Abstract
This article reviews the impact of diabetes and its treatment on vascular function with a focus on the reactivity of epineurial arterioles, blood vessels that provide circulation to the sciatic nerve. Another focus is the relationship between the dysregulation of neurovascular function and diabetic peripheral neuropathy. Diabetic peripheral neuropathy is a debilitating disorder that occurs in more than 50 percent of patients with diabetes. The etiology involves metabolic, vascular, and immunologic pathways besides neurohormonal growth factor deficiency and extracellular matrix remodeling. In the light of this complex etiology, an effective treatment for diabetic peripheral neuropathy has not yet been identified. Current opinion postulates that any effective treatment for diabetic peripheral neuropathy will require a combination of life style and therapeutic interventions. However, a more comprehensive understanding of the factors contributing to neurovascular and neural dysfunction in diabetes is needed before such a treatment strategy can be developed. After reading this review, the reader should have gained insight into the complex regulation of vascular function and blood flow to the sciatic nerve, and the impact of diabetes on numerous elements of vascular reactivity of epineurial arterioles of the sciatic nerve.
Keywords: diabetic peripheral neuropathy, epineurial arterioles, oxidative stress, inflammatory stress, vascular reactivity, calcitonin gene-related peptide, C-type natriuretic peptide, angiotensin converting enzyme, neutral endopeptidase
Abbreviations: ACE – angiotensin-converting enzyme; ADP – adenosine diphosphate; AGE – advanced glycation endproducts; ASIC-2 – acid-sensing ion channel 2; ATP – adenosine triphosphate; NAD(P)H – nicotinamide adenine dinucleotide phosphate; Nox – NAD(P)H oxidase; RAGE – receptor of advanced glycation endproducts; ROS – reactive oxygen species; SHR – spontaneously hypertensive rat; SOD – superoxide dismutase
1. Introduction
Peripheral neuropathy is a multi-faceted complication of diabetes that frequently leads to foot ulcers and may progress to ulceration and necrosis necessitating limb amputations [1]. Even though it is the most common complication of diabetes, the only recommended clinical treatment is good glycemic control, which only delays the onset and slows progression [2]. Diabetic peripheral neuropathy has been described by some investigators to be a disease of the vasculature leading to nerve ischemia and altered nerve function [3-6]. Other investigators have proposed that diabetic peripheral neuropathy is caused by a combination of metabolic defects associated with an increased flux of glucose through the aldose reductase pathway, leading to a defect in Na+/K+-ATPase activity and an alteration of signal transduction pathways in the nerve [7, 8]. Additional pathological contributors to diabetic peripheral neuropathy have been reported to include increased formation of advanced glycation endproducts, reduced neurotrophic support, and increased inflammatory and oxidative stress [9, 10]. Overall, these mechanisms and likely others cause damage to neurons, Schwann cells, and the vasculature. Ultimately, relentless damage to the nerve complex and surrounding vasculature leads to diabetic peripheral neuropathy. Given the complex etiology of diabetic peripheral neuropathy, a successful treatment will likely require a combination of early detection, life-style changes, and pharmaceutical interventions targeting the mechanisms deemed most responsible for the pathogenesis. Before this can occur, additional studies are needed to determine the most relevant and targetable pathways of diabetic peripheral neuropathy.
My laboratory has focused on the role of microvascular dysfunction in the development and progression of diabetic peripheral neuropathy. Our studies in both type 1 and type 2 diabetic rats have demonstrated that impaired vascular reactivity precedes the development of nerve dysfunction, as identified by reduced nerve conduction velocity [11, 12]. Moreover, our studies have indicated that increased oxidative stress is one factor leading to impaired neurovascular function in diabetes [13, 14]. Other studies have implicated the formation of excess advanced glycation endproducts (AGEs) in the onset of microvascular complications of diabetes [15-17]. Agents that block the formation of AGEs or its receptor (RAGE) are considered to be therapeutic targets for diabetic vascular complications [18-21]. Inhibition of AGE formation has also been shown to improve peripheral neuropathy in diabetic rodents [22-24]. Additional studies have shown that inhibition of aldose reductase can improve vascular dysfunction and peripheral neuropathy in diabetic rodents [11, 25-27]. Based on this work and others, it is clear that diabetic neurovascular dysfunction is a contributing factor to diabetic peripheral neuropathy [28]. Therefore, successful treatment of diabetic peripheral neuropathy needs to include interventions that improve vascular dysfunction. This article reviews the regulation of vascular reactivity of epineurial arterioles and blood vessels that provide circulation to the sciatic nerve, and discusses the current knowledge about diabetes-induced vascular impairment in these blood vessels.
2. Vascular reactivity of epineurial arterioles of the sciatic nerve
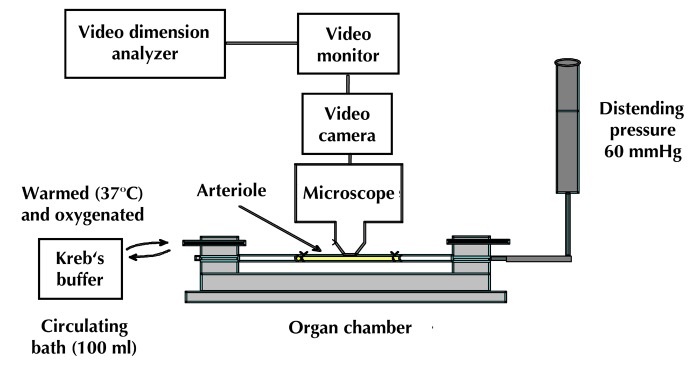
Determination of endoneurial nutritive blood flow has been a common endpoint to investigate the effect of diabetes on vascular function and diabetic peripheral neuropathy in rats, with many investigators finding an early reduction of endoneurial blood flow in the sciatic nerve of rodents with diabetes [29-35]. However, the method employed, hydrogen clearance, and the conditions maintained during the experimental procedure have contributed to controversy regarding the validity of this measurement and the contribution of changes in endoneurial blood flow to diabetic peripheral neuropathy [36-39]. Furthermore, measurement of endoneurial blood flow does not provide a direct examination of the impact of diabetes on vascular reactivity. This led us to pursue the use of video microscopy to investigate the in vitro vasodilatory responsiveness of arterioles vascularizing the region of the sciatic nerve [40]. Figure 1 provides an illustration of the epineurial arterioles we studied. These small vessels are generally oriented longitudinally in relation to the sciatic nerve and extend branches that penetrate the sciatic nerve. They are described as being epineurial rather than endoneurial to the sciatic nerve. The blood vessels used in our studies were second or third order branches of the internal pudendal and superior gluteal arteries [40]. The methodology used to isolate these blood vessels and to examine their vascular responsiveness in vitro using an organ chamber and video imaging has been previously described (Figure 2) [40, 41].
Figure 1. Epineurial arterioles innervating the sciatic nerve.
Epineurial arterioles are resistance size blood vessels branching from internal pudendal and superior gluteal arteries. These blood vessels regulate blood flow to the sciatic nerve, and have unique properties including innervation by sensory nerves that contain vasoactive peptides such as calcitonin gene-related peptide. The study of these blood vessels has yielded an array of interesting findings including early impairment of vascular relaxation by diabetes. Illustration provided by Soroku Yagihashi.
Figure 2. Vascular reactivity by videomicroscopy.
This device is used to examine vascular reactivity of epineurial arterioles of the sciatic nerve. Isolation of these blood vessel branches of the superior gluteal and internal pudendal arteries (50 to 150 µm internal diameter and 1-2 mm in length) is carried out by carefully dissecting and trimming fat and connective tissue. Both ends of the isolated vessel segment (in yellow) are then cannulated with glass micropipettes filled with physiological saline solution (PSS), and secured with nylon monofilament sutures. The pipettes are then attached to a single pressure reservoir (initially set at 0 mmHg) under conditions of "no flow". The organ chamber containing the cannulated vessels is fixed to the stage of an inverted microscope. Attached to the microscope are a closed-circuit television camera, a video monitor, and a video caliper. The organ chamber is connected to a rotary pump, which continuously circulates 37°C oxygenated PSS at 30 ml/min. The pressure within the vessel is increased to 60 mmHg over a period of 30 min. Internal vessel diameter (resolution of 2 µm) can be measured by manually adjusting the video micrometer. Vasodilation studies are performed with vessels preconstricted with U46619 to 30-50% of resting diameter.
Relaxation of epineurial arterioles of the sciatic nerve is mediated through a variety of mechanisms, suggesting that they have an important role in regulating blood flow to the sciatic nerve. Initial studies have demonstrated that acetylcholine-mediated vascular relaxation of pre-constricted epineurial arterioles is induced by two distinct endothelium-dependent mechanisms: 1) production of nitric oxide and 2) endothelium-derived hyperpolarizing factor [40]. Both diabetes and diet-induced obesity cause an impairment of acetylcholine-mediated vascular relaxation [42-44]. With diabetes, impairment of acetylcholine-mediated vascular relaxation occurred after 1-2 weeks of onset of hyperglycemia [11, 45]. Our studies have also identified C-type natriuretic peptide and calcitonin gene-related peptide as active vasodilators of epineurial arterioles [41, 46]. C-type natriuretic peptide is localized in endothelial cells of the vessel wall, and has properties consistent with endothelium-derived hyperpolarizing factor-like activity [46]. The exogenous vasodilator activity of C-type natriuretic peptide is decreased by diabetes. Epineurial arterioles of the sciatic nerve are innervated by nonadrenergic, noncholinergic nerves that immunostain for calcitonin gene-related peptide; exogenous calcitonin gene-related peptide is a very potent vasodilator. In type 1 diabetic rats, the calcitonin gene-related peptide content of sensory nerves innervating the epineurial arterioles and the vasodilation response to exogenous calcitonin gene-related peptide is decreased [41]. Insulin was also found to have a weak vasodilatory effect on epineurial arterioles [44].
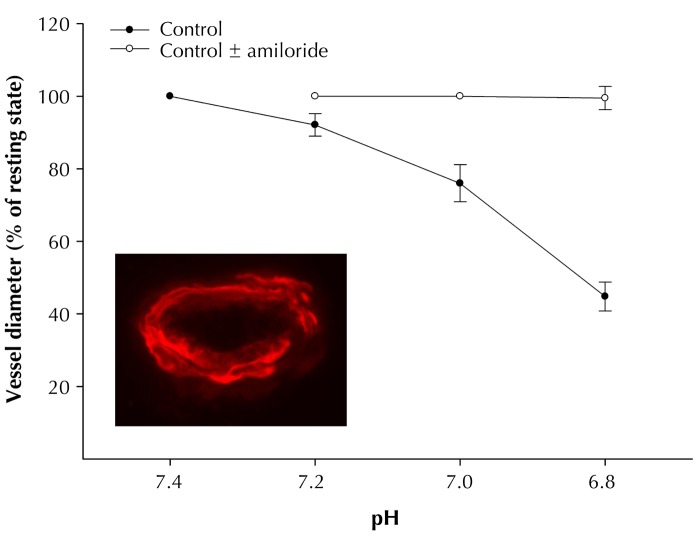
Vasoconstriction also contributes to the regulation of the vascular tone. Some compounds induce vasoconstriction in epineurial arterioles of the sciatic nerve, including vasopressin, neuropeptide Y, serotonin, and endothelin [47]. Epineurial arterioles are innervated by sensory nerves that contain neuropeptide Y [47]. When isolated epineurial arterioles were treated with capsaicin to induce vasodilation and the release of calcitonin gene-related peptide, it was surprising to find that capsaicin caused a concentration-dependent vasoconstriction [47]. The effect of capsaicin was transient, refractory, blocked by capsazepine, and duplicated by resiniferatoxin [47]. In long-term diabetic rats, capsaicin-induced vasoconstriction was attenuated. However, long-term diabetes did not impair vasoconstriction of epineurial arterioles in response to vasopressin, neuropeptide Y, serotonin, or endothelin [47]. Epineurial arterioles were also found to express receptors to vanilloid-1. In long-term diabetic rats, immunoreactivity for vanilloid receptor-1 was decreased [47]. Other interesting observations were that epineurial arterioles of the sciatic nerve expressed acid-sensing ion channel 2 (ASIC-2), and that decreasing pH caused vasoconstriction, which was prevented by pre-incubat-ing the vessels with amiloride (Figure 3). Based on these observations, it was concluded that vascular reactivity of epineurial arterioles that provide circulation to the sciatic nerve is regulated by several mecha-nisms, suggesting that control of blood flow to the sciatic nerve involves a complex interaction of vaso-regulatory compounds.
Figure 3. Effect of pH on vasoconstriction of epineurial arterioles of the sciatic nerve.
Vascular relaxation in response to pH is examined using the videomicroscopy system, as described in Figure 2. Epineurial arterioles are incubated with or without amiloride for 30 min before examining the effect of pH on vascular reactivity. As shown, a decrease in pH causes vasodilation of epineurial arterioles that is totally prevented by amiloride. The image is a representation of immunostaining for ASIC 2 in a cross section of an epineurial arteriole of the sciatic nerve. These data demonstrate the expression and functionality of ASIC in epineurial arterioles of the sciatic nerve.
3. Pathogenesis of diabetes and neurovascular tissue
3.1 Role of reactive oxygen species in diabetes-induced vascular dysfunction
Overabundance of reactive oxygen species (ROS) has long been considered to contribute to vascular dysfunction and diabetes complications [48-50]. Free radicals such as O2- and OH- cause vascular endothelial damage and reduced nitric oxide-mediated vasodilation. Angiotensin II formation or activity, AGE formation, and autoxidation are major sources of free radicals. Inhibition of these radicals by angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, aminoguanidine, transition metal chelators, antioxidants, or free radical scavengers have been shown to improve the diabetes-induced decrease in vascular function of epineurial arterioles, endoneurial blood flow, and neural dysfunction [13, 14, 51-58].
Impaired endothelium-dependent vasodilation has been demonstrated in various vascular beds of animal models of diabetes and humans with type 1 and type 2 diabetes [59, 60]. Some of the mechanisms attributed to diabetes-induced endothelium dysfunction include impaired signal transduction pathways or substrate availability, impaired release or increased metabolism of vasodilatory mediators, increased release of vascular constricting factors, and decreased reactivity of the smooth muscle to vasodilatory mediators [59, 60]. The mechanisms induced by hyperglycemia/diabetes considered to contribute to vascular dysfunction are the activation of protein kinase C, increased activity of the polyol pathway, increased formation of AGEs, increased levels of angiotensin II, and abnormal mitochondria activity. Interestingly, studies by Brownlee and colleagues have suggested hyperglycemia-induced production of O2- by mitochondria of endothelial cells as the common link for mechanisms of diabetes-induced vascular dysfunction [61, 62]. Our studies conducted with intact vascular tissue consisting of epineurial arterioles of the sciatic nerve support the studies by Brownlee and colleagues, which were conducted with cultured endothelial cells [63]. Studies by my laboratory have provided evidence that the generation of oxidative stress, through the production of O2- and peroxynitrite, impairs vascular function and endothelium-dependent vascular relaxation of epineurial arterioles of the sciatic nerve from diabetic rats, which precedes the slowing of motor nerve conduction velocity [11, 13, 14, 51, 52].
Studies designed to investigate the source of superoxide formation provided results suggesting that complex I of the mitochondrial electron transport chain and possibly NAD(P)H oxidase are responsible for the increase in O2- formation observed in epineurial arterioles derived from diabetic rats [63]. Furthermore, pretreatment of epineurial arterioles from diabetic rats with the protein kinase C inhibitor bisindolylmaleimide (GF 109203X) improved acetylcholine-mediated vascular relaxation, but did not prevent the increase in O2- formation, suggesting that activation of protein kinase C by oxidative stress is downstream of O2- formation [63]. We have also demonstrated that treatment of diabetic rats with three different types of antioxidants or chelators (α-lipoic acid, hydroxyethyl deferoxamine, or M40403) prevents the diabetes-induced increase in O2- and peroxynitrite formation in aorta and epineurial arterioles of the sciatic nerve and diabetes-induced vascular and neural dysfunction, thereby providing additional evidence that increased oxidative stress contributes to diabetes-induced vascular and neural disease [13, 14].
Studies from other laboratories have provided further evidence that antioxidants may prevent vascular complications in diabetes. Treating diabetic rats with tempol, a stable superoxide dismutase mimic compound, abolished the diabetes-induced increase in vascular O2-, malondialdehyde, and 8-epi-prostaglandin F(2α) production, and also the impairment in relaxation of aortic rings to acetylcholine [64]. Cameron and colleagues have demonstrated that treatment of diabetic rats with α-lipoic acid, or the metal chelators hydroxyethyl starch deferoxamine, or trientine prevent the diabetes-induced impairment in vascular relaxation associated with hyperalgesia and neurovascular deficits [54, 55, 65-67]. In addition, Keegan et al. found that treating diabetic rats with α-lipoic acid improves endothelium-dependent vascular relaxation of corpus cavernosum smooth muscle [57]. These studies imply that increased O2- formation via the mitochondrial electron transport chain and perhaps NAD(P)H oxidase are partially responsible for reduced vascular reactivity observed in epineurial arterioles of the sciatic nerve and other vascular tissues in diabetic rats [63]. Because metal chelators and OH- scavengers have been demonstrated to effectively prevent diabetes-induced vascular and neural dysfunction, it is likely that the formation of OH- may also contribute to impairment of vascular reactivity and nerve function in diabetes [51, 54, 55, 58, 65-68].
Besides antioxidants, therapies designed to improve nitric oxide formation have been shown to be beneficial in improving diabetes-induced vascular dysfunction. Treatment of diabetic rats with arginine or a tetrahydrobiopterin derivative improved vascular function in diabetic rats [69-75]. This implies that reduced availability of nitric oxide due to increased degradation, such as scavenging of nitric oxide by O2- to form peroxynitrite or reduced production of nitric oxide in the diabetic state, may lead to vascular impairment. Studies performed in my laboratory support the role of peroxynitrite in the pathogenesis of diabetic vascular and neural complications. Our studies demonstrated that treating diabetic rodents with a protein nitration inhibitor or peroxynitrite decomposition catalyst prevents vascular dysfunction and improves peripheral neuropathy in diabetic rodents [76, 77].
3.2 Prevention of oxidative stress as treatment for diabetic vascular and neural dysfunction
Many therapeutic approaches for the prevention of diabetic peripheral neuropathy have been applied successfully in animal models of diabetes, indicating that the etiology of the disease is multi-faceted and complex. This has led investigators to propose a wide range of mechanisms for the cause of diabetic peripheral neuropathy. Some of the different treatments that have been demonstrated to improve or prevent peripheral neuropathy in diabetic animal models include:
Aldose reductase inhibitors
Aminoguanidine
Antioxidants
Inhibitors of protein kinase C
Vasodilators
Nerve growth factor
γ-linolenic acid
Omega-3 polyunsaturated fatty acids derived from fish oils
Acetyl L-carnitine
Myo-inositol
Gangliosides
Inhibitors of poly(ADP-ribose) polymerase and others [78-92].
At this time, not all of these treatment protocols have been applied to human diabetes, although the results from those that have been used have been disappointing. This raises the question of why these treatment protocols generally failed in human trials after being successful in diabetic animal models. There are many reasons that could explain this unfavorable outcome, including the following:
Due to side effects or other concerns the dosage of the drugs used in human studies may have been inadequate and the efficacy too low to be effective against the targeted mechanism compared to animal studies. This is a valid concern and outcomes from studies using aldose reductase inhibitors, nerve growth factor, and antioxidants may have been influenced by this problem.
The targeted mechanisms for treating diabetic peripheral neuropathy in animal models may be incorrect, and have no direct contribution to diabetic peripheral neuropathy in humans. This appears unlikely as each of the compounds mentioned above, and their targeted mechanisms, are based on valid scientific data from both animal and human studies. However, whether each of the targeted mechanisms plays a significant role in the development of diabetic peripheral neuropathy in humans is a point of conjecture. Also, it is unlikely that one single mechanism is solely responsible for the development of diabetic peripheral neuropathy, and that combination therapy targeting two or more mechanisms will probably be necessary to treat diabetic peripheral neuropathy.
The patients participating in the human studies were inappropriate for the targeted mechanism because their complications have progressed to the point that the symptoms or endpoints being examined are not readily reversible. This is certainly a valid concern in studies of human diabetic peripheral neuropathy. Measurement of nerve conduction and symptom scores are the standard endpoints for many clinical trials. It takes many years of diabetes to develop deficits in nerve conduction velocity. It is possible that drug intervention in human diabetic peripheral neuropathy will likely have minimal benefit when patients have clinical symptoms that took many years to develop. The reason may be that most of these patients will probably have nerve damage that is either irreversible or only slowly reversed. Positive clinical outcomes would likely take longer to achieve than the period applied in the clinical trial. For instance, many of the clinical trials conducted with aldose reductase inhibitors were designed with a year of treatment or less. Considering the severity of the progression of diabetic peripheral neuropathy in patients who participated in these studies this treatment period is likely too short.
Outcomes were based on invalid endpoints. For instance, measurement of sorbitol for interventions using aldose reductase inhibitors may not be a good outcome predictor for the patient. Measurement of sorbitol may provide good evidence of the current metabolism, but may not reflect the severity or progression of the disease. In the case of human diabetic peripheral neuropathy, treatment with aldose reductase inhibitors may provide control of glucose flux through the aldose reductase pathway, and measurement of the level of sorbitol in tissue samples or cells may provide information on the efficacy of aldose reductase inhibitor treatment, but likely will not provide any indication of the present extent of nerve damage or progression of the disease. In order to better analyze the progression of diabetic peripheral neuropathy several relevant markers of nerve function and biological status including oxidative stress will probably be required.
These issues and failures of clinical trials have led to attempts to identify earlier markers for diabetic peripheral neuropathy. Promising research in animal models and humans have focused on examining density of the sensory nerves in the skin or cornea as an early and sensitive biomarker for diabetic peripheral neuropathy [93, 94]. Our work has indicated that loss of corneal nerves in the region of the whorl of the sub-basal plexus and penetration of the cornea epithelium are early markers for diabetic peripheral neuropathy [95, 96].
One of the most promising approaches for intervention and treatment of diabetic peripheral neuropathy and other diabetic complications is still the prevention of oxidative stress [97]. A variety of antioxidants including vitamin E have beneficial effects in treating diabetic peripheral neuropathy in diabetes patients and diabetic animal models [98-100]. More recently, α-lipoic acid has shown promise as a potential antioxidant treatment for diabetic peripheral neuropathy [101-103]. Our studies have demonstrated that α-lipoic acid provides a good protection against oxidative stress in diabetic rats of 4-6 week duration [13]. The treatment of diabetic rats with α-lipoic acid significantly improved the diabetes-induced decrease in endoneurial blood flow, endothelium-dependent vascular relaxation in arterioles that provide circulation to the region of the sciatic nerve, and motor nerve conduction velocity. α-lipoic acid treatment also reduced the production of superoxide by the aorta and superoxide and peroxynitrite by arterioles that provide circulation to the region of the sciatic nerve. Treatment of diabetic rats with α-lipoic acid prevented the diabetes-induced increase in thiobarbituric acid-reactive substances in serum, and significantly improved lens glutathione levels.
α-lipoic acid is a good metal chelator, and is capable of scavenging hydroxyl radicals, hypochlorous acid, and singlet oxygen, but not superoxide or peroxyl radicals [104-106]. However, in its reduced form, as dihydrolipoic acid, it is a good scavenger of superoxide and prevents initiation of lipid peroxidation [104-106]. In vivo, α-lipoic acid can be converted into dihydrolipoic acid [102, 104]. This reaction requires NAD(P)H, which is reduced in diabetes due to the increased flux of glucose through the aldose reductase pathway [107, 108]. Therefore, one potential form of combination therapy for the treatment of diabetic peripheral neuropathy may be combining an aldose reductase inhibitor with α-lipoic acid. This combination should promote the formation of dihydrolipoic acid, thereby enhancing the antioxidant potential of α-lipoic acid, and possibly providing a synergistic effect [25]. In a study of diabetic peripheral neuropathy in streptozotocin-induced diabetic rats conducted by Nakamura et al., the authors found that treating diabetic rats with the aldose reductase inhibitor NZ-314 improves nerve function and reduces oxidative stress [109]. They concluded that the efficacious effect of aldose reductase inhibition on diabetic peripheral neuropathy may be mediated by decreasing oxygen free radicals [109]. This would agree with our studies, demonstrating that treating diabetic rats with sorbinil improves glutathione levels presumably by correcting the redox imbalance [52]. We postulate that a similar mechanism may apply for the conversion of α-lipoic acid to dihydrolipoic acid. Correcting the redox imbalance in diabetic rats with an aldose reductase inhibitor may promote the production of dihydrolipoic acid in vivo [25].
The development of superoxide dismutase mimetics are another class of drugs with potential for treatment of diabetic complications, including diabetic peripheral neuropathy [14, 110, 111]. Because of limitations associated with enzyme therapies these non-peptidyl compounds may offer advantages resulting in better clinical therapies and outcomes for diseases mediated by O2- radicals such as diabetes [112]. In our studies, we demonstrated that treating diabetic rats with M40403 inhibits the generation of superoxide by aorta and epineurial arterioles of the sciatic nerve, the formation of peroxynitrite by epineurial arterioles of the sciatic nerve, the reduction in endoneurial blood flow, the slowing of motor nerve conduction velocity, and the impairment of endothelium-dependent vasodilation of epineurial arterioles. The treatment also improved the diabetes-induced increase in serum thio-barbituric-reactive substances and sciatic nerve-conjugated diene level, two additional markers of oxidative stress [14]. M40403 is a prototypic example of a stable, low molecular weight, manganese-containing, non-peptidic molecule possessing the function and catalytic rate of native superoxide dismutase (SOD) enzymes, but with the advantage of being a much smaller molecule (molecular weight 483 vs. 30,000 for M40403 and the native enzyme, respectively) [14, 112, 113].
Another form of treatment for diabetic peripheral neuropathy, yet to be thoroughly examined, is the intervention with ACE inhibitors and/or angiotensin receptor antagonists. It has been demonstrated that these drugs have antioxidant properties, neuroprotective potential, and may reduce the accumulation of AGEs [114-119]. Angiotensin II causes endothelium dysfunction by increasing NAD(P)H oxidase-mediated vascular 2- production [120, 121]. It has been shown that two isoforms of the NAD(P)H oxidase family (Nox), Nox1 and Nox4, are involved in the vascular oxidative stress pathways in response to angiotensin II [121]. The renin-angiotensin system is highly activated in patients with type 2 diabetes. Evidence suggests that intrarenal renin-angiotensin system activation is responsible for non-ACE-dependent angiotensin II production within the kidney and for the resulting low-renin state in patients with diabetes [122]. Hyperglycemia (both acute and sustained) and obesity activate the renin-angiotensin system [122]. A wide range of evidence now exists demonstrating that blocking of the renin-angiotensin system in diabetic patients reduces the progression of diabetic nephropathy [122]. However, little information is available whether treatment of diabetic patients with ACE inhibitors or angiotensin receptor antagonists improves or prevents the progression of diabetic neurovascular and neural disease. To provide an answer as to whether blocking the renin-angiotensin system can prevent diabetic peripheral neuropathy two issues must be addressed:
1. Does vascular dysfunction cause diabetic peripheral neuropathy?
2. Can ACE inhibitors and/or angiotensin receptor antagonists ameliorate diabetic vascular dysfunction and hence neuropathy [123]?
Existing data suggests that vascular dysfunction contributes significantly to the development and progression of diabetic peripheral neuropathy [13, 14, 52, 53]. It has also been demonstrated that treatment with ACE inhibitors improves endothelial dysfunction and reduces oxidative stress in diabetes [114, 118, 124]. Therefore, it seems likely that ACE inhibitors and/or angiotensin receptor antagonists may improve diabetic peripheral neuropathy. In studies by Cameron and colleagues with diabetic animal models, treatment of diabetic rats with an ACE inhibitor or with an angiotensin receptor antagonist improved motor and sensory nerve conduction velocities, increases endoneurial blood flow, and stimulated endoneurial angiogenesis [125, 126]. Likewise, Aggarwal et al. have demonstrated that treating streptozotocin-induced diabetic rats with lisinopril, an ACE inhibitor, improves diabetic peripheral neuropathy [127]. We have demonstrated that treating diet-induced obese and diabetic rats with enalapril improves glucose utilization and endpoints associated with diabetic peripheral neuropathy, including impaired vascular relaxation to acetylcholine and calcitonin gene-related peptide [128-133]. In studies using streptozotocin-induced diabetic rats, we demonstrated that treating diabetic rats with enalapril or the angiotensin receptor blocker L158809 improves diabetes-induced vascular and neural dysfunction [133]. Significant improvement was observed even when treatment was initiated 12 weeks after the onset of hyperglycemia.
We also found that treatment with enalapril is more effective in preventing/reversing diabetes-induced vascular and neural dysfunction than treatment with L158809. In contrast to reports suggesting synergy in treatment of diabetic nephropathy or hypertension by combination of ACE inhibitors with an angiotensin II receptor blocker, we found no evidence of synergy in the preventing/reversal of vascular and neural dysfunction related to diabetic peripheral neuropathy when we combined enalapril and L158809 [134-136]. We found that treatment of diabetic rats with enalapril or L158809 prevented/reversed superoxide formation by the aorta [132]. However, enalapril treatment was only partially effective and treatment with L158809 was non-effective in preventing/reversing superoxide formation in epineurial arterioles of the sciatic nerve. This is consistent with previous studies that have demonstrated that these drugs can prevent angiotensin II stimulation of NAD(P)H oxidase activity in large vessels associated with hypertension or diabetes, but subsequently increased the formation of superoxide [119, 120]. Our previous studies have suggested that the mitochondria may be the primary contributor to superoxide formation by epineurial arterioles derived from diabetic rats [63]. Therefore, it appears that enalapril and to a greater extent L158809 may have a limited capacity for preventing superoxide formation and oxidative stress in resistance size vessels.
Vasopeptidase inhibitors are a new class of drugs that simultaneously inhibit neutral endopeptidase and ACE activity [137]. Neutral endopeptidase is found in many tissues including vascular and renal tissue, and its activity is increased by fatty acids and glucose in human microvascular cells [138-142]. Interestingly, neutral endopeptidase is activated by protein kinase C, which is increased by diabetes in vascular tissues including endothelial cells [143, 144]. Neutral endopeptidase degrades natriuretic peptides, adrenomedullin, bradykinin, endothelin, and calcitonin gene-related peptide [145]. Therefore, the use of vasopeptidase inhibitors would likely promote expression of calcitonin gene-related peptide and C-type natriuretic peptide by blocking their degradation and thus, improving vascular functions. In this regard, vascular conductance in the femoral artery of streptozotocin-induced diabetic rats is improved by a vasopeptidase inhibitor [146]. Vasopeptidase inhibitors have also been reported to decrease matrix metalloproteinases and AGE accumulation/formation in type 2 diabetes and to improve wound healing [147-149]. Finally, it has been reported that neutral endopeptidase is responsible for 50% of the degradation of glucagon-like peptide 1 [150].
In summary, there is great potential for the treatment of diabetic complications with vasopeptidase inhibitors. However, there is no information about the potential benefits of vasopeptidase inhibitors in diabetic peripheral neuropathy. To address this issue we performed studies using diet-induced obese rats, streptozotocin-induced diabetic rats, and Zucker diabetic fatty rats treated with ilepatril, a vasopeptidase inhibitor [46, 128, 129, 151-154]. These studies demonstrated for the first time that treatment of obese or diabetic rats (modeling types 1 or 2 diabetes) with a vasopeptidase inhibitor significantly improved vascular and neural function. Correction of acetylcholine-mediated vascular relaxation in epineurial arterioles, endoneurial blood flow, and motor nerve conduction velocity was nearly 100%, while sensory nerve conduction velocity was improved, and thermal hypoalgesia was prevented by about 75%. Ilepatril also prevented loss of sensory nerves in the skin and cornea [155]. Vasopeptidase inhibitor treatment also significantly reduced oxidative stress in the aorta and epineurial arterioles. Overall, the level of efficacy with ilepatril was the greatest we have observed compared to a variety of other interventions ranging from antioxidants, ACE inhibitors, angiotensin receptor antagonists, aldose reductase inhibitors, aminoguanidine, or myo-inositol [11-14, 25].
There are likely several reasons for this level of efficacy:
1. As we have demonstrated, epineurial arterioles of the sciatic nerve express both C-type natriuretic peptide and neutral endopeptidase. Moreover, in diabetes the expression of neutral endopeptidase was increased [46]. It has been demonstrated that neutral endopeptidase decreases the bioactivity of C-type natriuretic peptide, and that local concentrations of vasoactive peptides in the vessel wall are regulated by the neutral endopeptidase cleavage pathway in the immediate vicinity of their target cells [145, 156]. Therefore, in epineurial arterioles of obese and diabetic rats increased expression of neutral endopeptidase can decrease C-type natriuretic peptide bioactivity, and treatment of diabetic rats with ilepatril protects C-type natriuretic peptide from degradation.
2. As we previously demonstrated, treatment with antioxidants protects vascular and neural function in streptozotocin-induced diabetic rats [13, 14]. Since vasopeptidase inhibitors contain ACE inhibitor activity and the ACE inhibitor enalapril improved diabetes-induced vascular and neural dysfunction and reduced oxidative stress in vascular tissue (as we have also demonstrated), it is likely that ilepatril efficacy is partially due to antioxidant properties [128, 129, 131, 133]. This was supported by studies demonstrating that increased formation of superoxide and nitrotyrosine in epineurial arterioles of diabetic rats is significantly reduced by treatment with ilepatril [46].
3. It has been demonstrated that another target for neutral endopeptidase is calcitonin gene-related peptide [157]. Neutral endopeptidase plays a major role in the inactivation of calcitonin gene-related peptide released from sensory fibers. It actively degrades calcitonin gene-related peptide in human skin, whereas inhibition of neutral endopeptidase increases circulating plasma levels of calcitonin gene-related peptide [158-160]. We have demonstrated that epineurial arterioles of the sciatic nerve are innervated by sensory nerves that contain calcitonin gene-related peptide, and that calcitonin gene-related peptide is an important regulator of vascular tone and blood flow in the sciatic nerve [41]. Furthermore, we have demonstrated that the bioactivity of exogenous calcitonin gene-related peptide and calcitonin gene-related peptide expression are decreased in type 1 and type 2 diabetic rats [41, 45]. Since neutral endopeptidase expression is increased in diabetes, the decrease in calcitonin gene-related peptide bioactivity in diabetes could partially be due to increased degradation. Overall, a decrease in C-type natriuretic peptide and calcitonin gene-related peptide bioactivity in vivo would likely compromise vascular tone, impair blood flow, induce ischemia, and subsequently decrease neural function. The likelihood of vasopeptidase inhibitors becoming a treatment option however is very small.
Ilepatril and other vasopeptidase inhibitors were found to cause angioedema in a small number of human subjects, and further development is unlikely. However, Novartis is developing LCZ696, a first-in class dual angiotensin II receptor blocker and neutral endopeptidase inhibitor [161]. This combination drug has been shown to reduce hypertension in spontaneously hypertensive rats (SHRs) [162]. In human studies, LCZ696 was found to be superior to enalapril in reducing the risks of death and hospitalizations for heart failure [163, 164]. The efficacy of LCZ696 in this study was so high that the clinical trial was stopped earlier than anticipated. It is believed that this drug may become available for testing of other purposes such as diabetes complications. My laboratory is currently negotiating with Novartis with the intent to test LCZ696 as a treatment for diabetic vascular and neural complications.
Another potential treatment for diabetic vascular and neural disease that may involve protection of neural tissue from oxidative stress is inhibition of protein kinase C and poly ADP ribose polymerase [165-172]. The protein kinase C family includes at least 12 isoforms and a number of other proteins with homology to either the phorbol ester/diacylglycerol domain or the protein kinase C-terminal region [165]. One specific isoform, protein kinase C-β2, has been found to be activated in retina, heart, and aorta of diabetic rats [165]. Using a specific inhibitor of the protein kinase C-β2 isoform, researchers found that retinal and renal vascular reactivity is normalized and renal function is improved in diabetic rats [165]. Recently, studies have demonstrated that protein kinase C-β2 inhibition also improves nerve conduction velocity and endoneurial blood flow in diabetic rats [167]. At present, the mechanism responsible for protein kinase C-β2-mediated vascular dysfunction in diabetes is unknown. However, it has been shown that high concentrations of vitamin E can reverse some of the changes in retinal and renal vessels caused by diabetes and inhibit the activation of protein kinase C-β2 by diabetes or hyperglycemia [166]. Furthermore, Brownlee and colleagues have demonstrated that the activation of protein kinase C by hyperglycemia in cultured bovine aorta endothelial cells is downstream of O2- generation, indicating that increased oxidative stress may influence the activation of protein kinase C in vascular tissue [62].
Another possible downstream mediator of hyperglycemia-induced oxidative stress is poly ADP ribose polymerase. Poly ADP ribose polymerase is an abundant nuclear enzyme of eukaryotic cells that participates in DNA repair in response to genotoxic stress [169, 170]. When activated by DNA single-stranded breaks, poly ADP ribose polymerase initiates an energy-dependent cycle by transferring ADP ribose units from NAD+ to nuclear proteins. This process results in rapid depletion of the intracellular NAD+ and ATP pools by slowing the rate of glycolysis and mitochondrial respiration, and eventually leading to cellular dysfunction and death [170]. Overactivation of poly ADP ribose polymerase represents an important mechanism of tissue damage in various pathological conditions associated with oxidant stress, including diabetes [169]. Recently, Pacher et al. have reported that the activation of poly ADP ribose polymerase contributes to the development of endothelium dysfunction in streptozotocin-induced diabetic mice [170]. In our studies, inhibition of poly ADP ribose polymerase reduced oxidative stress in epineurial arterioles of the sciatic nerve and aorta, and improved diabetic peripheral neuropathy [171, 172]. The development and use of specific inhibitors of poly ADP ribose polymerase have indicated that poly ADP ribose polymerase may be a novel target for the intervention of diabetes-induced endothelial dysfunction [169-174].
4. Conclusions
In summary, neurovascular dysfunction in diabetes is closely related to the development and progression of diabetic peripheral neuropathy. We have found that impaired endothelium-dependent vascular relaxation by epineurial arterioles of the sciatic nerve precedes the development of diabetic peripheral neuropathy. The regulation of vascular reactivity of epineurial arterioles of the sciatic nerve involves the interaction of a number of vasoactive mediators; many of which are affected by diabetes. The diabetes-induced dysregulation of vascular reactivity of epineural arterioles of the sciatic nerve is also complex, and likely involves an increase in oxidative stress and activation of ACE and neutral endopeptidase. Preventing diabetes-induced increase in oxidative stress using antioxidants or drugs having antioxidant properties and inhibiting downstream mediators of oxidative stress have been shown to improve vascular activity and neural function in diabetic animal models, indicating that an increase in oxidative stress in diabetes is associated with neural dysfunction.
Future studies should focus on determining the most effective therapeutic approach, timing and dosage of antioxidant therapy for preventing the diabetes-induced increase in oxidative stress and thus, the development and/or progression of diabetes complications including diabetic peripheral neuropathy [97]. In patients, an effective treatment for diabetic peripheral neural will likely require early intervention, prior to the development of severe clinical symptoms, with a combination of drugs that target several mechanisms most highly associated with the development and progression of diabetic peripheral neuropathy. The development of reliable early clinical endpoints or markers of diabetic peripheral neuropathy will also be required in order to effectively evaluate the efficacy of these drugs and outcomes during the clinical studies.
Acknowledgments
Disclosures
The author reports no conflict of interests.
References
- 1.Kim H, Kim JJ, Yoon YS. Emerging therapy for diabetic neuropathy: cell therapy targeting vessels and nerves. Endocr Metab Immune Disord Drug Targets. 2012;12:168–178. doi: 10.2174/187153012800493486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron NE, Cotter MA, Low PA. Nerve blood flow in early experimental diabetes in rats: relation to conduction deficits. Am J Physiol. 1991;261:E1–E8. doi: 10.1152/ajpendo.1991.261.1.E1. [DOI] [PubMed] [Google Scholar]
- 4.Nukada H, Dyck PJ. Microsphere embolization of nerve capillaries and fiber degeneration. Am J Pathol. 1984;115:275–287. [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron NE, Cotter MA, Dines KC, Maxfield EK, Carey F, Mirrlees DJ. Aldose reductase inhibition, nerve perfusion, oxygenation and function in streptozotocin-diabetic rats: dose-response considerations and independence from a myo-inositol mechanism. Diabetologia. 1994;37:651–663. doi: 10.1007/BF00417688. [DOI] [PubMed] [Google Scholar]
- 6.Cameron NE, Cotter MA, Archibald V, Dines KC, Maxfield EK. Anti-oxidant and pro-oxidant effects on nerve conduction velocity, endoneurial blood flow and oxygen tension in non-diabetic and streptozotocin-diabetic rats. Diabetologia. 1994;37:449–459. doi: 10.1007/s001250050131. [DOI] [PubMed] [Google Scholar]
- 7.Cotter MA, Dines KC, Cameron NE. Prevention and reversal of motor and sensory peripheral nerve conduction abnormalities in streptozotocin-diabetic rats by the prostacyclin analogue iloprost. Naunyn Schmiedebergs Archives Pharm. 1993;347:534–540. doi: 10.1007/BF00166747. [DOI] [PubMed] [Google Scholar]
- 8.Cameron NE, Cotter MA, Dines KC, Love A. Effects of aminoguanidine on peripheral nerve function and polyol pathway metabolites in streptozotocin-diabetic rats. Diabetologia. 1992;35:946–950. doi: 10.1007/BF00401423. [DOI] [PubMed] [Google Scholar]
- 9.Sima AA. Pathological mechanisms involved in diabetic neuropathy: can we slow the process? Curr Opin Invest Drugs. 2006;7:324–337. [PubMed] [Google Scholar]
- 10.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 11.Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int J Exp Diabetes Res. 2000;1:131–143. doi: 10.1155/EDR.2000.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Changes in endoneurial blood flow, motor nerve conduction velocity and vascular relaxation of epineurial arterioles of the sciatic nerve in ZDF-obese diabetic rats. Diabetes Metab Res Rev. 2002;18:449–456. doi: 10.1002/dmrr.257. [DOI] [PubMed] [Google Scholar]
- 13.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001;50:1927–1937. doi: 10.2337/diabetes.50.8.1927. [DOI] [PubMed] [Google Scholar]
- 14.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Salvemini D, Yorek A. Effect of M40403 treatment of diabetic rats on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Br J Pharmacol. 2001;134:21–29. doi: 10.1038/sj.bjp.0704216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chilelli NC, Burlina S, Lapolla A. AGEs, rather than hyperglycemia are responsible for microvascular complications in diabetes: a “glycoxidation-centric” point of view. Nutr Metab Cardiovasc Dis. 2013;23:913–919. doi: 10.1016/j.numecd.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi M, Takino J, Yamagishi S. Involvement of the toxic AGEs (TAGE)-RAGE system in the pathogenesis of diabetic vascular complications: a novel therapeutic strategy. Curr Drug Targets. 2010;11:1468–1482. doi: 10.2174/1389450111009011468. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi S, Nakamura K, Matsui T, Ueda S, Fukami K, Okuda S. Agents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: a novel therapeutic strategy for diabetic vascular complications. Expert Opin Investig Drugs. 2008;17:983–996. doi: 10.1517/13543784.17.7.983. [DOI] [PubMed] [Google Scholar]
- 18.Yamagishi S, Nakamura K, Matsui T, Noda Y, Imaizumi T. Receptor for advanced glycation end products (RAGE): a novel therapeutic target for diabetic vascular complication. Curr Pharm Des. 2008;14:487–495. doi: 10.2174/138161208783597416. [DOI] [PubMed] [Google Scholar]
- 19.Win MT, Yamamoto Y, Munesue S, Saito H, Han D, Motoyoshi S, Kamai T, Ohara T, Watanabe T, Yamamoto H. Regulation of RAGE for attenuating progression of diabetic vascular complications. Exp Diabetes Res. 2012;2012:894605. doi: 10.1155/2012/894605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagishi S, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr Diabetes Rev. 2005;1:93–106. doi: 10.2174/1573399052952631. [DOI] [PubMed] [Google Scholar]
- 21.Cameron NE, Gibson TM, Nangle MR, Cotter MA. Inhibitors of advanced glycation end product formation and neurovascular dysfunction in experimental diabetes. Ann N Y Acad Sci. 2005;1043:784–792. doi: 10.1196/annals.1333.091. [DOI] [PubMed] [Google Scholar]
- 22.Wada R, Yagihashi S. Role of advanced glycation end product and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci. 2005;1043:598–604. doi: 10.1196/annals.1338.067. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14:953–961. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- 24.Lukic IK, Humpert PM, Nawroth PP, Bierhaus A. The RAGE pathway: activation and perpetuation in the pathogenesis of diabetic neuropathy. Ann N Y Acad Sci. 2008;1126:76–80. doi: 10.1196/annals.1433.059. [DOI] [PubMed] [Google Scholar]
- 25.Yorek MA, Coppey LJ, Gellett JS, Davidson EP, Lund DD. Effect of fidarestat and alpha-lipoic acid on diabetes-induced epineurial arteriole vascular dysfunction. Exp Diabesity Res. 2004;5:123–135. doi: 10.1080/15438600490277824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung SS, Chung SK. Aldose reductase in diabetic microvascular complications. Curr Drug Targets. 2005;6:476–486. doi: 10.2174/1389450054021891. [DOI] [PubMed] [Google Scholar]
- 27.Obrosova IG, Pacher P, Szabo C, Zsengeller Z, Hirooka H, Stevens MJ, Yorek MA. Aldose reductase inhibition counteracts oxidative-nitrosative stress and poly(ADP-ribose) polymerase activation in tissue sites for diabetes complications. Diabetes. 2005;54:234–242. doi: 10.2337/diabetes.54.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kles KA, Vinik AI. Pathophysiology and treatment of diabetic peripheral neuropathy: the case for diabetic neurovascular function as an essential component. Curr Diabetes Rev. 2006;2:131–145. doi: 10.2174/157339906776818569. [DOI] [PubMed] [Google Scholar]
- 29.van Dam PS. Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2002;18:176–184. doi: 10.1002/dmrr.287. [DOI] [PubMed] [Google Scholar]
- 30.Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49:1006–1015. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- 31.Obrosova IG, Van Huysen C, Fathallah L, Cao XC, Greene DA, Stevens MJ. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2002;16:123–125. doi: 10.1096/fj.01-0603fje. [DOI] [PubMed] [Google Scholar]
- 32.Cotter MA, Cameron NE. Correction of neurovascular deficits in diabetic rats by beta2-adrenoceptor agonist and alpha1-adrenoceptor antagonist treatment: interactions with the nitric oxide system. Eur J Pharmacol. 1998;343:217–223. doi: 10.1016/s0014-2999(97)01533-1. [DOI] [PubMed] [Google Scholar]
- 33.Cotter MA, Cameron NE. Effects of dietary supplementation with arachidonic acid rich oils on nerve conduction velocity and blood flow in streptozotocin-diabetic rats. Prostaglandins Leukot Essent Fatty Acids. 1997;56(5):337–343. doi: 10.1016/s0952-3278(97)90581-0. [DOI] [PubMed] [Google Scholar]
- 34.Cameron NE, Cotter MA, Basso M, Hohman TC. Comparison of the effects of inhibitors of aldose reductase and sorbitol dehydrogenase on neurovascular function, nerve conduction and tissue polyol pathway metabolites in streptozotocin-diabetic rats. Diabetologia. 1997;40:271–281. doi: 10.1007/s001250050674. [DOI] [PubMed] [Google Scholar]
- 35.Cameron NE, Cotter MA. Comparison of the effects of ascorbyl gamma-linolenic acid and gamma-linolenic acid in the correction of neurovascular deficits in diabetic rats. Diabetologia. 1996;39:1047–1054. doi: 10.1007/BF00400653. [DOI] [PubMed] [Google Scholar]
- 36.Zochodne DW. Nerve and ganglion blood flow in diabetes: an appraisal. Int Rev Neurobiol. 2002;50:161–202. doi: 10.1016/s0074-7742(02)50077-5. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy JM, Zochodne DW. Influence of experimental diabetes on the microcirculation of injured peripheral nerve: functional and morphological aspects. Diabetes. 2002;51:2233–2240. doi: 10.2337/diabetes.51.7.2233. [DOI] [PubMed] [Google Scholar]
- 38.Theriault M, Dort J, Sutherland G, Zochodne DW. Local human sural nerve blood flow in diabetic and other polyneuropathies. Brain. 1997;120:1131–1138. doi: 10.1093/brain/120.7.1131. [DOI] [PubMed] [Google Scholar]
- 39.Zochodne DW, Ho LT. Normal blood flow but lower oxygen tension in diabetes of young rats: microenvironment and the influence of sympathectomy. Can J Physiol Pharmacol. 1992;70:651–659. doi: 10.1139/y92-083. [DOI] [PubMed] [Google Scholar]
- 40.Terata K, Coppey LJ, Davidson EP, Dunlap JA, Gutterman DD, Yorek MA. Acetylcholine-induced arteriolar dilation is reduced in streptozotocin-induced diabetic rats with motor nerve dysfunction. Br J Pharmacol. 1999;128:837–843. doi: 10.1038/sj.bjp.0702856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yorek MA, Coppey LJ, Gellett JS, Davidson EP. Sensory nerve innervation of epineurial arterioles of the sciatic nerve containing calcitonin gene-related peptide: effect of streptozotocin-induced diabetes. Exp Diabesity Res. 2004;5:187–193. doi: 10.1080/15438600490486732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coppey LJ, Gellett JS, Yorek MA. Mediation of vascular relaxation in epineurial arterioles of the sciatic nerve: effect of diabetes in type 1 and type 2 diabetic rat models. Endothelium. 2003;10:89–94. doi: 10.1080/10623320303366. [DOI] [PubMed] [Google Scholar]
- 43.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Yorek MA. Attenuation of vascular/neural dysfunction in Zucker rats treated with enalapril and rosuvastatin. Obesity. 2008;16:82–89. doi: 10.1038/oby.2007.19. [DOI] [PubMed] [Google Scholar]
- 44.Davidson EP, Coppey LJ, Calcutt NA, Oltman CL, Yorek MA. Diet-induced obesity in Sprague-Dawley rats causes microvascular and neural dysfunction. Diabetes Metab Res Rev. 2010;26:306–318. doi: 10.1002/dmrr.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oltman CL, Coppey LJ, Gellett JS, Davidson EP, Lund DD, Yorek MA. Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am J Physiol Endocrinol Metab. 2005;289:E113–E122. doi: 10.1152/ajpendo.00594.2004. [DOI] [PubMed] [Google Scholar]
- 46.Davidson EP, Kleinschmidt TL, Oltman CL, Lund DD, Yorek MA. Treatment of streptozotocin-induced diabetic rats with AVE7688, a vasopeptidase inhibitor: effect on vascular and neural disease. Diabetes. 2007;56:355–362. doi: 10.2337/db06-1180. [DOI] [PubMed] [Google Scholar]
- 47.Davidson EP, Coppey LJ, Yorek MA. Activity and expression of the vanilloid receptor 1 (TRPV1) is altered by long-term diabetes in epineurial arterioles of the rat sciatic nerve. Diabetes Metab Res Rev. 2006;22:211–219. doi: 10.1002/dmrr.599. [DOI] [PubMed] [Google Scholar]
- 48.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 50.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 51.Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes. 1997;46:S31–S37. doi: 10.2337/diab.46.2.s31. [DOI] [PubMed] [Google Scholar]
- 52.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Effect of treating streptozotocin-induced diabetic rats with sorbinil, myo-inositol or aminoguanidine on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Int J Exp Diab Res. 2002;3:21–36. doi: 10.1080/15604280212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yorek MA, Coppey LJ, Gellett JS, Davidson EP, Bing X, Lund DD, Dillon JS. Effect of treatment of diabetic rats with Dehydroepiandrosterone (DHEA) on vascular and neural function. Am J Physiol. 2002;283:E1067–E1075. doi: 10.1152/ajpendo.00173.2002. [DOI] [PubMed] [Google Scholar]
- 54.Cameron NE, Cotter MA, Maxfield EK. Antioxidant treatment prevents the development of peripheral nerve dysfunction in streptozotocin-diabetic rats. Diabetologia. 1993;36:299–304. doi: 10.1007/BF00400231. [DOI] [PubMed] [Google Scholar]
- 55.Cameron NE, Cotter MA. Neurovascular dysfunction in diabetic rats: potential contribution of autoxidation and free radicals examined using transition metal chelating agents. J Clin Invest. 1995;96:1159–1163. doi: 10.1172/JCI118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cameron NE, Cotter MA. Effects pf antioxidants on nerve and vascular dysfunction in experimental diabetes. Diabetes Res Clin Practice. 1999;45:137–146. doi: 10.1016/s0168-8227(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 57.Keegan A, Cotter MA, Cameron NE. Effects of diabetes and treatment with the antioxidant α-lipoic acid on endothelial and neurogenic responses of corpus cavernosum in rats. Diabetologia. 1999;42:343–350. doi: 10.1007/s001250051161. [DOI] [PubMed] [Google Scholar]
- 58.Karasu C, Dewhurst M, Stevens EJ, Tomlinson DR. Effects of antioxidant treatment on sciatic nerve dysfunction in streptozotocin-diabetic rats; comparison with essential fatty acids. Diabetologia. 1995;38:129–134. doi: 10.1007/BF00400086. [DOI] [PubMed] [Google Scholar]
- 59.Pieper GW, Siebeneich W. Diabetes-induced endothelial dysfunction is prevented by long-term treatment with the modified iron chelator, hydroxyethyl starch conjugated-deferoxamine. J Cardiovasc Pharmacol. 1997;30:734–738. doi: 10.1097/00005344-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 60.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 62.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 63.Coppey LJ, Gellett JS, Davidson EP, Yorek MA. Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radical Res. 2003;37:33–40. doi: 10.1080/1071576021000028442. [DOI] [PubMed] [Google Scholar]
- 64.Nassar T, Kadery B, Lotan C, Da'as N, Kleinman Y, Haj-Yehia A. Effects of the superoxide dismutase-mimetic compound tempol on endothelial dysfunction in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2002;436:111–118. doi: 10.1016/s0014-2999(01)01566-7. [DOI] [PubMed] [Google Scholar]
- 65.Cameron NE, Jack AM, Cotter MA. Effect of alpha-lipoic acid on vascular responses and nociception in diabetic rats. Free Radical Biol Med. 2001;31:125–135. doi: 10.1016/s0891-5849(01)00564-0. [DOI] [PubMed] [Google Scholar]
- 66.Cameron NE, Cotter MA. Effects of an extracellular metal chelator on neurovascular function in diabetic rats. Diabetologia. 2001;44:621–628. doi: 10.1007/s001250051669. [DOI] [PubMed] [Google Scholar]
- 67.Inkster ME, Cotter MA, Cameron NE. Effects of trientine, a metal chelator, on defective endothelium-dependent relaxation in the mesenteric vasculature of diabetic rats. Free Radic Res. 2002;36(10):1091–1099. doi: 10.1080/1071576021000028325. [DOI] [PubMed] [Google Scholar]
- 68.Cameron NE, Tuck Z, McCabe L, Cotter MA. Effect of the hydroxyl radical scavenger, dimethylthiourea, on peripheral nerve tissue perfusion, conduction velocity and nociception in experimental diabetes. Diabetologia. 2001;44:1161–1169. doi: 10.1007/s001250100626. [DOI] [PubMed] [Google Scholar]
- 69.Pieper GM, Dondlinger LA. Plasma and vascular tissue arginine are decreased in diabetes: acute supplementation restores endothelium-dependent relaxation by augmenting cGMP production. J Pharmacol Exp Ther. 1997;283(2):684–690. [PubMed] [Google Scholar]
- 70.Giugliano D, Marfella R, Coppola L, Verrazzo G, Acampora R, Giuta R, Nappo F, Lucarelli C, D'Onofrio F. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Circulation. 1997;95:1783–1790. doi: 10.1161/01.cir.95.7.1783. [DOI] [PubMed] [Google Scholar]
- 71.Pieper GM, Siebeneich W, Dondlinger LA. Short-term oral administration of L-arginine reverses defective endothelium-dependent relaxation and cGMP generation in diabetes. Eur J Pharmacol. 1996;317(2-3):317–320. doi: 10.1016/s0014-2999(96)00831-x. [DOI] [PubMed] [Google Scholar]
- 72.Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial junction. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.hyp.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 73.Pieper GM, Peltier BA. Amelioration by L-arginine of a dysfunctional arginine/nitric oxide pathway in diabetic endothelium. J Cardiovasc Pharmacol. 1995;25:397–403. doi: 10.1097/00005344-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Pieper GM. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J Cardiovasc Pharmacol. 1997;29:8–15. doi: 10.1097/00005344-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Katusic ZS. Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol. 2001;281:H981–H986. doi: 10.1152/ajpheart.2001.281.3.H981. [DOI] [PubMed] [Google Scholar]
- 76.Obrosova IG, Drel VR, Oltman CL, Mashtalir N, Tibrewala J, Groves JT, Yorek MA. Role of nitrosative stress in early neuropathy and vascular dysfunction in streptozotocin-diabetic-rats. Am J Physiol Endocrinol Metab. 2007;293:E1645–E1655. doi: 10.1152/ajpendo.00479.2007. [DOI] [PubMed] [Google Scholar]
- 77.Stavniichuk R, Shevalye H, Lupachyk S, Obrosov A, Groves JT, Obrosova IG, Yorek MA. Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2014;30:669–678. doi: 10.1002/dmrr.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boulton AJ, Malik RA. Diabetic neuropathy. Med Clin North Am. 1998;82(4):909–929. doi: 10.1016/s0025-7125(05)70029-8. [DOI] [PubMed] [Google Scholar]
- 79.Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43:957–973. doi: 10.1007/s001250051477. [DOI] [PubMed] [Google Scholar]
- 80.Ward JD. Biochemical and vascular factors in the pathogenesis of diabetic neuropathy. Clin Invest Med. 1995;18:267–274. [PubMed] [Google Scholar]
- 81.Tomlinson DR, Stevens EJ, Diemel LT. Aldose reductase inhibitors and their potential for the treatment of diabetic complications. Trends Pharmacol Sci. 1994;15(8):293–297. doi: 10.1016/0165-6147(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 82.Tomlinson DR, Willars GB, Carrington AL. Aldose reductase inhibitors and diabetic complications. Pharmacol Ther. 1992;54(2):151–194. doi: 10.1016/0163-7258(92)90031-t. [DOI] [PubMed] [Google Scholar]
- 83.Cameron NE, Cotter MA. The relationship of vascular changes to metabolic factors in diabetes mellitus and their role in the development of peripheral nerve complications. Diabetes Metab Rev. 1994;10:189–224. doi: 10.1002/dmr.5610100302. [DOI] [PubMed] [Google Scholar]
- 84.Sima AA, Sugimoto K. Experimental diabetic neuropathy: an update. Diabetologia. 1999;42:773–788. doi: 10.1007/s001250051227. [DOI] [PubMed] [Google Scholar]
- 85.Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Int Med. 1984;101:527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- 86.Tesfaye S, Malik R, Ward JD. Vascular factors in diabetic neuropathy. Diabetologia. 1994;37:847–854. doi: 10.1007/BF00400938. [DOI] [PubMed] [Google Scholar]
- 87.Feener EP, King GL. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350:9–13. doi: 10.1016/s0140-6736(97)90022-2. [DOI] [PubMed] [Google Scholar]
- 88.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 89.Feldman EL, Russell JW, Sullivan KA, Golovoy D. New insights into the pathogenesis of diabetic neuropathy. Curr Opin Neurol. 1999;12:553–563. doi: 10.1097/00019052-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 90.Schmidt AM, Stern D. A radical approach to the pathogenesis of diabetic complications. Trends Pharmacol Sci. 2000;21:367–369. doi: 10.1016/s0165-6147(00)01537-6. [DOI] [PubMed] [Google Scholar]
- 91.Coppey LJ, Davidson EP, Obrosov A, Yorek MA. Enriching the diet with menhaden oil improves peripheral neuropathy in streptozotocin-induced type 1 diabetic rats. J Neurophysiol. 2015;113:701–708. doi: 10.1152/jn.00718.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coppey LJ, Holmes A, Davidson EP, Yorek MA. Partial replacement with menhaden oil improves peripheral neuropathy in high fat fed low dose streptozotocin type 2 diabetic rat. J Nutr Metab. 2012;2012:950517. doi: 10.1155/2012/950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, Bruggermann J, Strom A, Peschel S, Kohler B, Stachs O, Guthoff RF, Roden M. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63:2454–2463. doi: 10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- 94.Malik RA. Which test for diagnosing early human diabetic neuropathy? Diabetes. 2014;63:2206–2208. doi: 10.2337/db14-0492. [DOI] [PubMed] [Google Scholar]
- 95.Davidson EP, Coppey LJ, Holmes A, Yorek MA. Changes in corneal innervation and sensitivity and acetylcholine-mediated vascular relaxation of the posterior ciliary artery in a type 2 diabetic rat. Invest Ophthalmol Vis Sci. 2012;53(3):1182–1187. doi: 10.1167/iovs.11-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davidson EP, Coppey LJ, Kardon RH, Yorek MA. Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Invest Ophthalmol Vis Sci. 2014;55:1222–1230. doi: 10.1167/iovs.13-13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marco ED, Jha JC, Sharma A, Wilkinson-Berka JL, Jandeleit-Dahm KA, de Haan JB. Are reactive oxygen species still the basis for diabetic complications? Clin Sci. 2015;129:199–216. doi: 10.1042/CS20150093. [DOI] [PubMed] [Google Scholar]
- 98.Manzella D, Barbieri M, Ragno E, Paolisso G. Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am J Clin Nutr. 2001;73:1052–1057. doi: 10.1093/ajcn/73.6.1052. [DOI] [PubMed] [Google Scholar]
- 99.van Dam PS, Bravenboer B, van Asbeck BS, Marx JJ, Gispen WH. High rat food vitamin E content improves nerve function in streptozotocin-diabetic rats. Eur J Pharmol. 1999;376:217–222. doi: 10.1016/s0014-2999(99)00376-3. [DOI] [PubMed] [Google Scholar]
- 100.Nicklander KK, Schmelzer JD, Rohwer DA, Low PA. Effect of alpha-tocopherol deficiency on indices of oxidative stress in normal and diabetic peripheral nerve. J Neurol Sci. 1994;126:6–14. doi: 10.1016/0022-510x(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 101.Kocak G, Aktan F, Canbolat O, Ozogul C, Elberg S, Yildizoglu N, Karasu C. Alpha-lipoic acid treatment ameliorates metabolic parameters, blood pressure, vascular reactivity and morphology of vessels already damaged by streptozotocin-diabetes. Diab Nutr Metab. 2000;13:308–318. [PubMed] [Google Scholar]
- 102.Coleman MD, Eason RC Bailey CJ. The therapeutic use of lipoic acid in diabetes: a current perspective. Environ Toxicol Pharmacol. 2001;10(4):167–172. doi: 10.1016/s1382-6689(01)00080-1. [DOI] [PubMed] [Google Scholar]
- 103.Papanas N, Ziegler D. Efficacy of alpha-lipoic acid in diabetic neuropathy. Expert Opin Pharmacother. 2014;15:2721–2731. doi: 10.1517/14656566.2014.972935. [DOI] [PubMed] [Google Scholar]
- 104.Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17:888–895. doi: 10.1016/s0899-9007(01)00658-x. [DOI] [PubMed] [Google Scholar]
- 105.Dincer Y, Telci A, Kayah R, Yilmaz IA, Cakatay U, Akcay T. Effect of α-lipoic acid on lipid peroxidation and anti-oxidant enzyme activities in diabetic rats. Clin Exp Pharmacol Physiol. 2002;29:281–284. doi: 10.1046/j.1440-1681.2002.03642.x. [DOI] [PubMed] [Google Scholar]
- 106.Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33(1):83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 107.Bravenboer B, Kappelle AC, Hamers FP, van Buren T, Erkelens DW, Gispen WH. Potential use of glutathione for the prevention and treatment of diabetic neuropathy in the streptozotocin-induced diabetic rat. Diabetologia. 1992;35:813–817. doi: 10.1007/BF00399926. [DOI] [PubMed] [Google Scholar]
- 108.van Dam PS. Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2002;18:176–184. doi: 10.1002/dmrr.287. [DOI] [PubMed] [Google Scholar]
- 109.Nakamura J, Hamada Y, Chaya S, Nakashima E, Naruse K, Kato K, Yasuda Y, Kamiya H, Sakakibara F, Koh N, Hotta N. Transition metals and polyol pathway in the development of diabetic neuropathy in rats. Diabetes Metab Res Rev. 2002;18:395–402. doi: 10.1002/dmrr.319. [DOI] [PubMed] [Google Scholar]
- 110.Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2011;53(1):135–159. [PubMed] [Google Scholar]
- 111.Doggrell SA. Therapeutic potential of selective superoxide dismutase mimetics. Drugs Future. 2002;27:385–390. [Google Scholar]
- 112.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 113.Salvemini D, Riley DP. M40403. Drugs Future. 2000;25(10):1027–1033. [Google Scholar]
- 114.de Cavanagh EMV, Inserra F, Toblli J, Stella I, Fraga G, Ferder L. Enalapril attenuates oxidative stress in diabetic rats. Hypertension. 2001;38:1130–1136. doi: 10.1161/hy1101.092845. [DOI] [PubMed] [Google Scholar]
- 115.Schiffrin EL. Vascular and cardiac benefits of angiotensin receptor blockers. Am J Med. 2002;113:409–418. doi: 10.1016/s0002-9343(02)01241-x. [DOI] [PubMed] [Google Scholar]
- 116.Forbes JM, Cooper ME, Thallas V, Burns WC, Thomas MC, Brammar GC, Lee F, Grant SL, Burrell LA, Jerums G, Osicka TM. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic neuropathy. Diabetes. 2002;51:3274–3282. doi: 10.2337/diabetes.51.11.3274. [DOI] [PubMed] [Google Scholar]
- 117.Brosnan MJ, Hamilton CA, Graham D, Lygate CA, Jardine E, Dominiczak AF. Irbesartan lowers superoxide levels and increases nitric oxide bioavailability in blood vessels from spontaneously hypertensive stroke-prone rats. J Hypertens. 2002;20(2):218–286. doi: 10.1097/00004872-200202000-00018. [DOI] [PubMed] [Google Scholar]
- 118.Munzel T, Keaney JF. Are ACE inhibitors a “magic bullet” against oxidative stress. Circulation. 2001;104:1571–1574. doi: 10.1161/hc3801.095585. [DOI] [PubMed] [Google Scholar]
- 119.Nickenig G, Harrison DG. The AT1-type angiotensin receptor in oxidative stress and atherogenesis. Circulation. 2002;105:393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 120.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H-oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 121.Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Rad Biol Med. 2001;31:1456–1464. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 122.Hollenberg NK. The renin-angiotensin system in the patient with diabetes: an evolution of understanding. Am J Clin Proc. 2000;3:15–20. [Google Scholar]
- 123.Malik RA. Can diabetic neuropathy be prevented by angiotensin-converting enzyme inhibitors? Ann Med. 2000;32:1–5. doi: 10.3109/07853890008995903. [DOI] [PubMed] [Google Scholar]
- 124.O'Driscoll G, Green D, Rankin J, Stanton K, Taylor R. Improvement in endothelial function by angiotensin converting enzyme inhibition in insulin-dependent diabetes mellitus. J Clin Invest. 1997;100:678–684. doi: 10.1172/JCI119580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cameron NE, Cotter MA, Robertson S. Angiotensin converting enzyme inhibition prevents development of muscle and nerve dysfunction and stimulates angiogenesis in streptozotocin-diabetic rats. Diabetologia. 1992;35:12–18. doi: 10.1007/BF00400846. [DOI] [PubMed] [Google Scholar]
- 126.Maxfield EK, Cameron NE, Cotter MA, Dines KC. Angiotensin II receptor blockade improves nerve function, modulates nerve blood flow and stimulates endoneurial angiogenesis in streptozotocin-diabetic rats. Diabetologia. 1193;36:1230–1237. doi: 10.1007/BF00400799. [DOI] [PubMed] [Google Scholar]
- 127.Aggarwal M, Singh J, Sood S, Arora B. Effects of lisinopril on streptozotocin-induced diabetic neuropathy in rats. Methods Find Exp Clin Pharamacol. 2001;23(3):131–134. doi: 10.1358/mf.2001.23.3.627945. [DOI] [PubMed] [Google Scholar]
- 128.Davidson EP, Coppey LJ, Holmes A, Yorek MA. Effect of inhibition of angiotensin converting enzyme and/or neutral endopeptidase on vascular and neural complications in high fat fed/low dose streptozotocin-diabetic rats. Eur J Pharmacol. 2012;677:180–187. doi: 10.1016/j.ejphar.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davidson EP, Coppey LJ, Dake B, Yorek MA. Effect of treatment of Sprague Dawley rats with AVE7688, enalapril, or candoxatril on diet-induced obesity. J Obes. 2011;2011:686952. doi: 10.1155/2011/686952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Yorek MA. Attenuation of vascular/neural dysfunction in Zucker rats treated with enalapril or rosuvastatin. Obesity. 2008;16:82–89. doi: 10.1038/oby.2007.19. [DOI] [PubMed] [Google Scholar]
- 131.Yorek MA. The potential role of angiotensin converting enzyme and vasopeptidase inhibitors in the treatment of diabetic neuropathy. Curr Drug Targets. 2008;9(1):77–84. doi: 10.2174/138945008783431736. [DOI] [PubMed] [Google Scholar]
- 132.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Adebara ET, Yorek MA. Vascular and neural dysfunction in Zucker diabetic fatty rats: a difficult condition of reverse. Diabetes Obes Metab. 2008;10:64–74. doi: 10.1111/j.1463-1326.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 133.Coppey LJ, Davidson EP, Rinehart TW, Gellett JS, Oltman CL, Lund DD, Yorek MA. ACE inhibitor or angiotensin II receptor antagonist attenuates diabetic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2006;55:341–348. doi: 10.2337/diabetes.55.02.06.db05-0885. [DOI] [PubMed] [Google Scholar]
- 134.Jacobsen P, Andersen S, Jensen BR, Parving HH. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. J Am Soc Nephrol. 2003;14(4):992–999. doi: 10.1097/01.asn.0000054495.96193.bf. [DOI] [PubMed] [Google Scholar]
- 135.Rosner MH, Okusa MD. Combination therapy with angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists in the treatment of patients with type 2 diabetes mellitus. Arch Intern Med. 2003;163:1025–1029. doi: 10.1001/archinte.163.9.1025. [DOI] [PubMed] [Google Scholar]
- 136.Panos J, Michelis MF, DeVita MV, Lavie RH, Wilkes BM. Combined converting enzyme inhibition and angiotensin receptor blockade reduce proteinuria greater than converting enzyme inhibition alone: insights into mechanism. Clin Nephrol. 2003;60:13–21. doi: 10.5414/cnp60013. [DOI] [PubMed] [Google Scholar]
- 137.Weber M. Emerging treatments for hypertension: potential role for vasopeptidase inhibition. Am J Hypertens. 1999;12:139S–147S. doi: 10.1016/s0895-7061(99)00205-8. [DOI] [PubMed] [Google Scholar]
- 138.Ebihara F, Di Marco GS, Juliano MA, Casarini DE. Neutral endopeptidase expression in mesangial cells. J Renin Angiotensin Aldosterone Syst. 2003;4(4):228–233. doi: 10.3317/jraas.2003.037. [DOI] [PubMed] [Google Scholar]
- 139.Vatter H, Schilling L, Schmiedek P, Ehrenreich H. Evidence for functional endothelin-converting enzyme activity in isolated rat basilar artery: effect of inhibitors. J Cardiovasc Pharmacol. 1998;31:S64–S67. doi: 10.1097/00005344-199800001-00021. [DOI] [PubMed] [Google Scholar]
- 140.Muangman P, Spenny ML, Tamura RN, Gibran NS. Fatty acids and glucose increase neutral endopeptidase activity in human microvascular endothelial cells. Shock. 2003;19:508–512. doi: 10.1097/01.shk.0000055815.40894.16. [DOI] [PubMed] [Google Scholar]
- 141.Edwards RM, Pullen M, Nambi P. Distribution of neutral endopeptidase activity along the rat and rabbit nephron. Pharmacology. 1999;59:45–50. doi: 10.1159/000028304. [DOI] [PubMed] [Google Scholar]
- 142.Gonzalez W, Soleilhac JM, Fournie-Zaluski MC, Roques BP, Michel JB. Characterization of neutral endopeptidase in vascular cells, modulation of vasoactive peptide levels. Eur J Pharmacol. 1998;345:323–331. doi: 10.1016/s0014-2999(98)00038-7. [DOI] [PubMed] [Google Scholar]
- 143.Kikkawa F, Shibata K, Suzuki T, Kajiyama H, Ino K, Nomura S, Mizutani S. Signal pathway involved in increased expression of neutral endopeptidase 24.11 by gonadotropin releasing hormone in choriocarcinoma cells. Placenta. 2004;25:176–183. doi: 10.1016/j.placenta.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 144.Suzki T, Ino K, Kikkawa F, Uehara C, Kajiyama H, Shibata K, Mizutani S. Neutral endopeptidase/CD10 expression during phorbol ester-induced differentiation of choriocarcinoma cells through the protein kinase C- and extracellular signal-regulated kinase-dependent signaling pathway. Placenta. 2002;23:475–482. doi: 10.1053/plac.2002.0820. [DOI] [PubMed] [Google Scholar]
- 145.Pu Q, Schiffrin EL. Effect of ACE/NEP inhibition on cardiac and vascular collagen in stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2001;14:1067–1072. doi: 10.1016/s0895-7061(01)02157-4. [DOI] [PubMed] [Google Scholar]
- 146.Arbin V, Claperon N, Fournie-Zaluski MC, Roques BP, Peyroux J. Effects of combined neutral endopeptidase 24-11 and angiotensin-converting enzyme inhibition on femoral vascular conductance in streptozotocin-induced diabetic rats. Br J Pharmacol. 2000;130:1297–1304. doi: 10.1038/sj.bjp.0703442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rizzone D, Rossi GP, Porteri E, Sticchi D, Rodella L, Rezzani R, Sleiman I, De Ciuceis C, Paiardi S, Bianchi R, Nussdorfer GG, Agabiti-Rosei E. Bradykinin and matrix metalloproteinases are involved the structural alterations of rat small resistance arteries with inhibition of ACE and NEP. J Hypertens. 2004;22:759–766. doi: 10.1097/00004872-200404000-00019. [DOI] [PubMed] [Google Scholar]
- 148.Wihler C, Schafer S, Schmid K, Deemer EK, Munch G, Bleich M, Busch AE, Dingermann T, Somoza V, Baynes JW, Huber J. Renal accumulation and clearance of advanced glycation end-products in type 2 diabetic nephropathy: effect of angiotensin-converting enzyme and vasopeptidase inhibition. Diabetologia. 2005;48:1645–1653. doi: 10.1007/s00125-005-1837-9. [DOI] [PubMed] [Google Scholar]
- 149.Spenny ML, Muangman P, Sullivan SR, Bunnett NW, Ansel JC, Olerud JE, Gibran NS. Neutral endopeptidase inhibition in diabetic wound repair. Wound Repair Regen. 2002;10(5):295–301. doi: 10.1046/j.1524-475x.2002.10504.x. [DOI] [PubMed] [Google Scholar]
- 150.Plamboeck A, Holst JJ, Carr RD, Deacon CF. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48:1882–1890. doi: 10.1007/s00125-005-1847-7. [DOI] [PubMed] [Google Scholar]
- 151.Davidson EP, Coppey LJ, Holmes A, Dake B, Yorek MA. Effect if treatment of high fat fed/low dose streptozotocin-diabetic rats with ilepatril on vascular and neural complications. Eur J Pharmacol. 2011;668:497–506. doi: 10.1016/j.ejphar.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Dake B, Yorek MA. Role of the effect of inhibition of neutral endopeptidase on vascular and neural complications in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2011;650:556–562. doi: 10.1016/j.ejphar.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Davidson EP, Coppey LJ, Kleinschmidt TL, Oltman CL, Yorek MA. Vascular and neural dysfunction in obese Zucker rats: effect of AVE7688. Exp Diabetes Res. 2009;2009:912237. doi: 10.1155/2009/912327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Yorek MA. Treatment of Zucker fatty rats with AVE7699 improves vascular and neral dysfunction. Diabetes Obese Metab. 2009;11:223–233. doi: 10.1111/j.1463-1326.2008.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Davidson EP, Coppey LJ, Yorek MA. Early loss of innervation of cornea epithelium in streptozotocin-induced type 1 diabetic rats: improvement with ilepatril treatment. Invest Ophthalmol Vis Sci. 2012;53(13):8067–8074. doi: 10.1167/iovs.12-10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Honing ML, Smits P, Morrison PJ, Burnett JC, Rabelink TJ Jr. C-type natriuretic peptide-induced vasodilation is dependent on hyperpolarization in human forearm resistance vessels. Hypertension. 2001;37:1179–1183. doi: 10.1161/01.hyp.37.4.1179. [DOI] [PubMed] [Google Scholar]
- 157.Katayama M, Nadel JA, Bunnett NW, Di Maria GU, Haxhiu M, Borson DB. Catabolism of calcitonin gene-related peptide and substance P by neutral endopeptidase. Peptides. 1991;12:563–567. doi: 10.1016/0196-9781(91)90102-u. [DOI] [PubMed] [Google Scholar]
- 158.McDowell G, Coutie W, Shaw C, Buchanan KD, Struthers AD, Nicholls DP. The effect of the neutral endopeptidase inhibitor drug, candoxatril, on circulating levels of two of the most potent vasoactive peptides. Br J Clin Pharmacol. 1997;43:329–332. doi: 10.1046/j.1365-2125.1997.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tramontana M, Del Bianco E, Ziche M, Santicioli P, Maggi CA, Geppetti P. The effect of thiorphan on release of sensory neuropeptides from guinea-pig cerebral venous sinuses. Pharmacol Res. 1991;23:285–294. doi: 10.1016/s1043-6618(05)80088-x. [DOI] [PubMed] [Google Scholar]
- 160.Kramer HH, Schmidt K, Leis S, Schmelz M, Sommer C, Birklein F. Inhibition of neutral endopeptidase (NEP) facilitates neurogenic inflammation. Exp Neurol. 2005;195:179–184. doi: 10.1016/j.expneurol.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 161.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 162.Kusaka H, Sueta D, Koibuchi N, Hasegawa Y, Nakagawa T, Lin B, Ogawa H, Kim-Mitsuyama S. LCZ696, angiotensin II receptor-neprilysin inhibitor, ameliorates high-salt-induced hypertension and cardiovascular injury more than valsartan alone. Am J Hypertens. 2015 doi: 10.1093/ajh/hpv015. In press. [DOI] [PubMed] [Google Scholar]
- 163.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 164.Pham AQ, Patel Y, Gallagher B. LCZ696 (angiotensin-neprilysin inhibition): the new kid on the heart failure block? J Pharm Pract. 2015;28(2):137–145. doi: 10.1177/0897190014568675. [DOI] [PubMed] [Google Scholar]
- 165.Corder R. New hope for the treatment of diabetic complications. Clin Sci. 2002;103:323–324. doi: 10.1042/cs1030323. [DOI] [PubMed] [Google Scholar]
- 166.Bursell SE, King GL. Can protein kinase C inhibition and vitamin E prevent the development of diabetic vascular complications? Diabetes Res Clin Pract. 1999;45:169–182. doi: 10.1016/s0168-8227(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 167.Cameron NE, Cotter MA. Effects of protein kinase Cβ inhibition on neurovascular dysfunction in diabetic rats: interaction with oxidative stress and essential fatty acid dysmetabolism. Diabetes Metab Res Rev. 2002;18:315–323. doi: 10.1002/dmrr.307. [DOI] [PubMed] [Google Scholar]
- 168.Feener EP, King GL. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350:9–13. doi: 10.1016/s0140-6736(97)90022-2. [DOI] [PubMed] [Google Scholar]
- 169.Soriano FG, Virag L, Jagtap P, Svabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Saizman AL, Southan GJ, Szabo C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose)polymerase activation. Nature Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 170.Patcher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose)polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 171.Obrosova IG, Drel VR, Pacher P, Ilnystska O, Wang ZQ, Stevens MJ, Yorek MA. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54:3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG. Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes. 2006;55:1686–1694. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Soriano FG, Virag l, Szabo C. Diabetic endothelial dysfunction: role of reactive oxygen and nitrogen species production and poly(ADP-ribose)polymerase activation. J Mol Med. 2001;79:437–448. doi: 10.1007/s001090100236. [DOI] [PubMed] [Google Scholar]
- 174.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose)polymerase inhibitors. Pharmacol Rev. 2002;54(3):375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]