Abstract
Cerebellar long-term depression (LTD) is a persistent attenuation of synaptic transmission at the parallel fiber–Purkinje cell synapse mediated by the removal of GluR2 subunit-containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. The removal of AMPA receptors requires protein kinase C phosphorylation of the GluR2 subunit within its carboxyl-terminal PSD-95/Discs Large/Zona Occludens-1 (PDZ) ligand and binding of the PDZ domain-containing protein, PICK1. The sequence of the GluR2 subunit is similar to that of the GluR3 and GluR4c subunits, which also contain PDZ ligands and protein kinase C consensus sites. Although GluR3 and GluR4c are also expressed in Purkinje cells, we have previously shown that cerebellar LTD is absent in GluR2–/– mice, suggesting that these subunits are unable to substitute functionally for GluR2. Here, we examine the apparent difference in the regulation of these AMPA receptor subunits by attempting to rescue LTD in GluR2–/– Purkinje cells with WT and mutant GluR2 and GluR3 subunits. Our results show that the selective interaction of the GluR2 subunit with the N-ethylmaleimide-sensitive factor protein is required for synaptic, but not extrasynaptic, incorporation of AMPA receptors as well as for their competence to undergo LTD. In addition, perfusion of a synthetic peptide that acutely disrupts the interaction of GluR2 with N-ethylmaleimide-sensitive factor selectively depletes GluR2-containing receptors from synapses and occludes LTD. These findings demonstrate that interaction of AMPA receptors with N-ethylmaleimide-sensitive factor plays a critical role in incorporation of AMPA receptors into synapses and for their subsequent removal during cerebellar LTD.
Keywords: cerebellar LTD, GluR2
Cerebellar long-term depression (LTD) at the parallel fiber–Purkinje cell synapse is a persistent form of synaptic plasticity that may be important for certain types of motor learning. Cerebellar LTD occurs when parallel fiber and climbing fiber inputs to a Purkinje cell are repeatedly coactivated and has been shown to involve the removal of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors from the plasma membrane of Purkinje cell dendrites (reviewed in refs. 1 and 2). Induction of cerebellar LTD is thought to require activation of AMPA receptors, voltage-gated calcium channels, and metabotropic mGluR1 receptors. Simultaneous activation of these receptors and channels induces a rise in postsynaptic Ca2+ and the liberation of diacylglycerol, which then work together to activate the α isoform of protein kinase C (3). This leads to phosphorylation of the AMPA receptor GluR2 subunit on Ser-880 within its carboxyl-terminal PSD-95/Discs Large/Zona Occludens-1 (PDZ) ligand and the consequent removal of AMPA receptors by clathrin-mediated endocytosis (4–7). The removal of AMPA receptors also appears to require interaction of the GluR2 subunit with the PDZ domain-containing protein, PICK1 (8). A model based on these findings proposes that PICK1 binds GluR2 after protein kinase C-mediated phosphorylation at Ser-880. PICK1 binding then facilitates endocytosis of GluR2-containing AMPA receptor complexes or, alternatively, their stabilization in internalized pools.
GluR2 belongs to a class of AMPA receptor subunits that contain short intracellular carboxyl-terminal domains. Among the others in this class are the GluR3 and GluR4c subunits. Sequence alignment indicates that both GluR3 and GluR4c contain protein kinase C phosphorylation consensus sites (Ser/Thr-X-Lys/Arg; ref. 9) similar to that surrounding GluR2 Ser-880. Moreover, like GluR2, the GluR3 and GluR4c subunits terminate in PDZ ligands, which serve as binding sites for the PDZ domain-containing proteins, PICK1 and GRIP1 and 2 (ref. 2). Given the similarity of GluR3 and GluR4c to GluR2 with regard to their putative protein kinase C phosphorylation sites and PICK1-binding capabilities, it remains unclear why LTD cannot be induced in cultured Purkinje cells prepared from GluR2–/– mice despite the continued expression of GluR3 and GluR4c (7).
One known difference between the GluR2 and GluR3/4c subunit carboxyl-terminal domains lies in the membraneproximal region. In this region, GluR2 contains a stretch of 10 amino acids that confer binding to the N-ethylmaleimidesensitive factor (NSF), a ubiquitous protein involved in vesicular trafficking events (10). Several studies in hippocampal slices or cultured hippocampal neurons have suggested that NSF binding to GluR2 is important for the maintenance of AMPA receptors at synapses. For example, intracellular perfusion of synthetic peptides that interfere with the NSF–GluR2 interaction has been shown to result in a rapid rundown of AMPA receptor-mediated synaptic currents (11–16). Moreover, in one study, viral expression of a fusion protein in hippocampal neurons designed to interfere with binding of NSF to GluR2 was found to reduce the surface expression of AMPA receptors visualized by immunocytochemistry (13).
To investigate the potential role of the GluR2 NSF-binding site in cerebellar LTD, we attempted to rescue LTD in cultured GluR2–/– Purkinje cells with WT and mutant GluR2 and GluR3 subunits. In addition, in GluR2+/+ Purkinje cells, we investigated the acute effects of interfering with the NSF–GluR2 interaction on synaptic transmission and LTD. Our results suggest that NSF binding to GluR2 is required for the mobilization of GluR2-containing receptors from the extrasynaptic to synaptic membrane and for the induction of cerebellar LTD.
Materials and Methods
Genotyping and Preparation of Dissociated Purkinje Cell Cultures. To determine the genetic identity of embryonic days 18–19 offspring from heterozygous breeding of GluR2 mutant mice (The Jackson Laboratory), small pieces of tail were isolated and subjected to proteinase K digestion. PCR-based genotyping was conducted with a primer (GCTGATATTGCCATTGCTCCATTAACTATC) annealing to the 5′ end of exon 11 of the mouse genomic GluR2 sequence, a primer (CTTTATAGTGAATTCATAGACACCATGAAT) annealing to an intronic region immediately 3′ of exon 11, and a primer (CACCAAAGAACGGAGCCGGTT) annealing to a Neo resistance gene. Dissociated cerebellar tissue from GluR2+/+ and GluR2–/– offspring resulting from the heterozygous intercrossing were pooled separately and plated as described (7). Cultures were maintained for 7–16 days in vitro before their use in patch-clamp experiments.
cDNA Mutagenesis. The GluR3 Q612R (GluR3 Q→R) pore mutant was created by using the QuikChange method (Stratagene) followed by subcloning into the cytomegalovirus-driven pRK5 mammalian expression vector. GluR3 Q612R/L853V/T854A/T857P (GluR3 Q→R NSF+) was then created by using splicing-by-overlap-extension PCR with pRK5/GluR3 Q612R as a template. GluR2 constructs were generated as described (7). All mutations were verified by sequence analysis.
Particle-Mediated Gene Delivery. For gene delivery, a gold/glycerol mixture was prepared as follows: 20 mg of 0.6-μm gold microcarriers were washed once in 70% ethanol and three times in sterile deionized, distilled water and resuspended in 0.5 ml of sterile 50% glycerol. For each transfection into Purkinje cell cultures, 5 μg of test plasmid cDNA, 1 μg of enhanced GFP plasmid (Clontech), 50 μl of 2.5 M CaCl2, and 10 μl of 1 M spermidine were sequentially added to 50 μl of the gold/glycerol mixture. The preparation was gently vortexed for 15 min, after which the gold particles were spun down and washed once with 70% ethanol and once with 100% ethanol. Washed gold particles were resuspended in 60 μl of 100% ethanol and pipetted onto macrocarrier disks (9 μl/disk). Using the Helios Gene Gun system (Bio-Rad), DNA-containing gold particles on the macrocarrier disks were delivered into Purkinje cell cultures on in vitro day 6. Cultures were then returned to the incubator for a minimum of 20 h before electrophysiological recording. Transfected Purkinje cells were identified by imaging GFP signals with 488-nm illumination.
Electrophysiology. Patch electrodes were filled with a solution containing 135 mM CsCl, 10 mM Hepes, 0.5 mM EGTA, 4 mM Na2-ATP, and 0.4 mM Na-GTP, adjusted to pH 7.35 with CsOH. In some experiments (Fig. 3), lyophylized peptides were directly dissolved in the internal saline. Cells were bathed in 140 mM NaCl/5mMKCl/2 mM CaCl2/0.8 mM MgCl2/10 mM Hepes/10 mM glucose/0.1 mM d-2-amino-5-phosphonovalerate/0.5 μM Tetrodotoxin/0.3 mM picrotoxin, adjusted to pH 7.35 with NaOH, which flowed at a rate of 0.5 ml/min. Some experiments (Fig. 3) used synaptic activation of granule cell–Purkinje cell pairs, as has been described (6, 7). For these experiments, Tetrodotoxin was omitted from the external saline, and granule cells were activated by using 0.5-ms long constant-voltage pulses through a loose-patch electrode (5–6 MΩ) filled with external saline. Patch electrodes were pulled from N51A glass and polished to yield a resistance of 2–4 MΩ. Iontophoresis electrodes (1-μm tip diameter) were filled with 10 mM glutamate (in 10 mM Hepes, pH 7.1) and were positioned ≈20 μm away from large-caliber dendrites. Test pulses were delivered by using negative current pulses (400–900 nA and 50- to 110-ms duration). For the experiments using exogenous glutamate test pulses, LTD-inducing pairing stimuli consisted of six, 3-s long depolarizations to 0 mV, each delivered together with a test pulse of glutamate. For experiments using granule cell–Purkinje cell pairs, LTD was induced by applying 60 pulses at 0.5 Hz at the test pulse stimulation strength to the granule cell, with each pulse paired with a 100-ms-long depolarization of the Purkinje neuron to 0 mV.
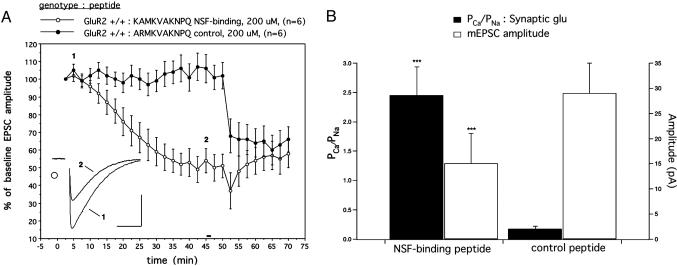
Fig. 3.
Acute peptide-mediated disruption of the NSF–GluR2 interaction causes gradual rundown of the synaptic EPSC amplitude during the baseline period and occludes subsequent LTD induction. (A) LTD was induced by pairing granule cell-evoked EPSCs with Purkinje cell depolarization after a stable baseline was achieved (horizontal bar at t = 50 min). Representative current traces are the averages of five consecutive responses. (Scale bars: 5 ms, 50 pA). (B) Basal synaptic properties of GluR2+/+ Purkinje cells perfused with NSF-binding or control peptides for ≥60 min (a separate population from that shown in A.PCa/PNa values are plotted on the left axis, whereas mEPSC amplitudes are plotted on the right axis. NSF-binding peptide, n = 10; control peptide, n = 9. ***, P < 0.001 compared with control.
Membrane currents were recorded with an Axopatch 200A amplifier and digitized at 10 kHz for exogenous glutamate responses and evoked excitatory postsynaptic currents (EPSCs) and 20 kHz for miniature EPSCs (mEPSCs). Signals were lowpass-filtered at 5 kHz [1 kHz for mEPSCs] and acquired by using axodata 1.2.2 software. Recordings in which Rinput or Rseries varied by >13% were excluded from the analysis. For analysis of mEPSCs, axograph 4.5 mini analysis software was used. This software detected events based on closeness of fit of the mEPSC to a sliding template. Events smaller than –3 pA were discarded. A separate template was created for each recording by averaging 30 of its most unambiguous mEPSCs as selected by eye. The mEPSC measures represent the means of 10 cells with 1,000 events recorded per cell.
Mean values of Rinput and mEPSC kinetics (10–90% rise time and 50% decay time) were not altered by any of the experimental treatments (data not shown).
To determine the Ca2+ permeability of AMPA receptorassociated channels in our cultured Purkinje cells, the reversal potentials of glutamate-evoked currents or mEPSCs were measured while varying [Ca2+]o. To quantify the relative permeability of Ca2+ and monovalent ions, the extended version of the Goldman–Hodgkin–Katz equation was used (17). We assumed Na+ and K+ to be equally permeant and both Cl– and Mg2+ to be impermeant. No allowance was made for the effects of surface charge, and calculations were made by using raw ionic concentrations rather than ionic activities.
Results
Overexpression of GluR3 Fails to Rescue Cerebellar LTD and Synaptic Responses in GluR2–/– Purkinje Cells. We investigated the ability of the GluR2 and GluR3 AMPA receptor subunits to rescue LTD in GluR2–/– Purkinje cells. To assay surface expression of transfected GluR3, we generated a mutant form of this subunit (GluR3 Q612R) that, when incorporated into functional receptor complexes in the plasma membrane, serves to restrict the entry of Ca2+ through the channel pore in a manner similar to GluR2 (18, 19). An analogous approach for electrophysiological “tagging” has been successfully used to assay surface delivery of recombinant AMPA receptors in virally infected hippocampal slices (reviewed in ref. 20). Because the relative Ca2+ permeability is markedly elevated in the GluR2–/– background (7, 21), plasma membrane incorporation of the GluR3 pore mutant (or WT GluR2) can be easily detected as a reduction in the Ca2+/Na+ permeability ratio of AMPA receptors.
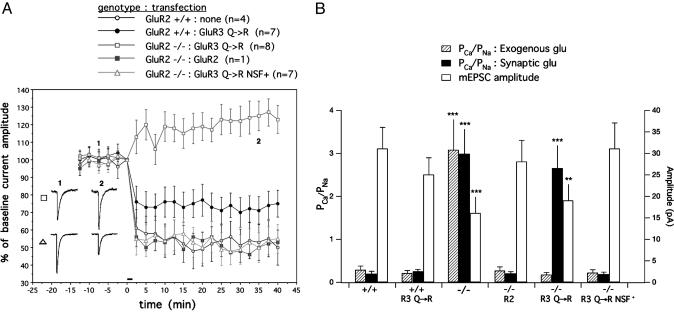
As previously reported (7), LTD was absent in Purkinje cells from GluR2–/– mice, and transfection of WT GluR2 cDNA into the GluR2–/– Purkinje cells produced a complete rescue (53% of baseline at t = 40 min, n = 1, compared with 50 ± 10% of baseline, mean ± SEM, n = 4 in GluR2+/+; Fig. 1A). However, transfection of a pore mutant form of GluR3 (GluR3 Q→R) into GluR2–/– Purkinje cells failed to restore LTD (123 ± 8.0% of baseline, n = 8). Moreover, when GluR3 Q→R was overexpressed in GluR2+/+ Purkinje cells, a significant attenuation of LTD was observed (75 ± 7.6% of baseline, n = 7).
Fig. 1.
Rescue of cerebellar LTD and synaptic Ca2+ permeability in GluR2–/– Purkinje cells requires NSF binding. (A) LTD was induced by iontophoretic glutamate in conjunction with depolarization at t = 0 min (horizontal bar). Error bars represent the SEM in this and all other graphs. Representative raw current traces were acquired at the indicated times. (Scale bars: 1 sec, 50 pA). (B) Basal synaptic properties of cultured Purkinje cells in each of the five conditions depicted in A.PCa/PNa values are plotted on the left axis, whereas mEPSC amplitudes are plotted on the right axis. **, P < 0.02 compared with GluR2+/+ and ***, P < 0.001 compared with GluR2+/+.
In accordance with previous results, the basal mean mEPSC amplitude was decreased and the Ca2+/Na+ permeability ratio for synaptic AMPA receptors was increased in GluR2–/– Purkinje cells due to loss of the GluR2 subunit (7). Moreover, these properties could be rescued by GluR2 transfection. However, as shown in Fig. 1B, transfection of the GluR3 Q→R subunit failed to rescue both the mEPSC amplitude (19 ± 4 pA in GluR3 Q→R transfected cells vs. 16 ± 4 pA in untransfected GluR2–/– and 31 ± 5 pA in GluR2+/+) and the Ca2+/Na+ permeability ratio for synaptic AMPA receptors (2.65 ± 0.55 in GluR3 Q→R transfected cells vs. 2.98 ± 0.60 in untransfected GluR2–/– and 0.19 ± 0.07 in GluR2+/+). Interestingly, GluR3 Q→R transfection completely restored a low Ca2+/Na+ permeability ratio of AMPA receptors in response to exogenously applied glutamate (0.17 ± 0.06 in GluR3 Q→R-transfected cells vs. 3.08 ± 0.73 in untransfected GluR2–/– and 0.28 ± 0.09 in GluR2+/+). Our interpretation of these findings is that the overexpressed GluR3 Q→ R subunit reaches the surface plasma membrane in GluR2–/– Purkinje cells, but fails to become incorporated into synapses.
Transfer of the NSF-Binding Sequence into GluR3 Allows Rescue of Basal Synaptic Properties and Cerebellar LTD in GluR2–/– Purkinje Cells. To test whether NSF binding could explain the functional differences between the GluR2 and GluR3 subunits, we created an NSF-binding site in GluR3 by mutating the membraneproximal region of the GluR3 Q→R construct to resemble that of GluR2 (GluR3 Q→R NSF+; Fig. 2). When transfected into GluR2–/– Purkinje cells, this construct provided a complete rescue of LTD (55 ± 8.2% of baseline, n = 7; Fig. 1 A) as well as the basal mean mEPSC amplitude (31 ± 6 pA in GluR3 Q→R NSF+ transfected cells vs. 16 ± 4 pA in untransfected GluR2–/– and 31 ± 5 pA in GluR2+/+; Fig. 1B). GluR3 Q→R NSF+ also completely rescued the Ca2+/Na+ permeability ratio to WT levels in response to both exogenous (0.22 ± 0.08 in GluR3 Q→R NSF+ transfected cells vs. 3.08 ± 0.73 in untransfected GluR2–/– and 0.28 ± 0.09 in GluR2+/+) and synaptic glutamate (0.18 ± 0.06 GluR3 Q→R NSF+ transfected cells vs. 2.98 ± 0.60 in untransfected GluR2–/– and 0.19 ± 0.07 in GluR2+/+; Fig. 1B). These data suggest a critical role for NSF binding in the movement of GluR2-containing receptors from the extrasynaptic to synaptic membrane and their subsequent removal upon induction of cerebellar LTD.
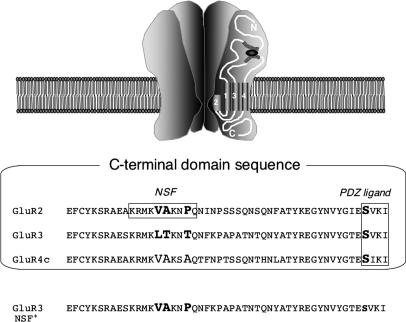
Fig. 2.
Sequence alignment of GluR2, GluR3, and GluR4c, shown for rat (≥98% identity to mouse homologs). Bold residues denote differences between GluR2 and GluR3 in the NSF-binding region. GluR3 NSF+ mutation = GluR3 L853V/T854A/T857P. Ser-880, located within the GluR2 PDZ ligand, as well as corresponding serines in other subunits, are also shown in bold.
Acute Peptide-Mediated Disruption of the GluR2–NSF Interaction in Purkinje Cells Reduces Synaptic Currents and Occludes Cerebellar LTD. To further probe the role of NSF binding, we acutely perfused peptides that interfere with the GluR2–NSF interaction into GluR2+/+ Purkinje cells in culture. When a previously characterized peptide specific for the NSF–GluR2 interaction (KAMKVAKNPQ; ref. 16) was introduced into Purkinje cells, a gradual rundown in EPSCs evoked by stimulation of granule cell–Purkinje cell pairs was observed, which became stable at ≈45 min (51 ± 6.7% of baseline at t = 50 min, n = 6; Fig. 3A). No rundown was observed with a control peptide (ARMKVAKNPQ; ref. 16) over this same time period (102 ± 7.5% of baseline, n = 6). Interestingly, the rundown in current observed with the NSF-interfering peptide after 45 min of recording was concomitant with a reduction in the mEPSC amplitude and an increase in the synaptic Ca2+/Na+ permeability ratio (measured in a separate population of cells), resulting in levels comparable to those observed in GluR2–/– Purkinje cells (mEPSC amplitude: 15 ± 6 pA for NSF-interfering peptide, n = 10 vs. 29 ± 6 pA for control peptide, n = 9; PCa/PNa: 2.45 ± 0.49 for NSF-interfering peptide vs. 0.17 ± 0.05 for control peptide; Fig. 3B). When LTD-inducing stimuli were applied to cell pairs after a stable baseline was achieved, no further depression was observed in Purkinje cells perfused with the NSF-interfering peptide (58 ± 8.0% of baseline vs. 66 ± 7.0% of baseline for control, measured at t = 70 min). Taken together, these results suggest that sustained perfusion of the NSF-interfering peptide eliminates GluR2-containing and LTD-competent AMPA receptors from the synapse.
Discussion
In this study, we investigated the differential ability of the GluR2 and GluR3 AMPA receptor subunits to support LTD in cerebellar Purkinje cells. WT and mutant GluR2 and GluR3 subunits were used to rescue LTD in GluR2–/– Purkinje cells. Altogether, these experiments show that the NSF-binding site on GluR2 is required for synaptic incorporation of AMPA receptors and induction of LTD. The GluR3 subunit, which is unable to bind NSF, is restricted to extrasynaptic sites when transfected into GluR2–/– Purkinje cells as demonstrated by the restoration of a low Ca2+/Na+ permeability ratio (by the Q→R pore mutant) in response to exogenous, but not synaptic, glutamate. However, when the NSF-binding sequence is inserted into GluR3, the transfected receptor can enter synapses and completely rescue the Ca2+/Na+ permeability ratio as well as LTD. These results are consistent with the peptide interference experiments in GluR2+/+ Purkinje cells in which sustained disruption of the GluR2–NSF interaction was found to deplete GluR2-containing receptors from the synaptic compartment. After a 45-min rundown period, synapses have electrophysiological properties similar to those of GluR2–/– Purkinje cells: The synaptic AMPA receptor Ca2+/Na+ permeability ratio is elevated and LTD cannot be induced.
Our results are consistent with previous studies showing that GluR3 fails to incorporate into synapses upon overexpression in virally infected hippocampal slice cultures (22). In this system, GFP-tagged GluR3 was visualized in spines but failed to change the rectification properties of synaptic AMPA receptors. Such a change would be expected with incorporation of GFP-GluR3-containing receptors into the synaptic plasma membrane because of the inward rectification properties of the GluR3 (Q) subunit (reviewed in ref. 20). Interestingly, although rectification was unchanged in this experiment, a decrease in AMPA receptor-mediated EPSC amplitude was observed. Our results in Purkinje cells suggest that NSF may be required for lateral migration of AMPA receptors from extrasynaptic to synaptic sites in hippocampal neurons as well. Although no direct role for NSF in lateral membrane diffusion has been identified, recent work indicates that NSF can dissociate GluR2–PICK1 complexes through its ATPase activity (23). In an analogous manner, it is conceivable that NSF permits AMPA receptor entry into synapses by disrupting GluR2 interactions with proteins, possibly PICK1, in regions outside the synapse.
Although GluR3 Q→R was delivered to the extrasynaptic plasma membrane in our experiments, it failed to rescue LTD that was both induced and measured by ionotophoretic glutamate application. In contrast, GluR3 Q→R NSF+ fully rescued LTD by using this same approach. Because ionotophoretic glutamate activates synaptic as well as extrasynaptic receptors, our results suggest that entry of AMPA receptors into synapses from extrasynaptic membranes may be a prerequisite for their removal during LTD. Alternatively, NSF may play some specific additional role in regulating the removal of AMPA receptors from the plasma membrane during LTD.
Previous studies performed in hippocampal slices have indicated that the NSF–GluR2 interaction regulates the same pool of receptors that become internalized during hippocampal LTD (14, 15). However, these studies relied on the use of peptides that block the interaction of GluR2 with NSF as well as the interaction of the AP2 clathrin adaptor. AP2 binds to GluR2 in a region that overlaps the NSF-binding site (16). Lee et al. (16) distinguished between the effects of NSF and AP2 by designing peptides that selectively interfere with the binding of either protein. When a peptide specific for the NSF–GluR2 interaction (KAMKVAKNPQ) was perfused into CA1 pyramidal cells, a rundown in EPSC amplitude was observed over a period of ≈20 min. In contrast to our results in Purkinje cell cultures by using the same peptide, Lee et al. (16) found that hippocampal LTD could still be induced following this rundown. With an AP-2-interfering peptide, Lee et al. (16) observed the opposite effect: no rundown of basal EPSCs but complete blockade of LTD induction. Although these results might initially suggest the existence of separate receptor pools regulated by NSF and AP2, Lee et al. (16) acknowledge the possibility that interference with NSF might indirectly lead to loss of LTD by means of occlusion. The LTD observed in their experiments by using the NSF-interfering peptide may represent incomplete occlusion, perhaps because of the different combinations of AMPA receptor subunits expressed in hippocampal cells as compared to Purkinje cells. This is consistent with the observation that hippocampal LTD is still present in GluR2–/– mice (21).
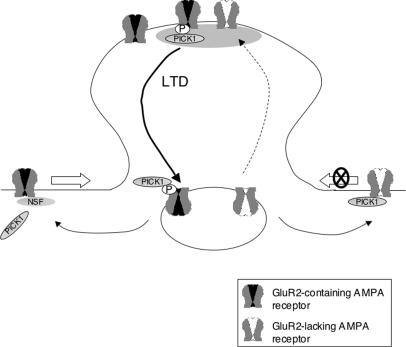
Although NSF appears to regulate entry of AMPA receptors into the synaptic compartment, our results indicate that at least some AMPA receptors become synaptically localized in an NSF-independent manner. This population of receptors corresponds to those contributing to the residual mEPSC response in GluR2–/– Purkinje cells and in GluR2+/+ Purkinje cells perfused with the NSF-interfering peptide after 45 min of rundown. These residual Ca2+-permeable AMPA receptors, however, cannot support LTD because no synaptic depression was observed in the absence of GluR2. This raises the possibility of an additional locus of action of NSF in receptor internalization during LTD, as mentioned above. Alternatively, these Ca2+-permeable AMPA receptors at synapses may contain subunits with long intracellular domains such as GluR4 that endow the receptors with other regulatory mechanisms that render them incompetent for LTD (Fig. 4).
Fig. 4.
A model of NSF and GluR2 action in cerebellar LTD based on rescue transfection and peptide perfusion experiments. GluR2-containing AMPA receptor complexes (drawn with a black pore) are Ca2+-impermeable, enter the synaptic compartment from extrasynaptic regions in a manner dependent on NSF, and are internalized upon induction of cerebellar LTD via phosphorylation and binding to PICK1. GluR2-lacking AMPA receptor complexes (drawn with a white pore) are Ca2+-permeable. They cannot bind NSF and remain largely extrasynaptic. A subset of GluR2-lacking AMPA receptors become synaptically localized in an NSF-independent manner (dashed arrow) but are incompetent for LTD.
Acknowledgments
We wish to thank R. Bock for technical assistance and S. Gardner and D. Bergles for helpful comments and advice. This work was supported by the National Institute of Neurological Disorders and Stroke, the Howard Hughes Medical Institute (to R.L.H.), the National Institute of Mental Health, and the Develbiss Fund (to D.J.L.).
Author contributions: J.P.S., R.L.H., and D.J.L. designed research; J.P.S. and D.J.L. performed research; J.P.S., R.L.H., and D.J.L. analyzed data; and J.P.S., R.L.H., and D.J.L. wrote the paper.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; LTD, long-term depression; EPSC, excitatory postsynaptic current; mEPSC, miniature EPSC; PICK1, protein interacting with C-kinase 1; NSF, N-ethylmaleimide-sensitive factor; PDZ, PSD-95/Discs Large/Zona Occludens-1.
References
- 1.Bear, M. F., Linden, D. J. (2001) in Synapses, eds. Cowan, W. M. & Davies, K. (Johns Hopkins Univ. Press, Baltimore), pp. 455–517.
- 2.Song, I. & Huganir, R. L. (2002) Trends Neurosci. 25, 578–588. [DOI] [PubMed] [Google Scholar]
- 3.Leitges, M., Kovac, J., Plomann, M. & Linden, D. J. (2004) Neuron 44, 585–594. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda, S., Launey, T., Mikawa, S. & Hirai, H. (2000) EMBO J. 19, 2765–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang, Y. T. & Linden, D. J. (2000) Neuron 25, 635–647. [DOI] [PubMed] [Google Scholar]
- 6.Linden, D. J. (2001) Proc. Natl. Acad. Sci. USA 98, 14066–14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung, H. J., Steinberg, J. P., Huganir, R. L. & Linden, D. J. (2003) Science 300, 1751–1755. [DOI] [PubMed] [Google Scholar]
- 8.Xia, J., Chung, H. J., Wihler, C., Huganir, R. L. & Linden, D. J. (2000) Neuron 28, 499–510. [DOI] [PubMed] [Google Scholar]
- 9.Kemp, B. E. & Pearson, R. B. (1990) Trends Biochem. Sci. 15, 342–346. [DOI] [PubMed] [Google Scholar]
- 10.Whiteheart, S. W. & Matveeva, E. A. (2004) J. Struct. Biol. 146, 32–43. [DOI] [PubMed] [Google Scholar]
- 11.Song, I., Kamboj, S., Xia, J., Dong, H., Liao, D. & Huganir, R. L. (1998) Neuron 21, 393–400. [DOI] [PubMed] [Google Scholar]
- 12.Nishimune, A., Isaac, J. T., Molnar, E., Noel, J., Nash, S. R., Tagaya, M., Collingridge, G. L., Nakanishi, S. & Henley, J. M. (1998) Neuron 21, 87–97. [DOI] [PubMed] [Google Scholar]
- 13.Noel, J., Ralph, G. S., Pickard, L., Williams, J., Molnar, E., Uney, J. B., Collingridge, G. L. & Henley, J. M. (1999) Neuron 23, 365–376. [DOI] [PubMed] [Google Scholar]
- 14.Luthi, A., Chittajallu, R., Duprat, F., Palmer, M. J., Benke, T. A., Kidd, F. L., Henley, J. M., Isaac, J. T. & Collingridge, G. L. (1999) Neuron 24, 389–399. [DOI] [PubMed] [Google Scholar]
- 15.Luscher, C., Xia, H., Beattie, E. C., Carroll, R. C., von Zastrow, M., Malenka, R. C. & Nicoll, R. A. (1999) Neuron 24, 649–658. [DOI] [PubMed] [Google Scholar]
- 16.Lee, S. H., Liu, L., Wang, Y. T. & Sheng, M. (2002) Neuron 36, 661–674. [DOI] [PubMed] [Google Scholar]
- 17.Jan, L. Y. & Jan Y. N. (1976) J. Physiol. (London) 262, 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hume, R. I., Dingledine, R. & Heinemann, S. F. (1991) Science 253, 1028–1031. [DOI] [PubMed] [Google Scholar]
- 19.Burnashev, N., Monyer, H., Seeburg, P. H. & Sakmann, B. (1992) Neuron 8, 189–198. [DOI] [PubMed] [Google Scholar]
- 20.Malinow, R. & Malenka, R. C. (2002) Annu. Rev. Neurosci. 25, 103–126. [DOI] [PubMed] [Google Scholar]
- 21.Jia, Z., Agopyan, N., Miu, P., Xiong, Z., Henderson, J., Gerlai, R., Taverna, F. A., Velumian, A., MacDonald, J., Carlen, P., et al. (1996) Neuron 17, 945–956. [DOI] [PubMed] [Google Scholar]
- 22.Shi, S., Hayashi, Y., Esteban, J. A. & Malinow, R. (2001) Cell 105, 331–343. [DOI] [PubMed] [Google Scholar]
- 23.Hanley, J. G., Khatri, L., Hanson, P. I. & Ziff, E. B. (2002) Neuron 34, 53–67. [DOI] [PubMed] [Google Scholar]