Abstract
Aims
To determine the effect of an Interactive Voice Response (IVR) brief intervention (BI) to reduce alcohol consumption among adults seeking primary care.
Methods
Patients (N = 1855) with unhealthy drinking were recruited from eight academic internal medicine and family medicine clinics and randomized to IVR-BI (n = 938) versus No IVR-BI control (n = 917). Daily alcohol consumption was assessed at baseline, 3- and 6-months using the Timeline Followback.
Results
The IVR-BI was completed by 95% of the 938 patients randomized to that condition, and 62% of them indicated a willingness to consider a change in their drinking. Participants in both conditions significantly reduced consumption over time, but changes were not different between groups. Regardless of condition, participants with alcohol use disorder (AUD) showed significant decreases in drinking outcomes. No significant changes were observed in patients without AUD, regardless of condition.
Conclusion
Although the IVR intervention was well accepted by patients, there was no evidence that IVR-BI was superior to No IVR-BI for reducing drinking in the subsequent 6 months. Because both the design and the intervention tested were novel, we cannot say definitively why this particular eHealth treatment lacked efficacy. It could be useful to evaluate the effect of the pre-randomization assessment alone on change in drinking. The high treatment engagement rate and successful implementation protocol are strengths, and can be adopted for future trials.
Short summary
We examined the efficacy of a novel BI for patient self-administration by automated telephone. Alcohol consumption decreased over time but there were no between-group changes in consumption. Regardless of treatment condition, participants with alcohol use disorder (AUD) showed significant reduction in drinking but participants without AUD showed no change.
INTRODUCTION
In-office clinician-initiated brief intervention (BI) is a recommended treatment for unhealthy drinking in primary care settings (World Health Assembly, 2005; Moyer, 2013; Substance Abuse and Mental Health Services Administration, 2015). The goals of BI are to reduce drinking by individuals whose alcohol consumption places them at elevated risk for health or social problems, recommend self-management strategies for quitting or cutting down, refer to specialty care if indicated, and continue to follow at subsequent visits (National Institute on Alcohol Abuse and Alcoholism, 2005; Kaner et al., 2009). In spite of strong evidence for efficacy (Whitlock et al., 2004; O'Donnell et al., 2014), implementation of BI in practice is far from universal. Data from the 2013 National Survey of Drug Use and Health showed that of respondents who had been to an outpatient clinic visit and screened positive for heavy episodic drinking, fewer than 5% had been advised to cut back (Glass et al., 2016). Barriers to providing appropriate care for unhealthy drinking include lack of time and administrative support for routine screening, providers’ resistance to addressing the topic of alcohol use and the stigma of alcoholism (Beich et al., 2002; Fortney et al., 2004; Johnson et al., 2011). Most primary care providers (PCPs) are not experienced in providing BIs for unhealthy alcohol use, let alone support for longer term interventions such as self-monitoring and feedback (Spandorfer et al., 1999; McCormick et al., 2006).
One strategy to overcome these barriers and provide needed information and advice to individuals who drink excessively is to offer low cost, scalable resources that can supplement or replace face-to-face screening and intervention by a healthcare provider. Electronic interventions are advantageous because they save time for providers, can deliver evidence-based content in a consistent way across patients, and may evoke more honest responses from users (Kobak et al., 1997). Meta-analyses and systematic reviews (Donoghue et al., 2014; Dedert et al., 2015) of electronic interventions for unhealthy drinking have demonstrated small but consistent effects on alcohol consumption across a range of settings, platforms and therapeutic complexity. For example, electronic BIs have been created for use in college campuses (Kypri et al., 2004; Neighbors et al., 2010), primary care (Gryczynski et al., 2015), trauma centers (Suffoletto et al., 2014) or the general population (Hester et al., 2005). Platforms include computers (Wagener et al., 2012), internet (Blankers et al., 2011), email (Araki et al., 2006), text messages (Suffoletto et al., 2014) or smartphones (Gustafson et al., 2014). Finally, therapeutic complexity has ranged from a single session of personalized feedback (Palfai et al., 2011), to a multi-session curriculum (Klein et al., 2012), and multicomponent system with 24-7 human support (Gustafson et al., 2014).
Interactive Voice Response (IVR) is an automated telephone technology system that has high potential as an eHealth platform because of its simplicity, accessibility and low cost. A number of studies have documented its utility as a self-monitoring device for symptoms associated with a variety of conditions, such as cancer, (Besse et al., 2016), cirrhosis (Thomson et al., 2015), HIV (Tucker et al., 2013) and depression (Fazzino et al., 2013), among others, in addition to problem drinking (Simpson et al., 2012; Cooney et al., 2015). In the field of alcohol and drug treatment, we and others have extended the functionality of IVR systems to include therapeutic content, not just self-monitoring (Mundt et al., 2006; Helzer et al., 2008; Rose et al., 2010, 2012; Simpson et al., 2012; Moore et al., 2013; Schroder et al., 2013; Hasin et al., 2014; Rose et al., 2015c). We are aware of only one other study of IVR being used to deliver a BI but it was in a non-clinical college student sample (Andersson, 2015), not primary care. That study compared IVR with both a web-based intervention and a control condition and demonstrated the feasibility of delivering a BI by IVR to a large sample. Compared to the control group, both web and IVR interventions significantly reduced estimated peak blood alcohol concentration and scores on the Alcohol Use Disorders Identification Test (AUDIT) at 6 weeks post-intervention, although effect sizes were small. Significant reductions in alcohol consumption were reported for the web condition but not the IVR condition.
The purpose of this study was to test the efficacy of a BI delivered by IVR to a large sample of primary care patients screened positive for unhealthy drinking prior to an office visit. The IVR-BI, based on guidelines from the National Institute on Alcohol Abuse and Alcoholism (National Institute on Alcohol Abuse and Alcoholism, 2005) for clinician-delivered BI, proved feasible in a previous non-randomized pilot study (Rose et al., 2010), with promising pre-post effects on drinking at 2 weeks. We hypothesized that patients randomized to IVR-BI would show greater reductions in drinking over the 6 months post-intervention, compared with those randomized to the control condition.
MATERIALS AND METHODS
Study design
This was a parallel group randomized controlled trial that tested the efficacy of alcohol IVR-BI. Participants were assigned to one of two arms by a random number generator. The active treatment condition received a pre-programmed single session BI delivered by IVR (IVR-BI condition) prior to their healthcare visit. The control condition did not receive IVR-BI. Participants were scheduled for telephone interviews to occur the day after the healthcare visit, and 3 and 6 months later. All participants received $10 for completing the IVR randomization call and $20 each for the post-visit interview and the 3- and 6-months telephone interviews which were ~30, 15 and 15 minutes in duration, respectively.
Sample and setting
Participants were recruited between June 2012 and January 2015, from eight primary care outpatient clinics affiliated with a single academic medical center in the Northeast. Sites were urban (n = 1), suburban (n = 5) and rural (n = 2), with 4–12 providers each. A total of 11,213 patients were assessed for the following eligibility criteria: age 18 or older, scheduled for a routine (non-acute) primary care visit in the next 3 days, English language proficiency, the absence of cognitive or hearing deficits, and positive pre-visit screening for unhealthy alcohol use based on the Single Alcohol Screening Question (SASQ), ‘How many times in the past year have you had X or more drinks in a day?’, where X is 5 for men and 4 for women (Smith et al., 2009).
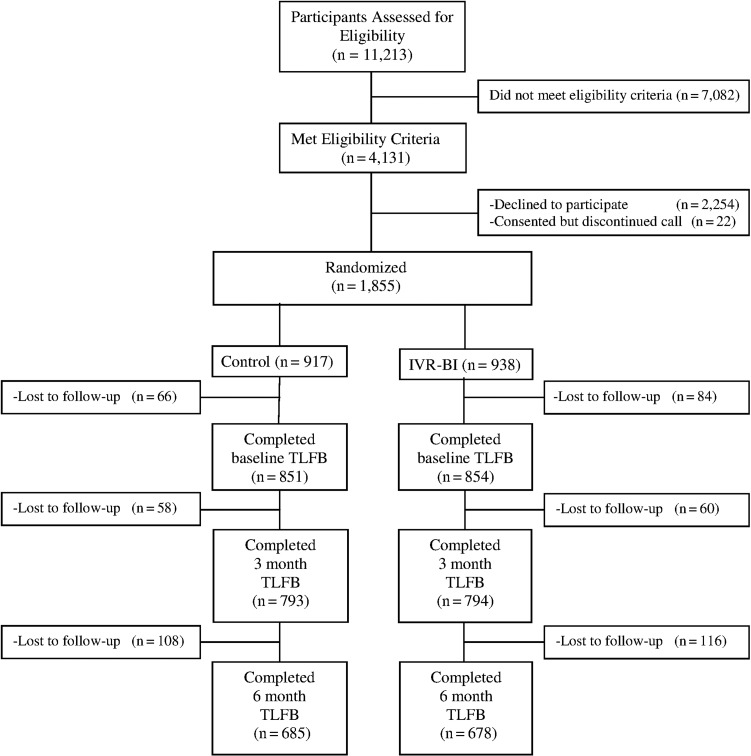
Of the 4131 patients meeting entry criteria, 1855 provided informed consent and were randomized to either IVR-BI or control conditions (Fig. 1). One hundred and fifty of these participants (8%) could not be contacted by phone or mail after their scheduled clinic visit and thus did not complete the first (post-visit) interview. They were excluded from all analyses as no baseline alcohol use data were available. There was no significant difference between the two conditions in the percentage of subjects lost to follow-up prior to the post-visit interview (IVR-BI = 7% vs control = 9%, P = 0.17). Demographic and alcohol use characteristics for the 1705 participants (92%) with available alcohol use data are displayed in Table 1. Participants (52% female) were predominantly Caucasian, middle aged and well-educated. Average weekly consumption was ~8 drinks per week with 28% having met criteria for past-year AUD. Over a third (37%) reported heavy episodic drinking within the month prior to randomization. There were no significant differences between conditions in either demographic or alcohol use characteristics.
Fig. 1.
CONSORT flow diagram.
Table 1.
Baseline characteristics of IVR-BI study participantsa
| Control (N = 851) | IVR-BI (N = 854) | P-value | |
|---|---|---|---|
| % Female | 53 | 52 | 0.48 |
| Marital status | |||
| % Single | 31 | 34 | 0.20 |
| % Married/civil union | 56 | 55 | |
| % Divorced/separated | 11 | 8 | |
| % Widowed | 2 | 3 | |
| % White | 94 | 96 | 0.11 |
| Age | |||
| % 18–29 | 20 | 19 | 0.96 |
| % 20–44 | 24 | 25 | |
| % 45–64 | 39 | 40 | |
| % 65+ | 16 | 16 | |
| Education | |||
| % ≤High school diploma or GED | 32 | 31 | 0.77 |
| % Some college/associates degree | 10 | 11 | |
| % Bachelor's degree or greater | 59 | 59 | |
| SASQ score median (IQR) | 5 (2–10) | 5 (2–12) | 0.35 |
| % AUD based on CIDI-SAM | 29 | 28 | 0.84 |
| % Reporting heavy episodic drinking in the past 30 days | 39 | 36 | 0.34 |
| Number of drinks per week (mean ± SD) | 8.1 ± 11.4 | 7.6 ± 9.4 | 0.36 |
| Number of drinking days per week (mean ± SD) | 2.9 ± 2.4 | 2.9 ± 2.3 | 0.90 |
| Number of drinks per drinking day (mean ± SD) | 2.7 ± 2.1 | 2.5 ± 1.7 | 0.17 |
asubjects completing baseline assessment of alcohol use.
Recruitment procedures
As detailed in a previous publication (Rose et al., 2015a), patients scheduled for routine office visits were notified by mail that a university research assistant (RA) would be calling to invite them to participate in a phone-based research study. An opt-out number was provided. Of the note, 2–3 days before the scheduled medical appointment, RAs called patients who had not opted out to request verbal consent to complete a brief pre-visit behavioral health screen by automated telephone. Patients who did not respond to the first contact attempt were re-contacted daily until the day of the appointment. The brief behavioral health screen included questions about pain, smoking, exercise, weight and mood, in addition to the SASQ (Smith et al., 2009). With patient consent, results of the behavioral health screen were automatically sent to his or her electronic health record.
Patients who scored one or more on the SASQ were eligible for the research. They were automatically transferred back to the RA after completing the screen and offered the chance to participate in the randomized trial. Consenting participants were first scheduled for the post-visit telephone interview and then were transferred to the IVR system for randomization and completion of the IVR-BI. Participants could transfer back to the RA if they had any questions but they otherwise disconnected from the IVR-BI when they were finished with the one-time intervention. Participants were required to complete the IVR-BI before their medical visit. All procedures were approved by the University of Vermont Committee on Human Research in the Medical Sciences.
Post-visit interviewers were not blind to study condition because the interview included questions about the IVR system. Follow-up (3 and 6 months) interviewers were blind to the subject's study condition. PCPs were blind to patients’ participation in the trial because such knowledge might affect the PCP's usual care—potentially increasing or decreasing their proclivity to intervene. Our research question was whether a patient-directed intervention could affect alcohol consumption, not whether providers could be externally influenced to make changes in their care. PCPs were free to provide their ‘usual care’, which may or may not include alcohol screening, discussion, BI or referral.
IVR-BI intervention
IVR is an automated telephone technology that enables users to interface with a computer by making touch-tone entries to pre-recorded prompts. The IVR-BI content is based on the four steps of NIAAA's (National Institute on Alcohol Abuse and Alcoholism, 2005) clinical recommendations for helping patients with unhealthy drinking: (a) Ask, (b) Assess, (c) Advise and Assist and (d) Follow-up Support. Step one, ‘Ask,’ is accomplished with the SASQ (Smith et al., 2009) from the pre-visit behavioral health screen. Therefore, both experimental conditions received that step.
The ‘Assess’ step consists of a short screen for AUD. The AUD screen includes two DSM-IV criteria shown to correlate highly with diagnosis (Vinson et al., 2007), (a) use in hazardous situations and (b) drinking larger amounts or for a longer period of time than intended, plus a question about prior withdrawal experiences. Positive responses to any of the three questions trigger a recommendation to seek an evaluation by a doctor or alcohol specialist. The recommendation is followed by a statement that doctors typically prescribe abstinence for people with these symptoms and that patients should discuss any quit attempt with a doctor to avoid dangerous withdrawal.
The ‘Advise and Assist’ step begins with a readiness to change assessment and then branches accordingly. The Not Ready branch offers three ‘Readiness Suggestions’ before terminating the call. The Ready branch leads to a choice to hear guidance on cutting down and/or quitting. Advice for Cutting Down includes goal-setting, planning for urges and high-risk situations, proactive avoidance of triggers, self-monitoring and other strategies. The Advice to Abstain section describes treatment and mutual help models commonly used to achieve abstinence, and includes information on local support and treatment resources.
The last step of the IVR-BI is ‘Follow-up Support,’ in which callers are encouraged to talk with their PCP about their alcohol use and to avail themselves of patient education materials at their doctor's office. A manipulation check on the intervention demonstrated that the IVR-BI had its intended effect on patient-provider communication: As reported in an earlier publication (Rose et al., 2016), individuals randomized to IVR-BI—compared to control—were more likely during the subsequent PCP visit to initiate conversations about alcohol use and to receive an alcohol-related recommendation. The full text of the IVR-BI is available from the authors.
All keypad entries during each participant's interface with the IVR-BI system, including call completion versus discontinuation and call duration, were captured real-time and stored in a secure, password-protected database without participant identifiers.
Assessments
Outcome measures. Alcohol consumption was assessed at each interview using the Timeline Followback (TLFB) (Sobell and Sobell, 1992) calendar method. The reliability of telephone administered TLFB has been established (Cohen and Vinson, 1995). At the follow-up interviews, TLFB assessment encompassed the entire time interval since the previous administration; however, 3- and 6-month outcome variables were constructed based on only data from the 30 days prior to the assessment. Outcome measures constructed from TLFB data were drinks per week, drinking days per week, drinks per drinking day and heavy episodic drinking, i.e. five or more drinks in a day for men or four or more for women (Chen et al., 2004/2005).
Baseline measures. Baseline alcohol consumption during the time period of 30 days prior to the primary care visit up through the day preceding the post-visit interview was ascertained using the TLFB. Past-year DSM-IV AUD was assessed using the AUDs section of the Composite International Diagnostic Interview–Substance Abuse Module (CIDI-SAM) (Cottler et al., 1989), a structured interview designed for administration by trained lay interviewers for the assessment of substance use disorders according to DSM-IV diagnostic criteria.
Statistical methods
Baseline comparisons between treatment conditions on demographic and alcohol use variables were performed using chi square, t-tests and Wilcoxon Rank Sum tests. Primary analyses were based on an intent-to-treat approach that utilized all subjects with baseline alcohol consumption data regardless of incomplete follow-up. Multilevel modeling for repeated measures data (SAS, PROC MIXED) were used to examine changes in alcohol consumption from baseline to 6-months post-randomization. Fixed factors in the model were treatment condition (IVR vs control), past-year DSM-IV AUD diagnosis based on CIDI-SAM, assessment time (baseline, 3- and 6-months) and their interactions. Subject was a random factor in the model. Parameter estimates were based on the restricted maximum likelihood method with the assumption of compound symmetry covariance structure. When significant interactions were detected (i.e. treatment by time, AUD by time or treatment by AUD by time), linear contrasts were constructed to determine whether there was evidence of change during specific periods (i.e. baseline to 3-months, 3–6 months, etc.) and whether changes were parallel across treatment and AUD conditions. Primary outcome measures were drinks per week, drinking days per week and drinks per drinking day. Treatment conditions were also compared on the percent of subjects reporting heavy episodic drinking in the past 30 days, which was analyzed using PROC GENMOD with a logit link function. Each outcome was computed based on TLFB done at the post-visit interview, 3- and 6-months assessments. For continuous outcomes, baseline data from the TLFB collected at the post-visit interview covered the 30-days prior to randomization while the 3- and 6-months TLFB encompassed the entire interval from the previous assessment. Our dichotomous outcome, percent of subjects who reported heavy episodic drinking in the past 30 days, was limited to a 30-day period for all three assessments to allow for appropriate comparisons. A parallel set of analyses were performed examining consumption in the subset of patients that who in excess of the 7/14 (female/male) drinks per week guideline during the 30 days prior to randomization as determined by their baseline TLFB. Statistical analyses were performed using SAS statistical software Version 9.4 (SAS Institute, Cary NC).
The study was designed to have 1400 evaluable subjects resulting in estimated power of 0.80 using α = 0.05 to detect a mean difference of 1.5 in the 6-month change in drinks per week between treatment conditions based on our a priori estimate of variability. Post hoc, based on the actual variability encountered in the sample, the estimated power was determined to be 0.80 using α = 0.05 to detect a mean difference of 1.2 between the two conditions in the 6-month change in drinks per week for the entire sample, 2.0 drinks per week within the AUD subgroup and 2.4 drinks per week in the subset of participants drinking in excess of the 7/14 per week threshold.
RESULTS
IVR-BI utilization
For subjects randomized to the IVR-BI arm, 96% completed the intervention. Median call duration was 6.5 minutes (IQR, 5.2–6.9 minutes). Sixty-two percent of participants indicated they were willing to consider making a change in their drinking. Of those, the vast majority (80%) chose to listen only to the recorded advice to cut down as opposed to advice on quitting their drinking. A full diagram of participant flow through the IVR-BI system branch points is available as supplemental material on the journal's website.
Drinking outcomes
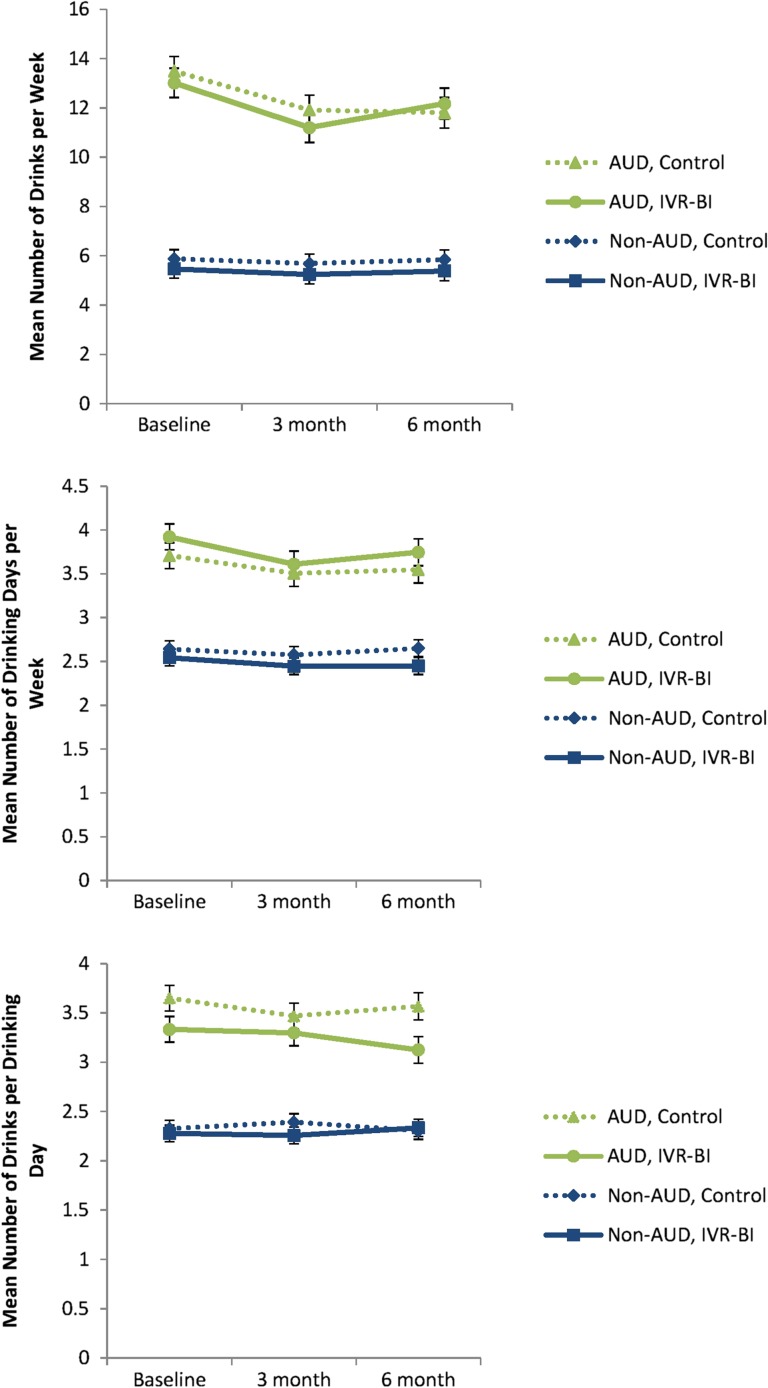
Mean values for each drinking outcome across treatment condition, assessment time and AUD diagnostic status are shown in Table 2 and graphically displayed in Figs 2 and 3. There were no significant differences between IVR-BI and control conditions in the changes in total drinks per week across assessments (group by time interaction, P = 0.41). Regardless of treatment condition, changes in consumption were dependent on whether subjects had a diagnosis of an AUD at the baseline post-visit interview (AUD by time interaction, P < 0.001). For those with an AUD diagnosis, significant decreases were observed from baseline to the 3-month assessment (P < 0.001). There were no further declines from 3 to 6 months (P = 0.21), but consumption levels remained significantly lower at 6 months than at baseline for the AUD condition (P < 0.001). No significant changes were observed in subjects who did not meet criteria for AUD (P = 0.60).
Table 2.
Drinking outcomes
| Outcome | Baseline | 3 Month | 6 Month | Time | Time*trt | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | IVR-BI | Control | IVR-BI | Control | IVR-BI | ||||
| n | n | n | n | n | n | ||||
| Mean | Mean | Mean | Mean | Mean | Mean | ||||
| SE | SE | SE | SE | SE | SE | ||||
| # Drinks per week | Overall | 851 | 854 | 793 | 794 | 685 | 678 | <0.01 | 0.41 |
| 9.68 | 9.24 | 8.80* | 8.22* | 8.82* | 8.78* | ||||
| (0.35) | (0.35) | (0.36) | (0.36) | (0.37) | (0.37) | ||||
| No AUD | 605 | 611 | 561 | 565 | 488 | 480 | |||
| 5.87 | 5.47 | 5.69 | 5.24 | 5.84 | 5.38 | ||||
| (0.38) | (0.38) | (0.38) | (0.38) | (0.40) | (0.40) | ||||
| AUD | 246 | 243 | 232 | 229 | 197 | 198 | |||
| 13.49 | 13.01 | 11.91* | 11.20* | 11.80* | 12.17* | ||||
| (0.59) | (0.60) | (0.60) | (0.60) | (0.62) | (0.62) | ||||
| # Drinking days per week | Overall | 851 | 854 | 793 | 794 | 685 | 678 | <0.01 | 0.64 |
| 3.17 | 3.23 | 3.04* | 3.03* | 3.10* | 3.10* | ||||
| (0.09) | (0.09) | (0.09) | (0.09) | (0.09) | (0.09) | ||||
| No AUD | 605 | 611 | 561 | 565 | 488 | 480 | |||
| 2.64 | 2.54 | 2.57 | 2.44 | 2.65 | 2.45 | ||||
| (0.09) | (0.09) | (0.09) | (0.09) | (0.10) | (0.10) | ||||
| AUD | 246 | 243 | 232 | 229 | 197 | 198 | |||
| 3.71 | 3.92 | 3.50* | 3.61* | 3.55* | 3.75* | ||||
| (0.15) | (0.15) | (0.15) | (0.15) | (0.15) | (0.15) | ||||
| # Drinks per drinking day | Overall | 782 | 808 | 757 | 762 | 648 | 650 | 0.39 | 0.86 |
| 2.99 | 2.80 | 2.93 | 2.78 | 2.93 | 2.73 | ||||
| (0.08) | (0.08) | (0.08) | (0.08) | (0.08) | (0.08) | ||||
| No AUD | 550 | 573 | 533 | 540 | 464 | 457 | |||
| 2.33 | 2.28 | 2.39 | 2.26 | 2.30 | 2.33 | ||||
| (0.08) | (0.08) | (0.08) | (0.08) | (0.09) | (0.09) | ||||
| AUD | 232 | 235 | 224 | 222 | 184 | 193 | |||
| 3.65 | 3.33 | 3.47 | 3.30 | 3.56 | 3.12 | ||||
| (0.13) | (0.13) | (0.13) | (0.13) | (0.14) | (0.14) | ||||
| % With heavy episodic drinking in past 30 days | Overall | 851 | 854 | 793 | 794 | 685 | 678 | <0.01 | 0.88 |
| 44.44 | 41.04 | 40.05* | 35.28* | 39.53* | 35.28* | ||||
| (1.95) | (1.89) | (1.94) | (1.87) | (2.10) | (2.01) | ||||
| No AUD | 605 | 611 | 561 | 565 | 488 | 480 | |||
| 29.75 | 29.30 | 28.70 | 25.31 | 26.84 | 27.08 | ||||
| (1.86) | (1.84) | (1.91) | (1.83) | (2.01) | (2.03) | ||||
| AUD | 246 | 243 | 232 | 229 | 197 | 198 | |||
| 60.16 | 53.91 | 52.59* | 46.72* | 53.81* | 44.44* | ||||
| (3.12) | (3.20) | (3.28) | (3.30) | (3.55) | (3.53) | ||||
Note: Tabled values are sample size, least square means and standard errors, except for excessive drinking in past 30 days which represent percent of subjects.
*Indicates significant decline from baseline independent of treatment group (P < 0.05).
Fig. 2.
Continuous measures of drinking outcomes across treatment conditions at baseline, 3- and 6-months.
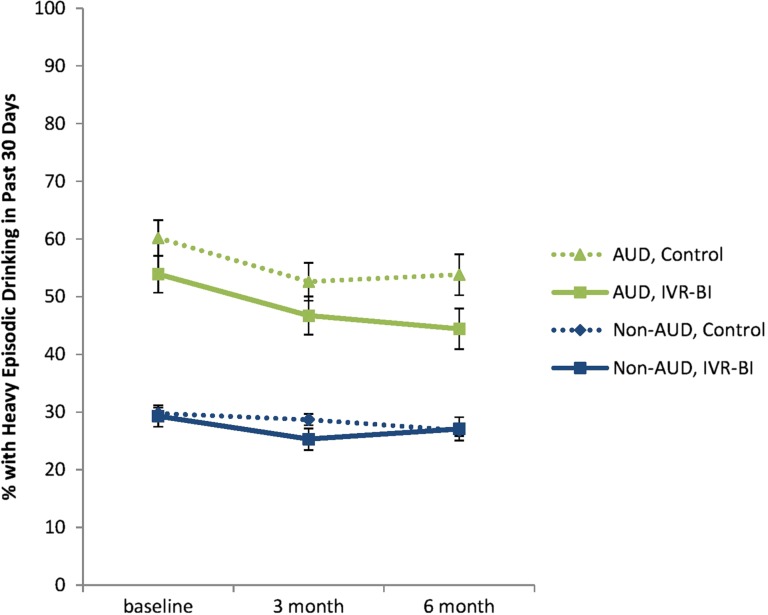
Fig. 3.
Percent of IVR-BI and control patients with heavy episodic drinking in the past 30 days at baseline, 3- and 6-months.
Similarly, no significant differences between IVR-BI and control conditions were observed in the changes in number of drinking days per week across time (time by group interaction, P = 0.64). Participants with AUD showed significant decreases from the baseline to 3-month assessments (P < 0.001), but not from 3- to 6-month assessments (P = 0.21). Number of drinking days remained significantly lower at 6 months than at baseline for this condition (P = 0.019). Subjects without a diagnosis of AUD showed no significant change in number of drinking days (P = 0.17).
Additionally, no significant differences between IVR-BI and control conditions were observed in the change in drinks per drinking day (time by group interaction, P = 0.86). Neither subjects with (P = 0.17) nor without AUD (P = 0.90) showed any change in drinks per drinking day across assessments.
Lastly, when conditions were compared on the proportion of subjects reporting heavy episodic drinking during the past 30 days, significant decreases from baseline were observed at the 3-month assessment (P < 0.001), but not from the 3- to 6-month assessments (P = 0.88; Fig. 3). There was no evidence that these changes were different between IVR and control conditions (time by group interaction, P = 0.88) or non-AUD and AUD conditions (time by AUD status interaction, P = 0.29). Additionally, changes in excessive drinking were not significantly different between treatment conditions within AUD (P = 0.59) or non-AUD subjects (P = 0.83).
To assess a potential floor effect, secondary analyses were performed in the subset of patients (n = 615) who drank in excess of the 7 (female)/14 (male) drinks per week criteria in the 30 days prior to study entry as determined by their baseline TLFB. The results in this subset paralleled the results for our full sample. That is, all treatment condition × time P-values were not significant (all P’s > 0.65). However, in this subset both AUD and non-AUD subjects significantly decreased their consumption over time (P < 0.05), independent of treatment condition.
DISCUSSION
In this trial, the IVR-BI and control conditions both showed reductions in drinking from baseline to follow-up assessments, but there were no differences between conditions on alcohol consumption at either 3- or 6-month follow-up. Independent of treatment condition, participants with AUD (but not those without AUD) showed a reduction in alcohol consumption over the study period. The failure of the IVR-BI to reduce drinking compared to control was unexpected because: (a) in-person BIs have demonstrated efficacy in primary care settings; (b) the IVR intervention closely matched the guidelines for provider-delivered interventions and (c) other electronic BIs have shown efficacy (e.g. Donoghue et al., 2014; Dedert et al., 2015). However, the IVR-BI differed from traditional BIs or other electronic BIs, not only in its delivery platform. Importantly, the IVR-BI occurred prior to the healthcare encounter instead of being part of a healthcare visit. Secondly, the patient's study participation (and randomization status) was not known to the medical provider, whereas in traditional studies it is the provider conducting the BI and thus the BI impacts the rest of the office visit and is potentially augmented by the patient-provider relationship.
Because the mean alcohol consumption of this sample at baseline was relatively low [average weekly consumption fell within the range of low risk drinking according to NIAAA (2005) guidelines], we conducted secondary analyses to determine if our null results were the result of a floor effect. Restricting our analysis to a subset of heavier drinkers showed only an effect of time and no differential treatment effect. Among these heavier drinkers, decreases over time were observed for participants with and without AUD.
This trial adds to a small but growing number of recently published negative BI trials. For example, the Screening and Intervention Programme for Sensible drinking multisite randomized pragmatic trial of BI in primary care showed that at 6 months post-intervention, advice or lifestyle counseling were no more effective than screening and simple feedback on self-reported hazardous or harmful drinking status as assessed with the AUDIT (Kaner et al., 2013). Other studies have reported null or inverse outcomes for both clinician-delivered and computerized BI in a variety of settings and with varied outcome measures (e.g. Hilbink et al., 2012; Duroy et al., 2016; Palm et al., 2016). Of course there are several positive outcome trials as well, leading us to conclude that the question of the effectiveness of both live and electronic alcohol BI remains unresolved.
One explanation for the observed pre-post reduction in drinking among patients with AUD and among heavier drinkers could be reactivity to the research assessment (Clifford et al., 2007). In other words, the pre-intervention contact with researchers and IVR-based screening may have been of sufficient potency to effect change in both the intervention and control conditions. This is conceivable because the assessment was ~5 times longer than the IVR-BI itself, which averaged <7 minutes in length. Our design did not include a no-assessment condition and therefore we are unable to rule out the effect of assessment alone. However, tests of assessment-only interventions are called for because they could offer a relatively low-cost, low-investment way of effecting positive movement on alcohol use for those at risk and could have large public health impacts.
Strengths
Despite demonstrating no significant effect on outcomes, this evaluation of an IVR intervention is important because of the rigor of its methodological approach. In this era of rapid technological advances and direct to consumer marketing of health related applications, it is important to carefully evaluate efficacy of novel, low cost approaches. This recruitment method is a promising strategy for possible future trials of alternative eHealth approaches, including minimal or assessment-only interventions. Furthermore, the intervention we tested here was empirically based and adherent to national guidelines for the treatment of unhealthy drinking in outpatient medical settings. The high completion rate of the intervention is important because it suggests that lack of exposure to the treatment is unlikely to account for our null results. Finally, the large sample size provided strong statistical power and confidence in the reproducibility of our results.
Weaknesses
Our inclusion criteria resulted in a sample of individuals whose average alcohol consumption was within NIAAA guidelines for low-risk drinking. While all participants screened positive for unhealthy drinking using an IVR-based SASQ, fewer than half of them met criteria for unhealthy drinking based on typical weekly consumption as reported in a subsequent telephone interview. Nonetheless, no differential effect of the intervention was observed within the subset of participants with AUD, who are on average heavier drinkers. In addition, we cannot rule out regression to the mean as an explanation for the pre-post reduction in drinking of alcohol dependent and heavier drinking patients.
Implications
While the results of this study did not support the efficacy of the intervention in this sample, we were able to demonstrate the feasibility of implementing this technology on a large scale. Importantly, the majority of participants exposed to the intervention indicated a willingness to consider a change in their drinking. As this and other studies have demonstrated (McCormick et al., 2006; Rose et al., 2015a, 2015b, 2016), there is a desire on the part of many primary care patients to receive drinking-related information and advice, suggesting continued investigation into optimal delivery systems is warranted.
One of the important advantages of eHealth approaches is the potential to scale to large populations with relatively small incremental costs. The next steps in evaluation of this approach would be to assess in other populations (e.g. heavier drinkers or specific demographic groups), other settings (emergency department, workplace or school), or to intensify the intervention (e.g. linking the electronic BI more tightly to a PCP intervention).
CONCLUSION
While large scale implementation of a pre-visit IVR screening and BI process in a primary care setting was successful, and within-subject reductions in drinking over time were observed in individuals with AUD diagnosis, the intervention had no significant effect on alcohol consumption at 3 or 6 months, compared with a control condition.
ACKNOWLEDGEMENTS
We are grateful to the participating providers and staff in the Departments of Family Medicine and Primary Care Internal Medicine at the University of Vermont Medical Center for facilitating subject recruitment for this project.
We thank Dr Amanda Kennedy for her comments on a previous version of this manuscript.
IVR development and hosting was provided by TeleSage, Inc.
FUNDING
This work was supported by the National Institute on Alcohol Abuse and Alcoholism grant R01AA018658 to G.L.R..
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- Andersson C. (2015) Comparison of WEB and interactive voice response (IVR) methods for delivering brief alcohol interventions to hazardous-drinking university students: a randomized controlled trial. Eur Addict Res 21:240–52. [DOI] [PubMed] [Google Scholar]
- Araki I, Hashimoto H, Kono K, et al. (2006) Controlled trial of worksite health education through face-to-face counseling vs. e-mail on drinking behavior modification. J Occup Health 48:239–45. [DOI] [PubMed] [Google Scholar]
- Beich A, Gannik D, Malterud K (2002) Screening and brief intervention for excessive alcohol use: qualitative interview study of the experiences of general practitioners. BMJ 325:870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse KT, Faber-Te Boveldt ND, Janssen GH, et al. (2016) Pain assessment with short message service and interactive voice response in outpatients with cancer and pain: a feasibility study. Pain Pract 16:320–6. [DOI] [PubMed] [Google Scholar]
- Blankers M, Koeter MW, Schippers GM (2011) Internet therapy versus internet self-help versus no treatment for problematic alcohol use: a randomized controlled trial. J Consult Clin Psychol 79:330–41. [DOI] [PubMed] [Google Scholar]
- Chen CM, Dufour MC, Yi H-Y (2004/2005) Alcohol consumption among young adults ages 18–24 in the United States: results from the 2001–2002 NESARC survey. Alcohol Res Health 28:269–80. [Google Scholar]
- Clifford PR, Maisto SA, Davis CM (2007) Alcohol treatment research assessment exposure subject reactivity effects: part I. Alcohol use and related consequences. J Stud Alcohol Drugs 68:519–28. [DOI] [PubMed] [Google Scholar]
- Cohen BB, Vinson DC (1995) Retrospective self-report of alcohol consumption: test-retest reliability by telephone. Alcohol Clin Exp Res 19:1156–61. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Sevarino KA, et al. (2015) Concurrent alcohol and tobacco treatment: effect on daily process measures of alcohol relapse risk. J Consult Clin Psychol 83:346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE (1989) The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br J Addict 84:801–14. [DOI] [PubMed] [Google Scholar]
- Dedert EA, McDuffie JR, Stein R, et al. (2015) Electronic interventions for alcohol misuse and alcohol use disorders: a systematic review. Ann Intern Med 163:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue K, Patton R, Phillips T, et al. (2014) The effectiveness of electronic screening and brief intervention for reducing levels of alcohol consumption: a systematic review and meta-analysis. J Med Internet Res 16:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duroy D, Boutron I, Baron G, et al. (2016) Impact of a computer-assisted screening, Brief Intervention and Referral to Treatment on reducing alcohol consumption among patients with hazardous drinking disorder in hospital emergency departments. The randomized BREVALCO trial. Drug Alcohol Depend 165:236–44. [DOI] [PubMed] [Google Scholar]
- Fazzino TL, Rabinowitz T, Althoff RR, et al. (2013) Monitoring daily affective symptoms and memory function using interactive voice response in outpatients receiving electroconvulsive therapy. J ECT 29:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortney J, Mukherjee S, Curran G, et al. (2004) Factors associated with perceived stigma for alcohol use and treatment among at-risk drinkers. J Behav Health Serv Res 31:418–29. [DOI] [PubMed] [Google Scholar]
- Glass JE, Bohnert KM, Brown RL (2016) Alcohol screening and intervention among United States adults who attend ambulatory healthcare. J Gen Intern Med 31:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczynski J, Mitchell SG, Gonzales A, et al. (2015) A randomized trial of computerized vs. in-person brief intervention for illicit drug use in primary care: outcomes through 12 months. J Subst Abuse Treat 50:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DH, McTavish FM, Chih MY, et al. (2014) A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA psychiatry 71:566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Aharonovich E, Greenstein E (2014) HealthCall for the smartphone: technology enhancement of brief intervention in HIV alcohol dependent patients. Addict Sci Clin Pract 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Rose GL, Badger GJ, et al. (2008) Using interactive voice response to enhance brief alcohol intervention in primary care settings. J Stud Alcohol Drugs 69:251–8. [DOI] [PubMed] [Google Scholar]
- Hester RK, Squires DD, Delaney HD (2005) The Drinker's Check-up: 12-month outcomes of a controlled clinical trial of a stand-alone software program for problem drinkers. J Subst Abuse Treat 28:159–69. [DOI] [PubMed] [Google Scholar]
- Hilbink M, Voerman G, van Beurden I, et al. (2012) A randomized controlled trial of a tailored primary care program to reverse excessive alcohol consumption. J Am Board Fam Med 25:712–22. [DOI] [PubMed] [Google Scholar]
- Johnson M, Jackson R, Guillaume L, et al. (2011) Barriers and facilitators to implementing screening and brief intervention for alcohol misuse: a systematic review of qualitative evidence. J Public Health 33:412–21. [DOI] [PubMed] [Google Scholar]
- Kaner E, Bland M, Cassidy P, et al. (2013) Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): pragmatic cluster randomised controlled trial. BMJ 346:e8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner EF, Newbury-Birch D, Heather N (2009) Brief intervention In Miller PMS.Evidence-based Addiction Treatment. Burlington, MA: Academic Press, 189–213. [Google Scholar]
- Klein AA, Slaymaker VJ, Dugosh KL, et al. (2012) Computerized continuing care support for alcohol and drug dependence: a preliminary analysis of usage and outcomes. J Subst Abuse Treat 42:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobak KA, Taylor LH, Dottl SL, et al. (1997) A computer-administered telephone interview to identify mental disorders. JAMA 278:905–10. [PubMed] [Google Scholar]
- Kypri K, Saunders JB, Williams SM, et al. (2004) Web-based screening and brief intervention for hazardous drinking: a double-blind randomized controlled trial. Addiction 99:1410–7. [DOI] [PubMed] [Google Scholar]
- McCormick KA, Cochran NE, Back AL, et al. (2006) How primary care providers talk to patients about alcohol: a qualitative study. J Gen Intern Med 21:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fazzino T, Barry DT, et al. (2013) The recovery line: a pilot trial of automated, telephone-based treatment for continued drug use in methadone maintenance. J Subst Abuse Treat 45:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer VA. (2013) Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med 159:210–8. [DOI] [PubMed] [Google Scholar]
- Mundt JC, Moore HK, Bean P (2006) An interactive voice response program to reduce drinking relapse: a feasibility study. J Subst Abuse Treat 30:21–9. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (2005) Helping Patients Who Drink Too Much: A Clinician's Guide. Washington, DC: National Institutes of Health. [Google Scholar]
- Neighbors C, Lewis MA, Atkins DC, et al. (2010) Efficacy of web-based personalized normative feedback: a two-year randomized controlled trial. J Consult Clin Psychol 78:898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell A, Anderson P, Newbury-Birch D, et al. (2014) The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol 49:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfai TP, Zisserson R, Saitz R (2011) Using personalized feedback to reduce alcohol use among hazardous drinking college students: the moderating effect of alcohol-related negative consequences. Addict Behav 36:539–42. [DOI] [PubMed] [Google Scholar]
- Palm A, Olofsson N, Danielsson I, et al. (2016) Motivational interviewing does not affect risk drinking among young women: A randomised, controlled intervention study in Swedish youth health centres. Scand J Public Health 44:611–8. [DOI] [PubMed] [Google Scholar]
- Rose GL, Badger GJ, Skelly JM, et al. (2016) A randomized controlled trial of ivr-based alcohol brief intervention to promote patient-provider communication in primary care. J Gen Intern Med 31:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GL, Ferraro TA, Skelly JM, et al. (2015. a) Feasibility of automated pre-screening for lifestyle and behavioral health risk factors in primary care. BMC Fam Pract 16:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GL, Guth SE, Badger GJ, et al. (2015. b) Brief intervention for heavy drinking in primary care: role of patient initiation. J Addict Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GL, MacLean CD, Skelly J, et al. (2010) Interactive voice response technology can deliver alcohol screening and brief intervention in primary care. J Gen Intern Med 25:340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GL, Skelly JM, Badger GJ, et al. (2015. c) Efficacy of automated telephone continuing care following outpatient therapy for alcohol dependence. Addict Behav 41:223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GL, Skelly JM, Badger GJ, et al. (2012) Interactive voice response for relapse prevention following cognitive-behavioral therapy for alcohol use disorders: a pilot study. Psychol Serv 9:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder KE, Tucker JA, Simpson CA (2013) Telephone-based self-change modules help stabilize early natural recovery in problem drinkers. J Stud Alcohol Drugs 74:902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Stappenbeck CA, Varra AA, et al. (2012) Symptoms of posttraumatic stress predict craving among alcohol treatment seekers: results of a daily monitoring study. Psychol Addict Behav 26:724–33. [DOI] [PubMed] [Google Scholar]
- Smith PC, Schmidt SM, Allensworth-Davies D, et al. (2009) Primary care validation of a single-question alcohol screening test. J Gen Intern Med 24:783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back: a technique for assessing self-reported alcohol consumption In Litten RZ, Allen JPS. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press, 41–74. [Google Scholar]
- Spandorfer JM, Israel Y, Turner BJ (1999) Primary care physicians’ views on screening and management of alcohol abuse: inconsistencies with national guidelines. J Fam Pract 48:899–902. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2015) About Screening, Brief Intervention, and Referral to Treatment (SBIRT), Vol. 2015.
- Suffoletto B, Kristan J, Callaway C, et al. (2014) A text message alcohol intervention for young adult emergency department patients: a randomized clinical trial. Ann Emerg Med 64:664–72.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson M, Volk M, Kim HM, et al. (2015) An automated telephone monitoring system to identify patients with cirrhosis at risk of re-hospitalization. Dig Dis Sci 60:3563–9. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Simpson CA, Huang J, et al. (2013) Utility of an interactive voice response system to assess antiretroviral pharmacotherapy adherence among substance users living with HIV/AIDS in the rural South. AIDS Patient Care STDS 27:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson DC, Kruse RL, Seale JP (2007) Simplifying alcohol assessment: two questions to identify alcohol use disorders. Alcohol Clin Exp Res 31:1392–8. [DOI] [PubMed] [Google Scholar]
- Wagener TL, Leffingwell TR, Mignogna J, et al. (2012) Randomized trial comparing computer-delivered and face-to-face personalized feedback interventions for high-risk drinking among college students. J Subst Abuse Treat 43:260–7. [DOI] [PubMed] [Google Scholar]
- Whitlock EP, Polen MR, Green CA, et al. (2004) Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 140:557–68. [DOI] [PubMed] [Google Scholar]
- World Health Assembly (2005) Public-health Problems Caused by Harmful Use of Alcohol. Geneva: World Health Organization. [Google Scholar]