Abstract
Background:
Myxoid and round cell liposarcomas (RCL) are low and high-grade counterparts of a common subtype of liposarcomas (LPS), representing a histologic continuum.
Aims:
The aim was to study the cytomorphology of hypercellular RCL and to look for features that differentiate RCL from other sarcomas displaying round cells with myxoid change.
Materials and Methods:
Eight cases of hypercellular RCL were identified retrospectively in which round cell component constituted greater than 75% of the area. Cytomorphological features were studied in detail.
Results:
Four cases were predominantly composed of round cells, out of which 2 were entirely composed of round cells. Myxoid metachromatic stroma was absent in 2 cases, while it was present in ample amounts in the remaining 6 cases. Spindle cells were completely absent in 5 cases and lipoblasts were observed in a single case of RCL. Plexiform vascular capillaries were seen in 4 cases. Adherence of neoplastic round cells around thin capillaries (ANAC) was observed in 7 cases.
Conclusions:
Plexiform capillary plexus and ANAC should be separately evaluated. The latter feature is the most consistent one, and is an important clue to the adipocytic nature of the neoplasm when hypercellular round cell areas are aspirated. Confident diagnosis of hypercellular RCL can be made on the basis of this feature. Lipoblasts, although specific, are seen in few cases. The rest of the features are nonspecific and useful only in combination.
Keywords: Capillaries, extracellular matrix, fine needle aspiration cytology, lipoblasts, myxoid liposarcoma, soft tissue
Introduction
Liposarcomas (LPS) are the second most common soft tissue sarcomas (STS). Myxoid and round cell variant occurs a decade or two earlier than other variants, accounting for 10% of all the STS and 30–35% of all the LPS. It usually presents in the deep soft tissues of lower extremities, upper extremities, and trunk.[1,2,3] The World Health Organization (WHO) classification in 2003 proposed round cell liposarcoma (RCL) to be a high-grade counterpart of myxoid liposarcoma (MLPS), lying on the poorly differentiated end of the spectrum with predominance of round cells, as evidenced by a common translocation abnormality, t(12;16) (q13;p11), that fuses the CHOP gene with the TLS gene.[3,4,5,6] Tumors with greater than 5% of round cell areas were considered RCL. The placement of these LPS into the RCL category relates to the poor prognosis of the disease which needs aggressive management including radiotherapy and chemotherapy.[7,8,9,10,11]
However, recent WHO classification in 2013 omitted the term RCL because high-grade MLPS can either have hypercellular spindle cells or round cells.[3] Round cell component in MLPS are more common than initially believed.[12,13] Fine needle aspiration (FNA) smears can have variable admixture of myxoid areas, round cells, and transistional areas in between, sometimes one predominating over others.[10] The aim of the study was to evaluate the cytomorphological features of RCL, which are dominated by round cells, such that a possible suspicion can be made regarding the possibility of RCL. We also looked for cytomorphological features that differentiate RCL from other soft tissue sarcomas having round cells with myxoid change.
Materials and Methods
Ten cases of RCL were identified, for which FNA smears were available. FNA smears from 8 cases were included, which constituted greater than 75% round cell areas on histology.[14] Each case was reviewed by at least 2 pathologists, and a provisional diagnosis was given at that time. All FNA were performed by 22–24 gauge needles, the number of passes ranged from 2 to 5 depending on the tumor size. Smears were stained with May–Grunewald–Giemsa (MGG), Papanicolaou (Pap), and Hematoxylin and Eosin stains (H and E).
All the 8 histological specimens received were nodular tissue masses. The round cell areas were separated from myxoid areas, which displayed round cell-to-stroma ratio exceeding 50% with no intervening myxoid areas. Transitional areas were excluded from round cell component. These are defined as areas of increased cellularity, in which cells remain spindled, do not have overlapping nuclear borders, an easily discernible plexiform vascular pattern, as well as intervening myxoid areas.[10] The exact percentage of round cell component was found to be greater than 75%.
Cytomorphological features of all the 8 cases included in the study were again reviewed by 3 independent pathologists using a double-blinded procedure without availability of histological diagnosis. Similar features were also evaluated for round cell tumors (RCT), which included 10 cases each of melanoma and lymphoma, 4 cases of alveolar rhabdomyosarcoma, 3 of Ewing's Sarcoma, and 2 were neuroblastomas. Eight cases of myxofibrosarcomas (MFS) and 3 of extraskeletal myxoid chondrosarcomas (ESMCS) were also evaluated because these myxoid sarcomas are the two most important differential diagnosis considered in cases of MLPS.
Results
The clinicopathological features of 10 patients are summarized in Table 1. Most of the patients presented with a slowly growing soft tissue mass. One of the patients had restriction of joint movement and pain with multifocal involvement of the left hand and foot. The age at presentation ranged from 35 to 60 years, with a male to female ratio of 2.3:1. Lower extremity was more commonly involved (6 cases) than the upper extremity (3 cases). The tumor size ranged from 5 to 20 cm.
Table 1.
Clinicopathological Features
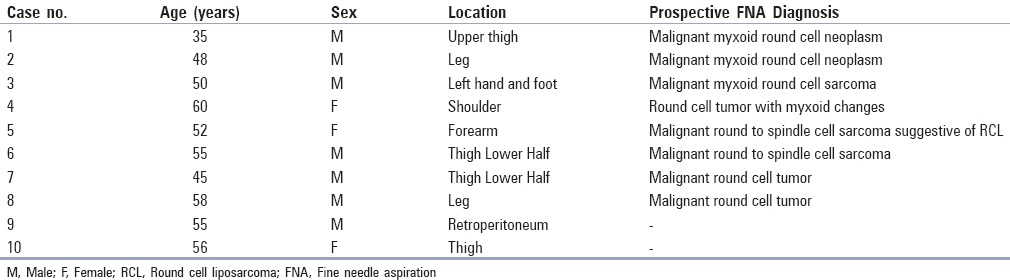
The important cytomorphological features of 8 cases are shown in Table 2. Four cases were predominantly composed of round cells, out of which 2 cases were entirely composed of round cells [Figure 1a] (case# 7 and 8). Thus, dense myxoid metachromatic stroma was absent in 2 cases whereas it was present in ample amount in the remaining 6 cases [Figure 1b]. The cells were predominantly dispersed with few discohesive sheets and irregular groups, displaying monomorphic round-to-oval nucleus, vesicular chromatin, indistinct-to-distinct nucleoli, and minimal-to-absent cytoplasm [Figure 1c]. Moderate amount of cytoplasm was also seen in 2 cases (case# 5 and 6). Spindle cells were completely absent in 5 cases, while in rest of the 3 cases, their cellularity was significantly lower than the round cells. The spindle cell groups and clusters had a bland appearance. Plexiform vascular capillaries were seen in 4 cases, whereas this feature was also observed in other myxoid soft tissue sarcomas, especially MFS, but absent in ESMCS [Figure 1b and d]. Adherence of neoplastic round cells around thin capillaries (ANAC) was observed in 7 cases [Figure 1c, e and f]. ANAC was also observed around plexiform vascular capillaries [Figure 1d]. Typical multivacuolated lipoblasts with a centrally placed scalloped nucleus or univacuolated signet ring shaped lipoblasts were observed in a single case of RCL (case# 5) [Figure 2a]. Vacuolated lipoid background was present in 3 cases.
Table 2.
Cytomorphological features of hypercellular RCL
Figure 1.
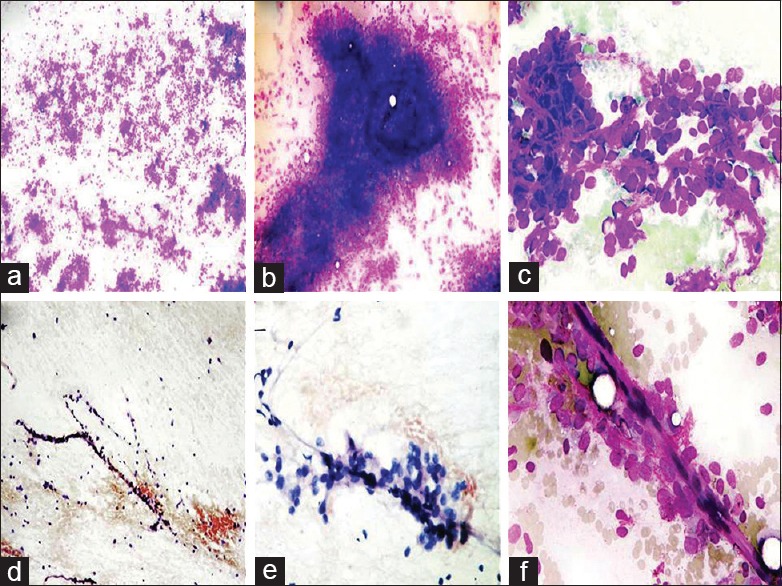
(a) Hypercellular smears displaying monomorphic round cells, (MGG ×40). (b) Smears displaying clumps of opaque myxoid material obscuring round cells. A plexiform capillary is still discernible, (MGG ×100). (c) Round monomorphic cells with indistinct-to-distinct nucleoli and fine thin capillaries in between, (MGG ×400). (d) Smears showing thin plexiform capillaries along with associated neoplastic round cell, (Pap stain ×40). (e, f) Higher magnification displaying thin capillaries with adherence of round neoplastic cells (ANAC), (e: Pap stain ×100; f : MGG × 400)
Figure 2.
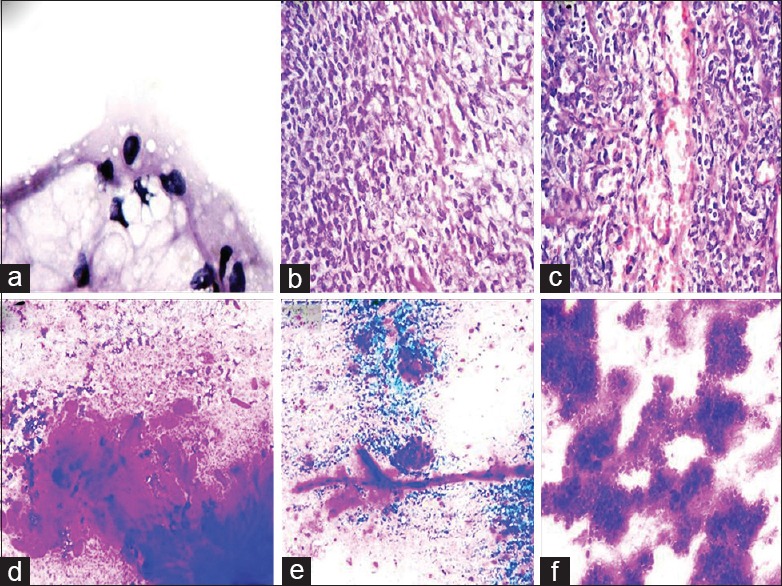
(a) Smears showing a multivacuolated lipoblast, (Pap stain ×400). (b) Sections on the right side shows transitional area. Left side of the section shows hypercellular round cells, (H and E stain ×100). (c) Sections showing thin compressed capillaries in between round cells, (H and E stain ×100). (d) Smears of MFS showing oval-to-spindle cells in opaque myxoid stroma obscuring cellular details. Note diffuse granular quality of myxoid material, (MGG ×100). (e) A thick plexiform capillary plexus against a myxoid background, (MGG × 100). (f) ESMCS. Smears show cord-like arrangement of round-to-oval cells in lacunae embedded in the myxoid matrix, (MGG ×100)
All the 8 cases on histology had hypercellular round cell areas greater than 75%, having lobulated growth pattern and infiltrative margins. There was a variable admixture of MLPS-like areas comprising spindle cells, lipoblasts, and myxoid stroma in 6 cases, with transitional areas in between them [Figure 1b]. Rest of the 2 cases were pure RCL with no intervening myxoid areas (case# 7 and 8). Thin branching capillary network were compressed in between round cells displaying typical crowfeet appearance in all the cases [Figure 2c].
Discussion
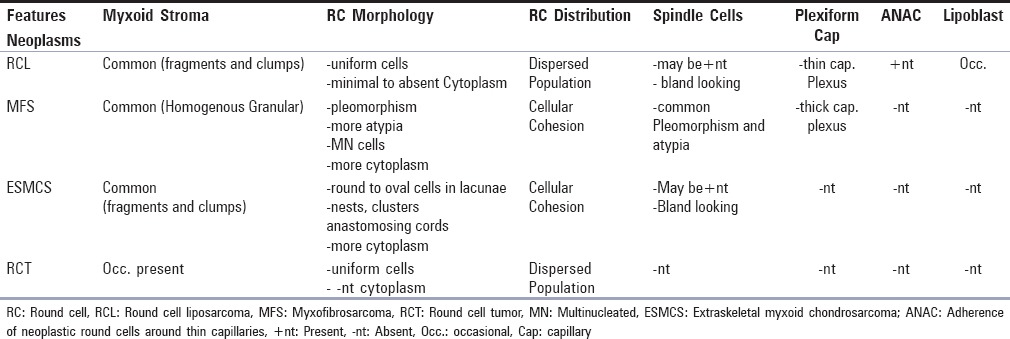
Most of the features were absent, with predominance of round cells in 4 cases, out of which in 2 cases even myxoid stroma was also absent (case# 1, 2, 7, and 8), causing diagnostic confusion with other RCT [Figure 1a].[14] The presence of occasional lipoblasts along with vacuolated background was the most specific diagnostic feature in ruling out other RCT, however, when lipoblasts were absent, presence of plexiform capillaries and ANAC in the background was an indication toward the adipocytic nature of the neoplasm. Differentiating RCL from RCT is important to decide the proper management of the tumor preoperatively.
In rest of the 4 cases, there was variable admixture of different features in addition to the predominance of round-to-oval cells and myxoid stroma (case# 3, 4, 5, and 6). Similar combination of features can be seen in MLPS, MFS, and ESMCS, although round cell cellularity is low as compared to RCL. Thus, MFS and ESCMS were the two most important myxoid sarcomas included in the differential diagnosis.[15,16,17,18,19,20,21]
The prominent features noted in MFS were round-to-oval and spindle cells, nuclear pleomorphism, multinucleated cells, and curvilinear vasculature. The background matrix in MFS had a diffuse granular quality spread over the entire smear instead of clumps and fragments in RCL [Figure 2d].[13,15,16,17,18,19] The more cellular uniformity, occasional presence of lipoblasts, plexiform thin capillaries instead of thick, and ANAC can rule out MFS [Figure 2e].
Smears of ESMCS lacked obvious chondroid differentiation on light microscopy with the presence of uniform monotonous round cell population embedded in myxoid metachromatic stroma; some of the cells had vacuolated cytoplasm. The cellular cohesion, cord-like arrangement of cells having grooved nuclei, and lack of plexiform vascular plexus goes against the diagnosis of RCL [Figure 2f].[20,21]
Features that distinguish RCL from MFS, ESMCS, and RCT are summarized in Table 3.
Table 3.
Differential diagnosis of RCL
The three translocation associated myxoid sarcomas with major cytomorphological overlap (MLPS, ESMCS, MFS) can also be separated on the basis of cytogenetic aberration as detected by fluorescence in situ hybridization (FISH).[22] Whether FISH is utilized or not, attempts to categorize these tumors based on morphology is inevitable, as FNA is the preferred first choice of investigation for most of the clinicians and cytological smears will always be the first sample for the pathologists to evaluate.
Rarely smears of MPNST can be dominated by monomorphic round cells with myxoid changes causing confusion with other RCT, however, the perivascular arrangement of cells on histology is imperceptible on cytology.[23]
Cytopathologists can encounter a variety of soft tissue neoplasms, both benign and malignant, with a background of myxoid matrix. They can include primary myxoid soft tissue neoplasms, whose nomenclature is based on their background matrix, as well as soft tissue neoplasms in which myxoid change is a secondary phenomenon. This secondary myxoid change can rarely be seen in neoplasms other than soft tissue.[24]
RCL are poorly differentiated form of MLPS, which are primary myxoid soft tissue neoplasms. The specificity of background myxoid metachromatic stroma in predicting LPS has been questioned because it has been observed in a number of other nonlipomatous sarcomas.[1] The amount of myxoid material on FNA observed by Kapila et al.,[12] Kilpatrick et al.,[13] Vicandi et al.[14] correlated with the grade of the tumor. It was abundant in low-grade MLPS and scarce to absent in high-grade poorly differentiated RCL, similar to the 2 cases in this study which were entirely composed of round cells with complete absence of myxoid material (case# 7 and 8). It was present in variable amounts in rest of the 6 cases. Thick opaque myxoid stroma in clumps and fragments was present in a majority of the cases similar to the findings by Wakely Jr et al.[25] who reported it in most of the malignant myxoid soft tissue neoplasms compared to benign neoplasms, which were dominated by thin semitransparent myxoid stroma. The later appearance of the stroma was observed focally in a single case (case# 5). Thus, the nature of myxoid stroma on light microscopy can suggest whether a tumor is benign or malignant. The composition of myxoid extracellular matrix also correlated with tumor type and grade detected by imaging mass spectroscopy in a study by Willems et al.[6]
While analyzing and interpreting FNA smears from this group of LPS, cytopathologists can come across different components in different proportions. It is easier to classify the neoplasm as MLPS if predominant myxoid areas are aspirated along with other components typical of liposarcoma. If hypercellular round cell areas are aspirated, then they should be interpreted with caution because of the following reasons. First, as the tumor goes into higher grade, round cell component predominates whereas lipoid component including lipoblasts, myxoid stroma decreases, and spindle cells, if present, are almost bland appearing. Thus, the probability of categorizing these neoplasms among myxoid soft tissue tumors becomes low. It encouraged its placement in the round cell group of neoplasms causing a wide range of diagnostic confusion, in contrast to other soft tissue neoplasms, in which higher grade rarely disqualify them as soft tissue tumors. Second, there can be variable admixture of MLPS-like areas in RCL because there is gradual transition towards poorly differentiated round cell morphology, which is evidenced by the presence of transitional areas on histology [Figure 2b] (case# 3, 4, 5, and 6).
Then the question arises, how can we identify these hypercellular round cell smears as LPS if lipoid vacuolated background or lipoblasts are absent? Adherence of round neoplastic cells around thin capillaries (ANAC) in the background was the most consistent as well as specific feature observed present either focally or widespread. It was still discernible in a case of RCL having pure round cell morphology with absence of other features (case# 7). These capillaries may not be typical plexiform. Although plexiform branching capillaries were observed in 4 cases, they can be a prominent feature in other high-grade soft tissue neoplasms. Kapila et al.[12] did not observe this pattern in a single case of RCL, whereas Wallas et al.[26] observed it in 2 cases and it was absent in all the 5 cases reported by Vicandi et al.[14] In their study on MLPS, Nemanqani et al.[27] reported plexiform pattern in 8 out of 10 cases and Kilpatrick et al.[13] noted this pattern in 3 out of 4 cases having hypercellular round cells.[11]
Typical plexiform capillaries or even simple capillaries were not discernible in highly cellular round cell areas, but those areas on the slide where the population of these round cells were present in dissociated form or in loosely cohesive irregular small groups one could easily identify ANAC [Figure 2e, f].
Lipoblasts are the most specific diagnostic feature for any subtype of liposarcoma, however, it is not the most consistent feature. Cellularity of spindle cells was significantly less than round cells with complete absence in 2 cases of pure RCL.
Conclusion
There is wide variation in cytological features of hypercellular RCL. A confident diagnosis on FNA is possible if attention is paid to individual cytolomorphological features.
Lipoblasts, although specific are not easily recognizable in smears, either they have a very low population or they are completely absent. Plexiform capillary plexus and ANAC should be separately evaluated. The latter feature can help in identifying the adipocytic nature of the neoplasm and is one of the most consistent cytologic features, which could suggest RCL. Myxoid stroma and spindle cells are nonspecific features that can help in reaching a diagnosis if studied in combination with other specific features.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nemanqani D, Mourad WA, Akhtar M, Moreau P, Rostom A, Ezzat A, et al. Liposarcoma: A clinicopathological study of 73 cases diagnosed at King Faisal specialist hospital and research centre. Ann Saudi Med. 1999;19:299–303. doi: 10.5144/0256-4947.1999.299. [DOI] [PubMed] [Google Scholar]
- 2.Fritchie KJ, Goldblum JR, Tubbs RR, Sun Y, Carver P, Billings SD, et al. The expanded histologic spectrum of myxoid liposarcoma with an emphasis on newly described patterns: Iimplications for diagnosis on small biopsy specimens. Am J Clin Pathol. 2012;137:229–39. doi: 10.1309/AJCP90YNOKBAGCDM. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LA. Sarcoma Classification: An update based on the 2013 World Health Organization Classification of Tumours of Soft tissue and Bone. Cancer. 2014;120:1763–74. doi: 10.1002/cncr.28657. [DOI] [PubMed] [Google Scholar]
- 4.Asano N, Susa M, Hosaka S, Nakayama R, Kobayashi E, Takeuchi K, et al. Metastatic patterns of myxoid/Round cell liposarcoma: A review of 25 years experience. Sarcoma 2012. 2012 doi: 10.1155/2012/345161. 345161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orvieto E, Furlanetto A, Laurino L, Dei Tos AP. Myxoid and round cell liposarcoma: A spectrum of myxoid adipocytic neoplasia. Semin Diagn Pathol. 2001;18:267–73. [PubMed] [Google Scholar]
- 6.Willems SM, van Remoortere A, van Zeijl R, Deelder AM, Mc Donnell LA, Hogendoorn PC. Imaging mass spectrometry of myxoid sarcomas identifies proteins and lipids specific to tumour type and grade and reveals biochemical intratumoral heterogeneity. J Pathol. 2010;222:400–9. doi: 10.1002/path.2771. [DOI] [PubMed] [Google Scholar]
- 7.Prestwich RJ, Taylor RE, Grimer R. Metastatic myxoid liposarcoma: Aggressive multimodality management. Clin Oncol. 2005;17:130. doi: 10.1016/j.clon.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Haniball J, Sumathi VP, Kindblom LG, Abudu A, Carter SR, Tillman RM, et al. Prognostic factors and metastatic patterns in primary myxoid/round cell liposarcoma. Sarcoma 2011. 2011 doi: 10.1155/2011/538085. 538085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ten Heuvel SE, Hoekstra HJ, van Ginkel RJ, Bastiaannet E, Suurmeijer AJ. Clinicopathologic prognostic factors in myxoid liposarcoma: A retrospective study of 49 patients with long term follow up. Ann Surg Oncol. 2007;14:222–9. doi: 10.1245/s10434-006-9043-7. [DOI] [PubMed] [Google Scholar]
- 10.Smith TA, Easley KA, Goldblum JR. Myxoid/Round cell liposarcoma of the extremeties. A clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am J Surg Pathol. 1996;20:171–80. doi: 10.1097/00000478-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Kilpatrick SE, Doyon J, Choong PF, Sim FH, Nascimento AG. The clinicopathologic spectrum of myxoid and round cell liposarcoma. A study of 95 cases. Cancer. 1996;77:1450–8. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1450::AID-CNCR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Kapila K, Ghosal N, Gill SS, Verma K. Cytomorphology of lipomatous tumours of soft tissue. Acta Cytol. 2003;47:555–62. doi: 10.1159/000326568. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick SE, Ward WG, Bos GD. The value of fine needle aspiration biopsy in the differential diagnosis of adult myxoid sarcomas. Cancer. 2000;90:167–77. doi: 10.1002/1097-0142(20000625)90:3<167::aid-cncr5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Vicandi B, Jimenez-Heffernan J, Lopez-Ferrer P, Gonzalez-Peramato P, Viguer JM. Cytologic features of round cell liposarcoma: A report on 5 patients. Cancer. 2003;99:28–32. doi: 10.1002/cncr.10925. [DOI] [PubMed] [Google Scholar]
- 15.Olson MT, Ali SZ. Myxofibrosarcoma: Cytomorphologic findings and differential diagnosis on fine needle aspiration. Acta Cytol. 2012;56:15–24. doi: 10.1159/000333134. [DOI] [PubMed] [Google Scholar]
- 16.Akerman M, Domanski HK. Soft tissue. In: Orell SR, Sterrett GF, editors. Orell and Sterrett's Fine needle aspiration cytology. 5th ed. Edinburgh: Churchill Livingstone Elsevier; 2012. pp. 387–411. [Google Scholar]
- 17.Domanski HA, Akerman M, Silverman J. Soft tissue and Musculoskeletal system. In: Gray W, Kocjan G, editors. Diagnostic Cytopathology. 3rd ed. Edinburgh: Churchill Livingstone Elsevier; 2010. pp. 755–807. [Google Scholar]
- 18.Czerniak B, Tuziak T. Soft tissue lesions. In: Koss LG, Melamed MR, editors. Koss's Diagnostic cytology and its histopathologic bases. 5th ed. Philadelphia: Lippincott Williams and Willkins; 2006. pp. 1302–48. [Google Scholar]
- 19.Colin P, Lagace R, Caillaud JM, Sastre Garau X, Klijanienko J. Fine needle aspiration in myxofibrosarcoma: Experience of institut curie. Diagn Cytopathol. 2010;38:343–6. doi: 10.1002/dc.21206. [DOI] [PubMed] [Google Scholar]
- 20.Wadhwa N, Arora VK, Singh N, Bhatia A. Fine needle aspiration cytology of primary extraskeletal myxoid chondrosarcoma. A case report. Acta Cytol. 2000;44:445–8. doi: 10.1159/000328496. [DOI] [PubMed] [Google Scholar]
- 21.Jakowski JD, Wakely PE., Jr Cytopathology of extraskeletal myxoid chondrosarcoma: Report of 8 cases. Cancer. 2007;111:298–305. doi: 10.1002/cncr.22948. [DOI] [PubMed] [Google Scholar]
- 22.Downs-Kelly E, Goldblum JR, Patel RM, Weiss SW, Folpe AL, Mertens F, et al. The utility of Fluoresence in situ hybridization (FISH) in the diagnosis of myxoid soft tissue neoplasms. Am J Surg Pathol. 2008;32:8–13. doi: 10.1097/PAS.0b013e3181578d5a. [DOI] [PubMed] [Google Scholar]
- 23.Wakely PE, Jr, Ali SZ, Bishop JA. The cytopathology of Malignant peripheral nerve sheath tumour: A report of 55 Fine- needle aspiration cases. Cancer. 2012;120:334–41. doi: 10.1002/cncy.21195. [DOI] [PubMed] [Google Scholar]
- 24.Elliot D, Pitman MB. Malignant melanoma with a myxoid stroma: A diagnostic pitfall on fine needle aspiration biopsy. Diagn Cytopathol. 2001;25:185–90. doi: 10.1002/dc.2034. [DOI] [PubMed] [Google Scholar]
- 25.Wakely PE, Jr, Geisinger KR, Cappellari JO, Silverman JF, Frable WJ. Fine needle aspiration cytopathology of soft tissue: Chondromyxoid and myxoid lesions. Diagn Cytopathol. 1995;12:101–5. doi: 10.1002/dc.2840120203. [DOI] [PubMed] [Google Scholar]
- 26.Walaas L, Kindblom LG. Lipomatous Tumours: A correlative cytologic and histologic study of 27 tumours examined by fine needle aspiration cytology. Hum Pathol. 1985;16:6–18. doi: 10.1016/s0046-8177(85)80208-2. [DOI] [PubMed] [Google Scholar]
- 27.Nemanqani D, Mourad WA. Cytomorphologic features of fine needle aspiration of liposarcoma. Diagn Cytopathol. 1999;20:67–9. doi: 10.1002/(sici)1097-0339(199902)20:2<67::aid-dc4>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]


