Abstract
A 43-year-old incarcerated man with AIDS was hospitalized for 30 pounds weight loss and diffuse pruritic rash. Three months prior, he was started on dapsone for Pneumocystis jiroveci pneumonia prevention. Biochemical evaluation was remarkable for eosinophilia, thrombocytopenia, acute renal insufficiency, transaminitis, thyrotoxicosis, and significant hyperglycemia (450 mg/dl; nl, 65–99). His hemoglobin A1c level was 5.9% (nl, 4.1–5.6). Thyroid-stimulating immunoglobulin, glutamic acid decarboxylase, and islet cell autoantibodies were within the normal range. He was found to have acute interstitial nephritis based on renal biopsy. He was diagnosed with hypersensitivity reaction due to dapsone. The patient was managed with a tapering dose of corticosteroid, beta-blocker, and multiple daily injections of insulin. The symptoms and biochemical disturbances including thyrotoxicosis resolved within a few weeks. Insulin requirements decreased but diabetes did not resolve with hemoglobin A1c of 6.1% a year after hospitalization. To our knowledge, this is the first case of hypersensitivity reaction due to dapsone causing simultaneous fulminant type 1 diabetes and thyroiditis.
Key words: Fulminant type 1 diabetes, hypersensitivity syndrome, thyroiditis, thyrotoxicosis
INTRODUCTION
Drug reaction with eosinophilia and systemic symptoms was named DRESS in 1996.[1] The incidence of this syndrome ranges from 1/1000–1/10,000 drug exposures.[2] The exact mechanism is unclear. However, many authors have suggested a relationship between these drug reactions and human herpes virus, Epstein–Barr virus, and Cytomegalovirus.[3] The most common involved organs are the liver and kidneys.[4] Autoimmune thyroiditis has been well reported in association with DRESS, but de novo fulminant type 1 diabetes (FT1D) has been described in only 11 individual cases.[5,6,7,8,9] We present a case of DRESS due to dapsone and subsequent development of thyroiditis and simultaneous FT1D.
CASE REPORT
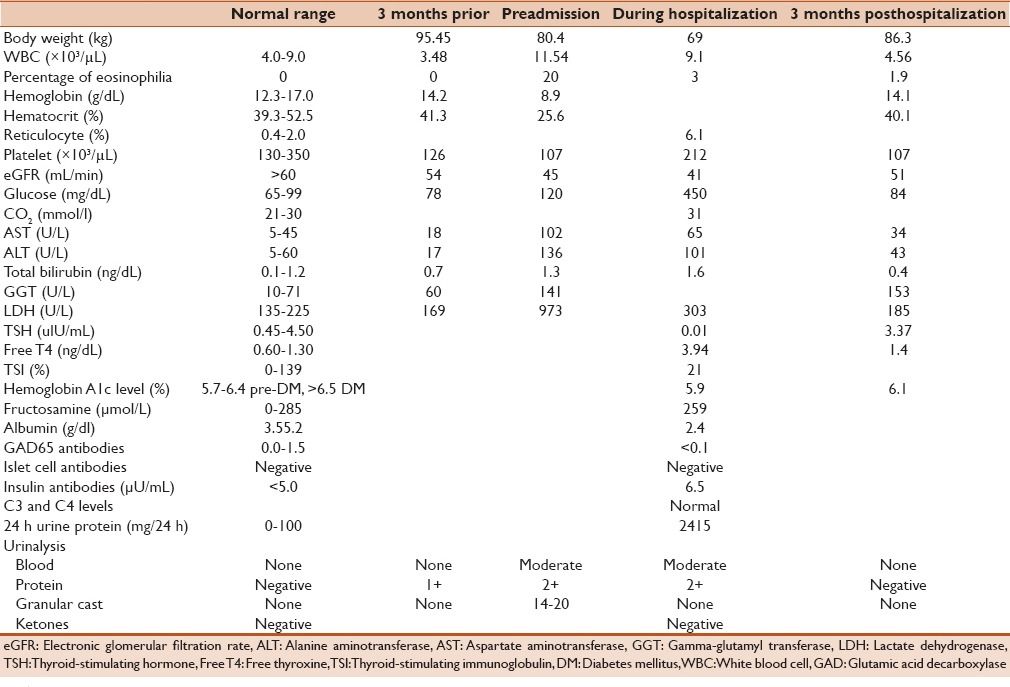
We present a case of a 43-year-old African-American male inmate who was referred to our hospital for worsening renal failure, proteinuria, and eosinophilia, with concern of acute interstitial nephritis. His medical history was remarkable for schizophrenia, hepatitis C genotype 3a, human immunodeficiency virus Centers for Disease Control Class A3, and Stage II chronic kidney disease. His medications were tenofovir, raltegravir, and trimethoprim-sulfamethoxazole for a history of Pneumocystis jiroveci pneumonia. The family history was positive for diabetes in a grandmother who was diagnosed at a young age. Three months prior to hospitalization, the patient was noted to have thrombocytopenia. Consequently, trimethoprim-sulfamethoxazole was switched to dapsone 100 mg daily. Two months later, the patient started to experience fevers, chills, fatigue, decreased appetite, nausea, vomiting, and diarrhea (4–6 episodes/day). He lost more than 30 pounds and developed a diffuse, dry pruritic rash.
Laboratory evaluation in the patient was remarkable for leukocytosis with 20% eosinophilia, transaminitis, and further worsening in thrombocytopenia and renal function [Table 1]. Nonfasting serum glucose was normal at 78 ng/dL. Urine analysis revealed moderate blood, 2+ protein (100 mg/dL) with 14–20 granular casts. Renal ultrasound revealed changes consistent with chronic renal disease with increased cortical echogenicity without obstruction.
Table 1.
The progression of the patient's weight and his biochemical profile over time
On presentation, the patient's temperature was 36.7°C, blood pressure was 104/57 mmHg, heart rate was 98 beats/min, respiratory rate was 17 with oxygenation 100% on room air, weight was 69 kg, and body mass index was 23.1. On physical examination, he appeared cachectic and fatigued with mild temporal wasting. He had dry mucous membranes and a nonpainful thyroid gland with no bruits or palpable nodules. Skin examination was notable for a diffuse, dry exfoliative dermatitis, mostly on the chest, abdomen, palms, and soles. The remainder of the physical examination was unremarkable.
Biochemical evaluation upon admission to the hospital was remarkable for thyrotoxicosis with thyroid-stimulating hormone of 0.01 uIU/ml (nl, 0.45–4.50), free thyroxine of 3.94 ng/dl (nl, 0.60–1.30), and thyroid-stimulating immunoglobulin (TSI) of 21% (nl, 0–139). A thyroid uptake and scan was not obtained because of a recent iodinated contrast administration during whole-body computed tomographic (CT) imaging. He was also noted to be hyperglycemic with serum glucose as high as 450 mg/dl without ketoacidosis [Table 1]. His hemoglobin A1c level was 5.9% (nl, 4.1–5.6), fructosamine was 259 umol/L (nl, 0–285) with an albumin of 2.4 g/dl (nl, 3.5–5.2), glutamic acid decarboxylase (GAD) and islet cell autoantibodies were undetectable, and insulin antibodies were mildly positive [Table 1]. Exocrine pancreatic enzymes were not checked, but the pancreas looked normal on CT scan imaging. The patient was tested for infectious etiologies including Cytomegalovirus, Cryptosporidium, Cyclospora, Parvovirus, histoplasmosis, Clostridium difficile, and Giardia infections, all of which were negative. A renal biopsy confirmed the diagnosis of acute interstitial nephritis, predominately with lymphocytes and eosinophils. He was diagnosed with DRESS, likely due to dapsone. Dapsone was stopped and the patient was started on a tapering dose of prednisone and given beta-blocker and multiple daily injections of insulin. The daily insulin requirement was 0.4 units/kg/day, which increased to 0.7 unit/kg/day on prednisone.
On follow-up, all of his symptoms including the rash had resolved within a few weeks. Leukocytosis, thrombocytopenia, eosinophilia, transaminitis, hyperbilirubinemia, and thyrotoxicosis had also resolved [Table 1]. GFR returned to baseline, and hemoglobin A1c level a year after hospitalization was 6.1%. The daily insulin requirement decreased to 0.25u/kg/day (insulin NPH twice daily).
DISCUSSION
DRESS syndrome is a severe adverse drug-induced reaction. It has been reported with multiple medications, most frequently with allopurinol and anticonvulsants such as phenytoin and carbamazepine.[10,11] In 2003, a scoring system to aid in the early detection and prompt treatment in these patients was developed. The scoring system is based on the distinct clinical reaction pattern of DRESS. This reaction often starts with a fever. Patients present with widespread skin eruptions often progressing to exfoliative dermatitis, multi-organ involvement, and hematologic abnormalities (eosinophilia and thrombocytopenia). Another noteworthy feature is a delayed onset, usually 2–6 weeks after the initiation of the drug therapy.[12] In one study, the overall mortality reached 10% from multi-organ failure and/or shock.[4]
Since the advent of the era of AIDS, dapsone has been increasingly utilized for prophylaxis treatment against Pneumocystis carinii infection. This has led to an increased incidence of dapsone-related complications including DRESS. Dapsone-induced DRESS accounts for about 17% of cases.[4]
There have been well-documented endocrinopathies including thyroiditis and FT1D in the setting of DRESS.[6,7,8,9] The interval between discontinuation of the medication and thyrotoxicosis diagnosis has been estimated to be approximately 5 months.[13] In our patient, thyroiditis was one of the presenting organ systems involved. Graves' disease and contrast-induced thyrotoxicosis are in the differential diagnosis for this case. However, absence of prior thyroid disease history, normal thyroid examination without palpable nodules, normal-looking thyroid on CT imaging, normal TSI level, and the resolution of thyrotoxicosis within a few weeks make the diagnosis of thyroiditis in the setting of DRESS the most likely etiology.
The mean duration from the onset of DRESS to the development of FT1D was 39.9 days, and in most cases, between 2 weeks and 2 months.[7,8] This entity associated with DRESS syndrome is very rare and only 11 cases have been reported.[7] However, none thus far has been associated with dapsone use. FT1D is a subset of type 1 diabetes that is not autoimmune in nature. It involves an abrupt onset of hyperglycemia, rapid progression to ketoacidosis (if left untreated), negative antibody screening, and normal or near-normal glycated hemoglobin A1c.[14] This patient's serum glucose levels before hospitalization were always normal. The remarkably elevated serum glucose during hospitalization (before starting corticosteroids) coupled with the mildly elevated hemoglobin A1c correlates with the rapid presentation of his diabetes. Further evidence against type 1 diabetes are the absence of GAD and islet cell autoantibodies. Insulin antibody was checked after starting insulin therapy which could have caused false result. C-peptide level was not unfortunately obtained. It is noteworthy though that dapsone has been reported to lead to falsely lower level of hemoglobin A1c by inducing hemolysis and promoting the oxidation of hemoglobin to methemoglobin, which interferes with the high-performance liquid chromatography assay used to measure hemoglobin A1c.[15,16] Our patient indeed had some findings which were suggestive of hemolysis such as anemia and elevated lactate dehydrogenase and reticulocyte count [Table 1]. While stress-induced hyperglycemia is a possibility, it usually resolves spontaneously as the acute illness or surgical stress abates. The diagnosis of FT1D was further strengthened by the prolonged requirement of insulin (0.25 μ/kg/day) even after the resolution of his symptoms and cessation of corticosteroid administration.
To our knowledge, this is the first case of DRESS due to dapsone, causing simultaneous FT1D and thyroiditis. As dapsone is being used more for chemoprophylaxis for P. carinii, clinicians need to be aware of this unusual but potentially serious presentation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS) Semin Cutan Med Surg. 1996;15:250–7. doi: 10.1016/s1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- 2.Gennis MA, Vemuri R, Burns EA, Hill JV, Miller MA, Spielberg SP. Familial occurrence of hypersensitivity to phenytoin. Am J Med. 1991;91:631–4. doi: 10.1016/0002-9343(91)90216-k. [DOI] [PubMed] [Google Scholar]
- 3.Descamps V, Valance A, Edlinger C, Fillet AM, Grossin M, Lebrun-Vignes B, et al. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol. 2001;137:301–4. [PubMed] [Google Scholar]
- 4.Chen YC, Chiu HC, Chu CY. Drug reaction with eosinophilia and systemic symptoms: A retrospective study of 60 cases. Arch Dermatol. 2010;146:1373–9. doi: 10.1001/archdermatol.2010.198. [DOI] [PubMed] [Google Scholar]
- 5.Dubois-Laforgue D, Moachon L, Laude H, Timsit J. Fulminant type 1 diabetes in the course of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. Diabetes Care. 2013;36:e68. doi: 10.2337/dc12-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imagawa A, Hanafusa T. Fulminant type 1 diabetes – An important subtype in East Asia. Diabetes Metab Res Rev. 2011;27:959–64. doi: 10.1002/dmrr.1236. [DOI] [PubMed] [Google Scholar]
- 7.Onuma H, Tohyama M, Imagawa A, Hanafusa T, Kobayashi T, Kano Y, et al. High frequency of HLA B62 in fulminant type 1 diabetes with the drug-induced hypersensitivity syndrome. J Clin Endocrinol Metab. 2012;97:E2277–81. doi: 10.1210/jc.2012-2054. [DOI] [PubMed] [Google Scholar]
- 8.Kano Y, Ishida T, Hirahara K, Shiohara T. Visceral involvements and long-term sequelae in drug-induced hypersensitivity syndrome. Med Clin North Am. 2010;94:743–59, xi. doi: 10.1016/j.mcna.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Ichiche M, Kiesch N, De Bels D. DRESS syndrome associated with HHV-6 reactivation. Eur J Intern Med. 2003;14:498–500. doi: 10.1016/j.ejim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J Clin Invest. 1988;82:1826–32. doi: 10.1172/JCI113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer JZ, Wallace SL. The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum. 1986;29:82–7. doi: 10.1002/art.1780290111. [DOI] [PubMed] [Google Scholar]
- 12.Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005;209:123–9. doi: 10.1016/j.tox.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Teo RY, Tay YK, Tan CH, Ng V, Oh DC. Presumed dapsone-induced drug hypersensitivity syndrome causing reversible hypersensitivity myocarditis and thyrotoxicosis. Ann Acad Med Singapore. 2006;35:833–6. [PubMed] [Google Scholar]
- 14.Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, et al. Fulminant type 1 diabetes: A nationwide survey in Japan. Diabetes Care. 2003;26:2345–52. doi: 10.2337/diacare.26.8.2345. [DOI] [PubMed] [Google Scholar]
- 15.Roxby A, Jain R. Dapsone interferes with hemoglobin A1c monitoring of diabetes in an HIV-infected patient. AIDS. 2013;27:299–301. doi: 10.1097/QAD.0b013e32835adde8. [DOI] [PubMed] [Google Scholar]
- 16.Shah AD, Fox RK, Rushakoff RJ. Falsely decreased HbA1c in a type 2 diabetic patient treated with dapsone. Endocr Pract. 2014;20:e229–32. doi: 10.4158/EP14291.CR. [DOI] [PMC free article] [PubMed] [Google Scholar]


