Abstract
Chronic hyperglycemia and its associated metabolic products are key factors responsible for the development and progression of diabetic chronic kidney disease (CKD). Endocrinologists are tasked with detection and management of early CKD before patients need referral to a nephrologist for advanced CKD or dialysis evaluation. Primary care physicians are increasingly becoming aware of the importance of managing hyperglycemia to prevent or delay progression of CKD. Glycemic control is an integral part of preventing or slowing the advancement of CKD in patients with diabetes; however, not all glucose-lowering agents are suitable for this patient population. The availability of the latest information on treatment options may enable physicians to thwart advancement of serious renal complication in patients suffering from diabetes. This review presents clinical data that shed light on the risk/benefit profiles of three relatively new antidiabetes drug classes, the dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 analogs, and sodium glucose co-transporter 2 inhibitors, particularly for patients with diabetic CKD, and summarizes the effects of these therapies on renal outcomes and glycemic control for endocrinologists and primary care physicians. Current recommendations for screening and diagnosis of CKD in patients with diabetes are also discussed.
Keywords: Diabetes, diabetic kidney disease, diabetic nephropathy, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 analogs, sodium glucose co-transporter 2 inhibitors
1. Introduction
The prevalence of diabetes mellitus is rapidly reaching epidemic proportions. Diabetic kidney disease (DKD) is a leading complication of diabetes, which can lead to serious consequences, including kidney failure and cardiovascular (CV) morbidity and mortality [1]. In the United States, an estimated 40% of patients with diabetes develop DKD [1]. Cross-sectional analyses of the National Health and Nutrition Examination Survey (NHANES) program revealed that during 1988–2008, the prevalence of DKD in the study population increased (2.2%–3.3%) in proportion to the prevalence of diabetes (6.0%–9.4%) [1]. Glycemic control is an integral part of preventing or reducing the risk of DKD. In this article, we chose to review renal outcomes of newer anti-hyperglycemic therapies in patients with type 2 diabetes mellitus (T2DM) and DKD.
1.1. Screening and Diagnosis of DKD
Current guidelines recommend annual quantitative testing of urinary albumin (e.g., urine albumin to creatinine ratio [UACR]) and calculation of estimated glomerular filtration rate (eGFR) in patients with type 1 diabetes (T1DM) beginning 5 years after diagnosis, and in all patients with T2DM starting at diagnosis [2,3]. A UACR of <30 mg/g is considered to be normal, whereas UACR of ≥30 mg/g is classified as albuminuria [2]. Proteinuria is defined as >300 mg/24 h of total urine protein (albumin and other nonselective proteins) [4]. UACR as measured by random spot urine sampling is commonly used to assess urinary albumin excretion (UAE) [2]. Because UACR can also vary due to factors such as vigorous exercise within 24 h, infection, fever, heart failure, pronounced hyperglycemia, and uncontrolled hypertension, it should be measured 2 to 3 times within 3 to 6 months to confirm increased UAE.
Whereas persistent albuminuria at UACR of 30–299 mg/g has been shown to be a marker of early DKD, lack of albuminuria may not reflect absence of progressive kidney disease. Studies in T1DM and T2DM, however, have found substantial proportions of patients with lower than normal GFR despite normal UAE [5,6]. In a cross-sectional study, 22% (n=23) of 105 normoalbuminuric patients with T1DM for ≥10 years had below normal GFR (<90 mL/min/1.73 m2) [5]. The low GFR group also had more advanced diabetic glomerular lesions as well as increased glomerular basement membrane thickness versus the normal GFR and non-diabetic control groups. Cross-sectional analysis of data from the third NHANES of patients with T2DM (n=1197) showed that of the 171 patients who had chronic kidney disease (CKD), 30% (n=51) were free of retinopathy and albuminuria [6]. Thus, screening for DKD based only on UACR would miss a substantial proportion of patients with progressive kidney disease.
Current American Diabetes Association (ADA) guidelines recommend using GFR in addition to UAE for staging CKD and state that “serum creatinine with eGFR should be assessed at least annually in all adults with diabetes, regardless of the degree of urine albumin excretion” [2]. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) staging of CKD is also based on eGFR. At stage 1 (when hyperfiltration may be present in early diabetes) the eGFR is normal to high (≥90 mL/min/1.73 m2) and progressively decreases to <15 mL/min/1.73 m2 with advancing CKD from stages 2 to 5. Table 1 shows likelihood of the diagnosis of DKD based on both variables, eGFR and albuminuria [3]. Concurrent diabetes and CKD may not always indicate DKD. According to the NKF KDOQI guidelines, CKD can be attributed to diabetes in patients presenting with macroalbuminuria or microalbuminuria with either diabetic retinopathy or T1DM of ≥10-year duration [3].
Table 1.
Likelihood of DKD based on UACR and GFR [3].
| Albuminuria | ||||
|---|---|---|---|---|
| GFR* | CKD Stage† | None‡ | UACR 30-299 mg/g | UACR ≥300 mg/g |
| >60 | 1+2 | At risk | Possible DKD | DKD |
| 30–60 | 3 | Unlikely DKD | Possible DKD | DKD |
| <30 | 4+5 | Unlikely DKD | Unlikely DKD | DKD |
*GFR expressed in mL/min.
†The NKF recommends using albuminuria data before initiation of RAAS inhibitor therapy to determine the staging.
‡UACR <30 mg/g.
CKD, chronic kidney disease; DKD, diabetic kidney disease; GFR, glomerular filtration rate; UACR, urine albumin to creatinine ratio.
1.2. Pathophysiology and Risk Factors for DKD
Long-standing hyperglycemia can potentially induce or deregulate several biochemical processes in the kidney, leading to increased reactive oxygen species, activation of protein kinase C, increased advanced glycation end products (AGEs), secretion of profibrotic cytokine, and low-grade chronic inflammation [7]. In early diabetes, renal hemodynamic changes may occur and are characterized by glomerular hyperfiltration. These processes ultimately lead to kidney injury and loss of renal function [7,8].
2. Pharmacotherapy for DKD
Pharmacological treatment of DKD is primarily targeted towards lowering HbA1c and blood pressure, with the goal of reducing UAE to prevent advancement of microalbuminuria to macroalbuminuria and subsequent progression to end-stage renal disease (ESRD). In hypertensive patients with DKD, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) – the two main classes of renin-angiotensin-aldosterone system (RAAS) blockers – are widely used [3]. According to the NKF KDOQI guidelines, strong class I evidence supports the benefit of ACEIs in T1DM and ARBs in T2DM in slowing the progression of DKD [3]. In normotensive, normoalbuminuric patients, use of ACEIs and ARBs is generally not recommended; however, the 2012 update to the KDOQI Clinical Practice Guideline for Diabetes and CKD suggests use of an ACEI or an ARB in normotensive patients with UAE ≥30 mg/g who are at high risk for DKD or its progression [9]. New therapeutic approaches under investigation for improving renal function in patients with DKD include endothelin receptor antagonists, AGEIs, AGE receptor antagonists, NADPH oxidase inhibitors, and anti-fibrotic agents [8].
2.1. Effect of Glycemic Control on DKD
Glycemic targets in the presence of DKD should be individualized depending on the patient characteristics. Based on several pivotal trials in T1DM and T2DM, the NKF KDOQI guidelines recommend intensive glycemic control (HbA1c ~53 mmol/mol [7%]) to prevent or delay the progression of albuminuria and other microvascular complications in T2DM [9]. Recent ADA guidelines also indicate that an HbA1c around or below 53 mmol/mol (7%) has been shown to reduce the risk of microvascular complications and suggest a general HbA1c goal of <53 mmol/mol (7%) for non-pregnant adults [2]. Landmark studies in patients with T2DM, including the Veterans Affairs Diabetes Trial (VADT) [10], the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial [11], and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial [12] have provided consistent evidence that intensive glycemic control (VADT, median HbA1c, 52 mmol/mol [6.9%]; ADVANCE, mean HbA1c, 48 mmol/mol [6.5%]; ACCORD, median HbA1c at transition to standard therapy, 45 mmol/mol [6.3%]) reduces albuminuria but with no significant improvement in DKD progression in patients with T2DM. In VADT, worsening of albuminuria occurred less frequently in the intensive treatment group (9.1%) compared with the standard therapy group (13.8%, p=0.01) at the end of 5 years, whereas decline in GFR was similar in both groups [10]. In the ADVANCE study, intensive glycemic control (HbA1c target of 48 mmol/mol [6.5%]) was associated with 30% reduction in development of macroalbuminuria and 21% reduced risk of renal events (macroalbuminuria, doubling of serum creatinine, ESRD, or death) at the end of 5.6 years [11]. The ACCORD study showed benefit of intensive therapy over standard therapy in reducing the incidence rates of micro- and macroalbuminuria (hazard ratio [95% confidence interval], 0.79 [0.69–0.90]; p=0.0005 and 0.69 [0.55–0.85]; p=0.0007, respectively) at early termination of the trial in 3.7 years. In these studies, however, the risk of CV or renal events (doubling of serum creatinine or ESRD) was not diminished by intensive glycemic control, and all-cause mortality was increased in ACCORD [10-12]. Therefore, an HbA1c target <53 mmol/mol (7%) is not recommended in patients who have risk factors for hypoglycemia (e.g., those taking insulin or sulphonylureas or having stage 4 or 5 CKD) [9]. Both ADA and NKF KDOQI guidelines suggest less stringent glucose control (HbA1c >53 mmol/mol [7%]) in patients with limited life expectancy, advanced microvascular or macrovascular complications, and long duration of T2DM who are unable to attain the general treatment goal despite diabetes self-management education, glucose monitoring, and use of multiple anti-diabetes drugs [2, 9].
2.2. Glucose-Lowering Agents
The adverse event (AE) profile of established glucose-lowering therapies, including metformin, sulphonylureas, thiazolidinediones, and insulin, can limit their use in patients with DKD; for example, according to the prescribing information, metformin is contraindicated in patients with renal disease or dysfunction (abnormal creatinine clearance [CrCl] or serum CrCl ≥1.5 mg/dL in men or ≥1.4 mg/dL in women), and recent Kidney Disease Improving Global Outcomes guidelines recommend discontinuation of metformin in those with GFR <30 mL/min [13]. Risk of hypoglycemia with sulphonylureas and insulin is worsened in patients with CKD because the reduced GFR prolongs their pharmacodynamic action, necessitating careful dose titrations [14]. Thiazolidinediones can be used without dose adjustment but are associated with a risk of weight gain, congestive heart failure, and bone fractures [15,16]. Treatment options with improved safety profiles, especially low risk of hypoglycemia and weight gain, are needed to manage hyperglycemia in patients with DKD. Incretin-enhancing therapies and sodium glucose co-transporter 2 (SGLT2) inhibitors are two of the most recent classes of anti-diabetes drugs. The efficacy of these recent drug classes in achieving glycemic control, their effects on renal function, and the potential for use in patients with DKD are discussed below. We have limited the discussion to drugs approved in the United States.
2.3. Incretin-Based Therapies
Incretin-based therapies follow two approaches to increase the in vivo activity of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) – the key incretins involved in regulation of plasma glucose levels [17]. One approach involves inhibition of the proteolytic enzyme dipeptidyl peptidase-4 (DPP-4) responsible for degradation of GLP-1 and GIP. DPP-4 inhibition leads to increased circulating incretin levels, and, ultimately, improved glycemic control (Fig. 1). The second approach involves providing exogenous, DPP-4 resistant GLP-1 receptor agonists to activate the GLP-1 receptor.
Fig. (1).
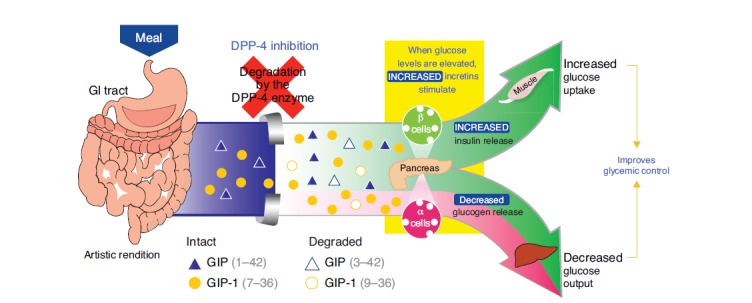
Effects of DPP-4 inhibition on regulation of plasma glucose. Reproduced with permission from Herman GA, Stein PP, Thornberry NA, Wagner JA. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Ther 2007; 81: 761-7. DPP-4, dipeptidyl peptidase-4; GIP, glucose-dependent insulinotropic peptide.
2.3.1. DPP-4 Inhibitors
Clinical studies have demonstrated anti-hyperglycemic efficacy of DPP-4 inhibitors alone or in combination with other anti-diabetes drugs (mean change in HbA1c, −3 to −19 mmol/mol [−0.3 to –1.7%]) [18] without excess risk of hypoglycemia (when the background therapy does not include sulphonylureas or insulin) or weight gain. Linagliptin is the only DPP-4 inhibitor excreted primarily via a non-renal route, and no dose adjustment is necessary in patients with CKD [19]. Other approved DPP-4 inhibitors (sitagliptin [20], saxagliptin [21], and alogliptin [22]) can be used in patients with CKD, but require dose adjustment for patients with moderate or severe CKD or ESRD (Table 4). Due to this requirement, assessment of renal function before initiating therapy and periodically thereafter is recommended for sitagliptin, saxagliptin, and alogliptin.
Table 4.
Recommended dosing of DPP-4 inhibitors in patients with T2DM and CKD.
| Severity of CKD | |||
|---|---|---|---|
|
No or Mild CKD
(CrCl ≥50 mL/min) |
Moderate
(CrCl ≥30 to <50 mL/min) |
Severe/ESRD
(CrCl <30 mL/min) |
|
| Alogliptin [22] | 25 mg qd* | 12.5 mg qd† | 6.25 mg qd |
| Linagliptin [19] | 5 mg qd | 5 mg qd | 5 mg qd |
| Saxagliptin [21] | 5 mg qd‡ | 2.5 mg qd | 2.5 mg qd |
| Sitagliptin [20] | 100 mg qd | 50 mg qd | 25 mg qd |
*CrCl ≥60 mL/min.
†For CrCl ≥30 to <60 mL/min.‡For CrCl >50 mL/min.
CKD, chronic kidney disease; CrCl, creatinine clearance; DPP-4, dipeptidyl peptidase-4 inhibitors; ESRD, end-stage renal disease; qd, once daily; T2DM, type 2 diabetes mellitus.
Glycemic efficacy of DPP-4 inhibitors as evaluated in clinical trials in patients with CKD is summarized in Table 2. In these individual trials and pooled analyses, DPP-4 inhibitors were found to be well tolerated. In a randomized controlled trial (RCT) involving patients with severe CKD, treatment with linagliptin for 52 weeks led to no clinically relevant change in mean eGFR, no drug-related renal failure, and comparable rates of renal and urinary adverse effects as with placebo (25.0% and 21.5%, respectively). Severe hypoglycemia occurred at low rates (4.4% with linagliptin; 4.6% with placebo) [23]. In another 52-week RCT in patients with moderate to severe CKD, linagliptin was compared with placebo or glimepiride (patients were randomized to placebo for 12 weeks and then switched to glimepiride for 40 weeks). Over the 40-week period, linagliptin was associated with less hypoglycemia (62/107, 57.9%) compared with glimepiride (79/114, 69.3%). After 52 weeks, linagliptin treatment resulted in a decrease in body weight relative to placebo/glimepiride treatment [24].
Table 2.
Glycemic efficacy of incretin-based therapies and SGLT2 inhibitors in patients with CKD.
|
Intervention
(N Randomized) |
Duration
(Weeks) |
Background
Therapy |
Baseline CKD Staging
(eGFR, mL/min/1.73 m2) |
HbA1c, mmol/mol (%) | ||
|---|---|---|---|---|---|---|
| Baseline | Adjusted Mean Change from Baseline | Difference vs Comparator | ||||
| Linagliptin 5 mg (68) Placebo (65) [23] |
52 | Other antidiabetes drugs, including insulin | 4/5 (<30)* | 66 (8.2) 66 (8.2) |
−8 (−0.7) 0 (0.0) |
−8 (−0.7) – |
| Linagliptin 5 mg (113) Placebo /glimepiride†(122) [24] |
52 | Insulin ± other OADs | ≥3 (<60) | 65 (8.1)‡ | −7 (−0.6) −6 (−0.5) |
NR – |
| Sitagliptin 50§or 25ǁ mg (65) Placebo/glipizide¶ (26) [27] |
54 | Insulin monotherapy (no other OADS) | Moderate§ to Severeǁ including ESRD | 60 (7.6) 62 (7.8) |
−8 (−0.7) −9 (−0.8) |
NR – |
| Sitagliptin 25 mg (64) Glipizide** (65) [28] |
54 | None | ESRD on dialysis | 63 (7.9) 62 (7.8) |
−8 (−0.7) −10 (−0.9) |
2 (0.2) – |
| Saxagliptin 2.5 mg (85) Placebo (85) [31] |
52 | Other antidiabetes drugs, including insulin | Moderateǁ to Severe¶
including ESRD |
68 (8.4) 65 (8.1) |
−12 (−1.1) −4 (−0.4) |
−8 (−0.7) – |
| Liraglutide 1.8 mg (140) Placebo (137) [NCT01620489] |
26 | Other OADs ± insulin | 3 (≥30 to <60) | 65 (8.1) 64 (8.0) |
−12 (−1.1) −4 (−0.4) |
−8 (−0.7) – |
| Albiglutide 30 mg (254)†† Sitagliptin (253)[40]†† |
26 | Other OADs‡‡ | Mild (≥60 to ≤89) Moderate (≥30 to ≤59) Severe (≥15 to ≤29) |
65 (8.1) 66 (8.2) |
−9 (−0.8) −6 (−0.5) |
−3 (−0.3) |
| Empagliflozin 25 mg (97/188/37)§§
Placebo (97/187/37) [46] |
24 | Other antidiabetes drugs, including insulin |
2 (≥60 to <90) | 64 (8.0) 65 (8.1) |
−7 (−0.6) 1 (0.1) |
−8 (−0.7) – |
| 3 (≥30 to <60) | 64 (8.0) 65 (8.1) |
−3 (−0.3) 1 (0.1) |
−4 (−0.4) – |
|||
| 4 (≥15 to <30) | 65 (8.1) 66 (8.2) |
1 (0.1) −4 (−0.4) |
NR – |
|||
| Canagliflozin 100 mg (90)ǁǁ Canagliflozin 300 mg (89)ǁǁ Placebo (90) [51]ǁǁ |
26 | None or other antidiabetes, therapies including insulin and GLP-1 analogs |
3 (≥30 to <50) | 63 (7.9) 64 (8.0) 64 (8.0) |
−3 (−0.3) −4 (−0.4) 0 (0.0) |
−3 (−0.3) −4 (−0.4) – |
*14.5% (19/133) of all randomized patients had baseline eGFR of 30 to 60 mL/min/1.73m2.
†Placebo for 12 weeks followed by glimepiride (1–4 mg/day) for 40 weeks.
‡Overall mean baseline value.
§CrCl ≥30 to <50 mL/min.
ǁCrCl <30 mL/min
¶ Placebo for 12 weeks followed by glipizide for 42 weeks.
**2.5 mg/day titrated to 10 mg twice daily.
††Albiglutide: initial dose 30 mg/week uptitrated to 50 mg/week as needed. Sitagliptin: 100 mg (mild CKD), 50 mg (moderate CKD), 25 mg (severe CKD).
‡‡ Patients with eGFR <60 mL/min/1.73m2 were washed off metformin therapy.
§§Stage 2 CKD patients were randomized to empagliflozin 10 mg, 25 mg or placebo, data for the 10 mg arm are not shown. The n values represent number of patients in each treatment group with stage 2/stage 3/stage 4 CKD.
ǁǁ Number of patients who received the study drug.
CKD, chronic kidney disease; ESRD, end-stage renal disease, GLP-1, glucagon-like peptide 1; NR, not reported; OAD, oral antidiabetes drug; SGLT2, sodium glucose co-transporter 2; CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate.
A pooled analysis in patients with albuminuria (UACR 30–3000 mg/g) on stable RAAS inhibitor treatment also showed benefit of linagliptin (Table 3). Relative to placebo, linagliptin reduced the HbA1c by 7 mmol/mol (0.6%) and UACR by 28%, with no significant differences in eGFR and systolic blood pressure (SBP) at 24 weeks [25]. In addition, the percent changes in geometric mean UACR were independent of changes in mean HbA1c or SBP over 24 weeks. The ongoing MARLINA-T2D™ trial (NCT01792518) is prospectively designed to evaluate modification of albuminuria with linagliptin in patients with T2DM and persistent albuminuria (UACR 30–3000 mg/g) after 24-week treatment (estimated enrollment is 404 patients). CARMELINA® (NCT01897532) is an ongoing large, long-term trial powered to prospectively compare renal outcomes (time to first occurrence of renal death, ESRD, or a sustained drop of ≥50% in eGFR) of linagliptin versus placebo. The estimated treatment period and enrollment are 48 months and 8300 patients, respectively.
Table 3.
Representative renal outcomes studies of incretin-based therapies and SGLT2 inhibitors.
| Intervention | Study Design (N) | Background Therapy | Baseline Renal Parameters | Key Renal Outcomes | ||
|---|---|---|---|---|---|---|
| Mean UACR (mg/g) | Mean eGFR (mL/min/1.73m2) |
Mean Change in UACR (95% CI)
(% Change or mg/g) |
Other | |||
| Linagliptin 5 mg Placebo [25] |
Pooled analysis of 4, 24-week, phase 3 RCTs (217) |
Stable ACEI/ARB therapy*± OADs | 73.8 (30.1–2534.4)† 80.5 (30.9–1538.2)† |
83.6 (35.8–189.7)† 87.9 (39.6–138.4)† |
−32 (−42, −21)‡ (linagliptin) −6 (−27, +23)‡ (placebo) |
eGFR§: −1.3 (linagliptin) −0.2 (placebo) |
| Alogliptin 25 mg Sitagliptin 50 mg [26] |
12-week, open-label, crossover study (8) | ARBs + other OADs | NR | 66.2 ± 9.3 | 33.9 ± 23.9ǁ (alogliptin) 81.0 ± 52.4ǁ (sitagliptin) |
eGFR: 64.7 ± 8.4 (alogliptin) 65.8 ± 8.0 (sitagliptin) sCr¶: 0.87 ± 0.21 (alogliptin) 0.84 ± 0.19 (sitagliptin) |
| Sitagliptin 50 mg [29] | 6-month observational cohort study (36) | Other OADs, ARBs, or statins | 11.6 ± 8.4 (normoalbuminuric) 98.4 ± 79 (microalbuminuric) 1263 ± 492 (macroalbuminuric) |
73.3 ± 16.3 | −4.5 ± 5.0 (normoalbuminuric) −24.9 ± 20 (microalbuminuric) −561 ± 89 (macroalbuminuric) |
eGFR: 77.0 ± 19.4 |
| Empagliflozin 25 mg** Placebo [46] |
52-week phase 3 RCT (741) | Antihypertensive + other anti-diabetes drugs | NR†† | Stage 2 CKD: 71.6 (10.6) stage 3 CKD: 44.9 (10.2) stage 4 CKD: 23.2 (4.9) |
Stage 2 CKD: −235.86 (−442.85, −28.86) stage 3 CKD: −183.78 (−305.18, −62.38) |
Slight decrease in eGFR for all stages of CKD that returned to baseline |
| Canagliflozin 100 mg Canagliflozin 300 mg Placebo [51] |
52-week, phase 3 RCT (269) | Antihypertensive + other anti-diabetes drugs | 255.8 221.6 257.7 |
39.8 38.8 40.0 |
−117.5 (canagliflozin 100 mg) −96.2 (canagliflozin 300 mg) 15.4 (placebo) |
eGFR‡‡: −3.6 (canagliflozin 100 mg) −3.9 (canagliflozin 300 mg) − 1.4 (placebo) |
*For ≥4 weeks before randomization.
†Median, range.
‡Percent change from baseline.
§Median change from baseline.
ǁMean values, mg/g.
¶sCr, baseline, 0.87 ± 0.20 mg/dL
**Patients with stage 2 CKD were randomized 1:1:1 to empagliflozin 10 mg, 25 mg, or placebo; data for 10 mg arm not shown.
††Numerical data not reported.
‡‡Mean change from baseline.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration; NR, not reported; OAD, oral antidiabetes drug; RCT, randomized controlled trial; sCr, serum creatinine; SGLT2, sodium glucose co-transporter 2; UACR, urine albumin to creatinine ratio.
Alogliptin was compared with sitagliptin (both given in addition to an ARB) in patients with T2DM and incipient nephropathy. In this crossover study, alogliptin (a more potent inhibitor of DPP-4 than sitagliptin) reduced albuminuria compared with sitagliptin (Table 3); however, no significant changes in eGFR, serum creatinine, or HbA1c were observed. The study showed significant increase in urinary cAMP and plasma stromal cell-derived factor-1α (SDF-1α) DPP-4 substrate – and decrease in urinary oxidative stress marker, 8-hydroxy-2’-deoxyguanosine, with alogliptin after crossover from sitagliptin. These findings suggest a possible glucose-independent renal protective effect via reduction of oxidative stress [26]. In patients with moderate to severe CKD including ESRD, 54-week treatment with sitagliptin was compared with placebo/glipizide (control arm: placebo for 12 weeks followed by glipizide for 42 weeks) [27]. Mean ± standard error (SE) changes in serum creatinine were –0.02 ± 0.06 mg/dL and 0.69 ± 0.58 mg/dL; mean ± SE UACR changes were −195 ± 331 mg/g and 457 ± 519 mg/g in the sitagliptin and control groups, respectively. The rates of renal and urinary AEs were similar between groups, and hypoglycemia was more frequent in the placebo/glipizide group (6/26, 23.1%) than in the sitagliptin group (3/65, 4.6%) [27]. In patients with ESRD and on dialysis, 54-week treatment with sitagliptin was well tolerated; the rates of overall AEs, and discontinuation due to AEs were similar between the sitagliptin and the comparator (glipizide) groups [28]. In this study, the rates of symptomatic and severe hypoglycemia were numerically lower with sitagliptin than with glipizide (6.3% and 0% versus 10.8% and 7.7%). In an open-label, observational 6-month study in patients with T2DM and varying degrees of albuminuria, sitagliptin significantly reduced urinary albumin excretion (Table 3), C-reactive protein, soluble vascular cell adhesion molecule 1, and HbA1c (baseline, 52 ± 9 mmol/mol [6.9% ± 0.8%] to 44 ± 7 mmol/mol [6.2% ± 0.6%]), with no significant change in eGFR. Patients with greater degree of albuminuria at baseline had more decline in mean UACR with sitagliptin [29]. In a 52-week single-arm study, sitagliptin added to sulphonylureas reduced HbA1c by 9 mmol/mol (0.8%), decreased UACR from 76.2 ± 95.6 to 33.0 ± 48.1 mg/g, and elicited a decrease in blood pressure [30]. An ongoing phase 4 RCT is designed to explore effect of sitagliptin given in addition to ACE inhibitor or ARB in reducing microalbuminuria in patients with T2DM (NCT02048904).
Saxagliptin treatment for 52 weeks in patients with T2DM and moderate to severe CKD, including ESRD on haemodialysis, resulted in comparable rates of AEs as placebo [31]. Patients with moderate to severe CKD experienced a slight decrease in mean eGFR from baseline to week 52; rates of hypoglycemia were comparable (28.2% and 29.4% with saxagliptin and placebo, respectively), as were the rates of serious adverse events (SAEs) (27.1% and 28.2%, respectively) and AEs leading to treatment discontinuation (11.8% and 8.2%, respectively).
2.3.2. GLP-1 Analogs
While safety and efficacy of GLP-1 analogs in DKD have been studied, data on long-term effect on renal outcomes (change in eGFR or albuminuria) are lacking. Exenatide is eliminated by the kidney; twice daily exenatide should not be used in patients with severe CKD (CrCl <30 mL/min) or ESRD and should be used with caution when initiating or escalating the doses from 5 µg to 10 µg in presence of moderate CKD (CrCl 30–50 mL/min) [32]. Similarly, once weekly exenatide should not be used in patients with severe CKD (CrCl <30 mL/min) or ESRD and should be used with caution in patients with moderate CKD (CrCl 30–50 mL/min) [33]. In patients taking exenatide, between 2005 and 2008, 78 cases of acute kidney injury (AKI) [34] were reported, likely as a result of volume depletion from nausea, vomiting, and reduced fluid intake. In a pooled analysis of 19 controlled trials, exenatide (5 µg or 10 µg twice daily) did not worsen CKD-related AEs, including AKI, when compared with placebo or insulin (incidence rate per 100 patient-years, 1.56 for both exenatide and pooled comparator groups) [35]. In a small randomized trial (n=31) patients with T2DM and microalbuminuria randomized to receive exenatide or glimepiride for 16 weeks demonstrated a numerically greater reduction from baseline in UAE with exenatide (mean ± SD, 107 ± 71 mg/day at baseline; 65 ± 47 mg/day after treatment; p<0.01 versus baseline) than with glimepiride (mean ± SD, 111 ± 74 mg/day at baseline; 106 ± 75 mg/day after treatment; p<0.01 versus baseline) [36]. In this study, glycemic efficacy of both treatments was comparable.
Data on the effects of liraglutide in patients with any degree of CKD are scarce. In a RCT (NCT01620489), in patients with T2DM and moderate CKD liraglutide added to other anti-diabetes treatments did not significantly change the mean eGFR compared with placebo (treatment ratio, 0.98; 95% CI, 0.94, 1.02; p=0.3575). Although liraglutide can be used in patients with CKD without dose adjustment, as it is metabolized by circulating endopeptidases, its use is advised with caution when initiating or escalating doses [37]. In a meta-analysis, liraglutide used alone or in combination with other oral anti-diabetes drugs elicited similar reductions from baseline in HbA1c (−14 mmol/mol [−1.3%] to −15 mmol/mol [−1.4%]) regardless of the CKD stage [38]. In this meta-analysis, liraglutide did not increase the incidence of AKI, hypoglycemia, or nausea in patients with T2DM and mild CKD (CrCl 60–89 mL/min) relative to placebo; however in a small number of patients with moderate to severe CKD (CrCl <60 mL/min) liraglutide increased frequency of nausea. No evidence of safety of liraglutide in patients with more severe CKD has been reported.
Albiglutide can be used in patients with CKD with caution advised for dose initiation and escalation; monitoring of renal function is required when patients report severe gastrointestinal (GI) reactions because GI events can potentially worsen renal function [39]. Clinical experience in patients with severe CKD is limited. Analysis of pooled data from eight randomized phase 3 trials revealed that frequencies of GI events, including diarrhea, nausea, and vomiting, increased as renal function declined [39]. In a 26-week RCT comparing efficacy and safety of albiglutide with sitagliptin in patients with mild to severe CKD and T2DM, both treatments had similar safety profiles [40]. The overall rates of GI AEs were 31.7% and 25.2% with albiglutide and sitagliptin respectively. The drug label for dulaglutide indicates that it can be used without dose adjustment in patients with CKD, including ESRD, but monitoring of renal function in patients reporting severe adverse GI reaction is recommended [41]. Experimental evidence suggests possible effects of GLP-1 analogs for improving DKD; however, results of clinical studies designed to support this hypothesis (for example, NCT01847313 – a phase 3 trial investigating effects of liraglutide on renal outcomes in people with DKD) are not yet available.
2.4. SGLT2 Inhibitors
SGLT2 is a membrane protein located in the proximal tubule of the kidney, which reabsorbs 90% of the glucose that is filtered across the glomerulus from plasma. In non-diabetic persons, renal glucose reabsorption increases with rising plasma glucose levels until the observed threshold of 180 mg/dL is reached, after which any excess glucose remains in the urine for excretion [42]. In the diabetic state, glucose excretion threshold is increased leading to sustained hyperglycemia. SGLT2 inhibition lowers the threshold for glycosuria, thereby increasing urinary glucose excretion (canagliflozin has been shown to maximally reduce the calculated renal threshold to glucose excretion to about 60 mg/dL in an ascending single-dose study in healthy men) [43]. However, urinary excretion of glucose in the presence of complete inhibition of SGLT2 does not result in 80% to 90% of filtered glucose appearing in the urine. This is because under conditions of SGLT2 inhibition, SGLT1 which is located downstream of SGLT2, is capable of increasing reabsorption such that only 35% to 40% of filtered glucose is excreted [42]. The glycemic efficacy of SGLT2 inhibitors diminishes with worsening degree of CKD. As filtration capacity of the glomerulus decreases, less glucose is filtered per day and more is retained in the circulation, which effectively diminishes the efficacy of SGLT2 inhibitors [44]. Generally, clinical trials have shown that at CKD stages 1 and 2 (eGFR >60 mL/min/1.73 m2) efficacy is comparable to that in patients with normal kidney function, and at eGFR ≤45 mL/min/1.73 m2 efficacy drops substantially or is completely lost [44]. SGLT2 inhibitors are currently not approved for the treatment of T1DM, and their off-label use in patients with T1DM cannot be recommended.
Empagliflozin can be used without dose adjustment in patients with T2DM and eGFR ≥45 mL/min/1.73 m2; however, it should be discontinued if eGFR falls persistently below 45 mL/min/1.73 m2. Evaluation of renal function is recommended before initiating therapy with empagliflozin and periodically thereafter, with more frequent monitoring in patients with eGFR <60 mL/min/1.73 m2 [45]. Upon treatment with empagliflozin 25 mg in stage 2 and 3 CKD patients, UACR decreased significantly (Table 3). Additionally, 32.6% of stage 3 CKD patients in the empagliflozin group improved from macroalbuminuria to microalbuminuria compared with 8.6% in the placebo group; 27.5% improved from microalbuminuria to normoalbuminuria with empagliflozin versus 21.4% with placebo. A significant improvement in SBP, diastolic blood pressure, and body weight may have partly contributed to these findings. In stage 2 and 3 CKD patients, the rates of AEs, hypoglycemia, and events consistent with urinary tract infection were similar in the empagliflozin and placebo groups [46]. In all patients, a small decrease in eGFR that occurred during the treatment period reverted to the baseline value at the end of the 3-week follow-up. This was postulated to be attributed to afferent arteriole vasoconstriction as a result of tubuloglomerular feedback that was reversible after the drug was stopped. An open-label, stratified clinical trial investigated the effect of 25 mg empagliflozin on hyperfiltration in patients with T1DM [47]. In the hyperfiltration group with GFR ≥135 mL/min/1.73 m2 after 8 weeks of treatment, GFR decreased by 33 and 44 mL/min/1.73 m2 under euglycaemic and hyperglycaemic clamp conditions, respectively; in addition, plasma nitric oxide and effective renal plasma flow also decreased. Notably, in patients with normal GFR (90–134 mL/min/1.73 m2), no significant change in GFR occurred. HbA1c was reduced significantly in both patient groups [47]. Thus, empagliflozin reduces the glomerular pressure in patients with hyperfiltration and may be of clinical benefit in this regard. The EMPA-REG OUTCOMETM trial (NCT01131676) is an ongoing, large, randomized controlled study (n=7042) designed to investigate long-term CV safety and potential CV benefit of empagliflozin in patients with T2DM and at high risk for CV disease. The majority of the study population (78%) has stage 2 or 3 CKD, and one of the prospective subgroup analyses involves analysis by renal function. Thus, the results of the EMPA-REG OUTCOME trial are expected to further clarify effects of empagliflozin on renal parameters in patients with CKD.
Dapagliflozin should not be used in patients with T2DM who have an eGFR <60 mL/min/1.73 m2. Evaluation of renal function is recommended before initiating therapy with dapagliflozin and periodically thereafter [48]. In a RCT, in stage 3 CKD patients, 24-week treatment with dapagliflozin (5 mg or 10 mg) did not significantly improve HbA1c compared with placebo [49]. Post hoc analysis of this study showed a slight drop in HbA1c (placebo-corrected change, 4 mmol/mol [−0.4%] and 4 mmol/mol [−0.3%] in the dapagliflozin 5 mg and 10 mg groups, respectively) in patients with eGFR 45–60 mL/min/1.73 m2, but no change with eGFR 30–45 mL/min/1.73 m2. In the dapagliflozin group, a mild initial decline in eGFR and CrCl at 1 week showed no worsening through the extension phase of this study (104 weeks), suggesting hemodynamic effect possibly caused by tubuloglomerular feedback.
Canagliflozin is not recommended in patients with T2DM and eGFR <45 mL/min/1.73 m2; when eGFR is below 60 mL/min/1.73 m2, the dose is limited to 100 mg/day and frequent monitoring of renal function is advised [50]. In patients with moderate CKD, frequencies of SAEs and AEs leading to study discontinuation with canagliflozin 100 mg, 300 mg, or placebo were comparable [51]. Although the mean UACR decreased, the mean eGFR was slightly lower with canagliflozin over 26 weeks, and this change was transient as the mean eGFR trended toward baseline at the end of the study (Table 3). Progression of albuminuria occurred in a lower proportion of patients treated with canagliflozin (5.1% and 8.3% with 100 mg and 300 mg, respectively) than with placebo (11.8%) [51]. An ongoing phase 4 RCT (CANVAS-R; NCT01989754) is designed to prospectively evaluate progression of albuminuria and changes in eGFR in patients with T2DM and increased CV risk. CREDENCE (NCT02065791) is another dedicated renal outcomes trial in progress, to study the effects of canagliflozin in reducing progression of renal impairment in patients with T2DM and DKD (stage 2 or 3 CKD and macroalbuminuria).
3. Key features of the three antidiabetes drug classes
3.1. DPP-4 Inhibitors
Clinical trials and pooled analyses have investigated the efficacy and safety of DPP-4 inhibitors in patients with
T2DM and varying degrees of CKD, including ESRD. In trials lasting ≥24 weeks, the mean reductions in HbA1c with a DPP-4 inhibitor were typically, 12 mmol/mol (1.1%) to 7 mmol/mol (0.6%). DPP-4 inhibitor treatment was generally associated with reduction in mean UACR, and no significant change in eGFR. Rates of hypoglycemia, and renal and urinary AEs were comparable between the DDP-4 inhibitor and placebo groups. Of all the DPP-4 inhibitors approved in the United States, linagliptin can be used without dose adjustment in patients with CKD, whereas sitagliptin, saxagliptin, and alogliptin require dose adjustment in CKD and continuous monitoring of renal function is recommended prior to therapy initiation and periodically thereafter.
3.2. GLP-1 Analogs
In clinical trials lasting ≥16 weeks in patients with CKD, GLP-1 analogs reduced the mean HbA1c from baseline typically by 15 mmol/mol (1.4%) to 8 mmol/mol (0.7%). Studies reporting the change in UACR and eGFR with GLP-1 analog treatment are limited–in 1 clinical trial in patients with microalbuminuria, exenatide caused greater reduction in UAE than glimepiride; in another clinical trial in patients with moderate CKD, liraglutide was found to cause no significant change in eGFR relative to placebo. In patients with CKD, GLP-1 analog treatment can cause GI reactions which may lead to worsening of renal function, therefore renal function should be monitored in this population.
3.3. SGLT2 Inhibitors
In clinical trials, in patients with up to CKD stage 3, SGLT2 inhibitors have shown moderate glucose-lowering efficacy with mean reduction in HbA1c ranging from 7 mmol/mol (0.6%) to 3 mmol/mol (0.3%). In patients with more advanced CKD use of SGLT2 inhibitors is not recommended due to loss efficacy. In general, SGLT2 inhibitor treatment led to reduction in UACR and slight initial decrease in eGFR, which either reverted to baseline or did not worsen by follow-up or in the study extension period.
4. Conclusion
Patients with T2DM are at risk for DKD, especially when hyperglycemia, hypertension, or both conditions are poorly controlled. Early detection and continuous monitoring for DKD are critical for slowing the progression of this complication. Periodic measurements of both UAE and eGFR in high-risk patients are advisable. Choosing the optimal glucose-lowering therapy in patients with DKD ultimately defines the success of the treatment regimen. Risks of adverse effects of established antihyperglycemic agents, including hypoglycemia, lactic acidosis, and weight gain, are likely augmented in the presence of DKD. Incretin-based therapies and SGLT2 inhibitors offer particular advantages for patients with T2DM because they are not inherently associated with these side effects. Clinical evidence has shown glucose-lowering efficacy of DPP-4 inhibitors without worsening of renal function while improving albuminuria in patients with varying degrees of CKD. Of all the approved DPP-4 inhibitors, linagliptin can be considered a convenient option in DKD because it does not need dose adjustment (Table 4). SGLT2 inhibitors can be used in patients at earlier stages of CKD (1 or 2) but are not currently recommended for patients at advanced stages (4 or 5) because of the loss of glycemic efficacy. Although a causal relationship between GLP-1 analogs and kidney injury or dysfunction has not been established, AE reports have led to recommendations for continuous monitoring in patients with CKD. The drugs reduce HbA1c, but no substantial clinical data supporting protective effects against hard renal outcomes are available at this time. Clinical trials addressing these potential renal benefits are planned or underway.
ACKNOWLEDGEMENTS
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to the development of the manuscript. Writing support was provided by Radha Narayan, PhD, of Envision Scientific Solutions, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
List of Abbreviations
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ACEIs
Angiotensin-converting enzyme inhibitors
- ADA
American Diabetes Association
- ADVANCE
Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation
- AE
Adverse event
- AGEs
Advanced glycation end products
- AKI
Acute kidney injury
- ARBs
Angiotensin II receptor blockers
- cAMP
Cyclic adenosine monophosphate
- CI
Confidence interval
- CKD
Chronic kidney disease
- CrCl
Creatinine clearance
- CV
Cardiovascular
- DKD
Diabetic kidney disease
- DPP-4
Dipeptidyl peptidase-4
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- GI
Gastrointestinal
- GIP
Glucose-dependent insulinotropic peptide
- GLP-1
Glucagon-like peptide-1
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NHANES
National health and nutrition examination survey
- NKF KDOQI
National Kidney Foundation Kidney Disease Outcomes Quality Initiative
- NR
Not reported
- OAD
Oral antidiabetes drug
- RAAS
Renin-angiotensin-aldosterone system
- RCT
Randomized controlled trial
- SAEs
Serious adverse events
- SBP
Systolic blood pressure
- sCr
Serum creatinine
- SDF-1α
Stromal cell-derived factor-1α
- SGLT2
Sodium glucose co-transporter 2
- T1DM
Type 1 diabetes
- T2DM
Type 2 diabetes mellitus
- UACR
Urine albumin to creatinine ratio
- UAE
Urinary albumin excretion
- VADT
Veterans Affairs Diabetes Trial
CONFLICT OF INTEREST
RJS is on the scientific advisory board of MDSci, Ischemix, MediBeacon and PLC Medical Systems, Inc. and is on the data and safety monitoring boards for Theracos, Inc. VA and CG report no conflicts of interest.
References
- 1.de Boer I.H., Rue T.C., Hall Y.N., et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes–2015. Diabetes Care. 2015;38:S1–S93. doi: 10.2337/dc14-2142. [DOI] [PubMed] [Google Scholar]
- 3.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease Am. J. Kidney Dis. 2007;49:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal V., Marinescu V., Agarwal M., McCullough P.A. Cardiovascular implications of proteinuria: an indicator of chronic kidney disease. Nat. Rev. Cardiol. 2009;6:301–311. doi: 10.1038/nrcardio.2009.11. [DOI] [PubMed] [Google Scholar]
- 5.Caramori M.L., Fioretto P., Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52:1036–1040. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 6.Kramer H.J., Nguyen Q.D., Curhan G., Hsu C.Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289:3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 7.Badal S.S., Danesh F.R. New insights into molecular mechanisms of diabetic kidney disease. Am. J. Kidney Dis. 2014;63:S63–S83. doi: 10.1053/j.ajkd.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal V., Kazilbash S.H., McCullough P.A. New therapeutic agents for diabetic kidney disease. Therapy. 2008;5:553–575. [Google Scholar]
- 9.KDOQI Clinical Practice Guideline for Diabetes and CKD 2012 Update. Am. J. Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Duckworth W., Abraira C., Moritz T., et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 11.ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 12.Ismail-Beigi F., Craven T., Banerji M.A., et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease 2013.
- 14.Bakris G.L. Recognition, pathogenesis, and treatment of different stages of nephropathy in patients with type 2 diabetes mellitus. Mayo Clin. Proc. 2011;86:444–456. doi: 10.4065/mcp.2010.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inzucchi S.E., Bergenstal R.M., Buse J.B., et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inzucchi S.E., Bergenstal R.M., Buse J.B., et al. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 17.Gallwitz B. The evolving place of incretin-based therapies in type 2 diabetes. Pediatr. Nephrol. 2010;25:1207–1217. doi: 10.1007/s00467-009-1435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheen A.J. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89–101. doi: 10.1016/j.diabet.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Boehringer Ingelheim Boehringer Ingelheim. Tradjenta (linagliptin) prescribing information. Available from: http://bidocs.boehringer-ingelheim.com/ BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Pres cribing+Information/PIs/Tradjenta/Tradjenta.pdf. Accessed March 16, 2015.
- 20.Merck & Co Merck & Co. Januvia (sitagliptin) prescribing information. Available from: http://www.januvia.com/sitagliptin/januvia/consumer/prescribing-information.jsp. Accessed March 16, 2015.
- 21.AstraZeneca AstraZeneca. Onglyza (saxagliptin) prescribing information. Available from: http://packageinserts.bms.com/pi/pi_onglyza.pdf. Accessed March 16, 2015.
- 22.Takeda Pharmaceuticals U.S. http://www.nesina.com/. Takeda Pharmaceuticals U.S.A. Inc. Nesina (alogliptin) prescribing information. Available from: Accessed March 16, 2014.
- 23.McGill J.B., Sloan L., Newman J., et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36:237–244. doi: 10.2337/dc12-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laakso M., Rosenstock J., Groop P.H., et al. Treatment with the dipeptidyl peptidase-4 inhibitor linagliptin or placebo followed by glimepiride in patients with type 2 diabetes with moderate to severe renal impairment: a 52-week, randomized, double-blind clinical trial. Diabetes Care. 2015;38:e15–e17. doi: 10.2337/dc14-1684. [DOI] [PubMed] [Google Scholar]
- 25.Groop P.H., Cooper M.E., Perkovic V., et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36:3460–3468. doi: 10.2337/dc13-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita H., Taniai H., Murayama H., et al. DPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1alpha in type 2 diabetic patients with incipient nephropathy. Endocr. J. 2014;61:159–166. doi: 10.1507/endocrj.ej13-0305. [DOI] [PubMed] [Google Scholar]
- 27.Chan J.C., Scott R., Arjona Ferreira J.C., et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes. Metab. 2008;10:545–555. doi: 10.1111/j.1463-1326.2008.00914.x. [DOI] [PubMed] [Google Scholar]
- 28.Arjona Ferreira J.C., Corry D., Mogensen C.E., et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am. J. Kidney Dis. 2013;61:579–587. doi: 10.1053/j.ajkd.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 29.Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr. J. 2011;58:69–73. doi: 10.1507/endocrj.k10e-382. [DOI] [PubMed] [Google Scholar]
- 30.Harashima S.I., Ogura M., Tanaka D., et al. Sitagliptin add-on to low dosage sulphonylureas: efficacy and safety of combination therapy on glycaemic control and insulin secretion capacity in type 2 diabetes. Int. J. Clin. Pract. 2012;66:465–476. doi: 10.1111/j.1742-1241.2012.02903.x. [DOI] [PubMed] [Google Scholar]
- 31.Nowicki M., Rychlik I., Haller H., et al. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int. J. Clin. Pract. 2011;65:1230–1239. doi: 10.1111/j.1742-1241.2011.02812.x. [DOI] [PubMed] [Google Scholar]
- 32.AstraZeneca AstraZeneca. Byetta (exenatide) prescribing information. Available from: http://www.byetta.com/. Accessed March 16, 2014.
- 33.AstraZeneca AstraZeneca. Bydureon (exenatide extended-release for injectable suspension) prescribing information. Available from:http://www.azpicentral.com/bydureon/pi_bydureon.pdf#page=1. Accessed March 19, 2014.
- 34.U.S. Food and Drug Administration U.S. Food and Drug Administration. Information for Healthcare Professionals: Reports of Altered Kidney Function in patients using Exenatide (Marketed as Byetta). 2009. Available from:http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm188656.htm. Accessed 15 December. 2009.
- 35.Macconell L., Brown C., Gurney K., Han J. Safety and tolerability of exenatide twice daily in patients with type 2 diabetes: integrated analysis of 5594 patients from 19 placebo-controlled and comparator-controlled clinical trials. Diabetes Metab. Syndr. Obes. 2012;5:29–41. doi: 10.2147/DMSO.S28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H., Zhang X., Hu C., Lu W. Exenatide reduces urinary transforming growth factor-beta1 and type IV collagen excretion in patients with type 2 diabetes and microalbuminuria. Kidney Blood Press. Res. 2012;35:483–488. doi: 10.1159/000337929. [DOI] [PubMed] [Google Scholar]
- 37.Novo Nordisk Novo Nordisk. Victoza (liraglutide) prescribing information. Available from: http://www.victoza.com/. Accessed March 16, 2015.
- 38.Davidson J.A., Brett J., Falahati A., Scott D. Mild renal impairment and the efficacy and safety of liraglutide. Endocr. Pract. 2011;17:345–355. doi: 10.4158/EP10215.RA. [DOI] [PubMed] [Google Scholar]
- 39.GlaxoSmithKline GlaxoSmithKline. Tanzeum (albiglutide) prescribing informtion. Available from: http://www.tanzeumrems.com/. Accessed March 16, 2015.
- 40.Leiter L.A., Carr M.C., Stewart M., et al. Efficacy and safety of the once-weekly GLP-1 receptor agonist albiglutide versus sitagliptin in patients with type 2 diabetes and renal impairment: a randomized phase III study. Diabetes Care. 2014;37:2723–2730. doi: 10.2337/dc13-2855. [DOI] [PubMed] [Google Scholar]
- 41. Eli Lilly and Company. Trulicity (dulaglutide) prescribing information. Available from: http://www.trulicity.com/. Accessed March 16, 2015.
- 42.Abdul-Ghani M.A., DeFronzo R.A., Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30-50% of filtered glucose load in humans. Diabetes. 2013;62:3324–3328. doi: 10.2337/db13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sha S., Devineni D., Ghosh A., et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes. Metab. 2011;13:669–672. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosenwasser R.F., Sultan S., Sutton D., Choksi R., Epstein B.J. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes Metab. Syndr. Obes. 2013;6:453–467. doi: 10.2147/DMSO.S34416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boehringer Ingelheim Jardiance (empagliflozin) prescribing information. Available from: https://www.jardiance.com. Accessed March 16, 2015.
- 46.Barnett A.H., Mithal A., Manassie J., et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 47.Cherney D.Z., Perkins B.A., Soleymanlou N., et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 48.AstraZeneca Farxiga (dapagliflozin) prescribing information. Available from:http://www.azpicentral.com/farxiga/pi_farxiga.pdf#page=1. Accessed March 16, 2015.
- 49.Kohan D.E., Fioretto P., Tang W., List J.F. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janssen Pharmaceuticals, Inc. Janssen Pharmaceuticals, Inc. Invokana (canagliflozin) prescribing information. Available from: http://www.invokanahcp.com/dosingand-prescribing-information. Accessed March 16, 2015.
- 51.Yale J.F., Bakris G., Cariou B., et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. 2013;15:463–473. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]


