Abstract
The burden of diabetes mellitus is relentlessly increasing. Diabetic nephropathy is the most common cause of end-stage renal disease (ESRD) worldwide and a major cause of morbidity and mortality in patients with diabetes. The current standard therapy of diabetic nephropathy involves intensive treatment of hyperglycemia and strict blood pressure control, mainly via blockade of the renin-angiotensin system (RAS). Attention has been drawn to additional beneficial effects of oral hypoglycemic drugs and fibrates on other aspects of diabetic nephropathy. On the other hand, antiproteinuric effects of RAS combination therapy do not seem to enhance the prevention of renal disease progression, and it has been associated with an increased rate of serious adverse events. Novel agents, such as bardoxolone methyl, pentoxifylline, inhibitors of protein kinase C (PKC), sulodexide, pirfenidone, endothelin receptor antagonists, vitamin D supplements, and phosphate binders have been associated with controversial outcomes or significant side effects. Although new insights into the pathogenetic mechanisms have opened new horizons towards novel interventions, there is still a long way to go in the field of DN research. The aim of this review is to highlight the recent progress made in the field of diabetes management based on the existing evidence. The article also discusses novel targets of therapy, with a special focus on the major pathophysiologic mechanisms implicated in the initiation and progression of diabetic nephropathy.
Keywords: diabetes mellitus, albuminuria, diabetic nephropathy, end-stage renal disease, ACE inhibitors
Abbreviations: ACEI – angiotensin-converting enzyme inhibitors; AGE – advanced glycosylation end products; ARB – angiotensin II receptor blockers; CKD – chronic kidney disease; DN – diabetic nephropathy; DPP-4 – dipeptidyl peptidase 4; ESH/ESC – European Societies of Hypertension and Cardiology; ESRD – end stage renal disease; ET-1 – endothelin 1; GFR – glomerular filtration rate; GLP-1 – glucagons-like peptide 1; JNC-8 – Eighth Joint National Committee; KDIGO – kidney disease improving global outcomes; NKF/KDOQI – National Kidney Foundation Kidney Disease Outcomes Quality Initiative; PPAR-α – peroxisome proliferator-activated receptor alpha; PPAR-γ – peroxisome proliferator activator receptor gamma; PKC – protein kinase C; RAS – rennin-angiotensin system; SGLT-2 – sodium-glucose co-transporter-2; TZD – thiazolidinediones; UAE – urine albumin excretion; VEGF – vascular endothelial growth factor
1. Introduction
The burden of diabetes mellitus is relentlessly increasing and the global prevalence is expected to rise from 6.4% in 2010 to 7.7% by 2030 [1]. Diabetic nephropathy which affects approximately one-third of individuals with diabetes is the most common cause of end-stage renal disease (ESRD) worldwide and a major cause of morbidity and mortality in patients with diabetes. This is due to the progression to ESRD and associated cardiovascular disease, especially in patients with type 2 diabetes [2, 3].
Diabetic nephropathy is a clinical syndrome characterized by persistent albuminuria (>300 mg/24 hr, or 300 mg/g creatinine), a progressive decline in glomerular filtration rate (GFR), arterial hypertension, and increased cardiovascular morbidity and mortality. It can also be defined as a spectrum of characteristic structural and functional changes, including glomerular hyperfiltration in the very early disease stage and the presence of moderately increased albuminuria. The latter is also called "microalbuminuria", which is defined as urinary albumin excretion between 30 and 300 mg/day or albumin-to-creatinine ratio between 2 and 28 mg albumin per mmol creatinine (mg/mmol) on a random urine sample [4, 5].
The current standard therapy of diabetic nephropathy involves intensive treatment of hyperglycemia and strict blood pressure control, mainly via blockade of the renin-angiotensin system (RAS). Major attention is currently focused on ongoing experimental studies and clinical trials with novel specific agents, which target the emerging pathophysiologic mechanisms involved in the progression of diabetic nephropathy. A few agents have shown beneficial effects in the experimental studies performed to date, although data regarding their clinical impact on diabetic patients remain ambiguous.
The aim of this review article is to highlight the recent progress made in the field of management of diabetic nephropathy based on the existing evidence. The article intends to provide evidence-based guidance on treatment options with reference to novel targets of therapy, while focusing on the major pathophysiologic mechanisms implicated in the initiation and progression of diabetic nephropathy which substantially constitute the targets for therapy.
2. Pathophysiological insights as potential therapeutic targets in diabetic nephropathy
Several pathogenetic processes are considered to be involved in diabetic nephropathy (Figure 1). Both intraglomerular hypertension induced by renal vasodilatation and ischemic injury induced by hyaline narrowing of the vessels supplying the glomeruli could lead to glomerulosclerosis [6]. Hyperglycemia may also directly induce mesangial expansion and injury, possibly via increased matrix production or glycation of matrix proteins [7]. Based on the observation that a decrease in cell surface heparan sulfate contributes to increased glomerular basement membrane permeability to albumin, the activation of protein kinase C and upregulation of heparanase expression may regarded as additional hyperglycemia-mediated mechanisms that are potentially pathogenic in diabetic nephropathy [8]. Activation of cytokines, profibrotic elements, inflammation, and vascular growth factors such as vascular endothelial growth factor (VEGF) may be involved in the process of matrix accumulation in diabetic nephropathy [9]. Defects in podocyte-specific insulin signaling may also contribute to the process. Therefore, the podocyte insulin receptor may provide a target for agents that prevent proteinuria and/or the development and progression of diabetic nephropathy [10].
Figure 1. Proposed pathophysiological mechanisms implicated in the pathogenesis of diabetic nephropathy.
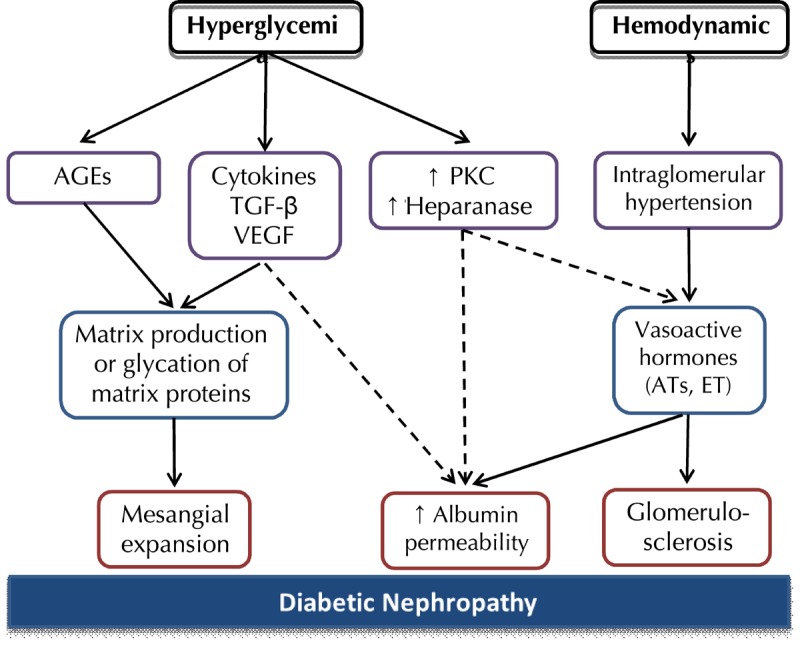
Hyperglycemia may induce mesangial expansion via the stimulation of cytokines and vascular growth factors or glycation of matrix proteins. Hyperglycemia-induced activation of protein kinase C and upregulation of heparanase expression result in increased glomerular permeability. Intraglomerular hypertension and subsequent stimulation of vasoactive hormones cause glomerulosclerosis. Substantial overlap exists between these different pathways.
While longitudinal studies have shown that microalbuminuria strongly predicts the development of diabetic nephropathy in type 1 diabetic patients [11, 12], there are biological mechanisms that initiate the early decline in kidney function, including oxidative, inflammatory, and fibrotic pathways [13]. These mechanisms should be considered in diagnosis and treatment besides the determination of microalbuminuria.
It has been suggested that a fraction of the microalbuminuric patients returns to normoalbuminuria. However, only treatment-induced and not spontaneous regression is associated with stable and long-lasting normalization in patients with type 1 diabetes [12]. In type 2 diabetes, moderately increased albuminuria is associated with declining kidney function, progression to severely increased albuminuria, and increased long-term mortality. Remission to normal albuminuria may occur as well [14, 15]. Additonally, microalbuminuria is a strong predictor of overall and cardiovascular mortality in diabetic patients, a finding which is valid for the general population as well [16]. Several other factors in addition to hyperglycemia are associated with microalbuminuria in diabetic patients, including arterial hypertension, obesity, heart failure, and other comorbidities [17, 18]. Until now, the exact mechanisms linking microalbuminuria to death from cardiovascular disease have been poorly understood. It is supposed that endothelial dysfunction resulting from endothelial-podocyte crosstalk across the glomerular filtration barrier is the underlying mechanism [19].
Finally, a subset of patients with diabetic nephropathy does not have overt proteinuria as a prerequisite to renal dysfunction. The exact pathogenetic mechanisms involved in this condition are also unknown [20].
3. Glycemic control
Timely and effective glycemic control may have a positive effect on the prevention of diabetic nephropathy. Evidence for the impact of strict glycemic control was first provided in type 1 diabetes. Two major clinical trials involving nearly 1,500 patients with type 1 diabetes demonstrated that intensive glycemic control reduces the incidence of micro- and macroalbuminuria by 39% and 54%, respectively. Intensive glycemic control resulted in a reduction of microalbuminuria by 45% after 18 years of follow-up, enabling a protection from kidney disease [21, 22].
Another important finding was that the long-term risk of a reduced glomerular filtration rate (GFR) was significantly lower among type 1 diabetes patients treated early in the course of the disease with intensive insulin therapy (HbA1c <6.05%, use of insulin pump) than among those treated with non-intensive insulin therapy [23]. Higher HbA1c concentrations are strongly associated with risk of chronic kidney disease (CKD), but a positive association seems to exist between higher HbA1c levels and incidence of CKD as well, even in the absence of albuminuria or other microvascular complications of diabetes [24]. According to the outcomes of several studies, which included patients who underwent pancreatic transplantation, strict glycemic control deccelerates the rate of progressive renal damage even in the presence of overt proteinuria [25-30].
Regarding type 2 diabetes, the two most important trials, UKPDS and ADVANCE, demonstrated that intensive glycemic control decreases the risks of moderately increased albuminuria [31, 32], overt proteinuria, and ESRD compared with standard control [32].
Poor glycemic control (HbA1c > 9%) appears to be common among patients with early stages of CKD; it is associated with a marked decline in clinical outcome and risk of progression to kidney disease. Thus, appropriate and timely control of HbA1c levesl in patients with diabetes and CKD is an essential step towards reducing diabetic complications. However, intensive glycemic control, with HbA1c <6.5%, may be associated with increased mortality [33]. A recent meta-analysis showed that, although intensive glucose control reduces the risk for micro- and macroalbuminuria, evidence regarding the effects on progression of CKD and end-stage renal disease remains ambiguous [34]. Another recent meta-analysis investigating the effect of intensive compared with conventional glycemic control on all-cause mortality showed that the HbA1c and all-cause mortality relationship in patients with type 2 diabetes is J-shaped, meaning that the relative risk for all-cause mortality increases with an increase in HbA1c above 7,5% and decreases with an increase in HbA1c below 7,5% [35]. Likewise, the ACCORD investigators found that HbA1c levels of 6.0% versus 7.0-7.9% resulted in excess mortality, thus suggesting that the benefits of intensive therapy regarding microvascular outcomes should be weighted against the increase in total and cardiovascular mortality and high risk for severe hypoglycemia [36]. Nevertheless, intensive glycemic control seemed to reduce the risk of microvascular complications in the ACCORD trail, albeit at the expense of an increased risk of hypoglycemia and higher all-cause mortality [36]. Another recent meta-analysis failed to demonstrate an all cause-mortality benefit of intensive glycemic control or a significant reduction in the rate of composite microvascular complications [37].
The current recommendation by the American Association of Clinical Endocrinologists is to target HbA1c <6.5%. In an attempt to balance out the risk of hypoglycemia with the clear benefit of renoprotection, the American Diabetes Association sets a goal of HbA1c <7% [38]. Accordingly, the recent KDIGO (Kidney Disease: Improving Global Outcomes) report on diabetic kidney disease highlights the fact that the beneficial effect of tight glycemic control on diabetic nephropathy is based almost exclusively on prevention of microalbuminuria and hindering its progression to overt albuminuria. The report suggest that the target HbA1c level may need to be adjusted upwards in patients with more advanced kidney disease, but particular attention should be paid to the augmented risk of severe hypoglycemia and death in these patients [39].
Besides glycemic control, attention has been paid to additional beneficial effects of oral hypoglycemic drugs on other aspects of diabetic nephropathy. These effects include the restoration of tubuloglomerular feedback mechanisms, lowering of glomerular hyperfiltration, and reduction of hyperglycemia-induced inflammatory and fibrotic markers by sodium-glucose co-transporter-2 (SGLT-2) inhibitors. Dipeptidyl peptidase 4 (DPP-4) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists are able to exert a renoprotective effect by reducing inflammation, fibrosis, and blood pressure [40]. Optimal prevention and treatment requires the implementation of therapies that interfere specifically with the pathogenesis of microvascular complications of diabetes. Therefore, glucose-lowering agents that provide renoprotection independent of their hypoglycemic effects may be considered as combined therapy. Simultaneous application of an SGLT-2 inhibitor and blockade of the renin-angiotensin-aldosterone system may be a more effective strategy to prevent the progression of diabetic nephropathy than either drug alone [40].
Abundant experimental data indicate that thiazolidinediones (TZD), a family of anti-diabetic drugs that activate the transcription factor peroxisome proliferator activator receptor gamma (PPAR-γ), have direct renoprotective effects [41-43]. These effects are most probably exerted by preventing diabetes-induced renal inflammatory processes. However, clinical studies have reported controversial outcomes, with some of them reporting significant antiproteinuric effects [44-46], and others demonstrating insignificant effects [47]. A meta-analysis of 15 studies, involving approximately 2,800 patients, showed that treatment with TZD significantly decreases urinary albumin and protein excretion [46]. A pilot study conducted in diabetic subjects with CKD showed a slower decline in renal function after initiation of TZD [48]. Another study compared the treatment with rosiglitazone, metformin, and glyburide in 4,351 recently diagnosed, drug-naïve type 2 diabetes patients. During a 5-year period after initial treatment with rosiglitazone, the increase in albuminuria was delayed (compared with metformin), renal function was preserved (compared with glyburide), and blood pressure control was improved relative to both other medications [49]. On the other hand, these agents raised some safety concerns, including an increased risk of cardiovascular disease, especially with rosiglitzone [50-52], and malignancy [53, 54]. In contrast, a recent study conducted by the Diabetes Shared Care Program (DSCP) in Taiwan showed an association between the use of TZD and reduced risk of cardiovascular events, including stroke and all-cause mortality [55].
Glomerular hyperfiltration is recognized as the first step in the progression of kidney disease in diabetic patients. At the onset of type 2 diabetes, hyperglycemia enhances proximal tubular reabsorption, thus leading to a decrease in solute load reaching the macula densa, with subsequent suppression of the tubuloglomerular feedback and increased glomerular filtration rate [56, 57]. As mentioned above, and evidenced by experimental studies, SGLT-2 inhibitors attenuate the progressive nature of diabetic nephropathy by preventing glomerular hyperfiltration independent of their blood glucose-lowering effects [58-60]. However, clinical data about the potential role of the proximal tubule in the pathophysiology of diabetic nephropathy and the nephroprotective effects of SGLT-2 inhibitors are currently insufficient [60]. Additionally, these agents have been tried in patients with type 1 diabetes, and short-term treatment with the SGLT-2 inhibitor empagliflozin was found to attenuate renal hyperfiltration [61, 62].
Studies have shown that DPP-4 inhibitors appear to possess anti-inflammatory properties and improve endothelial function, blood pressure control, lipid metabolism, and bone marrow function [63]. Additional experimental data reported direct favorable effects of DPP-4 inhibitors on microvascular complications of diabetes. However, the evidence is insufficient to confirm the preventive effect of this drug on the progression of diabetic microangiopathy in humans, independently of the effects on improved glucose control [63, 64].
4. Renin-angiotensin system (RAS)
Solid evidence from experimental studies in diabetic animals suggests that intraglomerular hypertension and glomerular hypertrophy play important roles in the onset of diabetic nephropathy as hyperglycemia induces renal vasodilation and a rise in GFR. Subsequent loss of nephrons accelerates the increase in intraglomerular pressure through the compensatory response of remaining nephrons. RAS inhibitors, including angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB), are widely used to control blood pressure in diabetic patients. These drugs have been extensively studied, and are considered superior to other antihypertensive drug categories in the treatment of diabetic nephropathy because of their capacity to reduce both intraglomerular pressure and proteinuria by preferentially dilating the efferent arteriole. The degree of proteinuria in glomerular disease tends to relate directly with the intraglomerular pressure; thus a treatment-induced reduction in protein excretion causes a desirable decline in intraglomerular pressure, and as a consequence improves renal outcome. There is now consensus that a decrease in protein excretion has predictive significance for improved renal prognosis [65-68]. Additionally, RAS components seem to be altered in diabetic podocytopathy, and their modulation may modify the progression of diabetic nephropathy [69].
The efficacy of ACEI therapy in type 1 diabetic patients with moderately increased albuminuria has been evaluated in several randomized prospective trials. In addition to the reduction of albuminuria, ACEI have significant long-term benefits [70]. In a systematic review of 11 trials of normotensive type 1 diabetic patients with moderately increased albuminuria, ACEI therapy significantly reduced the risk of progression to severe albuminuria, and significantly increased the chance of regression to normoalbuminuria [71]. Additionally, the beneficial response to ACEI seen in both hypertensive and normotensive subjects is consistent with several studies suggesting that these antihypertensive agents deccelerate the progression of diabetic nephropathy [72, 73]. Moreover, in some patients with type 1 diabetes, ACEI exhibit a marked antiproteinuric effect with sustained long-term remission or regression of nephropathy or the nephrotic syndrome; such patients appear to have better renal outcomes [74, 75]. Therefore, intensive control of systemic blood pressure, in particular with ACEI, may enable recovery from diabetic nephropathy in type 1 diabetes patients with advanced renal disease. Although there are few data on the efficacy of ARB in patients with type 1 diabetes and moderately increased albuminuria, based on their proven benefit in patients with type 2 diabetes, these drugs are probably as effective in type 1 diabetes patients as ACEI.
Regarding primary prevention of moderately increased albuminuria in patients with type 1 diabetes, several clinical trials have evaluated the efficacy of ACEI or ARB. However, three randomized, placebo-controlled trials in patients with type 1 diabetes and normoalbuminuria (EUCLID, RASS, DIRECT) showed no benefit from angiotensin inhibition [76-78]. Moreover, specific histologic findings from kidney biopsies of patients with diabetic nephropathy showed that treatment with these drugs had no significantly beneficial effects compared with placebo [77].
In contrast, the renoprotective effects observed with ACEI and ARB treatment have been substantiated in type 2 diabetic patients with moderately increased albuminuria [32, 79, 80]; both groups (ACEI and ARB) appeared to be equally effective [80]. Also, a clear renoprotective benefit of ACEI and ARB has been demonstrated in patients with type 2 diabetes and overt nephropathy; a larger reduction in albuminuria was correlated with a progressively lower risk of ESRD. It should be noted that these effects were independent of the difference in blood pressure reductions among the groups [81-88]. Lowering of albuminuria early in the course of the disease correlates with a decreased subsequent cardiovascular risk [89]. Nevertheless, patients with type 2 diabetes and advanced kidney disease are likely to progress to ESRD eventually, although more slowly, despite treatment with ACEI or ARBs.
Regarding primary prevention of moderately increased albuminuria, the effect of angiotensin inhibition in hypertensive patients with type 2 diabetes has been evaluated in four randomized, placebo controlled trials in patients with type 2 diabetes and normal albuminuria [90-94]. The trials suggest that ACEI and ARB are effective in preventing the new onset of moderately increased albuminuria in this group of patients. On the other hand, results from clinical trials in patients with type 2 diabetes and normal blood pressure remain controversial regarding the effectiveness of ACEI or ARB in primary prevention of moderately increased albuminuria; these results should thus be interpreted with caution [78, 94]. Therefore, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF/KDOQI) clinical practice guidelines have not proposed the implementation of ACEI or ARB in primary prevention of diabetic nephropathy in normotensive individuals with normo-albuminuria [95].
Combination therapy with ACEI and ARB compared with ACEI or ARB monotherapy was shown to reduce proteinuria in patients with type 1 and type 2 diabetes [96, 97]. However, the antiproteinuric effects of combination therapy do not seem to be sufficient for the prevention of renal disease progression or death. Moreover, combination therapy has been associated with an increased rate of serious adverse events. The VA NEPHRON-D trial was discontinued after approximately 2 years because of safety concerns; patients experienced acute kidney injury and severe hyperkalemia, conditions that were significantly more common with combination therapy [98]. The ONTARGET trial investigated the administration of combination therapy with telmisartan and ramipril in patients with diabetic nephropathy. It appeared that the therapy was associated with a non-significantly higher incidence of dialysis initiation or doubling of serum creatinine in comparison with monotherapy, as well as higher rates of acute kidney injury, hyperkalemia, and hypotension [99, 100]. A recent meta-analysis that evaluated the efficacy and safety of combination therapy in diabetic nephropathy suggested that it may be safely applied in early stages of the disease when there are signs of proteinuria, but should be cautiously used in the setting of advanced stages of renal dysfunction [101].
Aliskiren, an oral direct renin inhibitor, has a similar degree of blood pressure-lowering properties as other agents. In the AVOID trial, aliskiren combined with losartan was associated with a significantly greater reduction in proteinuria, but no significantly greater antihypertensive effect, than losartan monotherapy [102]. On the other hand, results from the multinational, randomized ALTITUDE trial, which included type 2 diabetic patients with pre-existing renal or cardiovascular disease assigned to aliskiren or placebo, showed that more patients in the aliskiren group reached the composite primary endpoint of renal and cardiovascular events, despite a similar incidence of renal events in both groups. Additionally, hyperkalemia was significantly more frequent with aliskiren. Therefore, the trial was prematurely terminated because of the lack of evidence regarding benefits and the higher risk of side effects [103].
Aldosterone antagonists seem to possess antiproteinuric effects when used alone and in combination with ACEI or ARB in both type 1 and type 2 diabetes, but involve a risk of hyperkalemia when applied in patients with reduced GFR [104-106]. However, there is no adequate long-term evidence of beneficial effects regarding the prevention of renal impairment through aldosterone antagonists [107, 108].
5. Blood pressure goals in diabetic nephropathy
It is well established that early treatment of hypertension is particularly important in diabetic patients both to prevent cardiovascular disease and to minimize the progression of microvascular complications of diabetes. Past major guidelines recommended that the target blood pressure in diabetic patients should be less than 130/80 mmHg. The ACCORD BP trial, which enrolled type 2 diabetic patients with increased cardiovascular risk, found no significant cardiovascular benefit, except for stroke, and more drug-related side effects at a mean systolic blood pressure of 119.3 than at 133.5 mmHg [109].
A recent meta-analysis of 40 trials examined the association between antihypertensive treatment and vascular disease in type 2 diabetes in 100,354 patients, including normotensive and hypertensive subjects [110]. Compared with placebo, antihypertensive therapy significantly reduced the rate of mortality, total cardiovascular disease, myocardial infarction, and stroke. However, analyses of patients classified according to their baseline systolic pressure level revealed that, with the exception of stroke, the benefit of antihypertensive therapy was limited to those whose initial systolic pressures were greater than 140 mmHg. Also, the different drug classes did not exhibit significant differences in their antihypertensive effects, except for stroke and heart failure [110].
The most recent major guidelines published by the eighth Joint National Committee (JNC 8) and the European Societies of Hypertension and Cardiology (ESH/ESC) recommend that all patients with diabetes should have a target blood pressure of less than 140/90 mmHg [111, 112].
6. Intensive lipid control
Intensive lipid lowering is an important part of diabetes management since diabetes is considered a coronary heart disease equivalent. In addition to promoting systemic atherosclerosis, an elevation in lipid levels may also contribute to the development of glomerulosclerosis in CKD [113]. Interventional studies suggest that anti-hyperlipidemic agents have a beneficial effect on diabetic nephropathy through improvement of albuminuria and renal function [114, 115]. Recent data show that fibrates are renoprotective, an effect independent of their antihyperlipidemic action [115, 116]. The benefits of fibrates may be associated with their anti-inflammatory properties and decreased production of type 1 collagen in the mesangium. Peroxisome proliferator-activated receptor α (PPAR-α) is expressed in several tissues including the kidney. Experimental data have suggested that fibrate-induced activation of PPAR-α, a member of a large nuclear receptor superfamily, plays a significant role in various metabolic and inflammatory signaling pathways that are involved in diabetic microvascular complications [117].
7. Novel targets in therapy
Bardoxolone methyl is an oral antioxidant. Its structure and activity profile is similar to cyclopentenone prostaglandins that exert anti-inflammatory effects by inhibiting the nuclear factor κB pathway. Experimental data of drug-induced or ischemic acute kidney injury have shown beneficial effects [118]. These positive effects were confirmed in the Bardoxolone Methyl in CKD and type 2 Diabetes (BEAM) trial, which enrolled 227 patients with type 2 diabetes and CKD stage III-IV. According to this trial, bardoxolone methyl therapy significantly increased estimated glomerular filtration rate (eGFR) at 1 year of follow-up, while placebo therapy did not have any effects on the eGFR [119].
The subsequent BEACON trial evaluated 2,185 patients with type 2 diabetes and stage 4 CKD, who were randomly assigned to bardoxolone methyl or placebo, while being under concomitant therapy with an ACEI or ARB [120]. The trial was stopped early after a median follow-up of nine months due to a significant increase in the incidence of cardiovascular events, although the primary endpoint which was a composite of end-stage renal disease and cardiovascular death was identical in both groups. Additionally, despite causing a significant rise in GFR, bardoxolone methyl significantly increased blood pressure and albuminuria, most probably via sodium and water retention [121].
Pentoxifylline is a non-specific phosphodiesterase inhibitor with anti-inflammatory properties; it has been used in patients with peripheral artery disease and alcoholic hepatitis. Several small studies including patients with diabetic nephropathy showed that pentoxifylline had antiproteinuric effects and reduced the rate of decline in eGFR [122-125]. Additional data are needed before pentoxifylline can be recommended as treatment for diabetic nephropathy.
Although the molecular mechanism of hyperglycemia-induced tissue injury still remains unclear, oxidative stress seems to play a key role, and exerts its harmful impact via the sorbitol pathway and accumulation of advanced glycosylation end (AGE) products. In experimental diabetes, sorbitol production is markedly enhanced by the intracellular conversion of glucose to sorbitol. Accumulation of sorbitol within the cells results in a rise in cell osmolality and a decrease in intracellular myoinositol, changes which lead to a decrease in Na-K-ATPase activity and a possible shift in the intracellular redox potential [126]. Aldose reductase inhibitors such as tolrestat were shown to improve some of the manifestations of diabetic nephropathy by reversing glomerular hyperfiltration and decreasing albuminuria, but their potential benefit remains in the initial stages of diabetic nephropathy in type 2 diabetes [127-129]. At present, aldose reductase inhibitors have shown evidence of benefit in patients with diabetic peripheral neuropathy [130].
Increased activity of protein kinase C (PKC) appears to contribute to the micro- and macrovascular complications of diabetes through changes in vascular permeability, angiogenesis, cell growth and apoptosis, vasodilation, cytokine activation, basement membrane thickening, and extracellular matrix expansion [131]. Lowering PKC may be possible via isoform-specific PKC inhibitors such as ruboxistaurin mesylate therapy. Therapies aimed at lowering PKC may be beneficial in slowing the progression of diabetic nephropathy, as shown in animal studies. However, studies in humans have provided ambiguous results to date; much larger trials are necessary to determine the potential clinical role of this agent [132-134].
Experimental data suggest that glomerular capillary wall and mesangial alterations in diabetic nephropathy involve alterations of glycoproteins in these structures [135]. Experimental data from diabetic animals reveal that the administration of anionic glycoproteins can effectively prevent the biochemical alterations that promote albuminuria [136]. Administration of sulodexide, a purified mixture of sulfated glycosaminoglycan polysaccharides, has been associated with a reduction in albuminuria in diabetic patients [137, 138]. However, a recent multicenter, placebo-controlled, double-blinded study showed that sulodexide did not decrease UAE in patients with diabetic nephropathy and microalbuminuria [139]. Based on these controversial data, further research is needed to clarify the potentially beneficial role of sulodexide in the early stages of diabetic nephropathy.
Pirfenidone is an oral synthetic antifibrotic agent that has demonstrated benefits in animal models of diabetic nephropathy and in patients with this syndrome by preventing the progression of renal impairment. Pirfenidone is thus a promising agent for the treatment of diabetic nephropathy, and should be further investigated and advanced [140, 141].
Experimental and clinical studies have shown that vitamin D has antiproteinuric effects via both RAS-dependent and RAS-independent pathways [142]. In experimental diabetic nephropathy, vitamin D receptor activation via calcitriol and paricalcitol was shown to decrease the expression of proinfammatory mediators in podocytes and tubular cells, and to prevent glomerular infiltration by macrophages, apoptosis, and extracellular matrix deposition [143]. These effects were observed even when proteinuria was not reduced. The vitamin D Receptor Activator in Albuminuria Lowering (VITAL) study, a multinational, placebo-controlled, double-blind trial, included albuminuric, type 2 diabetic patients receiving ACEI or ARB. It was found that the addition of paricalcitol to RAS inhibition reduced residual albuminuria in patients with diabetic nephropathy [144].
Endothelin-1 (ET-1) is a potent vasoconstrictory peptide with proinflammatory and profibrotic properties that exerts its biological effects through two receptor subtypes, namely ET(A) and ET(B). ET-1 promotes diuresis and natriuresis by local production and action through ET(B) receptors in the renal medulla, whereas activation of ET(A) receptors causes vasoconstriction, mesangial-cell proliferation, extracellular matrix production, and inflammation [145]. Endothelin-receptor antagonists are a promising therapeutic tool for diabetic nephropathy. However, their benefit remains controversial since administration of non-selective endothelin antagonists has been associated with adverse cardiovascular events, including congestive heart failure and fluid overload. Selective inhibition of ET(A) receptors appears not to interfere with the natriuretic, antihypertensive, and ET-clearing effects of ET(B) [146-149]. A recent meta-analysis of five randomized controlled trials on endothelin-receptor antagonists found reduced albuminuria in patients with diabetic nephropathy, but also an increased rate of serious adverse events compared with placebo [150]. It will be necessary to perform well-controlled, adequately powered trials with a longer duration to determine and weigh the potential benefits versus risks of endothelin inhibition in diabetic nephropathy. In an attempt to realize this proposal, the SONAR study, a currently ongoing, large, randomized, clinical trial, aims to determine the efficacy of atrasentan in preventing the progression of diabetic nephropathy [151].
Finally, the phosphate binder sevelamer carbonate, a currently used phosphate binder, was shown to significantly reduce HbA1c, fibroblast growth factor 23, and lipids, and to exhibit anti-inflammatory and antioxidant properties, independently of phosphate level reduction in patients with diabetes and early kidney disease [152]. However, a recent trial did not show any benefits of sevelamer regarding reductions in HbA1c or albuminuria overall in patients with type 2 diabetes and diabetic nephropathy, except for very specific groups of patients [153]. Thus, further studies may be warranted regarding this agent.
8. Conclusions and future challenges
The clinical course of diabetic nephropathy has changed significantly due to improvements in patient diagnosis, follow-up, and treatment. The availability and implementation of major guidelines play an important role in clinical treatment by supporting physicians to make evidence-based clinical decisions. Nowadays, the gold standard of diabetic nephropathy therapy involves intensive treatment of hyperglycemia and hypertension, mostly through RAS blockade. Additional beneficial effects on the pathophysiology of diabetic nephropathy could be ulitized by specific oral hypoglycemic drugs (such as PPAR-γ agonists, SGLT2 inhibitors, and DPP4 inhibitors) and fibrates. These agents are important therapeutic options.
Novel agents, such as bardoxolone methyl, pentoxifylline, PKC inhibitors, sulodexide, pirfenidone, endothelin receptor antagonists, vitamin D supplementation, and phosphate binders, have been associated with controversial results (Figure 2). Athough new insights into the pathogenetic mechanisms involved in diabetic nephropathy, including gene expression and identification of susceptibility loci, have opened new horizons towards novel intervention strategies, there is still a long way to go in the field of research into diabetic nephropathy.
Figure 2. Key elements in the management of diabetic nephropathy with established and potential novel therapeutic agents.
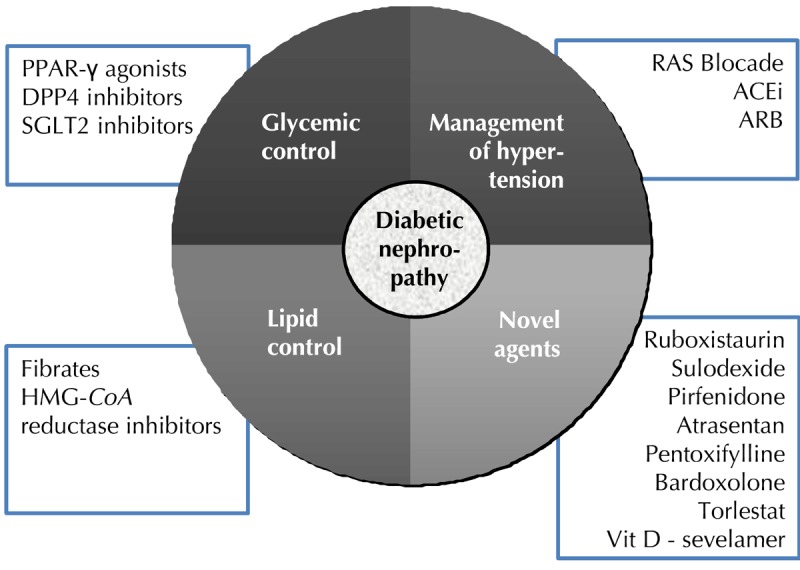
Specific classes of oral hypoglycemic and hypolipidemic agents are associated with renoprotective effects. RAS blockade remains the mainstay of treatment. Novel agents that target different pathophysiologic pathways in diabetic nephropathy are being investigated.
Acknowledgments
Disclosures
The authors report no conflict of interests.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Rossing P. Prediction, progression and prevention of diabetic nephropathy. The Minkowski Lecture 2005. Diabetologia. 2006;49(1):11–19. doi: 10.1007/s00125-005-0077-3. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD. et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64(4):510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Haneda M, Utsunomiya K, Koya D, Babazono T, Moriya T, Makino H, Kimura K, Suzuki Y, Wada T, Ogawa S. et al. A new Classification of Diabetic Nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. J Diabetes Investig. 2015;6(2):242–246. doi: 10.1111/jdi.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molitch ME, Adler AI, Flyvbjerg A, Nelson RG, So WY, Wanner C, Kasiske BL, Wheeler DC, de Zeeuw D, Mogensen CE. Diabetic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes. Kidney Int. 2015;87(1):20–30. doi: 10.1038/ki.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fliser D, Wagner KK, Loos A, Tsikas D, Haller H. Chronic angiotensin II receptor blockade reduces (intra)renal vascular resistance in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(4):1135–1140. doi: 10.1681/ASN.2004100852. [DOI] [PubMed] [Google Scholar]
- 7.Mishra R, Emancipator SN, Kern T, Simonson MS. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int. 2005;67(1):82–93. doi: 10.1111/j.1523-1755.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 8.van den Hoven MJ, Rops AL, Bakker MA, Aten J, Rutjes N, Roestenberg P, Goldschmeding R, Zcharia E, Vlodavsky I, van der Vlag J, Berden JH. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int. 2006;70(12):2100–2108. doi: 10.1038/sj.ki.5001985. [DOI] [PubMed] [Google Scholar]
- 9.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. Clin J Am Soc Nephrol. 2008;19(3):433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 10.Fornoni A. Proteinuria, the podocyte, and insulin resistance. N Engl J Med. 2010;363(21):2068–2069. doi: 10.1056/NEJMcibr1008395. [DOI] [PubMed] [Google Scholar]
- 11.Parving HH. Diabetic nephropathy: prevention and treatment. Kidney Int. 2001;60(5):2041–2055. doi: 10.1046/j.1523-1755.2001.00020.x. [DOI] [PubMed] [Google Scholar]
- 12.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348(23):2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 13.Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010;77(1):57–64. doi: 10.1038/ki.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araki S, Haneda M, Koya D, Hidaka H, Sugimoto T, Isono M, Isshiki K, Chin-Kanasaki M, Uzu T, Kashiwagi A. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes. 2007;56(6):1727–1730. doi: 10.2337/db06-1646. [DOI] [PubMed] [Google Scholar]
- 15.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR UKPDS GROUP. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 16.Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A, Jardine M, Gallagher M, Turnbull F, Chalmers J, Craig J, Huxley R. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. Plos Med. 2008;5(10):e207. doi: 10.1371/journal.pmed.0050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mogensen CE, Vestbo E, Poulsen PL, Christiansen C, Damsgaard EM, Eiskjaer H, Froland A, Hansen KW, Nielsen S, Pedersen MM. Microalbuminuria and potential confounders. Diabetes Care. 1995;18(4):572–581. doi: 10.2337/diacare.18.4.572. [DOI] [PubMed] [Google Scholar]
- 18.Bakris GL. Microalbuminuria: what is it? Why is it important? What should be done about it? J Clin Hypertens (Greenwich) 2001;3(2):99–102. doi: 10.1111/j.1524-6175.2001.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqi FS, Advani A. Endothelial-podocyte crosstalk: the missing link between endothelial dysfunction and albuminuria in diabetes. Diabetes. 2013;62(11):3647–3655. doi: 10.2337/db13-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dwyer JP, Lewis JB. Nonproteinuric diabetic nephropathy: when diabetics don't read the textbook. Med Clin North Am. 2013;97(1):53–58. doi: 10.1016/j.mcna.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 21.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 22.DCCT/EDIC research group. Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol. 2014;2:793–800. doi: 10.1016/S2213-8587(14)70155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DCCT/EDIC Research Group. de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365(25):2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2008;168(22):2440–2447. doi: 10.1001/archinte.168.22.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilous RW, Mauer SM, Sutherland DE, Najarian JS, Goetz FC, Steffes MW. The effects of pancreas transplantation on the glomerular structure of renal allografts in patients with insulin-dependent diabetes. N Engl J Med. 1989;321:80–85. doi: 10.1056/NEJM198907133210204. [DOI] [PubMed] [Google Scholar]
- 26.Fioretto P, Mauer SM, Bilous RW, Goetz FC, Sutherland DE, Steffes MW. Effects of pancreas transplantation on glomerular structure in insulin-dependent diabetic patients with their own kidneys. Lancet. 1993;342:1193–1196. doi: 10.1016/0140-6736(93)92183-t. [DOI] [PubMed] [Google Scholar]
- 27.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 28.Fioretto P, Sutherland DE, Najafian B, Mauer M. Remodeling of renal interstitial and tubular lesions in pancreas transplant recipients. Kidney Int. 2006;69:907–912. doi: 10.1038/sj.ki.5000153. [DOI] [PubMed] [Google Scholar]
- 29.Coppelli A, Giannarelli R, Vistoli F, Del Prato S, Rizzo G, Mosca F, Boggi U, Marchetti P. The beneficial effects of pancreas transplant alone on diabetic nephropathy. Diabetes Care. 2005;28:1366–1370. doi: 10.2337/diacare.28.6.1366. [DOI] [PubMed] [Google Scholar]
- 30.Khairoun M, de Koning EJ, van den Berg BM, Lievers E, de Boer HC, Schaapherder AF, Mallat MJ, Rotmans JI, van der Boog PJ, van Zonneveld AJ, de Fijter JW, Rabelink TJ, Reinders ME. Microvascular damage in type 1 diabetic patients is reversed in the first year after simultaneous pancreas-kidney transplantation. Am J Transplant. 2013;13(5):1272–1281. doi: 10.1111/ajt.12182. [DOI] [PubMed] [Google Scholar]
- 31.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 32.American Diabetes Association GENNID Study Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 33.Shurraw S, Hemmelgarn B, Lin M, Majumdar SR, Klarenbach S, Manns B, Bello A, James M, Turin TC, Tonelli M Alberta Kidney Disease Network. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171(21):1920–1927. doi: 10.1001/archinternmed.2011.537. [DOI] [PubMed] [Google Scholar]
- 34.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172(10):761–769. doi: 10.1001/archinternmed.2011.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold LW, Wang Z. The HbA1c and all-cause mortality relationship in patients with type 2 diabetes is J-shaped: a meta-analysis of observational studies. Rev Diabet Stud. 2014;11(2):138–152. doi: 10.1900/RDS.2014.11.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH Jr. et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ. 2011;343:d6898. doi: 10.1136/bmj.d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Diabetes Association. Standards of medical care in diabetes 2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 39.Diabetic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes. Kidney Int. 87(1):20–30. doi: 10.1038/ki.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gnudi L, Karalliedde J. Beat it early: putative renoprotective haemodynamic effects of oral hypoglycaemic agents. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv093. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calkin AC, Giunti S, Jandeleit-Dahm KA, Allen TJ, Cooper ME, Thomas MC. PPAR-alpha and -gamma agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol Dial Transplant. 2006;21(9):2399–2405. doi: 10.1093/ndt/gfl212. [DOI] [PubMed] [Google Scholar]
- 42.Isshiki K, Haneda M, Koya D, Maeda S, Sugimoto T, Kikkawa R. Thiazolidinedione compounds ameliorate glomerular dysfunction independent of their insulin-sensitizing action in diabetic rats. Diabetes. 2000;49(6):1022–1032. doi: 10.2337/diabetes.49.6.1022. [DOI] [PubMed] [Google Scholar]
- 43.Setti G, Hayward A, Dessapt C, Barone F, Buckingham R, White K, Bilous R, Hiroshi K, Gruden G, Viberti G, Gnudi L. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone prevents albuminuria but not glomerulosclerosis in experimental diabetes. Am J Nephrol. 2010;32(5):393–402. doi: 10.1159/000320129. [DOI] [PubMed] [Google Scholar]
- 44.Bakris G, Viberti G, Weston WM, Heise M, Porter LE, Freed MI. Rosiglitazone reduces urinary albumin excretion in type II diabetes. J Hum Hypertens. 2003;17(1):7–12. doi: 10.1038/sj.jhh.1001444. [DOI] [PubMed] [Google Scholar]
- 45.Bakris GL, Ruilope LM, McMorn SO, Weston WM, Heise MA, Freed MI, Porter LE. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens. 2006;24(10):2047–2055. doi: 10.1097/01.hjh.0000244955.39491.88. [DOI] [PubMed] [Google Scholar]
- 46.Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN, Lasaridis AN. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis. 2010;55(5):835–847. doi: 10.1053/j.ajkd.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal R, Saha C, Battiwala M, Vasavada N, Curley T, Chase SD, Sachs N, Semret MH. A pilot randomized controlled trial of renal protection with pioglitazone in diabetic nephropathy. Kidney Int. 2005;68(1):285–292. doi: 10.1111/j.1523-1755.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 48.Trivedi H, Lu N, Andresen BT, Whaley-Connell A. Slower decline of renal function after initiation of rosiglitazone in diabetics - a pilot study. Clin Nephrol. 2009;72(3):181–185. [PubMed] [Google Scholar]
- 49.Lachin JM, Viberti G, Zinman B, Haffner SM, Aftring RP, Paul G, Kravitz BG, Herman WH, Holman RR, Kahn SE ADOPT Study Group. Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin J Am Soc Nephrol. 2011;6(5):1032–1040. doi: 10.2215/CJN.09291010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 51.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304(4):411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 52.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170(14):1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 53.Turner RM, Kwok CS, Chen-Turner C, Maduakor CA, Singh S, Loke YK. Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78(2):258–273. doi: 10.1111/bcp.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ. 2012;184(12):E675–E683. doi: 10.1503/cmaj.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kornelius E, Chiou JY, Yang YS, Lu YL, Peng CH, Huang CN. The Diabetes Shared Care Program and Risks of Cardiovascular Events in Type 2 Diabetes. Am J Med. 2015 doi: 10.1016/j.amjmed.2015.03.025. In press. [DOI] [PubMed] [Google Scholar]
- 56.Persson P, Hansell P, Palm F. Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf) 2010;200(1):3–10. doi: 10.1111/j.1748-1716.2010.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moriya T, Tsuchiya A, Okizaki S, Hayashi A, Tanaka K, Shichiri M. Glomerular hyperfiltration and increased glomerular filtration surface are associated with renal function decline in normo- and microalbuminuric type 2 diabetes. Kidney Int. 2012;81(5):486–493. doi: 10.1038/ki.2011.404. [DOI] [PubMed] [Google Scholar]
- 58.De Nicola L, Gabbai FB, Liberti ME, Sagliocca A, Conte G, Minutolo R. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am J Kidney Dis. 2014;64(1):16–24. doi: 10.1053/j.ajkd.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Skrtic M, Cherney DZ. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2015;24(1):96–103. doi: 10.1097/MNH.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 60.Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm Metab Res. 2015 doi: 10.1055/s-0034-1395609. In press. [DOI] [PubMed] [Google Scholar]
- 61.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 62.Cherney DZ, Perkins BA. Sodium-glucose cotransporter 2 inhibition in type 1 diabetes: simultaneous glucose lowering and renal protection? Can J Diabetes. 2014;38(5):356–363. doi: 10.1016/j.jcjd.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Avogaro A, Fadini GP. The effects of dipeptidyl peptidase-4 inhibition on microvascular diabetes complications. Diabetes Care. 2014;37(10):2884–2894. doi: 10.2337/dc14-0865. [DOI] [PubMed] [Google Scholar]
- 64.Yamagishi SI, Fukami K, Matsui T. Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc Diabetol. 2015;14(1):2. doi: 10.1186/s12933-015-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lassila M, Cooper ME, Jandeleit-Dahm K. Antiproteinuric effect of RAS blockade: new mechanisms. Curr Hypertens Rep. 2004;6(5):383–392. doi: 10.1007/s11906-004-0058-9. [DOI] [PubMed] [Google Scholar]
- 66.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65(6):2309–2320. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 67.Lambers Heerspink HJ, Gansevoort RT. Albuminuria is an appropriate therapeutic target in patients with CKD: the pro view. Clin J Am Soc Nephrol. 2015 doi: 10.2215/CJN.11511114. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fried LF, Lewis J. Albuminuria is not an appropriate therapeutic target in patients with CKD: the con view. Clin J Am Soc Nephrol. 2015 doi: 10.2215/CJN.10681014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marquez E, Riera M, Pascual J, Soler MJ. Renin-angiotensin system within the diabetic podocyte. Am J Physiol Renal Physiol. 2015;308(1):F1–F10. doi: 10.1152/ajprenal.00531.2013. [DOI] [PubMed] [Google Scholar]
- 70.ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med. 2001;134(5):370–379. doi: 10.7326/0003-4819-134-5-200103060-00009. [DOI] [PubMed] [Google Scholar]
- 71.Newman DJ, Mattock MB, Dawnay AB, Kerry S, McGuire A, Yaqoob M, Hitman GA, Hawke C. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess. 2005;9(30):163. doi: 10.3310/hta9300. [DOI] [PubMed] [Google Scholar]
- 72.Parving HH, Hommel E, Jensen BR, Hansen HP. Long-term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int. 2001;60(1):228–234. doi: 10.1046/j.1523-1755.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- 73.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Remission and regression in the nephropathy of type 1 diabetes when blood pressure is controlled aggressively. Kidney Int. 2001;60 (1):277–283. doi: 10.1046/j.1523-1755.2001.00797.x. [DOI] [PubMed] [Google Scholar]
- 74.Hovind P, Rossing P, Tarnow L, Toft H, Parving J, Parving HH. Remission of nephrotic-range albuminuria in type 1 diabetic patients. Diabetes Care. 2001;24(11):1972–1977. doi: 10.2337/diacare.24.11.1972. [DOI] [PubMed] [Google Scholar]
- 75.Hovind P, Tarnow L, Rossing P, Carstensen B, Parving HH. Improved survival in patients obtaining remission of nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2004;66(3):1180–1186. doi: 10.1111/j.1523-1755.2004.00870.x. [DOI] [PubMed] [Google Scholar]
- 76.Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet. 1997;349(9068):1787–1792. [PubMed] [Google Scholar]
- 77.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bilous R, Chaturvedi N, Sjolie AK, Fuller J, Klein R, Orchard T, Porta M, Parving HH. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med. 2009;151(1):11–20. doi: 10.7326/0003-4819-151-1-200907070-00120. [DOI] [PubMed] [Google Scholar]
- 79.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 80.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351(19):1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 81.Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, Esmatjes E, Gilbert RE, Hunsicker LG, de Faria JB. et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16(10):3027–3037. doi: 10.1681/ASN.2004110919. [DOI] [PubMed] [Google Scholar]
- 82.Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45(2):281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 83.Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snapinn SM, Toto R RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int. 2003;63(4):1499–1507. doi: 10.1046/j.1523-1755.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- 84.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65(6):2309–2330. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 85.Eijkelkamp WB, Zhang Z, Remuzzi G, Parving HH, Cooper ME, Keane WF, Shahinfar S, Gleim GW, Weir MR, Brenner BM, de Zeeuw D. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007;18(5):1540–1546. doi: 10.1681/ASN.2006050445. [DOI] [PubMed] [Google Scholar]
- 86.Remuzzi G, Ruggenenti P, Perna A, Dimitrov BD, de Zeeuw D, Hille DA, Shahinfar S, Carides GW, Brenner BM RENAAL Study Group. Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: a post hoc analysis of the RENAAL trial results. J Am Soc Nephrol. 2004;15(12):3117–3125. doi: 10.1097/01.ASN.0000146423.71226.0C. [DOI] [PubMed] [Google Scholar]
- 87.Kaplan NM. Vascular outcome in type 2 diabetes: an ADVANCE? Lancet. 2007;370(9590):804–805. doi: 10.1016/S0140-6736(07)61304-X. [DOI] [PubMed] [Google Scholar]
- 88.Bakris GL, Berkwits M. Trials that matter: the effect of a fixed-dose combination of an Angiotensin-converting enzyme inhibitor and a diuretic on the complications of type 2 diabetes. Ann Intern Med. 2008;148(5):400–401. doi: 10.7326/0003-4819-148-5-200803040-00012. [DOI] [PubMed] [Google Scholar]
- 89.Holtkamp FA, de Zeeuw D, de Graeff PA, Laverman GD, Berl T, Remuzzi G, Packham D, Lewis JB, Parving HH, Lambers Heerspink HJ. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32(12):1493–1499. doi: 10.1093/eurheartj/ehr017. [DOI] [PubMed] [Google Scholar]
- 90.Patel A ADVANCE Collaborative Group. MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 91.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M. et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 92.Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM. et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 93.Ingelfinger JR. Preemptive olmesartan for the delay or prevention of microalbuminuria in diabetes. N Engl J Med. 2011;364(10):970–971. doi: 10.1056/NEJMe1014147. [DOI] [PubMed] [Google Scholar]
- 94.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61(3):1086–1097. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 95.National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RW, Cooper ME. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321(7274):1440–1444. doi: 10.1136/bmj.321.7274.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song JH, Cha SH, Lee HJ, Lee SW, Park GH, Lee SW, Kim MJ. Effect of low-dose dual blockade of renin-angiotensin system on urinary TGF-beta in type 2 diabetic patients with advanced kidney disease. Nephrol Dial Transplant. 2006;21(3):683–689. doi: 10.1093/ndt/gfi310. [DOI] [PubMed] [Google Scholar]
- 98.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM. et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–1903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 99.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 100.Mann JF, Anderson C, Gao P, Gerstein HC, Boehm M, Ryden L, Sleight P, Teo KK, Yusuf S ONTARGET investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens. 2013;31(2):414–421. doi: 10.1097/HJH.0b013e32835bf7b0. [DOI] [PubMed] [Google Scholar]
- 101.Ren F, Tang L, Cai Y, Yuan X, Huang W, Luo L, Zhou J, Zheng Y. Meta-analysis: the efficacy and safety of combined treatment with ARB and ACEI on diabetic nephropathy. Ren Fail. 2015;37(4):548–561. doi: 10.3109/0886022X.2015.1012995. [DOI] [PubMed] [Google Scholar]
- 102.Persson F, Lewis JB, Lewis EJ, Rossing P, Hollenberg NK, Parving HH. Aliskiren in combination with losartan reduces albuminuria independent of baseline blood pressure in patients with type 2 diabetes and nephropathy. Clin J Am Soc Nephrol. 2011;6(5):1025–1031. doi: 10.2215/CJN.07590810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M. et al. Cardiorenal End Points in a Trial of Aliskiren for Type 2 Diabetes. N Engl J Med. 2012;367(23):2204–2213. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 104.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20(12):2641–2650. doi: 10.1681/ASN.2009070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1(5):940–951. doi: 10.2215/CJN.00240106. [DOI] [PubMed] [Google Scholar]
- 106.Kato S, Maruyama S, Makino H, Wada J, Ogawa D, Uzu T, Araki H, Koya D, Kanasaki K, Oiso Y. et al. Anti-albuminuric effects of spironolactone in patients with type 2 diabetic nephropathy: a multicenter, randomized clinical trial. Clin Exp Nephrol. 2015 doi: 10.1007/s10157-015-1106-2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Esteghamati A, Noshad S, Jarrah S, Mousavizadeh M, Khoee SH, Nakhjavani M. Long-term effects of addition of mineralocorticoid receptor antagonist to angiotensin II receptor blocker in patients with diabetic nephropathy: a randomized clinical trial. Nephrol Dial Transplant. 2013;28(11):2823–2833. doi: 10.1093/ndt/gft281. [DOI] [PubMed] [Google Scholar]
- 108.Sato A. The necessity and effectiveness of mineralocorticoid receptor antagonist in the treatment of diabetic nephropathy. Hypertens Res. 2015 doi: 10.1038/hr.2015.19. In press. [DOI] [PubMed] [Google Scholar]
- 109.ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 111.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 112.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A. et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 113.Toyama T, Shimizu M, Furuichi K, Kaneko S, Wada T. Treatment and impact of dyslipidemia in diabetic nephropathy. Clin Exp Nephrol. 2014;18(2):201–205. doi: 10.1007/s10157-013-0898-1. [DOI] [PubMed] [Google Scholar]
- 114.Tonolo G, Velussi M, Brocco E, Abaterusso C, Carraro A, Morgia G, Satta A, Faedda R, Abhyankar A, Luthman H, Nosadini R. Simvastatin maintains steady patterns of GFR and improves AER and expression of slit diaphragm proteins in type II diabetes. Kidney Int. 2006;70(1):177–186. doi: 10.1038/sj.ki.5001515. [DOI] [PubMed] [Google Scholar]
- 115.Ansquer JC, Foucher C, Aubonnet P, Le Malicot K. Fibrates and microvascular complications in diabetes - insight from the FIELD study. Curr Pharm Des. 2009;15(5):537–552. doi: 10.2174/138161209787315701. [DOI] [PubMed] [Google Scholar]
- 116.Kouroumichakis I, Papanas N, Zarogoulidis P, Liakopoulos V, Maltezos E, Mikhailidis DP. Fibrates: therapeutic potential for diabetic nephropathy? Eur J Intern Med. 2012;23(4):309–316. doi: 10.1016/j.ejim.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 117.Park CW, Zhang Y, Zhang X, Wu J, Chen L, Cha DR, Su D, Hwang MT, Fan X, Davis L, Striker G, Zheng F, Breyer M, Guan Y. PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 2006;69(9):1511–1517. doi: 10.1038/sj.ki.5000209. [DOI] [PubMed] [Google Scholar]
- 118.Wu QQ, Wang Y, Senitko M, Meyer C, Wigley WC, Ferguson DA, Grossman E, Chen J, Zhou XJ, Hartono J, Winterberg P, Chen B, Agarwal A, Lu CY. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPAR gamma, and HO-1. Am J Physiol Renal Physiol. 2011;300(5):F1180–F1192. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 120.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369(26):2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chin MP, Reisman SA, Bakris GL, O'Grady M, Linde PG, McCullough PA, Packham D, Vaziri ND, Ward KW, Warnock DG, Meyer CJ. Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am J Nephrol. 2014;39(6):499–508. doi: 10.1159/000362906. [DOI] [PubMed] [Google Scholar]
- 122.Lin SL, Chen YM, Chiang WC, Wu KD, Tsai TJ. Effect of pentoxifylline in addition to losartan on proteinuria and GFR in CKD: a 12-month randomized trial. Am J Kidney Dis. 2008;52(3):464–474. doi: 10.1053/j.ajkd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 123.Perkins RM, Aboudara MC, Uy AL, Olson SW, Cushner HM, Yuan CM. Effect of pentoxifylline on GFR decline in CKD: a pilot, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2009;53(4):606–616. doi: 10.1053/j.ajkd.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 124.Goicoechea M, García de Vinuesa S, Quiroga B, Verdalles U, Barraca D, Yuste C, Panizo N, Verde E, Munoz MA, Luno J. Effects of pentoxifylline on inflammatory parameters in chronic kidney disease patients: a randomized trial. J Nephrol. 2012;25(6):969–975. doi: 10.5301/jn.5000077. [DOI] [PubMed] [Google Scholar]
- 125.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Chahin J, Mendez ML, Gallego E, Macia M, del Castillo N, Rivero A, Getino MA, Garcia P, Jarque A, Garcia J. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015;26(1):220–229. doi: 10.1681/ASN.2014010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Del-Corso A, Balestri F, Di Bugno E, Moschini R, Cappiello M, Sartini S, La-Motta C, Da-Settimo F, Mura U. A new approach to control the enigmatic activity of aldose reductase. Plos One. 2013;8(9):e74076. doi: 10.1371/journal.pone.0074076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iso K, Tada H, Kuboki K, Inokuchi T. Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in Type 2 diabetic patients. J Diabetes Complications. 2001;15(5):241–244. doi: 10.1016/s1056-8727(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 128.Azal O, Yönem A, Güler S, Cakir B, Baydar A, Corakci A, Kutlu M. Effects of aminoguanidine and tolrestat on the development of ocular and renal structural changes in experimental diabetic rats. Diabetes Obes Metab. 2002;4(1):75–79. doi: 10.1046/j.1463-1326.2002.00182.x. [DOI] [PubMed] [Google Scholar]
- 129.Tammali R, Reddy AB, Srivastava SK, Ramana KV. Inhibition of aldose reductase prevents angiogenesis in vitro and in vivo. Angiogenesis. 2011;14(2):209–221. doi: 10.1007/s10456-011-9206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schemmel KE, Padiyara RS, D'Souza JJ. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. J Diabetes Complications. 2010;24(5):354–360. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 131.Teng B, Duong M, Tossidou I, Yu X, Schiffer M. Role of protein kinase C in podocytes and development of glomerular damage in diabetic nephropathy. Front Endocrinol (Lausanne) 2014;5:179. doi: 10.3389/fendo.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cherney DZ, Konvalinka A, Zinman B, Diamandis EP, Soosaipillai A, Reich H, Lorraine J, Lai V, Scholey JW, Miller JA. Effect of protein kinase C beta inhibition on renal hemodynamic function and urinary biomarkers in humans with type 1 diabetes: a pilot study. Diabetes Care. 2009;32(1):91–93. doi: 10.2337/dc08-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW PKC-DRS, PKC-DMES, PKC-DRS 2 Study Groups. Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol. 2007;2(4):631–636. doi: 10.2215/CJN.00840207. [DOI] [PubMed] [Google Scholar]
- 134.Mehta NN, Sheetz M, Price K, Comiskey L, Amrutia S, Iqbal N, Mohler ER, Reilly MP. Selective PKC beta inhibition with ruboxistaurin and endothelial function in type-2 diabetes mellitus. Cardiovasc Drugs Ther. 2009;23(1):17–24. doi: 10.1007/s10557-008-6144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weiss R, Niecestro R, Raz I. The role of sulodexide in the treatment of diabetic nephropathy. Drugs. 2007;67(18):2681–2696. doi: 10.2165/00003495-200767180-00004. [DOI] [PubMed] [Google Scholar]
- 136.Yung S, Chau MK, Zhang Q, Zhang CZ, Chan TM. Sulodexide decreases albuminuria and regulates matrix protein accumulation in C57BL/6 mice with streptozotocin- induced type I diabetic nephropathy. Plos One. 2013;8(1):e54501. doi: 10.1371/journal.pone.0054501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gambaro G, Kinalska I, Oksa A, Pont'uch P, Hertlova M, Olsovsky J, Manitius J, Fedele D, Czekalski S, Perusicova J. et al. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the Di.N.A.S. randomized trial. J Am Soc Nephrol. 2002;13(6):1615–1625. doi: 10.1097/01.asn.0000014254.87188.e5. [DOI] [PubMed] [Google Scholar]
- 138.Heerspink HL, Greene T, Lewis JB, Raz I, Rohde RD, Hunsicker LG, Schwartz SL, Aronoff S, Katz MA, Eisner GM. et al. Effects of sulodexide in patients with type 2 diabetes and persistent albuminuria. Nephrol Dial Transplant. 2008;23(6):1946–1954. doi: 10.1093/ndt/gfm893. [DOI] [PubMed] [Google Scholar]
- 139.Lewis EJ, Lewis JB, Greene T, Hunsicker LG, Berl T, Pohl MA, de Zeeuw D, Heerspink HL, Rohde RD, Atkins RC. et al. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trial. Am J Kidney Dis. 2011;58(5):729–736. doi: 10.1053/j.ajkd.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 140.RamachandraRao SP, Zhu Y, Ravasi T, McGowan TA, Toh I, Dunn SR, Okada S, Shaw MA, Sharma K. Pirfenidone is renoprotective in diabetic kidney disease. J Am Soc Nephrol. 2009;20(8):1765–1775. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA. et al. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol. 2011;22(6):1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Humalda JK, Goldsmith DJ, Thadhani R, de Borst MH. Vitamin D analogues to target residual proteinuria: potential impact on cardiorenal outcomes. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfu404. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sanchez-Nino MD, Bozic M, Cordoba-Lanus E, Valcheva P, Gracia O, Ibarz M, Fernandez E, Navarro-Gonzalez JF, Ortiz A, Valdivielso JM. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2012;302(6):F647–F657. doi: 10.1152/ajprenal.00090.2011. [DOI] [PubMed] [Google Scholar]
- 144.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 145.Neuhofer W, Pittrow D. Endothelin receptor selectivity in chronic kidney disease: rationale and review of recent evidence. Eur J Clin Invest. 2009;39(Suppl 2):50–67. doi: 10.1111/j.1365-2362.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 146.Lenoir O, Milon M, Virsolvy A, Henique C, Schmitt A, Masse JM, Kotelevtsev Y, Yanagisawa M, Webb DJ, Richard S, Tharaux PL. Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J Am Soc Nephrol. 2014;25(5):1050–1062. doi: 10.1681/ASN.2013020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ritz E, Wenzel RR. Endothelin antagonist as add-on treatment for proteinuria in diabetic nephropathy: is there light at the end of the tunnel? J Am Soc Nephrol. 2011;22(4):593–595. doi: 10.1681/ASN.2011020158. [DOI] [PubMed] [Google Scholar]
- 148.Wenzel RR, Littke T, Kuranoff S, Jürgens C, Bruck H, Ritz E, Philipp T, Mitchell A SPP301 (Avosentan) Endothelin Antagonist Evaluation in Diabetic Nephropathy Study Investigators. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol. 2009;20(3):655–664. doi: 10.1681/ASN.2008050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H. et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25(5):1083–1093. doi: 10.1681/ASN.2013080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuan W, Li Y, Wang J, Li J, Gou S, Fu P. Endothelin-receptor antagonists for diabetic nephropathy: a meta-analysis. Nephrology (Carlton) 2015 doi: 10.1111/nep.12442. In press. [DOI] [PubMed] [Google Scholar]
- 151. https://clinicaltrials.gov/ct2/show/NCT01858532?term=sonar.
- 152.Vlassara H, Uribarri J, Cai W, Goodman S, Pyzik R, Post J, Grosjean F, Woodward M, Striker GE. Effects of sevelamer on HbA1c, inflammation, and advanced glycation end products in diabetic kidney disease. Clin J Am Soc Nephrol. 2012;7(6):934–942. doi: 10.2215/CJN.12891211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yubero-Serrano EM, Woodward M, Poretsky L, Vlassara H, Striker GE AGE-less Study Group. Effects of sevelamer carbonate on advanced glycation end products and antioxidant/pro-oxidant status in patients with diabetic kidney disease. Clin J Am Soc Nephrol. 2015;10(5):759–766. doi: 10.2215/CJN.07750814. [DOI] [PMC free article] [PubMed] [Google Scholar]


