Abstract
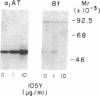
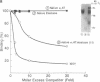
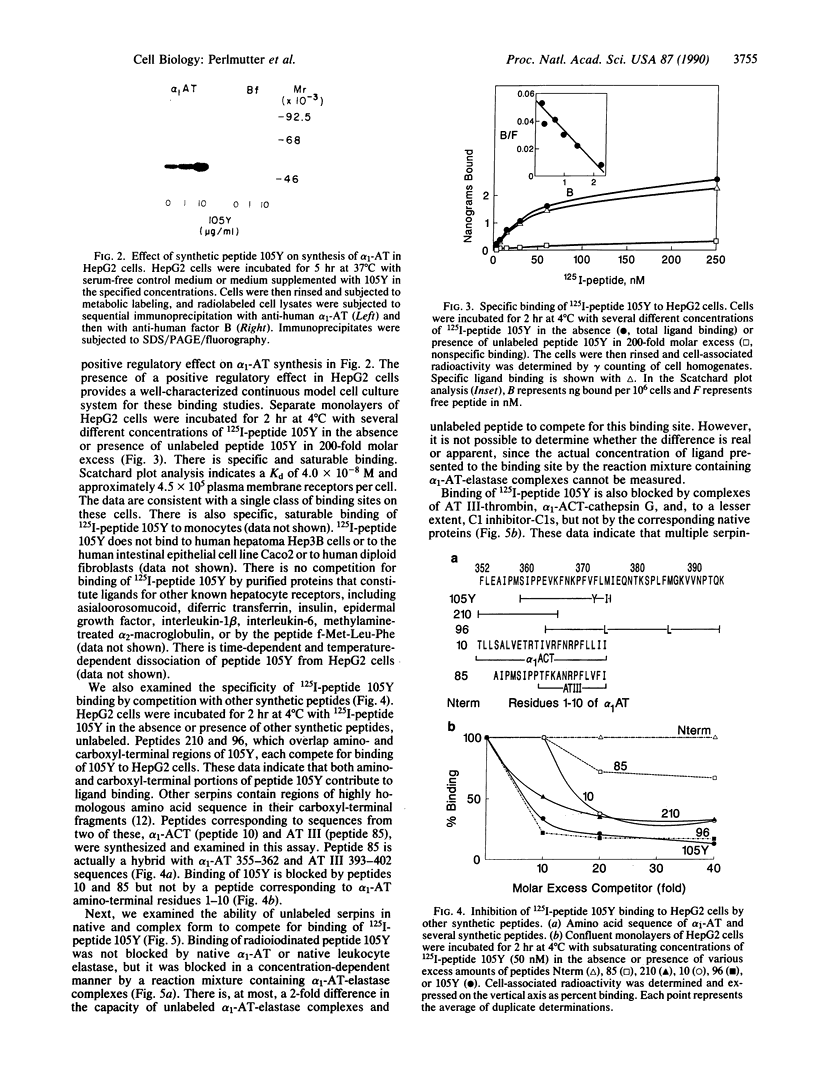
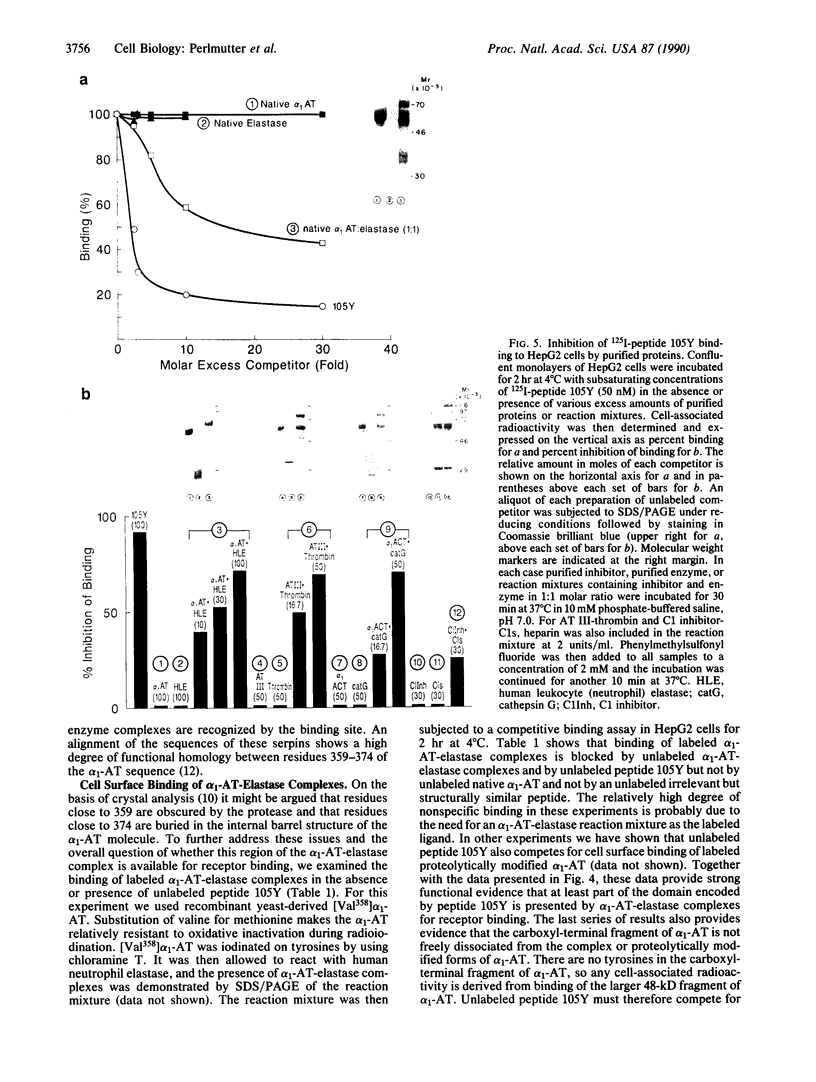
Formation of the covalently stabilized complex of alpha 1-antitrypsin (alpha 1-AT) with neutrophil elastase, the archetype of serine proteinase inhibitor serpin-enzyme complexes, is associated with structural rearrangement of the alpha 1-AT molecule and hydrolysis of a reactive-site peptide bond. An approximately 4-kDa carboxyl-terminal cleavage fragment is generated. alpha 1-AT-elastase complexes are biologically active, possessing chemotactic activity and mediating increases in expression of the alpha 1-AT gene in human monocytes and macrophages. This suggested that structural rearrangement of the alpha 1-AT molecule, during formation of a complex with elastase, exposes a domain that is recognized by a specific cell surface receptor or receptors. To test this hypothesis, the known three-dimensional structure of alpha 1-AT and comparisons of the primary structures of the serpins were used to select a potentially exteriorly exposed and highly conserved region in the complexed form of alpha 1-AT as a candidate ligand (carboxyl-terminal fragment, amino acids 359-374). We show here that synthetic peptides based on the sequence of this region bind specifically and saturably to human hepatoma cells and human monocytes (Kd = 4.0 X 10(-8) M, 4.5 X 10(5) plasma membrane receptors per cell) and mediate increases in synthesis of alpha 1-AT. Binding of peptide 105Y (Ser-Ile-Pro-Pro-Glu-Val-Lys-Phe-Asn-Lys-Pro-Phe-Val-Tyr-Leu-Ile) is blocked by alpha 1-AT-elastase complexes, antithrombin III (AT III)-thrombin complexes, alpha 1-antichymotrypsin (alpha 1-ACT)-cathepsin G complexes, and, to a lesser extent, complement component C1 inhibitor-C1s complexes, but not by the corresponding native proteins. Binding of peptide 105Y is also blocked by peptides with sequence corresponding to carboxy-terminal fragments of the serpins AT III and alpha 1-ACT, but not by peptides having the sequence of the extreme amino terminus of alpha 1-AT. The results also show that peptide 105Y inhibits binding of 125I-labeled alpha 1-AT-elastase complexes. Thus, these studies demonstrate an abundant, relatively high-affinity cell surface receptor which recognizes serpin-enzyme complexes (SEC receptor). This receptor is capable of modulating the production of at least one of the serpins, alpha 1-AT. Since the ligand specificity is similar to that previously described for in vivo clearance of serpin-enzyme complexes, the SEC receptor may also be involved in the clearance of certain serpin-enzyme complexes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banda M. J., Rice A. G., Griffin G. L., Senior R. M. Alpha 1-proteinase inhibitor is a neutrophil chemoattractant after proteolytic inactivation by macrophage elastase. J Biol Chem. 1988 Mar 25;263(9):4481–4484. [PubMed] [Google Scholar]
- Barbey-Morel C., Pierce J. A., Campbell E. J., Perlmutter D. H. Lipopolysaccharide modulates the expression of alpha 1 proteinase inhibitor and other serine proteinase inhibitors in human monocytes and macrophages. J Exp Med. 1987 Oct 1;166(4):1041–1054. doi: 10.1084/jem.166.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W. alpha 1-Antitrypsin: molecular pathology, leukocytes, and tissue damage. J Clin Invest. 1986 Dec;78(6):1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. J., Schwartz A. L. Regulation by phorbol esters of asialoglycoprotein and transferrin receptor distribution and ligand affinity in a hepatoma cell line. J Biol Chem. 1986 Nov 15;261(32):15081–15089. [PubMed] [Google Scholar]
- Glover G. I., Schasteen C. S., Liu W. S., Levine R. P. Synthetic peptide inhibitors of complement serine proteases--I. Identification of functionally equivalent protease inhibitor sequences in serpins and inhibition of C1s and D. Mol Immunol. 1988 Dec;25(12):1261–1267. doi: 10.1016/0161-5890(88)90040-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Morton P. A., Owensby D. A., Sobel B. E., Schwartz A. L. Catabolism of tissue-type plasminogen activator by the human hepatoma cell line Hep G2. Modulation by plasminogen activator inhibitor type 1. J Biol Chem. 1989 May 5;264(13):7228–7235. [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Pierce J. A. The alpha 1-antitrypsin gene and emphysema. Am J Physiol. 1989 Oct;257(4 Pt 1):L147–L162. doi: 10.1152/ajplung.1989.257.4.L147. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Punsal P. I. Distinct and additive effects of elastase and endotoxin on expression of alpha 1 proteinase inhibitor in mononuclear phagocytes. J Biol Chem. 1988 Nov 5;263(31):16499–16503. [PubMed] [Google Scholar]
- Perlmutter D. H., Travis J., Punsal P. I. Elastase regulates the synthesis of its inhibitor, alpha 1-proteinase inhibitor, and exaggerates the defect in homozygous PiZZ alpha 1 PI deficiency. J Clin Invest. 1988 Jun;81(6):1774–1780. doi: 10.1172/JCI113519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo S. V., Mast A. E., Feldman S. R., Salvesen G. In vivo catabolism of alpha 1-antichymotrypsin is mediated by the Serpin receptor which binds alpha 1-proteinase inhibitor, antithrombin III and heparin cofactor II. Biochim Biophys Acta. 1988 Nov 17;967(2):158–162. doi: 10.1016/0304-4165(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Pratt C. W., Church F. C., Pizzo S. V. In vivo catabolism of heparin cofactor II and its complex with thrombin: evidence for a common receptor-mediated clearance pathway for three serine proteinase inhibitors. Arch Biochem Biophys. 1988 Apr;262(1):111–117. doi: 10.1016/0003-9861(88)90173-7. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Lodish H. F. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J Biol Chem. 1982 Apr 25;257(8):4230–4237. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]