Abstract
The term "diabetic kidney" has recently been proposed to encompass the various lesions, involving all kidney structures that characterize protean kidney damage in patients with diabetes. While glomerular diseases may follow the stepwise progression that was described several decades ago, the tenet that proteinuria identifies diabetic nephropathy is disputed today and should be limited to glomerular lesions. Improvements in glycemic control may have contributed to a decrease in the prevalence of glomerular lesions, initially described as hallmarks of diabetic nephropathy, and revealed other types of renal damage, mainly related to vasculature and interstitium, and these types usually present with little or no proteinuria. Whilst glomerular damage is the hallmark of microvascular lesions, ischemic nephropathies, renal infarction, and cholesterol emboli syndrome are the result of macrovascular involvement, and the presence of underlying renal damage sets the stage for acute infections and drug-induced kidney injuries. Impairment of the phagocytic response can cause severe and unusual forms of acute and chronic pyelonephritis. It is thus concluded that screening for albuminuria, which is useful for detecting "glomerular diabetic nephropathy", does not identify all potential nephropathies in diabetes patients. As diabetes is a risk factor for all forms of kidney disease, diagnosis in diabetic patients should include the same combination of biochemical, clinical, and imaging tests as employed in non-diabetic subjects, but with the specific consideration that chronic kidney disease (CKD) may develop more rapidly and severely in diabetic patients.
Keywords: diabetes, chronic kidney disease, diabetic nephropathy, glomerulosclerosis, glomeruli, retinopathy
Abbreviations: ACE – angiotensin-converting enzyme; ADC - apparent diffusion coefficient; AFOG – acid fuchsin orange G; AIDS – acquired immune deficiency syndrome; AKI – acute kidney injury; ARB – angiotensin receptor blocker; CKD – chronic kidney disease; COX-2 – cyclooxygenase 2; CT – computed tomography; ERA-EDTA – European Renal Association / European Dialysis and Transplant Association; FSGS – focal segmental glomerulosclerosis; GFR – glomerular filtration rate; LDH – lactate dehydrogenase; MRI – magnetic resonance imaging; PAS – periodic acid-Schiff; PTH – parathyroid hormone; RAAS – renin-angiotensin-aldosterone system; UAE – urinary albumin excretion
1. Introduction
Diabetic nephropathy is a complex and multifaceted condition that can also be described as a syndrome with varying clinical manifestations and responses to therapy. It is related to different underlying pathophysiological mechanisms and to the effects of the different and changing treatments of diabetes itself [1-5].
The classic term "diabetic nephropathy" points to the presence of a single, well defined, and identifiable kidney disease. Because of the complexity and heterogeneity of renal impairment in diabetic patients, the classic term has been increasingly replaced by the more generic term "diabetic kidney disease" which is reminiscent of the term "chronic kidney disease". There are also other definitions in the context of kidney disease, including "diabetic glomerulopathy" and "diabetic podocytopathy", but they limit the field to a specific type or pathogenesis of kidney injury [6-14]. In this review, we use the classic term "diabetic nephropathy" to identify the typical progressive glomerular nephropathy in its stages and variants, while the term "diabetic kidney disease" is used in cases where kidney involvement affects other renal structures such as interstitium or blood vessels.
Over the past twenty years, the development and manifestation of diabetic nephropathy have changed, mainly because of improvements in diabetes treatment. In recent times, more attention has been given to type 2 rather than type 1 diabetes. While the original paradigm of diabetic nephropathy was first described in type 1 diabetes, nowadays the disease is more prevalent in type 2 diabetes, which is mainly ascribed to prolonged life expectancy of type 2 diabetes patients [4, 5, 15-21].
2. Disease of the "survivors": a parallel to dialysis
At the beginning of the 1980s, it was almost unexpected that dialysis patients survived the first decade of treatment. At the same time, a series of studies were conducted that attempted to identify the clinical and psychological features of the "best candidates" for long-term renal replacement therapy [22-25]. Two main issues emerged from these early studies:
The dialysis population had changed over time; the changes reflected the broader acceptance of elderly (attributed to increased life span in the overall population) and "high-risk" patients (diabetic patients were the prototype) [15-21, 26-29].
The treatment modified the clinical histories, and as a result of iatrogenesis or incomplete correction of uremia by dialysis, long-term survivors on dialysis presented with disease combinations that otherwise were exceptional [30-33].
Improvements in diabetic care increased survival rates. In younger type 1 diabetes patients, the improved survival prognosis caused a greater acceptance for treatment in dialysis facilities, which previously had not accepted younger patients because of the possible associated cardiovascular impairment. Increased long-term survival was achieved more frequently, at least in selected cases [34-40].
In the 1990s, diabetes patients became the predominant dialysis population, mainly because of the increase in type 2 diabetic patients. Due to the established longer survival in the predialysis phase, these patients could gain additional time until end-stage renal disease [4, 5, 15-20, 41-43].
The increased availability of kidney and pancreas transplants further changed the perspectives and management of diabetic patients with severe kidney disease, especially of those with type 1 diabetes. In the 1990s, researchers developed the idea of early combined kidney-pancreas transplantation at a stage in which it was possible to stop the progression of the concomitant retinal and neural damages [44-48].
In contrast, type 2 diabetes patients were frequently affected by "atypical" nephropathies, with combinations of early onset proteinuria (often explained as being due to undiagnosed kidney disease), scarce proteinuria, and diffuse vascular disease, or by a "stepwise" decrease in renal function that did not fit well with the classic description of the four stages of diabetic nephropathy. Typical lesions in diabetic patients coexisted with little proteinuria, while several authors reported non-diabetic kidney diseases as being more common [49-53].
The increase in type 2 diabetic patients visiting nephrology facilities was in part unexpected as it was the main goal of the so-called "Saint Vincent Declaration" (signed by a panel of European diabetes experts in 1989) to reduce end-stage diabetic nephropathy by one-third in five years [5, 16, 54-56]. However, the increase may be explained by improved survival and longer continuance in the dialysis stage before end-stage renal disease is reached. A similar phenomenon was observed in the overall dialysis population that continued to increase mainly because elderly patients with major comorbidities survived long enough to develop end-stage renal disease [27-29].
Almost 40 years after the widespread acceptance of diabetic patients in the dialysis programs, the increase in elderly patients on dialysis, a subset in which diabetic patients are highly represented, re-posed clinical and ethical problems associated with end-of-life issues and the difficulty in defining the limit between optimal care of frail patients and aggressive treatment [34-37, 57-62].
3. The profile of diabetic nephropathy in type 1 diabetic patients
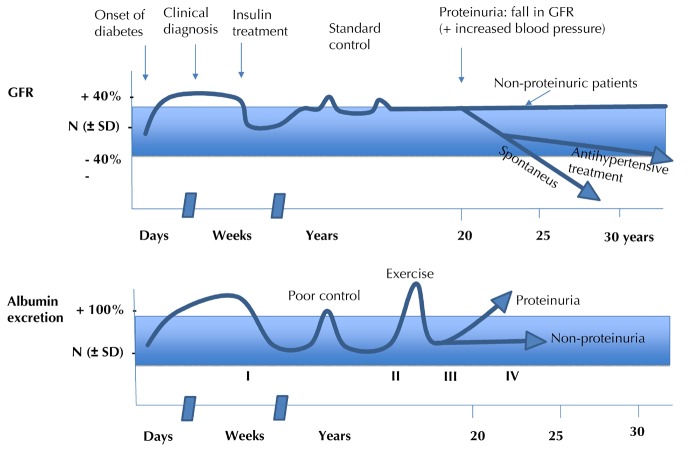
In their seminal work published in the early eighties, Mogensen and co-workers have developed a model describing the clinical history of nephropathy in type 1 diabetic patients over a course of five stages. Since then, it has been a referral model for assessing the progression and prognosis of diabetic nephropathy [63]. Figure 1 illustrates the five stages of renal damage in type 1 diabetes according to the model by Mogensen and co-workers:
Figure 1. Stages of diabetic nephropathy.
Figure creation according to the classic description of the stages of diabetic nephropathy by Mogensen et al. [63].
Stage 1: Early hyperfunction and hypertrophy, occurring before the start of insulin treatment; this condition is partly reversible by insulin treatment [63-64]. After this transitional phase, which can be avoided by starting timely and effective treatment, it follows a clinically "silent" stage 2.
-
Stage 2: In the original paper, the authors stated that this phase is "characterized by morphologic lesions without signs of clinical disease. However, kidney function tests and morphometry on biopsy specimens reveal changes". These changes include increased glomerular filtration rate (GFR) and albuminuria after physical exercise, which may be more prevalent in cases of poor diabetes control. The changes determined by kidney biopsy, which were initially reported by Osterby and co-workers and later confirmed by other groups, include thickening of the glomerular basement membrane and mesangial expansion; the latter is considered to be the hallmark of early diabetic nephropathy [65-70].
Albeit of great pathophysiological interest, these findings are of limited clinical use for the following reasons (as also stated in the original report):
This condition is not necessarily progressive such that several patients may remain in stage 2 throughout their lives.
The functional pattern is unremarkable such that kidney biopsy is not needed.
However, kidney biopsy may indeed be needed in the subsequent stages 3 and 4, especially in the case of early onset of renal damage.
Stage 3: Regarded as incipient diabetic nephropathy. Urinary albumin excretion (UAE) increases. It is thus also called the "microalbuminuric" phase. This stage merges into stage 4.
-
Stage 4: Overt diabetic nephropathy, with UAE slowly and gradually increasing over the years together with blood pressure. Stage 4 is characterized by persistent proteinuria (>0.5 g/24 h).
Microalbuminuria is thus the first sign of "true" diabetic nephropathy. Many authors still maintain this definition, in particular in specific situations such as pregnancy [71-74]. In the same paper, Mogensen and co-authors defined that diabetic nephropathy is present if blood pressure is high and untreated and GFR declines with a mean rate of about 1 ml/min/month [63].
Stage 5: End-stage renal failure.
In the Mogensen model, the development of overt proteinuria is accompanied by an increase in blood pressure; proteinuria (going up) and kidney function (going down) virtually cross. Afterwards, kidney failure occurs quickly. It is assumed that the process of decline in kidney function takes about 7.5 years from "normal" kidney function (GFR 100 ml/min) to end-stage renal disease (GFR 10 ml/min).
Controversy exists regarding the reversibility of microalbuminuria; the results of several studies over the last few decades remain inconclusive. The condition is potentially responsive to ACE inhibitors, angiotensin II receptor inhibitors, and their combination, but progressing obesity counteracts the effectiveness of this treatment strategy. It is alarming how many young diabetic patients are affected by obesity. Another treatment option is therefore to decelerate the progression of diabetic nephropathy through the restriction of protein intake [75-89].
Although the natural history of diabetic nephropathy, as reported over 30 years ago, has changed mainly because of the disease-modifying effects of therapy, some points still hold true. In the classic view of diabetic nephropathy, the microalbuminuric phase is followed by normalization of the GFR, which is considered the first sign of reduced nephron mass that is no longer able to accomplish a "hyperfiltration" response [63]. If GFR normalization is not linked to optimal diabetes control, it still represents the first sign of decreased renal functional reserve. Therefore, close attention should be paid when loss of GFR is relatively fast, regardless of baseline levels [90-91]. Interestingly, a similar interpretation was proposed for focal segmental lesions in obesity, a condition that is often associated with type 2 diabetes and the metabolic syndrome [92-95].
Nevertheless, the Mogensen model still applies to kidney disease in the setting of poorly controlled type 1 diabetes. Indeed, the natural history and sequence of normo- to micro- to macroalbuminuria has been integrated in the new classification of diabetic nephropathy published in 2014 [96].
4. Characteristics of the renal lesions in diabetic nephropathy
The main glomerular renal lesions in type 1 diabetes include a nodular, classical Kimmelstiel-Wilson lesion, a diffuse pattern, and the presence of non-specific exudative lesions [97-100]. Today, the accumulation of extracellular matrix is considered an indication of nephropathological changes. This accumulation may lead to mesangial expansion and reduction of filtration surface area, which is further disrupted by the thickening of glomerular basement membranes [69, 101-108]. Concomitant changes at the arteriolar level and in the renal interstitium contribute to the overall functional impairment. As in other primary kidney diseases, the severity of the vascular and interstitial lesions bears a strong negative effect on prognosis. The recent pathologic classification by Tervaert and the Renal Pathology Society does not differentiate between type 1 and type 2 diabetes, but provides a comprehensive effort to classify renal lesions from the mildest to the worst ones (Figure 2) [109].
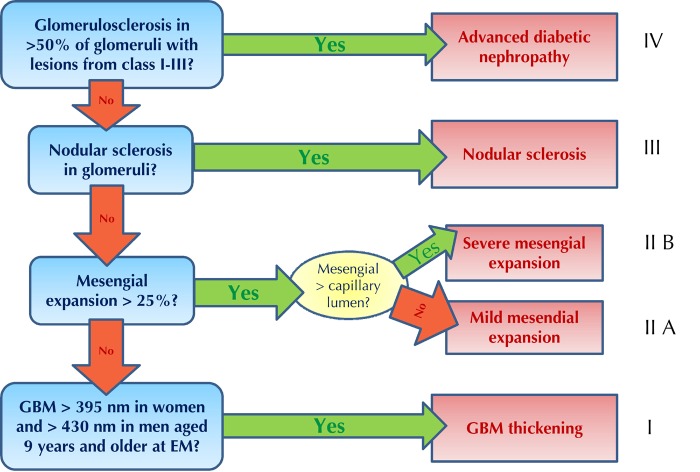
Figure 2. Pathologic classification of diabetic nephropathy.
GBM: glomerular basement membrane, EM: electron microscopy. Figure inspired by Tervaert et al. [109].
Figure 2 illustrates the four classes of diabetic nephropathy according to the extent of the pathologic findings [109]:
Class 1: Isolated glomerular basement membrane thickening and mild, non-specific changes, observable by light microscopy, at an extent at which they may not be applicable to the criteria of the other classes.
Class 2: Mesangial expansion, classified as mild or severe, but without nodular sclerosis or global glomerulosclerosis in more than 50% of glomeruli (class 2a: mild; class 2b: severe).
Class 3: Presence of nodular sclerosis in at least one glomerulus (Kimmelstiel-Wilson), without changes as described in class 4.
Class 4: Advanced diabetic glomerulosclerosis involving more than 50% of glomeruli.
Although controversial, the Tervaert classification is simple and incorporates prognostic factors. Also, the importance of its role was confirmed in a large recent case series, involving long-term follow-up investigations of diabetic patients [110-112].
Figure 3 shows examples of diabetic nephropathy in type 1 diabetic patients from Sardinia, a region with one of the highest incidence of type 1 diabetes world wide [113-115].
Figure 3. Examples of diabetic nephropathy in diabetic patients in Sardinia.
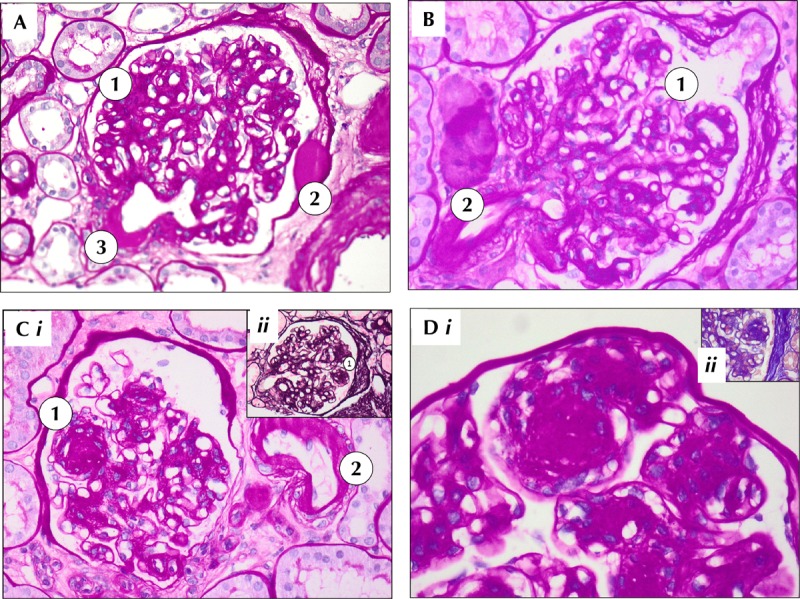
Sardinia is one of the regions with the highest incidence of diabetes in the world. A: Periodic acid-Schiff (PAS) 20 X. Diffuse glomerulosclerosis (1) with "capsular drops" from exudative lesions (2) and arteriolar hyalinosis (3). B: PAS staining, 20 X. Diffuse mesangial expansion with sclerosis (1) and arteriolar hyalinosis (2). C: i) PAS 20 X. Diabetic glomerulosclerosis, with nodular pattern (1). Arteriolar hyalinosis (2). ii) Methenamine silver 20 X. Diffuse and nodular glomerulosclerosis. D: i) Periodic acid-Schiff (PAS) 40X. Magnification of nodular glomerulosclerotic lesion surrounded by mesangial cells. ii) Acid fuchsin orange G (AFOG) 40 X. Diffuse and nodular sclerosis in diabetic nephropathy.
5. The "renal-retinal syndrome" and indications for kidney biopsy
As healthcare improved over time, type 2 diabetic patients survived long enough to become afflicted with diabetic nephropathy. Therefore, the first microscopic examinations of diabetic kidneys were performed at autopsies of these patients. In most cases, these patients died of cardiovascular diseases. Eight patients aged 48-68 years were described by Kimmelstiel and Wilson in 1935; these patients had a 3-15 year history of diabetes [116-117].
Once the first survivors of juvenile diabetes reached 10-15 years of follow-up, diabetic nephropathy became one of the most dangerous long-term complications of diabetes, tantamount to a disease of young diabetics. In type 2 diabetes, the role of diabetic nephropathy was overlooked for many decades [118-120].
There is still controversy regarding the question of whether to perform renal biopsy in all patients with diabetic nephropathy. In any case, the history of diabetic nephropathy in type 1 diabetes showed that there are atypical cases, which should undergo a different diagnostic pathway and include kidney biopsy [121-125]. Classically, the following five major criteria to identify atypical courses are reported:
Timing
Velocity of progression
Hematuria
Absent or low proteinuria
However, in routine clinical care it appeared that these criteria could not perform adequately in order to differentiate non-diabetic from diabetic nephropathy. This problem particularly referred to the increasing population of type 2 diabetic patients, where non-diabetic renal disease has increasingly been recognized [121, 127-135]. Furthermore, relating to the diagnosis of diabetic nephropathy, the morphological stage was not fully predictable by the clinical patterns. In detail, the basic principle applied was: diabetic nephropathy (defined as persistent microalbuminuria) usually occurs at least 10 years after the onset of type 1 diabetes. However, this is not equivalent to type 2 diabetes, where proteinuria is regarded as a marker of cardiovascular morbidity [136-140].
Hallmarks of non-diabetic nephropathy in type 1 diabetes patients include:
A shorter interval between diagnosis and onset of renal disease.
A stepwise or rapidly progressive increase in proteinuria or a decrease in kidney function.
Hematuria without or with low-grade proteinuria [121-125, 127-135, 137-142].
There are also exceptions from these criteria. The exceptions concern patients with atypical appearance of retinal and renal signs, which were once considered to be almost synchronous [143-146]. However, the association between renal and retinal disease is still regarded as close, with predicting power of diabetic retinopathy for the initiation of diabetic nephropathy [147]. Therefore, the combined consideration of both conditions as renal-retinal syndrome is still warranted. Due to the coincidence of renal and retinal diseases in diabetic patients some authors, interestingly including W.J. Kolff, the inventor of the artificial kidney, regarded dialysis in diabetic patients as a mere "palliative measure with little likelihood of long-term survival or improvement in quality of life" [34].
Despite the general agreement that renal biopsy is the gold standard for diagnosis and classification of diabetic nephropathy, some authors suggested different approaches, ranging from biopsy in all cases to the definition of selected subsets where biopsy should be performed. The proposed strategies may be summarized as follows:
All diabetic patients with kidney damage should undergo renal biopsy.
Only patients with suspicion of other nephropathies should undergo renal biopsy.
Only patients in whom the finding of a different kidney disease would lead to a specific treatment should undergo renal biopsy [53, 148-151].
In this regard, it is important to note that imaging profiles of kidneys in patients with advanced diabetic nephropathy may present without pathological findings (Figure 4).
Figure 4. Examples of diabetic nephropathy.
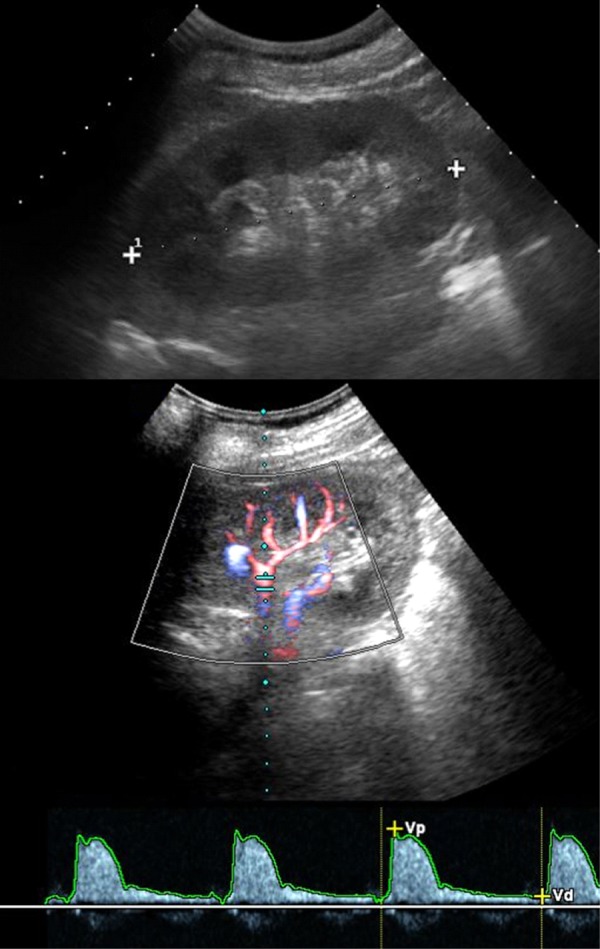
The figure shows ultrasound images of a 50-year old type 1 diabetic patient with severe renal failure. Ultrasonography (upper image) shows normal appearance of the left kidney with regular corticomedullary differentiation and size. Color-Doppler sonography and Doppler spectrum of an intrarenal artery demonstrate a normal waveform and a normal early systolic compliance peak without abnormal findings.
In the past, attention was given to the presence of overt nephropathy only, and other signs of diabetic end-stage kidney disease were frequently disregarded. Today, attention is focused on the onset and progression of CKD, even in the light of good glycemic control, and on the impairment of kidney function that occurs without overt albuminuria. These newer approaches aim to take into account alternative pathogenic pathways that may occur in some cases of CKD. Another important aspect still under investigation is the interplay between genetic background and epigenetic mechanisms, including DNA methylation, histone post-translational modifications in chromatin, and non-coding RNAs [152-156]. Different levels of hyperglycemia, their interplay with growth factors, and oxidant and inflammatory stress can alter gene expression in target cells, including podocytes and renal endothelial cells [154-156]. These alterations are persistent, and they are the basis of a "metabolic memory", by which previous, possibly unrecognized hyperglycemia may induce modifications that persist even after normoglycemia is restored.
Atypical relations between metabolic control and morphologic damage can exist. The interesting nosological entity of "diabetic nephropathy without diabetes" means that typical diabetic kidney lesions develop in patients without overt diabetes [157-159]. In about half of the reported cases, signs of glucose intolerance are found only after careful analysis. This finding confirms the importance of the interplay between genetic background and metabolic control, beyond a simplistic interpretation of diabetic nephropathy as a disease strictly related to sustained hyperglycemia [158-159].
6. Diabetic nephropathy in type 2 diabetes: diabetes as a disease or as a comorbid condition?
The morphology of the renal lesions in type 2 diabetes is considered to be almost indistinguishable from that in type 1 diabetes. In contrast, the natural history of kidney disease frequently differs. This discrepancy is mainly due to the shorter interval between diagnosis and overt renal disease in type 2 diabetes, an occurrence that was initially and simplistically explained by the subtle and non-symptomatic onset of type 2 diabetes. This has been increasingly described in young type 2 diabetics, where the genetic background may play an important role [160-167].
Typically in the elderly, the clinical picture of a diabetic patient may be dominated by diabetes and its complications. Alternatively, hypertension, lipid derangements, and obesity may progress into a dysmetabolic syndrome (the "metabolic syndrome" par excellence), in which diabetes is just one component. This concept is known as the "inactivity paradigm". Therein, diabetes is regarded as a "comorbid factor" in a disease that is dominated by obesity and vasculopathy. In this concept, the primary kidney diseases are renal vascular and nephroangiosclerotic diseases [168-171].
A few studies have addressed the following question in dialysis patients: is there any difference between diabetes as a comorbid condition (possibly with onset during dialysis) and diabetes as the cause of kidney disease? While smaller studies suggested that there is no difference, a recent large study based on the ERA-EDTA Registry found that mortality is higher in patients with diabetes as the primary renal disease compared to those with diabetes as a co-morbid condition. This finding suggests that survival is affected by the extent to which diabetes has caused organ damage, mainly within the cardiovascular system [172-174].
7. Progressive renal decline and non-albuminuric diabetic nephropathy: a new paradigm in type 1 diabetes
According to the classic concept, diabetic nephropathy is a disease marked by an increase in proteinuria during its progression. However, this concept has recently been challenged by the description of non-proteinuric (or non-nephrotic proteinuric) kidney disease in diabetic patients [71, 73, 41, 175-180]. The term "pauciproteinuria" has been implemented to describe these cases [179]. More than a decade ago, this clinical picture was described as a facet of the cardiovascular impairment in diabetic patients. The reasons for the change in recognition of this disease were due to the detection of various lesions and the establishment of alternative diagnoses that include the full spectrum of vascular nephropathies, including interstitial (and/or drug-induced) diseases, pyelonephritis, obstructive nephropathies, recurrent bouts of acute kidney injury, and change in the natural history of the disease due to improved therapies [176-181].
In some reports by our group, we described kidney disease in diabetic patients not characterized by proteinuria. These cases encompassed various conditions including metabolic derangements, genetic syndromes, and "primary" nephroangiosclerosis [182-185]. In a recent series of pregnant patients with "severe" kidney disease and type 1 diabetes, median proteinuria was 1.6 g/day at the start of pregnancy or at referral. While proteinuria ranged from 0.1 to 6.3 g/day, the lower level was observed in a diabetic patient with biopsy-proven diabetic nephropathy involving almost 50% of glomeruli. The median GFR was 67 ml/min. Retinopathy was present in all patients and clinical neuropathy in half of them [186]. This confirms that signs of diabetic nephropathy, as determined by kidney biopsy, can co-exist with minor clinical signs.
The changing pattern of nephropathy alongside the improvement in care suggests a similarity with AIDS-related nephropathies that increasingly present with less immune complex-mediated glomerular disease and more non-collapsing focal-segmental glomerulopathy. Currently these changing patterns cannot be explained; they are likely multifactorial. Antiretroviral therapies, renin-angiotensin-aldosterone system (RAAS) antagonists, earlier nephrology referral, and generally improved medical care may all play a role [187-188].
8. The changing pattern of glomerulosclerosis: a lesion rather than a disease
Our knowledge of several kidney diseases has improved and our treatment approaches are continuously being modified accordingly. This is also true for focal segmental glomerulosclerosis (FSGS); its disease profile consistently overlaps with diabetic nephropathy [189]. While skilled pathologists are able to distinguish between primary and secondary FSGS, the differences are not always clear. FSGS is one of the most frequently reported "non-diabetic" lesions in a recent study of a series of diabetic patients who underwent kidney biopsy [149, 190-191]. Curiously, this condition has not been reported previously, suggesting that FSGS and global glomerulosclerosis in diabetes may appear similarly, at least in some cases. In a series of such cases, patients that were classified as having diabetic nephropathy without retinopathy, were diagnosed with diffuse glomerulosclerosis, a non-nodular form of diabetic nephropathy [192].
Traditionally, secondary FSGS is regarded as a disease that is mediated hemodynamically. It is considered to be the result of a vast array of events, including drug effects, infections, and genetic mutations. Obesity has frequently been associated with this disease. Indeed, focal segmental glomerulosclerosis is the most common renal lesion observed in obese patients [189, 193-197]. As obese type 2 diabetic patients form a large subset of the entire population of diabetic subjects, diabetes and kidney disease may be associated with obesity as a comorbidity or, on the contrary, obesity-related glomerulopathy may have diabetes as a comorbid condition [190, 198, 199].
The search for a common pathway, which has already been attempted in FSGS, may be extended to some of the general glomerulosclerotic lesions in diabetes, and to their relationship with the focal lesions of FSGS. This consideration further highlights the discrepancy between metabolic demand and nephron mass.
9. Incidental association of glomerular diseases and diabetes: just a question of probability?
It has been difficult to find a cause-effect relationship or a common pathogenic link between the presence of diabetes and the prevalence of glomerular diseases, in particular membranous nephropathy [200-205]. The finding of (porcine) insulin deposits in the glomeruli in the context of membranous nephropathy may be regarded as evidence for such a link. However, there is also evidence for the lack of a direct relationship between diabetes and other glomerular diseases, namely the fact that the patterns of the biopsy-proven glomerular diseases vary over the world, and mainly reflect the most common glomerular diseases in the resident population [53, 124, 177-180, 190-192].
The assumption of a cause-effect relationship is supported by the simultaneously increasing prevalence of both diabetes and chronic kidney disease. Each of these diseases presently affects more than 10% of the population in high-income countries, with sharply rising incidences worldwide. The increased combined appearance of diabetes and kidney disease may be due to the rising attention for early diagnosis in diabetic patients, and concomitant early diagnosis of otherwise overlooked diseases such as CKD [206-212].
10. Impairment of kidney function in diabetic patients: metabolic aspects beyond GFR
While kidney damage is often defined by the presence of albuminuria and/or impaired GFR, several recent studies found early endocrine derangements in diabetic kidneys compared with non-diabetic kidney [212-214]. Indeed, erythropoietin synthesis has been reported to decline earlier in the diabetic than in non-diabetic CKD. This is an important finding since erythropoietin deficiency may enhance the vascular anoxic lesions in glomerulopathy or retinopathy [215-218].
Early derangements in the vitamin D-PTH axis have also been described. Vitamin D receptor polymorphisms have been implicated in the pathogenesis of diabetes and its complications [219-220]. Hyporeninemic hypoaldosteronism is another impairment that has been described in diabetic patients. The occurrence of this impairment may explain the disproportion between hyperkalemia and the rise in serum creatinine and/or in the absence of ACE-inhibitor and/or angiotensin receptors inhibitors [221-222].
11. Other chronic kidney diseases associated with diabetes: vascular kidney disease, renal infarction, and cholesterol emboli syndrome
The kidney can be regarded as an "atlas" of blood vessels of different size and specialization (filtration, concentration, higher or lower porosity). Diabetes may be described as a systemic metabolic disease with micro- and macrovascular complications. It is thus no surprise that all the vascular lesions that can occur in kidney diseases are also described in diabetes patients.
Atherosclerotic stenosis of the main renal arteries and their branches are a common and frequently overlooked component of severe diffuse atherosclerosis in diabetic and non-diabetic patients. Renal artery stenosis can be observed in about one third of cases with concomitant hypertension and/or kidney function impairment [224-226]. However, the old tenet that the presence of renal artery stenosis may be revealed by unstable blood pressure that requires more than two drugs for control or by severe hypertensive crises is unsustainable. Most of the patients with renal artery stenosis do not have clinical features specific enough to differentiate them from other hypertension and vasculopathy patients.
The proposed scores to identify patients with renal artery stenosis are indeed based on the presence of diffuse vascular disease, hypertension, and high serum creatinine [227-228]. However, diagnosis is complicated by frequent multiorgan involvement and the lack of information regarding the degree of revascularization. Whilst diagnosis is only the first step towards a tailored treatment, it is necessary to address the specific conditions in any single patient [229]. Furthermore, an acute increase in serum creatinine after intake of ACE inhibitors or angiotensin receptor blockers (ARBs) may be observed in the presence of microvascular disease. However, if a diabetic nephropathy patient has been on long-term therapy with ACE inhibitors or ARBs, an acute increase is unlikely, and a gradual increase may be overlooked because of a well-known underlying kidney disease [230-232].
In our experience, the occurrence of acute kidney injury (AKI) in the context of dehydration or overzealous diuretic use may be a complementary element in the diagnosis of renal artery stenosis. We believe that a systematic assessment of the renal arteries should be performed in all diabetic and vasculopathic patients. Also, the use of ACE inhibitors and ARBs should be limited, and only considered in selected patients with severe proteinuria, as these drugs per se represent a risk of inducing AKI [233-234].
Renal infarction is a rare complication that frequently involves stenosis of the large arterial vessels. It occurs usually within the intraparenchymal branches. This should be considered when a diabetic patient develops a clinical picture that is at first glance indistinguishable from that of acute pyelonephritis (described in the next paragraph). The main clues for acute infarction versus acute pyelonephritis derived from imaging investigation include:
Absence of perinephric involvement and renal swelling
Absence of spatial relationship with the calyces
Sharper differentiation of the lesion (if assessed at presentation)
The biochemical picture of renal infarction may be identical to that of pyelonephritis, but specific features include the slower decrease of C-reactive protein as a response to antibiotic therapy and the higher levels of lactate dehydrogenase (LDH), especially at first presentation (Figure 5) [235-237].
Figure 5. Unenhanced and multiphase-enhanced helical computed tomography in a 78 years old man with type 2 diabetes mellitus and renal infarction.
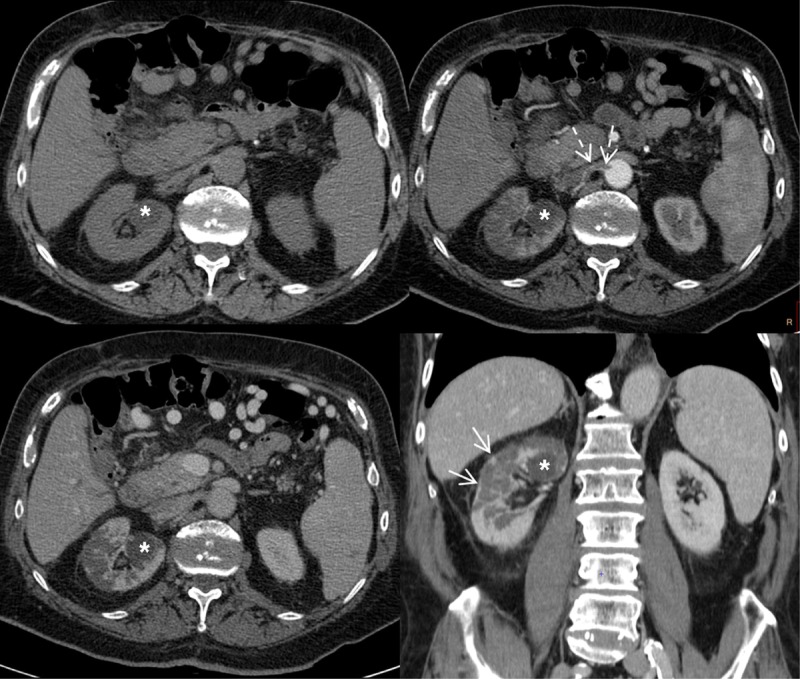
The pre-contrast scan shows homogeneous attenuation of the kidneys. The corticomedullary arterial phase and the nephrographic phase demonstrate multiple round- and wedge-shaped focal areas of decreased enhancement in the right kidney with loss of corticomedullary differentiation due to multiple infarctions. The arterial phase demonstrates a thrombus of the right renal artery as a filling defect extending from the ostium to the proximal segment of the main renal artery (dotted arrows). Coronal reformation of the nephrographic phase detects a thin rim of capsular enhancement (arrows) known as the "cortical rim sign", which suggests a diagnosis of renal infarction. A fluid cortical cyst is visible in the upper part of the right kidney (asterisks).
Involvement of medium-sized arteries in the kidney parenchyma has been extensively described as a frequent occurrence in diabetic kidney disease (e.g. in the recent Tervaert classification), and it has recently been associated with the "non-proteinuric" or "pauciproteinuric" pattern in diabetic kidney disease. The extensive involvement of the renal vasculature may explain the frequently observed stepwise increase in serum creatinine, which is less common in the "classic" forms of proteinuric diabetic nephropathy [109].
Small and medium-sized vessels are also a target of the cholesterol emboli syndrome, an emerging clinical condition that is frequently overlooked because of its clinical mimicry. Typical cases are linked to vascular manipulations or changes in anticoagulant-antiaggregant therapy, and may present with livedo, vasculitis-like skin lesions, and progressive renal impairment weeks or months after the manipulation or therapeutic change. Skin and kidney are frequently involved, but any organ can be affected. The so called "spontaneous" lesion is insidious and difficult to diagnose. It is usually linked to the presence of an ulcerated plaque and a rapid decrease in kidney function in the context of diffuse atherosclerosis [238-243].
12. Upper urinary tract infection as another acute and chronic kidney disease associated with diabetes
12.1 Upper urinary tract infections
The reason why urinary tract infections are frequent in diabetic patients has long been a matter of speculation. Glycosuria was initially considered a relevant factor as it constitutes a basis for bacterial growth. However, the risk of urinary tract infection is not higher in patients with isolated euglycemic glycosuria, thus suggesting a more complex relationship between host and local risk factors [244]. In any case, diabetic patients are at higher risk for severe parenchymal lesions, including unusual complicated urinary tract infections such as emphysematous pyelonephritis, malakoplakia, and "renal carbuncle" [245-250].
12.2. Acute pyelonephritis and upper urinary tract infection
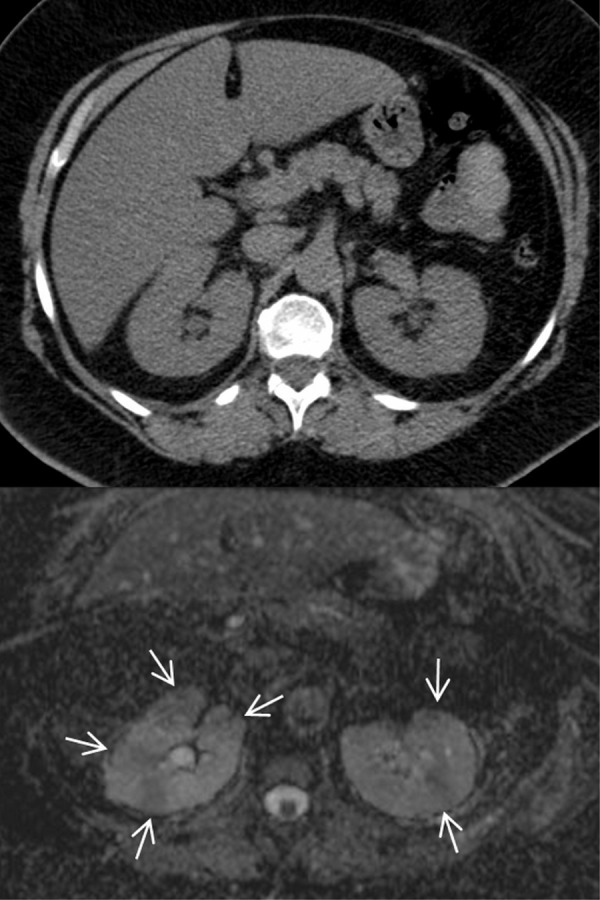
Acute pyelonephritis in diabetic patients is multifactorial and frequently severe, with a high incidence of abscessed lesions (Figures 6 and 7). Based on the experience of our group, diabetic patients presenting with a clinical picture of acute urinary tract infection should undergo a second-line imaging test (computed tomography (CT) scan with contrast media, or magnetic resonance imaging (MRI) with or without gadolinium). Long-term intravenous therapy is probably needed to avoid kidney scars [251-254]. The condition may also include subtle defects in the immunologic response, mainly involving phagocytosis. It may thus be regarded as a comorbidity of diabetes, and seems to be closely correlated to the quality of glucose control [255].
Figure 6. Computed tomography (CT) and diffusion-weighted magnetic resonance imaging (MRI) in a 60 years old type 2 diabetes patient who presented with bilateral acute flank pain at the emergency department.
Unenhanced helical CT scan of the abdomen (upper image) shows homogeneous attenuation of both kidneys, normally shaped and sized, without calcifications or stones. The appearance of fat around the kidneys is normal and homogeneous with no stranding or fluid collections throughout the perinephric space or within the peri-renal fascia. The apparent diffusion coefficient map obtained from diffusion-weighted MRI (lower image) at the same day as CT examination demonstrates multiple round- and wedge-shaped areas of hypo-intensity in the cortex of both kidneys (arrows), with restricted diffusion to the movement of water molecules (mean apparent diffusion coefficient (ADC) value of 1.38 x 10-3mm2s-1), which suggests foci of bilateral acute pyelonephritis.
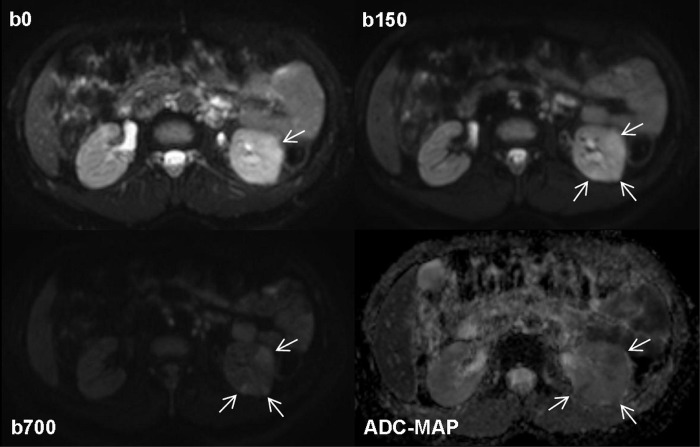
Figure 7. Diffusion-weighted MRI in a 38 years old patient with type 1 diabetes and acute bacterial pyelonephritis in the left kidney.
Native diffusion-weighted images obtained at b values of 0 and 150 sec/mm2 show some cortical wedge-shaped areas of faint hyper-intensity that are well detectable. The areas persist at the higher b value of 700 sec/mm2 (arrows) suggesting reduced diffusion due to inflammatory edema and acute pyelonephritis. This finding is confirmed by the apparent diffusion coefficient map obtained from b values of 150 and 700 sec/mm2, a procedure carried out to exclude vascular components detected by the MRI signal, which shows areas of low signal intensity (arrows; mean ADC value of 1.25 x 10-3mm2s-1) and healthy tissue for comparison reasons to see inflammatory parenchymal changes.
12.3 Adverse environmental and drug-disease effects and acute or chronic renal impairment
We are living in a polluted world. It is therefore not surprising that most patients are at an increased risk of renal toxicity. The risk is linked to the complex polypharmacy that is usually prescribed in patients with diabetes and advanced CKD [256-258]. A recent review summarized potential drug-disease interactions between drugs recommended in the guideline for type 2 diabetes and 11 comorbid conditions. While 32 potentially serious drug-disease interactions have been reported, the adverse interactions were most common in type 2 diabetes patients with chronic kidney disease (27/32) [258].
Adverse effects of drugs on the kidney are frequent; they can cause acute or chronic impairment of renal function, and their effect may be hemodynamic, toxic, or immunoallergic [259-262]. While virtually all drugs may cause one or more forms of renal impairment, baseline risks are enhanced by the presence of kidney disease, and increased in proportion to the number of prescribed drugs [263-264].
Many toxicity-related renal damages are direct and have clear symptoms. Therefore, they are rapidly evident, even in the absence of the classical hallmarks of skin rash, fever, and eosinophilia. They include the previously called "immuno-allergic" acute tubulo-interstitial nephritis (as described for antibiotics, acetaminophen, non-steroidal anti-inflammatory drugs, and allopurinol) and hemodynamic adverse effects (e.g. those caused by ACE-inhibitors and ARBs). However, one of the most alarming aspects of renal toxicity is that chronic damage may escape identification for a long time [265-269].
While a detailed discussion of these effects is beyond the scope of this review, it is worth noting that many drugs involved in biopsy-proven interstitial nephritis are commonly prescribed in elderly and diabetic patients (including proton pump inhibitors, allopurinol, anti COX-2, and antibiotics) [265-269].
Another important issue regards contrast media: the association between diabetes and risk for contrast media-induced nephropathy has already been described in the '80s, in particular in patients treated with metformin. These patients are at a higher risk of lactic acidosis. More recent data suggest that diabetic patients with proteinuria should receive particular attention as they are sensitive to kidney damage by contrast media. Also, high pre-procedural glucose blood levels are important in the prediction of adverse effects [270-276].
The actual incidence of these potentially severe diseases is not known, but they probably contribute significantly to the interstitial diseases observed as "non-proteinuric" renal diseases in diabetic patients.
Finally, we should not forget that the kidney has further crucial metabolic, endocrine, and enzymatic properties, including the previously mentioned role in erythropoiesis, vitamin D metabolism and renin-angiotensin axis, glycogen synthesis, and renalase or insulinase. The pathology-function relationship is presumably important, but its clinical role is not completely understood, and will surely be a matter of future research.
13. Conclusions
The old term "diabetic kidney" was recently re-proposed to encompass the various lesions that characterize the multifaceted, protean kidney damage in diabetic patients. The distribution of nodular and diffuse forms of glomerular lesions in diabetes patients has changed over time. While nodular glomerulosclerosis may be an indication of poorly controlled diabetes, and therefore improvement with better control is possible, both forms are characterized by an increase in proteinuria combined with a decrease in renal function.
However, the old tenet that proteinuria identifies "diabetic nephropathy" may no longer be true, or should be limited to glomerular lesions. The improvement in diabetic control may have reduced such typical lesions and may have revealed an increase in other types of renal damage, mainly vascular and interstitial types that typically present with little proteinuria. This has heralded the new trend of discussing the "new paradigm" of non-proteinuric kidney disease.
However, from a pathophysiological point of view, it is not surprising that all main kidney structures may be involved in kidney disease. Why? In a medicated society, in which the kidney is the target of drug and environmental toxicity, the presence of underlying renal damage through diabetes-related micro- and macrovascular kidney damage sets the stage for full-blown kidney disease and acute drug-induced kidney injury. In addition, impairment in the phagocytic response may be another factor causing the onset of severe and unusual forms of renal damage, including acute and chronic pyelonephritis. Therefore, a mere screening for albuminuria, although useful for detecting the "glomerular" forms of diabetic nephropathy, is not sufficient to detect all the potential nephropathies in diabetics. Further investigation needs to be carried out.
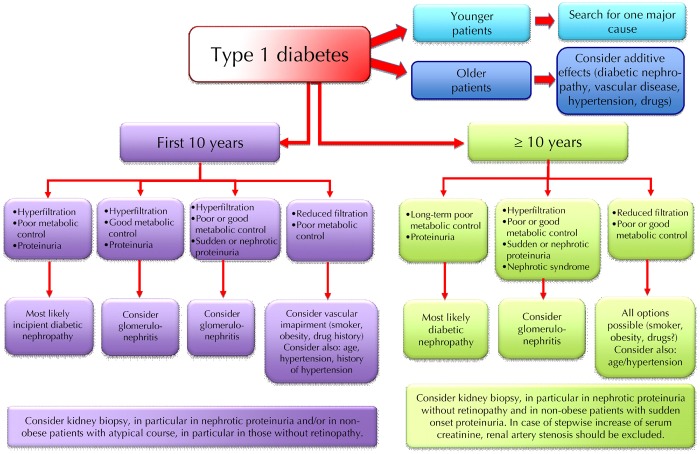
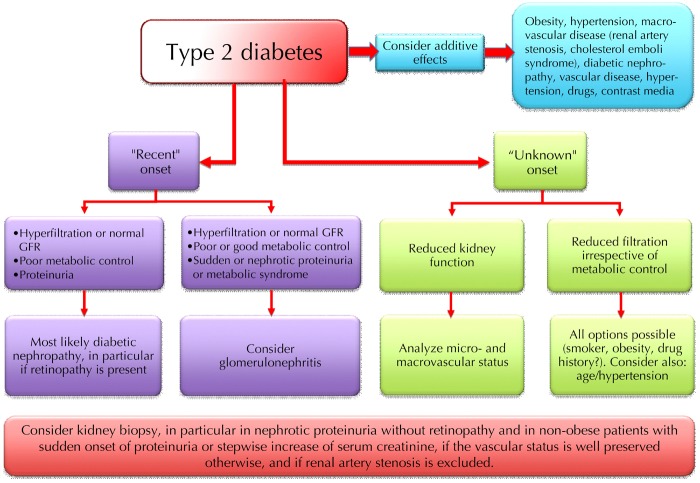
Diabetes is a risk factor for all forms of kidney disease. Diabetes patients are more prone to develop all kinds of clinical renal damage, and may suffer more severely from rapid progression. Kidney disease in diabetic patients should be identified by the same combination of biochemical, clinical, and imaging tests, as employed in the non-diabetic population. However, investigation methods including contrast medium can cause renal damage. Therefore, these methods should be used carefully in this fragile subset of patients who may have already lost their "renal reserve" (Figures 8 and 9).
Figure 8.
Schematic flow-chart for the diagnosis of kidney disease in type 1 diabetes.
Figure 9.
Schematic flow-chart for the diagnosis of kidney disease in type 2 diabetes.
Acknowledgments
Disclosures
The authors report no conflict of interests.
References
- 1.Kato M, Natarajan R. Diabetic nephropathy - emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517–530. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinke JM, Mauer M. International Diabetic Nephropathy Study Group. Lessons learned from studies of the natural history of diabetic nephropathy in young type 1 diabetic patients. Pediatr Endocrinol Rev. 2008;5(Suppl 4):958–963. [PubMed] [Google Scholar]
- 3.Jerums G, Premaratne E, Panagiotopoulos S, Clarke S, Power DA, MacIsaac RJ. New and old markers of progression of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S30–S37. doi: 10.1016/j.diabres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Jones CA, Krolewski AS, Rogus J, Xue JL, Collins A, Warram JH. Epidemic of end-stage renal disease in people with diabetes in the United States population: do we know the cause? Kidney Int. 2005;67(5):1684–1691. doi: 10.1111/j.1523-1755.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 5.Piccoli GB, Grassi G, Mezza E, Gai M, Iacuzzo C, Bechis F, Biancone L, Jeantet A, Dani F, Perin PC. et al. Early referral of Type 2 diabetic patients: are we ready for the assault? Nephrol Dial Transplant. 2002;17(7):1241–1247. doi: 10.1093/ndt/17.7.1241. [DOI] [PubMed] [Google Scholar]
- 6.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124(6):2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadler K, Goldberg IJ, Susztak K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep. 2015;15(7):40. doi: 10.1007/s11892-015-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanssen NM, Russell N, Cooper ME. Recent advances in glucose-lowering treatment to reduce diabetic kidney disease. Expert Opin Pharmacother. 2015;16(9):1325–1333. doi: 10.1517/14656566.2015.1041502. [DOI] [PubMed] [Google Scholar]
- 9.Takagi M, Babazono T, Uchigata Y. Differences in risk factors for the onset of albuminuria and decrease in glomerular filtration rate in people with Type 2 diabetes mellitus: implications for the pathogenesis of diabetic kidney disease. Diabet Med. 2015 doi: 10.1111/dme.12793. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4(1):39–45. doi: 10.2174/157339908783502370. [DOI] [PubMed] [Google Scholar]
- 11.Dei Cas A, Gnudi L. VEGF and angiopoietins in diabetic glomerulopathy: how far for a new treatment? Metabolism. 2012;61(12):1666–1673. doi: 10.1016/j.metabol.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Gnudi L. Cellular and molecular mechanisms of diabetic glomerulopathy. Nephrol Dial Transplant. 2012;27(7):2642–2649. doi: 10.1093/ndt/gfs121. [DOI] [PubMed] [Google Scholar]
- 13.Herman-Edelstein M, Thomas MC, Thallas-Bonke V, Saleem M, Cooper ME, Kantharidis P. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-beta: a model for diabetic podocytopathy. Diabetes. 2011;60(6):1779–1788. doi: 10.2337/db10-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marquez E, Riera M, Pascual J, Soler MJ. Renin-angiotensin system within the diabetic podocyte. Am J Physiol Renal Physiol. 2015;308(1):F1–F10. doi: 10.1152/ajprenal.00531.2013. [DOI] [PubMed] [Google Scholar]
- 15.Piccoli GB, Quarello F, Bonello F, Salomone M, Triolo G, Maffei S, Iadarola GM, Stramignoni E, Borca M, Beltrame G. et al. Diabetic patients on dialysis: a changing picture. Kidney Int Suppl. 1993;41:S14–S17. [PubMed] [Google Scholar]
- 16.Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34(5):795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 17.Bergrem H, Leivestad T. Diabetic nephropathy and end-stage renal failure: the Norwegian story. Adv Ren Replace Ther. 2001;8(1):4–12. doi: 10.1053/jarr.2001.21711. [DOI] [PubMed] [Google Scholar]
- 18.ESRD Incidence Study Group. Stewart JH, McCredie MR, Williams SM. Divergent trends in the incidence of end-stage renal disease due to type 1 and type 2 diabetes in Europe, Canada and Australia during 1998-2002. Diabet Med. 2006;23(12):1364–1369. doi: 10.1111/j.1464-5491.2006.01986.x. [DOI] [PubMed] [Google Scholar]
- 19.Assogba FG, Couchoud C, Hannedouche T, Villar E, Frimat L, Fagot-Campagna A, Jacquelinet C, Stengel B. French Renal Epidemiology and Information Network Registry. Trends in the epidemiology and care of diabetes mellitus-related end-stage renal disease in France, 2007-2011. Diabetologia. 2014;57(4):718–728. doi: 10.1007/s00125-014-3160-9. [DOI] [PubMed] [Google Scholar]
- 20.Williams ME. Diabetic CKD/ESRD 2010: a progress report? Semin Dial. 2010;23(2):129–133. doi: 10.1111/j.1525-139X.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- 21.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172(10):761–769. doi: 10.1001/archinternmed.2011.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poznanski EO, Miller E, Salguero C, Kelsh RC. Quality of life for long-term survivors of end-stage renal disease. JAMA. 1978;239(22):2343–2347. doi: 10.1001/jama.239.22.2343. [DOI] [PubMed] [Google Scholar]
- 23.Neff MS, Eiser AR, Slifkin RF, Baum M, Baez A, Gupta S, Amarga E. Patients surviving 10 years of hemodialysis. Am J Med. 1983;74(6):996–1004. doi: 10.1016/0002-9343(83)90799-4. [DOI] [PubMed] [Google Scholar]
- 24.Gutman RA. Characteristics of long-term (14 years) survivors of maintenance dialysis. Nephron. 1983;33(2):111–115. doi: 10.1159/000182923. [DOI] [PubMed] [Google Scholar]
- 25.Piccoli GB, Mezza E, Anania P, Iadarola AM, Vischi M, Torazza MC, Fop F, Guarena C, Martina G, Messina M. et al. Patients on renal replacement therapy for 20 or more years: a clinical profile. Nephrol Dial Transplant. 2002;17(8):1440–1449. doi: 10.1093/ndt/17.8.1440. [DOI] [PubMed] [Google Scholar]
- 26.Rao TK, Nathanson G, Avram M, Manis T, Kountz SL, Friedman EA. Improved survival in older, sicker patients begun on maintenance hemodialysis. Proc Clin Dial Transplant Forum. 1976;6:62–66. [PubMed] [Google Scholar]
- 27.Joly D, Anglicheau D, Alberti C, Nguyen AT, Touam M, Grünfeld JP, Jungers P. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14(4):1012–1021. doi: 10.1097/01.asn.0000054493.04151.80. [DOI] [PubMed] [Google Scholar]
- 28.Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 29.Singh P, Germain MJ, Cohen L, Unruh M. The elderly patient on dialysis: geriatric considerations. Nephrol Dial Transplant. 2014;29(5):990–996. doi: 10.1093/ndt/gft246. [DOI] [PubMed] [Google Scholar]
- 30.Burdese M, Mezza E, Rabbia C, Merlo M, Savio D, Bermond F, Soragna G, Davini O, Piccoli GB. The early vascular ageing of long-term RRT patients: endoprosthetic repair of an aortic aneurysm in a young patient on RRT for over 20 years. Nephrol Dial Transplant. 2005;20(1):239–240. doi: 10.1093/ndt/gfg542. [DOI] [PubMed] [Google Scholar]
- 31.Triga K, Dousdampanis P, Aggelakou-Vaitsi S, Gellner K. Thirty years survivor on hemodialysis: a case report. Saudi J Kidney Dis Transpl. 2014;25(5):1056–1058. doi: 10.4103/1319-2442.139939. [DOI] [PubMed] [Google Scholar]
- 32.Chazot C, Laurent G, Charra B, Blanc C, VoVan C, Jean G, Vanel T, Terrat JC, Ruffet M. Malnutrition in long-term haemodialysis survivors. Nephrol Dial Transplant. 2001;16(1):61–69. doi: 10.1093/ndt/16.1.61. [DOI] [PubMed] [Google Scholar]
- 33.Otsubo S, Kimata N, Okutsu I, Oshikawa K, Ueda S, Sugimoto H, Mitobe M, Uchida K, Otsubo K, Nitta K. et al. Characteristics of dialysis-related amyloidosis in patients on hemodialysis therapy for more than 30 years. Nephrol Dial Transplant. 2009;24:1593–1598. doi: 10.1093/ndt/gfn706. [DOI] [PubMed] [Google Scholar]
- 34.Ghavamian M, Gutch CF, Kopp KF, Kolff WJ. The sad truth about hemodialysis in diabetic nephropathy. JAMA. 1972;222(11):1386–1389. [PubMed] [Google Scholar]
- 35.White N, Snowden SA, Parsons V, Sheldon J, Bewick M. The management of terminal renal failure in diabetic patients by regular dialysis therapy. Nephron. 1973;11(5):261–275. doi: 10.1159/000180234. [DOI] [PubMed] [Google Scholar]
- 36.Blumenkrantz MJ, Shapiro DJ, Mimura N, Greopoulus DG, Griedler RM, Levin S, Tenckhoff H, Coburn JW. Maintenance peritoneal dialysis as an alternative in the patient with diabetes mellitus and end-stage uremia. Kidney Int Suppl. 1974;1:108–114. [PubMed] [Google Scholar]
- 37.Zimmerman SW, Glass N, Sollinger H, Miller D, Belzer F. Treatment of end-stage diabetic nephropathy: over a decade of experience at one institution. Medicine (Baltimore) 1984;63(5):311–317. doi: 10.1097/00005792-198409000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Hirschl MM, Heinz G, Sunder-Plassmann G, Derfler K. Renal replacement therapy in type 2 diabetic patients: 10 years' experience. Am J Kidney Dis. 1992;20(6):564–568. doi: 10.1016/s0272-6386(12)70219-6. [DOI] [PubMed] [Google Scholar]
- 39.Catalano C, Goodship TH, Tapson JS, Venning MK, Taylor RM, Proud G, Tunbridge WM, Elliot RW, Ward MK, Alberti KG. Renal replacement treatment for diabetic patients in Newcastle upon Tyne and the Northern region, 1964-88. BMJ. 1990;301(6751):535–540. doi: 10.1136/bmj.301.6751.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piccoli GB, Mesiano P, Mezza E, Pacitti A, Burdese M, Bermond F, Jeantet A, Segoloni GP. Twenty years of renal replacement therapy in a type 1 diabetic patient: advantages of a multiple choice dialysis system. Int J Artif Organs. 2003;26(5):442–445. doi: 10.1177/039139880302600511. [DOI] [PubMed] [Google Scholar]
- 41.Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66(4):1596–1605. doi: 10.1111/j.1523-1755.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 42.Andresdottir G, Jensen ML, Carstensen B, Parving HH, Rossing K, Hansen TW, Rossing P. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care. 2014;37(6):1660–1667. doi: 10.2337/dc13-2036. [DOI] [PubMed] [Google Scholar]
- 43.Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, Zagar T, Poole CD. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, Mauer SM, Kennedy WR, Goetz FC, Robertson RP. et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233(4):463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman AL. Appropriateness and timing of kidney and/or pancreas transplants in type 1 and type 2 diabetes. Adv Ren Replace Ther. 2001;8(1):70–82. doi: 10.1053/jarr.2001.21709. [DOI] [PubMed] [Google Scholar]
- 46.Piccoli GB, Mezza E, Gino M, Grassi G, Soragna G, Fop F, Burdese M, Gai M, Motta D, Malfi B. et al. Referral of type 1 diabetic patients to a nephrology unit: will pre-emptive transplantation change our life? J Nephrol. 2004;17(2):275–283. [PubMed] [Google Scholar]
- 47.Luan FL, Samaniego M. Transplantation in diabetic kidney failure patients: modalities, outcomes, and clinical management. Semin Dial. 2010;23(2):198–205. doi: 10.1111/j.1525-139X.2010.00708.x. [DOI] [PubMed] [Google Scholar]
- 48.Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR) Rev Diabet Stud. 2011;8(1):6–16. doi: 10.1900/RDS.2011.8.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taft JL, Billson VR, Nankervis A, Kincaid-Smith P, Martin FI. A clinical-histological study of individuals with diabetes mellitus and proteinuria. Diabet Med. 1990;7(3):215–221. doi: 10.1111/j.1464-5491.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. The Collaborative Study Group. Nephrol Dial Transplant. 1998;13(10):2547–2552. doi: 10.1093/ndt/13.10.2547. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki Y, Ueno M, Hayashi H, Nishi S, Satou H, Karasawa R, Inn H, Suzuki S, Maruyama Y, Arakawa M. A light microscopic study of glomerulosclerosis in Japanese patients with noninsulin-dependent diabetes mellitus: the relationship between clinical and histological features. Clin Nephrol. 1994;42(3):155–162. [PubMed] [Google Scholar]
- 52.Serra A, Romero R, Bayes B, Lopez D, Bonet J. Is there a need for changes in renal biopsy criteria in proteinuria in type 2 diabetes? Diabetes Res Clin Pract. 2002;58(2):149–153. doi: 10.1016/s0168-8227(02)00131-6. [DOI] [PubMed] [Google Scholar]
- 53.Richards NT, Greaves I, Lee SJ, Howie AJ, Adu D, Michael J. Increased prevalence of renal biopsy findings other than diabetic glomerulopathy in type II diabetes mellitus. Nephrol Dial Transplant. 1992;7(5):397–399. [PubMed] [Google Scholar]
- 54.Diabetes care and research in Europe: the Saint Vincent declaration. Diabet Med. 7(4):360. [PubMed] [Google Scholar]
- 55.Piwernetz K, Home PD, Snorgaard O, Antsiferov M, Staehr-Johansen K, Krans M. Monitoring the targets of the St Vincent Declaration and the implementation of quality management in diabetes care: the DIABCARE initiative. The DIABCARE Monitoring Group of the St Vincent Declaration Steering Committee. Diabet Med. 1993;10(4):371–377. doi: 10.1111/j.1464-5491.1993.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 56.Leese B. Diabetes mellitus and the St Vincent Declaration. The economic implications. Pharmacoeconomics. 1995;7(4):292–307. doi: 10.2165/00019053-199507040-00004. [DOI] [PubMed] [Google Scholar]
- 57.Johansen K, Dalrymple LS, Delgado C, Kaysen GA, Kornak J, Grimes B, Chertow GM. Association between body composition and frailty among prevalent hemodialysis patients: a US Renal Data System special study. J Am Soc Nephrol. 2014;25(2):381–389. doi: 10.1681/ASN.2013040431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95(2):C40–C46. doi: 10.1159/000073708. [DOI] [PubMed] [Google Scholar]
- 59.O'Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012;15(2):228–235. doi: 10.1089/jpm.2011.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brunori G, Viola BF, Maiorca P, Cancarini G. How to manage elderly patients with chronic renal failure: conservative management versus dialysis. Blood Purif. 2008;26(1):36–40. doi: 10.1159/000110561. [DOI] [PubMed] [Google Scholar]
- 61.Jones JW, McCullough LB. Extending life or prolonging death: when is enough actually too much? J Vasc Surg. 2014;60(2):521–522. doi: 10.1016/j.jvs.2014.05.054. [DOI] [PubMed] [Google Scholar]
- 62.Brennan F, Brown M. An ethical approach to dialysis - an alliance of nephrology, palliative medicine and ethics. QJM. 2013;106(5):397–400. doi: 10.1093/qjmed/hct066. [DOI] [PubMed] [Google Scholar]
- 63.Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 64.Mogensen CE, Osterby R, Gundersen HJ. Early functional and morphologic vascular renal consequences of the diabetic state. Diabetologia. 1979;17(2):71–76. doi: 10.1007/BF01222205. [DOI] [PubMed] [Google Scholar]
- 65.Osterby R, Gundersen HJ, Horlyck A, Kroustrup JP, Nyberg G, Westberg G. Diabetic glomerulopathy. Structural characteristics of the early and advanced stages. Diabetes. 1983;32(Suppl 2):79–82. doi: 10.2337/diab.32.2.s79. [DOI] [PubMed] [Google Scholar]
- 66.Osterby R. Glomerular structural changes in type 1 (insulin-dependent) diabetes mellitus: causes, consequences, and prevention. Diabetologia. 1992;35(9):803–812. doi: 10.1007/BF00399925. [DOI] [PubMed] [Google Scholar]
- 67.Bangstad HJ, Osterby R, Dahl-Jorgensen K, Berg KJ, Hartmann A, Nyberg G, Frahm Bjorn S, Hanssen KF. Early glomerulopathy is present in young, type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1993;36(6):523–529. doi: 10.1007/BF02743268. [DOI] [PubMed] [Google Scholar]
- 68.Rudberg S, Osterby R, Dahlquist G, Nyberg G, Persson B. Predictors of renal morphological changes in the early stage of microalbuminuria in adolescents with IDDM. Diabetes Care. 1997;20(3):265–271. doi: 10.2337/diacare.20.3.265. [DOI] [PubMed] [Google Scholar]
- 69.Matsumae T, Jimi S, Uesugi N, Takebayashi S, Naito S. Clinical and morphometrical interrelationships in patients with overt nephropathy induced by non-insulin-dependent diabetes mellitus. A light- and electron-microscopy study. Nephron. 1999;81(1):41–48. doi: 10.1159/000045244. [DOI] [PubMed] [Google Scholar]
- 70.Dalla Vestra M, Saller A, Bortoloso E, Mauer M, Fioretto P. Structural involvement in type 1 and type 2 diabetic nephropathy. Diabetes Metab. 2000;26(Suppl 4):8–14. [PubMed] [Google Scholar]
- 71.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1(8287):1430–1432. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 72.Viberti G, Pickup JC, Bilous RW, Keen H, Mackintosh D. Correction of exercise-induced microalbuminuria in insulin-dependent diabetics after 3 weeks of subcutaneous insulin infusion. Diabetes. 1981;30(10):818–823. doi: 10.2337/diab.30.10.818. [DOI] [PubMed] [Google Scholar]
- 73.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311(2):89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 74.Piccoli GB, Clari R, Ghiotto S, Castelluccia N, Colombi N, Mauro G, Tavassoli E, Melluzza C, Cabiddu G, Gernone G. et al. Type 1 diabetes, diabetic nephropathy, and pregnancy: a systematic review and meta-study. Rev Diabet Stud. 2013;10(1):6–26. doi: 10.1900/RDS.2013.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med. 2001;134(5):370–379. doi: 10.7326/0003-4819-134-5-200103060-00009. [DOI] [PubMed] [Google Scholar]
- 76.Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. Am J Med. 1995;99(5):497–504. doi: 10.1016/s0002-9343(99)80226-5. [DOI] [PubMed] [Google Scholar]
- 77.Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006;2006(4):CD006257. doi: 10.1002/14651858.CD006257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2009;2009(3):CD007004. doi: 10.1002/14651858.CD007004.pub2. [DOI] [PubMed] [Google Scholar]
- 79.Perico N, Ruggenenti P, Remuzzi G. Losartan in diabetic nephropathy. Expert Rev Cardiovasc Ther. 2004;2(4):473–483. doi: 10.1586/14779072.2.4.473. [DOI] [PubMed] [Google Scholar]
- 80.Jacobsen P, Andersen S, Rossing K, Jensen BR, Parving HH. Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 2003;63(5):1874–1880. doi: 10.1046/j.1523-1755.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- 81.Jacobsen P, Rossing K, Parving HH. Single versus dual blockade of the renin-angiotensin system (angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers) in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2004;13(3):319–324. doi: 10.1097/00041552-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Otoda T, Kanasaki K, Koya D. Low-protein diet for diabetic nephropathy. Curr Diab Rep. 2014;14(9):523. doi: 10.1007/s11892-014-0523-z. [DOI] [PubMed] [Google Scholar]
- 83.Robertson L, Waugh N, Robertson A. Protein restriction for diabetic renal disease. Cochrane Database Syst Rev. 2007;2007(4):CD002181. doi: 10.1002/14651858.CD002181.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piccoli GB, Motta D, Martina G, Consiglio V, Gai M, Mezza E, Maddalena E, Burdese M, Colla L, Tattoli F. et al. Low-protein vegetarian diet with alpha-chetoanalogues prior to pre-emptive pancreas-kidney transplantation. Rev Diabet Stud. 2004;1(2):95–102. doi: 10.1900/RDS.2004.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giordano M, Ciarambino T, Castellino P, Paolisso G. Light and shadows of dietary protein restriction in elderly with chronic kidney disease. Nutrition. 2013;29(9):1090–1093. doi: 10.1016/j.nut.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 86.Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2015;22(4):277–282. doi: 10.1097/MED.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 87.Reinehr T, Holl RW, Roth CL, Wiesel T, Stachow R, Wabitsch M, Andler W, DPV-Wiss Study Group. Insulin resistance in children and adolescents with type 1 diabetes mellitus: relation to obesity. Pediatr Diabetes. 2005;6(1):5–12. doi: 10.1111/j.1399-543X.2005.00093.x. [DOI] [PubMed] [Google Scholar]
- 88.Chillaron JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism. 2014;63(2):181–187. doi: 10.1016/j.metabol.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Pinhas-Hamiel O, Levek-Motola N, Kaidar K, Boyko V, Tisch E, Mazor-Aronovitch K, Graf-Barel C, Landau Z, Lerner-Geva L, Frumkin Ben-David R. Prevalence of overweight, obesity and metabolic syndrome components in children, adolescents and young adults with type 1 diabetes mellitus. Diabetes Metab Res Rev. 2015;31(1):76–84. doi: 10.1002/dmrr.2565. [DOI] [PubMed] [Google Scholar]
- 90.Zhang L, Krzentowski G, Albert A, Lefebvre PJ. Factors predictive of nephropathy in DCCT Type 1 diabetic patients with good or poor metabolic control. Diabet Med. 2003;20(7):580–585. doi: 10.1046/j.1464-5491.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- 91.Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ, Maahs DM. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv121. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 93.Tsuboi N, Utsunomiya Y, Hosoya T. Obesity-related glomerulopathy and the nephron complement. Nephrol Dial Transplant. 2013;28(Suppl 4):108–113. doi: 10.1093/ndt/gft258. [DOI] [PubMed] [Google Scholar]
- 94.Srivastava T. Nondiabetic consequences of obesity on kidney. Pediatr Nephrol. 2006;21(4):463–470. doi: 10.1007/s00467-006-0027-4. [DOI] [PubMed] [Google Scholar]
- 95.Amann K, Benz K. Structural renal changes in obesity and diabetes. Semin Nephrol. 2013;33(1):23–33. doi: 10.1016/j.semnephrol.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 96.Haneda M, Utsunomiya K, Koya D, Babazono T, Moriya T, Makino H, Kimura K, Suzuki Y, Wada T, Ogawa S. et al. A new classification of diabetic nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. Clin Exp Nephrol. 2015;19(1):1–5. doi: 10.1007/s10157-014-1057-z. [DOI] [PubMed] [Google Scholar]
- 97.Fisher ER, Perez-Stable E, Amidi M, Sarver ME, Danowski TS. Ultrastructural renal changes in juvenile diabetics. JAMA. 1967;202(4):291–295. [PubMed] [Google Scholar]
- 98.Gauld WR, Stalker AL, Lyall A. Renal complications in diabetes mellitus with special reference to the Kimmelstiel-Wilson lesion. Br Med J. 1948;2(4568):194–200. doi: 10.1136/bmj.2.4568.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Epstein FH, Zupa VJ. Clinical correlates of the Kimmelstiel-Wilson lesion. N Engl J Med. 1956;254(19):896–900. doi: 10.1056/NEJM195605102541904. [DOI] [PubMed] [Google Scholar]
- 100.Horsfield GI, Lannigan R. Exudative lesions in diabetes mellitus. J Clin Pathol. 1965;18:47–53. doi: 10.1136/jcp.18.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Drummond K, Mauer M International Diabetic Nephropathy Study Group. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51(5):1580–1587. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 102.Bangstad HJ, Osterby R, Hartmann A, Berg TJ, Hanssen KF. Severity of glomerulopathy predicts long-term urinary albumin excretion rate in patients with type 1 diabetes and microalbuminuria. Diabetes Care. 1999;22(2):314–319. doi: 10.2337/diacare.22.2.314. [DOI] [PubMed] [Google Scholar]
- 103.Fioretto P, Steffes MW, Sutherland DE, Mauer M. Sequential renal biopsies in insulin-dependent diabetic patients: structural factors associated with clinical progression. Kidney Int. 1995;48(6):1929–1935. doi: 10.1038/ki.1995.493. [DOI] [PubMed] [Google Scholar]
- 104.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ellis EN, Steffes MW, Goetz FC, Sutherland DE, Mauer SM. Glomerular filtration surface in type I diabetes mellitus. Kidney Int. 1986;29(4):889–894. doi: 10.1038/ki.1986.82. [DOI] [PubMed] [Google Scholar]
- 106.Steffes MW, Bilous RW, Sutherland DE, Mauer SM. Cell and matrix components of the glomerular mesangium in type I diabetes. Diabetes. 1992;41(6):679–684. doi: 10.2337/diab.41.6.679. [DOI] [PubMed] [Google Scholar]
- 107.Lane PH, Steffes MW, Fioretto P, Mauer SM. Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int. 1993;43(3):661–667. doi: 10.1038/ki.1993.95. [DOI] [PubMed] [Google Scholar]
- 108.Harris RD, Steffes MW, Bilous RW, Sutherland DE, Mauer SM. Global glomerular sclerosis and glomerular arteriolar hyalinosis in insulin dependent diabetes. Kidney Int. 1991;40(1):107–114. doi: 10.1038/ki.1991.187. [DOI] [PubMed] [Google Scholar]
- 109.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E. et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 110.Mise K, Hoshino J, Ubara Y, Sumida K, Hiramatsu R, Hasegawa E, Yamanouchi M, Hayami N, Suwabe T, Sawa N. et al. Renal prognosis a long time after renal biopsy on patients with diabetic nephropathy. Nephrol Dial Transplant. 2014;29(1):109–118. doi: 10.1093/ndt/gft349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shimizu M, Furuichi K, Toyama T, Kitajima S, Hara A, Kitagawa K, Iwata Y, Sakai N, Takamura T, Yoshimura M. et al. Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Diabetes Care. 2013;36(11):3655–3662. doi: 10.2337/dc13-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.An Y, Xu F, Le W, Ge Y, Zhou M, Chen H, Zeng C, Zhang H, Liu Z. Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant. 2015;30(2):257–266. doi: 10.1093/ndt/gfu250. [DOI] [PubMed] [Google Scholar]
- 113.Songini M, Lombardo C. The Sardinian way to type 1 diabetes. J Diabetes Sci Technol. 2010;4(5):1248–1255. doi: 10.1177/193229681000400526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Contu L, Carcassi C, Trucco M. Diabetes susceptibility in Sardinia. Lancet. 1991;338(8758):65. doi: 10.1016/0140-6736(91)90066-x. [DOI] [PubMed] [Google Scholar]
- 115.Bruno G, Pagano G, Faggiano F, De Salvia A, Merletti F. Effect of Sardinian heritage on risk and age at onset of type 1 diabetes: a demographic case-control study of Sardinian migrants. Int J Epidemiol. 2000;29(3):532–535. [PubMed] [Google Scholar]
- 116.Cameron JS. The discovery of diabetic nephropathy: from small print to centre stage. J Nephrol. 2006;19(Suppl 10):S75–S87. [PubMed] [Google Scholar]
- 117.Kimmelstiel P, Wilson C. Intercapillary lesions in the glomeruli of the kidney. Am J Pathol. 1936;12:83–97. [PMC free article] [PubMed] [Google Scholar]
- 118.Caramori ML, Kim Y, Huang C, Fish AJ, Rich SS, Miller ME, Russell G, Mauer M. Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes. 2002;51:506–513. doi: 10.2337/diabetes.51.2.506. [DOI] [PubMed] [Google Scholar]
- 119.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes. 1994;43:1358–1364. doi: 10.2337/diab.43.11.1358. [DOI] [PubMed] [Google Scholar]
- 120.Berg UB, Torbjörnsdotter TB, Jaremko G, Thalme B. Kidney morphological changes in relation to long-term renal function and metabolic control in adolescents with IDDM. Diabetologia. 1998;41:1047–1056. doi: 10.1007/s001250051029. [DOI] [PubMed] [Google Scholar]
- 121.Gonzalez Suarez ML, Thomas DB, Barisoni L, Fornoni A. Diabetic nephropathy: Is it time yet for routine kidney biopsy? World J Diabetes. 2013;4(6):245–255. doi: 10.4239/wjd.v4.i6.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Penescu M, Mandache E. The value of kidney biopsy in diabetes mellitus. Rom J Morphol Embryol. 2010;51:13–19. [PubMed] [Google Scholar]
- 123.Zhang PP, Ge YC, Li SJ, Xie HL, Li LS, Liu ZH. Renal biopsy in type 2 diabetes: timing of complications and evaluating of safety in Chinese patients. Nephrology (Carlton) 2011;16:100–105. doi: 10.1111/j.1440-1797.2010.01369.x. [DOI] [PubMed] [Google Scholar]
- 124.Lin YL, Peng SJ, Ferng SH, Tzen CY, Yang CS. Clinical indicators which necessitate renal biopsy in type 2 diabetes mellitus patients with renal disease. Int J Clin Pract. 2009;63(8):1167–1176. doi: 10.1111/j.1742-1241.2008.01753.x. [DOI] [PubMed] [Google Scholar]
- 125.Dhaun N, Bellamy CO, Cattran DC, Kluth DC. Utility of renal biopsy in the clinical management of renal disease. Kidney Int. 2014;85(5):1039–1048. doi: 10.1038/ki.2013.512. [DOI] [PubMed] [Google Scholar]
- 126.Soni SS, Gowrishankar S, Kishan AG, Raman A. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 2006;11(6):533–537. doi: 10.1111/j.1440-1797.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 127.Zhuo L, Zou G, Li W, Lu J, Ren W. Prevalence of diabetic nephropathy complicating non-diabetic renal disease among Chinese patients with type 2 diabetes mellitus. Eur J Med Res. 2013;18:4. doi: 10.1186/2047-783X-18-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Teng J, Dwyer KM, Hill P, See E, Ekinci EI, Jerums G, MacIsaac RJ. Spectrum of renal disease in diabetes. Nephrology (Carlton) 2014;19(9):528–536. doi: 10.1111/nep.12288. [DOI] [PubMed] [Google Scholar]
- 129.Chong YB, Keng TC, Tan LP, Ng KP, Kong WY, Wong CM, Cheah PL, Looi LM, Tan SY. Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: a single centre review. Ren Fail. 2012;34(3):323–328. doi: 10.3109/0886022X.2011.647302. [DOI] [PubMed] [Google Scholar]
- 130.Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27(3):322–328. doi: 10.1159/000102598. [DOI] [PubMed] [Google Scholar]
- 131.Tone A, Shikata K, Matsuda M, Usui H, Okada S, Ogawa D, Wada J, Makino H. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract. 2005;69(3):237–242. doi: 10.1016/j.diabres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 132.Mak SK, Gwi E, Chan KW, Wong PN, Lo KY, Lee KF, Wong AK. Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant. 1997;12(12):2588–2591. doi: 10.1093/ndt/12.12.2588. [DOI] [PubMed] [Google Scholar]
- 133.Huang F, Yang Q, Chen L, Tang S, Liu W, Yu X. Renal pathological change in patients with type 2 diabetes is not always diabetic nephropathy: a report of 52 cases. Clin Nephrol. 2007;67(5):293–297. doi: 10.5414/cnp67293. [DOI] [PubMed] [Google Scholar]
- 134.Chang TI, Park JT, Kim JK, Kim SJ, Oh HJ, Yoo DE, Han SH, Yoo TH, Kang SW. Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes Res Clin Pract. 2011;92(2):198–204. doi: 10.1016/j.diabres.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 135.Zhou J, Chen X, Xie Y, Li J, Yamanaka N, Tong X. A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant. 2008;23(6):1940–1945. doi: 10.1093/ndt/gfm897. [DOI] [PubMed] [Google Scholar]
- 136.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M. et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 137.Menne J, Izzo JL Jr, Ito S, Januszewicz A, Katayama S, Chatzykirkou C, Mimran A, Rabelink TJ, Ritz E, Ruilope LM. et al. Prevention of microalbuminuria in patients with type 2 diabetes and hypertension. J Hypertens. 2012;30(4):811–818. doi: 10.1097/HJH.0b013e328351856d. [DOI] [PubMed] [Google Scholar]
- 138.Chatzikyrkou C, Menne J. Update on the ROADMAP clinical trial report: olmesartan for the prevention or delay of microalbuminuria development in type 2 diabetes. Expert Rev Cardiovasc Ther. 2012;10(9):1087–1092. doi: 10.1586/erc.12.100. [DOI] [PubMed] [Google Scholar]
- 139.Deckert T, Egeberg J, Frimodt-Moller C, Sander E, Svejgaard A. Basement membrane thickness, insulin antibodies and HLA-antigens in long standing insulin dependent diabetics with and without severe retinopathy. Diabetologia. 1979;17(2):91–96. doi: 10.1007/BF01222208. [DOI] [PubMed] [Google Scholar]
- 140.Dornan TL, Jenkins S, Cotton RE, Tattersall RB, Burden RP. The nephrotic syndrome at presentation of insulin-dependent diabetes mellitus; cause or coincidence? Diabet Med. 1988;5(4):387–390. doi: 10.1111/j.1464-5491.1988.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 141.Hommel E, Carstensen H, Skott P, Larsen S, Parving HH. Prevalence and causes of microscopic haematuria in type 1 (insulin-dependent) diabetic patients with persistent proteinuria. Diabetologia. 1987;30(8):627–630. doi: 10.1007/BF00277319. [DOI] [PubMed] [Google Scholar]
- 142.Chobanian MC, Chevalier RL, Sturgill BC, Bolton WK. Early onset of clinical diabetic nephropathy in children - a new subgroup? Int J Pediatr Nephrol. 1984;5(1):23–29. [PubMed] [Google Scholar]
- 143.Friedman EA, L'Esperance FA Jr. Diabetic renal-retinal syndrome. The prognosis improves. Arch Intern Med. 1980;140(9):1149–1150. [PubMed] [Google Scholar]
- 144.Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. The Collaborative Study Group. Nephrol Dial Transplant. 1998;13(10):2547–2552. doi: 10.1093/ndt/13.10.2547. [DOI] [PubMed] [Google Scholar]
- 145.Wolf G, Müller N, Mandecka A, Müller UA. Association of diabetic retinopathy and renal function in patients with types 1 and 2 diabetes mellitus. Clin Nephrol. 2007;68(2):81–86. doi: 10.5414/cnp68081. [DOI] [PubMed] [Google Scholar]
- 146.Lövestam-Adrian M, Agardh E, Agardh CD. The temporal development of retinopathy and nephropathy in type 1 diabetes mellitus during 15 years diabetes duration. Diabetes Res Clin Pract. 1999;45(1):15–23. doi: 10.1016/s0168-8227(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 147.He F, Xia X, Wu XF, Yu XQ, Huang FX. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia. 2013;56:457–466. doi: 10.1007/s00125-012-2796-6. [DOI] [PubMed] [Google Scholar]
- 148.Zhuo L, Ren W, Li W, Zou G, Lu J. Evaluation of renal biopsies in type 2 diabetic patients with kidney disease: a clinicopathological study of 216 cases. Int Urol Nephrol. 2013;45:173–179. doi: 10.1007/s11255-012-0164-6. [DOI] [PubMed] [Google Scholar]
- 149.Sharma SG, Bomback AS, Radhakrishnan J, Herlitz LC, Stokes MB, Markowitz GS, D'Agati VD. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol. 2013;8:1718–1724. doi: 10.2215/CJN.02510213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wong TY, Choi PC, Szeto CC, To KF, Tang NL, Chan AW, Li PK, Lai FM. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care. 2002;25:900–905. doi: 10.2337/diacare.25.5.900. [DOI] [PubMed] [Google Scholar]
- 151.Biesenbach G, Bodlaj G, Pieringer H, Sedlak M. Clinical versus histological diagnosis of diabetic nephropathy - is renal biopsy required in type 2 diabetic patients with renal disease? QJM. 2011;104:771–774. doi: 10.1093/qjmed/hcr059. [DOI] [PubMed] [Google Scholar]
- 152.Park TS. How much glycemic control is needed to prevent progression of diabetic nephropathy? J Diabetes Investig. 2012;3(5):411–412. doi: 10.1111/j.2040-1124.2012.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shurraw S, Hemmelgarn B, Lin M, Majumdar SR, Klarenbach S, Manns B, Bello A, James M, Turin TC, Tonelli M. et al. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171(21):1920–1927. doi: 10.1001/archinternmed.2011.537. [DOI] [PubMed] [Google Scholar]
- 154.Kato M, Natarajan R. Diabetic nephropathy - emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517–530. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–455. doi: 10.1007/s00125-014-3462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Keating ST, El-Osta A. Glycemic memories and the epigenetic component of diabetic nephropathy. Curr Diab Rep. 2013;13(4):574–581. doi: 10.1007/s11892-013-0383-y. [DOI] [PubMed] [Google Scholar]
- 157.Innes A, Furness PN, Cotton RE, Burden RP, Morgan AG. Diabetic glomerulosclerosis without diabetes mellitus - two case reports and a review of the literature. Nephrol Dial Transplant. 1992;7(7):642–646. doi: 10.1093/ndt/7.7.642. [DOI] [PubMed] [Google Scholar]
- 158.Navaneethan SD, Singh S, Choudhry W. Nodular glomerulosclerosis in a non-diabetic patient: case report and review of literature. J Nephrol. 2005;18(5):613–615. [PubMed] [Google Scholar]
- 159.Lopez-Revuelta K, Abreu AA, Gerrero-Marquez C, Stanescu RI, Marin MI, Fernandez EP. Diabetic Nephropathy without Diabetes. J Clin Med. 2015;4(7):1403–1427. doi: 10.3390/jcm4071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ismail N, Becker B, Strzelczyk P, Ritz E. Renal disease and hypertension in non-insulin-dependent diabetes mellitus. Kidney Int. 1999;55(1):1–28. doi: 10.1046/j.1523-1755.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- 161.Yokoyama H, Okudaira M, Otani T, Takaike H, Miura J, Saeki A, Uchigata Y, Omori Y. Existence of early-onset NIDDM Japanese demonstrating severe diabetic complications. Diabetes Care. 1997;20(5):844–847. doi: 10.2337/diacare.20.5.844. [DOI] [PubMed] [Google Scholar]
- 162.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296(4):421–426. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- 163.Solis-Herrera C, Triplitt CL, Lynch JL. Nephropathy in youth and young adults with type 2 diabetes. Curr Diab Rep. 2014;14(2):456. doi: 10.1007/s11892-013-0456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35(6):1265–1271. doi: 10.2337/dc11-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wilson C. Paediatrics: kidney disease in youth-onset type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(6):319. doi: 10.1038/nrendo.2012.55. [DOI] [PubMed] [Google Scholar]
- 166.Franceschini N, Shara NM, Wang H, Voruganti VS, Laston S, Haack K, Lee ET, Best LG, Maccluer JW, Cochran BJ. et al. The association of genetic variants of type 2 diabetes with kidney function. Kidney Int. 2012;82(2):220–225. doi: 10.1038/ki.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Freedman BI, Bostrom M, Daeihagh P, Bowden DW. Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol. 2007;2(6):1306–1316. doi: 10.2215/CJN.02560607. [DOI] [PubMed] [Google Scholar]
- 168.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 169.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 170.Greenfield JR, Campbell LV. Relationship between inflammation, insulin resistance and type 2 diabetes: 'cause or effect'? Curr Diabetes Rev. 2006;2(2):195–211. doi: 10.2174/157339906776818532. [DOI] [PubMed] [Google Scholar]
- 171.Shin JA, Lee JH, Lim SY, Ha HS, Kwon HS, Park YM, Lee WC, Kang MI, Yim HW, Yoon KH. et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. 2013;4(4):334–343. doi: 10.1111/jdi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Catalano C, Postorino M, Marino C. The impact of diabetes on patients' survival in dialysis patients with non-diabetic renal disease and in patients who develop diabetes during chronic dialysis. Nephrol Dial Transplant. 1996;11(6):1124–1128. [PubMed] [Google Scholar]
- 173.Schroijen MA, Dekkers OM, Grootendorst DC, Noordzij M, Romijn JA, Krediet RT, Boeschoten EW, Dekker FW NECOSAD Study Group. Survival in dialysis patients is not different between patients with diabetes as primary renal disease and patients with diabetes as a co-morbid condition. BMC Nephrol. 2011;12:69. doi: 10.1186/1471-2369-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schroijen MA, van de Luijtgaarden MW, Noordzij M, Ravani P, Jarraya F, Collart F, Prütz KG, Fogarty DG, Leivestad T, Prischl FC. et al. Survival in dialysis patients is different between patients with diabetes as primary renal disease and patients with diabetes as a co-morbid condition. Diabetologia. 2013;56(9):1949–1957. doi: 10.1007/s00125-013-2966-1. [DOI] [PubMed] [Google Scholar]
- 175.Parving HH, Oxenboll B, Svendsen PA, Christiansen JS, Andersen AR. Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh) 1982;100:550–555. doi: 10.1530/acta.0.1000550. [DOI] [PubMed] [Google Scholar]
- 176.Dwyer JP, Lewis JB. Nonproteinuric diabetic nephropathy: when diabetics don't read the textbook. Med Clin North Am. 2013;97(1):53–58. doi: 10.1016/j.mcna.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 177.Chawla V, Roshan B. Non-proteinuric diabetic nephropathy. Curr Diab Rep. 2014;14(10):529. doi: 10.1007/s11892-014-0529-6. [DOI] [PubMed] [Google Scholar]
- 178.Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis JB. Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: results from the DEMAND Study. Cardiorenal Med. 2012;2(1):1–10. doi: 10.1159/000333249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Halimi JM. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab. 2012;38(4):291–297. doi: 10.1016/j.diabet.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 180.Nakao K, Uzu T, Araki S, Kume S, Deji N, Chin-Kanasaki M, Araki H, Isshiki K, Sugimoto T, Kawai H. et al. Arterial stiffness and renal impairment in non-proteinuric type 2 diabetic patients. J Diabetes Investig. 2012;3(1):86–91. doi: 10.1111/j.2040-1124.2011.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gimeno-Orna JA, Lou-Arnal LM, Boned-Juliani B, Molinero-Herguedas E. Mild renal insufficiency as a cardiovascular risk factor in non-proteinuric type II diabetes. Diabetes Res Clin Pract. 2004;64(3):191–199. doi: 10.1016/j.diabres.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 182.Piccoli GB, Mezza E, Burdese M, Terzolo M, Grassi G, Bermond F, Soragna G, Gai M, Dani F, Jeantet A. et al. Progression of renal failure without proteinuria in a patient with type 1 diabetes. Nephrol Dial Transplant. 2004;19(12):3197–3199. doi: 10.1093/ndt/gfh251. [DOI] [PubMed] [Google Scholar]
- 183.Piccoli GB, Mezza E, Jeantet A, Segoloni GP. An uncommon genetic syndrome with acute renal failure in a 30-year-old diabetic patient. Nephrol Dial Transplant. 2003;18(1):206–208. doi: 10.1093/ndt/18.1.206. [DOI] [PubMed] [Google Scholar]
- 184.Piccoli GB, Bonino LD, Campisi P, Vigotti FN, Ferraresi M, Fassio F, Brocheriou I, Porpiglia F, Restagno G. Chronic kidney disease, severe arterial and arteriolar sclerosis and kidney neoplasia: on the spectrum of kidney involvement in MELAS syndrome. BMC Nephrol. 2012;13:19. doi: 10.1186/1471-2369-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Burdese M, Consiglio V, Mezza E, Bergamo D, Grassi G, Soragna G, Rossetti M, Segoloni GP, Mazzucco G, Piccoli GB. 'Primary' nephrosclerosis in a type 1 diabetic patient. Nephrol Dial Transplant. 2005;20(4):817–819. doi: 10.1093/ndt/gfh659. [DOI] [PubMed] [Google Scholar]
- 186.Piccoli GB, Tavassoli E, Melluzza C, Grassi G, Monzeglio C, Donvito V, Leone F, Attini R, Ghiotto S, Clari R. et al. Severe diabetic nephropathy in type 1 diabetes and pregnancy - a case series. Rev Diabet Stud. 2013;10(1):68–78. doi: 10.1900/RDS.2013.10.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lescure FX, Flateau C, Pacanowski J, Brocheriou I, Rondeau E, Girard PM, Ronco P, Pialoux G, Plaisier E. HIV-associated kidney glomerular diseases: changes with time and HAART. Nephrol Dial Transplant. 2012;27(6):2349–2355. doi: 10.1093/ndt/gfr676. [DOI] [PubMed] [Google Scholar]
- 188.Mohan S, Herlitz LC, Tan J, Cheng JT, Anderson HL, Stokes MB, Markowitz GS, D'Agati VD, Radhakrishnan J. The changing pattern of glomerular disease in HIV and hepatitis C co-infected patients in the era of HAART. Clin Nephrol. 2013;79(4):285–291. doi: 10.5414/CN107774. [DOI] [PubMed] [Google Scholar]
- 189.Sethi S, Zand L, Nasr SH, Glassock RJ, Fervenza FC. Focal and segmental glomerulosclerosis: clinical and kidney biopsy correlations. Clin Kidney J. 2014;7(6):531–537. doi: 10.1093/ckj/sfu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zwi LJ, Yiu TS, Marshall MR, Lam-Po-Tang MK. Non-diabetic renal diseases in a multi-ethnic New Zealand cohort with type 2 diabetes mellitus: clinical and histopathological features. Pathology. 2014;46(5):424–432. doi: 10.1097/PAT.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 191.Prakash J. Non-diabetic renal disease (NDRD) in patients with type 2 diabetes mellitus (type 2 DM) J Assoc Physicians India. 2013;61(3):194–199. [PubMed] [Google Scholar]
- 192.Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int. 2000;58(4):1719–1731. doi: 10.1046/j.1523-1755.2000.00333.x. [DOI] [PubMed] [Google Scholar]
- 193.Verani RR. Obesity-associated focal segmental glomerulosclerosis: pathological features of the lesion and relationship with cardiomegaly and hyperlipidemia. Am J Kidney Dis. 1992;20(6):629–634. doi: 10.1016/s0272-6386(12)70230-5. [DOI] [PubMed] [Google Scholar]
- 194.Praga M, Hernandez E, Morales E, Campos AP, Valero MA, Martínez MA, Leon M. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16(9):1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- 195.Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 196.Darouich S, Goucha R, Jaafoura MH, Zekri S, Ben Maiz H, Kheder A. Clinicopathological characteristics of obesity-associated focal segmental glomerulosclerosis. Ultrastruct Pathol. 2011;35(4):176–182. doi: 10.3109/01913123.2011.584657. [DOI] [PubMed] [Google Scholar]
- 197.Chen HM, Li SJ, Chen HP, Wang QW, Li LS, Liu ZH. Obesity-related glomerulopathy in China: a case series of 90 patients. Am J Kidney Dis. 2008;52(1):58–65. doi: 10.1053/j.ajkd.2008.02.303. [DOI] [PubMed] [Google Scholar]
- 198.Martin-Rodriguez E, Guillen-Grima F, Marti A, Brugos-Larumbe A. Comorbidity associated with obesity in a large population: The APNA study. Obes Res Clin Pract. 2015 doi: 10.1016/j.orcp.2015.04.003. In press. [DOI] [PubMed] [Google Scholar]
- 199.Tryggestad JB, Willi SM. Complications and comorbidities of T2DM in adolescents: findings from the TODAY clinical trial. J Diabetes Complications. 2015;29(2):307–312. doi: 10.1016/j.jdiacomp.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Furuta T, Seino J, Saito T, Sato H, Agatsuma J, Ootaka T, Satoh T, Yoshinaga K. Insulin deposits in membranous nephropathy associated with diabetes mellitus. Clin Nephrol. 1992;37(2):65–69. [PubMed] [Google Scholar]
- 201.Abrass CK, Cohen AH. Accelerated glomerulosclerosis in diabetic rats with immune complex injury. Diabetes. 1987;36(11):1246–1253. doi: 10.2337/diab.36.11.1246. [DOI] [PubMed] [Google Scholar]
- 202.Ahuja TS, Velasco A, Deiss W Jr, Indrikovs AJ, Rajaraman S. Diabetic nephropathy with anti-GBM nephritis. Am J Kidney Dis. 1998;31(1):127–130. doi: 10.1053/ajkd.1998.v31.pm9428463. [DOI] [PubMed] [Google Scholar]
- 203.Venkateswara K, Crosson JT. Idiopathic membranous glomerulonephritis in diabetic patients: report of three cases and review of the literature. Arch Intern Med. 1980;140(5):624–627. doi: 10.1001/archinte.140.5.624. [DOI] [PubMed] [Google Scholar]
- 204.Jennette JC, Huffman KA. Concurrent membranous glomerulopathy and diabetes mellitus. Arch Intern Med. 1981;141(10):1386–1387. [PubMed] [Google Scholar]
- 205.Rao KV. Concurrent membranous glomerulopathy and diabetes mellitus - reply. Arch Intern Med. 1981;141(10):1387. [PubMed] [Google Scholar]
- 206.Nissenson AR, Pereira BJ, Collins AJ, Steinberg EP. Prevalence and characteristics of individuals with chronic kidney disease in a large health maintenance organization. Am J Kidney Dis. 2001;37(6):1177–1183. doi: 10.1053/ajkd.2001.24520. [DOI] [PubMed] [Google Scholar]
- 207.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 208.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X. et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 209.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y. et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13(6):621–630. doi: 10.1007/s10157-009-0199-x. [DOI] [PubMed] [Google Scholar]
- 211.Plantinga LC, Crews DC, Coresh J, Miller ER 3rd, Saran R, Yee J, Hedgeman E, Pavkov M, Eberhardt MS, Williams DE. et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5(4):673–682. doi: 10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, Fluck N, MacLeod A, McNamee P, Prescott G. et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess. 2010;14(21):1–184. doi: 10.3310/hta14210. [DOI] [PubMed] [Google Scholar]
- 213.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 214.Akbari A, Clase CM, Acott P, Battistella M, Bello A, Feltmate P, Grill A, Karsanji M, Komenda P, Madore F. et al. Canadian Society of Nephrology commentary on the KDIGO clinical practice guideline for CKD evaluation and management. Am J Kidney Dis. 2015;65(2):177–205. doi: 10.1053/j.ajkd.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 215.Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care. 2001;24(3):495–499. doi: 10.2337/diacare.24.3.495. [DOI] [PubMed] [Google Scholar]
- 216.Thomas MC. The high prevalence of anemia in diabetes is linked to functional erythropoietin deficiency. Semin Nephrol. 2006;26(4):275–282. doi: 10.1016/j.semnephrol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 217.Mercadal L, Metzger M, Casadevall N, Haymann JP, Karras A, Boffa JJ, Flamant M, Vrtovsnik F, Stengel B, Froissart M. et al. Timing and determinants of erythropoietin deficiency in chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(1):35–42. doi: 10.2215/CJN.04690511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Khoshdel A, Carney S, Gillies A, Mourad A, Jones B, Nanra R, Trevillian P. Potential roles of erythropoietin in the management of anaemia and other complications diabetes. Diabetes Obes Metab. 2008;10(1):1–9. doi: 10.1111/j.1463-1326.2007.00711.x. [DOI] [PubMed] [Google Scholar]
- 219.Liu Z, Liu L, Chen X, He W, Yu X. Associations study of vitamin D receptor gene polymorphisms with diabetic microvascular complications: a meta-analysis. Gene. 2014;546(1):6–10. doi: 10.1016/j.gene.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 220.Tizaoui K, Kaabachi W, Hamzaoui A, Hamzaoui K. Contribution of VDR polymorphisms to type 1 diabetes susceptibility: Systematic review of case-control studies and meta-analysis. J Steroid Biochem Mol Biol. 2014;143:240–249. doi: 10.1016/j.jsbmb.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 221.Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases. 2014;2(10):488–496. doi: 10.12998/wjcc.v2.i10.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Karet FE. Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol. 2009;20(2):251–254. doi: 10.1681/ASN.2008020166. [DOI] [PubMed] [Google Scholar]
- 223.van Nieuwkoop C, Ijpelaar DH, Bolk JH. Treating proteinuria in a diabetic patient despite hyperkalaemia due to hyporeninaemic hypoaldosteronism. Neth J Med. 2007;65(2):75–77. [PubMed] [Google Scholar]
- 224.de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens. 2009;27(7):1333–1340. doi: 10.1097/HJH.0b013e328329bbf4. [DOI] [PubMed] [Google Scholar]
- 225.Postma CT, Klappe EM, Dekker HM, Thien T. The prevalence of renal artery stenosis among patients with diabetes mellitus. Eur J Intern Med. 2012;23(7):639–642. doi: 10.1016/j.ejim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 226.Alexander N, Matsushita K, Sang Y, Ballew S, Mahmoodi BK, Astor BC, Coresh J. Kidney Measures with Diabetes and Hypertension on Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Am J Nephrol. 2015;41(4-5):409–417. doi: 10.1159/000433450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ma Q, Zheng B, Meng K, Yong Q, He Y, Wang J, Li S, Zhao D, Xu Z, Hao P. et al. A simple score for predicting renal artery stenosis in patients with ischemic heart disease. Int J Clin Exp Med. 2015;8(3):4302–4310. [PMC free article] [PubMed] [Google Scholar]
- 228.Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens. 2010;23(11):1159–1169. doi: 10.1038/ajh.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Textor SC, Misra S, Oderich GS. Percutaneous revascularization for ischemic nephropathy: the past, present, and future. Kidney Int. 2013;83(1):28–40. doi: 10.1038/ki.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160(5):685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 231.Ahmed A. Use of angiotensin-converting enzyme inhibitors in patients with heart failure and renal insufficiency: how concerned should we be by the rise in serum creatinine? J Am Geriatr Soc. 2002;50(7):1297–1300. doi: 10.1046/j.1532-5415.2002.50321.x. [DOI] [PubMed] [Google Scholar]
- 232.Jackevicius CA, Wong J, Aroustamian I, Gee M, Mody FV. Rates and predictors of ACE inhibitor discontinuation subsequent to elevated serum creatinine: a retrospective cohort study. BMJ Open. 2014;4(8):e005181. doi: 10.1136/bmjopen-2014-005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ohta Y, Fujii K, Arima H, Matsumura K, Tsuchihashi T, Tokumoto M, Tsuruya K, Kanai H, Iwase M, Hirakata H. et al. Increased renal resistive index in atherosclerosis and diabetic nephropathy assessed by Doppler sonography. J Hypertens. 2005;23(10):1905–1911. doi: 10.1097/01.hjh.0000181323.44162.01. [DOI] [PubMed] [Google Scholar]
- 234.Calabia J, Torguet P, Garcia I, Martin N, Mate G, Marin A, Molina C, Valles M. The relationship between renal resistive index, arterial stiffness, and atherosclerotic burden: the link between macrocirculation and microcirculation. J Clin Hypertens (Greenwich) 2014;16(3):186–191. doi: 10.1111/jch.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Piccoli GB, Priola AM, Vigotti FN, Guzzo G, Veltri A. Renal infarction versus pyelonephritis in a woman presenting with fever and flank pain. Am J Kidney Dis. 2014;64(2):311–314. doi: 10.1053/j.ajkd.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 236.Antopolsky M, Simanovsky N, Stalnikowicz R, Salameh S, Hiller N. Renal infarction in the ED: 10-year experience and review of the literature. Am J Emerg Med. 2012;30(7):1055–1060. doi: 10.1016/j.ajem.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 237.Huang CC, Chen WL, Chen JH, Wu YL, Shiao CJ. Clinical characteristics of renal infarction in an Asian population. Ann Acad Med Singapore. 2008;37(5):416–420. [PubMed] [Google Scholar]
- 238.Pennington M, Yeager J, Skelton H, Smith KJ. Cholesterol embolization syndrome: cutaneous histopathological features and the variable onset of symptoms in patients with different risk factors. Br J Dermatol. 2002;146(3):511–517. doi: 10.1046/j.1365-2133.2002.04611.x. [DOI] [PubMed] [Google Scholar]
- 239.Spring MW, Hartley B, Scoble JE, Viberti GC. A man with diabetes and unexplained renal failure. Lancet. 1998;352(9132):956. doi: 10.1016/s0140-6736(98)07198-0. [DOI] [PubMed] [Google Scholar]
- 240.Kashyap AS, Kashyap S. Diabetes and renal cholesterol emboli. Lancet. 1998;352(9141):1711. doi: 10.1016/s0140-6736(05)61493-6. [DOI] [PubMed] [Google Scholar]
- 241.Scolari F, Tardanico R, Zani R, Pola A, Viola BF, Movilli E, Maiorca R. Cholesterol crystal embolism: A recognizable cause of renal disease. Am J Kidney Dis. 2000;36(6):1089–1109. doi: 10.1053/ajkd.2000.19809. [DOI] [PubMed] [Google Scholar]
- 242.Mittal BV, Alexander MP, Rennke HG, Singh AK. Atheroembolic renal disease: a silent masquerader. Kidney Int. 2008;73(1):126–130. doi: 10.1038/sj.ki.5002433. [DOI] [PubMed] [Google Scholar]
- 243.Piccoli GB, Sargiotto A, Burdese M, Colla L, Bilucaglia D, Magnano A, Consiglio V, Piccoli G, Picciotto G. Cholesterol emboli syndrome in type 2 diabetes: the disease history of a case evaluated with renal scintigraphy. Rev Diabet Stud. 2005;2(2):92–96. doi: 10.1900/RDS.2005.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Patterson JE, Andriole VT. Bacterial urinary tract infections in diabetes. Infect Dis Clin North Am. 1997;11(3):735–750. doi: 10.1016/s0891-5520(05)70383-4. [DOI] [PubMed] [Google Scholar]
- 245.Liao WC, Chou JW. Emphysematous pyelonephritis, ureteritis and cystitis in a diabetic patient. QJM. 2010;103(11):893–894. doi: 10.1093/qjmed/hcp180. [DOI] [PubMed] [Google Scholar]
- 246.Eid YM, Salam MM. Diabetic ketoacidosis presenting with emphysematous pyelonephritis. J Diabetes Complications. 2010;24(3):214–216. doi: 10.1016/j.jdiacomp.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 247.Gupta P, Gupta R, Jandial K, Samotra S, Rana V, Gupta R, Gupta S, Singh J. Emphysematous pyelonephritis in the setting of diabetes mellitus. J Assoc Physicians India. 2011;59:119–120. [PubMed] [Google Scholar]
- 248.Pontin AR, Barnes RD, Joffe J, Kahn D. Emphysematous pyelonephritis in diabetic patients. Br J Urol. 1995;75(1):71–74. doi: 10.1111/j.1464-410x.1995.tb07237.x. [DOI] [PubMed] [Google Scholar]
- 249.Burdese M, Repetto L, Lasaponara F, Maass J, Bergamo D, Mezza E, Jeantet A, Segoloni GP, Piccoli GB. The deceiving image: asymptomatic renal malakoplakia in a patient with chronic renal failure. Nephrol Dial Transplant. 2003;18(8):1675–1676. doi: 10.1093/ndt/gfg138. [DOI] [PubMed] [Google Scholar]
- 250.Eichbauer-Sturm G, Janko O, Grafinger P, Höpfl I, Syre G, Biesenbach G, Zazgornik . Malacoplakia: a possible complication of poorly controlled diabetes mellitus? Dtsch Med Wochenschr. 1999;124(48):1453–1455. doi: 10.1055/s-2008-1035681. [DOI] [PubMed] [Google Scholar]
- 251.Piccoli GB, Consiglio V, Deagostini MC, Serra M, Biolcati M, Ragni F, Biglino A, De Pascale A, Frascisco MF, Veltri A. et al. The clinical and imaging presentation of acute "non-complicated" pyelonephritis: a new profile for an ancient disease. BMC Nephrol. 2011;12:68. doi: 10.1186/1471-2369-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rollino C, Beltrame G, Ferro M, Quattrocchio G, Sandrone M, Quarello F. Acute pyelonephritis in adults: a case series of 223 patients. Nephrol Dial Transplant. 2012;27(9):3488–3493. doi: 10.1093/ndt/gfr810. [DOI] [PubMed] [Google Scholar]
- 253.De Pascale A, Piccoli GB, Priola SM, Rognone D, Consiglio V, Garetto I, Rizzo L, Veltri A. Diffusion-weighted magnetic resonance imaging: new perspectives in the diagnostic pathway of non-complicated acute pyelonephritis. Eur Radiol. 2013;23(11):3077–3086. doi: 10.1007/s00330-013-2906-y. [DOI] [PubMed] [Google Scholar]
- 254.Rathod SB, Kumbhar SS, Nanivadekar A, Aman K. Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol. 2015;56(2):244–249. doi: 10.1177/0284185114520862. [DOI] [PubMed] [Google Scholar]
- 255.Restrepo BI, Twahirwa M, Rahbar MH, Schlesinger LS. Phagocytosis via complement or Fc-gamma receptors is compromised in monocytes from type 2 diabetes patients with chronic hyperglycemia. Plos One. 2014;9(3):e92977. doi: 10.1371/journal.pone.0092977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Diez-Manglano J, Gimenez-Lopez M, Garces-Horna V, Sevil-Puras M, Castellar-Otin E, Gonzalez-Garcia P, Fiteni-Mera I, Morlanes-Navarro T PLUPAR Study Researchers. Excessive polypharmacy and survival in polypathological patients. Eur J Clin Pharmacol. 2015;71(6):733–739. doi: 10.1007/s00228-015-1837-8. [DOI] [PubMed] [Google Scholar]
- 257.Marengoni A, Onder G. Guidelines, polypharmacy, and drug-drug interactions in patients with multimorbidity. BMJ. 2015;350:H1059. doi: 10.1136/bmj.h1059. [DOI] [PubMed] [Google Scholar]
- 258.Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW, Alderson P, Thompson A, Payne K, Guthrie B. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015;350:H949. doi: 10.1136/bmj.h949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Taber SS, Pasko DA. The epidemiology of drug-induced disorders: the kidney. Expert Opin Drug Saf. 2008;7(6):679–690. doi: 10.1517/14740330802410462. [DOI] [PubMed] [Google Scholar]
- 260.Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457–468. doi: 10.2147/IJNRD.S39747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Paller MS. Drug-induced nephropathies. Med Clin North Am. 1990;74(4):909–917. doi: 10.1016/s0025-7125(16)30525-9. [DOI] [PubMed] [Google Scholar]
- 262.Schetz M, Dasta J, Goldstein S, Golper T. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11(6):555–565. doi: 10.1097/01.ccx.0000184300.68383.95. [DOI] [PubMed] [Google Scholar]
- 263.Blix HS, Viktil KK, Reikvam A, Moger TA, Hjemaas BJ, Pretsch P, Vraalsen TF, Walseth EK. The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. Eur J Clin Pharmacol. 2004;60(9):651–658. doi: 10.1007/s00228-004-0830-4. [DOI] [PubMed] [Google Scholar]
- 264.Blix HS, Viktil KK, Moger TA, Reikvam A. Use of renal risk drugs in hospitalized patients with impaired renal function - an underestimated problem? Nephrol Dial Transplant. 2006;21(11):3164–3171. doi: 10.1093/ndt/gfl399. [DOI] [PubMed] [Google Scholar]
- 265.Kodner CM, Kudrimoti A. Diagnosis and management of acute interstitial nephritis. Am Fam Physician. 2003;67(12):2527–2534. [PubMed] [Google Scholar]
- 266.Ulinski T, Sellier-Leclerc AL, Tudorache E, Bensman A, Aoun B. Acute tubulointerstitial nephritis. Pediatr Nephrol. 2012;27(7):1051–1057. doi: 10.1007/s00467-011-1915-9. [DOI] [PubMed] [Google Scholar]
- 267.Praga M, Gonzalez E. Acute interstitial nephritis. Kidney Int. 2010;77(11):956–961. doi: 10.1038/ki.2010.89. [DOI] [PubMed] [Google Scholar]
- 268.Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, Nasr SH. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558–666. doi: 10.1053/j.ajkd.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 269.Ito T, Watanabe S, Tsuruga K, Aizawa T, Hirono K, Ito E, Joh K, Tanaka H. Severe intrinsic acute kidney injury associated with therapeutic doses of acetaminophen. Pediatr Int. 2015;57(2):E53–E55. doi: 10.1111/ped.12607. [DOI] [PubMed] [Google Scholar]
- 270.Manske CL, Sprafka JM, Strony JT, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89(5):615–620. doi: 10.1016/0002-9343(90)90180-l. [DOI] [PubMed] [Google Scholar]
- 271.McCartney MM, Gilbert FJ, Murchison LE, Pearson D, McHardy K, Murray AD. Metformin and contrast media - a dangerous combination? Clin Radiol. 1999;54(1):29–33. doi: 10.1016/s0009-9260(99)91236-9. [DOI] [PubMed] [Google Scholar]
- 272.Rasuli P, Hammond DI. Metformin and contrast media: where is the conflict? Can Assoc Radiol J. 1998;49(3):161–166. [PubMed] [Google Scholar]
- 273.Thomsen HS, Morcos SK. Contrast media and metformin: guidelines to diminish the risk of lactic acidosis in non-insulin-dependent diabetics after administration of contrast media. ESUR Contrast Media Safety Committee. Eur Radiol. 1999;9(4):738–740. doi: 10.1007/s003300050746. [DOI] [PubMed] [Google Scholar]
- 274.Stolker JM, McCullough PA, Rao S, Inzucchi SE, Spertus JA, Maddox TM, Masoudi FA, Xiao L, Kosiborod M. Pre-procedural glucose levels and the risk for contrast-induced acute kidney injury in patients undergoing coronary angiography. J Am Coll Cardiol. 2010;55(14):1433–1440. doi: 10.1016/j.jacc.2009.09.072. [DOI] [PubMed] [Google Scholar]
- 275.Yang JQ, Ran P, Chen JY, He YT, Li LW, Tan N, Li G, Sun S, Liu Y, Zhan JX. et al. Development of contrast-induced acute kidney injury after elective contrast media exposure in patients with type 2 diabetes mellitus: effect of albuminuria. Plos One. 2014;9(9):E106454. doi: 10.1371/journal.pone.0106454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rose TA Jr, Choi JW. Intravenous Imaging Contrast Media Complications: The Basics That Every Clinician Needs to Know. Am J Med. 2015 doi: 10.1016/j.amjmed.2015.02.018. In press. [DOI] [PubMed] [Google Scholar]