Abstract
Distal symmetric sensorimotor polyneuropathy (DSPN) is the most common neurological manifestation in diabetes. Major risk factors of DSPN include diabetes duration, hyperglycemia, and age, followed by prediabetes, hypertension, dyslipidemia, and obesity. Height, smoking, insulin resistance, hypoinsulinemia, and others represent an additional risk. Importantly, hyperglycemia, hypertension, dyslipidemia, obesity, and smoking are modifiable. Stringent glycemic control has been shown to be effective in type 1, but not to the same extent in type 2 diabetes. Antilipidemic treatment, especially with fenofibrate, and multi-factorial intervention have produced encouraging results, but more experience is necessary. The major comorbidities of DSPN are depression, autonomic neuropathy, peripheral arterial disease, cardiovascular disease, nephropathy, retinopathy, and medial arterial calcification. Knowledge of risk factors and comorbidities has the potential to enrich the therapeutic strategy in clinical practice as part of the overall medical care for patients with neuropathy. This article provides an updated overview of DSPN risk factors and comorbidities.
Keywords: diabetes, diabetic neuropathy, hyperglycemia, prevalence, prediabetes, risk factors, hypertension
Abbreviations: ABI – ankle-brachial index; CAN – diabetic cardiovascular autonomic neuropathy; CGM – continuous glucose monitoring; DAN – diabetic autonomic neuropathy; CI – confidence interval; DCCT – diabetes control and complications trial; DNA – deoxyribonucleic acid; DSPN – distal symmetric sensorimotor polyneuropathy; HDLc – high-density lipoprotein cholesterol; HR – hazard ratio; HRV – heart rate variability; IFG – impaired fasting glucose; IGF1 – insulin-like growth factor 1; IGT – impaired glucose tolerance; IL – interleukin; KORA – co-operative research in the region of Augsburg; LDLc – low-density lipoprotein cholesterol; MAC – medial arterial calcification; MDA – malondialdehyde; MPV – mean platelet volume; NCV – nerve conduction velocity; NGF – nerve growth factor; NGT – normal glucose tolerance; OR – odds ratio; OSA – obstructive sleep apnea; PAD – peripheral artery disease; sICAM-1 – soluble intercellular adhesion molecule-1; T1D – type 1 diabetes mellitus; T2D – type 2 diabetes mellitus; UKPDS – United Kingdom prospective diabetes study; VPT – vibration perception threshold
1. Introduction
In diabetes, the most common neurological complication is distal symmetric sensorimotor polyneuropathy (DSPN), often simply referred to as diabetic polyneuropathy [1, 2]. Its prevalence is approximately 30% in hospitalized diabetes patients and 20-30% in community-based patients [1]. It is now increasingly accepted that it starts to develop earlier than previously considered, namely as early as prediabetes [3]. The main clinical features of DSPN include symmetrical, predominantly sensory deficits in the distal lower extremities, and neuropathic pain [1, 2]. Moreover, DSPN is a pivotal risk factor for diabetic foot ulceration due to the loss of protective sensation [2, 4, 5]. Due to its important clinical impact, there has been considerable effort to ensure and improve its early diagnosis, including the development of new screening tests [6-9]. At the same time, research has been aimed at a deeper understanding of the pathogenesis and risk factors, since this knowledge may help towards preventing DSPN [1]. Therefore, the aim of the present review was to provide an update on these developments, with a focus on the risk factors of DSPN.
2. Search strategy
The electronic search for relevant literature was based on PubMed, Embase, and Google scholar databases up to March 2015 using combinations of the following keywords: age, cardiovascular, comorbidities, depression, diabetes, diabetic neuropathy, height, hyperglycemia, insulin, platelets, polyneuropathy, risk factors, smoking. All types of articles written in the English language were included, whereas those written in other languages were studied in abstract form only.
3. Risk factors of distal symmetric sensorimotor polyneuropathy
The risk factors of DSPN, including its degree of association with DSPN, are summarized in Table 1.
Table 1. Risk factors of distal symmetric sensorimotor polyneuropathy.
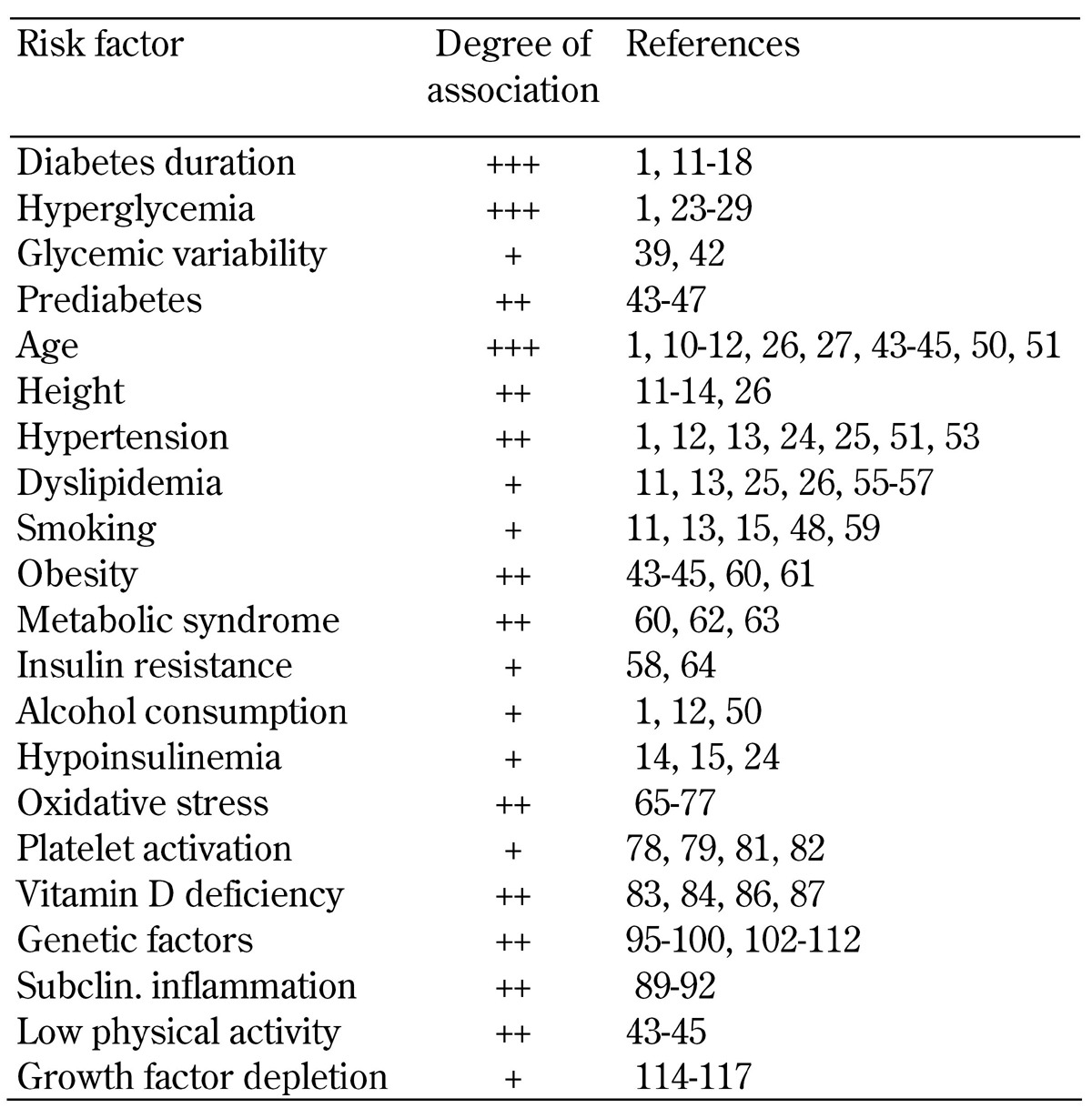
Legend: Moderate association (+), stronger association (++), very strong association (+++).
3.1 Duration of diabetes
Diabetes duration is a major and well-recognized risk factor of DSPN [1, 10]. In both diabetes types, the association of DSPN with diabetes duration is independent of patients’ age [1, 11-15]. Although the exact prevalence of DSPN differs according to the diagnostic methodology used and the population selected (for example, hospital-based vs. outpatient-based vs. community-based) [1], its association with diabetes duration remains significant [1, 11-18]. However, the following two important observations should not escape our notice:
Long-term stringent metabolic control may reduce the prevalence of DSPN, despite longer diabetes duration, especially in type 1 diabetes (T1D) [19, 20]. In a 24-year follow-up of patients with T1D, the development of confirmed clinical DSPN in inadequately controlled patients was 64%, as opposed to 0% in stringently controlled patients [20].
The distribution in age of onset is broad, with some patients developing DSPN after long diabetes duration, while others exhibit this complication as early as the prediabetic stage [3, 21].
Interestingly, Delcourt et al. have reported that the correlation of DSPN with diabetes duration in France was strong until the age of 54 only, but this finding has never been replicated [22].
3.2 Hyperglycemia
Hyperglycemia is the other major risk factor of DSPN [1, 23-26]. Its paramount importance has been documented in both T1D and type 2 diabetes (T2D) [12, 24-29]. It has been calculated that every 1% increment in HbA1c is connected with approximately 10-15% higher frequency of DSPN [1].
Therefore, the effectiveness of strict glycemic control in reducing the incidence and progression of DSPN has been the object of several ambitious studies in both types of diabetes [16, 19, 20, 29-33]. However, an important difference has emerged between T1D and T2D. As detected in a meta-analysis, optimized glycemic control in T1D exerts significantly beneficial effects in preventing the development of clinical DSPN and reducing neurological deficits, while in T2D this effect was not (wholly) significant (p = 0.06) [34]. Another meta-analysis concluded that intensive glucose-lowering treatment was not successful in reducing DSPN in patients with T2D [35]. These findings point to a potential difference in terms of the pathogenesis of DSPN between the two types of diabetes. However, the data are far from being conclusive, because the trials in T2D have only secondarily looked at DSPN, and included only clinical evaluation measures. Therefore, the identification of a beneficial treatment effect was more difficult [1].
3.3 Glycemic variability
Patients with similar HbA1c and mean blood glucose levels can have markedly different daily glucose excursions. The role of glucose variability in pathophysiological pathways is a subject of debate [36]. Recent observational studies suggest an association between high HbA1c variability and all-cause mortality in T2D subjects, particularly in individuals with low mean HbA1c levels of <7.3% [37] or <8% [38]. There is little information on the role of glycemic variability as a risk factor for the development of DSPN. While one study concluded that blood glucose variability may constitute an important risk factor in the development of neuropathy in patients with T1D [39], the Diabetes Control and Complications Trial (DCCT) failed to confirm this association for DSPN and cardiac autonomic neuropathy (CAN) [40]. In a recent smaller study from Korea, variability in continuous glucose monitoring (CGM) and all parameters of HbA1c variability were independently associated with the presence of CAN in T2D subjects [41]. Another small study from China reported a close relationship between glycemic variability and DSPN in T2D patients with HbA1c levels <7% [42].
It has been reasoned that there is no "gold standard" for determining glucose variability and that the only way to determine the utility of targeting glycemic variability would be to conduct studies specifically aimed at lowering glucose variability to assess its influence on the development of diabetic neural and vascular complications [36]. In summary, the role of glucose variability in causing neuropathy remains unclear. Reducing glucose variability could become an important part of diabetes management, should the association with diabetic complications be established. At present, there is little supportive evidence for targeting glucose variability separately from mean glucose and/or HbA1c values [36]. More research is necessary to clarify this important question.
3.4 Prediabetes
There is accumulating evidence suggesting that the combined prevalence of DSPN and CAN is increased in individuals with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) as compared to those with normal glucose tolerance (NGT) [21]. In the KORA (Cooperative Research in the Region of Augsburg) study, the prevalence of DSPN was 13% in IGT, lying between diabetes (28%) and NGT (7.4%) [43]. Neuropathic pain was as frequent as 8.7% in IGT vs. 1.2% in NGT [44]. In subjects with prior acute myocardial infarction, the rate of neuropathic pain was 14.8% in IGT vs. 21% in diabetes and 3.7% in NGT [45]. This pattern was confirmed by the KORA F4 Survey using an alternative definition for DSPN [46]. Among prediabetes subgroups, IFG+IGT, but not isolated IFG or IGT, was associated with a higher risk of DSPN, compared with normal glucose tolerance [46]. A J-shaped relationship was shown between DSPN and quartiles of 2-h post-challenge glucose, but not with fasting glucose and HbA1c levels [46]. Moreover, in the KORA S4 survey, the prevalence of CAN was increased in individuals with diabetes and in those with IFG+IGT and, to a lesser degree, in those with isolated IFG [47]. Thus, combined IFG+IGT in particular is associated with an increased risk of DSPN and CAN, almost reaching the rates seen in patients with known diabetes. However, some authors argue against an increased prevalence of DSPN and small fiber neuropathy in prediabetes [48, 49].
3.5 Age
Age has long been regarded as a risk factor of DSPN [1, 10-12, 26, 27, 43-45, 50, 51]. Several groups have shown that age exerts an independent effect on DSPN, leading to a progressive increase in its prevalence for approximately every decade of life [10, 27, 50, 51]. The independent effect of age has also been shown for prediabetes in epidemiological surveys [43-45]. However, there is an important caveat in these observations. As age per se causes a progressive deterioration in neurological functions, independent of diabetes, the symptoms call for a differentiated interpretation [14, 52]. Delcourt et al. carried out an adjustment for age and found that DSPN no longer correlated with age [22]. On the other hand, it is conceivable that the impact of age on nerve function outweighs the influence of diabetes in the elderly population in which the prevalence of DSPN in milder degrees of hyperglycemia (prediabetes) may approach that of known diabetes [43-45]. Regrettably, no age-adjusted normal values have been used in the vast majority of studies.
3.6 Height
Height has been implicated in its pathogenesis of DSPN because of the length-dependent pattern of the disease, as a measure of nerve fiber length. Evidence for an association of DSPN with height comes from studies in both diabetes types [11-14, 26]. In a population study in Mauritius, height was a significant (p < 0.001) independent risk factor of DSPN, increasing its prevalence by 36% for every 5 cm increment (odds ratio (OR): 1.36, 95% confidence interval (CI): 1.19-1.57) in the cross-sectional analysis [14]. In the same study, prospective data confirmed this association (OR: 1.23, 95% CI: 1.03-1.45 per 5 cm, p = 0.02) [14]. By contrast, Franklin et al. found no association between height and prevalence of DSPN in a population study in Southern Colorado [51].
3.7 Hypertension
Hypertension is another risk factor for DSPN [1, 12, 13, 24, 25, 51, 53], but there appears to be a difference between the two diabetes types. In T1D, the data is affirmative [13, 25]. Forrest et al. have identified hypertension as the strongest predictor of DSPN, as it increased the relative risk approximately four times in a 6-year period [13]. Similarly, Tesfaye et al. have reported that systolic hypertension was an independent predictor after adjustment for age, duration of diabetes, and metabolic control [25]. In contrast, studies in T2D have been negative [12, 24, 51, 53]. Of note, tight blood pressure control in the United Kingdom Prospective Diabetes Study did not reduce deterioration of DSPN [54].
3.8 Dyslipidemia
Dyslipidemia is an additional risk factor, according to some studies. After adjustment for metabolic control, age, and other covariates, DSPN has been shown to correlate with high cholesterol [26] and triglycerides [11]. In T1D, increased low-density lipoprotein cholesterol (LDLc) [13] and triglycerides [25] have been identified as predictors of DSPN. However, in the first study, LDLc lost significance as a risk factor following adjustment for metabolic control and other covariates [13]. In T2D, fibrate use (hazard ratio (HR): 0.52, 95% CI: 0.27-0.98) and statin use (HR: 0.65, 95% CI: 0.46-0.93) significantly reduced the incidence of DSPN over 5 years [26]. Similarly, Keech et al. have reported improvement of protective sensation in DSPN following fenofibrate treatment [55]. More impressively, the same group has shown a significant (p = 0.027) reduction in minor lower-extremity amputations associated with DSPN (HR: 0.53, 95% CI: 0.30-0.94) [56].
Wiggin et al. have reanalyzed data from two randomized, placebo-controlled, clinical trials of patients with mild/moderate DSPN, and found that reduction of sural nerve myelinated fibre density at 52 weeks was independently correlated with high serum triglycerides (p = 0.04) [57]. More recently, a 6-year follow-up study of 48 Korean patients revealed that reduced high-density lipoprotein cholesterol (HDLc) (OR: 5.292, 95% CI: 1.001-27.989, p = 0.05) and high triglycerides (OR: 6.129, 95% CI: 1.057-35.528, p = 0.043) significantly increased the risk of DSPN, after adjusting for age and gender [58]. On the other hand, there is also some evidence against the association of DSPN with serum lipids [14, 24].
3.9 Smoking
There is some evidence that smoking is an independent risk factor of DSPN in T1D [11, 13]. In T2D, smoking may also be a risk factor [15], but its contribution may be weak and not independent [14]. Surprisingly, a protective effect of smoking has been reported in US veterans [12]. More recently, a meta-analysis including 10 prospective and 28 cross-sectional studies has found that smoking had an unadjusted OR of 1.26 for prospectively developing DSPN (95% CI: 0.86-1.85) [59]. In the cross-sectional studies, the pooled OR for DSPN due to smoking was 1.42 (95% CI: 1.21-1.65) [48]. For both analyses, evidence was graded as low-strength [59].
3.10 Obesity
In the Southern German population, obesity has been identified as a risk factor of DSPN [43-45]. In the general US population ≥40 years, obesity and the presence of at least 2 cardiovascular risk factors (triglycerides or plasma glucose, reduced HDLc, increased waist circumference, hypertension) increases the likelihood of peripheral neuropathy (OR: 2.20, 95% CI: 1.43-3.39) [60]. Subjects with morbid obesity have been found to exhibit features of small nerve fiber dysfunction (impaired pain perception and diminished reflex vasodilatation) [61].
3.11 Metabolic syndrome
With or without obesity, presence of the metabolic syndrome has been found to increase the likelihood of peripheral neuropathy (OR: 2.20 and 1.5, respectively) in the general population [60]. Among patients with idiopathic neuropathy, more frequent and more prominent features of the metabolic syndrome other than hyperglycemia have been found to influence the disease, as compared with those free from neuropathy [62, 63].
3.12 Insulin resistance
A study in 86 T2D patients has identified insulin resistance as a major independent risk factor of DSPN (p < 0.001) [64]. Of these patients, 48 were followed for 6 years [58]. It was found that initial insulin resistance was positively associated with impairment of sural sensory nerve action potential at 6 years (r = 0.629, p = 0.001), after adjustment for age, gender, and height [58].
3.13 Alcohol
Some studies [12, 50] have reported an association between DSPN and alcohol consumption, but others have not [15, 43-45]. In general, it may be difficult to differentiate between DSPN with alcohol as a risk factor and alcoholic neuropathy in a person with diabetes [1]. It would be useful to address this differentiation by careful patient selection in a prospective cohort study.
3.14 Hypoinsulinemia
In Finnish male patients recently diagnosed with T2D, low insulin in the fasting state and at 2 hours after oral glucose administration was associated (p = 0.03) with incident DSPN, irrespective of glycemic control (evaluated as fasting glucose and glycated hemoglobin) [24]. Likewise, Sands et al. have found an association between low C-peptide and DSPN, but this association lost significance after adjustment for diabetes duration [15]. A similar but weak association has also been found in the Mauritius population [14]. Generally, the data on hypoinsulinemia are interesting, but it is important to remind that long diabetes duration per se is an important confounder, and needs to be taken into account when interpreting study results.
3.15 Oxidative stress
Experimental evidence suggests that oxidative stress is connected with DSPN [65-68]. Numerous studies have reported associations between systemic markers of oxidative stress and DSPN in diabetic patients. For example, lower values of reduced glutathione and reduced glutathione/oxi-dized glutathione ratio in association with DSPN have been reported [69]. Lower plasma concentrations of nitric oxide and higher endogenous oxidative DNA damage in T2D patients with DSPN have also been observed [70]. Bierhaus et al. have reported increased plasma methylglyoxal concentrations in association with painful DSPN [71]. Migdalis et al. have found reduced thiobarbitouric acid-reacting substances in T2D patients with DSPN [72]. An overexpression of cutaneous mitochondrial superoxide dismutase was described in skin biopsies from recent-onset T2D subjects, suggesting enhanced local compensatory antioxidative defense at an early stage of T2D [73].
However, little information is available from prospective studies. In a 6-year study, Ziegler et al. found that increased plasma superoxide generation was associated with a decline in median sensory nerve conduction velocity (NCV) and a deterioration in heart rate variability (HRV) at rest [74]. Low vitamin E/lipid ratio tended to predict a decrease in peroneal motor NCV and an increase in malleolar VPT. Hoeldtke et al. showed that malondialdehyde (MDA) excretion in urine, a marker of lipid peroxidation, and plasma nitrite and nitrate, markers of nitrosative stress, were associated with sweating abnormalities, while sensory function tests correlated inconsistently with MDA excretion [75-77]. Further studies are required to determine whether optimized patient selection strategies showing increased levels of oxidative stress biomarkers such as superoxide generation could be of value in identifying those patients who may respond better to antioxidant treatment.
3.16 Platelet activation
Patients with DSPN and retinopathy may suffer from increased platelet aggregation [78]. This is even more pronounced in the setting of marked chronic hyperglycemia [79]. Mean platelet volume (MPV) is a marker of platelet activation, and may be increased in diabetes patients, especially in the context of microvascular complications [80]. Elevated MPV has indeed been reported in T2D patients with DSPN [81, 82].
Accumulating evidence indicates that platelet activation plays a contributory role in impaired microcirculation and peripheral nerve function [1, 2]. However, there are no prospective data to confirm or refute the role of increased MPV and/or other platelet activation markers in the development of DSPN. Similarly, there is no information on potential cut-off levels of these platelet markers, which may quantitatively express increased risk of DSPN development.
3.17 Low vitamin D
In T2D, the level of vitamin D was found to be significantly lower in those patients with DSPN [83]. Of patients with DSPN, 81.5% had vitamin D deficiency compared with 60.4% of those without DSPN [83]. DSPN was significantly associated with vitamin D deficiency after adjustment for diabetes duration, glycated hemoglobin, and LDLc (OR: 3.47. 95% CI: 1.04-11.56, p = 0.043) [83]. In a short, 8-week prospective study, the same authors demonstrated a significant (p < 0.001) improvement of neuropathic symptoms with oral vitamin D supplementation vs. placebo [84]. There is also recent evidence of a significant association between low vitamin D and impaired heart rate variability [85]. At present, there is growing interest in the contribution of vitamin D to the prevention of DSPN [86, 87] and peripheral artery disease [88].
3.18 Inflammation
Several population-based studies conducted in different KORA cohorts have documented associations between markers of systemic subclinical inflammation and the presence of DSPN [89-92]. In the KORA F3 cohort, high levels of C-reactive protein (CRP) and interleukin 6 (IL-6) were positively associated with the presence and severity of DSPN [92]. In the KORA F4 cohort, serum concentrations of the anti-inflammatory IL-1 receptor antagonist (IL-1RA), IL-6, IL-18, and soluble intercellular adhesion molecule-1 (sICAM-1) were positively associated with the severity of neuropathic deficits, suggesting that DSPN is linked to pro-inflammatory and anti-inflammatory, possibly compensatory, processes in the older general population [91].
It was also reported that serum concentrations of IL-6 and sICAM-1 are positively associated with painful DSPN [90]. Moreover, serum levels of omentin, an adipokine with anti-inflammatory, insulin-sensitizing, and cardioprotective properties, were reduced in T2D individuals with DSPN, independently of established risk factors of polyneuropathy [89]. In T2D, there is also evidence of increased serum uric acid in the presence of DSPN [93, 94]. Future studies should clarify the temporal sequence and causality of these associations.
3.19 Genetic factors
Genetic factors have been implicated in the pathogenesis of DSPN, to account for the clinical observation that DSPN may, occasionally, be encountered in subjects with short diabetes duration and adequate glycemic control [1]. In this context, reduced/impaired Na+/K+-ATPase activity and increased aldose reductase activity may play a supplementary role [97-100]. Na+/K+-ATPase may be influenced by metabolic control and C-peptide secretion [101]. Some single nucleotide polymorphisms (SNPs) have been studied in Russian T1D patients with DSPN: these relate to the poly(ADP-ribose)polymerase-1 gene, the catalase gene, and genes encoding the enzymes superoxide dismutase extracellular superoxide dismutase [102-104]. In Greek T2D patients, the D allele of the Alpha2B adrenoceptor has been found to be associated with presence and severity of DSPN [105]. In German T1D and T2D patients, 2 single nucleotide polymorphisms (Asp299Gly and Thr399Ile) of the toll-like receptor 4 gene were found to be related with reduced DSPN in T2D, but (importantly) not in T1D [106].
Apolipoprotein E polymorphisms have been studied in a number of trials [107-110]. Results regarding the role of the ε4 allele as a risk factor have been conflicting, but some studies may be criticized because of insufficient patient homogeneity, inconsistent DSPN definition, and small sample size [111]. Monastiriotis et al. have reported a 5-fold increased risk of severe DSPN in the presence the ε4 allele (adjusted OR: 5.26, 95% CI: 2.24-12.31, p = 0.0001) in Greek T2D patients [112]. However, the exact supplementary role of these SNPs in the development and progression of DSPN remains obscure until confirmatory prospective studies in sufficiently large representative cohorts are available.
3.20 Low physical activity
Low physical activity has been linked with DSPN in the Southern German population [43-45]. In this context, a recent systematic review has examined the therapeutic importance of physical activity for treating pain in DSPN [113]. The evidence of improvement is insufficient; this may be due to limited data (2 studies), but also methodological limitations and risk of bias [113].
3.21 Growth factor depletion
There has been some discussion about reduced levels and impaired function of nerve growth factor (NGF) and insulin-like growth factor 1 (IGF-1) in patients with DSPN [114-116]. NGF induces neuronal growth and differentiation, and protects neural cells from apoptosis [114]. In DSPN, preliminary clinical evidence with NGF has been encouraging [114]. However, a large randomized prospective phase 3 clinical trial failed to show a significant beneficial effect of recombinant NGF on neuropathic deficits [117]. It demonstrated some symptomatic improvement only [117]. As this trial has been criticized for NGF formulation and dosage and for a strong placebo effect, further progress with this agent was discouraged [114].
In DSPN, Migdalis et al. have found a negative correlation of DSPN severity with IGF-1 (r = -0.39, p < 0.01) and IGF-1 receptors (r = -0.34, p < 0.01) [115]. Guo et al. have also reported significantly diminished IGF-1 levels in DSPN (p < 0.05) as compared with diabetes patients free from this complication [116]. These studies point out the need for prospective data on the role of IGF-1 depletion and its potential therapeutic effectiveness in the onset and progression of DSPN.
4. Comorbidities of DSPN
The comorbidities of DSPN are summarized in Table 2.
Table 2. Comorbidities of distal symmetric sensorimotor polyneuropathy.

Legend: Moderate association (+), stronger association (++), very strong association (+++).
4.1 Depression
Depression is an important comorbidity of DSPN [118-121]. Among neuropathic symptoms, it is pain and, primarily, postural instability (leading to gait uncertainty) that are associated with depression [119, 120]. In the same context, DSPN may be associated with poor quality of life [121], and painful symptoms may be linked with a reduced well-being index [122].
4.2 Cognitive dysfunction
Cognitive dysfunction is a further comorbidity that is being increasingly analyzed [123-128]. In the experimental model of DSPN, reduced pain perception may be associated with memory dysfunction [123]. Patients with DSPN may suffer from cognitive dysfunction [125-127], which affects verbal, visuospatial, and multitasking measures of executive function [124], and this may result in gait disturbances [125, 126] and increased risk of falls [126].
4.3 Autonomic neuropathy
Patients with DSPN frequently suffer from diabetic autonomic neuropathy (DAN) as well; approximately 50% have concomitant CAN [1, 64, 129-132]. The co-existence of DSPN and DAN increases with progressive diabetes duration and poor metabolic control, although this varies with the cohort studied and method of assessment [132]. In an Italian study, the majority of subjects with DAN exhibited DSPN [133]. The presence of concomitant DAN is important for prognosis, given that it is a risk factor of mortality via cardiovascular disease [134].
4.4 Peripheral artery disease
Patients with DSPN may also have concomitant peripheral artery disease (PAD) [60, 135]. US subjects ≥40 years with obesity and ≥2 cardiovascular risk factors exhibited a 2.4% frequency of simultaneous DSPN and PAD [60]. Obesity increased the likelihood of the presence of both complications (OR: 6.91, 95% CI: 2.64-18.06) [60]. In the general diabetic population of Augsburg, Germany, there was a significant association between DSPN and PAD (p < 0.05) [43]. In the same population, PAD was associated with painful DSPN [44, 45]. In Sweden, T2D patients had a three-fold higher frequency of PAD than those without diabetes (52% vs. 16%, p = 0.001) [118]. DSPN was independently associated with PAD (OR: 2.31, 95% CI: 1.25-4.25, p = 0.007) [136]. In a Greek clinic-based study, ankle-brachial index (ABI) was significantly lower in T2D patients with DSPN than in those without this complication (p = 0.001) [137]. ABI <0.9 exhibited 47% sensitivity and 90.7% specificity for DSPN [137].
4.5 Medial arterial calcification
Medial arterial calcification (MAC), or Mönckeberg's sclerosis, represents calcification of the tunica media. It is a well-known cause of spurious ABI elevation and mainly observed in the infra-popliteal arteries of diabetes patients [137, 138]. In a case series, Edmonds et al. showed that MAC was seen almost exclusively in patients with DSPN [139]. The same group reported that MAC was more common and severe in DSPN patients with neuropathy than in those without this complication (p < 0.001); there was a significant association between MAC and DSPN (p < 0.001) [140]. Other studies have confirmed the association of MAC with DSPN [141, 142]. Young et al. observed that MAC correlated with vibration perception threshold (r = 0.35, p < 0.01) [142]. Vibration perception threshold and diabetes duration were identified as independent predictors of MAC [142].
4.6 Cardiovascular disease
DSPN is frequently associated with cardiovascular disease [134, 135, 143]. Interestingly, a prospective, primary care study found that incident DSPN was significantly more frequent in subjects with cardiovascular disease at baseline (p = 0.01) [144]. Cardiovascular disease was an independent predictor of DSPN development after 10 years (OR: 2.32, 95% CI: 1.03-5.22) [144].
4.7 Nephropathy
Nephropathy may be more frequent in the presence of DSPN [145, 146]. Conversely, patients with diabetic nephropathy may exhibit more pronounced DSPN, and may therefore be at increased risk of severe diabetic foot lesions [147]. This information is important for the organization of an overall strategy for detecting, monitoring, and treating chronic microvascular complications.
4.8 Retinopathy
Similarly to nephropathy, retinopathy may also be regarded as a comorbidity of DSPN [146, 148, 149]. A study has shown progressive deterioration of corneal nerve fiber pathology with increasing severity of retinopathy [149]. Another group has shown progression of corneal nerve fiber pathology in parallel with diabetic retinopathy and DSPN [150].
4.9 Obstructive sleep apnea
Obstructive sleep apnea (OSA) has recently been recognized as a comorbidity of DSPN [151, 152]. A significantly higher frequency of DSPN in T2D patients with OSA than in those without OSA has been reported (p < 0.001) [151]. In a meta-analysis, DSPN was linked with a pooled OR 1.95 (95% CI: 1.03-3.70) for OSA [152]. A very recent, small case study series has suggested that severe OSA may be responsible for the failure to heal diabetic foot ulcers [153]. Therefore, OSA should be considered in the differential diagnoses of non-healing foot ulcers. More experience in this area is awaited [154]. In the light of these considerations, it may be useful to screen for OSA in patients with DSPN and neuropathic foot ulceration [155].
5. Discussion
Obviously, knowledge of risk factors for DSPN is clinically useful, because it offers the opportunity for delay and prevention of this complication. Some risk factors are modifiable and should receive the clinician's attention. These factors include primarily hyperglycemia, hypertension, dyslipidemia, and obesity [1, 13, 23-29, 51, 55, 56, 60]. Secondary risk factors are smoking and prediabetes [1, 21, 60, 63]. Platelet activation [79-82], oxidative stress [65-74], low vitamin D [83, 84], insulin resistance [58], and genetic factors [100-106] play another supplementary role, but, for the time being, there are limited options for intervention. A suggestion for targeting insulin resistance originates from the study by Pop-Busui et al., showing that reduced incident DSPN is less frequent in T2D patients receiving insulin-sensitizing oral agents (66%) than in those receiving insulin-providing treatment (66% vs. 72%, respectively, p = 0.02) [155]. This effect was more pronounced in men (HR: 0.75, 99% CI: 0.58-0.99, p < 0.01).
Obviously, the greatest challenge is to improve outcomes with long-term consistent, stringent glycemic control [1]. The beneficial effect of this strategy is undeniable in T1D [19, 20, 29, 156], but has hitherto not proved significant in T2D [33-35]. This may reflect differences in etiology, but may also be used to motivate further improvements in anti-diabetic regimens and screening for DSPN in T2D. Of note, antilipidemic treatment, especially fenofibrate, has shown encouraging results [26, 55, 56], but these benefits have not yet been realized and confirmed in everyday reality.
Probably, the most ambitious approach involves multi-factorial intervention, addressing several major risk factors [157-159]. In T2D, Gaede et al. have employed an intensified approach, including strict metabolic control, aspirin, statins, angiotensin-converting enzyme inhibitors, anti-oxidants, and cessation of smoking [157]. They managed to demonstrate significant improvements in cardiovascular disease, retinopathy, nephropathy, and CAN, but not in DSPN [157]. In screen-detected diabetes, early intensive, multi-factorial therapy did not reduce incident DSPN and prevalent CAN compared with routine care [158, 159]. However, the feasibility of such studies is questionable. If participants have once been allocated to the routine clinical care arm of the trial, their degree of CVD risk factor control approaches that of the intensively treated group. Indeed, routine clinical care in expert centers is so effective nowadays that the randomization procedure leaves only marginal differences between the groups regarding blood pressure, HbA1c, and serum lipids. Therefore, this procedure minimizes the likelihood to detect differences in clinical neuropathy outcomes.
6. Conclusions
The major risk factors of DSPN include diabetes duration, hyperglycemia, and age, followed by hypertension, dyslipidemia, obesity, and metabolic syndrome [1, 11-15, 24, 25, 29-35, 36, 43-45, 50, 51, 55, 60]. Additional risk factors include height, smoking, insulin resistance, hypoinsulinemia, prediabetes, and several others [1, 15, 21, 64]. Of these, hyperglycemia, hypertension, dyslipidemia, obesity, prediabetes, and metabolic syndrome are modifiable, providing some opportunity to prevent and/or reduce the progression of DSPN. Stringent glycemic control has been shown to be effective in T1D [20, 29], but not or only marginally so in T2D [33-35].
Antilipidemic treatment, especially fenofibrate, has shown encouraging results [26, 55, 56], but more knowledge is needed. Multi-factorial intervention also appeared very promising initially, but this optimism has not been confirmed [157-159]. Emerging risk factors, notably vitamin D deficiency, inflammation, and oxidative stress also need to be therapeutically addressed, but we are currently at the very beginning of our knowledge in this area.
The major comorbidities of DSPN are depression, autonomic neuropathy, peripheral artery disease, cardiovascular disease, nephropathy, retinopathy, and medial arterial calcification [1, 60, 113-122, 129-131, 135, 137, 139-142, 146]. Given that these conditions increase morbidity and mortality, it is advisable for the clinician to screen for and monitor them as part of the overall management plan for DSPN.
Acknowledgments
Disclosures
The authors report no conflict of interests.
References
- 1.Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014;126:3–22. doi: 10.1016/B978-0-444-53480-4.00001-1. [DOI] [PubMed] [Google Scholar]
- 2.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A. et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol. 2011;7(11):682–690. doi: 10.1038/nrendo.2011.113. [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(Suppl 1):S3–S6. doi: 10.1002/dmrr.833. [DOI] [PubMed] [Google Scholar]
- 5.Papanas N, Maltezos E. The diabetic foot: established and emerging treatments. Acta Clin Belg. 2007;62(4):230–238. doi: 10.1179/acb.2007.037. [DOI] [PubMed] [Google Scholar]
- 6.Papanas N, Ziegler D. New vistas in the diagnosis of diabetic polyneuropathy. Endocrine. 2014;47(3):690–698. doi: 10.1007/s12020-014-0285-z. [DOI] [PubMed] [Google Scholar]
- 7.Papanas N, Ziegler D. Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Curr Diab Rep. 2013;13(4):488–499. doi: 10.1007/s11892-013-0390-z. [DOI] [PubMed] [Google Scholar]
- 8.Papanas N, Boulton AJ, Malik RA, Manes C, Schnell O, Spallone V, Tentolouris N, Tesfaye S, Valensi P, Ziegler D. et al. A simple new non-invasive sweat indicator test for the diagnosis of diabetic neuropathy. Diabet Med. 2013;30(5):525–534. doi: 10.1111/dme.12000. [DOI] [PubMed] [Google Scholar]
- 9.Körei AE, Istenes I, Papanas N, Kempler P. Small-fiber neuropathy: a diabetic microvascular complication of special clinical, diagnostic, and prognostic importance. Angiology. 2015 doi: 10.1177/0003319715583595. In press. [DOI] [PubMed] [Google Scholar]
- 10.Young MJ, Breddy JL, Veves A, Boulton AJ. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17(6):557–560. doi: 10.2337/diacare.17.6.557. [DOI] [PubMed] [Google Scholar]
- 11.Tesfaye S, Stevens LK, Stephenson JM, Fuller JH, Plater M, Ionescu-Tirgoviste C, Nuber A, Pozza G, Ward JD. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39(11):1377–1384. doi: 10.1007/s001250050586. [DOI] [PubMed] [Google Scholar]
- 12.Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20(7):1162–1167. doi: 10.2337/diacare.20.7.1162. [DOI] [PubMed] [Google Scholar]
- 13.Forrest KY, Maser RE, Pambianco G, Becker DJ, Orchard TJ. Hypertension as a risk factor for diabetic neuropathy: a prospective study. Diabetes. 1997;46(4):665–670. doi: 10.2337/diab.46.4.665. [DOI] [PubMed] [Google Scholar]
- 14.Shaw JE, Hodge AM, de Courten M, Dowse GK, Gareeboo H, Tuomilehto J, Alberti KG, Zimmet PZ. Diabetic neuropathy in Mauritius: prevalence and risk factors. Diabetes Res Clin Pract. 1998;42(2):131–139. doi: 10.1016/s0168-8227(98)00100-4. [DOI] [PubMed] [Google Scholar]
- 15.Sands ML, Shetterly SM, Franklin GM, Hamman RF. Incidence of distal symmetric (sensory) neuropathy in NIDDM. The San Luis Valley Diabetes Study. Diabetes Care. 1997;20(3):322–329. doi: 10.2337/diacare.20.3.322. [DOI] [PubMed] [Google Scholar]
- 16.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, Waberski BH, Lachin JM Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33(5):1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4000 patients observed between 1947 and 1973. Diabetes Care. 1978;1(1):168–188. [Google Scholar]
- 18.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4000 patients observed between 1947 and 1973. Diabetes Care. 1978;1(2):252–263. [Google Scholar]
- 19.Martin CL, Albers JW, Pop-Busui R DCCT/EDIC Research Group. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):31–38. doi: 10.2337/dc13-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler D, Behler M, Schroers-Teuber M, Roden M. Near-normoglycaemia and development of neuropathy: a 24-year prospective study from diagnosis of type 1 diabetes. BMJ Open. 2015;5:e006559. doi: 10.1136/bmjopen-2014-006559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papanas N, Ziegler D. Polyneuropathy in impaired glucose tolerance: is postprandial hyperglycemia the main culprit? A mini-review. Gerontology. 2013;59(3):193–198. doi: 10.1159/000343988. [DOI] [PubMed] [Google Scholar]
- 22.Delcourt C, Vauzelle-Kervroedan F, Cathelineau G, Papoz L. Low prevalence of long-term complications in non-insulin-dependent diabetes mellitus in France: a multicenter study. CODIAB-INSERM-ZENECA Pharma Study Group. J Diabetes Complications. 1998;12(2):88–95. doi: 10.1016/s1056-8727(97)98005-3. [DOI] [PubMed] [Google Scholar]
- 23.Migdalis I, Leslie D, Mavrogiannaki A, Papanas N, Valensi P, Vlassara H. Diabetes Mellitus 2014. Int J Endocrinol. 2015;2015:845759. doi: 10.1155/2015/845759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(2):89–94. doi: 10.1056/NEJM199507133330203. [DOI] [PubMed] [Google Scholar]
- 25.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 26.Davis TM, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2008;51(4):562–566. doi: 10.1007/s00125-007-0919-2. [DOI] [PubMed] [Google Scholar]
- 27.Herman WH, Aubert RE, Engelgau MM, Thompson TJ, Ali MA, Sous ES, Hegazy M, Badran A, Kenny SJ, Gunter EW. et al. Diabetes mellitus in Egypt: glycaemic control and microvascular and neuropathic complications. Diabet Med. 1998;15(12):1045–1051. doi: 10.1002/(SICI)1096-9136(1998120)15:12<1045::AID-DIA696>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O'Brien PC, Melton LJ 3rd, Service FJ. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 29.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 30.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 31.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 32.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 33.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr. et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. doi: 10.1002/14651858.CD007543.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, Kassai B, Erpeldinger S, Wright JM, Gueyffier F. et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31(2):171–182. doi: 10.1210/er.2009-0021. [DOI] [PubMed] [Google Scholar]
- 37.Ma WY, Li HY, Pei D, Hsia TL, Lu KC, Tsai LY, Wei JN, Su CC. Variability in hemoglobin A1c predicts all-cause mortality in patients with type 2 diabetes. J Diabetes Complications. 2012;26(4):296–300. doi: 10.1016/j.jdiacomp.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Skriver MV, Sandbaek A, Kristensen JK, Stovring H. Relationship of HbA1c variability, absolute changes in HbA1c, and all-cause mortality in type 2 diabetes: a Danish population-based prospective observational study. BMJ Open Diabetes Res Care. 2015;3:e000060. doi: 10.1136/bmjdrc-2014-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bragd J, Adamson U, Bäcklund LB, Lins PE, Moberg E, Oskarsson P. Can glycaemic variability, as calculated from blood glucose self-monitoring, predict the development of complications in type 1 diabetes over a decade? Diabetes Metab. 2008;34(6 Pt 1):612–616. doi: 10.1016/j.diabet.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Siegelaar SE, Kilpatrick ES, Rigby AS, Atkin SL, Hoekstra JB, Devries JH. Glucose variability does not contribute to the development of peripheral and autonomic neuropathy in type 1 diabetes: data from the DCCT. Diabetologia. 2009;52(10):2229–2232. doi: 10.1007/s00125-009-1473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jun JE, Jin SM, Baek J, Oh S, Hur KY, Lee MS, Lee MK, Kim JH. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:70. doi: 10.1186/s12933-015-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu F, Zhao LH, Su JB, Chen T, Wang XQ, Chen JF, Wu G, Jin Y, Wang XH. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6(1):139. doi: 10.1186/1758-5996-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A KORA Study Group. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3):464–469. doi: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A KORA Study Group. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. 2009;10(2):393–400. doi: 10.1111/j.1526-4637.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- 45.Ziegler D, Rathmann W, Meisinger C, Dickhaus T, Mielck A KORA Study Group. Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre-diabetes and diabetes. The KORA Myocardial Infarction Registry. Eur J Pain. 2009;13(6):582–587. doi: 10.1016/j.ejpain.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Bongaerts BW, Rathmann W, Kowall B, Herder C, Stöckl D, Meisinger C, Ziegler D. Post-challenge hyperglycemia is positively associated with diabetic polyneuropathy: the KORA F4 study. Diabetes Care. 2012;35(9):1891–1893. doi: 10.2337/dc11-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziegler D, Voss A, Rathmann W, Strom A, Perz S, Roden M, Peters A, Meisinger C KORA Study Group. Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: the KORA S4 survey. Diabetologia. 2015;58(5):1118–1128. doi: 10.1007/s00125-015-3534-7. [DOI] [PubMed] [Google Scholar]
- 48.Dyck PJ, Clark VM, Overland CJ, Davies JL, Pach JM, Dyck PJ, Klein CJ, Rizza RA, Melton LJ 3rd, Carter RE. et al. Impaired glycemia and diabetic polyneuropathy: The OC IG Survey. Diabetes Care. 2012;35(3):584–591. doi: 10.2337/dc11-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kassardjian CD, Dyck PJ, Davies JL, Carter RE, Dyck PJ. Does prediabetes cause small fiber sensory polyneuropathy? Does it matter? J Neurol Sci. 2015;355(1-2):196–198. doi: 10.1016/j.jns.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walters DP, Gatling W, Mullee MA, Hill RD. The prevalence of diabetic distal sensory neuropathy in an English community. Diabet Med. 1992;9(4):349–353. doi: 10.1111/j.1464-5491.1992.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 51.Franklin GM, Shetterly SM, Cohen JA, Baxter J, Hamman RF. Risk factors for distal symmetric neuropathy in NIDDM. The San Luis Valley Diabetes Study. Diabetes Care. 1994;17(10):1172–1177. doi: 10.2337/diacare.17.10.1172. [DOI] [PubMed] [Google Scholar]
- 52.Martina IS, van Koningsveld R, Schmitz PI, van der Meche FG, van Doorn PA. Measuring vibration threshold with a graduated tuning fork in normal aging and in patients with polyneuropathy. European Inflammatory Neuropathy Cause and Treatment (INCAT) group. J Neurol Neurosurg Psychiatry. 1998;65(5):743–747. doi: 10.1136/jnnp.65.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savage S, Estacio RO, Jeffers B, Schrier RW. Urinary albumin excretion as a predictor of diabetic retinopathy, neuropathy, and cardiovascular disease in NIDDM. Diabetes Care. 1996;19(11):1243–1248. doi: 10.2337/diacare.19.11.1243. [DOI] [PubMed] [Google Scholar]
- 54.The UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 55.Keech AC, Rajamani JK, Best JD, Hankey G, Donoghoe MW, Li L. Predictors of peripheral neuropathy and effects of fenofibrate among 9,795 subjects with type 2 diabetes: the fenofibrate intervention and event lowering in diabetes (FIELD) study. Diabetologia. 2011;54(Suppl 1):A180. doi: 10.1007/s00125-010-1951-1. [DOI] [PubMed] [Google Scholar]
- 56.Rajamani K, Colman PG, Li LP, Best JD, Voysey M, D'Emden MC, Laakso M, Baker JR, Keech AC FIELD study investigators. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet. 2009;373(9677):1780–1788. doi: 10.1016/S0140-6736(09)60698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58(7):1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho YN, Lee KO, Jeong J, Park HJ, Kim SM, Shin HY, Hong JM, Ahn CW, Choi YC. The role of insulin resistance in diabetic neuropathy in Koreans with type 2 diabetes mellitus: a 6-year follow-up study. Yonsei Med J. 2014;55(3):700–708. doi: 10.3349/ymj.2014.55.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clair C, Cohen MJ, Eichler F, Selby KJ, Rigotti NA. The effect of cigarette smoking on diabetic peripheral neuropathy: a systematic review and meta-analysis. J Gen Intern Med. 2015;30(8):1193–1203. doi: 10.1007/s11606-015-3354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ylitalo KR, Sowers M, Heeringa S. Peripheral vascular disease and peripheral neuropathy in individuals with cardiometabolic clustering and obesity: National Health and Nutrition Examination Survey 2001-2004. Diabetes Care. 2011;34(7):1642–1647. doi: 10.2337/dc10-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herman RM, Brower JB, Stoddard DG, Casano AR, Targovnik JH, Herman JH, Tearse P. Prevalence of somatic small fiber neuropathy in obesity. Int J Obes (Lond) 2007;31(2):226–235. doi: 10.1038/sj.ijo.0803418. [DOI] [PubMed] [Google Scholar]
- 62.Smith AG, Rose K, Singleton JR. Idiopathic neuropathy patients are at high risk for metabolic syndrome. J Neurol Sci. 2008;273(1-2):25–28. doi: 10.1016/j.jns.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pittenger GL, Mehrabyan A, Simmons K, Amandarice , Dublin C, Barlow P, Vinik AI. Small fiber neuropathy is associated with the metabolic syndrome. Metab Syndr Relat Disord. 2005;3(2):113–121. doi: 10.1089/met.2005.3.113. [DOI] [PubMed] [Google Scholar]
- 64.Lee KO, Nam JS, Ahn CW, Hong JM, Kim SM, Sunwoo IN, Moon JS, Na SJ, Choi YC. Insulin resistance is independently associated with peripheral and autonomic neuropathy in Korean type 2 diabetic patients. Acta Diabetol. 2012;49(2):97–103. doi: 10.1007/s00592-010-0176-6. [DOI] [PubMed] [Google Scholar]
- 65.Schmelzer JD, Zochodne DW, Low PA. Ischemic and reperfusion injury of rat peripheral nerve. Proc Natl Acad Sci USA. 1989;86(5):1639–1642. doi: 10.1073/pnas.86.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herbst U, Toborek M, Kaiser S, Mattson MP, Hennig B. 4-Hydroxynonenal induces dysfunction and apoptosis of cultured endothelial cells. J Cell Physiol. 1999;181(2):295–303. doi: 10.1002/(SICI)1097-4652(199911)181:2<295::AID-JCP11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 67.Al-Rejaie SS, Aleisa AM, Abuohashish HM, Parmar MY, Ola MS, Al-Hosaini AA, Ahmed MM. Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol Res. 2015 doi: 10.1179/1743132815Y.0000000079. In press. [DOI] [PubMed] [Google Scholar]
- 68.Varkonyi T, Putz Z, Keresztes K, Martos T, Lengyel C, Stirban A, Jermendy G, Kempler P. Current options and perspectives in the treatment of diabetic neuropathy. Curr Pharm Des. 2013;19(27):4981–5007. doi: 10.2174/13816128113199990310. [DOI] [PubMed] [Google Scholar]
- 69.Mendez MM, Folgado J, Tormo C, Artero A, Ascaso M, Martinez-Hervas S, Chaves FJ, Ascaso JF, Real JT. Altered glutathione system is associated with the presence of distal symmetric peripheral polyneuropathy in type 2 diabetic subjects. J Diabetes Complications. 2015 doi: 10.1016/j.jdiacomp.2015.05.023. In press. [DOI] [PubMed] [Google Scholar]
- 70.Kasznicki J, Kosmalski M, Sliwinska A, Mrowicka M, Stanczyk M, Majsterek I, Drzewoski J. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol Biol Rep. 2012;39(9):8669–8678. doi: 10.1007/s11033-012-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnölzer M, Lasitschka F. et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18(6):926–933. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 72.Migdalis IN, Triantafilou P, Petridou E, Varvarigos N, Totolos V, Rigopoulos A. Lipid peroxides in type 2 diabetic patients with neuropathy. Res Commun Mol Pathol Pharmacol. 2005;117-118:5–12. [PubMed] [Google Scholar]
- 73.Ziegler D, Strom A, Brüggemann J, Ziegler I, Ringel B, Püttgen S, Roden M GDS Group. Overexpression of cutaneous mitochondrial superoxide dismutase in recent-onset type 2 diabetes. Diabetologia. 2015;58(7):1621–1625. doi: 10.1007/s00125-015-3609-5. [DOI] [PubMed] [Google Scholar]
- 74.Ziegler D, Buchholz S, Sohr C, Nourooz-Zadeh J, Roden M. Oxidative stress predicts progression of peripheral and cardiac autonomic nerve dysfunction over 6 years in diabetic patients. Acta Diabetol. 2015;52(1):65–72. doi: 10.1007/s00592-014-0601-3. [DOI] [PubMed] [Google Scholar]
- 75.Hoeldtke RD, Bryner KD, Hoeldtke ME, Christie I, Ganser G, Hobbs G, Riggs J. Sympathetic sudomotor disturbance in early type 1 diabetes mellitus is linked to lipid peroxidation. Metabolism. 2006;55(11):1524–1531. doi: 10.1016/j.metabol.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 76.Hoeldtke RD, Bryner KD, Corum LL, Hobbs GR, Van Dyke K. Lipid peroxidation in early type 1 diabetes mellitus is unassociated with oxidative damage to DNA. Metabolism. 2009;58(5):731–734. doi: 10.1016/j.metabol.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 77.Hoeldtke RD, Bryner KD, Van Dyke K. Oxidative stress and autonomic nerve function in early type 1 diabetes. Clin Auton Res. 2011;21(1):19–28. doi: 10.1007/s10286-010-0084-4. [DOI] [PubMed] [Google Scholar]
- 78.Kajita K, Ishizuka T, Miura A, Kanoh Y, Ishizawa M, Kimura M, Yasuda K. Increased platelet aggregation in diabetic patients with microangiopathy despite good glycemic control. Platelets. 2001;12(6):343–351. doi: 10.1080/09537100120078386. [DOI] [PubMed] [Google Scholar]
- 79.Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complications. 2009;23(2):89–94. doi: 10.1016/j.jdiacomp.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T, Lakasas G. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15(8):475–478. doi: 10.1080/0953710042000267707. [DOI] [PubMed] [Google Scholar]
- 81.Xiao W, Huang Y, Dong J, Zhang X, Hu J. Relationship between platelet volume indices with macrovascular and peripheral neuropathy complications in type 2 diabetic patients. J Diabetes. 2014;6(4):298–303. doi: 10.1111/1753-0407.12104. [DOI] [PubMed] [Google Scholar]
- 82.Papanas N, Mavridis G, Karavageli E, Symeonidis G, Maltezos E. Peripheral neuropathy is associated with increased mean platelet volume in type 2 diabetic patients. Platelets. 2005;16(8):498–499. doi: 10.1080/09537100500384723. [DOI] [PubMed] [Google Scholar]
- 83.Shehab D, Al-Jarallah K, Mojiminiyi OA, Al Mohamedy H, Abdella NA. Does vitamin D deficiency play a role in peripheral neuropathy in type 2 diabetes? Diabet Med. 2012;29(1):43–49. doi: 10.1111/j.1464-5491.2011.03510.x. [DOI] [PubMed] [Google Scholar]
- 84.Shehab D, Al-Jarallah K, Abdella N, Mojiminiyi OA, Al Mohamedy H. Prospective evaluation of the effect of short-term oral vitamin D supplementation on peripheral neuropathy in type 2 diabetes mellitus. Med Princ Pract. 2015;24(3):250–256. doi: 10.1159/000375304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung CH, Jung SH, Kim KJ, Kim BY, Kim CH, Kang SK, Mok JO. The relationship between vitamin D status and cardiac autonomic neuropathy in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2015;12(5):342–351. doi: 10.1177/1479164115588546. [DOI] [PubMed] [Google Scholar]
- 86.Hitman GA. Vitamin D, diabetic neuropathy and supplementation post-gestational diabetes. Diabet Med. 2012;29(1):1. doi: 10.1111/j.1464-5491.2011.03524.x. [DOI] [PubMed] [Google Scholar]
- 87.Putz Z, Martos T, Nemeth N, Körei AE, Vagi OE, Kempler MS, Kempler P. Is there an association between diabetic neuropathy and low vitamin D levels? Curr Diab Rep. 2014;14(10):537. doi: 10.1007/s11892-014-0537-6. [DOI] [PubMed] [Google Scholar]
- 88.Gouveri E, Papanas N, Hatzitolios AI, Maltezos E. Hypovitaminosis D and peripheral arterial disease: emerging link beyond cardiovascular risk factors. Eur J Intern Med. 2012;23(8):674–681. doi: 10.1016/j.ejim.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Herder C, Bongaerts BW, Ouwens DM, Rathmann W, Heier M, Carstensen-Kirberg M, Koenig W, Thorand B, Roden M, Meisinger C. et al. Low serum omentin levels in the elderly population with type 2 diabetes and polyneuropathy. Diabet Med. 2015 doi: 10.1111/dme.12761. In press. [DOI] [PubMed] [Google Scholar]
- 90.Herder C, Bongaerts BW, Rathmann W, Heier M, Kowall B, Koenig W, Thorand B, Roden M, Meisinger C, Ziegler D. Differential association between biomarkers of subclinical inflammation and painful polyneuropathy. Results from the KORA F4 Study. Diabetes Care. 2015;38(1):91–96. doi: 10.2337/dc14-1403. [DOI] [PubMed] [Google Scholar]
- 91.Herder C, Bongaerts BW, Rathmann W, Heier M, Kowall B, Koenig W, Thorand B, Roden M, Meisinger C, Ziegler D. Association of subclinical inflammation with polyneuropathy in the older population: KORA F4 Study. Diabetes Care. 2013;36(11):3663–3670. doi: 10.2337/dc13-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herder C, Lankisch M, Ziegler D, Rathmann W, Koenig W, Illig T, Döring A, Thorand B, Holle R, Giani G. et al. Subclinical inflammation and diabetic polyneuropathy: MONICA/KORA Survey F3 (Augsburg/Germany) Diabetes Care. 2009;32(4):680–682. doi: 10.2337/dc08-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Papanas N, Katsiki N, Papatheodorou K, Demetriou M, Papazoglou D, Gioka T, Maltezos E. Peripheral neuropathy is associated with increased serum levels of uric acid in type 2 diabetes mellitus. Angiology. 2011;62(4):291–295. doi: 10.1177/0003319710394164. [DOI] [PubMed] [Google Scholar]
- 94.Papanas N, Demetriou M, Katsiki N, Papatheodorou K, Papazoglou D, Gioka T, Kotsiou S, Maltezos E, Mikhailidis DP. Increased serum levels of uric acid are associated with sudomotor dysfunction in subjects with type 2 diabetes mellitus. Exp Diabetes Res. 2011;2011:346051. doi: 10.1155/2011/346051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leung L, Cahill CM. TNF-alpha and neuropathic pain - a review. J Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamakawa I, Kojima H, Terashima T, Katagi M, Oi J, Urabe H, Kawai H, Chan L, Yasuda H, Maegawa H. et al. Inactivation of TNF-α ameliorates diabetic neuropathy in mice. Am J Physiol Endocrinol Metab. 2011;301(5):E844–E852. doi: 10.1152/ajpendo.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vague P, Dufayet D, Coste T, Moriscot C, Jannot MF, Raccah D. Association of diabetic neuropathy with Na/K ATPase gene polymorphism. Diabetologia. 1997;40(5):506–511. doi: 10.1007/s001250050708. [DOI] [PubMed] [Google Scholar]
- 98.Raccah D, Gallice P, Pouget J, Vague P. Hypothesis: low Na/K-ATPase activity in the red cell membrane, a potential marker of the predisposition to diabetic neuropathy. Diabete Metab. 1992;18(3):236–241. [PubMed] [Google Scholar]
- 99.Heesom AE, Millward A, Demaine AG. Susceptibility to diabetic neuropathy in patients with insulin dependent diabetes mellitus is associated with a polymorphism at the 5' end of the aldose reductase gene. J Neurol Neurosurg Psychiatry. 1998;64(2):213–216. doi: 10.1136/jnnp.64.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chung SS, Chung SK. Genetic analysis of aldose reductase in diabetic complications. Curr Med Chem. 2003;10(15):1375–1387. doi: 10.2174/0929867033457322. [DOI] [PubMed] [Google Scholar]
- 101.Vague P, Coste TC, Jannot MF, Raccah D, Tsimaratos M. C-peptide, Na+,K(+)-ATPase, and diabetes. Exp Diabesity Res. 2004;5:37–50. doi: 10.1080/15438600490424514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nikitin AG, Chudakova DA, Strokov IA, Bursa TR, Chistiakov DA, Nosikov VV. Leu54Phe and Val762Ala polymorphisms in the poly(ADP-ribose)polymerase-1 gene are associated with diabetic polyneuropathy in Russian type 1 diabetic patients. Diabetes Res Clin Pract. 2008;79(3):446–452. doi: 10.1016/j.diabres.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 103.Chistiakov DA, Zotova EV, Savost'anov KV, Bursa TR, Galeev IV, Strokov IA. The 262T>C promoter polymorphism of the catalase gene is associated with diabetic neuropathy in type 1 diabetic Russian patients. Diabetes Metab. 2006;32(1):63–68. doi: 10.1016/s1262-3636(07)70248-3. [DOI] [PubMed] [Google Scholar]
- 104.Strokov IA, Bursa TR, Drepa OI, Zotova EV, Nosikov VV, Ametov AS. Predisposing genetic factors for diabetic polyneuropathy in patients with type 1 diabetes: a population-based case-control study. Acta Diabetol. 2003;40(Suppl 2):S375–S379. doi: 10.1007/s00592-003-0123-x. [DOI] [PubMed] [Google Scholar]
- 105.Papanas N, Papatheodorou K, Papazoglou D, Kotsiou S, Christakidis D, Maltezos E. An insertion/deletion polymorphism in the alpha2B adrenoceptor gene is associated with peripheral neuropathy in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2007;115(5):327–330. doi: 10.1055/s-2007-967084. [DOI] [PubMed] [Google Scholar]
- 106.Rudofsky G Jr, Reismann P, Witte S, Humpert PM, Isermann B, Chavakis T, Tafel J, Nosikov VV, Hamann A, Nawroth P. et al. Asp299Gly and Thr399Ile genotypes of the TLR4 gene are associated with a reduced prevalence of diabetic neuropathy in patients with type 2 diabetes. Diabetes Care. 2004;27(1):179–183. doi: 10.2337/diacare.27.1.179. [DOI] [PubMed] [Google Scholar]
- 107.Tsuzuki S, Murano T, Watanabe H, Itoh Y, Miyashita Y, Shirai K. The examination of apoE phenotypes in diabetic patients with peripheral neuropathy. Rinsho Byori. 1998;46(8):829–833. [PubMed] [Google Scholar]
- 108.Bedlack RS, Edelman D, Gibbs JW 3rd, Kelling D, Strittmatter W, Saunders AM, Morgenlander J. APOE genotype is a risk factor for neuropathy severity in diabetic patients. Neurology. 2003;60(6):1022–1024. doi: 10.1212/01.wnl.0000056689.50682.94. [DOI] [PubMed] [Google Scholar]
- 109.Zhou Z, Hoke A, Cornblath DR, Griffin JW, Polydefkis M. APOE epsilon4 is not a susceptibility gene in idiopathic or diabetic sensory neuropathy. Neurology. 2005;64(1):139–141. doi: 10.1212/01.WNL.0000148587.97690.4E. [DOI] [PubMed] [Google Scholar]
- 110.Voron'ko OE, Yakunina NY, Strokov IA, Lavrova IN, Nosikov VV. Association of polymorphic markers of the lipid metabolism genes with diabetic neuropathy in type 1 diabetes mellitus. Mol Biology. 2005;39(3):206–209. [PubMed] [Google Scholar]
- 111.Monastiriotis C, Papanas N, Veletza S, Maltezos E. APOE gene polymorphisms and diabetic peripheral neuropathy. Arch Med Sci. 2012;8(4):583–588. doi: 10.5114/aoms.2012.30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Monastiriotis C, Papanas N, Trypsianis G, Karanikola K, Veletza S, Maltezos E. The ε4 allele of the APOE gene is associated with more severe peripheral neuropathy in type 2 diabetic patients. Angiology. 2013;64(6):451–455. doi: 10.1177/0003319712453645. [DOI] [PubMed] [Google Scholar]
- 113.Davies B, Cramp F, Gauntlett-Gilbert J, Wynick D, McCabe CS. The role of physical activity and psychological coping strategies in the management of painful diabetic neuropathy - A systematic review of the literature. Physiotherapy. 2015 doi: 10.1016/j.physio.2015.04.003. In press. [DOI] [PubMed] [Google Scholar]
- 114.Tiaka EK, Papanas N, Manolakis AC, Maltezos E. The role of nerve growth factor in the prophylaxis and treatment of diabetic foot ulcers. Int J Burns Trauma. 2011;1(1):68–76. [PMC free article] [PubMed] [Google Scholar]
- 115.Migdalis IN, Kalogeropoulou K, Kalantzis L, Nounopoulos C, Bouloukos A, Samartzis M. Insulin-like growth factor-I and IGF-I receptors in diabetic patients with neuropathy. Diabet Med. 1995;12(9):823–827. doi: 10.1111/j.1464-5491.1995.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 116.Guo H, Yang Y, Geng Z, Zhu L, Yuan S, Zhao Y, Gao Y, Fu H. The change of insulin-like growth factor-1 in diabetic patients with neuropathy. Chin Med J (Engl) 1999;112(1):76–79. [PubMed] [Google Scholar]
- 117.Apfel SC, Schwartz S, Adornato BT, Freeman R, Biton V, Rendell M, Vinik A, Giuliani M, Stevens JC, Barbano R. et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: A randomized controlled trial. rhNGF Clinical Investigator Group. JAMA. 2000;284(17):2215–2221. doi: 10.1001/jama.284.17.2215. [DOI] [PubMed] [Google Scholar]
- 118.Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, Garrow A, Waterman C, Cavanagh PR, Boulton AJ. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- 119.Vileikyte L, Peyrot M, Gonzalez JS, Rubin RR, Garrow AP, Stickings D, Waterman C, Ulbrecht JS, Cavanagh PR, Boulton AJ. Predictors of depressive symptoms in persons with diabetic peripheral neuropathy: a longitudinal study. Diabetologia. 2009;52(7):1265–1273. doi: 10.1007/s00125-009-1363-2. [DOI] [PubMed] [Google Scholar]
- 120.Vileikyte L, Gonzalez JS. Recognition and management of psychosocial issues in diabetic neuropathy. Handb Clin Neurol. 2014;126:195–209. doi: 10.1016/B978-0-444-53480-4.00013-8. [DOI] [PubMed] [Google Scholar]
- 121.Vileikyte L, Peyrot M, Bundy C, Rubin RR, Leventhal H, Mora P, Shaw JE, Baker P, Boulton AJ. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care. 2003;26(9):2549–2555. doi: 10.2337/diacare.26.9.2549. [DOI] [PubMed] [Google Scholar]
- 122.Papanas N, Tsapas A, Papatheodorou K, Papazoglou D, Bekiari E, Sariganni M, Paletas K, Maltezos E. Glycaemic control is correlated with well-being index (WHO-5) in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2010;118(6):364–367. doi: 10.1055/s-0029-1243623. [DOI] [PubMed] [Google Scholar]
- 123.Patel SS, Udayabanu M. Effect of Urtica dioica on memory dysfunction and hypoalgesia in an experimental model of diabetic neuropathy. Neurosci Lett. 2013;552:114–119. doi: 10.1016/j.neulet.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 124.Rucker JL, Jernigan SD, McDowd JM, Kluding PM. Adults with diabetic peripheral neuropathy exhibit impairments in multitasking and other executive functions. J Neurol Phys Ther. 2014;38(2):104–110. doi: 10.1097/NPT.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roman de Mettelinge T, Delbaere K, Calders P, Gysel T, Van Den Noortgate N, Cambier D. The impact of peripheral neuropathy and cognitive decrements on gait in older adults with type 2 diabetes mellitus. Arch Phys Med Rehabil. 2013;94(6):1074–1079. doi: 10.1016/j.apmr.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 126.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 127.Ba-Tin L, Strike P, Tabet N. Diabetic peripheral microvascular complications: relationship to cognitive function. Cardiovasc Psychiatry Neurol. 2011;2011:723434. doi: 10.1155/2011/723434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paul L, Ellis BM, Leese GP, McFadyen AK, McMurray B. The effect of a cognitive or motor task on gait parameters of diabetic patients, with and without neuropathy. Diabet Med. 2009;26(3):234–239. doi: 10.1111/j.1464-5491.2008.02655.x. [DOI] [PubMed] [Google Scholar]
- 129.Kim SH, Lee KA, Jin HY, Baek HS, Park TS. Relationship between the Korean version survey of the autonomic symptoms score and cardiac autonomic neuropathy parameters in patients with diabetic peripheral neuropathy. Diabetes Metab J. 2014;38(5):349–355. doi: 10.4093/dmj.2014.38.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ratzmann KP, Raschke M, Gander I, Schimke E. Prevalence of peripheral and autonomic neuropathy in newly diagnosed type II (noninsulin-dependent) diabetes. J Diabet Complications. 1991;5(1):1–5. doi: 10.1016/0891-6632(91)90002-7. [DOI] [PubMed] [Google Scholar]
- 131.Ziegler D, Gries FA, Mühlen H, Rathmann W, Spüler M, Lessmann F. Prevalence and clinical correlates of cardiovascular autonomic and peripheral diabetic neuropathy in patients attending diabetes centers. The Diacan Multicenter Study Group. Diabetes Metab. 1993;19(1 Pt 2):143–151. [PubMed] [Google Scholar]
- 132.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 133.Gentile S, Bizzarro A, Marmo R, de Bellis A, Orlando C. Medial arterial calcification and diabetic neuropathy. Acta Diabetol Lat. 1990;27(3):243–253. doi: 10.1007/BF02581336. [DOI] [PubMed] [Google Scholar]
- 134.Vinik AI, Maser RE, Ziegler D. Neuropathy: the crystal ball for cardiovascular disease? Diabetes Care. 2010;33(7):1688–1690. doi: 10.2337/dc10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brownrigg JR, de Lusignan S, McGovern A, Hughes C, Thompson MM, Ray KK, Hinchliffe RJ. Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart. 2014;100(23):1837–1843. doi: 10.1136/heartjnl-2014-305657. [DOI] [PubMed] [Google Scholar]
- 136.Kärvestedt L, Martensson E, Grill V, Elofsson S, von Wendt G, Hamsten A, Brismar K. Peripheral sensory neuropathy associates with micro- or macroangiopathy: results from a population-based study of type 2 diabetic patients in Sweden. Diabetes Care. 2009;32(2):317–322. doi: 10.2337/dc08-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Papanas N, Symeonidis G, Mavridis G, Georgiadis GS, Papas TT, Lazarides MK, Maltezos E. Ankle-brachial index: a surrogate marker of microvascular complications in type 2 diabetes mellitus? Int Angiol. 2007;26(3):253–257. [PubMed] [Google Scholar]
- 138.Edmonds ME. Medial arterial calcification and diabetes mellitus. Z Kardiol. 2000;89(Suppl 2):101–104. doi: 10.1007/s003920070107. [DOI] [PubMed] [Google Scholar]
- 139.Edmonds ME, Roberts VC, Watkins PJ. Blood flow in the diabetic neuropathic foot. Diabetologia. 1982;22(1):9–15. doi: 10.1007/BF00253862. [DOI] [PubMed] [Google Scholar]
- 140.Edmonds ME, Morrison N, Laws JW, Watkins PJ. Medial arterial calcification and diabetic neuropathy. Br Med J (Clin Res Ed) 1982;284(6320):928–930. doi: 10.1136/bmj.284.6320.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corbin DO, Young RJ, Morrison DC, Hoskins P, McDicken WN, Housley E, Clarke BF. Blood flow in the foot, polyneuropathy and foot ulceration in diabetes mellitus. Diabetologia. 1987;30(7):468–473. doi: 10.1007/BF00279614. [DOI] [PubMed] [Google Scholar]
- 142.Young MJ, Adams JE, Anderson GF, Boulton AJ, Cavanagh PR. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia. 1993;36(7):615–621. doi: 10.1007/BF00404070. [DOI] [PubMed] [Google Scholar]
- 143.Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. 2011;34(10):2244–2249. doi: 10.2337/dc11-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ybarra-Munoz J, Jurado-Campos J, Garcia-Gil M, Zabaleta-Del-Olmo E, Mir-Coll T, Zabalegui A, Vidal J, Romeo JH. Cardiovascular disease predicts diabetic peripheral polyneuropathy in subjects with type 2 diabetes: A 10-year prospective study. Eur J Cardiovasc Nurs. 2014 doi: 10.1177/1474515114565215. In press. [DOI] [PubMed] [Google Scholar]
- 145.Low SK, Sum CF, Yeoh LY, Tavintharan S, Ng XW, Lee SB, Tang WE, Lim SC. Prevalence of chronic kidney disease in adults with type 2 diabetes mellitus. Ann Acad Med Singapore. 2015;45(5):164–171. [PubMed] [Google Scholar]
- 146.Prasannakumar M, Rajput R, Seshadri K, Talwalkar P, Agarwal P, Gokulnath G, Kotak B, Raza A, Vasnawala H, Teli C. et al. An observational, cross-sectional study to assess the prevalence of chronic kidney disease in type 2 diabetes patients in India (START-India) Indian J Endocrinol Metab. 2015;19(4):520–523. doi: 10.4103/2230-8210.157857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Papanas N, Liakopoulos V, Maltezos E, Stefanidis I. The diabetic foot in end stage renal disease. Ren Fail. 2007;29(5):519–528. doi: 10.1080/08860220701391662. [DOI] [PubMed] [Google Scholar]
- 148.Agrawal RP, Ola V, Bishnoi P, Gothwal S, Sirohi P, Agrawal R. Prevalence of micro and macrovascular complications and their risk factors in type-2 diabetes mellitus. J Assoc Physicians India. 2014;62(6):504–508. [PubMed] [Google Scholar]
- 149.Bitirgen G, Ozkagnici A, Malik RA, Kerimoglu H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet Med. 2014;31:431–438. doi: 10.1111/dme.12324. [DOI] [PubMed] [Google Scholar]
- 150.Nitoda E, Kallinikos P, Pallikaris A, Moschandrea J, Amoiridis G, Ganotakis ES, Tsilimbaris M. Correlation of diabetic retinopathy and corneal neuropathy using confocal microscopy. Curr Eye Res. 2012;37:898–906. doi: 10.3109/02713683.2012.683507. [DOI] [PubMed] [Google Scholar]
- 151.Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Mughal S, Jose B, Piya MK, Barnett AH, Stevens MJ. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med. 2012;186:434–441. doi: 10.1164/rccm.201112-2135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fujihara K, Kodama S, Horikawa C, Yoshizawa S, Sugawara A, Hirasawa R, Shimano H, Yachi Y, Suzuki A, Hanyu O. et al. The relationship between diabetic neuropathy and sleep apnea syndrome: a meta-analysis. Sleep Disord. 2013;2013:150371. doi: 10.1155/2013/150371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vas PR, Ahluwalia R, Manas AB, Manu CA, Kavarthapu V, Edmonds ME. Undiagnosed severe sleep apnoea and diabetic foot ulceration - a case series based hypothesis: a hitherto under emphasized factor in failure to heal. Diabet Med. 2015 doi: 10.1111/dme.12813. In press. [DOI] [PubMed] [Google Scholar]
- 154.Papanas N, Steiropoulos P. Obstructive sleep apnoea and diabetic foot: new responsibilities? Angiology. 2015 doi: 10.1177/0003319715599469. In press. [DOI] [PubMed] [Google Scholar]
- 155.Pop-Busui R, Lu J, Brooks MM, Albert S, Althouse AD, Escobedo J, Green J, Palumbo P, Perkins BA, Whitehouse F. et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. 2013;36:3208–3215. doi: 10.2337/dc13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Calles-Escandon J, Lovato LC, Simons-Morton DG, Kendall DM, Pop-Busui R, Cohen RM, Bonds DE, Fonseca VA, Ismail-Beigi F, Banerji MA. et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:721–727. doi: 10.2337/dc09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 158.Sandbaek A, Griffin SJ, Sharp SJ, Simmons RK, Borch-Johnsen K, Rutten GE, van den Donk M, Wareham NJ, Lauritzen T, Davies MJ et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial: the ADDITION-Europe Study. Diabetes Care. 2014;37:2015–2023. doi: 10.2337/dc13-1544. [DOI] [PubMed] [Google Scholar]
- 159.Charles M, Fleischer J, Witte DR, Ejskjaer N, Borch-Johnsen K, Lauritzen T, Sandbaek A. Impact of early detection and treatment of diabetes on the 6-year prevalence of cardiac autonomic neuropathy in people with screen-detected diabetes: ADDITION-Denmark, a cluster-randomised study. Diabetologia. 2013;56:101–108. doi: 10.1007/s00125-012-2744-5. [DOI] [PubMed] [Google Scholar]


