Abstract
Metazoan cells have two pathways for intron removal involving the U2- and U12-type spliceosomes, which contain mostly nonoverlapping sets of small nuclear ribonucleoproteins. We show that in vitro splicing of a U12-type intron assembles an exon junction complex (EJC) that is comparably positioned and contains many of the same components as that deposited by the U2-type spliceosome. The presence of a U12-type intron downstream of a premature termination codon within an open reading frame (ORF) induces nonsense-mediated decay of the mRNA in vivo. These findings suggest a common pathway for EJC assembly by the two spliceosomes and highlight the evolutionary age of the EJC and its downstream functions in gene expression.
Keywords: spliceosome, mRNA surveillance, RNA processing
The removal of introns from pre-mRNAs is carried out by a large RNA–protein complex called the spliceosome. Recently, it has been realized that during the course of splicing in metazoan cells, the spliceosome deposits on the nascent mRNA a set of protein factors that shape subsequent events in gene expression (1, 2). The so-called exon junction complex (EJC) resides 20–24 nt upstream of the exon–exon junction that results from completion of the pre-mRNA splicing reaction (1). The EJC has been reported to contain at least seven proteins: REF/Aly, SRm160, Y14, RNPS1, Magoh, hUpf3, and UAP56 (3). Recently, Pinin (4) and eIF4A3 (5–8) have been added to the list. Among the EJC components, REF/Aly, Y14, and/or Magoh have been observed to bind the mRNA export receptor TAP (9, 10), whereas RNPS1 and Y14 interact with hUpf3, a factor required for nonsense-mediated mRNA decay (NMD) (11, 12). These observations suggest a central role for the EJC in the nuclear export and quality control of processed mRNAs (13). In addition, the EJC has been implicated in enhancing translation and in the localization of specific transcripts within the cytoplasm (14, 15). Recently, RNA interference knockdown of many of the above EJC components in Drosophila revealed a defect in mRNA export only for UAP56 (16, 17), arguing for the existence of mRNA export adaptors in addition to constituents of the EJC (18).
Not only the functions of the EJC but also its pathway of assembly remain to be described in molecular detail. Specifically, it is unclear how the spliceosome orchestrates the deposition of EJC components at a specific position upstream of the exon–exon junction. Site-specific crosslinking and proteomic analyses (19) have suggested that some EJC components bind at the C complex stage, which encompasses the first step of the splicing reaction. Recently, eIF4A3, a member of the DExH/D-box family of RNA helicases, has been implicated as the component most likely to be responsible for the tight but sequence-independent binding of the EJC to 5′ exon sequences (5–8). Immunoprecipitation (IP) experiments conducted in a simple in vitro splicing system indicated stepwise binding of successive EJC components to the mRNA (20). However, it is not yet known which splicing factors are involved in their recruitment.
In a wide variety of higher eukaryotes, there exist two distinct classes of introns, U2- and U12-dependent, which are excised by two distinct spliceosomes (21). Like the well characterized U2-dependent (major) spliceosome, the U12-dependent (minor) spliceosome excises introns by a two-step transesterification reaction (22). The U12-type spliceosome contains a divergent set of small nuclear ribonucleoproteins (snRNPs) with U11, U12, U4atac, and U6atac replacing U1, U2, U4, and U6, respectively; the U5 snRNP is common to both spliceosomes. U11 and U12 form a stable di-snRNP that synergistically recognizes the 5′ splice site and the branch site of U12-type introns, different from the apparently independent binding of the U1 and U2 snRNPs early in the U2-dependent splicing pathway (23). Recently, Lührmann and coworkers (24–26) analyzed the protein components of the snRNPs in the minor spliceosome, revealing that a number of proteins are shared with the major spliceosome. Analysis of the 18S U11/U12 di-snRNP identified seven proteins specific to the U12-type spliceosome (27). In contrast, the U4/U6.U5 and U4atac/U6atac.U5 tri-snRNPs exhibit remarkably similar protein compositions (26, 28, 29).
Here, we have examined the ability of the U12-dependent (minor) spliceosome to assemble an EJC upstream of exon–exon junctions. This analysis was expected to shed light on the mechanism of EJC formation: if the same set of EJC proteins is deposited by both spliceosomes, then factor(s) common to the two spliceosomes would be implicated, whereas different EJC components would suggest the involvement of factor(s) specific to each spliceosome. Our studies in an in vitro system competent for minor-class intron splicing indicate that the U12-type spliceosome indeed assembles an EJC that contains seven proteins in common with that deposited by the major spliceosome: REF/Aly, SRm160, Y14, RNPS1, Magoh, Upf3, and UAP56. The presence of Upf3 suggested that this EJC also shares at least one downstream function, the ability to direct NMD. We show by in vivo analyses that a premature termination codon (PTC) upstream of a U12-type intron in a reporter construct reduces accumulation of the spliced mRNA.
Methods
Plasmids. P120E2 (30) was linearized by HindIII digestion and used for in vitro transcription. To construct the human MATN1 minigene, a genomic fragment of human MATN1 containing the region from exons 6–8 [including the last 152 bp of exon 6, the 829-bp intron (major), the 81 bp of exon 7, the 615-bp intron 7 (minor), and the first 87 bp of exon 8] was amplified by PCR from human genomic DNA, followed by cloning into pcDNA-flag vector (12). The PTC-containing MATN1 construct was created by altering a C to A to create a UGA codon 62 nt upstream of the 5′ splice site of intron 7 by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene). The intronless MATN1 cDNA fragments were amplified by RT-PCR from total RNAs prepared from human embryonic kidney (HEK)293 cells transfected with either the WT MATN1 plasmid (for WTΔIn) or the PTC MATN1 plasmid (for PTCΔIn), followed by cloning into the pcDNA-flag vector.
Nuclear Extracts. Preparation of nuclear extract containing each epitope-tagged EJC component was carried out essentially as described (31). Briefly, HEK293 cells grown in 15-cm dishes were transfected with an expression plasmid (12.5 μg) encoding a flag-tagged EJC component by using TransIT-293 Transfection Kit (Mirus, Madison, WI), according to the instruction manual. After 48 h, the transfected cells were washed twice with PBS buffer and harvested with a rubber policeman. The harvested cells were resuspended in a volume of buffer A (32) equal to the packed volume of the harvested cells and incubated on ice for 15 min. The swollen cells were disrupted by passage through a syringe as described, followed by spinning at 15,000 × g for 30 sec. The precipitate was resuspended in cold buffer C (32) and gently mixed at 4°C for 30 min. The extracted nuclear components were recovered by spinning at 14,000 × g for 5 min and dialyzed against buffer D (32) at 4°C for 3 h. Western blotting to detect the levels of flag-tagged proteins was performed by using an Owl semidry electroblotter (Owl Scientific, Woburn, MA).
In Vitro Splicing, RNase H Cleavage, and IP. In vitro splicing of the minor-class intron of the P120 pre-mRNA was carried out as described by Tarn and Steitz (30). Nuclear extracts from transfected HEK293 cells were mixed with standard HeLa cell nuclear extract (1:1). For IP experiments, 5× reactions were carried out for 4 h. After in vitro splicing, an optimized concentration (1–10 μM) of a DNA oligonucleotide complementary to the P120 pre-mRNA was added and incubated at 30°C for 10 min. Nine percent of the reaction mixture was removed (input sample), and 90% was used for IP. IP was carried out essentially as described (31). A 40-ml suspension of anti-flag antibody–agarose conjugate (Sigma) was washed three times with NET2 buffer (31) and added to the reaction mixture, followed by vigorous mixing at 4°C for 3 h. The beads were washed eight times with 1 ml of NET2 buffer, and bound RNAs were recovered by Trizol extraction. The extracted RNA samples were run on 6% polyacrylamide gels with 7 M urea. The IP pattern was analyzed by PhosphorImager (Molecular Dynamics).
Transfection and RNA Analyses. Transfection of the MATN1 minigene into HeLa cells was carried out essentially as described (33). Either the flag-MATN1 WT or that containing a PTC was cotransfected with a β-globin control plasmid and with either the WT Upf1 expression plasmid or that containing a point mutation (R844C; ref. 34) using TransIT-HeLaMONSTER Transfection kit (Mirus). After 24 h, RNA was prepared, and Northern blot analysis carried out as described (33).
Results and Discussion
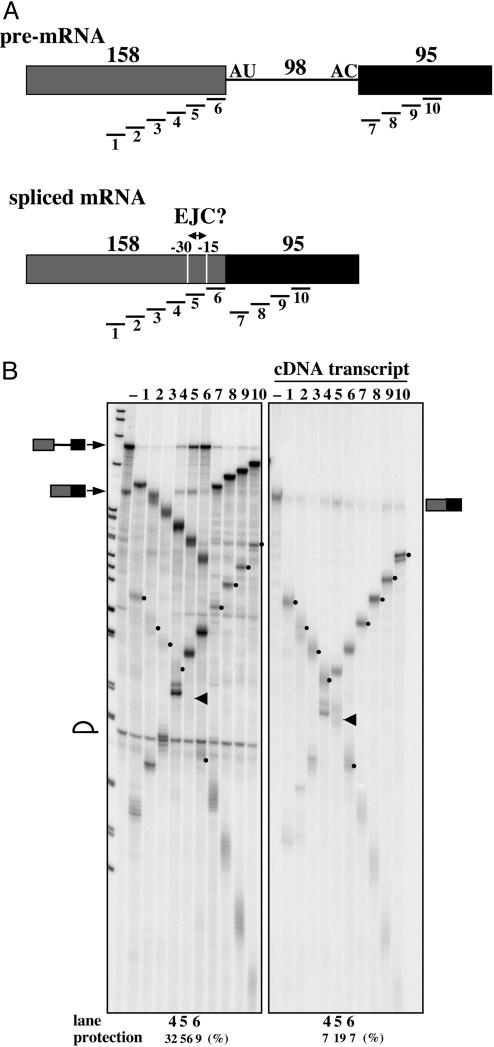
RNase H Protection Patterns Indicate the Deposition of an EJC-Like Complex by the U12-Type Spliceosome. To ask whether an EJC is deposited by the U12-type spliceosome, we adopted the RNase H protection assay originally used by Le Hir et al. (1) to detect the presence of proteins bound to the 5′ exon of mRNAs generated by removal of a major-class intron. We examined instead the P120 pre-mRNA, which contains a U12-type intron, after splicing in a HeLa cell extract (30). Specifically, in vitro splicing of the P120 pre-mRNA was carried out for 4 h in the presence of a 2′-O-methyl oligonucleotide (U2b); this oligonucleotide not only blocks cryptic U2-type splicing but enhances U12-type splicing as much as 10-fold (ref. 30; data not shown). Then, one of 10 different deoxyoligonucleotides (15 nt long), complementary along the length of the P120 spliced RNA (Fig. 1A) was added and incubation continued for 10 min. The stable binding of a protein complex (such as the EJC) interferes with oligonucleotide annealing to the RNA, thereby yielding protection of the corresponding region from cleavage by RNase H endogenous to the extract.
Fig. 1.
Detection of an EJC on the spliced P120 mRNA by RNase H protection. (A) Schematics of the P120 pre-mRNA and spliced mRNA added to the in vitro splicing reaction, showing the lengths of each exon and of the U12-type intron. Positions of the oligonucleotides used for RNase H digestion are shown below, with the putative EJC-binding region (–15 to –30) indicated on the spliced mRNA. (B) In vitro splicing of the P120 pre-mRNA followed by RNase H protection analysis. (Left) Pattern for the P120 pre-mRNA. (Right) a comparable analysis of the transcript made from P120 cDNA. The oligonucleotide added to each sample is shown above. The identities of the bands in the minus (–) lanes are given on the left for Left and on the right for Right. RNase H cleavage products arising from the spliced mRNA that are distinct from those arising from the pre-mRNA are indicated by dots, with the arrows pointing to bands that are underrepresented because of protection from RNase H. The levels of protected mRNAs in lanes 4–6 relative to lanes – (no targeting oligonucleotide) for Left and Right are shown below. For the pre-mRNA, the protections in percent were estimated to be: lane 4, <5; lane 5, ≈30; lane 6, ≈70; these values are therefore considerably above those for the same sequences in the cDNA transcript.
As shown in Fig. 1B, the spliced P120 mRNA was significantly protected from RNase H cleavage (bands indicated by arrows) when oligo 5 (complementary to region 5, spanning –15 to –30 nts relative to the 5′ splice site) was added after in vitro splicing. The cleavage pattern of the comparable intronless P120 cDNA transcript, added to the same splicing extract, confirms the identity of the products (indicated by dots) as arising from the spliced mRNA. The percent of spliced product protected from cleavage (i.e., the remaining uncleaved spliced RNA relative to the corresponding no-oligonucleotide controls, lanes –) is shown below lanes 4, 5, and 6 and reveals that the adjacent region 4 (–30 to –45) is also protected but more weakly. These results indicate that splicing-dependent factors stably associate with a region of the P120 spliced RNA analogous to that occupied by the EJC deposited by the U2-type spliceosome (1).
Interestingly, strong protection of region 6 (with some region 5) from RNase H cleavage was also observed for the unspliced P120 pre-mRNA (Fig. 1B). This observation suggests a specific interaction between the upstream exon and the U12-type spliceosome early in splicing, consistent with evidence that the 5′ exon is required for establishing the catalytic core of the U12-type spliceosome (35). This unique feature of the U12-type spliceosome is believed to reflect differences in the initial recognition of the intron ends by the U11/U12 di-snRNP (23). Accordingly, the corresponding regions of pre-mRNAs containing U2-type introns have not been reported as being protected from RNase H cleavage (1).
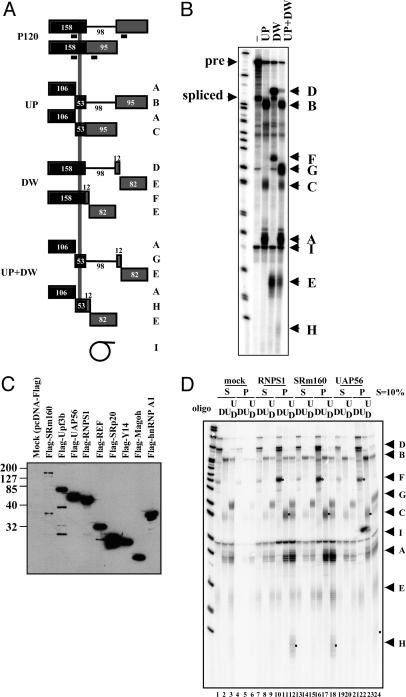
Known EJC Components Are Detected in Association with P120 mRNA. To characterize the EJC-like complex assembled on the spliced P120 substrate, we carried out IP experiments, examining components known to be associated with the EJC formed by major-class intron splicing. To obtain a high and uniform efficiency of IP, instead of using protein-specific antibodies, flag-tagged EJC proteins expressed in transfected HEK293 cells were precipitated with an anti-flag monoclonal antibody (12, 20). Flag-tagged EJC proteins exhibit weak nonspecific interactions independent of in vitro splicing with both U2- and U12-type substrates; the problem is especially severe for pre-mRNAs containing U2-type introns (our unpublished data). Thus, the spliced P120 substrate was fragmented into three pieces with RNase H before being subjected to co-IP (1). Fragmentation was carried out by adding complementary deoxyoligonucleotides [either oligoUP, oligoDW, or both (UP+DW); Fig. 2A] after in vitro splicing followed by further incubation for 10 min. Of the RNA fragments produced (labeled in Fig. 2 A), fragments C, F, and H arise from the spliced mRNA and contain the EJC-binding site (Fig. 2 A and B); they should be preferentially coprecipitated with a tagged EJC component. Fragments A and E are produced from both pre-mRNA and spliced mRNA and cannot be distinguished.
Fig. 2.
Co-IP of P120 spliced RNA fragments with flag-tagged EJC components after in vitro splicing. (A) Schematic of the P120 pre-mRNA and its spliced product, showing the RNA fragments (A–H) expected from RNase H cleavage in the presence of UP, DW, or both UP and DW oligonucleotides. The vertical gray line represents the region where the EJC is expected to assemble. The electrophoretic fractionation of these RNase H-digested fragments, as well as that of I, the intron lariat, is shown in B.(C) Western blotting of flag-tagged EJC components present in the nuclear extracts prepared from transfected HEK293 cells. (D) Co-IP of RNA fragments with flag-RNPS1, flag-SRm160, and flag-UAP56. Ten percent of the supernatant (S) was loaded; pellets are in the P lanes. The oligonucleotides used are shown above (D, DW; U, UP; UD, DW+UP). Each band is identified on the right. The spliced mRNA fragments containing the EJC-binding site (C, F, and H) are indicated by dots in the pellet lanes (lanes 10–12, 16–18, and 22–24).
Expression plasmids encoding flag-tagged versions of seven known EJC components and of two control RNA-binding proteins were separately transfected into HEK293 cells and nuclear extracts prepared. Western blots probed with anti-flag antibody (Fig. 2C) revealed excellent expression levels for most of the flag-tagged proteins. Flag-SRm160 is detectably but less well expressed; its low signal is probably in part artifactual due to poor transfer to the nitrocellulose membrane because of both its large size and the blotting conditions used (S. McCracken and B. J. Blencowe, personal communication; our unpublished observations; see Methods).
Fig. 2D shows representative co-IP results obtained using anti-flag antibody on RNase H fragments generated after in vitro splicing of the P120 substrate. Fragments C, F, and H are preferentially associated with flag-RNPS1 (lanes 10–12) and flag-SRm160 (lanes 13–15). The pattern obtained with flag-UAP56 (lanes 22–24) also shows enhancement, but not as great, of these three fragments.
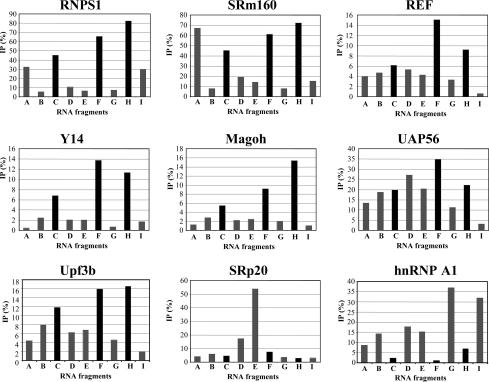
The efficiency of co-IP of each P120 spliced RNA fragment by all of the flag-tagged proteins tested (data not shown) was quantified and plotted in Fig. 3. These graphs show that the seven flag-tagged EJC components yield much higher levels of fragments C, F, and H relative to other fragments than the two control RNA-binding proteins, flag-SRp20 and flag-hnRNP A1. The differences are particularly striking in the case of flag-RNPS1, flag-Y14, and flag-Magoh. Because C, F, and H include the region upstream of the exon–exon junction in the spliced P120 RNA, their enhanced co-IP relative to fragments arising from other parts of the mRNA (A and E) or from the pre-mRNA (B, D, and G) indicates the assembly of these seven tagged proteins into an EJC-like complex.
Fig. 3.
Quantitation of RNA fragments coimmunoprecipited with flag-tagged EJC components. IP efficiency (%) was calculated as (P-BGp)/{(P-BGp)+(S-BGs)×10}×100 (%), where P, band intensity of the pellet; S, band intensity of the supernatant; BGp, background of the pellet lane; and BGs, background of the supernatant lane. Each bar in the graph is the mean of three independent experiments. The true standard deviation is difficult to assess because of differences in the splicing efficiency from experiment to experiment. The spliced mRNA fragments containing the EJC-binding site are highlighted in black.
The co-IP efficiency (Fig. 3) of P120 fragments containing the EJC-binding site was very high with flag-RNPS1 and flag-SRm160 (50–80% precipitation) but lower for flag-tagged REF, Y14, Magoh, and Upf3b (<20% precipitation). Even for this latter group, the specificity for fragments C, F, and H was quite high (Fig. 3). The different efficiencies are unlikely to be caused by differential dissociation of components from the EJC, because Y14 and Magoh are considered to be at the core of the complex (36), remaining bound until the mRNA is exported to cytoplasm. In contrast, RNPS1, UAP56, and SRm160 have been suggested to dissociate from the EJC earlier (19). It may be that the flag epitopes in Y14 and Magoh are simply less accessible than those of the tagged RNPS1 and SRm160 proteins. Upf3 is suspected to associate with the EJC via RNPS1 and/or Y14 (11, 12), suggesting that its binding should depend on the presence of RNPS1 and Y14 in the EJC. Thus, the observed inefficient co-IP of RNA fragments with flag-Upf3b (Fig. 3) may be due to its indirect binding to the EJC. Although TAP is another protein that should associate with the EJC via REF, Y14, and/or Magoh (9, 10), we were unable to detect co-IP of fragments C, F, and H with flag-tagged TAP (our unpublished data). This suggests either that TAP binding is unstable under our in vitro conditions or that remodeling of the EJC might be required for TAP association.
In the flag-SRm160 pattern (Fig. 3), fragment A, as well as C, F, and H, is coprecipitated. This may be due to the binding of other SR proteins. SRm160 is an SR-related protein, known to interact with various SR proteins, and fragment A contains exonic splicing enhancer (ESE)-like stretches [10 putative SF2-binding sites, 8 putative SC35-binding sites, 3 putative SRp40-binding sites, and 2 putative SRp55-binding sites, according to a search with ESE finder (http://exon.cshl.edu/ESE)]. An alternative possibility is that SRm160 interacts with the cap-binding complex, which would associate with the 5′ end of fragment A. Indeed, the co-IP of SRm160 with CBP80 has been observed (ref. 37 and our unpublished data).
The pattern of fragment coprecipitation by flag-UAP56 is less specific for the predicted EJC-bound fragments than those of the other EJC components (Fig. 3). UAP56 was originally identified as a splicing factor interacting with U2AF65 (38). Recently, it has been reported that UAP56 is an essential mRNA export factor in Drosophila (17), as well as a binding partner of REF/Aly (39). Moreover, UAP56 is recruited to both intron-containing and intronless mRNAs during transcription, mediated by the TREX complex (40). Because of these multiple interactions during mRNA biogenesis, it is possible that several UAP56 molecules are bound by one (pre-)mRNA molecule, perhaps explaining the coprecipitation of several RNA fragments with lower specificity.
The co-IP patterns of the two control proteins (Fig. 3) are also interesting. Flag-hnRNP A1 coprecipitated the various P120 RNA fragments with opposite preference to those of EJC components; namely, C, F, and H were the most poorly precipitated. This result is consistent with a recently proposed scenario whereby hnRNP proteins bound to the pre-mRNA are largely exchanged upon completion of the splicing reaction (41). SRp20 has been observed to become crosslinked to the EJC-binding site of an adenovirus mRNA (19). In contrast, with the P120 mRNA, flag-SRp20 was not observed to select EJC-specific fragments but instead preferentially bound fragment E. We searched for an SRp20-binding sequence (42) in fragment E, but no match could be found, suggesting that SRp20 may associate with this fragment by interaction with another protein(s).
NMD Is Induced by Splicing of a U12-Type Intron. Because our biochemical analyses indicated that hUpf3 is a component of the EJC deposited upon excision of the P120 minor-class intron, we investigated whether NMD is induced by the presence of a PTC upstream of a U12-type intron in vivo. NMD is a cellular process whereby introduction of a PTC into an open reading frame (ORF) >55 nt upstream of the last exon–exon junction induces translation-dependent decay of the mRNA (reviewed in ref. 3). Three interacting factors recruited by the EJC and Upf3 (most directly), followed by Upf2 and Upf1, are essential for communicating to the degradation machinery that a ribosome has stalled at an upstream nonsense codon.
We initially examined constructs containing the P120 intron used in the in vitro splicing experiments but found that it was too poorly spliced in vivo to observe NMD. This observation is in accord with the report of Patel et al. (43) that U12-type introns are removed significantly more slowly than U2-type introns. We therefore searched the database for a U12-type intron that is the last in its host gene; this would exclude the possibility that a downstream major-class intron might induce NMD even if U12-type introns were inactive in this regard. The human MATN1 gene, encoding Matrilin1 (cartilage matrix protein; ref. 33), fulfills this criterion. Its single minor-class intron is the seventh and last in its pre-mRNA.
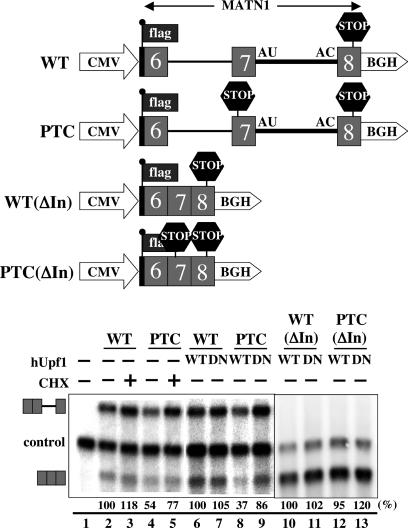
A portion of the MATN1 gene including exons 6–8 was cloned downstream of the cytomegalovirus promoter. Translation of the resulting spliced RNA initiates within the flag-tag sequence located just upstream and in the same reading frame as the MATN1 coding region (Fig. 4). A PTC (UGA) was created 62 nt upstream of the 5′ splice site of the U12-type seventh intron. The mutant and WT MATN1 plasmids were transfected into HeLa cells. NMD was assayed by observing the effect of an overexpressed hUpf1 dominant-negative mutant (R844C) or of cycloheximide (34) on the level of PTC-containing MATN1 spliced mRNA. The control was cotransfected β-globin mRNA.
Fig. 4.
Nonsense-mediated decay induced by the splicing of a U12-type intron. The constructs used for transfection are shown above. The WT construct contains a portion of the human MATN1 gene (from exon 6 to exon 8) driven by the cytomegalovirus promoter. The PTC construct contains a PTC in exon 7, as indicated. The termini of the minor (7th) intron are represented by AU-AC. The 5′ end of the MATN1 fragment was fused to the flag-tag sequence. The poly(A) signal is derived from that of the bovine growth hormone gene (BGH). The ΔIn constructs are comparable to the WT and PTC but lack both introns. In the Northern blot shown below, hUpf1, either WT or dominant-negative mutant (DN), was cotransfected with the MATN1 minigene in lanes 6–9. Cycloheximide (CHX) was present in lanes 3 and 5. The identities of the pre- and spliced MATN1 bands are shown on the right; “control” indicates the cotransfected β-globin mRNA. To calculate the relative MATN1 mRNA levels, the values in lanes 2, 6, and 10 were set at 100% for lanes 3–5, 7–9, and 11–13, respectively, followed by normalization to the level of β-globin mRNA in each lane. The same experiments were carried out three times, with the mean (shown below) and standard deviations for the calculated levels of MATN1 spliced mRNA in percent as follows: lane 2, 100; lane 3, 118 ± 24; lane 4, 54 ± 6; lane 5, 77 ± 20; lane 6, 100; lane 7, 105 ± 6; lane 8, 37 ± 7; lane 9, 86 ± 17; lane 10, 100; lane 11, 102 ± 10; lane 12, 95 ± 6; and lane 13, 120 ± 16. Lanes 1–9 and 10–13 are derived from two different experiments. The level of the unspliced MATN1 pre-mRNA was decreased somewhat by creation of the PTC, but the effect was smaller than for the spliced mRNA (70–80%). The apparent elevation of pre-mRNA levels by addition of cycloheximide is likely indirect, because it occurs for both the WT and the PTC RNAs.
As shown in Fig. 4B, Northern blot analyses revealed that the U12-type intron of the MATN1 pre-mRNA is poorly spliced (30–40% mRNA) compared with either the U2-type first intron of the MATN1 construct or the control β-globin mRNA, which are virtually completely spliced. Nonetheless, it is clear that the level of spliced PTC-containing MATN1 RNA is markedly lower than that of the spliced WT mRNA (40–50%; Fig. 4, compare lanes 2 and 4 and lanes 6 and 8). As has been well documented for mRNAs containing major-class introns (3), this indicates that the presence of a PTC in the spliced MATN1 mRNA induces NMD.
The addition of cycloheximide, which blocks translation elongation or expression of a dominant-negative Upf1 mutant, stabilized the PTC-containing spliced MATN1 mRNA (Fig. 4, compare lanes 4 and 5 and lanes 8 and 9). Although the effect of the Upf1 mutant was stronger than that of cycloheximide, both results are significant and were reproducibly obtained.
Finally, we checked that the apparent NMD seen with a PTC inserted upstream of the U12-type intron in the MATN1 construct is not due to the fortuitous presence of a cis-acting sequence that acts as a fail-safe mechanism (44). Were this the case, the level of the PTC-containing transcript from an intronless version of the construct shown in Fig. 4 would be expected to be lower than that of the comparable WT construct. Fig. 4 (lanes 10–13) shows no significant difference in the WT vs. PTC intronless transcript levels in the absence or presence of the dominant-negative Upf1. Together, the data presented in Fig. 4 argue that a PTC located upstream of a minor-class intron can induce splicing-dependent NMD, supporting our biochemical characterization of the U12-type EJC presented in Figs. 1, 2, 3.
Conclusion
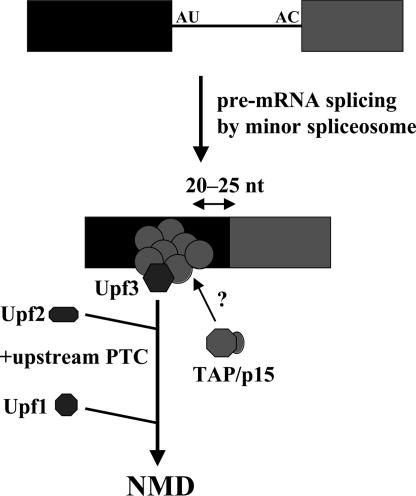
The findings reported here add another common feature to the list of properties shared by the U2- and U12-dependent spliceosomes. Both deposit an EJC containing at least seven previously characterized proteins, including Upf3, ≈20–25 nts upstream of the exon–exon junction (Fig. 5). We have confirmed the ability of the U12-type EJC to direct nonsense-mediated decay when a PTC is located at a suitable distance upstream in the mRNA. These commonalities argue that splicing factors responsible for initiating assembly of the EJC will be the same for the two spliceosomes. It will be interesting to establish whether any additional components and functions identified for the EJC will also be shared by the two types of introns in metazoan cells. If it is the case that the U2- and U12-type spliceosomes evolved from a common progenitor, as has been proposed (45), then splicing by the progenitor spliceosome may also have led to deposition of an EJC-like complex. It is of course possible that the EJC evolved later and was inherited from one spliceosome by the other. In either case, the links between splicing and NMD, as well as to other downstream events in mRNA biogenesis and function, are very ancient indeed.
Fig. 5.
Model for the deposition of an EJC by splicing of a U12-type intron. The ability of the EJC to recruit TAP remains to be determined.
Acknowledgments
We thank Kazio Tycowski, Shobha Vasudevan, Rachel Mitton-Fry, Dennis Mishler, and other members of the Steitz laboratory for stimulating discussions and comments on the manuscript; Adrian Krainer and Jens Lykke-Andersen for suggestions on the manuscript; and Victor Fok for help with the figures. This work was supported by a Human Frontiers Science Program long-term fellowship (to T.H.) and Grant GM26154 from the National Institutes of Health (to J.A.S.). J.A.S. is an Investigator of the Howard Hughes Medical Institute.
Author contributions: T.H., M.-D.S., and J.A.S. designed research; T.H. and M.-D.S. performed research; T.H. and M.-D.S. contributed new reagents/analytic tools; T.H., M.-D.S., and J.A.S. analyzed data; and T.H. and J.A.S. wrote the paper.
Abbreviations: EJC, exon junction complex; NMD, nonsense-mediated mRNA decay; snRNP, small nuclear ribonucleoprotein; PTC, premature termination codon; HEK, human embryonic kidney; IP, immunoprecipitation.
References
- 1.Le Hir, H., Izaurralde, E., Maquat, L. E. & Moore, M. J. (2000) EMBO J. 19, 6860–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Hir, H., Moore, M. J. & Maquat, L. E. (2000) Genes Dev. 14, 1098–1108. [PMC free article] [PubMed] [Google Scholar]
- 3.Maquat, L. E. (2004) Nat. Rev. Mol. Cell. Biol. 5, 89–99. [DOI] [PubMed] [Google Scholar]
- 4.Li, C., Lin, R.-I., Lai, M.-C., Ouyang, P. & Tarn, W.-Y. (2003) Mol. Cell. Biol. 23, 7363–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, C. C., Dostie, J., Diem, M. D., Feng, W., Mann, M., Rappsilber, J. & Dreyfuss, G. (2004) RNA 10, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibuya, T., Tange, T., Sonenberg, N. & Moore, M. J. (2004) Nat. Struct. Mol. Biol. 4, 346–351. [DOI] [PubMed] [Google Scholar]
- 7.Palacios, I. M., Gatfield, D., St. Johnston, D. & Izaurralde, E. (2004) Nature 427, 753–757. [DOI] [PubMed] [Google Scholar]
- 8.Ferraiuolo, M. A., Lee, C.-S., Ler, L. W., Hsu, J. L., Costa-Mattioli, M., Luo, M.-J., Reed, R. & Sonenberg, N. (2004) Proc. Natl. Acad. Sci. USA 101, 4118–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou, Z., Luo, M. J., Straesser, K., Katahira, J., Hurt, E. & Reed, R. (2000) Nature 407, 401–405. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka, N., Diem, M. D., Kim, V. N., Yong, J. & Dreyfuss, G. (2001) EMBO J. 20, 6424–6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, V. N., Kataoka, N. & Dreyfuss, G. (2001) Science 293, 1832–1836. [DOI] [PubMed] [Google Scholar]
- 12.Lykke-Andersen, J., Shu, M.-D. & Steitz, J. A. (2001) Science 293, 1836–1839. [DOI] [PubMed] [Google Scholar]
- 13.Le Hir, H., Gatfield, D., Izaurralde, E. & Moore, M. J. (2001) EMBO J. 20, 4987–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiegand, H. L., Lu, S. & Cullen, B. R. (2003) Proc. Natl. Acad. Sci. USA 100, 11327–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nott, A., Le Hir, H. & Moore, M. J. (2004) Genes Dev. 18, 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatfield, D. & Izaurralde, E. (2002) J. Cell Biol. 159, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herold, A., Teixeira, L. & Izaurralde, E. (2003) EMBO J. 22, 2472–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, Y., Gattoni, R., Stevenin, J. & Steitz, J. A. (2003) Mol. Cell 11, 837–843. [DOI] [PubMed] [Google Scholar]
- 19.Reichert, V. L., Le Hir, H., Jurica, M. S. & Moore, M. J. (2002) Genes Dev. 16, 2778–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kataoka, N. & Dreyfuss, G. (2004) J. Biol. Chem. 279, 7009–7013. [DOI] [PubMed] [Google Scholar]
- 21.Patel, A. A. & Steitz, J. A. (2003) Nat. Rev. Mol. Cell. Biol. 4, 960–970. [DOI] [PubMed] [Google Scholar]
- 22.Tarn, W.-Y. & Steitz, J. A. (1997) Trends Biochem. Sci. 22, 132–137. [DOI] [PubMed] [Google Scholar]
- 23.Frilander, M. J. & Steitz, J. A. (1999) Genes Dev. 13, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Will, C. L., Schneider, C., Reed, R. & Lührmann, R. (1999) Science 284, 2003–2005. [DOI] [PubMed] [Google Scholar]
- 25.Will, C. L., Schneider, C., MacMillan, A. M., Katopodis, N. F., Neubauer, G., Wilm, M., Lührmann, R. & Query, C. C. (2001) EMBO J. 20, 4536–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider, C., Will, C. L., Makarova, O. V., Makarov, E. M. & Luhrmann, R. (2002) Mol. Cell. Biol. 22, 3219–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Will, C. L., Schneider, C., Hossbach, M., Urlaub, H., Rauhut, R., Elbashir, S., Tuschl, T. & Lührmann, R. (2004) RNA 10, 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo, H. R., Moreau, G. A., Levin, N. & Moore, M. J. (1999) RNA 5, 893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makarov, E. M., Makarova, O. V., Urlaub, H., Gentzel, M., Will, C. L., Wilm, M. & Lührmann, R. (2002) Science 298, 2205–2208. [DOI] [PubMed] [Google Scholar]
- 30.Tarn, W.-Y. & Steitz, J. A. (1996) Cell 84, 801–811. [DOI] [PubMed] [Google Scholar]
- 31.Hirose, T., Shu, M. D. & Steitz, J. A. (2003) Mol. Cell 12, 113–123. [DOI] [PubMed] [Google Scholar]
- 32.Dignam, J. D., Lebovitz, R. M. & Roeder, R. G. (1983) Nucleic Acids Res. 11, 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins, R. N., Osborne-Lawrence, S. L., Sinclair, A. K., Eddy, R. L., Jr., Byers, M. G., Shows, T. B. & Duby, A. D. (1990) J. Biol. Chem. 265, 19624–19631. [PubMed] [Google Scholar]
- 34.Lykke-Andersen, J., Shu, M.-D. & Steitz, J. A. (2000) Cell 103, 1121–1131. [DOI] [PubMed] [Google Scholar]
- 35.Frilander, M. J. & Steitz, J. A. (2001) Mol. Cell 7, 217–226. [DOI] [PubMed] [Google Scholar]
- 36.Dostie, J. & Dreyfuss, G. (2002) Curr. Biol. 12, 1060–1067. [DOI] [PubMed] [Google Scholar]
- 37.Lejeune, F., Ishigaki, Y., Li, X. & Maquat, L. E. (2002) EMBO J. 21, 3536–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleckner, J., Zhang, M., Valcarcel, J. & Green, M. R. (1997) Genes Dev. 11, 1864–1872. [DOI] [PubMed] [Google Scholar]
- 39.Luo, M. L., Zhou, Z., Magni, K., Christoforides, C., Rappsilber, J., Mann, M. & Reed, R. (2001) Nature 413, 644–647. [DOI] [PubMed] [Google Scholar]
- 40.Strasser, K., Masuda, S., Mason, P., Pfannstiel, J., Oppizzi, M., Rodriguez-Navarro, S., Rondon, A. G., Aguilera, A., Struhl, K., Reed, R., et al. (2002) Nature 417, 304–308. [DOI] [PubMed] [Google Scholar]
- 41.Reed, R. & Magni, K. (2001) Nat. Cell Biol. 3, E201–E204. [DOI] [PubMed] [Google Scholar]
- 42.Schaal, T. D. & Maniatis, T. (1999) Mol. Cell. Biol. 19, 1705–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel, A. A., McCarthy, M. & Steitz, J. A. (2002) EMBO J. 21, 3804–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, J., Sun, X., Qian, Y. & Maquat, L. E. (1998) RNA 4, 801–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burge, C. B., Padgett, R. A. & Sharp, P. A. (1998) Mol. Cell 2, 773–785. [DOI] [PubMed] [Google Scholar]