Abstract
CrkII is an adaptor protein possessing oncogenic potential despite the lack of an enzymatic domain. We investigated here the physiological functions of CrkII by studying its ability to induce anchorage-independent cell growth. We found that inhibition or null mutation of focal adhesion kinase (FAK) blocked the anchorage-independent growth induced by CrkII overexpression, indicating that FAK is a critical determinant of the transforming activity of CrkII. CrkII overexpression enhanced the autophosphorylation of FAK at Tyr-397 and tyrosine phosphorylation of p130Cas (Crk-associated substrate, Cas) upon stimulation of integrin by fibronectin. Moreover, the constitutive phosphorylation of FAK and Cas was observed in CrkII-overexpressing cells, even when they were in the suspended condition, consistent with the ability of CrkII to induce anchorage-independent growth. Using Cas-deficient cells, we showed Cas function to be essential for both the CrkII-induced phosphorylation of FAK (Tyr-397) and anchorage-independent cell growth. The CrkII-induced FAK autophosphorylation depended upon CrkII–Cas complex formation. Furthermore, we showed that CrkII knockdown resulted in defects in integrin-mediated events, such as cell spreading, haptotactic migration, and FAK autophosphorylation. The integrin-mediated FAK autophosphorylation was also reduced in Cas-deficient cells. These results suggest that the CrkII–Cas complex functions in integrin-mediated FAK activation signaling. Our findings show the importance of CrkII in integrin-mediated events, acting upstream of FAK to affect the activation of this kinase, which appears to have a central role in this pathway.
Keywords: anchorage-independent growth, integrin, tyrosine phosphorylation
The adaptor protein CrkII, which contains one Src homology (SH) 2 and two SH3 domains, was originally identified as a protooncogene product of the avian sarcoma virus, v-Crk (1). Our recent studies showed that although CrkII is a cellular protein, it can induce anchorage independent growth of NIH 3T3 cells when it is overexpressed in them (2). In fact, it has been reported that increased expression of cellular Crk is associated with human cancers, such as lung adenocarcinomas with an aggressive phenotype and is involved in migration and invasiveness in glioblastomas (3–5). Because Grb2, which is another SH2-SH3 adaptor protein and a well-known critical mediator of proliferation signals, does not show such activity (2), the oncogenic potential of CrkII is likely due to its unique physiological function(s). In this study, we wish to clarify which of the cellular interactions mediated by CrkII is most critical in relation to such oncogenic activity.
CrkII is considered to be involved in integrin-mediated signals because the focal adhesion components p130Cas (Crk-associated substrate, Cas) and paxillin can couple to the Crk-SH2 domain (6, 7), and these CrkII–Cas complexes regulate integrin-mediated cell migration (8, 9). Therefore, it has been speculated that integrin-mediated signals are involved in the oncogenic activity of CrkII. The transmembrane integrins mediate the adhesion of a cell to the extracellular matrix, which regulates cell growth, migration, and survival (10). Integrin-mediated cell adhesion creates many of the intracellular signals in which focal adhesion kinase (FAK) plays a central role. Upon integrin stimulation, FAK is rapidly autophosphorylated at its Tyr-397, an event that is correlated with its increased catalytic activity (11) and provides a binding site for SH2-containing proteins such as the Src family kinases (SFKs) (12–15). According to the current hypothesis, SFK is recruited to the phosphorylated Tyr-397 of FAK and then further phosphorylates FAK on Tyr-407, Tyr-576/Tyr-577, Tyr-863, and Tyr-925 (16). Phosphorylation at Tyr-576/Tyr-577, located in the activation loop of the catalytic domain, has been shown to enhance the kinase activity of FAK (11, 17). FAK and SFK also mediate the phosphorylation of FAK-binding proteins such as Cas and paxillin, which can recruit other signaling molecules, thus leading to multiple downstream signaling pathways (7, 18, 19). The molecular mechanisms that regulate the early events in FAK autophosphorylation processes and connect the focal adhesion molecules to the clustered integrins are unclear.
Whereas CrkII is believed to act as a downstream mediator of the tyrosine-phosphorylated focal adhesion molecules, v-Crk-expressing cells showed strong tyrosine phosphorylation of FAK (20–23). However, it is still unclear whether FAK is essential for the Crk-induced transformation. In this study, we demonstrated that FAK is a critical determinant of the transforming activity of CrkII. CrkII overexpression promoted FAK autophosphorylation even with the cells in the suspended condition. In addition, CrkII knockdown abolished integrin-mediated responses, including the FAK phosphorylation. We also found that Cas was involved in CrkII regulation of FAK phosphorylation. Our findings point to a site of physiological function of cellular CrkII upstream of FAK activation.
Materials and Methods
Plasmids and Antibodies. Flag-tagged CrkII (Flag-CrkII) (2) and v-Src were subcloned into the retroviral vector pCX4bsr (24). cDNAs of HA-tagged WT and mutant FAK (Y397F, Y407F, Y576/Y577F, mPR1.2, and KD) were kindly provided by S. Hanks (Vanderbilt University, Nashville, TN) (11). FAK-related nonkinase (FRNK) cDNA was created by PCR with WT FAK as a template. The FAK and FRNK cDNAs were subcloned into the retroviral vector pCX4puro. cDNAs of Cas and Cas mutant with a deleted substrate domain (ΔSD) were kindly provided by H. Hirai (University of Tokyo, Tokyo) (25). To make the Cas mutant with a deleted SH3 domain (ΔSH3) construct, we deleted the nucleotides corresponding to amino acid residues 10–59 by PCR. The Cas constructs were subcloned into pCX4puro. The following antibodies were purchased: anti-Crk (Transduction Laboratories, Lexington, KY); anti-Cas, anti-FAK, and anti-CrkL (Santa Cruz Biotechnology); anti-phospho-FAK Y397 and Y577 (BioSource International, Camarillo, CA); anti-HA (3F10, Roche Molecular Biochemicals); and anti-pTyr (4G10, Upstate Biotechnology, Lake Placid, NY).
Cell Culture. NIH 3T3 and HeLa cells were maintained in DMEM containing 5% calf serum and 10% FCS, respectively. The FAK-deficient fibroblasts and Cas-deficient fibroblast cell lines (kindly donated by T. Yamamoto, University of Tokyo; M. Hamaguchi, Nagoya University, Nagoya, Japan; and H. Hirai, University of Tokyo, respectively) were cultured as described in refs. 26 and 27.
Cell Culture in Anchorage-Free Semisolid Medium. Cells were maintained in DMEM containing 1.5% methylcellulose, supplemented with 10% FCS, and collected by centrifugation as described by Assoian et al. (28) with some modification.
Retroviral Expression. Ecotropic retroviruses were produced by transient transfection of Plat-E cells (29) with the viral vectors as described in ref. 2.
Soft-Agar Colony Formation Assay. For the soft-agar colony formation assay, cells were plated into a soft-agar matrix and incubated for 3–4 weeks as described in ref. 24.
Immunoprecipitation and Immunoblotting. Cells were lysed in ice-cold RIPA buffer [50 mM Tris (pH 7.4) containing 1% Nonidet P-40, 0.1% SDS, 1% sodium deoxycholate, 150 mM NaCl, 5 mM EDTA, 50 mM NaF, 2 mM Na3VO4, 10 μg/ml each of leupeptin and aprotinin, and 1 mM PMSF]. The lysates were immunoprecipitated with the indicated antibodies for 2 h at 4°C and subsequently incubated with Protein G Sepharose (Amersham Pharmacia) for 1 h. The precipitated protein complexes were washed three times with lysis buffer without sodium deoxycholate or SDS and then suspended in Laemmli SDS/PAGE sample buffer. For immunoblotting, the samples were subjected to SDS/PAGE and transferred to an Immobilon membrane (Millipore) as described in ref. 2.
Cell Stimulation with Fibronectin. Cells were serum-starved overnight and harvested by 0.05% trypsin treatment. The treatment was stopped with 0.5 mg/ml soybean trypsin inhibitor in DMEM. After washing, cells were resuspended in DMEM and held in suspension for 1 h at 37°C. Culture dishes were coated (overnight, 4°C) with 10 μg/ml fibronectin (Chemicon). The suspended cells were then plated onto the fibronectin (FN)-coated dishes and incubated at 37°C for various times.
RNA Interference. To construct the retroviral vectors for expression of small interfering RNAs, we used pSUPER.retro (OligoEngine, Seattle). Chemically synthesized oligonucleotides (5′-gatccccGCCTGAAGAGCAGTGGTGGAATgtgtgctgtccATTC CACCACTGCTCTTCAGGCtttttggaaa-3′ and 5′-agcttttccaaaaaGCCTGAAGAGCAGTGGTGGAATggacagcacacATTCCACCACTGCTCTTCAGGCggg-3′); were annealed and ligated into the vector digested with BglII and HindIII. The 22-nt CrkII target sequences are indicated in capital letters in the oligonucleotide sequence.
Adhesion and Spreading Assay. For adhesion experiments, 96-well plates were coated with FN (10 μg/ml). Serum-starved cells were plated for the desired times. After washing, the adherent cells left in each well were estimated by using WST-1 reagent (Roche Molecular Biochemicals). For spreading analysis, serum-starved cells were plated onto FN-coated (10 μg/ml) dishes for the desired times and then chilled on ice for 15 min. Spread cells were determined by dark-phase counting from five fields.
Cell Migration. Cell migration assays were performed by using Transwell chambers (Costar) as described by Yano et al. (30). In brief, the undersurface of the polycarbonate membrane of the chambers was coated with FN (10 μg/ml in PBS). The lower chamber was filled with 400 μl of assay medium (0.2% BSA DMEM). Serum-starved cells were suspended in assay medium, and 8 × 104 of them in 0.1 ml were added to the upper chamber. After 3 h at 37°C, cells that had migrated to the undersurface of the upper chamber were fixed in 4% paraformaldehyde, stained with crystal violet, and counted.
Results
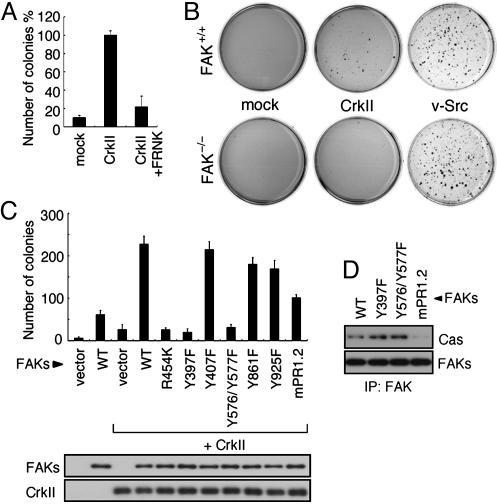
FAK Is Required for CrkII-Induced Anchorage-Independent Cell Growth. To determine the significance of FAK in CrkII-induced cell transformation, we infected NIH 3T3 cells with retroviruses expressing CrkII; some of the cultures were simultaneously infected with FRNK, which functions as a potent dominant-negative inhibitor of FAK activity (31, 32). As shown in Fig. 1A, FRNK expression markedly reduced the CrkII-induced colony formation in soft agar, suggesting that the FAK activity is required for the ability of CrkII to induce anchorage-independent cell growth. Moreover, CrkII did not induce colony formation by FAK-null fibroblasts (FAK–/–), although it did by the FAK+/+ control cells (Fig. 1B). In contrast, v-Src showed no difference in colony formation by either type of cell (Fig. 1B), indicating that FAK is not a critical determinant of v-Src-induced cell growth in soft agar, as reported in refs. 33–35. These results clearly show that FAK is essential for CrkII-induced anchorage-independent cell growth.
Fig. 1.
FAK activity is necessary for CrkII-mediated transformation. (A) NIH 3T3 cells expressing Flag-CrkII were infected with retroviruses expressing FRNK (CrkII+FRNK) or control vector (CrkII). Control cells were infected with empty vector alone (mock). The soft-agar colony formation assay was performed by plating the indicated cells into a soft agar containing 10% serum. Three weeks later, the numbers of colonies were counted. Bars represent the averages of three independent experiments. Error bars represent standard deviations. The number of colonies of CrkII cells was taken as 100%. (B) FAK+/+ or FAK–/– fibroblasts were infected with retrovirus expressing Flag-CrkII (CrkII), v-Src, or the control vector (mock) and subjected to the soft-agar colony formation assay as in A. Colonies were stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, and photographs were taken. Representative dishes from three independent experiments are shown. (C) FAK–/– fibroblasts were infected with retroviruses expressing the indicated combinations of HA-FAK constructs and CrkII and subjected to the soft-agar colony formation assay as in A. The expression levels of FAK mutants and CrkII in cell lysates were analyzed by immunoblotting with anti-FAK and anti-Crk antibody, respectively (Lower). (D) FAK–/– fibroblasts expressing the indicated FAK molecules were lysed with RIPA buffer. FAKs were immunoprecipitated from the lysates with anti-HA and then immunoblotted with anti-Cas (Upper) or anti-FAK (Lower).
Next, we introduced a series of FAK mutant into the CrkII-overexpressing FAK–/– cells and examined the soft-agar colony-forming activity of CrkII (Fig. 1C). Although the introduction of WT FAK (WT) into the FAK-null cells restored the CrkII-induced colony formation, a catalytically defective mutant of FAK mutated at the ATP-binding site (R454K) had no effect on the colony formation (Fig. 1C). Two mutants important for the catalytic activity of FAK, Tyr-397 (Y397F) and a double mutant for both Tyr-576 and Tyr-577 (Y576/Y577F) (11, 17) also could not restore the colony formation, either. The results obtained with these mutants (R454K, Y397F, and Y576/Y577F) thus indicate that the CrkII-induced anchorage-independent cell growth depended on the FAK kinase activity. In contrast, expression of other Tyr mutants of FAK, including the Grb2 binding-site mutant (Y925F), did not result in any significant decrease in the colony formation compared with the number for the WT.
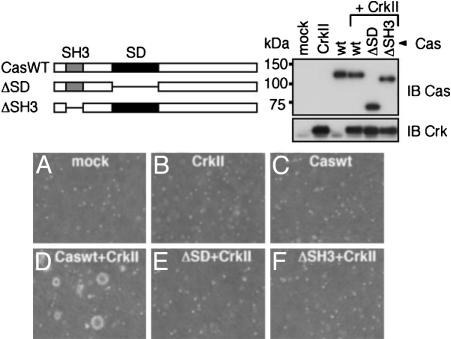
Cas Is Involved in CrkII-Induced Anchorage-Independent Cell Growth. Within its C-terminal region, FAK contains two proline-rich regions (PR-1 and PR-2), which are reported to be binding sites for the SH3 domain of Cas. Introduction of an mPR1.2 mutant of FAK, with mutations in both PR-1 and PR-2, into CrkII-overexpressing FAK–/– cells resulted in a 55.5% reduction in the number of colonies formed (Fig. 1C), and their size was smaller than that of the WT ones (data not shown). Because mPR1.2 showed no interaction with Cas (36) (Fig. 1D), this result suggests a critical role for Cas in the CrkII-induced anchorage-independent cell growth. To better clarify the involvement of Cas in the CrkII-induced anchorage independent cell growth, we next used Cas-null fibroblasts (Cas–/–). As shown in Fig. 2B, colony formation was not observed in Cas–/– cells overexpressing CrkII, but the colony-forming activity of CrkII was restored by the expression of WT Cas (Caswt) (Fig. 2D). This result indicates the requirement of Cas for the CrkII-induced anchorage-independent growth. In contrast, a Cas mutant having a deletion in its SH3 domain, which is required for binding to FAK (21, 37), failed to restore the colony formation (Fig. 2F). In addition, expression of a Cas mutant with a deleted SD, which consists of a cluster of YxxP motifs and offers binding sites for the Crk-SH2 domain in a phosphorylation-dependent manner (8, 38, 39), also failed to recover the colony formation (Fig. 2E). These results indicate that the presence of Cas with both its SH3 domain and substrate domain (SD) intact is essential for the anchorage-independent growth of cells overexpressing CrkII.
Fig. 2.
Cas is required for the CrkII-induced anchorage-independent growth. (Upper Left) Cas mutants are schematically shown (ΔSD and ΔSH3). (A–F) Cas–/– fibroblasts were infected with retroviruses expressing control (mock) (A), CrkII (B), WT Cas (Caswt) (C), Caswt and CrkII (D), ΔSD and CrkII (E), and ΔSH3 and CrkII (F) and subjected to the soft-agar colony formation assay as in Fig. 1. Images show typical fields at 40×.(Upper Right) The expression of Cas mutants and CrkII was detected by immunoblot analysis of cell lysates by using anti-Cas and anti-Crk, respectively.
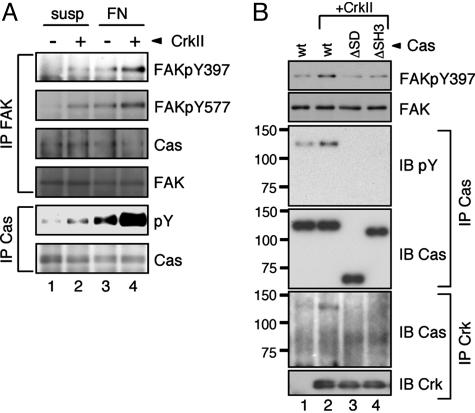
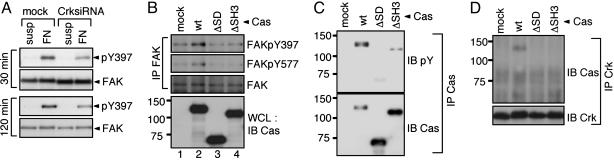
CrkII Overexpression Induces Tyrosine Phosphorylation of FAK and Cas Without Stimulation of Integrin. Because FAK activity is required for the anchorage-independent growth by CrkII, we next analyzed the effect of CrkII overexpression on the activation of FAK. We measured the status of FAK activation by monitoring its tyrosine phosphorylation at the Tyr-397 (pY397), the major autophosphorylation site of FAK, and the Tyr-577 (pY577) residue within the FAK domain. After cell attachment to FN, CrkII-overexpressing cells showed enhanced phosphorylation of FAK at both its Tyr-397 and the Tyr-577 compared with mock-infected NIH 3T3 cells (Fig. 3A, lanes 3 and 4). Moreover, constitutive phosphorylation of FAK at both sites was clearly observed in the CrkII cells, even in the absence of FN stimulation (Fig. 3A, lanes 1 and 2). The phosphorylation level of FAK in the suspended CrkII cells was comparable with that of the control cells attached to the FN (Fig. 3A, lanes 2 and 3). These results suggest that CrkII overexpression induces the constitutive activation of FAK in an anchorage-independent manner.
Fig. 3.
CrkII overexpression enhances FAK phosphorylations. (A) CrkII promotes tyrosine phosphorylations of FAK and Cas. NIH 3T3 cells expressing Flag-CrkII (+) or empty vector (–) were cultured in serum-containing medium in suspension (susp) or on FN-coated dishes (FN) for 30 min, harvested, washed, and lysed with RIPA buffer. FAK was immunoprecipitated with anti-FAK (IP FAK), and subsequently immunoblotted with site-specific anti-phospho-FAK Y397 (FAKpY397), Y577 (FAKpY577), anti-FAK (FAK), or anti-Cas (Cas). Cas was immunoprecipitated with anti-Cas (IP Cas) and then immunoblotted with anti-phosphotyrosine (pY) or anti-Cas. (B) Cas is involved in CrkII-induced phosphorylation of FAK Tyr-397. Cas–/– fibroblasts expressing the indicated combinations of Cas constructs and Flag-CrkII, described in Fig. 2, were cultured in suspension for 48 h in 1.5% methylcellulose-DMEM supplemented with 10% FCS. The cells were then harvested, washed, and lysed with RIPA buffer. The lysates were probed with anti-phospho-FAK Y397 (pY397) or anti-FAK (FAK). Cas immunoprecipitates (IP Cas) from the lysates were probed with 4G10 (IB pY) or anti-Cas (IB Cas). Crk immunoprecipitates (IP Crk) were probed with anti-Cas (IB Cas) or anti-Crk (IB Crk). The position of molecular mass markers (in kDa) is shown at left.
We confirmed that Cas was coprecipitated with endogenous FAK, as previously reported (Fig. 3A), indicating the formation of the FAK–Cas complex. However, there was no significant difference in the complex formation among cells under our experimental conditions. Upon stimulation of integrin, Cas is rapidly tyrosine phosphorylated and subsequently localized to sites of focal adhesion (37, 40–42). In CrkII cells, the FN-induced phosphorylation of Cas was markedly augmented compared with that in the control cells (Fig. 3A, lanes 3 and 4). In addition, even in the suspended condition, CrkII cells showed an increased level of tyrosine phosphorylation of Cas (Fig. 3A, lanes 1 and 2). Taken together, these results suggest that CrkII overexpression can bypass the requirement of cell attachment to FN for FAK activation and Cas phosphorylation.
Cas Is Involved in CrkII-Induced Anchorage-Independent Autophosphorylation of FAK. To address the mechanism of the CrkII-induced constitutive autophosphorylation of FAK, we investigated the functional role of Cas in the FAK phosphorylation by using Cas–/– cells expressing a series of Cas constructs as mentioned in Fig. 2. As shown in Fig. 3B, for the cells in suspension cultures, CrkII overexpression enhanced phosphorylation of FAK at Tyr-397 only in the cells expressing WT (wt) Cas (Fig. 3B, lane 2) but not in those expressing the ΔSD or ΔSH3 (Fig. 3B, lanes 3 and 4). These results are closely correlated with those for the soft-agar colony formation shown in Fig. 2. Under the same experimental conditions, the tyrosine phosphorylation level of ΔSD and ΔSH3 was much lower than that of wt-Cas (Fig. 3B, lanes 2–4). Furthermore, wt-Cas, but neither the ΔSD nor ΔSH3, was coprecipitated with the overexpressed CrkII (Fig. 3B). Taken together, these results suggest that the association of phosphorylated Cas with CrkII is critical for the CrkII-induced anchorage-independent phosphorylation of FAK.
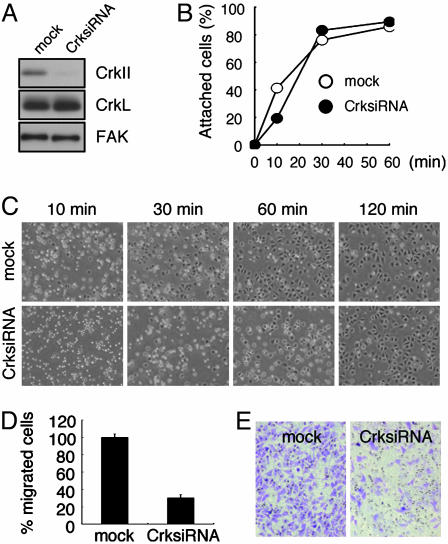
CrkII Is Required for Integrin Function. Our finding that CrkII overexpression enhanced the adhesion-dependent phosphorylation of FAK (Fig. 3A) raises another interesting possibility that CrkII might be physiologically implicated in the integrin-mediated signaling pathway leading to the activation of FAK. To investigate the physiological role of CrkII, we used a short interfering dsRNA approach to achieve knockdown of endogenous CrkII levels. Using a retroviral vector designed to express a short interfering dsRNA for CrkII, we stably inhibited the expression of endogenous CrkII in a bulk cell population of HeLa cells (Fig. 4A). Because FAK is a key mediator of the extracellular matrix-integrin signaling, which regulates cell spreading and migration, the CrkII-knockdown cells were assessed for their ability to spread and migrate. As shown in Fig. 4C, CrkII-knockdown cells showed drastically delayed cell spreading on FN compared with mock-infected cells. Most of the mock cells spread with polarized extension between 30 and 60 min. In contrast, the CrkII-knockdown cells were still round, and only a portion of the cells spread even at 60 min (Fig. 4C). The majority of CrkII-knockdown cells had eventually spread by 120 min (Fig. 4C). This delay in spreading on FN is also observed in FRNK-expressing cells and FAK–/– (17, 32, 43). The addition of serum could promote the cell spreading but not rescue the delayed spreading of the knockdown cells (data not shown). The CrkII-knockdown cells also showed reduced adhesion to FN at an early time point (Fig. 4B). However, the knockdown and control cells could no longer be distinguished from one another after 30 min (Fig. 4B), suggesting that cell adhesion was delayed but not blocked. The migration ability of the CrkII-knockdown cells was analyzed by measuring haptotactic migration toward FN in a Transwell chamber assay. The CrkII-knockdown cells showed significantly impaired migration (31%) relative to the control cells (Fig. 4 D and E), indicating a defect in responses to FN stimulation. Taken together, these results demonstrate that CrkII is required for integrin function.
Fig. 4.
CrkII knockdown results in impaired cellular responses on FN. HeLa cells expressing the murine ecotropic receptor were infected with a retrovirus expressing a short interfering dsRNA for CrkII (CrksiRNA) or the control vector (mock). Infected cells were subjected to drug selection, and the selected bulk populations were then used for the following experiments. (A) Infected cells were analyzed for CrkII, a closely related protein CrkL, or FAK expression by immunoblotting. (B and C) After serum starvation, the cells were replated on FN-coated dishes. (B) Adherent cells were estimated at the indicated times after replating. (C) Phase-contrast images (40×) were recorded at the indicated times to evaluate cell spreading. Representative fields from five experiments are shown. At 30 min, 65% of control cells were spread, whereas 25% of the knockdown cells were spread. At 60 min, 85% of control cells were spread, whereas 47% of the knockdown cells were spread. By 120 min, 95% of the cells were spread in all cultures. (D) The serum-starved cells were analyzed for migration toward FN. Bars represent the averages of three independent experiments plus or minus standard deviation. (E) Representative images (40×) of the FN-coated lower porous membrane surface from the cell migration assays. Cells were stained with crystal violet.
Regulation of Integrin-Stimulated Autophosphorylation of FAK by CrkII and Cas. To test whether a defect in FAK activation is involved in the above-mentioned delayed spreading and impaired migration, we next examined the integrin-stimulated FAK autophosphorylation in the CrkII-knockdown cells. As shown in Fig. 5A, upon FN-induced integrin activation, constitutively reduced phosphorylation of Tyr-397 was observed in the CrkII-knockdown cells compared with the level for the control cells. This result further indicates that CrkII is an important regulator of FAK phosphorylation, which is implicated in the regulation of cell spreading and migration.
Fig. 5.
Integrin-stimulated FAK Tyr-397 phosphorylation is impaired in CrkII-knockdown cells and in Cas-null cells. (A) Serum-starved CrkII-knockdown cells (CrksiRNA) and control cells (mock), described in Fig. 4, were kept in suspension (susp) or plated on FN-coated dishes for indicated times, harvested, and lysed with RIPA buffer. The lysates were probed with anti-phospho-FAK Y397 (pY397) or anti-FAK (FAK). (B–D) After serum-starvation, Cas–/– fibroblasts expressing the indicated Cas proteins were replated on FN-coated dishes for 30 min and lysed with RIPA buffer. (B) FAK immunoprecipitates from the lysates (IP FAK) were probed with anti-FAKpY397 (FAKpY397), anti-FAKpY577 (FAKpY577) or anti-FAK (FAK). Whole-cell lysates (WCL) were immunoblotted with anti-Cas (IB Cas). (C) Cas immunoprecipitates (IP Cas) from the same lysates were probed with 4G10 (IB pY) or anti-Cas (IB Cas). (D) Crk immunoprecipitates (IP Crk) were probed with anti-Cas (IB Cas) or anti-Crk (IB Crk).
Because the result shown in Fig. 3B suggests the involvement of Cas in the regulation of FAK phosphorylation by CrkII, we tested the possibility that Cas also plays an important role in integrin-stimulated FAK Tyr phosphorylation. As expected, after having been plated on FN, Cas–/– cells showed reduced FAK phosphorylation at both Tyr-397 and Tyr-577, compared with the null cells infected with wtCas (Fig. 5B, lanes 1 and 2). In contrast, introduction of neither the ΔSD nor the ΔSH3 into the null cells restored FAK phosphorylation at either site, showing that both the SD and the SH3 domains of Cas were critical for the integrin-induced FAK phosphorylation (Fig. 5B, lanes 3 and 4). Furthermore, consistent with the results shown in Fig. 3B, the FN-stimulated FAK phosphorylation could be correlated with Cas phosphorylation and CrkII–Cas complex formation (Fig. 5 C and D). These results strongly suggest that CrkII and Cas physiologically regulate the integrin-stimulated FAK activation through CrkII–Cas complex formation.
Discussion
v-Crk and CrkII overexpression lead to anchorage-independent but serum-dependent cell growth (44) (data not shown), suggesting that the Crk selectively activates an integrin-mediated signaling pathway in the transformation process. In addition, previous studies showed that hypertyrosine phosphorylation of FAK was observed in v-Crk-transformed cells (20–23), prompting us to test whether FAK is a critical determinant of the CrkII transformation. Here, by using a dominant-negative FAK mutant as well as FAK-null cells, we clearly showed that FAK activity was required for the anchorage-independent cell growth induced by CrkII overexpression. Furthermore, we found that even in the suspended condition, CrkII-overexpressing cells showed significant phosphorylation of FAK on its Tyr-397, the major autophosphorylation site, and on Tyr-577, located in the activation loop, indicating that FAK is constitutively activated. There are several lines of evidence suggesting that constitutive FAK activation plays an important role in cell transformation (45–47). The introduction of a constitutively activated variant of FAK was earlier shown to induce anchorage-independent growth and survival of MDCK cells (47). Moreover, increased expression and activation of FAK have been reported to correlate with increased cancer cell motility, invasion, and proliferation (48–51). In view of all of the data taken together, we propose that CrkII overexpression induces anchorage-independent growth by FAK activation bypassing the requirement of cell attachment to the extracellular matrix.
Because oncogenes are thought to be points on normal growth-signaling pathways that are constitutively activated by mutation or overexpression (52), the fact that CrkII-induced transformation totally depended on FAK, which is in stark contrast to v-Src-induced transformation, strongly suggests the role of CrkII as an upstream regulator of FAK activation. In fact, we showed that the CrkII knockdown reduced the integrin-stimulated FAK Tyr-397 autophosphorylation (Fig. 5A). In addition, as was observed for FAK-deficient cells and FRNK-expressing cells (17, 26, 32, 43, 53), CrkII-knockdown cells delayed cell spreading on FN and migrated poorly in response to haptotactic signals (Fig. 4), clearly demonstrating that CrkII is physiologically involved in the integrin-stimulated FAK activation signal.
We found that Cas is involved in CrkII-regulated FAK phosphorylation. The Cas mutants having a deleted SD domain or SH3 domain showed reduced ability of CrkII to induce the constitutive phosphorylation of FAK Tyr-397 as well as the anchorage-independent cell growth (Figs. 2 and 3B). These mutants also displayed reduced integrin-stimulated autophosphorylation of FAK (Fig. 5B), implying that Cas is physiologically involved in the integrin-stimulated FAK activation signal. Because the SD and the SH3 domains are mapped as the binding regions for CrkII and FAK, respectively, the formation of the CrkII–Cas–FAK molecular complex may be important for the FAK autophosphorylation. In fact, we observed that the FAK phosphorylation was well correlated with Cas tyrosine phosphorylation and formation of the CrkII–Cas complex in the case of both CrkII overexpression and integrin stimulation (Figs. 3B and 5 C and D). The phosphorylation of Cas is thought to be regulated by its SH3 domain with which FAK and its related kinase, Pyk2, are associated. Consistently, the ΔSH3 mutant was not phosphorylated by either CrkII overexpression or integrin stimulation and did not coprecipitate with CrkII (Figs. 3B and 5 C and D). These observations suggest that the Cas–FAK complex is required for phosphorylation of Cas and for CrkII–Cas complex formation. Existing models for FAK and Cas functions in integrin signaling have placed Cas downstream of FAK. It has been reported that Cas is a mediator of FAK-promoted cell migration (53, 54) and that cell migration depends on the formation of Crk–Cas complexes (8, 54). Our present observations provide a model in which the CrkII–Cas–FAK complex may not only serve as a downstream mediator of activated FAK but also affect the activation of FAK as an upstream regulator.
FAK autophosphorylation at Tyr-397 is an initial event leading to full activation of this kinase. However, the signaling events that occur immediately after integrin clustering and lead to autophosphorylation of FAK Tyr-397 are still unknown. Recently, it was reported that defective function of SFKs reduces (but not abolishes) integrin-stimulated FAK autophosphorylation (55, 56). Furthermore, we previously reported that v-Crk-induced FAK autophosphorylation was reduced by expression of Csk, a negative regulator of SFKs (23). These observations suggest that SFK activation is possibly involved in CrkII-regulated FAK phosphorylation. In addition, because SFKs have also been implicated in integrin-dependent phosphorylation of Cas, they may be required for promoting CrkII–Cas complex formation. However, we were unable to detect significant Src activation in the CrkII-overexpressing NIH 3T3 cells (data not shown). Whereas SFKs have been clearly shown to play roles in downstream signaling of integrin stimulation, several studies were unable to demonstrate integrin-dependent activation of Src (56–59). Cary et al. (56) proposes that basal kinase activity of Src is clearly essential for integrin-mediated events but that regulation of Src activity by phosphorylation of Tyr-416 in its activation loop may not occur. We speculate that CrkII overexpression promotes the efficiency of SFK-mediated phosphorylation of substrates, such as Cas and FAK, through its scaffolding function rather than by changing the activity of SFKs. Interestingly, some scaffolding events are clearly essential for integrin-mediated FAK autophosphorylation. Cary et al. (56) also reported that a kinase-inactive Src mutant expressed in SFK-defective cells could rescue integrin-stimulated autophosphorylation of FAK Tyr-397 and that its overexpression permitted FAK phosphorylation even in the absence of integrin stimulation. This observation is very similar to the case with CrkII. Although CrkII has not been observed to associate directly with FAK, the scaffolding function of CrkII, such as in the CrkII–Cas complex formation, might be essential for promoting efficient autophosphorylation of FAK.
From studying the ability of CrkII to induce anchorage-independent cell growth, we found the physiological function of cellular CrkII upstream of FAK activation. We propose that the CrkII–Cas complex acts as a previously unidentified mechanism to promote the autophosphorylation of FAK after integrin engagement. Further studies to understand the function of CrkII in FAK autophosphorylation will provide more details on this mechanism of FAK activation.
Acknowledgments
We thank S. Hanks, H. Hirai, T. Kitamura, T. Yamamoto, and M. Hamaguchi for providing reagents; M. Yutsudo for the use of facilities for the viral infections; and members of the Hanafusa laboratory for technical assistance. T.I. thanks T. Yamamoto for valuable discussions. This work was supported by grants-in-aid for Specially Promoted Research of the Ministry of Education, Sports, and Culture of Japan.
Author contributions: T.I., T.A., and H.H. designed research; T.I. and Y.F. performed research; T.I. and T.A. contributed new reagents/analytic tools; T.I., T.A., and H.H. analyzed data; and T.I., T.A., and H.H. wrote the paper.
Abbreviations: Cas, Crk-associated substrate; Cas–/–, Cas-null fibroblasts; FAK, focal adhesion kinase; FAK–/–, FAK-null fibroblasts; Flag-CrkII, Flag-tagged CrkII; FN, fibronectin; FRNK, FAK-related nonkinase; SD, substrate domain; ΔSD, Cas mutant with a deleted SD; SFK, Src family kinases; SH, Src homology; ΔSH3, Cas mutant having a deletion in its SH3 domain.
References
- 1.Mayer, B. J., Hamaguchi, M. & Hanafusa, H. (1988) Nature 332, 272–275. [DOI] [PubMed] [Google Scholar]
- 2.Iwahara, T., Akagi, T., Shishido, T. & Hanafusa, H. (2003) Oncogene 22, 5946–5957. [DOI] [PubMed] [Google Scholar]
- 3.Nishihara, H., Tanaka, S., Tsuda, M., Oikawa, S., Maeda, M., Shimizu, M., Shinomiya, H., Tanigami, A., Sawa, H. & Nagashima, K. (2002) Cancer Lett. (Shannon, Irel.) 180, 55–61. [DOI] [PubMed] [Google Scholar]
- 4.Miller, C. T., Chen, G., Gharib, T. G., Wang, H., Thomas, D. G., Misek, D. E., Giordano, T. J., Yee, J., Orringer, M. B., Hanash, S. M. & Beer, D. G. (2003) Oncogene 22, 7950–7957. [DOI] [PubMed] [Google Scholar]
- 5.Takino, T., Nakada, M., Miyamori, H., Yamashita, J., Yamada, K. M. & Sato, H. (2003) Cancer Res. 63, 2335–2337. [PubMed] [Google Scholar]
- 6.Sakai, R., Iwamatsu, A., Hirano, N., Ogawa, S., Tanaka, T., Mano, H., Yazaki, Y. & Hirai, H. (1994) EMBO J. 13, 3748–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaller, M. D. & Parsons, J. T. (1995) Mol. Cell. Biol. 15, 2635–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klemke, R. L., Leng, J., Molander, R., Brooks, P. C., Vuori, K. & Cheresh, D. A. (1998) J. Cell Biol. 140, 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert, M. L., Kim, J. I. & Birge, R. B. (2000) Nat. Cell Biol. 2, 899–905. [DOI] [PubMed] [Google Scholar]
- 10.Hynes, R. O. (1992) Cell 69, 11–25. [DOI] [PubMed] [Google Scholar]
- 11.Calalb, M. B., Polte, T. R. & Hanks, S. K. (1995) Mol. Cell. Biol. 15, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, H. C. & Guan, J. L. (1994) Proc. Natl. Acad. Sci. USA 91, 10148–10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobb, B. S., Schaller, M. D., Leu, T. H. & Parsons, J. T. (1994) Mol. Cell. Biol. 14, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaller, M. D., Hildebrand, J. D., Shannon, J. D., Fox, J. W., Vines, R. R. & Parsons, J. T. (1994) Mol. Cell. Biol. 14, 1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing, Z., Chen, H. C., Nowlen, J. K., Taylor, S. J., Shalloway, D. & Guan, J. L. (1994) Mol. Biol. Cell 5, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlaepfer, D. D., Hauck, C. R. & Sieg, D. J. (1999) Prog. Biophys. Mol. Biol. 71, 435–478. [DOI] [PubMed] [Google Scholar]
- 17.Owen, J. D., Ruest, P. J., Fry, D. W. & Hanks, S. K. (1999) Mol. Cell. Biol. 19, 4806–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuori, K., Hirai, H., Aizawa, S. & Ruoslahti, E. (1996) Mol. Cell. Biol. 16, 2606–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlaepfer, D. D., Broome, M. A. & Hunter, T. (1997) Mol. Cell. Biol. 17, 1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanner, S. B., Reynolds, A. B., Vines, R. R. & Parsons, J. T. (1990) Proc. Natl. Acad. Sci. USA 87, 3328–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polte, T. R. & Hanks, S. K. (1995) Proc. Natl. Acad. Sci. USA 92, 10678–10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altun-Gultekin, Z. F., Chandriani, S., Bougeret, C., Ishizaki, T., Narumiya, S., de Graaf, P., Van Bergen en Henegouwen, P., Hanafusa, H., Wagner, J. A. & Birge, R. B. (1998) Mol. Cell. Biol. 18, 3044–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akagi, T., Murata, K., Shishido, T. & Hanafusa, H. (2002) Mol. Cell. Biol. 22, 7015–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akagi, T., Shishido, T., Murata, K. & Hanafusa, H. (2000) Proc. Natl. Acad. Sci. USA 97, 7290–7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamoto, T., Sakai, R., Ozawa, K., Yazaki, Y. & Hirai, H. (1996) J. Biol. Chem. 271, 8959–8965. [DOI] [PubMed] [Google Scholar]
- 26.Ilic, D., Furuta, Y., Kanazawa, S., Takeda, N., Sobue, K., Nakatsuji, N., Nomura, S., Fujimoto, J., Okada, M. & Yamamoto, T. (1995) Nature 377, 539–544. [DOI] [PubMed] [Google Scholar]
- 27.Honda, H., Oda, H., Nakamoto, T., Honda, Z., Sakai, R., Suzuki, T., Saito, T., Nakamura, K., Nakao, K., Ishikawa, T., et al. (1998) Nat. Genet. 19, 361–365. [DOI] [PubMed] [Google Scholar]
- 28.Assoian, R. K., Boardman, L. A. & Drosinos, S. (1989) Anal. Biochem. 177, 95–99. [DOI] [PubMed] [Google Scholar]
- 29.Morita, S., Kojima, T. & Kitamura, T. (2000) Gene Ther. 7, 1063–1066. [DOI] [PubMed] [Google Scholar]
- 30.Yano, H., Uchida, H., Iwasaki, T., Mukai, M., Akedo, H., Nakamura, K., Hashimoto, S. & Sabe, H. (2000) Proc. Natl. Acad. Sci. USA 97, 9076–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaller, M. D., Borgman, C. A. & Parsons, J. T. (1993) Mol. Cell. Biol. 13, 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson, A. & Parsons, T. (1996) Nature 380, 538–540. [DOI] [PubMed] [Google Scholar]
- 33.Hauck, C. R., Hsia, D. A., Puente, X. S., Cheresh, D. A. & Schlaepfer, D. D. (2002) EMBO J. 21, 6289–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy, S., Ruest, P. J. & Hanks, S. K. (2002) J. Cell. Biochem. 84, 377–388. [DOI] [PubMed] [Google Scholar]
- 35.Moissoglu, K. & Gelman, I. H. (2003) J. Biol. Chem. 278, 47946–47959. [DOI] [PubMed] [Google Scholar]
- 36.Polte, T. R. & Hanks, S. K. (1997) J. Biol. Chem. 272, 5501–5509. [DOI] [PubMed] [Google Scholar]
- 37.Harte, M. T., Hildebrand, J. D., Burnham, M. R., Bouton, A. H. & Parsons, J. T. (1996) J. Biol. Chem. 271, 13649–13655. [DOI] [PubMed] [Google Scholar]
- 38.Matsuda, M., Nagata, S., Tanaka, S., Nagashima, K. & Kurata, T. (1993) J. Biol. Chem. 268, 4441–4446. [PubMed] [Google Scholar]
- 39.Feller, S. M., Knudsen, B. & Hanafusa, H. (1994) EMBO J. 13, 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nojima, Y., Morino, N., Mimura, T., Hamasaki, K., Furuya, H., Sakai, R., Sato, T., Tachibana, K., Morimoto, C., Yazaki, Y., et al. (1995) J. Biol. Chem. 270, 15398–15402. [DOI] [PubMed] [Google Scholar]
- 41.Vuori, K. & Ruoslahti, E. (1995) J. Biol. Chem. 270, 22259–22262. [DOI] [PubMed] [Google Scholar]
- 42.Petch, L. A., Bockholt, S. M., Bouton, A., Parsons, J. T. & Burridge, K. (1995) J. Cell Sci. 108, 1371–1379. [DOI] [PubMed] [Google Scholar]
- 43.Richardson, A., Malik, R. K., Hildebrand, J. D. & Parsons, J. T. (1997) Mol. Cell. Biol. 17, 6906–6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stam, J. C., Geerts, W. J., Versteeg, H. H., Verkleij, A. J. & van Bergen en Henegouwen, P. M. (2001) J. Biol. Chem. 276, 25176–25183. [DOI] [PubMed] [Google Scholar]
- 45.Freedman, V. H. & Shin, S. I. (1974) Cell 3, 355–359. [DOI] [PubMed] [Google Scholar]
- 46.Guan, J. L. & Shalloway, D. (1992) Nature 358, 690–692. [DOI] [PubMed] [Google Scholar]
- 47.Frisch, S. M., Vuori, K., Ruoslahti, E. & Chan-Hui, P. Y. (1996) J. Cell Biol. 134, 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owens, L. V., Xu, L., Craven, R. J., Dent, G. A., Weiner, T. M., Kornberg, L., Liu, E. T. & Cance, W. G. (1995) Cancer Res. 55, 2752–2755. [PubMed] [Google Scholar]
- 49.Wang, D., Grammer, J. R., Cobbs, C. S., Stewart, J. E., Jr., Liu, Z., Rhoden, R., Hecker, T. P., Ding, Q. & Gladson, C. L. (2000) J. Cell Sci. 113, 4221–4230. [DOI] [PubMed] [Google Scholar]
- 50.Slack, J. K., Adams, R. B., Rovin, J. D., Bissonette, E. A., Stoker, C. E. & Parsons, J. T. (2001) Oncogene 20, 1152–1163. [DOI] [PubMed] [Google Scholar]
- 51.Kahana, O., Micksche, M., Witz, I. P. & Yron, I. (2002) Oncogene 21, 3969–3977. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz, M. A. (1997) J. Cell Biol. 139, 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sieg, D. J., Hauck, C. R. & Schlaepfer, D. D. (1999) J. Cell Sci. 112, 2677–2691. [DOI] [PubMed] [Google Scholar]
- 54.Cary, L. A., Han, D. C., Polte, T. R., Hanks, S. K. & Guan, J. L. (1998) J. Cell Biol. 140, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salazar, E. P. & Rozengurt, E. (2001) J. Biol. Chem. 276, 17788–17795. [DOI] [PubMed] [Google Scholar]
- 56.Cary, L. A., Klinghoffer, R. A., Sachsenmaier, C. & Cooper, J. A. (2002) Mol. Cell. Biol. 22, 2427–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark, E. A., King, W. G., Brugge, J. S., Symons, M. & Hynes, R. O. (1998) J. Cell Biol. 142, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sieg, D. J., Ilic, D., Jones, K. C., Damsky, C. H., Hunter, T. & Schlaepfer, D. D. (1998) EMBO J. 17, 5933–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh, E. S., Gu, H., Saxton, T. M., Timms, J. F., Hausdorff, S., Frevert, E. U., Kahn, B. B., Pawson, T., Neel, B. G. & Thomas, S. M. (1999) Mol. Cell. Biol. 19, 3205–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]