainly accomplished by carboxylesterases (CarE) and glutathione-S-transferase (GSTs)32. Our findings showed an increase in CarE and GSTs activities during the initial 4 days following matrine treatment. This increase in enzyme activity in response shows the possible involvement of these enzymes in the insecticide detoxification mechanism of B. tabaci23. Luo & Zhang33 also observed similar fluctuation of CarE and GSTs in Plutella xylostella in response to matrine treatment. Matrine treatment also resulted in inhibition of CarE in turnip aphid (Lipaphis erysimi)34. Although few reports are available on changes in CarE and GSTs activity following matrine treatment, the extent of changes can vary with change in the insect species targeted and the concentration of matrine used. In this study, we found that CarE and GSTs increased during initial periods following L. muscarium treatment which is similar to the findings of Tian et al.35 who also observed similar changes in CarE and GSTs activity of B. tabaci following Isaria fumosorosea applications. They observed increases in activity of the said enzymes until 72 h post fungal application after which enzyme activities were restrained leading to metabolic imbalance and insect death35. The treatment of B. tabaci with a combination of matrine and L. muscarium resulted in a significant reduction of CarE and GSTs activities throughout the experimental period. These results are in line with the findings of Jia et al.23 who have shown a similar reduction of enzyme activity in Locusta migratoria following joint application of chlorantriniliprole and Metarhizium anisopliae. The changes in activities of detoxifying enzymes explained above can make the target pest more susceptible to fungal infection36. The reduction in CarE and GSTs activities in response to joint application of insecticide (matrine) and the entomopathogenic fungi (L. muscarium) throughout the experimental period can be related to the sequence of chemical’s/pathogen’s action against B. tabaci. Insecticides normally act as a stressor increasing the susceptibility of a target pest to an entomopathogenic fungus37. The enzyme acetylcholinestrase (AchE) rapidly terminates nerve impulses by catalyzing the hydrolysis of the neurotransmitter, acetylcholine at peripheral and central synapses of the insects’ nervous system38. AchE is also an important target site for insecticide action in the central nervous system of insects39. During this study, AchE activities of B. tabaci decreased throughout the experimental period following treatment with matrine and L. muscarium alone or in combination. The decrease in AchE activities can be related to the mode of action of both agents. Matrine, an alkaloid extracted from S. flavescens is known to target insect acetylcholine (Ach) receptors which in turn effects AchE production40. Luo et al.34 observed similar inhibition of AchE activity in turnip aphid (L. erysimi) following matrine application. The reduction in AchE activity of B. tabaci following L. muscarium application is in line with the findings of Zibaee et al.24 who observed similar inhibition of AchE activity when Beauveria bassiana and its secondary metabolites were applied against sunn pest (Eurygaster integriceps). These changes in AchE activity of B. tabaci can be related to the production of a secondary metabolite named bassianolide by L. muscarium. Bassianolide can inhibit acetylcholine receptors of insect muscles reducing the production of AchE26,27.
Chitinase (CHI) is an important component of insect growth and development. Chitinases degrade chitin present in an insect cuticle and peritrophic membrane41. Chitinases have also been reported to be involved in insect defence against entomopathogenic fungi and parasites42. Our results showed an increase in CHI activitythroughout the experimental period following L. muscarium application whereas reduction in CHI activities was observed after 3 days of matrine application or joint application of matrine and L. muscarium. We speculate that the joint application of matrine and L. muscarium disrupted the insect moulting process by creating an imbalance of chitinase production which is an essential enzyme required for moulting23.
Antioxidant enzymes (SOD, CAT, and POD) are also called enzymes of the protective cellular system as superoxide dismutase can enhance H2O2 production from O2 through dismutation while catalase and peroxidase are known to catalyze H2O production from H2O2. These reactions can result in the elimination of bio-membrane damage by reactive oxygen species (ROS)43. In our study, the activities of antioxidant enzymes (SOD, CAT, and POD) increased during the initial 3 days followed by a decrease when treated with L. muscarium alone. On the other hand, when matrine was applied in combination with L. muscarium, SOD and POD activities increased during the initial 3 days but CAT activities started to decrease after 24 h. This decrease in CAT activity can have a direct relationship with the accumulation of superoxide radicals produced during the destruction process23. The decreased activities of antioxidant enzymes (SOD, CAT, and POD) during the later experimental period can result in a reduced elimination of ROS which in turn can denature different biomolecules of the insect body. The denaturation of biomolecules can stop all the cellular processes, so leading to the death of the insect44.
To summarize, the enzymatic response of B. tabaci to combined matrine and L. muscarium treatment displays a reduction in CarE, GSTs and CHI during the initial infection period, whereas SOD, POD and CAT activities decreased during the later infection period. The changes in enzymatic activities suggest that the probability of L. muscarium infection was increased by matrine and L. muscarium which in turn enervated the whitefly defence against matrine. Based on the results of current and previous studies, we can hypothesize that the enzymatic defence of B. tabaci is activated by the assault of either matrine or L. muscarium and that the combination of these two agents can overcome this defence strategy through a strong synergistic effect. The chemical basis of this strong synergistic effect is possibly related to the disturbance of the acetylcholine balance and changes in AchE activities of the whitefly as both matrine and L. muscarium can target insect acetylcholine (Ach) receptors which in turn effects AchE production24,40. Therefore, our results have revealed the complex biochemical processes involved in the synergistic action of matrine and L. muscarium against B. tabaci. These findings will be helpful in designing effective means of integrated whitefly control in the future.
Materials and Methods
Insect cultures
MEAM1 whitefly were collected in Guangzhou from cotton plants and reared at the Engineering Research Center of Biological Control, Ministry of Education, South China Agricultural University. The MEAM1 B. tabaci was identified by using mitochondrial COI (mtCOI) sequencing as described by Khasdan et al.45. MEAM1 B. tabaci was reared in a glasshouse on Gossypium hirsutum (Malvaceae), Lu-Mian 32 under the following conditions 26 ± 1 °C, 70 ± 10% R.H. and 14:10-h light: dark photoperiod.
Fungus and insecticides
For all assays, the fungal strain L. muscarium V20 originally isolated from Trialeurodes sp., deposited to the collection at Key laboratory of biopesticides innovation and application of Guangdong Province, South China Agricultural University, Guangzhou, P.R. China was used during these studies.
Matrine powder (95%) was provided by Guangdong New Scene Bioengineering, Yangjiang, China. Preliminary trials conducted to test the possible influence of matrine on fungal growth and fungal enzymes showed no positive or negative effect on fungal growth and enzyme production (Ali et al. unpublished data).
Bioassays
Bioassay I: Efficacy of L. muscarium against B. tabaci
Five different conidial concentrations of L. muscarium (1 × 104, 1 × 105, 1 × 106, 1 × 107 and 1 × 108 conidia/ml) were prepared by following the method of Ali et al.46. The fungal conidia cultured on Potato Dextrose Agar (PDA) were harvested with deionized water containing 0.01% Tween 80 and sieved using filter paper (Whatman No. 2; Science Kit & Boreal Laboratories, New York, NY, USA) into sterile vials. Conidia were counted using a compound microscope and a hemocytometer (0.0625 m2; Fuchs-Rosenthal Merck Euro Lab, Darmstadt, Germany) to calibrate a suspension of 1 × 108 conidia/ml. Spore viability was determined before preparation of suspension by spreading 0.2 ml of suspension on PDA and estimating the number of germinated propagules after 24 h of incubation at room temperature. Spores were considered viable when the germ tube length was equal to or greater than the width. The viability of conidia was assessed immediately before each experiment was started and germination was estimated to >95% for all experiments. The conidial suspension (1 × 108 conidia/ml) was used as a stock solution and lower concentrations (1 × 107, 1 × 106, 1 × 105 and 1 × 104 conidia/ml) were prepared through serial dilutions by using deionized water containing 0.01% Tween 80.
Newly molted 2nd instar B. tabaci nymphs were treated with different concentrations by dipping the infested leaves47 in each conidial concentration for 30 s, and then removing them to air dry before being transferred to clean glass petri dishes (ø 9 cm). Control insect groups were treated with deionized water containing 0.01% Tween 80. A piece of filter paper was placed at the bottom of the dish with 200 μl water for moisture maintenance. Each treatment (each conidial concentration) and control was repeated three times with fresh batch of insects and fresh conidial suspension. Each repetition contained four leaves with 100 individuals per leaf. Mortality was recorded at 24 h intervals for 8 days. Infected nymphs were identified by their red colour and later on by outgrowth of mycelia while naturally dead nymphs were yellowish in colour with flattened bodies. The cadavers were removed and cultured separately at 26 °C and >90% R.H. to observe the fungal sporulation. If sporulation was observed, death was considered as a result of L. muscarium infection.
To obtain L. muscarium infected whitefly nymphs for enzymatic studies, B. tabaci nymphs were treated with three conidial concentrations (1 × 106, 1 × 107 and 1 × 108 conidia/ml) by following the method used above.
Bioassay II: Efficacy of matrine against B. tabaci
Matrine (95% pure) was dissolved in ddH2O to prepare a stock solution of 50 mg/L. Five different concentrations of matrine (15 mg/L, 5 mg/L, 0.5 mg/L, 0.05 mg/L and 0.005 mg/L) were prepared by serial dilutions. Newly molted 2nd instar B. tabaci nymphs were treated with different concentrations by dipping the infested leaves in each concentration for 30 s, and then removing them to air dry before being transferred to clean glass petri dishes (ø 9 cm). Control insect groups were treated with ddH2O. A piece of filter paper was place at the bottom of the dish with 200 μl water for moisture maintenance. Each treatment (each concentration) and control was repeated three times with a fresh batch of insects. Each repetition contained four leaves with 100 individuals per leaf. Mortality was recorded at 24 h intervals for 8 days. Infected nymphs were identified by their red colour while naturally dead nymphs were yellowish in colour with flattened bodies. To obtain insecticide challenged whitefly nymphs for enzymatic studies, B. tabaci nymphs were treated with three concentrations of matrine (5, 0.5 and 0.05 mg/L) by following the method used above.
Bioassay III: Efficacy of L. muscarium combined with matrine against B. tabaci
Lecanicillium muscarium was combined with matrine in four dose combinations for each of three different proportions (Table 3). Newly molted 2nd instar B. tabaci nymphs were treated with different concentrations by again dipping the infested leaves in each concentration for 30 s, and then removing them to air dry before being transferred to clean glass petri dishes (ø 9 cm). Control insect groups were treated with deionized water containing 0.01% Tween 80. A piece of filter paper was placed at the bottom of the dish with 200 μl water to maintain moisture. Each treatment (each dose combination) and control was repeated three times with both a fresh batch of insects and conidial suspension. Each repetition contained four leaves with 100 individuals per leaf. Mortality was recorded at 24 h intervals for 8 days. Infected nymphs were identified by their red colour and later on by outgrowth of mycelia while naturally dead nymphs were yellowish in colour with flattened bodies. To obtain L. muscarium + matrine challenged whitefly nymphs for enzymatic studies, B. tabaci nymphs were treated with three dose combinations (5 mg/L + 1 × 108 conidia/ml; 0.5 mg/L + 1 × 107 conidia/ml; and 0.05 mg/L + 1 × 106 conidia/ml) by following the method used above.
Table 3. Different concentrations of Lecanicillium muscarium and matrine used in the experiments.
| Bioassay I | Bioassay II | Bioassay III |
Bioassay IV |
||||||
|---|---|---|---|---|---|---|---|---|---|
| L. muscarium (conidia/mL) | Matrine (mg/L) | CT1 (Mt* + Lm**2:8) | CT2 (Mt + Lm 5:5) | CT3 (Mt + Lm 8:2) | CT4 (Mt + Lm) | ||||
| 1 × 104 | 0.005 mg/L | 0.001 | 0.8 × 105 | 0.0025 | 0.5 × 105 | 0.004 | 0.2 × 105 | 0.005 | 1 × 105 |
| 1 × 105 | 0.05 mg/L | ||||||||
| 1 × 106 | 0.5 mg/L | 0.01 | 0.8 × 106 | 0.025 | 0.5 × 106 | 0.04 | 0.2 × 106 | 0.05 | 1 × 106 |
| 1 × 107 | 5 mg/L | 0.1 | 0.8 × 107 | 0.25 | 0.5 × 107 | 0.4 | 0.2 × 107 | 0.5 | 1 × 107 |
| 1 × 108 | 15 mg/L | 0.75 | 0.8 × 108 | 1.88 | 0.5 × 108 | 4.5 | 0.2 × 108 | 5 | 1 × 108 |
*Mt = Matrine, **Lm = Lecanicillium muscarium.
Bioassay IV: Susceptibility of B. tabaci to matrine after 24 h exposure to L. muscarium
Newly molted 2nd instar B. tabaci nymphs were treated with different L. muscarium concentrations as shown in Table 3 and as described above. Treated nymphs on leaves were placed into clean glass petri dishes (ø 20 cm). A piece of filter paper was placed at the bottom of the dish with 200 μl of water to maintain moisture. After 24 h, leaves bearing whitefly nymphs infected with L. muscarium were dipped in different concentrations of matrine (shown in Table 3) for 30 s. Each treatment (each dose combination) and control was repeated three times with both a fresh batch of insects and conidial suspension. Each repetition contained four leaves with 100 individuals per leaf. Mortality was recorded at 24 h intervals for 8 days. Infected nymphs were identified by their red colour and later on by outgrowth of mycelia while naturally dead nymphs were yellowish in colour with flattened bodies.
Enzyme preparation
Second instar whitefly nymphs (100 individuals) inoculated with different doses of L. muscarium, matrine, and L. muscarium + matrine dose-combinations were removed from leaf surfaces on a daily basis for a period of 7 days. Collected nymphs were homogenized with ice cold 0.05 M Sodium phosphate buffer at different pH levels as follows: pH 7.0 (AchE and CarE: 1.5 ml PBS), pH 7.3 (SOD, POD and CAT: 1.5 ml PBS) and pH 7.5 (CHI and GSTs: 1.5 ml PBS). The homogenized samples were centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatants were transferred to new tubes and centrifuged at 12,000 rpm for 15 min at 4 °C. The final supernatants were used as the enzyme preparation for the different enzyme and protein assays.
Protein assay
Total protein contents of supernatants from insect homogenates were quantified following Bradford48 by using bovine albumin serum (BSA) as standard.
Enzyme activity assays
Carboxylestrease (CarE) was quantified by using 1- naphthyl acetate as substrate49. The reaction mixture (0.45 ml 0.05 mol/L sodium phosphate buffer, 1.8 ml 0.00003 mol/L 1- naphthyl acetate and 0.05 ml sample) was incubated in water at 30 °C for 15 min followed by addition of 0.9 ml 1% fast blue RR salt to terminate the reaction. The change in absorbance was measured at 600 nm. Enzyme activity was determined by the protein changes per unit over time.
The glutathione S- transferase (GSTs) was conducted using the procedures developed by Habig50. The changes in absorbance were measured at 340 nm. The enzyme activity was measured by protein changes per unit over time (U/mg per min).
Acetylcholinestrase (AChE) activity was assayed by following Ellman et al.51 with some slight modifications. The reaction mixture contained 50 μL sample solution, 100 μL 45 μM 5-5-dithiobis-(2-nitrobenzoic acid), 100 μL acetylthiocholine iodide and 90 μL sodium phosphate buffer. The change in absorbance at 405 nm was recorded for 40 min. Enzyme activity was calculated as the rate of absorbancechange per mg protein (mOD/min/mg).
Superoxide dismutase (SOD) activity was measured in supernatants by nitro blue tetrazolium (NBT) reduction52. One unit of SOD activity was defined as the amount of SOD required for inhibition of the reduction of NBT by 50% (A560) and was expressed as units per mg protein (U/mg protein).
Catalase activity was assayed by the method described by Beers & Sizer53, in which the decomposition of hydrogen peroxide (H2O2) was analyzed spectrophotometrically at 240 nm. One unit of catalase activity was defined as the amount of enzyme that decomposes1 mmol H2O2/min at an initial H2O2 concentration of 30 mM at pH 7.0 and 25 °C.
The activity of peroxidase (POD) was assessed by following the method of Simon et al.54. The changes in absorbance were measured at 470 nm. Enzyme activity was determined by the protein changes per unit (U/min per min).
The chitinase (CHI) activity in the culture supernatant was estimated as described earlier using acid swollen chitin as the substrate. To prepare acid swollen chitin, the chitin (10 g), was suspended in chilled O-phosphoric acid (88%, w/v) and left at 0 °C for 1 h with stirring. The acid swollen chitin was repeatedly washed with chilled distilled water, followed with a 1% (w/v) NaHCO3 solution which was further dialyzed against cold distilled water. After homogenization in a Waring® blender (1 min), 50 mM acetate buffer, pH 5.0, was added to the suspension so that 1 ml of suspension contained 7 mg of chitin. The reaction mixture for the chitinase assay contained 1 ml 0.7% acid swollen chitin, 1 ml 50 mM acetate buffer, pH 5.0 and 1 ml enzyme solution that was incubated at 50 °C for 1 h. The N-acetylglucosamine (GlcNAc) produced was estimated colorimetrically with p-dimethyl amino benzaldehyde (DMAB)55. One international unit was defined as the activity that produced 1 μmol of GlcNAc per min.
Data analysis
Median lethal concentration (LC50) values for each bioassay were calculated through probit analysis in SAS 9.1 software for windows56. LC50 values for different bioassays were subjected to one –way ANOVA at 5% level of significance followed by mean comparison through Tukey’s HSD test.
Coefficients of co-toxicity were calculated by following Sun & Johnson57. The co-toxicity coefficients for the mixed formulation were calculated after calculating the LC50 of each component in the mixture. Calculations were performed by using the following equations (Equations 1) described by Jia et al.23:
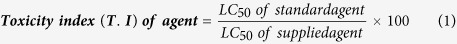 |
 |
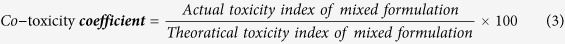 |
Co-toxicity coefficient greater than 100 showed a synergistic effect while the mixture with co-toxicity coefficient less than 100 corresponded to antagonistic effect. A Co-toxicity coefficient of 100 indicated that the effect of the mixture was similar to that predicted from the proportions of the two components.
In the figures presented, each point represents the average x-fold change in values from five sub-replicates of enzyme activities compared with the control.
Additional Information
How to cite this article: Ali, S. et al. Toxicological and biochemical basis of synergism between the entomopathogenic fungus Lecanicillium muscarium and the insecticide matrine against Bemisia tabaci (Gennadius). Sci. Rep. 7, 46558; doi: 10.1038/srep46558 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This research was funded by the grants from Science and Technology Program of Guangzhou, P.R. China (201509010023), the Development of Guangdong Engineering Research Centre of Microbial Pesticides (2015B090903042), the National Department Public Benefit Research Foundation (201303019) and the Science and Technology Program of Guangzhou, P.R. China (201604020180).
Footnotes
The authors declare no competing financial interests.
Author Contributions S.A. and B.Q. designed the study. S.A., C.Z., Z.S., X.W. and J.W. performed all experiments. S.A., Z.W., analyzed data and wrote the manuscript. A.C. edited the manuscript. All authors read and approved the manuscript.
References
- Barbosa L. D. et al. Indigenous American species of the Bemisia tabaci complex are still widespread in the Americas. Pest Manag. Sci. 70, 1440–1445 (2014). [DOI] [PubMed] [Google Scholar]
- De Barro P. J., Liu S.-S., Boykin L. M. & Dinsdale A. B. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19 (2011). [DOI] [PubMed] [Google Scholar]
- Oliveira M. R. V., Henneberry T. J. & Anderson P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 20, 709–723 (2001). [Google Scholar]
- Perring T. M. The Bemisia tabaci species complex. Crop Prot. 20, 725–737 (2001). [Google Scholar]
- Lapidotand M. & Polston J. Biology and epidemiology of Bemisia–vectored viruses in Bemisia: Bionomics and Management of a Global Pest (eds Stansly P. & Naranjo S.) 233–339 (Springer, 2010). [Google Scholar]
- Liang P., Tian Y.-A., Biondi A., Desneux N. & Gao X. W. Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicol. 21, 1889–1898 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Z., Yan H., Yang Y. & Wu Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China. Pest Manag. Sci. 66, 1360–1366 (2010). [DOI] [PubMed] [Google Scholar]
- Devine G. J. & Furlong M. J.. Insecticide use: Contexts and ecological consequences. Agri. Hum. Val. 24, 281–306 (2007). [Google Scholar]
- Revathi K., Chandrasekaran R., Thanigaivel A., Kirubakaran S. A. & Nathan S. S. Biocontrol efficacy of protoplast fusants between Bacillus thuringiensis and Bacillus subtilis against Spodoptera litura Fabr. Arch. Phytopathol. Plant Prot. 47, 1365–1375 (2014). [Google Scholar]
- Zare R. & Gams W. A revision of Verticillium section Prostrata IV. The genera Lecanicillium and Simplicillium gen nov. Nova. Hedwigia. 73, (1–2), 1–50 (2001). [Google Scholar]
- Fatiha L., Huang Z., Ali S. & Ren S. X. Effect of Lecanicillium muscarium on Eretmocerus sp. nr.furuhashii (Hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (Hemiptera: Aleyrodidae). J. Pest Sci. 82, 27–32 (2009). [Google Scholar]
- Ren S. X., Ali S., Huang Z. & Wu J. H. Lecanicillium muscarium as microbial insecticide against whitefly and its interaction with other natural enemies. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial BiotechnologyVol. 1 (eds Méndez-Vilas A.) 339–348 (Formatex, 2010). [Google Scholar]
- Wang L., Huang J., You M. & Liu B. Time-dose-mortality modeling and virulence indices for six strains of Verticillium lecanii against sweetpotato whitefly, Bemisia tabaci (Gennadius). J. Appl. Entomol. 128, 494–500 (2004). [Google Scholar]
- Mao L. & Henderson G. Antifeedant activity and acute and residual toxicity of alkaloids from Sophora flavescens (Leguminosae) against Formosan subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 100, 866–870 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang S. et al. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and akt inactivation. PLoS One 7, 1–11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. L., Goh S. H. & Ho S. H. Screening of Chinese medicinal herbs for bioactivity against Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J. Stored Prod. Res. 43, 290–296 (2007). [Google Scholar]
- Wang Y. L., Guan Z. G., Jia X. S., Wu S. Y. & Wei H. G. Study progress of matrine application in farming pest control. J. Shanxi Agric. Sci. 40, 424–428 (2007). [Google Scholar]
- Zanardi O. Z. et al. Bioactivity of a matrine-based biopesticide against four pest species of agricultural importance. Crop Prot. 67, 160–167 (2015). [Google Scholar]
- Bakr E. M., Soliman Z. R., Hassan M. F. & Tawadrous S. S. D. Biological activity of the organic pesticide Baicao No. 1 against the red spider mite Tetranychusurticae Koch. Acarines 6, 35–39 (2012). [Google Scholar]
- Hwang I. C. et al. Evaluation of toxicity of plant extract made by neem and matrine against main pests and natural enemies. Korean. J. Appl. Entomol. 48, 87–94 (2009). [Google Scholar]
- Dubovskiy I. M. et al. The activity of nonspecific esterases and glutathione- S-transferase in Locusta migratoria larvae infected with the fungus Metarhizium anisopliae (Ascomycota, Hypocreales). Entomol. Rev. 92, 27–31 (2012). [Google Scholar]
- Li X., Schuler M. A. & Berenbaum M. R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 52, 231–253 (2007). [DOI] [PubMed] [Google Scholar]
- Jia M. et al. Biochemical basis of synergism between pathogenic fungus Metarhizium anisopliae and insecticide chlorantraniliprole in Locusta migratoria (Meyen). Sci. Rep. 6, 28424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibaee A., Bandani A. R. & Tork M. Effect of the entomopathogenic fungus, Beauveria bassiana, and its secondary metabolite on detoxifying enzyme activities and acetylcholinesterase (AChE) of the Sunn pest, Eurygaster integriceps (Heteroptera: Scutellaridae). Biocontrol Sci. Techn. 19, 485–498 (2009). [Google Scholar]
- Xing J., Liang P. & Gao X. W. Effects of sublethal concentrations of chlorantraniliprole on insecticide susceptibility and detoxifying enzyme activity in Plutella xylostella. Chinese J. Pestic. Sci. 13, 464–470 (2011). [Google Scholar]
- Nakajyo S. et al. On the inhibitory mechanism of bassianolide, a cyclodepsipeptide, in acetylcholine-induced contraction in guinea pig taenia coli. Jap. J. Pharmacol. 33, 573–582 (1983). [DOI] [PubMed] [Google Scholar]
- Xu Y. Q., Orozco R., Wijerante E. M. K., Espinosa-Artiles P., Lesile Gunatilaka A. A., Stock S. P. & Molnar I. Biosynthesis of the cyclooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana. Fungal Gen. and Biol. 46, 353–364. [DOI] [PubMed] [Google Scholar]
- Cuthbertson A. G. S. Update on the status of Bemisia tabaci in the UK and the use of entomopathogenic fungi within eradication programs. Insects, 4, 198–205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson A. G. S. & Walters K. F. A. Pathogenecity of the entomopathogenic fungus, Lecanicillium muscarium, against the sweetpotato whitefly, Bemisia tabaci, under laboratory and glasshouse conditions. Mycopathol. 160, 315–319 (2005). [DOI] [PubMed] [Google Scholar]
- Cuthbertson A. G. S., Blackburn L. F., Northing P., Luo W., Cannon R. J. C. & Walters K. F. A. Further compatability tests of the entomopathogenic fungus, Lecanicillium muscarium with conventional insecticide products for control of sweetpotato whitefly, Bemisia tabaci on poinsettia plants. Insect Sci. 15, 355–360 (2008). [Google Scholar]
- Sharififard M., Mossadegh M. S., Vazirianzadeh B. & Zarei-Mahmoudabadi A. Interactions between Entomopathogenic fungus, Metarhizium anisopliae and sublethal doses of spinosad for control of house fly, Musca domestica. Iran. J. Arthropod-Bor. 5, 28–36 (2011). [PMC free article] [PubMed] [Google Scholar]
- Claudianoc C. et al. A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 15, 615–636 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W. C. & Zhang Q. The effects of Sophora alopecuroids alkaloids on metabolic esterases of the diamondback moth. Acta. Entomol. Sin. 46, 122–125 (2003). [Google Scholar]
- Luo W. C., Li Y. S. & Mu L. Y. The toxicities of alkaloids from Sophora alopecuroids against turnip aphids and effects on several estereases. Acta Entomol. Sin. 40, 358–365, (in Chinese) (1997). [Google Scholar]
- Tian J., Diao H. L., Liang L., Hao C., Arthurs S. & Ma R. Y. Pathogenicity of Isaria fumosorosea to Bemisia tabaci, with some observations on the fungal infection process and host immune response. J. Inverteb. Pathol. 130, 147–153 (2015). [DOI] [PubMed] [Google Scholar]
- Hall I. M. Microbial Control in Insect Pathology An Advanced Treatise (ed. Steinhaus E. A.) 477–511 (Academic, 1963). [Google Scholar]
- Furlong M. J. & Groden E. Evaluation of synergistic interactions between the Colorado potato beetle (Coleoptera: Chrysomelidae) pathogen Beauveria bassiana and the insecticides, imidacloprid, and cyromazine. J. Econ. Entomol. 94, 344–356. [DOI] [PubMed] [Google Scholar]
- Bourne Y., Sharpless K. B., Taylor P. & Marchot P. Steric and dynamic parameters influencing in situ cyclo additions to form triazole inhibitors with crystalline acetylcholinestrase. J. Amer. Chem. Soc. 138, 1611–1621 (2016). [DOI] [PubMed] [Google Scholar]
- Senthil N. S. et al. Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown planthopper Nilaparvata lugens (Stål). Ecotox. Environ. Safe. 70, 244–250 (2008). [DOI] [PubMed] [Google Scholar]
- Liu L. J., Alam M. S., Hirata K., Matsuda K. & Ozoe Y. Actions of quinolizidine alkaloids on Periplanta Americana nicotinic acetylcholine receptors. Pest Manag. Sci. 64, 1222–1228 (2008). [DOI] [PubMed] [Google Scholar]
- Ali S., Wu J. H., Huang Z. & Ren S. X. Production and regulation of extracellular chitinase from the entomopathogenic fungus Isaria fumosorosea. Biocont. Sci. Technol. 20, 723–738 (2010). [Google Scholar]
- Wang X. et al. Characterization of a 46 kDa insect chitinase from transgenic tobacco. Insect Biochem. Molec. 26, 1055–1064 (1996). [Google Scholar]
- Ding S. Y., Li H. Y., Li X. F. & Zhang Z. Y. Effects of two kinds of transgenic poplar on protective enzymes system in the midgut of larvae of American white moth. J. For. Res. Jpn. 12, 119–122 (2001). [Google Scholar]
- Felton G. Antioxidant defenses of invertebrates and vertebrates in Oxidative Stress and Antioxidant Defenses in Biology (ed. Ahmad S.) 356–434 (Springer, 1995).
- Khasdan V. et al. DNA markers for identifying biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) and studying population dynamics. Bull. Entomol. Res. 95, 605–613 (2005). [DOI] [PubMed] [Google Scholar]
- Ali S., Huang Z. & Ren S. X. Media composition influences on growth, enzyme activity and virulence of the entomopathogen hyphomycete Isaria fumosoroseus. Entomol. Exp. Appl. 131, 30–38 (2009). [Google Scholar]
- Cuthbertson A. G. S., Blackburn L. F., Northing P., Luo W., Cannon R. J. C. & Walters K. F. A. Leaf dipping as an environmental screening measure to test chemical efficacy against Bemisiatabaci on poinsettia plants. Inter. J. Environ. Sci. Tech. 6, 347–352 (2009). [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- Byrne F. J., Gorman K. J., Cahill M., Denholm I. & Devonshire A. L. The role of B-type esterases in conferring insecticide resistance in the tobacco whitefly, Bemisia tabaci (Genn). Pest Manag. Sci. 56, 867–874 (2000). [Google Scholar]
- Habig W. H., Assays for differentiation of glutathione S-transferase In Method in Enzymology (eds Willian B. J.) 398–405 (Academic Press, 1981). [DOI] [PubMed] [Google Scholar]
- Ellman G. L., Coutney K. D., Andre V. & Featherstone R. M. A new and rapid calorimetric determination of acetylcholinestrase activity. Biochem. Pharmacol. 7, 88–95 (1961). [DOI] [PubMed] [Google Scholar]
- Beauchamp C. & Fridovich I. Superoxide dismutase-improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 (1971). [DOI] [PubMed] [Google Scholar]
- Beers R. F. & Sizer I. W. A Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140 (1952). [PubMed] [Google Scholar]
- Simon L. M., Fatrai Z., Jonas D. E. & Matkovics B. Study of metabolism enzymes during the development of Phaseolus vulgaris L. Physiol. der Pflanzen. 166, 387–392 (1974). [Google Scholar]
- Reissing J. L., Stornminger J. L. & Leloir L. F. A modified colorimetric method for the estimation of N-acetylamin sugars. J. Biol. Chem. 217, 959–966 (1955). [PubMed] [Google Scholar]
- SAS Institute. SAS user’s guide. StatisticsSAS Institute, Cary, North Carolina, USA (2000) [Google Scholar]
- Sun Y. P. & Johnson E. R. Analysis of joint action of insecticides against houseflies. J. Econ. Entomol. 53, 887–892 (1960). [Google Scholar]