Abstract
There is a global diabetes epidemic correlating with an increase in obesity. This coincidence may lead to a rise in the prevalence of type 2 diabetes. There is also an as yet unexplained increase in the incidence of type 1 diabetes, which is not related to adiposity. Whilst improved diabetes care has substantially improved diabetes outcomes, the disease remains a common cause of working age adult-onset blindness. Diabetic retinopathy is the most frequently occurring complication of diabetes; it is greatly feared by many diabetes patients. There are multiple risk factors and markers for the onset and progression of diabetic retinopathy, yet residual risk remains. Screening for diabetic retinopathy is recommended to facilitate early detection and treatment. Common biomarkers of diabetic retinopathy and its risk in clinical practice today relate to the visualization of the retinal vasculature and measures of glycemia, lipids, blood pressure, body weight, smoking, and pregnancy status. Greater knowledge of novel biomarkers and mediators of diabetic retinopathy, such as those related to inflammation and angiogenesis, has contributed to the development of additional therapeutics, in particular for late-stage retinopathy, including intra-ocular corticosteroids and intravitreal vascular endothelial growth factor inhibitors ('anti-VEGFs') agents. Unfortunately, in spite of a range of treatments (including laser photocoagulation, intraocular steroids, and anti-VEGF agents, and more recently oral fenofibrate, a PPAR-alpha agonist lipid-lowering drug), many patients with diabetic retinopathy do not respond well to current therapeutics. Therefore, more effective treatments for diabetic retinopathy are necessary. New analytical techniques, in particular those related to molecular markers, are accelerating progress in diabetic retinopathy research. Given the increasing incidence and prevalence of diabetes, and the limited capacity of healthcare systems to screen and treat diabetic retinopathy, there is need to reliably identify and triage people with diabetes. Biomarkers may facilitate a better understanding of diabetic retinopathy, and contribute to the development of novel treatments and new clinical strategies to prevent vision loss in people with diabetes. This article reviews key aspects related to biomarker research, and focuses on some specific biomarkers relevant to diabetic retinopathy.
Keywords: diabetic retinopathy, macular edema, electroretinogram, mydriatic retinal photography, AGE
Abbreviations: ACCORD – Action to Control Cardiovascular Risk in Diabetes; ACE – angiotensin-converting enzyme; AGE – advanced glycation end-product; Apo – apolipoprotein; ARIC – Atherosclerosis Risk in Communities; AVR – arterio-venous ratio; CAMs – cell adhesion molecules; CCM – corneal confocal microscopy; CEL – carboxy ethyl lysine; CGM – continuous glucose monitoring; CML – carboxy methyl lysine; CRP – C-reactive protein; CTGF – connective tissue growth factor; CVD – cardiovascular disease; DCCT – Diabetes Control and Complications Trial; DME – diabetic macular edema; DRIL – disorganization of retinal inner layers; EDIC – Epidemiology of Diabetes Interventions and Complications; EDTA – ethylenediamine tetraacetic acid; EET – epoxyeicosatrienoic acid; EPO – erythropoietin; ERG – electroretinogram; ETDRS – Early Treatment of Diabetic Retinopathy Study ; FGF – fibroblast growth factor; FIELD – Fenofibrate Intervention and Event Lowering in Diabetes; FMD – flow-mediated dilation; GC/MS – gas chromatography mass spectroscopy; G-gap – glycation gap; GV – glycemic variability; HGI – hemoglobin glycation index ; HMGB1 – high-mobility group box 1; HMG-CoA – 3-hydroxy-3-methyl-glutaryl coenzyme A; HIF-1 – hypoxia-inducible factor 1; HPLC – high-pressure liquid chromatography; MCP-1 – monocyte chemotactic protein 1; MIF – macrophage migration inhibitory factor; MPO – myeloperoxidase; NMR – nuclear magnetic resonance; OCT – ocular computerized tomogram; PAI-1 – plasminogen activator inhibitor 1; PDR – proliferative diabetic retinopathy; PEDF – pigment epithelium-derived factor; PKC – protein kinase C; PON-1 – paraoxonase 1; RAAS – renin-angiotensin-aldosterone system; RAGE – receptor for AGE; SDF-1 – stromal-derived factor 1; sE-selectin – soluble E-selectin; sICAM-1 – soluble intercellar cell adhesion molecule 1 ; sVAP – soluble vascular adhesion protien; sVCAM-1 – soluble vascular cell adhesion molecule 1; sRAGE – soluble receptor for AGEs; UKPDS – United Kingdom Prospective Diabetes Study; VEGF – vascular endothelial growth factor; VER – visual-evoked response; WESDR – Wisconsin Epidemiologic Study of Diabetic Retinopathy; WNT – wingless-related integration site; WOSCOPS – West of Scotland Coronary Prevention Study
1. Introduction
Diabetes mellitus is an increasingly prevalent, chronic condition characterized by an absolute or relative lack of insulin, as well as hyperglycemia, dyslipidemia, and neurovascular damage. The damage can affect every organ system in the body of patients, impair their quality of life, and cause a burden to the local and global community and economy. Diabetic retinopathy is a common complication in type 1 and type 2 diabetes. The personal and socioeconomic costs of the condition are high. Diabetes is the most common cause of working-age adult onset blindness [1, 2]. The risk of a person with diabetes losing vision is 25-fold that of people without diabetes [2].
Clinical, biochemical, and molecular factors, including genetic and epigenetic factors, contribute to the risk of diabetic retinopathy. Several factors are also therapeutic targets and some, such as pigment epithelium-derived factor (PEDF) [3] and microRNAs [4], may be future therapeutic agents. Knowledge of traditional and novel biomarkers may improve prognostication and the development and personalization of primary and secondary prevention strategies for diabetic retinopathy, including novel even more effective therapeutics.
In this review, we briefly discuss diabetes and diabetic retinopathy, give an overview of biomarkers, and discuss traditional and novel biomarkers in diabetic retinopathy in relation to clinical practice and research.
2. Diabetes mellitus and diabetic retinopathy
The incidence and prevalence of Type 2 diabetes is increasing, predominantly related to greater rates of obesity and sedentary lifestyles. Five to ten percent of all diabetes patients are affected by type 1 diabetes in high-prevalence countries such as Finland [5]. In Africa, the prevalence of type 1 diabetes is difficult to determine because of undersupply with insulin, high mortality, unregistered rural cases, and low adherence to therapy demands [6]. It is anticipated that by 2030 over 80% of people with diabetes will live in underdeveloped countries [1].
Gestational diabetes is another common form of diabetes [5, 7], but as it resolves post-pregnancy, the diabetes duration is too short to cause diabetic retinopathy. However, these women are at high risk for type 2 diabetes, and therefore diabetic retinopathy, later in life.
The different forms of diabetes can be associated with acute metabolic complications, including fluid and electrolyte disturbances, hyperglycemia, hypoglycemia, increased risk of sepsis, and delayed wound healing. The chronic complications of diabetes predominantly relate to vascular damage of the microvasculature in the retina and kidney, accelerated cardiovascular disease, and neural damage, including peripheral and autonomic neuropathy. Diabetic retinopathy has both vascular and neural components; hence biomarkers relevant to both vascular and neural retinal tissues are potentially important. People who develop diabetic retinopathy are also at high risk of other vascular complications, including diabetic nephropathy and cardiovascular disease [8-10]. This may relate to common risk factors for tissue damage such as hyperglycemia, dyslipidemia, obesity, and smoking.
The current global prevalence of diabetic retinopathy is estimated to be 126 million of the 382 million people with diabetes. Thirty seven million people around the world are estimated to have vision threatening diabetic retinopathy [2].
There are various stages of diabetic retinopathy (described in more detail in other articles in this RDS Special Edition). Determination of the relevant stage depends on the absence or presence of the clinical ocular biomarkers of retinal vascular lesions, such as microaneurysms, hemorrhages, soft and hard exudates, edema, and neovascularization. Diabetic retinopathy stages range from normal (or apparently normal) across background diabetic retinopathy (mild, moderate, or severe) to proliferative diabetic retinopathy (PDR), which includes subgrades, such as vision threatening retinopathy and complicated retinopathy, e.g. retinal detachment. The diabetic macular edema component of diabetic retinopathy can occur at any stage of background or proliferative retinopathy, and can be subdivided into focal/center-involved, diffuse/non-center involved, ischemic, and clinically significant categories [11].
In people with type 1 diabetes, diabetic retinopathy is usually not evident until five or more years after onset, but after 20 years of type 1 diabetes 99% have some form of diabetic retinopathy. In people with type 2 diabetes, diabetic retinopathy can be present directly at diabetes diagnosis; this may be due to years of undiagnosed diabetes. By 20 years of type 2 diabetes, about 60% of people have some level of diabetic retinopathy [11]. People with young onset type 2 diabetes are at high risk of diabetic vascular complications, even higher risk than people with similar duration of type 1 diabetes [12-14]. This may relate to the young onset type 2 diabetes patients having more traditional risk factors such as obesity, insulin resistance, dyslipidemia, and hypertension, and related novel risk factors such as (obesity-induced) inflammation.
In a 2012 meta-analysis of almost 23,000 people with diabetes from 35 countries (which did not include Asia), the prevalence of any type of diabetic retinopathy was 35% [15]. The prevalence of PDR was 7%, of diabetic macular edema 7%, and of vision-threatening diabetic retinopathy 10%. Major associates of diabetic retinopathy in this study were diabetes duration, diabetes type (type 1 greater than type 2 diabetes), level of glycemic control, and blood pressure [15]. Due to the high and rising rates of diabetes and challenged/overwhelmed health care systems it is important to develop strategies that address diabetic retinopathy in both affluent and less well-resourced regions.
In more affluent regions, the prevalence of severe diabetic retinopathy has declined [16], despite increasing prevalence of both type 1 and type 2 diabetes. However, the expected number of patients to be screened and treated is not likely to decline. In people with type 1 diabetes, PDR has decreased over 10-fold in people diagnosed before 1975 vs. those diagnosed after 1985 [16]. Better control of risk factors, extended screening, and improved treatments for diabetic retinopathy account for this great clinical improvement [11, 17-19]. These improvements, and hopefully further gains in prediction, prevention, detection, and treatment of diabetic retinopathy, are strongly related to biomarkers. As an example, the recognition of the role of vascular endothelial growth factor (VEGF) in angiogenesis, including retinal neovascularization, led to the development and use of intraocular anti-VEGF therapy for sight-threatening diabetic retinopathy [18, 19]. Unfortunately, not all patients respond well to this treatment. Therefore, other treatments for late-stage diabetic retinopathy are needed.
3. Biomarkers
3.1 Definition
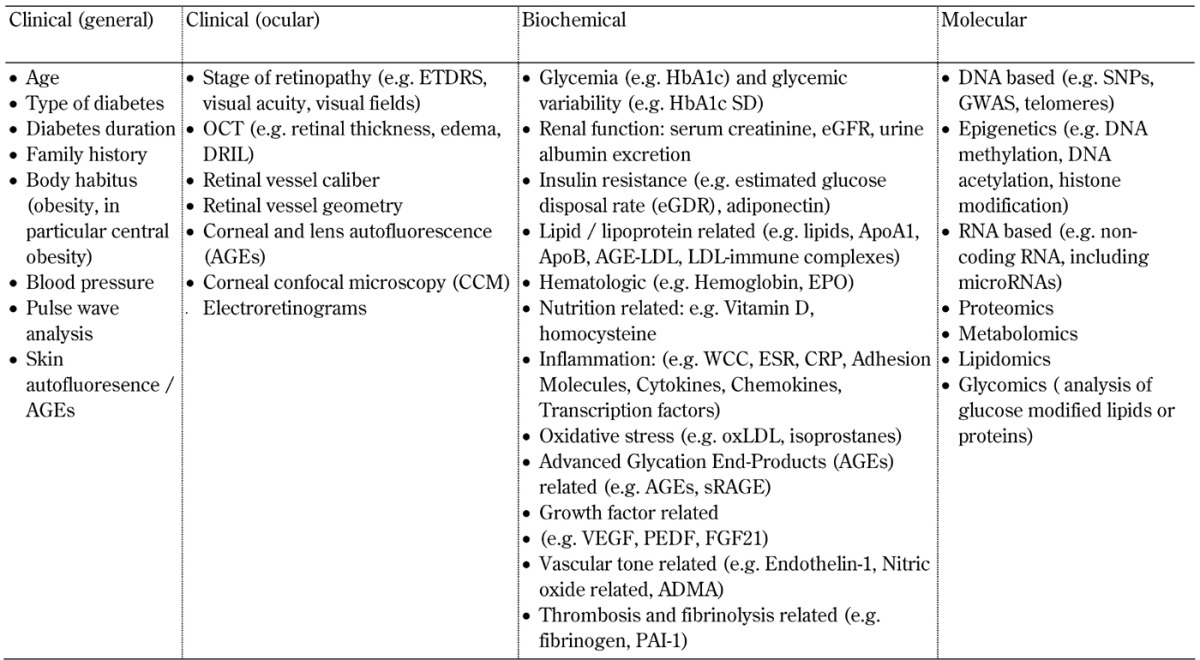
A biomarker can be defined as a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention [20]. Examples of biomarkers in clinical medicine include HbA1c levels, carotid intima media thickness, serum creatinine levels, Early Treatment of Diabetic Retinopathy Study (ETDRS) score, and genotypes or tumor markers such as for breast cancer. Diabetic retinopathy-related biomarkers, including those in clinical practice and research, are summarized in Table 1.
Table 1. Traditional and novel biomarkers related to diabetic retinopathy differentiated by clinical, biochemical, and molecular categories.
3.2 Differences between a biomarker, a risk factor, and a determinant
The terms biomarker, risk factor, and determinant are often used interchangeably. The World Health Organization defines a risk factor as any attribute, characteristic, or exposure of an individual that increases the likelihood of developing a disease or injury. These factors include for example smoking, hypertension, and hyperglycemia. The first use of this term traced back to cardiovascular epidemiologist Dr. William Kannel in a 1961 paper [21]. Sometimes, the term "determinant" is used, meaning a variable that is associated with either increased or decreased risk. As an example: while high HDL-C levels are associated with lower risk of cardiovascular disease, and therefore are considered a "determinant", low HDL-C levels constitute a "risk factor". Some risk factors can be associated with a reduction of disease severity, but not all risk factors can be altered; the latter include age and diabetes duration.
3.3 Types of biomarkers
Biomarkers and risk factors can be subdivided in various ways. Commonly there are traditional and novel biomarkers and risk factors. Traditional biomarkers are those that are well established in clinical practice and research. Novel biomarkers are generally regarded as those of interest. They are possibly associated with or predictive of the disease or its response to treatments, but are neither proven, nor widely accepted or used in clinical practice.
Traditional and novel risk factors may relate to the same category and the same process. For example, HbA1c, a measure of glycemic control, is strongly associated with diabetic retinopathy, and is a traditional risk factor [22]. Other aspects of glucose control include glycemic variability and alternate assays, e.g. to determine the level of plasma 1'5 anhydroglucitrol [23], a dietary monosaccharide excreted in urine. These aspects may also be regarded as biomarkers, but they are not well studied in relation to diabetic retinopathy. They are thus regarded as novel biomarkers. The development of new biomarkers is a refinement process depending on the degree of etiological disclosure. A similar process can be observed for lipid levels. Whilst high total and LDL-cholesterol (LDL-C), triglycerides, and low HDL-C levels are traditional risk factors for cardiovascular disease, and possibly also for diabetic retinopathy [15, 24], lipoprotein subclass profiles based on lipoprotein particle size [25], determined by nuclear magnetic resonance (NMR) and measures of circulating oxidized LDL [26], are more recently developed, and may thus be regarded as novel biomarkers.
Biomarkers and risk factors can also be categorized based on the type of test, which may include the following:
Clinical tests, biochemical markers, and molecular factors, with the latter including genetic and epigenetic markers [4].
'Omics' studies, including proteomics [27, 28] and lipidomics [29], which are beginning to be applied to diabetic retinopathy.
While individual markers are sometimes used in clinical practice, a panel of biomarkers, including biomarkers from different categories, is used on other occasions. For example, many cardiovascular risk calculators use clinical factors such as age, presence or absence of diabetes, smoking status, and blood pressure, and they use biochemical factors such as cholesterol levels as well. Similar to nephropathy and cardiovascular disease, retinopathy has multiple risk factors. It is thus likely that a panel of biomarkers rather than an individual biomarker will prove useful in clinical research and practice. Such multi-biomarker panels are already used in some countries for cardiovascular disease [30], and panels using clinically available biomarkers have been recently suggested for a composite of diabetic micro- and macrovascular complications [31]. Retinopathy-specific risk algorithms suitable for use in diverse patient groups are highly desirable. It will be important to determine the predictive value of additional parameters, particularly those that are not readily available and are costly, such as the results of electroretinograms (ERGs), genome wide association studies (GWAS), and microRNA profiles (discussed below).
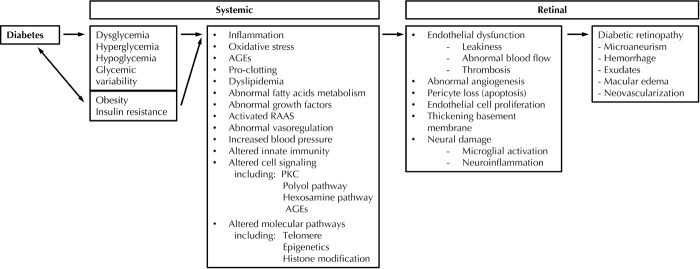
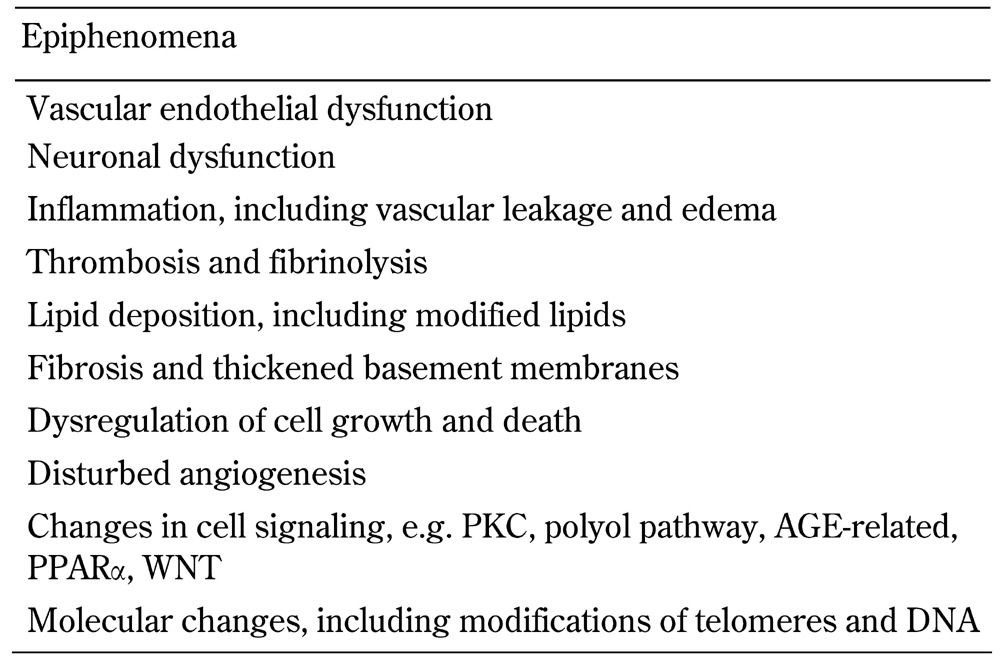
The pathway linking diabetes, risk markers, and mediators with diabetic retinopathy is shown in Figure 1. Examples of clinical, biochemical, and molecular biomarkers of proven or potential relevance to diabetic retinopathy are shown in Table 1. These biomarkers are related to traditional risk factors for diabetic retinopathy, shown in Table 2 (including the major risk factors of poor glycemic control, longer diabetes duration, hypertension, and smoking), and to the pathophysiology of diabetic retinopathy, shown in Table 3 (including vascular and neural dysfunction, inflammation, oxidative stress, and dyslipoproteinemia). Central to many of the processes promoting diabetic retinopathy is hyperglycemia, which is a major risk factor for both onset and progression of diabetic retinopathy (discussed further below). Whilst effective at reducing the development and progression of diabetic retinopathy [22], tight glycemic control is often difficult to achieve in clinical practice in both type 1 and type 2 diabetes.
Figure 1.
Systemic and retinal biomarkers for diabetic retinopathy.
Table 2. Traditional risk factors for diabetic retinopathy.
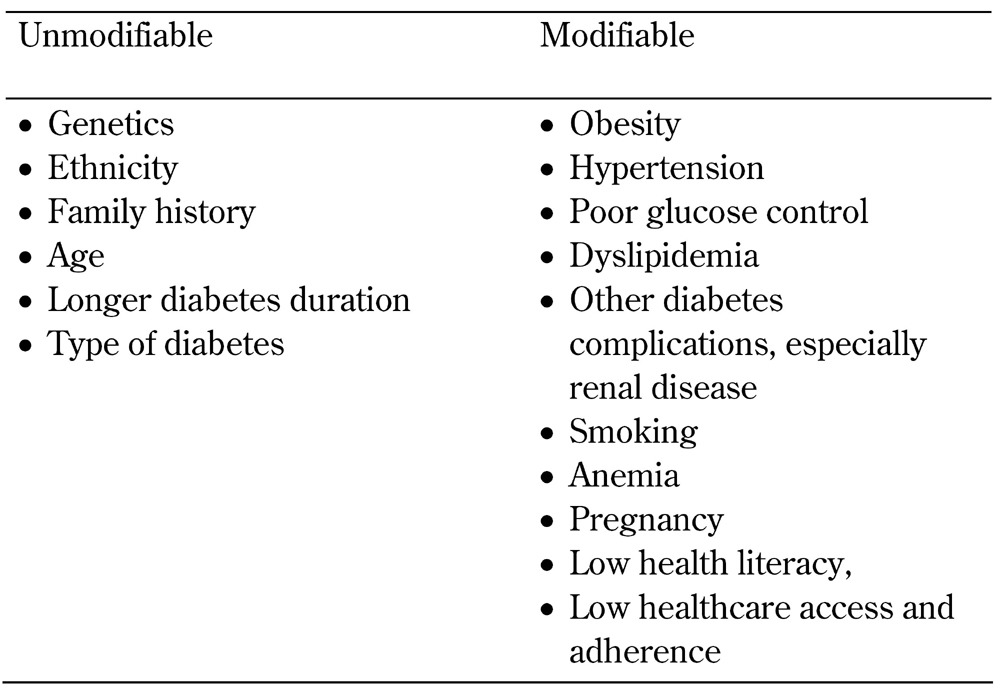
Table 3. Pathophysiology of diabetic retinopathy.
3.4 Why use biomarkers?
The reasons for using biomarkers are summarized in Table 4, and discussed below. Whilst major risk factors for the development and progression of diabetic retinopathy are available (summarized in Table 2), well-proven tools for early diagnosis and prediction of overt disease are currently missing. The latter also applies to the determination of who will respond to a specific treatment or combinations thereof. Validated biomarkers for diabetic retinopathy would likely facilitate its early diagnosis, and enable early treatment. Biomarkers may also guide treatment choice. This includes the identification of subgroups of patients with diabetes and retinopathy according to their different responses to treatment such as intraocular anti-VEGF treatment. There may be three subgroups represented by those who respond either well, negatively, or not at all. This use of biomarkers is a common practice in some areas of medicine, in particular oncology [32], but not yet in diabetes.
Table 4. Reasons to use biomarkers.
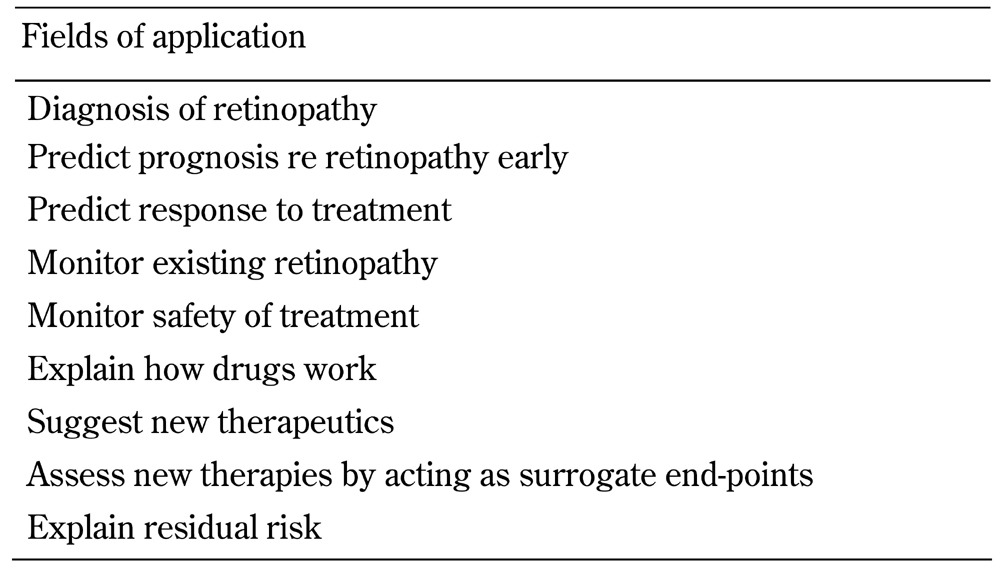
The early prediction of diabetes complications is desired by people with diabetes, clinicians, and health care providers. The use of multiple factors, including clinical, biochemical, and molecular components, will likely better predict diabetic retinopathy. It will also determine the need for therapy and predict the response to therapies. The capacity to accurately target treatments may improve treatment-effect size, and reduce the risk of individuals who may not benefit from certain therapies as they may undergo treatments with potential side-effects, inconvenience, and cost. Greater knowledge of biomarkers will enhance the understanding of the mechanisms of retinopathy and of effective treatments, explain residual risk, and facilitate disease monitoring.
It has been calculated that the major clinical risk factors for diabetic retinopathy explain only a fraction of the variation in the risk of retinopathy. For example, in the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) Study, HbA1c, blood pressure, and total cholesterol only accounted for about 10% of the variability in retinopathy risk [33]. However, it has been suggested that biomarkers related to these major retinopathy risk factors can be used to guide retinopathy screening intervals so as to increase the cost-efficiency of this resource. Recent reviews support the extension of retinal screening intervals from the generally recommended annual review to every two years for people with well-controlled type 2 diabetes and no diabetic retinopathy [34, 35]. Also, personalized screening intervals have been suggested. These should be estimated using a mathematical algorithm considering gender, type and duration of diabetes, glycemic control, blood pressure, and current retinopathy grade [36].
Biomarkers and related basic science studies are important to identify molecular pathways in disease and therapy; they have been major contributors in the development of anti-VEGF treatments. Biomarkers can also be used in the development and testing of novel therapeutics, including surrogate end-points and safety tests.
4. Challenges in the development and use of biomarkers
The following factors need to be considered in the detection and evaluation of (new) biomarkers for diabetic retinopathy:
Velocity of disease development in individual patients
Variations in phenotyping
Subclinical ocular damage
Metabolic memory
Usefulness of biomarkers to be confirmed in independent populations
Predictors at population level may not apply to all individuals
Large-scale studies are frequently needed, e.g. for genetic investigations
Microenvironments at tissue, cellular, and molecular level
Readily accessible tissues (blood, urine) are not the site of disease
Dissecting cause and effect, causative or epiphenomenon
Funding for biomarkers often low priority
Assay availability, cost, quality
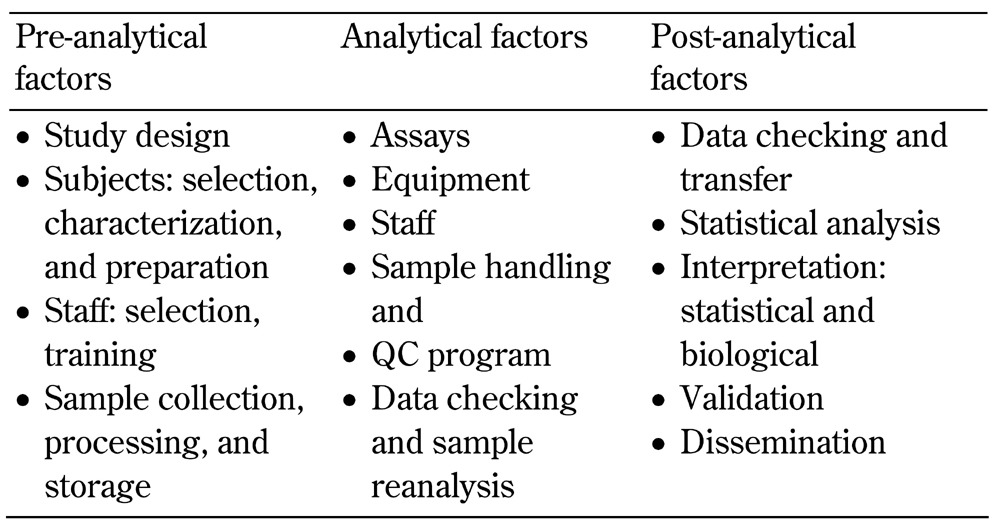
Assay related factors: pre-analytical, analytical, post-analytical (see Table 5)
Table 5. Assay-related factors that can impact biomarker levels.
4.1 Multiple risk factors and diagnostic criteria
A major challenge in the use of biomarkers is that multiple risk factors and pathways impact the development and progression of diabetic retinopathy (Figure 1, Tables 1-3). Hence, multiple component biomarker panels may be more useful than separate biomarkers.
There are also variations in the criteria for diagnosis and staging of diabetic retinopathy. Adequate, detailed, and ideally the same diagnostic and staging criteria should be used. Variations may exist based on the instrumentation used for retinal imaging (e.g. standard or wide-screen retinal cameras [37, 38], mydriatic or non-mydriatic views, optical coherence tomography (OCT) [39, 40]) and for staging of diabetic retinopathy.
4.2 Slow development of diabetic retinopathy
Another challenge is the slow development of diabetic retinopathy; it may proceed over years and decades. There is likely retinal vascular and neural damage before overt diabetic retinopathy becomes clinical evident by the appearance of microaneurysms and exudates or obvious retinal edema detected by ophthalmoscopy or retinal photography evaluation. Tests that prove useful in clinical practice include:
4.3 Subclinical retinal damage and metabolic memory
Subclinical retinal damage can have late adverse consequences. Therefore, there is a need for early-detection biomarkers of diabetic retinopathy. The diabetic dog experiments by Engerman and Kern provide a rationale for the new biomarkers for early detection [43]. After 2.5 years of poor glycemic control, at a time when there was no evident retinopathy, the diabetic dogs were switched to good glycemic control for a further 2.5 years. At the five year time point, these dogs had severe diabetic retinopathy, just as severe as those dogs that had had poor glucose control throughout [43]. This phenomenon is known as metabolic memory or the 'legacy effect'. It refers to the phenomenon by which the body’s tissues, including the retina, continue to respond to poor or good glycemic control for years after the glucose control has improved or worsened. Our human clinical studies, discussed later in this article, showed that retinal vessel caliber in 'normal' retina in children and adolescents with type 1 diabetes were predictive of the subsequent appearance of microaneurysms.
Metabolic memory may modulate the relationship between biomarkers and clinical status. Due to the clinical importance of metabolic memory, biomarkers for this phenomenon are of intensive research interest. Tissues shown to be susceptible to glucose-induced memory effects include retinae, kidneys, nerves, and arteries [44, 45]. In humans, metabolic memory of glycemia was first observed in type 1 diabetes by the DCCT/EDIC study [46-48] and in type 2 diabetes by the 10-year post-completion UKPDS study [49]. In the DCCT/EDIC study, metabolic memory for intensive diabetes management, mainly reflecting a 2% lower HbA1c level for a mean of 6.9 years, has been shown to protect against the development of diabetic retinopathy for over a decade after the study end and for need for ocular surgery 15 years later [46-48]. Biomarkers related to metabolic memory undergoing investigation include advanced glycation end-products (AGEs) [50] and epigenetics [51, 52].
Follow-up of other clinical trials suggest that there is vascular metabolic memory for lipids, blood pressure, and glycemia [53]. Due to metabolic memory and subclinical retinal damage biomarkers must be assessed in and proven relevant to different stages and types of diabetes.
4.4 Usefulness of biomarkers in independent populations
The usefulness of a biomarker in diverse populations needs to be demonstrated. There may be differences in the association between biomarkers and diabetic retinopathy in people with type 1 and type 2 diabetes, and between people at different stages of diabetes, of different ages, ethnicities, and gender. Comorbidities, medications, and environmental factors may also alter biomarker levels and their relationship with diabetic retinopathy. A comorbidity of particular relevance to diabetic retinopathy is that of nephropathy as the two microvascular complications are clinically related, in that patients with retinopathy are at greater risk of nephropathy, and also of cardiovascular disease [8-10]. Usually, some level of renal damage, such as hyperfiltration and/or increased albuminuria, is concurrent with diabetic retinopathy. The presence of renal disease, even at an early stage, is associated with multiple adverse changes in vascular disease risk factors. These changes include those related to lipoproteins, clotting and fibrinolysis, and inflammatory factors, particularly those produced by the liver [54-56]. This may relate to a compensatory increase in albumin and other proteins synthesized by the liver to preserve plasma oncotic pressure in the face of albuminuria. Statistical adjustment for nephropathy may ‘lose’ important relationships between biomarkers for retinopathy due to their strong relationship and collinearity.
4.5 Predictors at population level may not apply to all individuals
Another problem arises from the evaluation of biomarkers in large studies, namely that predictors at population level may not be applicable to individuals. Biomarkers relevant to both are important to achieve improved outcomes related to diabetic retinopathy. However, large scale studies are needed, particularly for the assessment of genotypes related to diabetic retinopathy. International collaborations are usually essential. Such genome wide association studies (GWAS) have accelerated identification of retinopathy-related genes [57-60].
4.6 Microenvironments at tissue, cellular, and molecular level
There are many different microenvironments in the body; every organ and tissue can have its own microenvironment, including blood and cells. A biomarker may be present at multiple sites, and its relationship with retinopathy status may differ. For example, circulating levels of PEDF are increased in subjects with compared to those without proliferative retinopathy [61, 62], but PEDF levels in ocular tissue are decreased in people with late stage diabetic retinopathy relative to those without retinopathy [63-65].
4.7 Readily accessible tissues and the site of disease
Another issue in the selection of suitable biomarkers is that relevant tissues for retinopathy evaluation may not be readily accessible. Fortunately, the retinal vasculature is accessible to visualization, and modern cameras are increasing the available field of view. OCTs [40], now in clinical use, also enable a detailed assessment of retinal structure and the presence of edema. Clinical research tools include ocular and skin autoflourescence measures, which reflect AGEs [66, 67]. Many studies have shown these non-invasively assessed skin AGEs to be independently associated with diabetic retinopathy [66-72]. Another non-invasive clinical tool can evaluate nerve structure in the cornea (by corneal confocal microscopy), which may relate to retinal health [39]. ERGs that test retinal neural function are mostly used in clinical research [42], but a portable ERG device that can detect sight-threatening diabetic retinopathy has recently been validated and approved for use in clinical practice [73].
4.8 Relevant tissue accessibility and assay availability
Blood and urine are most frequently used for biomarker analyses, but the relevant biomarkers may not be present or detectable, or its relationship with retinal status may differ from that in the retina, as mentioned previously for PEDF. As another example, increased intraocular VEGF is strongly implicated in macular edema and retinal angiogenesis, yet circulating levels are at or below the limit of detection of many ELISAs.
Vitreous fluid is sometimes available for clinical research related to biomarkers [65, 74]. More recently tears, which are more clinically accessible, are being used to assess biomarkers related to diabetic retinopathy [27, 75-77]. Further clinical studies are of great interest.
4.9 Dissecting cause and effect
Even when an association between diabetic retinopathy risk or status and a biomarker is identified, it can be difficult to discern cause and effect, or whether the observation is an epiphenomenon. This is where longitudinal studies and relevant basic science studies can be helpful.
5. Factors that may affect biomarker assay results
There are multiple assay-related factors, including pre-analytical, analytical, and post-analytical factors (discussed below and summarized in Table 5). These factors need to be carefully considered in biomarker studies.
5.1 Pre-analytical factors
Study design must be considered as to the kind of samples and their time and manner of collection, and where and how they are to be analyzed. For example, if a longitudinal study is planned, then sample stability over the required storage time must be considered. Local versus central site analyses must be considered at the study design stage to ensure sample stability during shipping and storage, and to avoid assay differences between sites. Power calculations should be performed prior to the study to ensure adequate sample size. Given the potential for environment and treatments to alter molecular markers, consent for repeated collection of samples for genetic analyses should be obtained. With the rapid rate of development of new analytical techniques for biomarkers, studies should ideally include the collection of additional samples for storage and future analyses. These additional samples also can provide a reserve in the case that samples for pre-specified biochemical or molecular biomarker analyses are lost, which may happen during transport or because of instrument malfunction.
Factors related to subject selection and characterization are also important. For example, study participants need to be well characterized according to pre-specified and agreed criteria such as:
Type of diabetes
Age
Diabetes duration
Definitions of diabetes complications
Concurrent conditions/diseases
Medication (including over-the-counter drugs)
Ethnicity
Inclusion and exclusion criteria
Also, adequate details of complication definitions should be reported so as to enable comparisons with other studies.
Study staff is another key-factor as the collection of the relevant clinical information and samples determined in the protocol requires skills and training. The staff also needs to be observant and detail-orientated regarding subject preparation and sample handling. Sample collection, processing, transport, and storage can impact biomarker results.
The right sample must be collected, e.g. retinal photo field as well as venous, arterial, or capillary blood. Venous occlusion time for 1-3 minutes, which can occur if the phlebotomist is having difficulty finding a vein or many blood samples must be collected, can increase levels of even 'simple' tests such as lipids and protein levels by 5-10%. The first of many blood tubes will be most 'blood like', with differences between tubes of the same analyte being 5-15% [78]. Fist pumping to increase blood flow during sample collection can lower sample pH and increase potassium and lactate levels. Hemolysis and severe hypertriglyceridemia can interfere with some biomarker assays [79].
Collection tubes must be in-date, so any preservatives are active, and of the right type. For example, EDTA, a commonly used anti-coagulant and antioxidant for plasma collection, binds calcium, so that it cannot be used for assays that are calcium-dependent, such as paraoxonase activity [80]. Lithium heparin, sometimes used for blood/plasma collection, is not appropriate for biomarkers to be analyzed by PCR as heparin inhibits PCR reactions [81]. Blood samples to generate serum need to be stored in serum type collection tubes, and be given long enough, usually at room temperature, to clot; otherwise a more plasma-like sample is obtained.
Centrifugation should be performed at appropriate g-force, time, and temperature. If blood is not spun at a speed high enough or long enough less plasma or serum will be yielded, and also cellular components such as platelets may not be fully pelleted and removed from the fluid to be analyzed. For example, platelets contain large amounts of PAI-1, so incompletely spun plasma or serum will give an artifact of a very high levels of PAI-1 if residual platelets, which lyse on thawing, are included [82, 83].
Samples should be aliqouted into in clearly labeled freezer-safe or liquid nitrogen-safe tubes of appropriate size and material, and frozen, usually at -70°C or below until thawed for analysis. Attention to sample shipping temperatures will prevent inadvertent freeze-thaws. Inventories of the samples collected, any issues with their collection, and their storage site should be kept. There is commercially available software which can be purchased for this purpose, but for smaller studies, properly recorded in-house spreadsheets can by sufficient.
5.2 Analytical factors
Once in the laboratory, many factors influence biomarker levels such as the type of assay chosen. Ideally, the assay will have been well validated against the gold standard, and assay performance and reliability for the sample type and storage time optimized. For example, the gold standard for quantifying the AGE carboxy methyl lysine (CML) is gas chromatography - mass spectroscopy (GC/MS), yet many groups use ELISA for convenience, availability, and cost purposes. However, ELISA has greater cross-reactivity with non-CML AGEs and potential other products [84].
Also, the instruments used can impact biomarker levels. For example, we recently published a comparison of five different platforms to detect and quantify a microRNA signature for diabetic retinopathy. The different platforms were associated with different sensitivities, sample volumes required, and cost per sample [85]. Maintenance should be kept up to date as poorly maintained or faulty instruments can generate erroneous results.
Sample handling both before and during assays can impact biomarker levels. Sample handling factors include the following aspects:
Sample storage conditions and duration
Thawing protocol
Whether samples were centrifuged after thawing to pellet and remove debris and cryoprecipitate
Number of previous freeze thaw processes
Time and temperature at which a sample is left thawed prior to assays
For example, in our laboratory, plasma Ox-LDL levels by ELISA increased significantly after three freeze thaw cycles, whilst serum paraoxonase 1 (PON-1) activity was stable for at least eight freeze thaw cycles.
The following assay-related factors are also important:
Intra- and inter-assay coefficients of variation (CVs)
Assay controls
Whether there are any effects caused by the sample position in the assay run, e.g. some ELISA kits have an ‘edge effect’ with different absorbance rates in wells on the edge of the plate [86].
A good biomarker laboratory will have characterized these aspects of each of its assays, and should make such knowledge available to their collaborators.
Acceptability of each assay result, including standard curve, controls, and repeatability, should be determined at the time the assay is in process. Any high-level samples above the standard curve range should be reanalyzed in a greater dilution, and technically unsuitable samples, or those data points not included in the subsequent data analysis, should be reanalyzed where possible. Kit lot numbers, operators, and comments regarding potential confounders such as hemolysis or lipemia should be recorded, and transferred into the database.
5.3 Post-analytical factors
Care must be taken in data checking, particularly if there has been manual data entry rather than electronic file transfer. Assay drift over time, best seen from tracking of the controls, and any differences between operators and kit batches should be assessed.
Robust statistical techniques should be used, ideally with data validation by a separate statistician. With modern analytical techniques such as GWAS, proteomics, and metabolomics, skilled bioinformaticians and special software are needed. The data must then be interpreted, not just in the statistical context, but also in the biological context. The following statistical measures are of relevance to biomarkers (or biomarker panels):
Sensitivity: the proportion of individuals with a disease (e.g. diabetic retinopathy) who test positive for that particular biomarker. This is also known as the true positive rate.
Specificity: the proportion of subjects without the disease who test negative. This is also known as the true negative rate.
Positive predictive power: the proportion of subjects with a positive test who are correctly diagnosed.
Negative predictive power: the proportion of subjects with a negative test who are correctly diagnosed.
Relative risk or risk ratio (RR): the ratio of the probability of an event (e,g, developing retinopathy) occurring in an exposed group to the probability of the event occurring in a comparison, non-exposed group.
The results must be disseminated, and ideally, validated in other subject groups.
5.4 Funding and biobanking
A very practical consideration in biomarker analyses is its funding in clinical research, and once validated, in clinical practice. With increasingly sophisticated instrumentation and assays, and generally declining medical research funding, it can be difficult to obtain funding for biomarker-related studies.
Whilst biobanking of suitable samples is not cost-free, it should be considered in clinical studies and trials for subsequently funded biomarker studies. Biobanking can also help ‘future-proof’ research by providing relevant data and samples in a time- and cost-effective manner for analysis of biomarkers by techniques that may not have existed at the time of the original study. For example, many of the specific biomarkers evaluated in the DCCT and FIELD studies, and some of the analytical techniques used, did not exist at the time of planning and conducting of these trials.
Collaborations should be considered so as to increase research study sample size, and therefore statistical power, and to facilitate access to analytical platforms or expertise, thus expediting progress in diabetic retinopathy related research.
Finally, for a biomarker to be used in clinical practice, the assay must be widely available, acceptable to patients, reproducible, and cost-effective.
6. Characteristics of a reliable biomarker
A reliable biomarker or biomarker panel should predict retinopathy risk or response to treatment very early, and with low false positive and false negative rates. The biomarkers should be valid in men and women of different ages, ethnicities, types, and stages of diabetes, and in diverse health states, diets, and drug treatments. The required samples must be easily attainable, stable when stored, and reproducible with different operators and instruments. Any required instrument or analyst must be widely available, and the test must be affordable.
7. Biomarkers of interest in diabetic retinopathy
Having considered important aspects of selection, use, and interpretation of biomarkers in research and in clinical practice above, we will now discuss several classes of biomarkers and individual biomarkers relevant to diabetic retinopathy research and clinical practice.
7.1 Ocular-based biomarkers
Vision. Visual acuity and visual fields are biomarkers of diabetic retinopathy, though they are usually not abnormal until the latest stage of the disease.
Classification of retinopathy. The absence or presence, type, and severity of retinal vessel lesions diagnosed by ophthalmoscopy or by mydriatic or non-mydriatic retinal photography are biomarkers of diabetic retinopathy status. These markers are used in routine clinical practice and in research [34, 87-89]. Diabetic retinopathy may be asymptomatic for years, even at an advanced stage, so screening is essential to identify, monitor, and guide the treatment of retinopathy.
There are various retinal imaging and grading systems, equivalent to different biomarker 'assays'. New wide-angle imaging systems using scanning laser ophthalmoscopes can visualize up to 200 degrees (82%) of the retina; this improves coverage of the mid and peripheral retina and offers improved prognostic value in diabetic retinopathy over conventional 7-field ETDRS photography [90, 91]. Using wide-angle fluorescein angiography, correlations between peripheral retinal ischemia and diabetic macular edema have been identified [89, 91], which may have therapeutic implications. A systematic review and meta-analysis of publications supports the use of digital retinal imaging for diabetic retinopathy screening, preferably mydriatic imaging with 100-200° views [92].
Blood flow changes. Commonly described, early functional defects in diabetes are altered retinal blood flow and loss of normal autoregulatory capacity. Whilst first recognized in the 1930's, this observation was validated and extended by Kohner in the 1970's only. Kohner suggested that blood flow abnormalities were pathological, and an early biomarker for progression of diabetic retinopathy [93-95]. Using more modern techniques, such as Doppler flow velocity waveform analysis, even earlier changes in blood flow have been identified, prior to the onset of clinically overt retinopathy, and even in prediabetic (impaired glucose tolerance) subjects [96]. Decreased total retinal blood flow and arteriolar vasoconstriction have been confirmed by several other groups [97-99]. Later in the retinal disease process, retinal arterioles dilate, which causes increased blood flow [98, 100] and accelerated progression to diabetic macular edema (DME) and PDR [101]. Increased blood pressure induced by exercise, acute hyperinsulinemia, and the degree of diabetic retinopathy present can alter retinal vessel vasodilatory capacity, which can be measured at rest or after exercise [102-104].
Retinal vessel flicker response and blood flow. Brachial artery flow-mediated dilation (FMD) in response to several minutes of arm ischemia induced by a blood pressure cuff is a functional measure of systemic arterial function. The measure has been associated with atherosclerosis and cardiovascular disease, and has been found to be predictive of these diseases. Brachial artery FMD and related abnormalities detected by pulsewave analysis are usually decreased in people with diabetes compared to those without diabetes [105, 106]; this is thought to be related to impaired nitric oxide (NO) bioavailability in diabetes patients [107]. A similar phenomenon can be observed in retinal vessels, with flickering light applied as the stressor. Vasodilation is a normal physiological response to flickering light, and leads to increased blood flow, providing additional oxygen and nutrients to meet the increased requirements of metabolically active cells. Retinal hyperemia in response to flickering light is regarded as a function of ganglion cell activity and NO release. Several groups have shown the retinal flicker response to be reduced in type 1 and type 2 diabetes per se [108, 109], and to be progressively worse with more severe diabetic retinopathy [110]. The flicker response may also be affected by blood pressure, acute hyperinsulinemia, and level of diabetic retinopathy [103].
The flicker response has also been demonstrated to correlate with altered retinal neural function detected by electroretinogram [111], and with changes in retinal vessel caliber [112].
We have used the retinal flicker response as a biomarker of drug-induced changes in prostaglandins and epoxyeicosatrienoic acids (EETs) in adults with diabetes, but we did not find any statistically significant correlations [113]. We are not aware of any longitudinal studies relating retinal flicker responses and subsequent diabetic retinopathy outcomes.
Oxygen saturation in retinal vasculature. The non-invasively quantified oxygenation of systemic blood is clinically used, particularly in intensive care and emergency departments. Oxygenation of blood in retinal vessels can also be quantified non-invasively based on the differential light absorbance of oxyhemoglobin and deoxyhaemoglobin [114, 115]. In human cross-sectional studies, oxygen saturation has been found to be higher in retinal venules of diabetic than non-diabetic subjects [114, 116, 117], and to decline further with increasing severity of diabetic retinopathy [114, 117]. This may relate to loss of metabolically active retina, anatomical damage to retinal vessels, and arterio-venous shunt formation in the retina, all of which reducing oxygen consumption. It would be interesting to evaluate retinal oxygenation as a biomarker for the progression of retinopathy, and response to treatments such as fenofibrate, in longitudinal studies. Whilst dynamic tools such as retinal blood flow and oxygenation can be useful clinical research tools and biomarkers of response to stressors and therapeutics, retinal vessel caliber, assessed by retinal photography, which is commonly used for diabetic retinopathy screening and management, has greater potential to be used as a biomarker in clinical practice. This is due to easy accessibility of the site of evaluation, cost-effectiveness of the diagnostic procedure, and low specialization of the workforce needed for retinal photography and retinal caliber measurement.
Retinal vessel caliber. Retinal arterial and venous caliber and their ratio, even in the presence of an apparently normal retina, are potential early biomarkers of subsequent risk for diabetic retinopathy, and also for diabetic nephropathy [41, 118, 119]. Relationships between retinal vessel characteristics such as retinal venule caliber were reported as a biomarker of subsequent vision loss 30 years ago [120]. However, due to challenges in the precise measurement of retinal vessel caliber then, the more readily and reliably assessed retinal venous beading was incorporated into the Airlie House diabetic retinopathy classification system [121]. Retinal vessel caliber was not extensively explored until the development of retinal photography. With the advent of digital retinal photos and semi-automated software to grade retinal calibers, many cross-sectional and longitudinal studies, relating vessel caliber to retinal and renal outcomes, have been conducted (reviewed in [41, 118, 119]), including by the author and her colleagues [122-124]. Most studies of retinal caliber are at rest, but some investigators also evaluate the retinal vessel caliber response to exercise [102-104].
Many studies have investigated the association of retinal vessel caliber with diabetic retinopathy in both type 1 and type 2 diabetes patients [41, 118, 119]. Both cross-sectional [99, 125] and prospective studies [126-128] showed strong evidence of venular widening to be associated with diabetic retinopathy. Reduced retinal arteriole caliber has also been associated with diabetic retinopathy [99, 127] in a cross-sectional study, and in several prospective studies in type 1 diabetes [122, 124]. In a longitudinal study of 64 type 2 diabetes patients (followed up over a mean of 6.8 years), resting retinal arteriole diameter decreased significantly in patients with improved diabetic retinopathy [102]. To the best of our knowledge, the longest longitudinal study to date is the 16-year Danish Cohort of Pediatric Diabetes study, in which wider veins and smaller arterioles were independent predictors of proliferative retinopathy, nephropathy, and neuropathy [126].
Reduced retinal arteriole caliber has also been associated with moderate to severe diabetic macular ischemia in adults with type 2 diabetes [129]. In the WESDR study, retinal vessel caliber was independently associated with the risk of incident nephropathy, lower extremity amputation, and stroke mortality in persons with type 2 diabetes [130]. In a longitudinal study of type 1 diabetes, retinal arteriolar narrowing was independently associated with nephropathy and cardiovascular disease [131].
Most longitudinal studies report changes from baseline, usually starting in children and adolescents without clinically evident retinopathy or early or moderate retinopathy. It will be of particular interest to learn whether baseline retinal vessel caliber measures at this and later ages are associated with subsequent late-stage diabetic retinopathy, need for treatment, vitrectomy, or loss of visual acuity. Furthermore, we wish to learn what effects medical therapies, such as improved glycemic control, renin-angiotensin-aldosterone system (RAAS) blockade, or fenofibrate treatment, may have on retinal vessel caliber and retinopathy.
Various measurement systems and software packages are available to assist with retinal vessel caliber measurement. The similarities and differences between the various platforms are of interest, and matter of future research. Likewise, it will be interesting to learn what clinical, biochemical, and molecular factors modulate retinal caliber. We have reported associations between circulating PAI-1 activity and retinal vessel caliber in type 2 diabetes patients [132]. A cross-sectional study of 112 community-based persons with type 2 diabetes, aged 44-83 years, reported on retinal arteriolar and venular caliber and their arterio-venous ratio (AVR) determined from fundus photography using a validated computer-assisted method. It was shown that vessel caliber and AVR were correlated with PAI-1 activity. In adjusted linear regression models, PAI-1 activity was positively associated with retinal arteriole caliber and AVR, and inversely correlated with venules. Also, wider arterioles were independently associated with other vascular risk factors such as waist-to-hip ratio, HDL-C, and lower systolic blood pressure, whereas narrower venules were associated with older age and higher albuminuria. These findings support the role for PAI-1 activity in the retinal microvasculature of patients with type 2 diabetes, and may partially explain the link between retinal vascular caliber and cardiovascular disease [132].
Given the many positive clinical studies, the widespread availability and acceptability of digital retinal photography, and the ability to grade retinal photos remotely using computer assisted diagnosis, retinal vessel caliber may soon be a biomarker in clinical practice. Even today, it is frequently used as a surrogate end-point in clinical trials.
Retinal vessel geometry. Retinal vessel geometry comprises another group of retinal vessel-based biomarkers. It includes measures of vessel branching angles, branching complexity, and fractals. We have evaluated these novel clinical biomarkers, which can also be derived from retinal photos in relation to diabetic retinopathy and nephropathy [133-138]. In our cross-sectional study of young type 1 diabetes patients, greater retinal arteriole tortuosity was independently associated with retinopathy and early stage of nephropathy [138]. In our longitudinal studies in a pediatric cohort of type 1 diabetes patients, greater simple tortuosity and lower arteriolar length to diameter ratio were independently associated with incident retinopathy [133].
In our cross-sectional study of young type 1 diabetes patients, traditional vascular risk factors correlated with novel retinal geometry measures. Older age was associated with decreased arteriolar and venular tortuosity, and females had larger arteriolar branching angles than males. After adjusting for age and sex, the following associations were found:
Longer diabetes duration was associated with larger arteriolar branching angle and increased arteriolar optimality deviation
Higher HbA1c levels were associated with increased arteriolar tortuosity
Higher systolic blood pressure was associated with decreased arteriolar length to diameter ratio
Higher total cholesterol levels were associated with increased arteriolar length to diameter ratio and decreased venular optimality deviation
These associations remained after controlling for HbA1c, retinal vessel caliber, and retinopathy status, and were seen in subjects with early retinopathy and without retinopathy [137].
Fractal analysis was independently associated with diabetic retinopathy in our cross-sectional analysis of young subjects with type 1 diabetes [136], but fractal analyses were not predictive of the subsequent development of diabetic retinopathy [135]. It may thus be of less clinical value than predictive markers such as retinal vessel calibers, at least in this type of diabetic cohort.
Neural retina assessments: electroretinograms and visual evoked responses. There is general agreement that both neural and vascular changes are linked with diabetic retinopathy, but there has been controversy as to which occurs first. Availability and sensitivity of the tools used to assess retinal vascular and neural structure and function are important to answer this question. The links between retinal-neural and retinal-vascular function and structure, and the changes in diabetes, are not yet fully understood. Retinal glial cells are involved in the interaction between vascular, neural, and retinal compartments. Müller cells that surround retinal blood vessels are assumed to modulate vascular permeabilty, blood flow, and vascular cell survival [139-143].
Retinal neural health can be assessed in vivo, in animal models, and in man, using electroretinography (ERG) and visual evoked responses (VER). In diabetes, these methods are predominantly research tools, but are sometimes used in clinical practice, in particular in non-diabetic ocular diseases. ERG involves electrical stimulation, and has several subtypes, including flash and multifocal ERGs. Using these tools, differences have been identified in (neural) retinal function and at different disease stages between subjects with and those without diabetes, with the abnormality increasing with severity of the (more readily classified) vascular stages of retinopathy. Abnormal ERG can be present in diabetic subjects even in the setting of apparently normal retina [143]. In cross-sectional studies, ERG changes have been noted in type 1 diabetes patients with a disease duration of only one year [144]. In longitudinal, clinical, observational studies, multifocal ERG-readings predicted the location of new retinopathy that developed 1-3 years later [145, 146]. Therefore, neurodegeneration could be a useful biomarker to predict the future development of microvascular damage in the diabetic retina. A portable ERG device has recently been released for non-mydriatic use in the primary care setting for the detection of late-stage diabetic retinopathy [73].
Ocular coherence tomography (OCT). The OCT is another clinical biomarker that is now widely used in clinical practice. OCT provides images of the multiple retinal layers, and measures the thickness of the various layers. In diabetes-related retinal-neural degeneration, retinal ganglion cells and nerve fiber layers are thinned. The OCT is also very useful in identifying and quantifying retinal edema, including macular edema, which can be difficult to detect by retinal photography or fundoscopy. Abnormalities in OCT can be detected even in diabetic patients with a normal fundoscopic examination [143].
In a recent systematic review including 14 studies of OCT in type 1 and type 2 diabetes patients, retinal neurodegenerative changes were noted, even in the absence of diabetic retinopathy. Several layers in the retina and the mean retinal nerve fiber layer around the optic nerve head were significantly thinner in people with type 2 diabetes than in non-diabetic subjects. In type 1 diabetes patients with no retinopathy, the OCT was normal, but abnormal in those with retinopathy [39].
In a 2015 Cochrane systematic review of 10 studies published between 1998 and 2012 (830 participants, 1,387 eyes), OCT was suggested as the new standard for the diagnosis of diabetic macular edema [40]. In a recent study in the Veterans Affairs Diabetes Trial including adults with type 2 diabetes and current or prior diabetic macular edema, early (4-month) changes in the disorganization of the retinal inner layer (DRIL) were detected by OCT, and were independent predictors of subsequent changes in visual acuity in a 1-year follow-up [147].
Corneal confocal microscopy (CCM). CCM is used to examine the corneal structure, including corneal nerve fibers. Whilst used in clinical practice in other ophthalmic conditions, CCM is an emerging clinical research tool in diabetes. Nerve fiber density, branch density, and length can be quantified by related software. In a recent meta-analysis of 16 clinical trials in type 1 and type 2 diabetes subjects using CCM, both groups of diabetic subjects had abnormal corneal nerve structure compared with non-diabetic subjects, even in the absence of diabetic peripheral neuropathy. CCM-detected nerve damage was more severe when diabetic retinopathy or neuropathy was present [39].
As yet, we are not aware of studies relating CCM to retinal vessel caliber and ERGs. CCM is being used in clinical research, including as a surrogate end-point in some clinical studies. However, further research and standardization of protocols are needed before it is disposed to widespread clinical use as a marker of diabetic neural damage and diabetic retinopathy. We will now discuss biochemical biomarkers of diabetic retinopathy.
7.2 Glucose-related biomarkers
HbA1c. Whilst some of the lesions observable in diabetic retinopathy, such as microaneurysms, exist in other non-diabetic conditions (e.g. hypertension), diabetic retinopathy development and progression is very strongly related to glycemic control, which is best reflected by HbA1c levels. The HbA1c assay, which is widely used in clinical practice and research, is based on the non-enzymatic glycation of hemoglobin, and reflects average blood glucose levels over the preceding 2-3 months. In addition to HbA1c itself, clinicians and researchers often use other HbA1c-related measures, including time-averaged HbA1c levels, HbA1c variability, and other measures such as the 'glycation gap' (discussed later in this section).
It is now well-established that tight blood glucose control early in the course of diabetes, and sustaining it over time, are major benefits in the protection against diabetic retinopathy. This knowledge was provided by the randomized controlled intervention trial in type 1 diabetes, the DCCT [47], and in type 2 diabetes by the United Kingdom Prospective Diabetes Study (UKPDS) [148]. In the DCCT, intensive diabetes management resulted in HbA1c levels ~2% lower than conventional diabetes care. This treatment reduced the primary end-point of diabetic retinopathy development (primary prevention) by 76% and diabetic retinopathy progression (secondary prevention) by 54%, with an initial transient (one year) worsening of retinopathy prior to improvement [47]. The DCCT was followed by an even longer and ongoing observational follow-up study, the Epidemiology of Diabetes Intervention and Complications (EDIC) study, which led to the now widespread recognition of metabolic memory for glycemic control (as discussed previously), for which biomarkers and mechanisms are being sought.
In a reanalysis of the DCCT diabetic retinopathy results, exploring the features of metabolic memory relative to HbA1c, Lind et al. demonstrated that HbA1c levels dating back 2-3 years had the greatest relative risk contribution to current progression of retinopathy. Furthermore, HbA1c levels dating back up to five years made a greater contribution than concurrent HbA1c values, while values from eight years earlier still had an important impact [149].
In UKPDS, newly-diagnosed type 2 diabetes patients were randomized to intensive vs. standard glucose control (which differed in HbA1c levels by 0.9%), using various glucose control agents. The patients were followed for two decades [148]. At study-end, intensive therapy significantly reduced cumulative microvascular end-points by 25%. For retinopathy, intensive therapy reduced progression. This became evident within six years of randomization, and was sustained. The reduction in risk amounted to 21% after 12 years. Ten years after UKPDS close-out, about one third of the original study subjects were available for follow-up. It was demonstrated that prior intensive HbA1c control reduced risk of a composite microvascular end-point by 24%; retinopathy-specific data were not provided [49].
Hypoglycemia. The relationship between hyperglycemia and diabetic retinopathy is well-known. Hence, biomarkers of hyperglycemia are important in both clinical practice and research. More recently, links between hypoglycemia and vascular complications of diabetes have been recognized [150]. These complications are perhaps mediated by induction of oxidative stress and proinflammatory processes. Specific analysis of the DCCT Cohort data regarding diabetic retinopathy did not find an association between hypoglycemia and diabetic retinopathy [151]. Further studies and consideration of ambient blood glucose levels are of interest as hypoglycemia has been found to block an observed decrease in diurnal macular thickness in people with diabetic macular edema assessed by OCT [152].
Glycemic variability. Another glycemic related group of biomarkers is that of glycemic variability (GV). Glycemic variability is reflected by either short-term or long-term blood glucose variability. Short-term GV is usually based on fingerprick blood glucose tests or on days of continuous glucose monitoring (CGM) of subcutaneous interstitial fluid glucose levels. Long-term GV is usually based on standard deviation of serial HbA1c levels (HbA1c SD). These measures have been explored as biomarkers of increased risk of diabetic micro- and macrovascular complications. Most, but not all, studies found positive associations between various measures of GV, including the HbA1c SD [153-155], with more consistently positive outcomes in type 2 than in type 1 diabetes [154, 155]. However, publication bias may have led to an over-representation of positive studies published. Also, long-term metabolic memory for glycemia may have impacted retinopathy, but have not been included in the study time or considered in the discussion.
Hemoglobin glycation index (HGI) and glycation gap. The HGI is calculated as follows: HGI = observed HbA1c - predicted HbA1c. It is based on differences between mean blood glucose levels and predicted vs. actual HbA1c levels, and was developed in the DCCT and applied to the DCCT cohort. At seven years' follow-up, type 1 diabetic patients with higher-than-predicted HbA1c levels (high HGI group) had three times greater risk of retinopathy (30% vs. 9%, p < 0.001) than patients in the low-HGI group [156].
The Glycation Gap (G-gap) is a variant of the HGI measure. It refers to the potential difference between measured HbA1c levels and HbA1c levels predicted from levels of fructosamine, a measure of glycated circulating proteins, predominantly albumin. A predicted HbA1c (FHbA1c) is calculated from the simultaneously measured fructosamine standardized to the HbA1c distribution: FHbA1c = (((fructosamine - mean fructosamine)/SD fructosamine) x 3 SD HbA1c) + mean HbA1c. The G-gap is the difference between the true HbA1c and the fructosamine-derived standardized predicted FHbA1c (G-gap = HbA1c - FHbA1c). A negative G-gap denotes the true (intracellular glycation marker) HbA1c being lower than the FHbA1c, and a positive G-gap denotes the true HbA1c being higher than that predicted by fructosamine. Some [157, 158], but not all [159], studies have found that the glycation gap is an independent predictor of diabetic retinopathy.
As mentioned earlier, there are many microenvironments to consider in biomarker analysis. To understand and interpret HbA1c, fructosamine, and glycation gap measures correctly and to consider them as biomarkers of diabetic complications we have to consider their specific properties, which include:
These biomarkers are indirect measures of blood glucose
They have greatly different half-lives (2-3 months for HbA1c and 2-weeks for fructosamine)
They are subject to other influencing factors, such as renal dysfunction and hemoglobinopathies (for HbA1c)
Their microenvironments differ; hemoglobin is intracellular and fructosamine is extracellular
Protein glycation is a non-enzymatic reaction dependent on glucose concentrations, whilst intracellularly enzymatic deglycation of proteins can occur [159, 160]. The key deglycating enzyme, fructosamine-3-kinase, has isoforms and a genetic polymorphism suggested to influence HbA1c and complication risk.
Currently, HbA1c and fructosamine are used clinically. Further clinical studies are needed to discern the role of these and other novel biomarkers of glycemia in diabetic retinopathy and its management.
7.3 Factors related to advanced glycation end-products (AGEs)
AGEs comprise a family of compounds formed from complex chemical reactions between proteins and glucose. The compounds can also form during oxidation of polyunsaturated fatty acids in the presence of protein [161]. Whilst early glycation products such as HbA1c are reversible once formed, AGEs are not spontaneously reversible. AGEs form naturally in many tissues throughout the body, including on short-lived proteins such as albumin, immunoglobulins, hemoglobin, and lipoproteins, and on long-lived proteins such as collagens and ocular lens crystallins. Collagen-based AGEs increase with aging. Well-known AGEs are carboxymethyllysine (CML), carboxyethyllyine (CEL), and pentosidine. Some of them are fluorescent (e.g. pentosidine), whilst others (CML) are not. AGE formation is thought to be increased by oxidative stress, and accelerated in diabetes and renal disease, even in the absence of diabetes. Smoke and many foods (e.g. pizza, toast, coffee, cola, and Peking duck) contain AGEs and contribute to AGE levels in the body.
AGEs can be measured specifically by gas chromatography / mass spectroscopy (CML, CEL), high-pressure liquid chromatography (HPLC) (pentosidine), or less-specific ELISAs. AGEs in skin, cornea, and lenses can be measured non-invasively, clinically based on tissue auto-fluorescence [66, 67, 70-72].
We have previously reviewed this issue [162-164]. In general, higher levels of circulating AGEs are frequently, but not always, associated with diabetic vascular complications, including retinopathy. However, concurrent renal disease, which generally increases AGEs, should be considered. The type of AGE, the method used to assay it, and the site of where the AGE is, in particular if it is short- or long-lived tissue, can influence the relationship of AGE levels to diabetic retinopathy status.
AGEs have many adverse effects of relevance to vascular (and neural) complications of diabetes, including:
Inflammation
Oxidative stress
Blood clotting
Fibrosis
Cytotoxicity
Pro- and anti-angiogenic actions
Effects on cell-signaling and molecular pathways
AGE-based modification of circulating proteins and extravasated proteins such as LDL enhances their pathogenicity, also in the retina (reviewed in [162-167]). In the DCCT/EDIC cohort, we demonstrated that baseline levels of AGE-LDL and oxidized LDL (oxLDL) within circulating immune complexes were independent predictors for retinopathy progression several years later [26]. Circulating AGEs are relatively short-lived. AGEs in long-lived tissues such as skin collagen have a validated non-invasive assessment tool. There is increasing evidence for its association with and prediction of diabetic retinopathy.
Clinically the use of skin collagen-based AGEs is increasingly being used in clinical research, with more recent non-invasive skin AGE readers replacing the need for full thickness skin biopsy and complex biochemical analyses. Correlations between skin biopsy AGE levels and non-invasively assessed skin (auto-flourescence) AGEs have been demonstrated [168]. Several groups have shown skin AGEs to be strongly related to prior glycemic control reflected by serial HbA1c levels over many (up to 15) years [68, 69, 169, 170].
Many studies in diverse ethnic groups have demonstrated correlations between skin AGEs measured non-invasively or biochemically and concurrent retinopathy status, including its presence and/or severity [66, 70, 169, 171-173]. Non-invasively assessed skin AGEs were also associated with retinopathy in the DCCT/EDIC cohort [173]. Skin collagen AGEs measured biochemically on skin biopsies were independent predictors of diabetic retinopathy (and other microvascular complications) in the DCCT/EDIC cohort [174-175]. Recently, a panel of 10 AGEs measured chemically in skin biopsy collagen taken near DCCT close-out has been shown to be predictive of the subsequent retinopathy status [50]. These studies support that a major driver of long-lived AGEs is glycemia, and that skin AGEs, which takes minutes to measure using non-invasive tools, may be a suitable biomarker for years of glycemic control, and for diabetic retinopathy risk.
Factors related to the receptor for AGEs (RAGE). RAGE is member of a family of receptors for AGEs. It is a 35 kDa transmembrane receptor from the immunoglobulin superfamily, and also known as a pattern recognition receptor. RAGE binds AGEs and other ligands, including high-mobility group protein B1 (HMGB1), an intracellular DNA-binding protein important in chromatin remodeling. RAGE activation is proinflammatory. It is of particular relevance to diabetic retinopathy that higher levels of AGEs, including soluble RAGE (sRAGE) and pentosidine, have been associated with diabetic retinopathy in cross-sectional studies [176].
sRAGE is a circulating form or a receptor for AGEs. Isoforms of the RAGE protein, which lack the transmembrane and signaling domain, exist in man and are thought to be protective against AGE- and RAGE-related damage, perhaps by acting as a decoy, binding and inactivating AGEs [176]. RAGE is expressed by many cells in the retina, with the highest expression achieved in Müller glia [177]. Blockade of RAGE may be a useful therapeutic strategy. sRAGE has been shown to prevent diabetes-related Müller glia dysfunction during diabetes [177], and to inhibit retinal vessel leukostasis in AGE-infused (non-diabetic) mice [178]. Importantly, RAGE antagonists can prevent acellular capillary formation in diabetic mice [179], and mice, in which RAGE has been genetically deleted, show significant protection against diabetic retinopathy [180]. Together, these clinical and related basic science studies show the value of such biomarkers in developing therapeutics.
7.4 Lipid- and lipoprotein-related biomarkers
There are many cross-sectional and longitudinal studies showing an adverse profile of ‘traditional’ lipid levels, including total cholesterol, triglycerides, HDL-cholesterol, and (usually calculated) LDL-cholesterol (LDL-C). These levels have been associated with retinopathy in young and adult type 1 and type 2 diabetes patients. However, not all studies are confirmatory, which may be due to variability in lipid levels, the use of lipid levels at only a single time point, metabolic memory, weaker associations than with hyperglycemia, or publication bias. We have previously reviewed this issue [25, 162, 163].
In a cross-sectional study of the type 1 diabetic DCCT/EDIC cohort, retinopathy was positively associated with serum triglycerides and negatively with HDL-C [23]. In the type 1 diabetic Pittsburgh Epidemiology of Diabetes Complications study [181], serum triglycerides, and to some extent LDL-C, were associated cross-sectionally with retinopathy, and longitudinally with progression to PDR. In the recent, very large META-EYE study, high total cholesterol level was a risk factor for diabetic retinopathy, specifically for diabetic macular edema [15]. In contrast, a 13-country study of long type 2 diabetes duration showed that plasma lipid levels (high triglycerides and low HDL-C levels) were associated with retinopathy on univariate analysis, but not at full adjustment for potential confounders [24]. In recent studies, we found that circulating levels of apolipoprotein A1 (associated with HDL) and apolipoprotein B (found in LDL, lipoprotein(a), VLDL, and chylomicrons) were stronger predictors of diabetic retinopathy than traditional lipid levels [182].
Retinal exudates, which include extravasated lipids, are sometimes used as a surrogate end-point for retinopathy. In the type 2 diabetic Early Treatment Diabetic Retinopathy Study (ETDRS) including 2,709 patients [183], high LDL-C levels at baseline doubled the risk of developing retinal hard exudates. In the Hoorn study, which included 2,484 diabetic and non-diabetic individuals, diabetic retinopathy was positively associated with total cholesterol and triglyceride levels, and retinal hard exudates were associated with LDL-C levels [184]. In the Atherosclerosis Risk In Communities (ARIC) study, only retinal hard exudates were associated with LDL-C and lipoprotein(a) levels [185]. Other features of retinopathy were not associated with lipid levels. Studies of lipid levels are of interest, in particular if they include OCT and other surrogate end-points such as retinal vessel caliber and geometry.
In general, the links between traditional lipid levels and diabetic retinopathy are not particularly strong, particularly not at the individual basis. In contrast, the impact of some lipid-lowering drugs on diabetic retinopathy are considerable. 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are potent LDL-C-lowering drugs. They protect against cardiovascular events equally well in both adults with and without type 1 or type 2 diabetes and in primary and secondary prevention settings [186]. However, in statin studies to date (in which diabetic retinopathy was usually a secondary end-point), statins did not alter retinopathy [187]. In contrast, fenofibrate, an orally active PPAR-alpha agonist, primarily used to lower triglyceride levels, reduced diabetic retinopathy by 31-40% in two large randomized controlled trials in type 2 diabetes, the Fenofibrate Intervention and Event Lowering in Diabetes Trial (FIELD) [188] and the ACCORD Lipid Trial [189]. In both trials, the clinical benefit (over 5 years of intervention) was not related to the effects on traditional lipid levels. As we [190] and others [191] have recently reviewed, pleiotropic effects of fenofibrate, including anti-inflammatory, anti-angiogenic, and cell signaling (PPAR-alpha and WNT pathways) effects, may be major driving forces. However, other novel lipoprotein-related markers may be contributory and merit further evaluation.
Novel lipoprotein biomarkers include modified lipoproteins such as oxidized LDL, modified LDL in immune complexes, and lipoprotein subclasses. The lipoproteins are defined by size (and detected by NMR), or by density (and detected by density gradient ultracentrifugation), or by apolipoprotein content (and detected by antibody affinity-based assays).
In a cross-sectional study of the DCCT/EDIC cohort, NMR analysis of circulating lipoproteins identified stronger associations with subclasses of small LDL and small HDL (more so in men than in women) [23]. Longitudinal studies are of interest, and are planned for follow-up of these baseline data. In a longitudinal DCCT/EDIC cohort study on both unadjusted and covariate-adjusted models, higher levels of AGE-LDL and oxLDL in circulating immune complexes were associated with progression of diabetic retinopathy [26].
Modified lipoproteins such as oxidized LDL are thought to be more atherogenic than unmodified lipoproteins. Modified lipoproteins are found in higher concentrations in the vessel wall than in the antioxidant-rich high-turnover plasma. In diabetes, the blood retinal barriers become leaky, and lipoproteins are extravasated. They are likely remodified whilst trapped in the retina. Extensive human, animal, and cell culture studies carried out by Lyons et al. demonstrated that lipoproteins and modified lipoproteins exist in the retina, and that they contribute to retinal damage. Lyons’ team has shown that extravasated and modified LDL and immune-complexed oxLDL are present in the retina of diabetic patients. They have also demonstrated that glycated LDL and/or oxLDL exert cytotoxicity to cultured human retinal capillary endothelial cells [192], pericytes [166, 192-195], retinal pigment epithelium [165], and Muller glial cells [196]. Finally, these glycated LDL could be shown to cause changes in cell signaling, gene expression, cell apoptosis, and autophagy [165, 192, 193, 195, 196].
Lipids, including fatty acids and sphingolipids, may also play a role in diabetic retinopathy. In the DCCT/EDIC cohort, decreased plasma levels of a subset of very long chain ceramide species, measured by HPLC-tandem mass spectroscopy, were associated with the development of proteinuria after 14-20 years of follow-up [197]. Similar lipidomic-based studies in diabetic retinopathy would be interesting.
In summary, while traditional lipid levels in blood are somewhat linked with diabetic retinopathy, lipoproteins in the retina, particularly modified lipoproteins and those identified in the circulation using more novel techniques, may play an important role in retinopathy, and may become therapeutic targets.
7.5 Blood pressure
Blood pressure (BP) is a clinical biomarker. It is highly variable both within and between individuals. Variations in the relationship of BP with retinopathy between studies may relate to different measurement techniques of BP and different cut-points for normal vs. hypertension. The various measures include:
'In clinic' and 'at home'
Manual and automated sphygmomanometers
Single vs. mean of multiple BP readings
24-hour BP readings (which are more reliable, but also more costly)
Systolic, diastolic, mean, and lying and standing BP differences may be measured. The presence or absence of (normal) nocturnal falls in BP, called dipping, is also implicated in diabetic vascular complication risk. Whilst best studied in diabetic nephropathy [198], non-dipping overnight blood pressure has also been associated with increased risk of diabetic retinopathy in type 2 diabetes subjects [199].
The UKPDS demonstrated that high BP is an important risk factor for diabetic retinopathy [148]. However, BP levels on average in the UKPDS were higher than in many diabetic patients today; this is due to lower treatment targets and improved blood pressure-lowering drugs. In the large individual participant data meta-analysis, the META-EYE Study, diabetic patients with BP >140/90 mmHg, or those who were on anti-hypertensive drugs, were more likely to develop diabetic retinopathy than those with normal BP. Also, all stages of diabetic retinopathy, from mild to vision-threatening-related end-points, were higher in people with higher BP levels [15].
In contrast, a recent Cochrane review of BP control over 4.5 years in type 2 diabetic patients showed benefits of antihypertensive agents on preventing retinopathy onset, but no effect on diabetic retinopathy progression [200]. However, the time frame and vascular memory for BP and BP drugs may have modulated the study outcome. Trials have shown diabetic patients may benefit from early use of anti-hypertensive agents, even in the setting of normal BP levels. Particular beneficial are those drugs related to the RAAS system, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin-2 receptor blockers (ARB) [143]. These benefits may relate to the presence of a RAAS system in the eye [201].
7.6 Inflammation
The persistence of chronic inflammation in the retina from diabetes onset until late-stage sight-threatening retinopathy is now well-recognized, and has been reviewed in several publications [202, 203].
There are multiple biomarkers within the inflammatory response, many of which can be detected at altered, usually increased, levels in the vitreous, in the retina, and frequently in the systemic circulation. The categories of inflammatory biomarkers are summarized in Table 6. They include adhesion molecules, which are known to be relevant very early in retinopathy, and growth factors, which are best known in late-stage retinopathy. A key role is played by elevated VEGF levels, which form the basis of anti-VEGF intraocular injections, now widely used clinically. Other anti-angiogenic peptide-based therapies are in development [202].
Table 6. Inflammatory biomarkers in diabetic retinopathy.
Legend: CTGF – connective tissue growth factor, EPO – erythropoietin, HIF-1 – hypoxia-inducible factor 1, HMGB1 – high-mobility group box 1, ICAM – intercellular adhesion molecule, IGF1 – insulin-like growth factor 1, IL – interleukin, IP10 – interferon gamma-induced protein 10, MCP-1 – monocyte chemotactic protein 1, MIF – macrophage migration inhibitory factor, MIG – monokine induced by gamma interferon, NF-ĸB – nuclear factor kappa B, PEDF – pigment epithelium-derived factor, PGF – placental growth factor, SDF-1 – stromal cell-derived factor 1, sVAP – soluble vascular adhesion protein 1, TNFα – tumor necosis factor alpha, VCAM-1 – vascular cell adhesion protein 1, VEGF - vascular endothelial growth factor.
Vascular cell adhesion molecules (CAMs) are usually increased in retinal and extraocular vessels even in the early stages of diabetes and diabetic retinopathy, leading to increased leukocyte adhesion (leukostasis of neutrophils and monocytes) and interactions. The leukocytes can become trapped in retinal capillaries which may cause occlusion and non-perfusion [204]. They are indeed also found within the retina itself, particularly near blood vessels [205]. Leukostasis occurs in diabetic animal models [206] and in people with diabetes [207]. Adherent leukocytes can themselves damage the retinal vascular endothelium [208]. Leukocyte adherence is a biomarker of diabetic retinopathy and often used as surrogate end-point in animal model research, as we did in demonstrating the benefit of fenofibrate in reducing retinal inflammation and diabetic retinopathy [209].
Evidence of extraocular (systemic) inflammation may be provided by:
Elevated levels of soluble forms of CAMs
Increased circulating neutrophil levels in late-stage diabetic retinopathy [213]
The neutrophils express more myeloperoxidase (MPO), and generate greater amounts of cytotoxic hydrogen peroxide in diabetic than in non-diabetic subjects [214].
In some clinical studies, serum levels of soluble tumor necrosis factor (TNF) receptors 1 and 2 [215], TNFα [216], soluble vascular cell adhesion molecule 1 (sVCAM-1), soluble intercellar cell adhesion molecule 1 (sICAM-1), and soluble E-selectin (sE-selectin) [217] have been associated with the presence of diabetic retinopathy. However, other studies have not found such associations between retinopathy and inflammatory markers, particularly if corrected for confounders [218, 219]. Differences may relate to study size, subject characteristics, and statistical analyses. A genetic inflammation biomarker, genotypes for C-reactive protein (CRP), has been associated with increased risk (OR 1.3) for diabetic retinopathy in Chinese subjects with type 2 diabetes [220]. Of greater interest are longitudinal studies. Whilst some studies have not found predictive power of baseline inflammatory systemic markers for future retinopathy [218], some have detected links. In a substudy of 1,391 subjects from DCCT-EDIC cohort, circulating levels of sE-selectin at baseline was an independent predictor for the development of diabetic retinopathy (3-step ETDRS score) over 16 years of follow-up [221].
Pro-inflammatory factors, such as cytokines and chemokines, are shown in Table 6. They have been identified at increased levels in the retina and/or in the vitreous fluid [203, 222]. Whilst the level of inflammation in diabetic retinopathy is often regarded as low, the levels for some inflammatory factors have been found to be quite high; for example, IL-8 levels in vitreous fluid were comparable to that found in pleural effusions of patients with pneumonia [223]. High levels of vitreous fluid interleukins (IL-6 and IL-8) have been correlated with diabetic retinopathy progression in PDR and vitreous surgery outcomes [224, 225]. Hence, pro-inflammatory factors provide useful as biomarkers, at least in clinical research as vitreous fluid is not a readily accessible sample for non-surgical clinical practice.
Diabetic retinopathy also involves the neural and the vascular retina. Inflammation may stem from neural Müller cells and microglia which may be useful biomarkers [226-228].
Many inflammatory biomarkers are used as surrogate end-points in preclinical studies that test novel therapeutics for diabetic retinopathy, such as FT011, a novel anti-inflammatory and anti-fibrotic agent. In the hypertensive Ren-2 rat model of diabetic retinopathy, intravitreal FT011 reduced retinal leukostasis, microglial density, and ICAM-1 mRNA levels [229]. Similarly, systemic and ocular fenofibrate reduced retinal inflammation (and angiogenesis) in rodent models of diabetic retinopathy [209].
7.7 Thrombosis-related biomarkers
Microthrombi are a feature of diabetic retinopathy. Therefore, biomarkers related to clotting and fibrinolysis are relevant to diabetic retinopathy. A potential modulating factor is that many clotting- and fibrinolysis-related parameters, particularly those produced by the liver, increase in the presence of renal damage, even at early stages. Therefore, association of these (and other) biochemical biomarkers may be confounded by coexistent renal disease.
A prothrombotic marker that has been associated with increased risk of both cardiovascular disease (CVD) and diabetic retinopathy is plasminogen activator inhibitor 1 (PAI-1). However, the results relating to PAI-1 genotype and risk of diabetic retinopathy (and other vascular complications) are contrasting. Therefore, a meta-analysis was performed to assess the association between the PAI-1 -675 4G/5G polymorphism and susceptibility to diabetic retinopathy. There were 10 studies with 1,327 cases and 1,557 controls; none of them found a significant association with retinopathy [230]. With regard to baseline circulating protein PAI-1 (activity) levels in both the type 1 diabetic DCCT [221] and type 2 diabetic VADT study [231], higher PAI-1 levels were associated with increased risk of diabetic retinopathy many years into the future.
Fibrinogen levels are also associated with increased inflammation and hypercoagulability. They have also been associated with increased risk of retinopathy. In the prospective VADT, subjects who were assigned to intensive glucose control and who had lower baseline levels of fibrinogen had a lower risk of retinopathy progression [231]. In a very small cross-sectional study of type 1 diabetic subjects, fibrinogen levels did not differ between those with and those without retinopathy [232]. Therefore, larger and prospective studies with adjustment for multiple covariates are needed.
7.8 Angiogenesis-related biomarkers
VEGF is a potent inducer of retinal vascular leakage and angiogenesis. The identification of the key role of this biomarker in diabetic retinopathy has led to the development of anti-VEGF treatments. There are several review articles regarding the role of VEGF and anti-VEGF agents in diabetic retinopathy [233-235]. Whilst VEGF genotype is linked with retinopathy risk [234], it is not yet used in clinical practice.
Pigment epithelium-derived factor (PEDF) is an endogenous antagonist of VEGF. It was first identified in the retinal pigment epithelium, but is now known to be widely expressed in the body, including eye, kidney, arteries, liver, and adipocytes. Most circulating PEDF is thought to arise from liver and adipocytes. PEDF is excreted in the urine; circulating levels rise with renal disease. PEDF has potent antiangiogenic properties as well as anti-inflammatory and anti-oxidant effects [3, 236-238]. It is detectable at high levels in blood.
In cross-sectional studies of adults with type 1 diabetes, we demonstrated higher circulating PEDF levels in those with than those without microvascular complications, including PDR [61]. In another small cross-sectional study, we did not find a difference in circulating PEDF levels between those with and those without diabetic retinopathy [239]. However, in our larger study of the FIELD type 2 diabetes study, circulating PEDF levels were higher in those with than those without retinopathy, once adjustments were made for renal dysfunction and other covariates. Also, higher PEDF levels at baseline were associated with lower risk of retinopathy, and fenofibrate treatment increased PEDF levels (personal communication Jenkins A, Januszewsi A, Keech A). These clinical biomarker studies are supported by positive preclinical studies that tested the development of PEDF eye-drops for the treatment of diabetic retinopathy [240].
Fibroblast growth factor 21 (FGF-21) is another growth factor with effects on glucose levels, lipid levels, and thermogenesis; it also has pro-angiogenic effects [241, 242]. In a cross-sectional study, FGF-21 levels in the circulation have been independently associated with the severity of diabetic retinopathy in type 2 diabetes patients [243]. In the FIELD study, baseline FGF-21 levels were also associated with microvascular complications at baseline. and with their on-trial development, though retinopathy specific outcomes were not reported [244]. Fenofibrate greatly increased plasma FGF-21 levels by approximately 80% [244].
Many other growth factors, including hepatic growth factor and connective tissue growth factor (CTGF), are also implicated in diabetic retinopathy. They are useful as biomarkers in basic science studies exploring mechanisms of retinal damage and protection, and as a surrogate end-point [235, 245]. Whilst there are some (positive) human diabetes studies (in the DCCT-EDIC cohort) linking CTGF genotype and urinary levels, they relate to nephropathy and surrogate end-points of cardiovascular disease [246, 247] rather than retinopathy. Studies analyzing these markers in relation to retinopathy would be of interest.
7.9 Oxidative stress markers
Oxidative stress is implicated in the development of diabetic vascular complications [162]. Oxidative stress is very difficult to measure. It has a wide range of biomarkers, which are usually very short lived, and it is highly variable within and between individuals. Serum levels may not be representative of what occurs at the site of disease as serum is full of antioxidants, including fat and water-soluble antioxidants such as vitamin E and vitamin C, respectively. Studies of ocular tissue are desirable. In a study of serum and vitreous fluid, higher levels of oxidative stress markers (lipid peroxides, malondialdehyde) and lower levels of superoxide dismutase were identified in the vitreous of diabetic vs. non-diabetic eye disease patients. Correlations between vitreous and serum levels of lipid peroxides and superoxide dismutase were seen in diabetic retinopathy patients [74]. LDL and oxLDL have been identified in human diabetic retina, with increasing amounts in more severe diabetes retinopathy, and no oxLDL evident in non-diabetic retina [248]. As retinal tissue has a high blood flow and high tissue turnover it would be likely susceptible to oxidative stress. This issue merits further research.
7.10 Nutrition-related biomarkers
Nutrition related biomarkers are implicated in several forms of ocular disease, including diabetic retinopathy. Multi-vitamins are used to treat some non-diabetic forms of eye disease, such as age-related macular degeneration.
Vitamin D. There are vitamin D receptors in the eye. Vitamin D receptor-related genotypes have been associated with diabetic retinopathy in type 1 and type 2 diabetic subjects [249-254]. Similar to its known role in bone and calcium metabolism, vitamin D also has roles in insulin resistance and immune function, and it has anti-inflammatory, anti-angiogenic, and anti-fibrotic effects. In preclinical studies, topical vitamin D inhibits retinal and corneal angiogenesis and intraocular pressure. A review of vitamin D in ocular health has been published by Nebbioso and colleagues [253].
Cross-sectional studies have been conducted in many different ethnic groups. Most of them [255-265], including the larger ones, but not all [258, 264], found associations between low vitamin D levels and the presence and severity of diabetic retinopathy. In a cross-sectional study of the Children's Hospital at Westmead type 1 diabetes cohort, vitamin D levels were inversely associated with the presence diabetic retinopathy, which was assessed based on vascular lesions [261]. However, vitamin D levels were not associated with earlier biomarkers of retinal vascular geometry [266]. In the few longitudinal studies available, including the FIELD (type 2 diabetes) study [267], the VA Diabetes Trial [268], and a type 1 diabetes cohort, lower vitamin D levels at baseline were not an independent predictor of microvascular complications after adjustment for confounders [269].
Homocysteine. This is an essential amino acid, which is synthesized in the body from methionine, and it is lowered by higher levels of folic acid and vitamin B12. Homocysteine is associated with increased clotting, inflammation, endothelial dysfunction, disturbed angiogenesis, and vascular matrix composition. High homocysteine levels are associated with increased risk of vascular disease in the general population [270, 271]. On the other hand, lowering homocysteine levels (by B-group vitamin supplements) is not associated with a reduction in vascular disease risk [272].
Most [273-277], but not all [219], cross-sectional studies found higher levels of homocysteine in the blood to be associated with greater risk of diabetic retinopathy. A meta-analysis of 31 studies confirmed this association [272]. In a small cross-sectional study of plasma, aqueous, and vitreous fluid, high homocysteine levels in the eye were also associated with diabetic retinopathy [275]. However, there are few longitudinal studies. In a longitudinal type 1 diabetic patient study, homocysteine levels were only associated with the development of diabetic macular edema, but not other forms of retinopathy [218]. Further longitudinal studies and intervention studies are of interest, particularly against the background that metformin use is associated with lower B-group vitamin levels and homocysteine elevation [278, 279].
8. Molecular biomarkers
Diabetic retinopathy is thought to have both genetic and acquired (environmental) modulators. This assumption is supported by twin and family studies showing concordance for diabetic retinopathy [280]. As yet, no genetic markers are used in clinical practice in diabetes, and there are few genetic studies for diabetic retinopathy compared with the number of genetic studies for diabetes per se, diabetic nephropathy, or non-diabetic-related ocular diseases. The field of genetics and diabetic retinopathy will benefit from:
Detailed phenotyping (enabling comparisons between stages of retinopathy and between different studies)
Large studies in different ethnic groups
Collaborative endeavors
Adjustment for potential confounders and validation studies
Inclusion of biomarkers relevant to the genes
Use of more recently available molecular techniques
8.1 GWAS and SNPs
Earlier genetic studies, including twin and family studies, candidate gene and linkage studies, and small GWAS analyses are well reviewed and summarized by Kuo et al. [280]. Genes related to pathways and other biomarkers implicated in retinopathy have been identified in some, but not all studies, including genes related to:
VEGF
Aldose reductase
RAAS (ACE)
Lipoproteins (ApoE, PON)
EPO
Inflammation (TNF, ICAM1)
Unlike candidate gene studies, a genome-wide association study (GWAS) provides an unbiased assessment of genes implicated in a disease process. GWAS in diabetic retinopathy are emerging, and have suggested several loci related to diabetic retinopathy risk. For example, a Japanese study (<1000 cases and controls) reported associations between the long non-coding (lnc) RNA RP1-90L14 and susceptibility to diabetic retinopathy [281]. These studies are relatively small as measured by GWAS standard (ranging from several hundred to several thousand subjects), do not include optimally characterized subjects, and await validation. In some cases, validation has not confirmed the original observation. Further and much larger studies are necessary; these should have adequately large sample size, standard definitions for retinopathy, and adjustment for confounders. This is a costly, but important endeavor. Even if susceptible or protective genes are identified, alterations in gene expression may occur because of environmental factors. Thus, studies of epigenetics are also important.
8.2 Telomeres
Telomeres are repetitive specialized DNA protein loop structures at chromosome ends that help stabilize chromosomes via prevention of degradation, end-to-end fusion, and abnormal DNA recombination. Telomeres shorten, approximately 30-200 nucleotides get lost with each cell cycle, and once they reach a critical length, cell cycle arrest or apoptosis is activated [283-285]. Telomere length is highly heritable [286], and the telomere shortening process is thought to be accelerated by hyperglycemia, insulin resistance, oxidative stress, and inflammation [283-288].
Shorter telomeres have been suggested as both a marker and mediator of aging and age-related diseases, including cardiometabolic disorders [283-288], and may integrate both genetic and environmental risk markers. Telomeres are more readily measured in circulating white blood cells, but are also relevant to studies of cultured retinal cells and ocular tissues. There are several studies reporting:
Shorter telomeres in people with diabetes
Associations of shorter telomeres with the presence of diabetes complications [287, 288]
Predictive power for future nephropathy [289] and mortality [290]
However, to the best of our knowledge, there are no specific telomere length- and diabetic retinopathy-related studies.
In the West of Scotland Coronary Prevention Study (WOSCOPS), a large clinical study, statins attenuated the cardiovascular risk associated with shorter telomeres [291]. Therefore, telomere length may be a therapeutic target. It may be targeted via modulating telomerase activity that impacts telomere length. However, one of the challenges in this field of research is the measurement of telomere length. Most studies use a qPCR based method, where differences in the standard used can affect study results and make comparisons between studies and laboratories difficult. Alternate approaches comprise the measurement of absolute telomere length by pulsed field gel electrophoresis [292], which is more labor-intensive and therefore less readily applicable to large studies, and computation [293].
8.3 Epigenetics
Epigenetics is an exciting and relatively new field in molecular biology. It is aimed at providing insight into interactions between environmental factors and genes, which may also contribute to metabolic memory [294-299]. Epigenetic studies are relevant to cultured cells, tissue studies, as well as animal and human studies. Epigenetic biomarkers include DNA methylation, modification of histones, and non-coding RNAs, including microRNAs [298]. Further clinical and basic science studies in diabetic retinopathy are merited.
Histones are highly basic proteins found in eukaryotic nuclei that allow the negatively-charged DNA to be wrapped around them, thus enabling a higher order of packaging. Methylation or acetylation of specific histone tails changes their net charge, thereby influencing interactions with DNA. Histone methylation at various promoter regions can significantly alter transcriptional regulation at defined promoters, and cause suppression or inappropriately sustained gene expression, thus impacting normal cell function. Increased methylation of DNA and of specific amino acids on histone tails is inversely related to gene expression, leading to lower levels of RNA. Environmental factors, including changes in glycemia, smoking, obesity, lipids, maternal and early life, nutrition, and drugs can directly influence DNA/histone methylation, leading to alterations in gene expression [294-297, 300]. DNA and histone methylation and heritable changes to the genome (without any changes in DNA sequence) have been associated with CVD and diabetic vascular damage [294, 295, 297-299].
Histone methylation via histone methyltransferase (HMT) enzymes at lysine residues has been implicated in diabetic vascular complications [294, 295]. High glucose exposure of retinal capillary endothelial cells induces histone methylation, which may lead to:
Altered transcriptional activity of Sp1 at the Keap1 promoter
Transcriptional activity of Nrf2
Protection from anti-oxidant gene expression [297]
Of relevance to metabolic memory, only 16 hours of high glucose exposure of vascular endothelial cells may cause epigenetic changes that activate NF-κB at the p65 promoter and increase oxidative stress, even after six days of return to normal glucose levels [295]. There is evidence from animal models that a histone deacetylase inhibitor (a drug class being tested in human cancer trials) ameliorates diabetic nephropathy [15, 16]. Also, cultured retinal cells exposed to fenofibrate show epigenetic changes, including anti-inflammatory effects. Fenofibrate suppresses cellular metabolic memory of high glucose in diabetic retinopathy via a sirtuin 1-dependent signaling pathway [301]. However, as yet, there are no publications regarding the metabolic memory for fenofibrate exposure in the FIELD or ACCORD trials. Further clinical and ocular studies are of great interest.
MicroRNAs are another group of biomarkers of great interest in diabetic retinopathy. Whilst gene sequences cannot be changed, the expression of genes can be altered by microRNAs, with each microRNA influencing the expression of multiple genes.
MicroRNAs are a group of small (~22 nucleotide) single-stranded RNA molecules that do not code for protein, but act post-transcriptionally to modulate expression of target genes, including genes involved in the following physiological and disease processes through a mechanism that inhibits protein expression by interfering with mRNA translation and stability [302]:
Angiogenesis
Blood flow
Neural dysfunction
Tissue-specific inflammation
Glucose metabolism
MicroRNAs can cross cellular boundaries and communicate with other cells via gap junctions, through exosome-mediated transfer, and via ruptured cell membranes (as occurs in cell death). Thus, microRNAs can exist outside cells, and be efficiently detected in the circulation (free, associated with proteins, or in membrane bound particles) [303], and in ocular tissues, including vitreous [304] and aqueous [305] fluids. Their value as biomarker is increased by the fact that they are resistant to degradation by endogenous nucleases and by repeated freeze-thawing. They also have high detection capacity due to their long half-life (days to weeks) in serum.
Recently, three miRNAs in serum of patients with diabetic retinopathy have been reported as potential biomarkers of the disease [4, 302, 306]. Our team is currently taking a discovery rather than a candidate approach to microRNAs for late-stage diabetic retinopathy, and recently published a paper highlighting the impact of the instrumentation used for detection of these molecular biomarkers [85]. This new research area holds considerable clinical potential for diagnosis, prognosis, and identifying of potential drug-targets, and even for microRNAs being therapeutic agents themselves.
Using recently available and evolving molecular tools, the investigation of genetic SNPs, telomeres, and epigenetic profiles as predictors and modulators of diabetic retinopathy requires large cohorts with many events to confer sufficient power for a reliable identification of moderate associations, and for the exploration of markers of treatment benefits. Such studies are costly, but worthwhile, as results may also facilitate identification of new mechanisms of retinal damage and protection. Coupled with excellent phenotyping, study results should help advancing our understanding of the disease process, and provide new ways to prevent vascular damage and protect vision.
9. Emerging biomarkers
9.1 'Omics' platforms
Proteomics and metabolomics are emerging analytical platforms in diabetic retinopathy. They are of interest, particularly when linked with related studies of the genome and of biochemical and clinical biomarkers.
Proteomics is the large scale study of protein structure and function. It can be applied to cultured cells, and to plasma, serum, urine, and tissues, including ocular tissues such as the vitreous in animals and humans. Proteomics studies are starting to emerge in the field of diabetic retinopathy [27, 28, 307-313]. Usually, dozens of differentially expressed proteins are identified, but subject numbers are usually small, and confounders such as intraocular blood and clinical factors are often not well considered.
Metabolomics is the "systematic study of the unique chemical fingerprints that specific cellular processes leave behind". It is the study of the small molecule metabolite profiles. A range of analytical techniques for fluids or tissues are available, often via shared (core) resources. In many cases, the multiple, highly complex substance to be analyzed within a sample are separated (e.g. by capillary electrophoresis, HPLC, or gas chromatography). The separation is carried out prior to detection by different methods, including NMR, various types of mass spectroscopy, ion-mobility spectrometry, electrochemical detection, and radiolabel detection. As with proteomics, the data output is complex, and requires specific software and data analysis.
As yet, there are few and small metabolomics studies in diabetic retinopathy. A small Chinese study (n = 89) compared plasma from non-diabetic subjects and diabetic patients without retinopathy with plasma from patients with non-proliferative and proliferative retinopathy. The authors reported differences in fatty acid, amino acids, and glucose metabolites [314]. In another study of vitreous from type 1 diabetic vs. non-diabetic patients, PDR-related vitreous had higher levels of glucose and lactate and lower levels of ascorbic acid [315]. Further studies in the field are needed.
9.2 Tear analysis
An evolving area for seeking biomarkers for diabetic retinopathy is tear analysis. Like blood, tears are readily accessible. A small prospective study, with 16 subjects per group, found higher levels of TNFα in tears from subjects with vs. without type 2 diabetes and with vs. without diabetic retinopathy [75]. Ideally, circulating levels should also be quantified, and corrected for potential confounders such as glycemia and nephropathy status.
Tear fluid proteomics has also been investigated as an alternative tool for diabetic retinopathy screening. In a cross-sectional study of tear proteomics, levels of lipocalin 1, lactotransferrin, lacritin, lysozyme C, lipophilin A, and the immunoglobulin lambda chain were at significantly higher relative levels in the tears of patients with diabetic retinopathy [316]. As this is also a new and promising field of studies, we expect a wealth of new research, also into diabetic retinopathy, which would be warranted.
10. Conclusions and future directions
Whilst the prognosis and number of treatments available for diabetic retinopathy have increased over the past few decades, at least in affluent regions, diabetic retinopathy remains a common and feared cause of vision loss. With the global diabetes epidemic, the number of people at risk of diabetic retinopathy is likely to increase substantially.
Biomarkers are already used widely in clinical research and clinical practice, and have contributed to improved treatment strategies. However, we still cannot predict accurately who will develop clinically significant diabetic retinopathy, nor can we reliably predict treatment responses. All treatments are associated with potential side-effects, which could be monitored by biomarkers. With an increasingly sophisticated array of clinical, biochemical, and molecular biomarker assays available, clinical and basic research and clinical practice could make further progress. Therefore, further biomarker research is a worthwhile endeavor to improve our understanding of diabetic retinopathy and clinical outcomes.
Biobanking, collaborations, and careful consideration of pre-analytic, analytic, and post-analytic factors in biomarker assays are important considerations in progress towards reducing the burden of diabetic retinopathy.
Acknowledgments
Acknowledgments
The authors wish to express their thanks to their many collaborators, clinical and research colleagues, patients, and research study participants from around the world. They all have contributed greatly to improved knowledge of diabetic retinopathy. We also wish to acknowledge funding support from the NHMRC Australia, the Sydney Medical School Foundation, the Fred Hollows Foundation, and the Juvenile Diabetes Research Foundation.
Disclosures
The authors report no conflict of interests.
References
- 1.IDF. 6th Atlas IDF, International Diabetes Federation, Diabetes Atlas. 2011. [Google Scholar]
- 2.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elahy M, Baindur-Hudson S, Cruzat VF, Newsholme P, Dass CR. Mechanisms of PEDF-mediated protection against reactive oxygen species damage in diabetic retinopathy and neuropathy. J Endocrinol. 2014;222(3):R129–R139. doi: 10.1530/JOE-14-0065. [DOI] [PubMed] [Google Scholar]
- 4.Qing S, Yuan S, Yun C, Hui H, Mao P, Wen F, Ding Y, Liu Q. Serum miRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell Physiol Biochem. 2014;34(5):1733–1740. doi: 10.1159/000366374. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes: Australian facts 2008. WELFARE A I O H A, Editor 2008, Diabetes series no. 8. Cat. no. CVD 40. AIHW; Canberra: [Google Scholar]
- 6.Elamin A, Altahir H, Ismail B, Tuvemo T. Clinical pattern of childhood type 1 (insulin-dependent) diabetes mellitus in the Sudan. Diabetologia. 1992;35(7):645–648. doi: 10.1007/BF00400256. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8(11):639–649. doi: 10.1038/nrendo.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunwald JE, Ying GS, Maguire M, Pistilli M, Daniel E, Alexander J, Whittock-Martin R, Parker C, Mohler E, Lo JC. et al. Association between retinopathy and cardiovascular disease in patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort (CRIC) Study) Am J Cardiol. 2012;110(2):246–253. doi: 10.1016/j.amjcard.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahr AJ, Molitch ME. Diabetes, cardiovascular risk and nephropathy. Cardiol Clin. 2010;28(3):467–475. doi: 10.1016/j.ccl.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Son JW, Jang EH, Kim MK, Kim IT, Roh YJ, Baek KH, Song KH, Yoon KH, Cha BY, Lee KW. et al. Diabetic retinopathy is associated with subclinical atherosclerosis in newly diagnosed type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;91(2):253–259. doi: 10.1016/j.diabres.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Guidelines for Diabetic Retinopathy Management. https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/di15.pdf.
- 12.Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, Twigg SM, Yue DK, Wong J. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36(12):3863–3869. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eppens MC, Craig ME, Cusumano J, Hing S, Chan AK, Howard NJ, Silink M, Donaghue KC. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29(6):1300–1306. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 14.Wong J, Constantino M, Yue DK. Morbidity and mortality in young-onset type 2 diabetes in comparison to type 1 diabetes: where are we now? Curr Diab Rep. 2015;15(1):566. doi: 10.1007/s11892-014-0566-1. [DOI] [PubMed] [Google Scholar]
- 15.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J. et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyto JP, Harjutsalo V, Forsblom C, Hietala K, Summanen PA, Groop PH. Decline in the cumulative incidence of severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care. 2011;34(9):2005–2007. doi: 10.2337/dc10-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans JR, Michelessi M, Virgili G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2014;11:CD011234. doi: 10.1002/14651858.CD011234.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Zapata MJ, Marti-Carvajal AJ, Sola I, Pijoan JI, Buil-Calvo JA, Cordero JA, Evans JR. Anti-vascular endothelial growth factor for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2014;11:CD008721. doi: 10.1002/14651858.CD008721.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simo R, Hernandez C. Advances in the medical treatment of diabetic retinopathy. Diabetes Care. 2009;32(8):1556–1562. doi: 10.2337/dc09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J 3rd. Factors of risk in the development of coronary heart disease - six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 22.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 23.Januszewski AS, Karschimkus C, Davis KE, O'Neal D, Ward G, Jenkins AJ. Plasma 1,5 anhydroglucitol levels, a measure of short-term glycaemia: assay assessment and lower levels in diabetic vs. non-diabetic subjects. Diabetes Res Clin Pract. 2012;95(1):e17–e19. doi: 10.1016/j.diabres.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Hermans MP, Fioretto P, Valensi P, Davis T, Horton E, Wanner C, Al-Rubeaan K, Aronson R, Barzon I. et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation. 2014;129(9):999–1008. doi: 10.1161/CIRCULATIONAHA.113.002529. [DOI] [PubMed] [Google Scholar]
- 25.Lyons TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT, Klein RL. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45(3):910–918. doi: 10.1167/iovs.02-0648. [DOI] [PubMed] [Google Scholar]
- 26.Lopes-Virella MF, Baker NL, Hunt KJ, Lyons TJ, Jenkins AJ, Virella G. High concentrations of AGE-LDL and oxidized LDL in circulating immune complexes are associated with progression of retinopathy in type 1 diabetes. Diabetes Care. 2012;35(6):1333–1340. doi: 10.2337/dc11-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torok Z, Peto T, Csosz E, Tukacs E, Molnar A, Maros-Szabo Z, Berta A, Tozser J, Hajdu A, Nagy V. et al. Tear fluid proteomics multimarkers for diabetic retinopathy screening. BMC Ophthalmol. 2013;13(1):40. doi: 10.1186/1471-2415-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torok Z, Peto T, Csosz E, Tukacs E, Molnar AM, Berta A, Tozser J, Hajdu A, Nagy V, Domokos B. et al. Combined methods for diabetic retinopathy screening, using retina photographs and tear fluid proteomics biomarkers. J Diabetes Res. 2015;2015:623619. doi: 10.1155/2015/623619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartzman ML, Iserovich P, Gotlinger K, Bellner L, Dunn MW, Sartore M, Grazia Pertile M, Leonardi A, Sathe S, Beaton A. et al. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes. 2010;59(7):1780–1788. doi: 10.2337/db10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Australian absolute cardiovascular disease risk calculator. http://www.cvdcheck.org.au/
- 31.Soedamah-Muthu SS, Vergouwe Y, Costacou T, Miller RG, Zgibor J, Chaturvedi N, Snell-Bergeon JK, Maahs DM, Rewers M, Forsblom C. et al. Predicting major outcomes in type 1 diabetes: a model development and validation study. Diabetologia. 2014;57(11):2304–2314. doi: 10.1007/s00125-014-3358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unger FT, Witte I, David KA. Prediction of individual response to anticancer therapy: historical and future perspectives. Cell Mol Life Sci. 2015;72(4):729–757. doi: 10.1007/s00018-014-1772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115(11):1859–1868. doi: 10.1016/j.ophtha.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stratton IM, Aldington SJ, Taylor DJ, Adler AI, Scanlon PH. A simple risk stratification for time to development of sight-threatening diabetic retinopathy. Diabetes Care. 2013;36(3):580–585. doi: 10.2337/dc12-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor-Phillips S, Mistry H, Leslie R, Todkill D, Tsertsvadze A, Connock M, Clarke A. Extending the diabetic retinopathy screening interval beyond 1 year: systematic review. Br J Ophthalmol. 2015 doi: 10.1136/bjophthalmol-2014-305938. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aspelund T, Thornorisdottir O, Olafsdottir E, Gudmundsdottir A, Einarsdottir AB, Mehlsen J, Einarsson S, Palsson O, Einarsson G, Bek T. et al. Individual risk assessment and information technology to optimise screening frequency for diabetic retinopathy. Diabetologia. 2011;54(10):2525–2532. doi: 10.1007/s00125-011-2257-7. [DOI] [PubMed] [Google Scholar]
- 37.Price LD, Au S, Chong NV. Optomap ultrawide field imaging identifies additional retinal abnormalities in patients with diabetic retinopathy. Clin Ophthalmol. 2015;9:527–531. doi: 10.2147/OPTH.S79448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva PS, Cavallerano JD, Haddad NM, Kwak H, Dyer KH, Omar AF, Shikari H, Aiello LM, Sun JK, Aiello LP. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122(5):949–956. doi: 10.1016/j.ophtha.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 39.De Clerck EE, Schouten JS, Berendschot TT, Kessels AG, Nuijts RM, Beckers HJ, Schram MT, Stehouwer CD, Webers CA. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3(8):653–663. doi: 10.1016/S2213-8587(15)00136-9. [DOI] [PubMed] [Google Scholar]
- 40.Virgili G, Menchini F, Casazza G, Hogg R, Das RR, Wang X, Michelessi M. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD008081. doi: 10.1002/14651858.CD008081.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen TT, Wong TY. Retinal vascular changes and diabetic retinopathy. Curr Diab Rep. 2009;9(4):277–283. doi: 10.1007/s11892-009-0043-4. [DOI] [PubMed] [Google Scholar]
- 42.Pescosolido N, Barbato A, Stefanucci A, Buomprisco G. Role of electrophysiology in the early diagnosis and follow-up of diabetic retinopathy. J Diabetes Res. 2015;2015:319692. doi: 10.1155/2015/319692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36(7):808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins A, O'Neal D. The legacy effect in type 2 diabetes. Diabetes Manag (Lond) 2010;30:6–7. [Google Scholar]
- 45.Thomas MC. Glycemic exposure, glycemic control, and metabolic karma in diabetic complications. Adv Chronic Kidney Dis. 2014;21(3):311–317. doi: 10.1053/j.ackd.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Aiello LP, Sun W, Das A, Gangaputra S, Kiss S, Klein R, Cleary PA, Lachin JM, Nathan DM. Intensive diabetes therapy and ocular surgery in type 1 diabetes. N Engl J Med. 2015;372(18):1722–1733. doi: 10.1056/NEJMoa1409463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64(2):631–642. doi: 10.2337/db14-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White NH, Sun W, Cleary PA, Danis RP, Davis MD, Hainsworth DP, Hubbard LD, Lachin JM, Nathan DM. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol. 2008;126(12):1707–1715. doi: 10.1001/archopht.126.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 50.Genuth S, Sun W, Cleary P, Gao X, Sell DR, Lachin J, Monnier VM. Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes. 2015;64(1):266–278. doi: 10.2337/db14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cencioni C, Spallotta F, Greco S, Martelli F, Zeiher AM, Gaetano C. Epigenetic mechanisms of hyperglycemic memory. Int J Biochem Cell Biol. 2014;51:155–158. doi: 10.1016/j.biocel.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Miao F, Chen Z, Genuth S, Paterson A, Zhang L, Wu X, Li SM, Cleary P, Riggs A, Harlan DM. et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes. 2014;63(5):1748–1762. doi: 10.2337/db13-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jermendy G. Vascular memory: can we broaden the concept of the metabolic memory? Cardiovasc Diabetol. 2012;11:44. doi: 10.1186/1475-2840-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenkins AJ, Steele JS, Janus ED, Best JD. Increased plasma apolipoprotein(a) levels in IDDM patients with microalbuminuria. Diabetes. 1991;40(6):787–790. doi: 10.2337/diab.40.6.787. [DOI] [PubMed] [Google Scholar]
- 55.Jenkins AJ, Steele JS, Janus ED, Santamaria JD, Best JD. Plasma apolipoprotein (a) is increased in type 2 (non-insulin-dependent) diabetic patients with microalbuminuria. Diabetologia. 1992;35(11):1055–1059. doi: 10.1007/BF02221681. [DOI] [PubMed] [Google Scholar]
- 56.Lee P, Jenkins A, Bourke C, Santamaria J, Paton C, Janus E, Best J. Prothrombotic and antithrombotic factors are elevated in patients with type 1 diabetes complicated by microalbuminuria. Diabet Med. 1993;10(2):122–128. doi: 10.1111/j.1464-5491.1993.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 57.Agardh E, Lundstig A, Perfilyev A, Volkov P, Freiburghaus T, Lindholm E, Ronn T, Agardh CD, Ling C. Genome-wide analysis of DNA methylation in subjects with type 1 diabetes identifies epigenetic modifications associated with proliferative diabetic retinopathy. BMC Med. 2015;13:182. doi: 10.1186/s12916-015-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burdon KP, Fogarty RD, Shen W, Abhary S, Kaidonis G, Appukuttan B, Hewitt AW, Sharma S, Daniell M, Essex RW. et al. Genome-wide association study for sight-threatening diabetic retinopathy reveals association with genetic variation near the GRB2 gene. Diabetologia. 2015;58(10):2288–2297. doi: 10.1007/s00125-015-3697-2. [DOI] [PubMed] [Google Scholar]
- 59.Hosseini SM, Boright AP, Sun L, Canty AJ, Bull SB, Klein BE, Klein R, Paterson AD. The association of previously reported polymorphisms for microvascular complications in a meta-analysis of diabetic retinopathy. Hum Genet. 2015;134(2):247–257. doi: 10.1007/s00439-014-1517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwak SH, Park KS. Genetic studies on diabetic microvascular complications: focusing on genome-wide association studies. Endocrinol Metab (Seoul) 2015;30(2):147–158. doi: 10.3803/EnM.2015.30.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jenkins AJ, Zhang SX, Rowley KG, Karschimkus CS, Nelson CL, Chung JS, O'Neal DN, Januszewski AS, Croft KD, Mori TA. et al. Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in Type 1 diabetes. Diabet Med. 2007;24(12):1345–1351. doi: 10.1111/j.1464-5491.2007.02281.x. [DOI] [PubMed] [Google Scholar]
- 62.Li S, Fu XA, Zhou XF, Chen YY, Chen WQ. Angiogenesis-related cytokines in serum of proliferative diabetic retinopathy patients before and after vitrectomy. Int J Ophthalmol. 2012;5(6):726–730. doi: 10.3980/j.issn.2222-3959.2012.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boehm BO, Lang G, Feldmann B, Kurkhaus A, Rosinger S, Volpert O, Lang GK, Bouck N. Proliferative diabetic retinopathy is associated with a low level of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor. A pilot study. Horm Metab Res. 2003;35(6):382–386. doi: 10.1055/s-2003-41362. [DOI] [PubMed] [Google Scholar]
- 64.Boehm BO, Lang G, Volpert O, Jehle PM, Kurkhaus A, Rosinger S, Lang GK, Bouck N. Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia. 2003;46(3):394–400. doi: 10.1007/s00125-003-1040-9. [DOI] [PubMed] [Google Scholar]
- 65.McAuley AK, Sanfilippo PG, Hewitt AW, Liang H, Lamoureux E, Wang JJ, Connell PP. Vitreous biomarkers in diabetic retinopathy: a systematic review and meta-analysis. J Diabetes Complications. 2014;28(3):419–425. doi: 10.1016/j.jdiacomp.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Yasuda M, Shimura M, Kunikata H, Kanazawa H, Yasuda K, Tanaka Y, Konno H, Takahashi M, Kokubun T, Maruyama K. et al. Relationship of skin autofluorescence to severity of retinopathy in type 2 diabetes. Curr Eye Res. 2015;40(3):338–345. doi: 10.3109/02713683.2014.918152. [DOI] [PubMed] [Google Scholar]
- 67.Januszewski AS, Sachithanandan N, Karschimkus C, O'Neal DN, Yeung CK, Alkatib N, Jenkins AJ. Non-invasive measures of tissue autofluorescence are increased in Type 1 diabetes complications and correlate with a non-invasive measure of vascular dysfunction. Diabet Med. 2012;29(6):726–733. doi: 10.1111/j.1464-5491.2011.03562.x. [DOI] [PubMed] [Google Scholar]
- 68.Araszkiewicz A, Naskret D, Zozulinska-Ziolkiewicz D, Pilacinski S, Uruska A, Grzelka A, Wegner M, Wierusz-Wysocka B. Skin autofluorescence is associated with carotid intima-media thickness, diabetic microangiopathy, and long-lasting metabolic control in type 1 diabetic patients. Results from Poznan Prospective Study. Microvasc Res. 2015;98:62–67. doi: 10.1016/j.mvr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Genevieve M, Vivot A, Gonzalez C, Raffaitin C, Barberger-Gateau P, Gin H, Rigalleau V. Skin autofluorescence is associated with past glycaemic control and complications in type 1 diabetes mellitus. Diabetes Metab. 2013;39(4):349–354. doi: 10.1016/j.diabet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 70.Hirano T, Iesato Y, Toriyama Y, Imai A, Chiba D, Murata T. Correlation between diabetic retinopathy severity and elevated skin autofluorescence as a marker of advanced glycation end-product accumulation in type 2 diabetic patients. J Diabetes Complications. 2014;28(5):729–734. doi: 10.1016/j.jdiacomp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Noordzij MJ, Mulder DJ, Oomen PH, Brouwer T, Jager J, Castro Cabezas M, Lefrandt JD, Smit AJ. Skin autofluorescence and risk of micro- and macrovascular complications in patients with type 2 diabetes mellitus - a multi-centre study. Diabet Med. 2012;29(12):1556–1561. doi: 10.1111/dme.12005. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka K, Tani Y, Asai J, Nemoto F, Kusano Y, Suzuki H, Hayashi Y, Asahi K, Nakayama M, Miyata T. et al. Skin autofluorescence is associated with severity of vascular complications in Japanese patients with type 2 diabetes. Diabet Med. 2012;29(4):492–500. doi: 10.1111/j.1464-5491.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- 73. http://www.lkc.com/products/RETeval/index-non-us.html.
- 74.Brzovic-Saric V, Landeka I, Saric B, Barberic M, Andrijasevic L, Cerovski B, Orsolic N, Dikic D. Levels of selected oxidative stress markers in the vitreous and serum of diabetic retinopathy patients. Mol Vis. 2015;21:649–664. [PMC free article] [PubMed] [Google Scholar]
- 75.Costagliola C, Romano V, De Tollis M, Aceto F, dell'Omo R, Romano MR, Pedicino C, Semeraro F. TNF-alpha levels in tears: a novel biomarker to assess the degree of diabetic retinopathy. Mediators Inflamm. 2013;2013:629529. doi: 10.1155/2013/629529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen-Khuong T, Everest-Dass AV, Kautto L, Zhao Z, Willcox MD, Packer NH. Glycomic characterization of basal tears and changes with diabetes and diabetic retinopathy. Glycobiology. 2015;25(3):269–283. doi: 10.1093/glycob/cwu108. [DOI] [PubMed] [Google Scholar]
- 77.von Thun und Hohenstein-Blaul N, Funke S, Grus FH. Tears as a source of biomarkers for ocular and systemic diseases. Exp Eye Res. 2013;117:126–137. doi: 10.1016/j.exer.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 78.McNair P, Nielsen SL, Christiansen C, Axelsson C. Gross errors made by routine blood sampling from two sites using a tourniquet applied at different positions. Clin Chim Acta. 1979;98(1-2):113–118. doi: 10.1016/0009-8981(79)90171-2. [DOI] [PubMed] [Google Scholar]
- 79.Kroll MH, Elin RJ. Interference with clinical laboratory analyses. Clin Chem. 1994;40(11 Pt 1):1996–2005. [PubMed] [Google Scholar]
- 80.Hofer SE, Bennetts B, Chan AK, Holloway B, Karschimkus C, Jenkins AJ, Silink M, Donaghue KC. Association between PON 1 polymorphisms, PON activity and diabetes complications. J Diabetes Complications. 2006;20(5):322–328. doi: 10.1016/j.jdiacomp.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 81.Garcia ME, Blanco JL, Caballero J, Gargallo-Viola D. Anticoagulants interfere with PCR used to diagnose invasive aspergillosis. J Clin Microbiol. 2002;40(4):1567–1568. doi: 10.1128/JCM.40.4.1567-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Juhan-Vague I, Alessi MC, Fossat C, Declerck PJ, Kruithof EK. Plasma determination of plasminogen activator inhibitor 1 antigen must be performed in blood collected on antiplatelet/anticoagulant mixture. Thromb Haemost. 1987;58(4):1096. [PubMed] [Google Scholar]
- 83.Kruithof EK, Nicolosa G, Bachmann F. Plasminogen activator inhibitor 1: development of a radioimmunoassay and observations on its plasma concentration during venous occlusion and after platelet aggregation. Blood. 1987;70(5):1645–1653. [PubMed] [Google Scholar]
- 84.Degenhardt TP, Grass L, Reddy S, Thorpe SR, Diamandis EP, Baynes JW. Technical note. The serum concentration of the advanced glycation end-product N epsilon-(carboxymethyl)lysine is increased in uremia. Kidney Int. 1997;52(4):1064–1067. doi: 10.1038/ki.1997.429. [DOI] [PubMed] [Google Scholar]
- 85.Farr RJ, Januszewski AS, Joglekar MV, Liang H, McAulley AK, Hewitt AW, Thomas HE, Loudovaris T, Kay TW, Jenkins A. et al. A comparative analysis of high-throughput platforms for validation of a circulating microRNA signature in diabetic retinopathy. Sci Rep. 2015;5:10375. doi: 10.1038/srep10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. https://www.abdserotec.com/helpful-elisa-hints.html.
- 87.Looker HC, Nyangoma SO, Cromie DT, Olson JA, Leese GP, Philip S, Black MW, Doig J, Lee N, Briggs A. et al. Predicted impact of extending the screening interval for diabetic retinopathy: the Scottish Diabetic Retinopathy Screening programme. Diabetologia. 2013;56(8):1716–1725. doi: 10.1007/s00125-013-2928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stefansson E, Bek T, Porta M, Larsen N, Kristinsson JK, Agardh E. Screening and prevention of diabetic blindness. Acta Ophthalmol Scand. 2000;78(4):374–385. doi: 10.1034/j.1600-0420.2000.078004374.x. [DOI] [PubMed] [Google Scholar]
- 89.Thomas RL, Dunstan FD, Luzio SD, Chowdhury SR, North RV, Hale SL, Gibbins RL, Owens DR. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol. 2015;99(1):64–68. doi: 10.1136/bjophthalmol-2013-304017. [DOI] [PubMed] [Google Scholar]
- 90.Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587–2595. doi: 10.1016/j.ophtha.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 91.Wessel MM, Aaker GD, Parlitsis G, Cho M, D'Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785–791. doi: 10.1097/IAE.0b013e3182278b64. [DOI] [PubMed] [Google Scholar]
- 92.Shi L, Wu H, Dong J, Jiang K, Lu X, Shi J. Telemedicine for detecting diabetic retinopathy: a systematic review and meta-analysis. Br J Ophthalmol. 2015;99(6):823–831. doi: 10.1136/bjophthalmol-2014-305631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohner EM. The retinal blood flow in diabetes. Diabete Metab. 1993;19(5):401–404. [PubMed] [Google Scholar]
- 94.Kohner EM, Hamilton AM, Saunders SJ, Sutcliffe BA, Bulpitt CJ. The retinal blood flow in diabetes. Diabetologia. 1975;11(1):27–33. doi: 10.1007/BF00422814. [DOI] [PubMed] [Google Scholar]
- 95.Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44(6):603–607. doi: 10.2337/diab.44.6.603. [DOI] [PubMed] [Google Scholar]
- 96.Lockhart CJ, McCann AJ, Pinnock RA, Hamilton PK, Harbinson MT, McVeigh GE. Multimodal functional and anatomic imaging identifies preclinical microvascular abnormalities in type 1 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2014;307(12):H1729–H1736. doi: 10.1152/ajpheart.00372.2014. [DOI] [PubMed] [Google Scholar]
- 97.Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37(5):886–897. [PubMed] [Google Scholar]
- 98.Ciulla TA, Harris A, Latkany P, Piper HC, Arend O, Garzozi H, Martin B. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002;80(5):468–477. doi: 10.1034/j.1600-0420.2002.800503.x. [DOI] [PubMed] [Google Scholar]
- 99.Klein R, Klein BE, Moss SE, Wong TY, Hubbard L, Cruickshanks KJ, Palta M. Retinal vascular abnormalities in persons with type 1 diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology. 2003;110(11):2118–2125. doi: 10.1016/S0161-6420(03)00863-7. [DOI] [PubMed] [Google Scholar]
- 100.Schmetterer L, Wolzt M. Ocular blood flow and associated functional deviations in diabetic retinopathy. Diabetologia. 1999;42(4):387–405. doi: 10.1007/s001250051171. [DOI] [PubMed] [Google Scholar]
- 101.Klein R, Myers CE, Lee KE, Gangnon R, Klein BE. Changes in retinal vessel diameter and incidence and progression of diabetic retinopathy. Arch Ophthalmol. 2012;130(6):749–755. doi: 10.1001/archophthalmol.2011.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frederiksen CA, Jeppesen P, Knudsen ST, Poulsen PL, Mogensen CE, Bek T. The blood pressure-induced diameter response of retinal arterioles decreases with increasing diabetic maculopathy. Graefes Arch Clin Exp Ophthalmol. 2006;244(10):1255–1261. doi: 10.1007/s00417-006-0262-1. [DOI] [PubMed] [Google Scholar]
- 103.Jeppesen P, Knudsen ST, Poulsen PL, Hessellund A, Schmitz O, Bek T. Acute hyperinsulinemia increases the contraction of retinal arterioles induced by elevated blood pressure. Am J Physiol Heart Circ Physiol. 2013;305(11):H1600–H1604. doi: 10.1152/ajpheart.00560.2013. [DOI] [PubMed] [Google Scholar]
- 104.Pedersen L, Jeppesen P, Knudsen ST, Poulsen PL, Bek T. Improvement of mild retinopathy in type 2 diabetic patients correlates with narrowing of retinal arterioles. A prospective observational study. Graefes Arch Clin Exp Ophthalmol. 2014;252(10):1561–1567. doi: 10.1007/s00417-014-2614-6. [DOI] [PubMed] [Google Scholar]
- 105.Meeking DR, Cummings MH, Thorne S, Donald A, Clarkson P, Crook JR, Watts GF, Shaw KM. Endothelial dysfunction in type 2 diabetic subjects with and without microalbuminuria. Diabet Med. 1999;16(10):841–847. doi: 10.1046/j.1464-5491.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 106.Wilson AM, O'Neal D, Nelson CL, Prior DL, Best JD, Jenkins AJ. Comparison of arterial assessments in low and high vascular disease risk groups. Am J Hypertens. 2004;17(4):285–291. doi: 10.1016/j.amjhyper.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 107.McVeigh GE. Arterial compliance in hypertension and diabetes mellitus. Am J Nephrol. 1996;16(3):217–222. doi: 10.1159/000169001. [DOI] [PubMed] [Google Scholar]
- 108.Bek T, Hajari J, Jeppesen P. Interaction between flicker-induced vasodilatation and pressure autoregulation in early retinopathy of type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2008;246(5):763–769. doi: 10.1007/s00417-008-0766-y. [DOI] [PubMed] [Google Scholar]
- 109.Pemp B, Garhofer G, Weigert G, Karl K, Resch H, Wolzt M, Schmetterer L. Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Invest Ophthalmol Vis Sci. 2009;50(9):4029–4032. doi: 10.1167/iovs.08-3260. [DOI] [PubMed] [Google Scholar]
- 110.Nguyen TT, Kawasaki R, Wang JJ, Kreis AJ, Shaw J, Vilser W, Wong TY. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care. 2009;32(11):2075–2080. doi: 10.2337/dc09-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lecleire-Collet A, Audo I, Aout M, Girmens JF, Sofroni R, Erginay A, Le Gargasson JF, Mohand-Said S, Meas T, Guillausseau PJ. et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011;52(6):2861–2867. doi: 10.1167/iovs.10-5960. [DOI] [PubMed] [Google Scholar]
- 112.Nguyen TT, Kawasaki R, Kreis AJ, Wang JJ, Shaw J, Vilser W, Wong TY. Correlation of light-flicker-induced retinal vasodilation and retinal vascular caliber measurements in diabetes. Invest Ophthalmol Vis Sci. 2009;50(12):5609–5613. doi: 10.1167/iovs.09-3442. [DOI] [PubMed] [Google Scholar]
- 113.Noonan JE, Dusting GJ, Nguyen TT, Jenkins AJ, Man RE, Best WJ, Dias DA, Jayasinghe NS, Roessner U, Lamoureux EL. Flicker light-induced retinal vasodilation is unaffected by inhibition of epoxyeicosatrienoic acids and prostaglandins in humans. Invest Ophthalmol Vis Sci. 2014;55(10):7007–7013. doi: 10.1167/iovs.14-14947. [DOI] [PubMed] [Google Scholar]
- 114.Hammer M, Vilser W, Riemer T, Mandecka A, Schweitzer D, Kuhn U, Dawczynski J, Liemt F, Strobel J. Diabetic patients with retinopathy show increased retinal venous oxygen saturation. Graefes Arch Clin Exp Ophthalmol. 2009;247(8):1025–1030. doi: 10.1007/s00417-009-1078-6. [DOI] [PubMed] [Google Scholar]
- 115.Hardarson SH. Retinal oximetry. Acta Ophthalmol. 2013;91(5):489–490. doi: 10.1111/aos.12239. [DOI] [PubMed] [Google Scholar]
- 116.Hardarson SH, Stefansson E. Retinal oxygen saturation is altered in diabetic retinopathy. Br J Ophthalmol. 2012;96(4):560–563. doi: 10.1136/bjophthalmol-2011-300640. [DOI] [PubMed] [Google Scholar]
- 117.Jorgensen CM, Hardarson SH, Bek T. The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision-threatening retinopathy. Acta Ophthalmol. 2014;92(1):34–39. doi: 10.1111/aos.12283. [DOI] [PubMed] [Google Scholar]
- 118.Ikram MK, Cheung CY, Lorenzi M, Klein R, Jones TL, Wong TY. Retinal vascular caliber as a biomarker for diabetes microvascular complications. Diabetes Care. 2013;36(3):750–759. doi: 10.2337/dc12-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wong TY. Retinal vessel diameter as a clinical predictor of diabetic retinopathy progression: time to take out the measuring tape. Arch Ophthalmol. 2011;129(1):95–96. doi: 10.1001/archophthalmol.2010.347. [DOI] [PubMed] [Google Scholar]
- 120.Rand LI, Krolewski AS, Aiello LM, Warram JH, Baker RS, Maki T. Multiple factors in the prediction of risk of proliferative diabetic retinopathy. N Engl J Med. 1985;313(23):1433–1438. doi: 10.1056/NEJM198512053132302. [DOI] [PubMed] [Google Scholar]
- 121.Goldberg M, Fine S. Symposium on the treatment of diabetic retinopathy. Airlie House, Department of Health E a W, Editor; Warrenton, VA, USA: 1968. pp. 1–913. [Google Scholar]
- 122.Alibrahim E, Donaghue KC, Rogers S, Hing S, Jenkins AJ, Chan A, Wong TY. Retinal vascular caliber and risk of retinopathy in young patients with type 1 diabetes. Ophthalmology. 2006;113(9):1499–1503. doi: 10.1016/j.ophtha.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 123.Benitez-Aguirre P, Craig ME, Cass HG, Sugden CJ, Jenkins AJ, Wang JJ, Cusumano J, Hodgson LA, Lee K, Wong TY. et al. Sex differences in retinal microvasculature through puberty in type 1 diabetes: are girls at greater risk of diabetic microvascular complications? Invest Ophthalmol Vis Sci. 2015;56(1):571–577. doi: 10.1167/iovs.14-15147. [DOI] [PubMed] [Google Scholar]
- 124.Cheung N, Rogers SL, Donaghue KC, Jenkins AJ, Tikellis G, Wong TY. Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care. 2008;31(9):1842–1846. doi: 10.2337/dc08-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsai AS, Wong TY, Lavanya R, Zhang R, Hamzah H, Tai ES, Cheung CY. Differential association of retinal arteriolar and venular caliber with diabetes and retinopathy. Diabetes Res Clin Pract. 2011;94(2):291–298. doi: 10.1016/j.diabres.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 126.Broe R, Rasmussen ML, Frydkjaer-Olsen U, Olsen BS, Mortensen HB, Hodgson L, Wong TY, Peto T, Grauslund J. Retinal vessel calibers predict long-term microvascular complications in type 1 diabetes: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987) Diabetes. 2014;63(11):3906–3914. doi: 10.2337/db14-0227. [DOI] [PubMed] [Google Scholar]
- 127.Klein R, Klein BE, Moss SE, Wong TY, Hubbard L, Cruickshanks KJ, Palta M. The relation of retinal vessel caliber to the incidence and progression of diabetic retinopathy: XIX: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2004;122(1):76–83. doi: 10.1001/archopht.122.1.76. [DOI] [PubMed] [Google Scholar]
- 128.Roy MS, Klein R, Janal MN. Retinal venular diameter as an early indicator of progression to proliferative diabetic retinopathy with and without high-risk characteristics in African Americans with type 1 diabetes mellitus. Arch Ophthalmol. 2011;129(1):8–15. doi: 10.1001/archophthalmol.2010.340. [DOI] [PubMed] [Google Scholar]
- 129.Liew G, Sim DA, Keane PA, Tan AG, Mitchell P, Wang JJ, Wong TY, Fruttiger M, Tufail A, Egan CA. Diabetic macular ischaemia is associated with narrower retinal arterioles in patients with type 2 diabetes. Acta Ophthalmol. 2015;93(1):e45–e51. doi: 10.1111/aos.12519. [DOI] [PubMed] [Google Scholar]
- 130.Klein R, Klein BE, Moss SE, Wong TY. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007;114(10):1884–1892. doi: 10.1016/j.ophtha.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 131.Grauslund J, Hodgson L, Kawasaki R, Green A, Sjolie AK, Wong TY. Retinal vessel calibre and micro- and macrovascular complications in type 1 diabetes. Diabetologia. 2009;52(10):2213–2217. doi: 10.1007/s00125-009-1459-8. [DOI] [PubMed] [Google Scholar]
- 132.Brazionis L, Yau J, Rowley K, Itsiopoulos C, O'Dea K, Wong TY, Jenkins A. Plasminogen activator inhibitor-1 (PAI-1) activity and retinal vascular calibre in type 2 diabetes. Diabetes Res Clin Pract. 2010;87(2):192–199. doi: 10.1016/j.diabres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 133.Benitez-Aguirre P, Craig ME, Sasongko MB, Jenkins AJ, Wong TY, Wang JJ, Cheung N, Donaghue KC. Retinal vascular geometry predicts incident retinopathy in young people with type 1 diabetes: a prospective cohort study from adolescence. Diabetes Care. 2011;34(7):1622–1627. doi: 10.2337/dc10-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Benitez-Aguirre PZ, Sasongko MB, Craig ME, Jenkins AJ, Cusumano J, Cheung N, Wong TY, Donaghue KC. Retinal vascular geometry predicts incident renal dysfunction in young people with type 1 diabetes. Diabetes Care. 2012;35(3):599–604. doi: 10.2337/dc11-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cheung N, Donaghue KC, Liew G, Rogers SL, Wang JJ, Lim SW, Jenkins AJ, Hsu W, Li Lee M, Wong TY. Quantitative assessment of early diabetic retinopathy using fractal analysis. Diabetes Care. 2009;32(1):106–110. doi: 10.2337/dc08-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lim SW, Cheung N, Wang JJ, Donaghue KC, Liew G, Islam FM, Jenkins AJ, Wong TY. Retinal vascular fractal dimension and risk of early diabetic retinopathy: A prospective study of children and adolescents with type 1 diabetes. Diabetes Care. 2009;32(11):2081–2083. doi: 10.2337/dc09-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sasongko MB, Wang JJ, Donaghue KC, Cheung N, Benitez-Aguirre P, Jenkins A, Hsu W, Lee ML, Wong TY. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care. 2010;33(6):1331–1336. doi: 10.2337/dc10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sasongko MB, Wong TY, Donaghue KC, Cheung N, Jenkins AJ, Benitez-Aguirre P, Wang JJ. Retinal arteriolar tortuosity is associated with retinopathy and early kidney dysfunction in type 1 diabetes. Am J Ophthalmol. 2012;153(1):176–183. doi: 10.1016/j.ajo.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 139.Bringmann A, Wiedemann P. Muller glial cells in retinal disease. Ophthalmologica. 2012;227(1):1–19. doi: 10.1159/000328979. [DOI] [PubMed] [Google Scholar]
- 140.Feng Y, Busch S, Gretz N, Hoffmann S, Hammes HP. Crosstalk in the retinal neurovascular unit - lessons for the diabetic retina. Exp Clin Endocrinol Diabetes. 2012;120(4):199–201. doi: 10.1055/s-0032-1304571. [DOI] [PubMed] [Google Scholar]
- 141.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26(11):2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Metea MR, Newman EA. Signalling within the neurovascular unit in the mammalian retina. Exp Physiol. 2007;92(4):635–640. doi: 10.1113/expphysiol.2006.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Simo R, Hernandez C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab. 2014;25(1):23–33. doi: 10.1016/j.tem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 144.Parisi V, Uccioli L. Visual electrophysiological responses in persons with type 1 diabetes. Diabetes Metab Res Rev. 2001;17(1):12–18. doi: 10.1002/dmrr.177. [DOI] [PubMed] [Google Scholar]
- 145.Han Y, Adams AJ, Bearse MA Jr, Schneck ME. Multifocal electroretinogram and short-wavelength automated perimetry measures in diabetic eyes with little or no retinopathy. Arch Ophthalmol. 2004;122(12):1809–1815. doi: 10.1001/archopht.122.12.1809. [DOI] [PubMed] [Google Scholar]
- 146.Harrison WW, Bearse MA Jr, Ng JS, Jewell NP, Barez S, Burger D, Schneck ME, Adams AJ. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci. 2011;52(2):772–777. doi: 10.1167/iovs.10-5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun JK, Radwan SH, Soliman AZ, Lammer J, Lin MM, Prager SG, Silva PS, Aiello LB, Aiello LP. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015;64(7):2560–2570. doi: 10.2337/db14-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 149.Lind M, Oden A, Fahlen M, Eliasson B. The shape of the metabolic memory of HbA1c: re-analysing the DCCT with respect to time-dependent effects. Diabetologia. 2010;53(6):1093–1098. doi: 10.1007/s00125-010-1706-z. [DOI] [PubMed] [Google Scholar]
- 150.Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther. 2012;14(Suppl 1):S51–S58. doi: 10.1089/dia.2012.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kilpatrick ES, Rigby AS, Atkin SL, Frier BM. Does severe hypoglycaemia influence microvascular complications in Type 1 diabetes? An analysis of the Diabetes Control and Complications Trial database. Diabet Med. 2012;29(9):1195–1198. doi: 10.1111/j.1464-5491.2012.03612.x. [DOI] [PubMed] [Google Scholar]
- 152.Feldman-Billard S, Dupas B, Sedira N, Bitu J, Erginay A, Guillausseau PJ, Massin P. Hypoglycaemia is associated with the absence of a decrease in diurnal macular thickness in patients with diabetic macular oedema. Diabetes Metab. 2013;39(2):169–173. doi: 10.1016/j.diabet.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 153.Cavalot F. Do data in the literature indicate that glycaemic variability is a clinical problem? Glycaemic variability and vascular complications of diabetes. Diabetes Obes Metab. 2013;15(Suppl 2):3–8. doi: 10.1111/dom.12140. [DOI] [PubMed] [Google Scholar]
- 154.McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care. 2004;27(6):1259–1264. doi: 10.2337/diacare.27.6.1259. [DOI] [PubMed] [Google Scholar]
- 155.Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010;12(4):288–298. doi: 10.1111/j.1463-1326.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 156.Lachin JM, Genuth S, Nathan DM, Rutledge BN. The hemoglobin glycation index is not an independent predictor of the risk of microvascular complications in the Diabetes Control and Complications Trial. Diabetes. 2007;56(7):1913–1921. doi: 10.2337/db07-0028. [DOI] [PubMed] [Google Scholar]
- 157.Cosson E, Banu I, Cussac-Pillegand C, Chen Q, Chiheb S, Jaber Y, Nguyen MT, Charnaux N, Valensi P. Glycation gap is associated with macroproteinuria but not with other complications in patients with type 2 diabetes. Diabetes Care. 2013;36(7):2070–2076. doi: 10.2337/dc12-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nayak AU, Nevill AM, Bassett P, Singh BM. Association of glycation gap with mortality and vascular complications in diabetes. Diabetes Care. 2013;36(10):3247–3253. doi: 10.2337/dc12-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Szwergold BS, Beisswenger PJ. Enzymatic deglycation - a new paradigm or an epiphenomenon? Biochem Soc Trans. 2003;31(Pt 6):1428–1432. doi: 10.1042/bst0311428. [DOI] [PubMed] [Google Scholar]
- 160.Conner JR, Beisswenger PJ, Szwergold BS. Some clues as to the regulation, expression, function, and distribution of fructosamine-3-kinase and fructosamine-3-kinase-related protein. Ann N Y Acad Sci. 2005;1043:824–836. doi: 10.1196/annals.1333.095. [DOI] [PubMed] [Google Scholar]
- 161.Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271(17):9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 162.Jenkins AJ, Best JD, Klein RL, Lyons TJ. Lipoproteins, glycoxidation and diabetic angiopathy. Diabetes Metab Res Rev. 2004;20(5):349–368. doi: 10.1002/dmrr.491. [DOI] [PubMed] [Google Scholar]
- 163.Jenkins AJ, Rowley KG, Lyons TJ, Best JD, Hill MA, Klein RL. Lipoproteins and diabetic microvascular complications. Curr Pharm Des. 2004;10(27):3395–3418. doi: 10.2174/1381612043383188. [DOI] [PubMed] [Google Scholar]
- 164.Stitt AW, Jenkins AJ, Cooper ME. Advanced glycation end products and diabetic complications. Expert Opin Investig Drugs. 2002;11(9):1205–1223. doi: 10.1517/13543784.11.9.1205. [DOI] [PubMed] [Google Scholar]
- 165.Du M, Wu M, Fu D, Yang S, Chen J, Wilson K, Lyons TJ. Effects of modified LDL and HDL on retinal pigment epithelial cells: a role in diabetic retinopathy? Diabetologia. 2013;56(10):2318–2328. doi: 10.1007/s00125-013-2986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fu D, Wu M, Zhang J, Du M, Yang S, Hammad SM, Wilson K, Chen J, Lyons TJ. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 2012;55(11):3128–3140. doi: 10.1007/s00125-012-2692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yu JY, Lyons TJ. Modified lipoproteins in diabetic retinopathy: a local action in the retina. J Clin Exp Ophthalmol. 2013;4(6):314. doi: 10.4172/2155-9570.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans RO, Smit AJ. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47(7):1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 169.Kerkeni M, Saidi A, Bouzidi H, Ben Yahya S, Hammami M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvasc Res. 2012;84(3):378–383. doi: 10.1016/j.mvr.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 170.Sugisawa E, Miura J, Iwamoto Y, Uchigata Y. Skin autofluorescence reflects integration of past long-term glycemic control in patients with type 1 diabetes. Diabetes Care. 2013;36(8):2339–2345. doi: 10.2337/dc12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu C, Xu L, Gao H, Ye J, Huang Y, Wu M, Xie T, Ni P, Yu X, Cao Y, Lu S. The association between skin autofluorescence and vascular complications in Chinese patients with diabetic foot ulcer: an observational study done in Shanghai. Int J Low Extrem Wounds. 2015;14(1):28–36. doi: 10.1177/1534734614568375. [DOI] [PubMed] [Google Scholar]
- 172.Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, Cleary PA, Lachin J, Genuth S. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes. 1999;48(4):870–880. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Orchard TJ, Lyons TJ, Cleary PA, Braffett BH, Maynard J, Cowie C, Gubitosi-Klug RA, Way J, Anderson K, Barnie A. et al. The association of skin intrinsic fluorescence with type 1 diabetes complications in the DCCT/EDIC study. Diabetes Care. 2013;36(10):3146–3153. doi: 10.2337/dc12-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, Monnier VM. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes. 2005;54(11):3103–3111. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Monnier VM, Sell DR, Strauch C, Sun W, Lachin JM, Cleary PA, Genuth S. The association between skin collagen glucosepane and past progression of microvascular and neuropathic complications in type 1 diabetes. J Diabetes Complications. 2013;27(2):141–149. doi: 10.1016/j.jdiacomp.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dong N, Shi H, Xu B, Cai Y. Increased plasma S100A12 levels are associated with diabetic retinopathy and prognostic biomarkers of macrovascular events in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2015;56(8):4177–4185. doi: 10.1167/iovs.15-16470. [DOI] [PubMed] [Google Scholar]
- 177.Zong H, Ward M, Madden A, Yong PH, Limb GA, Curtis TM, Stitt AW. Hyperglycaemia-induced pro-inflammatory responses by retinal Muller glia are regulated by the receptor for advanced glycation end-products (RAGE) Diabetologia. 2010;53(12):2656–2666. doi: 10.1007/s00125-010-1900-z. [DOI] [PubMed] [Google Scholar]
- 178.Barile GR, Pachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H. et al. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46(8):2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- 179.Moore TC, Moore JE, Kaji Y, Frizzell N, Usui T, Poulaki V, Campbell IL, Stitt AW, Gardiner TA, Archer DB, Adamis AP. The role of advanced glycation end products in retinal microvascular leukostasis. Invest Ophthalmol Vis Sci. 2003;44(10):4457–4464. doi: 10.1167/iovs.02-1063. [DOI] [PubMed] [Google Scholar]
- 180.Li G, Tang J, Du Y, Lee CA, Kern TS. Beneficial effects of a novel RAGE inhibitor on early diabetic retinopathy and tactile allodynia. Mol Vis. 2011;17:3156–3165. [PMC free article] [PubMed] [Google Scholar]
- 181.McVicar CM, Ward M, Colhoun LM, Guduric-Fuchs J, Bierhaus A, Fleming T, Schlotterer A, Kolibabka M, Hammes HP, Chen M, Stitt AW. Role of the receptor for advanced glycation endproducts (RAGE) in retinal vasodegenerative pathology during diabetes in mice. Diabetologia. 2015;58(5):1129–1137. doi: 10.1007/s00125-015-3523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sasongko MB, Wong TY, Nguyen TT, Kawasaki R, Jenkins A, Shaw J, Wang JJ. Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids. Diabetes Care. 2011;34(2):474–479. doi: 10.2337/dc10-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chew EY, Klein ML, Ferris FL 3rd, Remaley NA, Murphy RP, Chantry K, Hoogwerf BJ, Miller D. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114(9):1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 184.van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Polak BC. Blood pressure, lipids, and obesity are associated with retinopathy: the hoorn study. Diabetes Care. 2002;25(8):1320–1325. doi: 10.2337/diacare.25.8.1320. [DOI] [PubMed] [Google Scholar]
- 185.Klein R, Sharrett AR, Klein BE, Moss SE, Folsom AR, Wong TY, Brancati FL, Hubbard LD, Couper D. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology. 2002;109(7):1225–1234. doi: 10.1016/s0161-6420(02)01074-6. [DOI] [PubMed] [Google Scholar]
- 186.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 187.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 188.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E. et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 189.Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH. et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Noonan JE, Jenkins AJ, Ma JX, Keech AC, Wang JJ, Lamoureux EL. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes. 2013;62(12):3968–3975. doi: 10.2337/db13-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Simo R, Roy S, Behar-Cohen F, Keech A, Mitchell P, Wong TY. Fenofibrate: a new treatment for diabetic retinopathy. Molecular mechanisms and future perspectives. Curr Med Chem. 2013;20(26):3258–3266. doi: 10.2174/0929867311320260009. [DOI] [PubMed] [Google Scholar]
- 192.Song W, Barth JL, Yu Y, Lu K, Dashti A, Huang Y, Gittinger CK, Argraves WS, Lyons TJ. Effects of oxidized and glycated LDL on gene expression in human retinal capillary pericytes. Invest Ophthalmol Vis Sci. 2005;46(8):2974–2982. doi: 10.1167/iovs.04-1505. [DOI] [PubMed] [Google Scholar]
- 193.Barth JL, Yu Y, Song W, Lu K, Dashti A, Huang Y, Argraves WS, Lyons TJ. Oxidised, glycated LDL selectively influences tissue inhibitor of metalloproteinase-3 gene expression and protein production in human retinal capillary pericytes. Diabetologia. 2007;50(10):2200–2208. doi: 10.1007/s00125-007-0768-z. [DOI] [PubMed] [Google Scholar]
- 194.Diffley JM, Wu M, Sohn M, Song W, Hammad SM, Lyons TJ. Apoptosis induction by oxidized glycated LDL in human retinal capillary pericytes is independent of activation of MAPK signaling pathways. Mol Vis. 2009;15:135–145. [PMC free article] [PubMed] [Google Scholar]
- 195.Song W, Barth JL, Lu K, Yu Y, Huang Y, Gittinger CK, Argraves WS, Lyons TJ. Effects of modified low-density lipoproteins on human retinal pericyte survival. Ann N Y Acad Sci. 2005;1043:390–395. doi: 10.1196/annals.1333.045. [DOI] [PubMed] [Google Scholar]
- 196.Wu M, Yang S, Elliott MH, Fu D, Wilson K, Zhang J, Du M, Chen J, Lyons T. Oxidative and endoplasmic reticulum stresses mediate apoptosis induced by modified LDL in human retinal Muller cells. Invest Ophthalmol Vis Sci. 2012;53(8):4595–4604. doi: 10.1167/iovs.12-9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Klein RL, Hammad SM, Baker NL, Hunt KJ, Al Gadban MM, Cleary PA, Virella G, Lopes-Virella MF. Decreased plasma levels of select very long chain ceramide species are associated with the development of nephropathy in type 1 diabetes. Metabolism. 2014;63(10):1287–1295. doi: 10.1016/j.metabol.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Knudsen ST, Jeppesen P, Frederiksen CA, Andersen NH, Bek T, Ingerslev J, Mogensen CE, Poulsen PL. Endothelial perturbation: a link between non-dipping and retinopathy in type 2 diabetes? J Am Soc Hypertens. 2007;1(3):208–215. doi: 10.1016/j.jash.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 199.Magri CJ, Xuereb RG, Fava S. Non-dipping heart rate and microalbuminuria in type 2 diabetes mellitus. Cardiology. 2014;129(1):28–35. doi: 10.1159/000362714. [DOI] [PubMed] [Google Scholar]
- 200.Do DV, Wang X, Vedula SS, Marrone M, Sleilati G, Hawkins BS, Frank RN. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD006127. doi: 10.1002/14651858.CD006127.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wilkinson-Berka JL, Tan G, Jaworski K, Miller AG. Identification of a retinal aldosterone system and the protective effects of mineralocorticoid receptor antagonism on retinal vascular pathology. Circ Res. 2009;104(1):124–133. doi: 10.1161/CIRCRESAHA.108.176008. [DOI] [PubMed] [Google Scholar]
- 202.Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res. 2015;2015:582060. doi: 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Valle A, Giamporcaro GM, Scavini M, Stabilini A, Grogan P, Bianconi E, Sebastiani G, Masini M, Maugeri N, Porretti L. et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes. 2013;62(6):2072–2077. doi: 10.2337/db12-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28(5):348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 206.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B. et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 207.Chibber R, Ben-Mahmud BM, Chibber S, Kohner EM. Leukocytes in diabetic retinopathy. Curr Diabetes Rev. 2007;3(1):3–14. doi: 10.2174/157339907779802139. [DOI] [PubMed] [Google Scholar]
- 208.Talahalli R, Zarini S, Tang J, Li G, Murphy R, Kern TS, Gubitosi-Klug RA. Leukocytes regulate retinal capillary degeneration in the diabetic mouse via generation of leukotrienes. J Leukoc Biol. 2013;93(1):135–143. doi: 10.1189/jlb.0112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, Mott R, Lyons TJ, Ma JX. Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes. 2013;62(1):261–272. doi: 10.2337/db11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes. 2004;53(8):1979–1986. doi: 10.2337/diabetes.53.8.1979. [DOI] [PubMed] [Google Scholar]
- 211.Devaraj S, Tobias P, Jialal I. Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine. 2011;55(3):441–445. doi: 10.1016/j.cyto.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 212.Josefsen K, Nielsen H, Lorentzen S, Damsbo P, Buschard K. Circulating monocytes are activated in newly diagnosed type 1 diabetes mellitus patients. Clin Exp Immunol. 1994;98(3):489–493. doi: 10.1111/j.1365-2249.1994.tb05517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Woo SJ, Ahn SJ, Ahn J, Park KH, Lee K. Elevated systemic neutrophil count in diabetic retinopathy and diabetes: a hospital-based cross-sectional study of 30,793 Korean subjects. Invest Ophthalmol Vis Sci. 2011;52(10):7697–7703. doi: 10.1167/iovs.11-7784. [DOI] [PubMed] [Google Scholar]
- 214.Gorudko IV, Kostevich VA, Sokolov AV, Shamova EV, Buko IV, Konstantinova EE, Vasiliev VB, Cherenkevich SN, Panasenko OM. Functional activity of neutrophils in diabetes mellitus and coronary heart disease: role of myeloperoxidase in the development of oxidative stress. Bull Exp Biol Med. 2012;154(1):23–26. doi: 10.1007/s10517-012-1865-7. [DOI] [PubMed] [Google Scholar]
- 215.Kuo JZ, Guo X, Klein R, Klein BE, Cui J, Rotter JI, Ipp E, Chen YD. Systemic soluble tumor necrosis factor receptors 1 and 2 are associated with severity of diabetic retinopathy in Hispanics. Ophthalmology. 2012;119(5):1041–1046. doi: 10.1016/j.ophtha.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zorena K, Mysliwska J, Mysliwiec M, Balcerska A, Hak L, Lipowski P, Raczynska K. Serum TNF-alpha level predicts nonproliferative diabetic retinopathy in children. Mediators Inflamm. 2007;2007:92196. doi: 10.1155/2007/92196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nowak M, Wielkoszynski T, Marek B, Kos-Kudla B, Swietochowska E, Sieminska L, Kajdaniuk D, Glogowska-Szelag J, Nowak K. Blood serum levels of vascular cell adhesion molecule (sVCAM-1), intercellular adhesion molecule (sICAM-1) and endothelial leucocyte adhesion molecule-1 (ELAM-1) in diabetic retinopathy. Clin Exp Med. 2008;8(3):159–164. doi: 10.1007/s10238-008-0173-z. [DOI] [PubMed] [Google Scholar]
- 218.Klein BE, Knudtson MD, Tsai MY, Klein R. The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy: Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2009;127(9):1175–1182. doi: 10.1001/archophthalmol.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nguyen TT, Alibrahim E, Islam FM, Klein R, Klein BE, Cotch MF, Shea S, Wong TY. Inflammatory, hemostatic, and other novel biomarkers for diabetic retinopathy: the multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32(9):1704–1709. doi: 10.2337/dc09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Peng D, Wang J, Zhang R, Tang S, Jiang F, Chen M, Yan J, Sun X, Wang T, Wang S. et al. C-reactive protein genetic variant is associated with diabetic retinopathy in Chinese patients with type 2 diabetes. BMC Endocr Disord. 2015;15:8. doi: 10.1186/s12902-015-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rajab HA, Baker NL, Hunt KJ, Klein R, Cleary PA, Lachin J, Virella G, Lopes-Virella MF. The predictive role of markers of Inflammation and endothelial dysfunction on the course of diabetic retinopathy in type 1 diabetes. J Diabetes Complications. 2015;29(1):108–114. doi: 10.1016/j.jdiacomp.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Simo-Servat O, Hernandez C, Simo R. Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediators Inflamm. 2012;2012:872978. doi: 10.1155/2012/872978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hernandez C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simo R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22(6):719–722. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- 224.Murugeswari P, Shukla D, Kim R, Namperumalsamy P, Stitt AW, Muthukkaruppan V. Angiogenic potential of vitreous from Proliferative Diabetic Retinopathy and Eales' Disease patients. Plos One. 2014;9(10):e107551. doi: 10.1371/journal.pone.0107551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales' disease. Retina. 2008;28(6):817–824. doi: 10.1097/IAE.0b013e31816576d5. [DOI] [PubMed] [Google Scholar]
- 226.Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46(6):2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 227.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126(2):227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 228.Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000;17(3):463–471. doi: 10.1017/s0952523800173122. [DOI] [PubMed] [Google Scholar]
- 229.Deliyanti D, Zhang Y, Khong F, Berka DR, Stapleton DI, Kelly DJ, Wilkinson-Berka JL. FT011, a novel cardiorenal protective drug, reduces inflammation, gliosis and vascular injury in rats with diabetic retinopathy. Plos One. 2015;10(7):e0134392. doi: 10.1371/journal.pone.0134392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Xu K, Liu X, Yang F, Cui D, Shi Y, Shen C, Tang W, Yang T. PAI-1 -675 4G/5G polymorphism in association with diabetes and diabetic complications susceptibility: a meta-analysis study. Plos One. 2013;8(11):e79150. doi: 10.1371/journal.pone.0079150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Azad N, Agrawal L, Emanuele NV, Klein R, Bahn GD, McCarren M, Reaven P, Hayward R, Duckworth W. Association of PAI-1 and fibrinogen with diabetic retinopathy in the Veterans Affairs Diabetes Trial (VADT) Diabetes Care. 2014;37(2):501–506. doi: 10.2337/dc13-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Polat SB, Ugurlu N, Yulek F, Simavli H, Ersoy R, Cakir B, Erel O. Evaluation of serum fibrinogen, plasminogen, alpha2-anti-plasmin, and plasminogen activator inhibitor levels (PAI) and their correlation with presence of retinopathy in patients with type 1 DM. J Diabetes Res. 2014;2014:317292. doi: 10.1155/2014/317292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res. 2015;99:137–148. doi: 10.1016/j.phrs.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 234.Han L, Zhang L, Xing W, Zhuo R, Lin X, Hao Y, Wu Q, Zhao J. The associations between VEGF gene polymorphisms and diabetic retinopathy susceptibility: a meta-analysis of 11 case-control studies. J Diabetes Res. 2014;2014:805801. doi: 10.1155/2014/805801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Praidou A, Androudi S, Brazitikos P, Karakiulakis G, Papakonstantinou E, Dimitrakos S. Angiogenic growth factors and their inhibitors in diabetic retinopathy. Curr Diabetes Rev. 2010;6(5):304–312. doi: 10.2174/157339910793360815. [DOI] [PubMed] [Google Scholar]
- 236.Craword SE, Fitchev P, Veliceasa D, Volpert OV. The many facets of PEDF in drug discovery and disease: a diamond in the rough or split personality disorder? Expert Opin Drug Discov. 2013;8(7):769–792. doi: 10.1517/17460441.2013.794781. [DOI] [PubMed] [Google Scholar]
- 237.He X, Cheng R, Benyajati S, Ma JX. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond) 2015;128(11):805–823. doi: 10.1042/CS20130463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yamagishi S, Matsui T. Pigment epithelium-derived factor (PEDF) and cardiometabolic disorders. Curr Pharm Des. 2014;20(14):2377–2386. doi: 10.2174/13816128113199990473. [DOI] [PubMed] [Google Scholar]
- 239.Jenkins A, Zhang SX, Gosmanova A, Aston C, Dashti A, Baker MZ, Lyons T, Ma JX. Increased serum pigment epithelium derived factor levels in type 2 diabetes patients. Diabetes Res Clin Pract. 2008;82(1):e5–e7. doi: 10.1016/j.diabres.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Liu Y, Leo LF, McGregor C, Grivitishvili A, Barnstable CJ, Tombran-Tink J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol Med. 2012;18:1387–1401. doi: 10.2119/molmed.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kim KH, Lee MS. FGF21 as a mediator of adaptive responses to stress and metabolic benefits of anti-diabetic drugs. J Endocrinol. 2015;226(1):R1–R16. doi: 10.1530/JOE-15-0160. [DOI] [PubMed] [Google Scholar]
- 242.Liu JJ, Foo JP, Liu S, Lim SC. The role of fibroblast growth factor 21 in diabetes and its complications: A review from clinical perspective. Diabetes Res Clin Pract. 2015;108(3):382–389. doi: 10.1016/j.diabres.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 243.Lin Y, Xiao YC, Zhu H, Xu QY, Qi L, Wang YB, Li XJ, Zheng ML, Zhong RS, Zhang Y. et al. Serum fibroblast growth factor 21 levels are correlated with the severity of diabetic retinopathy. J Diabetes Res. 2014;2014:929756. doi: 10.1155/2014/929756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ong KL, Januszewski AS, O'Connell R, Buizen L, Jenkins AJ, Xu A, Sullivan DR, Barter PJ, Scott RS, Taskinen MR. et al. Relationship of fibroblast growth factor 21 with baseline and new on-study microvascular disease in the Fenofibrate Intervention and Event Lowering in Diabetes study. Diabetologia. 2015;58(9):2035–2044. doi: 10.1007/s00125-015-3652-2. [DOI] [PubMed] [Google Scholar]
- 245.Klaassen I, van Geest RJ, Kuiper EJ, van Noorden CJ, Schlingemann RO. The role of CTGF in diabetic retinopathy. Exp Eye Res. 2015;133:37–48. doi: 10.1016/j.exer.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 246.Jaffa AA, Usinger WR, McHenry MB, Jaffa MA, Lipstiz SR, Lackland D, Lopes-Virella M, Luttrell LM, Wilson PW. Connective tissue growth factor and susceptibility to renal and vascular disease risk in type 1 diabetes. J Clin Endocrinol Metab. 2008;93(5):1893–1900. doi: 10.1210/jc.2007-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang B, Carter RE, Jaffa MA, Nakerakanti S, Lackland D, Lopes-Virella M, Trojanowska M, Luttrell LM, Jaffa AA. Genetic variant in the promoter of connective tissue growth factor gene confers susceptibility to nephropathy in type 1 diabetes. J Med Genet. 2010;47(6):391–397. doi: 10.1136/jmg.2009.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wu M, Chen Y, Wilson K, Chirindel A, Ihnat MA, Yu Y, Boulton ME, Szweda LI, Ma JX, Lyons TJ. Intraretinal leakage and oxidation of LDL in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(6):2679–2685. doi: 10.1167/iovs.07-1440. [DOI] [PubMed] [Google Scholar]
- 249.Bucan K, Ivanisevic M, Zemunik T, Boraska V, Skrabic V, Vatavuk Z, Galetovic D, Znaor L. Retinopathy and nephropathy in type 1 diabetic patients - association with polymorphysms of vitamin D-receptor, TNF, Neuro-D and IL-1 receptor 1 genes. Coll Antropol. 2009;33(Suppl 2):99–105. [PubMed] [Google Scholar]
- 250.Cyganek K, Mirkiewicz-Sieradzka B, Malecki MT, Wolkow P, Skupien J, Bobrek J, Czogala M, Klupa T, Sieradzki J. Clinical risk factors and the role of VDR gene polymorphisms in diabetic retinopathy in Polish type 2 diabetes patients. Acta Diabetol. 2006;43(4):114–119. doi: 10.1007/s00592-006-0225-3. [DOI] [PubMed] [Google Scholar]
- 251.Nebbioso M, Buomprisco G, Pascarella A, Pescosolido N. Modulatory effects of 1,25-dihydroxyvitamin D3 on eye disorders: a critical review. Crit Rev Food Sci Nutr. 2015 doi: 10.1080/10408398.2014.893504. In press. [DOI] [PubMed] [Google Scholar]
- 252.Taverna MJ, Selam JL, Slama G. Association between a protein polymorphism in the start codon of the vitamin D receptor gene and severe diabetic retinopathy in C-peptide-negative type 1 diabetes. J Clin Endocrinol Metab. 2005;90(8):4803–4808. doi: 10.1210/jc.2004-2407. [DOI] [PubMed] [Google Scholar]
- 253.Taverna MJ, Sola A, Guyot-Argenton C, Pacher N, Bruzzo F, Slama G, Reach G, Selam JL. Taq I polymorphism of the vitamin D receptor and risk of severe diabetic retinopathy. Diabetologia. 2002;45(3):436–442. doi: 10.1007/s00125-001-0769-2. [DOI] [PubMed] [Google Scholar]
- 254.Zhong X, Du Y, Lei Y, Liu N, Guo Y, Pan T. Effects of vitamin D receptor gene polymorphism and clinical characteristics on risk of diabetic retinopathy in Han Chinese type 2 diabetes patients. Gene. 2015;566(2):212–216. doi: 10.1016/j.gene.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 255.Ahmadieh H, Azar ST, Lakkis N, Arabi A. Hypovitaminosis d in patients with type 2 diabetes mellitus: a relation to disease control and complications. ISRN Endocrinol. 2013;2013:641098. doi: 10.1155/2013/641098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Aksoy H, Akcay F, Kurtul N, Baykal O, Avci B. Serum 1,25 dihydroxy vitamin D (1,25(OH)2D3), 25 hydroxy vitamin D (25(OH)D) and parathormone levels in diabetic retinopathy. Clin Biochem. 2000;33(1):47–51. doi: 10.1016/s0009-9120(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 257.Alcubierre N, Valls J, Rubinat E, Cao G, Esquerda A, Traveset A, Granado-Casas M, Jurjo C, Mauricio D. Vitamin D deficiency Is associated with the presence and severity of diabetic retinopathy in type 2 diabetes mellitus. J Diabetes Res. 2015;2015:374178. doi: 10.1155/2015/374178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bonakdaran S, Shoeibi N. Is there any correlation between vitamin D insufficiency and diabetic retinopathy? Int J Ophthalmol. 2015;8(2):326–331. doi: 10.3980/j.issn.2222-3959.2015.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.He R, Shen J, Liu F, Zeng H, Li L, Yu H, Lu H, Lu F, Wu Q, Jia W. Vitamin D deficiency increases the risk of retinopathy in Chinese patients with type 2 diabetes. Diabet Med. 2014;31(12):1657–1664. doi: 10.1111/dme.12581. [DOI] [PubMed] [Google Scholar]
- 260.Jee D, Han K, Kim EC. Inverse association between high blood 25-hydroxyvitamin D levels and diabetic retinopathy in a representative Korean population. Plos One. 2014;9(12):e115199. doi: 10.1371/journal.pone.0115199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kaur H, Donaghue KC, Chan AK, Benitez-Aguirre P, Hing S, Lloyd M, Cusumano J, Pryke A, Craig ME. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care. 2011;34(6):1400–1402. doi: 10.2337/dc11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Patrick PA, Visintainer PF, Shi Q, Weiss IA, Brand DA. Vitamin D and retinopathy in adults with diabetes mellitus. Arch Ophthalmol. 2012;130(6):756–760. doi: 10.1001/archophthalmol.2011.2749. [DOI] [PubMed] [Google Scholar]
- 263.Payne JF, Ray R, Watson DG, Delille C, Rimler E, Cleveland J, Lynn MJ, Tangpricha V, Srivastava SK. Vitamin D insufficiency in diabetic retinopathy. Endocr Pract. 2012;18(2):185–193. doi: 10.4158/EP11147.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Reddy GB, Sivaprasad M, Shalini T, Satyanarayana A, Seshacharyulu M, Balakrishna N, Viswanath K, Sahay M. Plasma vitamin D status in patients with type 2 diabetes with and without retinopathy. Nutrition. 2015;31(7-8):959–963. doi: 10.1016/j.nut.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 265.Shimo N, Yasuda T, Kaneto H, Katakami N, Kuroda A, Sakamoto F, Takahara M, Irie Y, Horikawa K, Miyashita K. et al. Vitamin D deficiency is significantly associated with retinopathy in young Japanese type 1 diabetic patients. Diabetes Res Clin Pract. 2014;106(2):e41–e43. doi: 10.1016/j.diabres.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 266.Poon M, Craig ME, Kaur H, Cusumano J, Sasongko MB, Wong TY, Donaghue KC. Vitamin D deficiency is not associated with changes in retinal geometric parameters in young people with type 1 diabetes. J Diabetes Res. 2013;2013:280691. doi: 10.1155/2013/280691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Herrmann M, Sullivan DR, Veillard AS, McCorquodale T, Straub IR, Scott R, Laakso M, Topliss D, Jenkins AJ, Blankenberg S. et al. Serum 25-hydroxyvitamin D: a predictor of macrovascular and microvascular complications in patients with type 2 diabetes. Diabetes Care. 2015;38(3):521–528. doi: 10.2337/dc14-0180. [DOI] [PubMed] [Google Scholar]
- 268.Alele JD, Luttrell LM, Hollis BW, Luttrell DK, Hunt KJ. Relationship between vitamin D status and incidence of vascular events in the Veterans Affairs Diabetes Trial. Atherosclerosis. 2013;228(2):502–507. doi: 10.1016/j.atherosclerosis.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 269.Joergensen C, Hovind P, Schmedes A, Parving HH, Rossing P. Vitamin D levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care. 2011;34(5):1081–1085. doi: 10.2337/dc10-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.McCully KS. Chemical pathology of homocysteine. I. Atherogenesis. Ann Clin Lab Sci. 1993;23(6):477–493. [PubMed] [Google Scholar]
- 271.Ueland PM, Refsum H. Plasma homocysteine, a risk factor for vascular disease: plasma levels in health, disease, and drug therapy. J Lab Clin Med. 1989;114(5):473–501. [PubMed] [Google Scholar]
- 272.Marti-Carvajal AJ, Sola I, Lathyris D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2015;1:CD006612. doi: 10.1002/14651858.CD006612.pub4. [DOI] [PubMed] [Google Scholar]
- 273.Bulum T, Blaslov K, Duvnjak L. Plasma homocysteine is associated with retinopathy in type 1 diabetic patients in the absence of nephropathy. Semin Ophthalmol. 2015;2015:1–5. doi: 10.3109/08820538.2014.912338. [DOI] [PubMed] [Google Scholar]
- 274.Cho HC. The relationship among homocysteine, bilirubin, and diabetic retinopathy. Diabetes Metab J. 2011;35(6):595–601. doi: 10.4093/dmj.2011.35.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lim CP, Loo AV, Khaw KW, Sthaneshwar P, Khang TF, Hassan M, Subrayan V. Plasma, aqueous and vitreous homocysteine levels in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96(5):704–707. doi: 10.1136/bjophthalmol-2011-301044. [DOI] [PubMed] [Google Scholar]
- 276.Satyanarayana A, Balakrishna N, Pitla S, Reddy PY, Mudili S, Lopamudra P, Suryanarayana P, Viswanath K, Ayyagari R, Reddy GB. Status of B-vitamins and homocysteine in diabetic retinopathy: association with vitamin-B12 deficiency and hyperhomocysteinemia. Plos One. 2011;6(11):e26747. doi: 10.1371/journal.pone.0026747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Xu C, Wu Y, Liu G, Liu X, Wang F, Yu J. Relationship between homocysteine level and diabetic retinopathy: a systematic review and meta-analysis. Diagn Pathol. 2014;9:167. doi: 10.1186/s13000-014-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kilic S, Yilmaz N, Zulfikaroglu E, Erdogan G, Aydin M, Batioglu S. Inflammatory-metabolic parameters in obese and nonobese normoandrogenemic polycystic ovary syndrome during metformin and oral contraceptive treatment. Gynecol Endocrinol. 2011;27(9):622–629. doi: 10.3109/09513590.2010.530706. [DOI] [PubMed] [Google Scholar]
- 279.Sahin M, Tutuncu NB, Ertugrul D, Tanaci N, Guvener ND. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J Diabetes Complications. 2007;21(2):118–123. doi: 10.1016/j.jdiacomp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 280.Kuo JZ, Wong TY, Rotter JI. Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 2014;132(1):96–107. doi: 10.1001/jamaophthalmol.2013.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Awata T, Yamashita H, Kurihara S, Morita-Ohkubo T, Miyashita Y, Katayama S, Mori K, Yoneya S, Kohda M, Okazaki Y. et al. A genome-wide association study for diabetic retinopathy in a Japanese population: potential association with a long intergenic non-coding RNA. Plos One. 2014;9(11):e111715. doi: 10.1371/journal.pone.0111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Chang YC, Chang EY, Chuang LM. Recent progress in the genetics of diabetic microvascular complications. World J Diabetes. 2015;6(5):715–725. doi: 10.4239/wjd.v6.i5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.McCord RA, Broccoli D. Telomeric chromatin: roles in aging, cancer and hereditary disease. Mutat Res. 2008;647(1-2):86–93. doi: 10.1016/j.mrfmmm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 285.Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100(1):15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- 286.Al-Attas OS, Al-Daghri NM, Alokail MS, Alkharfy KM, Alfadda AA, McTernan P, Gibson GC, Sabico SB, Chrousos GP. Circulating leukocyte telomere length is highly heritable among families of Arab descent. BMC Med Genet. 2012;13:38. doi: 10.1186/1471-2350-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Balasubramanyam M, Adaikalakoteswari A, Monickaraj SF, Mohan V. Telomere shortening and metabolic/vascular diseases. Indian J Med Res. 2007;125(3):441–450. [PubMed] [Google Scholar]
- 288.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111(17):2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 289.Fyhrquist F, Tiitu A, Saijonmaa O, Forsblom C, Groop PH. Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. J Intern Med. 2010;267(3):278–286. doi: 10.1111/j.1365-2796.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 290.Astrup AS, Tarnow L, Jorsal A, Lajer M, Nzietchueng R, Benetos A, Rossing P, Parving HH. Telomere length predicts all-cause mortality in patients with type 1 diabetes. Diabetologia. 2010;53(1):45–48. doi: 10.1007/s00125-009-1542-1. [DOI] [PubMed] [Google Scholar]
- 291.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 292.Brugere JF, Cornillot E, Metenier G, Vivares CP. Occurence of subtelomeric rearrangements in the genome of the microsporidian parasite Encephalitozoon cuniculi, as revealed by a new fingerprinting procedure based on two-dimensional pulsed field gel electrophoresis. Electrophoresis. 2000;21(12):2576–2581. doi: 10.1002/1522-2683(20000701)21:12<2576::AID-ELPS2576>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 293.Nersisyan L, Arakelyan A. Computel: computation of mean telomere length from whole-genome next-generation sequencing data. Plos One. 2015;10(4):e0125201. doi: 10.1371/journal.pone.0125201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cooper ME, El-Osta A. Epigenetics: mechanisms and implications for diabetic complications. Circ Res. 2010;107(12):1403–1413. doi: 10.1161/CIRCRESAHA.110.223552. [DOI] [PubMed] [Google Scholar]
- 295.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Keating ST, El-Osta A. Epigenetics and metabolism. Circ Res. 2015;116(4):715–736. doi: 10.1161/CIRCRESAHA.116.303936. [DOI] [PubMed] [Google Scholar]
- 297.Mishra M, Zhong Q, Kowluru RA. Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55(11):7256–7265. doi: 10.1167/iovs.14-15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Perrone L, Matrone C, Singh LP. Epigenetic modifications and potential new treatment targets in diabetic retinopathy. J Ophthalmol. 2014;2014:789120. doi: 10.1155/2014/789120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–455. doi: 10.1007/s00125-014-3462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Hardikar AA, Satoor SN, Karandikar MS, Joglekar MV, Puranik AS, Wong W, Kumar S, Limaye A, Bhat DS, Januszewski AS. et al. Multigenerational undernutrition increases susceptibility to obesity and diabetes that is not reversed after dietary recuperation. Cell Metab. 2015;22(2):312–319. doi: 10.1016/j.cmet.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 301.Zhao S, Li J, Wang N, Zheng B, Li T, Gu Q, Xu X, Zheng Z. Fenofibrate suppresses cellular metabolic memory of high glucose in diabetic retinopathy via a sirtuin 1-dependent signalling pathway. Mol Med Rep. 2015;12(4):6112–6118. doi: 10.3892/mmr.2015.4164. [DOI] [PubMed] [Google Scholar]
- 302.Mastropasqua R, Toto L, Cipollone F, Santovito D, Carpineto P, Mastropasqua L. Role of microRNAs in the modulation of diabetic retinopathy. Prog Retin Eye Res. 2014;43:92–107. doi: 10.1016/j.preteyeres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 303.Weiland M, Gao XH, Zhou L, Mi QS. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol. 2012;9(6):850–859. doi: 10.4161/rna.20378. [DOI] [PubMed] [Google Scholar]
- 304.Ragusa M, Caltabiano R, Russo A, Puzzo L, Avitabile T, Longo A, Toro MD, Di Pietro C, Purrello M, Reibaldi M. MicroRNAs in vitreus humor from patients with ocular diseases. Mol Vis. 2013;19:430–440. [PMC free article] [PubMed] [Google Scholar]
- 305.Dunmire JJ, Lagouros E, Bouhenni RA, Jones M, Edward DP. MicroRNA in aqueous humor from patients with cataract. Exp Eye Res. 2013;108:68–71. doi: 10.1016/j.exer.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 306.Li J, Chen Y, Qin X, Wen J, Ding H, Xia W, Li S, Su X, Wang W, Li H. et al. MiR-138 downregulates miRNA processing in HeLa cells by targeting RMND5A and decreasing Exportin-5 stability. Nucleic Acids Res. 2014;42(1):458–474. doi: 10.1093/nar/gkt839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Liu J, Feener EP. Plasma kallikrein-kinin system and diabetic retinopathy. Biol Chem. 2013;394(3):319–328. doi: 10.1515/hsz-2012-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lu CH, Lin ST, Chou HC, Lee YR, Chan HL. Proteomic analysis of retinopathy-related plasma biomarkers in diabetic patients. Arch Biochem Biophys. 2013;529(2):146–156. doi: 10.1016/j.abb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 309.Monteiro JP, Santos FM, Rocha AS, Castro-de-Sousa JP, Queiroz JA, Passarinha LA, Tomaz CT. Vitreous humor in the pathologic scope: insights from proteomic approaches. Proteomics Clin Appl. 2015;9(1-2):187–202. doi: 10.1002/prca.201400133. [DOI] [PubMed] [Google Scholar]
- 310.Murthy KR, Goel R, Subbannayya Y, Jacob HK, Murthy PR, Manda SS, Patil AH, Sharma R, Sahasrabuddhe NA, Parashar A. et al. Proteomic analysis of human vitreous humor. Clin Proteomics. 2014;11(1):29. doi: 10.1186/1559-0275-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Semba RD, Huang H, Lutty GA, Van Eyk JE, Hart GW. The role of O-GlcNAc signaling in the pathogenesis of diabetic retinopathy. Proteomics Clin Appl. 2014;8(3-4):218–231. doi: 10.1002/prca.201300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tian H, Wang L, Cai R, Zheng L, Guo L. Identification of protein network alterations upon retinal ischemia-reperfusion injury by quantitative proteomics using a Rattus norvegicus model. Plos One. 2014;9(12):e116453. doi: 10.1371/journal.pone.0116453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Wang H, Feng L, Hu J, Xie C, Wang F. Differentiating vitreous proteomes in proliferative diabetic retinopathy using high-performance liquid chromatography coupled to tandem mass spectrometry. Exp Eye Res. 2013;108:110–119. doi: 10.1016/j.exer.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 314.Li X, Luo X, Lu X, Duan J, Xu G. Metabolomics study of diabetic retinopathy using gas chromatography-mass spectrometry: a comparison of stages and subtypes diagnosed by Western and Chinese medicine. Mol Biosyst. 2011;7(7):2228–2237. doi: 10.1039/c0mb00341g. [DOI] [PubMed] [Google Scholar]
- 315.Barba I, Garcia-Ramirez M, Hernandez C, Alonso MA, Masmiquel L, Garcia-Dorado D, Simo R. Metabolic fingerprints of proliferative diabetic retinopathy: an 1H-NMR-based metabonomic approach using vitreous humor. Invest Ophthalmol Vis Sci. 2010;51(9):4416–4421. doi: 10.1167/iovs.10-5348. [DOI] [PubMed] [Google Scholar]
- 316.Csosz E, Boross P, Csutak A, Berta A, Toth F, Poliska S, Torok Z, Tozser J. Quantitative analysis of proteins in the tear fluid of patients with diabetic retinopathy. J Proteomics. 2012;75(7):2196–2204. doi: 10.1016/j.jprot.2012.01.019. [DOI] [PubMed] [Google Scholar]