Abstract
Objective
Symptoms of emotional distress related to diabetes have been associated with inadequate self-care behaviors, medication non-adherence, and poor glycemic control that may predispose patients to premature death. African American women, in whom diabetes is more common and social support is often insufficient, may be at particularly high risk. The objective of this study was to examine the impact of lowering diabetes-related emotional distress on glycemic control and associated behavioral correlates in rural African American women with uncontrolled type 2 diabetes (T2D).
Design
Post-hoc analysis of prospective, randomized, controlled trial.
Setting
Rural communities in the southeastern United States.
Patients
129 rural middle-aged African American women with uncontrolled type 2 diabetes (T2D)(A1C ≥ 7.0).
Primary Independent Variable
Diabetes-related distress.
Main Outcome Measures
Changes from baseline to 12-month follow-up in diabetes-related distress, and associated changes in medication adherence, self-care activities, self-efficacy, and glycemic control (A1C).
Results
Patients with a reduction in diabetes-related distress (n=79) had significantly greater improvement in A1C, medication adherence, self-care activities, and self-efficacy compared with those in whom diabetes distress worsened or was unchanged (n=50). Changes in distress were also significantly and inversely correlated with improvements in medication adherence, self-care activities, and self-efficacy.
Conclusions
Among rural African American women, reductions in diabetes-related distress may be associated with lower A1C and improvements in self-efficacy, self-care behaviors, and medication adherence.
Keywords: Diabetes-related Distress, A1C, Glycemic Control, Self-Care, Self-Efficacy
Introduction
The management of type 2 diabetes (T2D) involves adopting and maintaining a variety of lifestyle and self-care behaviors including dietary, physical activity, glucose monitoring, and medication adherence that may be new for patients.1 As glycemic control becomes more difficult with increasing duration of disease, treatment intensification involving additional medication, lifestyle changes, and monitoring is often recommended. These behavioral changes are inter-related and can result in ever-increasing complexity for the patient, potentially impacting quality of life. These complex behavioral changes may impact role functioning, a concern among African American women who often care for others in their family, often with limited social and financial support. Due to this increasing complexity and burden of self-care behaviors, many female patients experience increased emotional distress that has been reported in more than 40% of patients with T2D.2-5 This emotional distress related to diabetes has been associated with inadequate self-care behaviors, medication non-adherence, and poor glycemic control.2,3,6 Diabetes-related distress has also been associated with an increased risk for depression in a longitudinal study of patients with diabetes.7 Further, recent evidence from our prior work suggests that stress and depressive symptoms in patients with diabetes may be associated with a higher incidence of cardiovascular events and death, especially among African American women.8
While elevated levels of diabetes-related distress have been associated with inadequate self-care behaviors and adverse cardiovascular outcomes, it is unclear if improving adverse distress levels in at-risk African American women with T2D can result in improved glycemic outcomes. One study by Fisher et al9 describes a pragmatic trial called the REDEEM study that was specifically designed to reduce diabetes-related distress using telephone-delivered support. Early findings from a study by Hessler et al10 demonstrated that improving regimen-related distress in particular resulted in improved self-care behaviors and glycemic control. In addition, the impact of a specific distress–reducing cognitive behavioral treatment program in European patients with diabetes and subclinical depression was recently described by Hermann et al,11 demonstrating improvements in both measures of distress and depressive symptoms. However, self-care behaviors and glycemic control were not significantly improved despite improvement in the psychological measures. These results are conflicting and may relate to the nature of the intervention strategy and to the populations studied. Further, most prior studies of diabetes-related distress have occurred in large metropolitan communities with predominantly Caucasian populations and the potential impact of lowering distress levels has not been well-studied in rural African American women with T2D, a population with elevated levels of distress and different social support structures but also with potential strengths and resiliency.12,13
The purpose of our present study was to examine the impact of lowering diabetes-related emotional distress on glycemic control and associated behavioral correlates in at-risk rural African American women with uncontrolled T2D. Further, we examined if changes in diabetes-related distress were associated with changes in self-efficacy, self-care behaviors, or medication adherence as potential correlates of improvement in glycemic control.
Methods
Research Design
This study is a post-hoc analysis of a prospective, randomized, controlled trial, the EMPOWER study, to examine the impact of changes in diabetes-related distress. The design and baseline characteristics of the EMPOWER diabetes study have been previously described.12 Briefly, EMPOWER is a randomized prospective clinical trial of a peer advisor telephone-delivered 16-session small-changes lifestyle intervention (small self-determined nutrition and physical activity goals relative to baseline behaviors) and medication adherence support compared with a control group receiving mailed diabetes educational materials (number of mailings matched for the number of contacts), in adult African American women with uncontrolled T2D. The phone was chosen as the primary mode of intervention specifically because of the lack of transportation and distance to community centers noted in the three rural counties identified for our study. The peer advisor facilitated the development, implementation, and monitoring of specific small changes goals related to diet, physical activity, and medication adherence. Specific treatment topics addressed by the peer advisor included self-management behaviors that focus on portion control, sweetened beverage consumption, leisure time physical activity, medication adherence, self-monitoring of weight and blood sugar, stress management, and similar T2D management behaviors. The peer advisor-facilitated goal selection was designed to be relative to baseline behaviors (to emphasize lifestyle change), determined by the patient instead of externally set by staff (to optimize goal ownership and self-efficacy), and small and manageable to minimize the likelihood of feeling overwhelmed with goals that were “unreachable.” The goal was to facilitate patients accumulating and sustaining a number of small lifestyle changes and improved health behaviors over time, resulting in weight reduction and improved glycemic control.
Sample and Setting
Patients recruited included adult (aged 18 – 75 years) African American women with uncontrolled T2D (A1C ≥ 7.0%) and without end-stage complications/advanced disease (determined by self-report and medical records review). Study patients were recruited from both regional primary care medical practices and from community sites located in the rural southeastern United States. All patients signed a statement of informed consent that was approved by the study university’s Institutional Review Board. The trial was registered with ClinicalTrials.gov [NCT01806194]. Only patients with complete data for all measures at all time points were included in our present study.
Patients
Our present study included 129 patients from both treatment arms who had complete clinical and behavioral measures available at baseline and at all follow-up time points (ie, no missing data). This included 71 patients from the intervention arm (55%); and 58 patients from the control arm (45%). There were no major differences in baseline characteristics between this study sample with complete follow-up data and the larger enrollment sample.
Outcome Measures and Data Collection Procedures
The independent variable of interest was the change in diabetes-related distress, measured using the Diabetes Distress Scale developed by Polonsky et al.4 Specifically, the Diabetes Distress Scale (DDS), a well-validated 17-item measure of disease-specific distress,4 was administered at baseline and at 12-month follow-up via a face-to-face research visit in the community with one of the investigators. The mean distress scores and mean change from baseline to 12-month follow-up were computed. For example, the DDS asks respondents to rate the extent to which they “feel overwhelmed by the demands of living with diabetes,” “feel they are often failing in their diabetes routine,” and “don’t feel confident in their day-to-day ability to manage their diabetes.” The investigators also measured several potential correlates of change in glycemic control at the same baseline and 12-month follow-up visits including self-reported medication adherence, measured using the validated 8-item Morisky Medication Adherence Scale (MMAS-8).14 Values for the MMAS-8 score were recoded as described by Morisky14 to achieve a score ranging from 0 to 8, with higher scores indicative of greater medication adherence. Diabetes self-care behaviors were assessed and scored using the Summary of Diabetes Self-Care Activities measure, a validated instrument described by Toobert et al.15 Empowerment and self-efficacy were assessed and scored via validated instruments including the Diabetes Empowerment Scale – Short Form, described by Anderson et al,16 and by the Diabetes Self-Efficacy Scale,17 respectively. Responses for each instrument were collected using paper and pencil surveys completed by the patient at each research visit. Change in A1C from baseline to 12-month follow-up was measured via fingerstick at the same research visits. (DCA Vantage, Siemens Healthcare Diagnostics, Inc., Tarrytown, NY)
Statistics
Sample size for this study was estimated using a two-tailed alpha of .05, a beta of .20, an effect size of .5, and a standard deviation of 1.0 resulting in a sample size needed of 63 patients per group (total n=126). The investigators examined the relationship between changes in diabetes-related distress (mean DDS score) and changes in A1C using various statistical comparisons. To facilitate an initial comparison of demographic, clinical and behavioral parameters at baseline only by distress level, patients were dichotomized as follows: those with a baseline mean DDS of ≥3.0 were considered to have elevated distress while patients with a mean DDS of <3.0 were considered to have a normal distress level. Demographic (education, income, employment, food assistance), clinical (A1C) and behavioral (self-care behaviors, self-efficacy, medication adherence) parameters at baseline were then compared in these two distress level groups using independent samples T-tests and Chi square comparisons. Mean changes in A1C and in mean DDS scores across time from baseline to 12-month follow-up were also initially evaluated as continuous measures using Pearson correlation analysis.
To characterize the associations with change in DDS scores from baseline to 12-month follow-up, all patients were subsequently dichotomized into two groups based on whether their diabetes-related distress improved vs worsened or stayed the same across the 12 months. Mean changes from baseline to 12-month follow-up in A1C, self-efficacy, self-care behaviors, and medication adherence were then compared between the two dichotomized distress groups (distress improved vs worsened/stayed the same) using a student’s T test. In addition, changes in the continuous measures for distress, self-efficacy, self-care behaviors, and medication adherence from baseline to 12-month follow-up were evaluated using Pearson correlation analysis. All data were analyzed using SPSS vs. 22 (IBM, Chicago, IL).
Results
Baseline Parameters by Level of Distress
Approximately 35% of women (n=45) had elevated levels of diabetes-related distress at baseline. Table 1 provides a comparison of demographic, clinical, and behavioral parameters at baseline for women with low/normal levels of distress (< 3.0) vs those with elevated levels of distress (≥ 3.0). These findings demonstrate that those with elevated distress levels at baseline had significantly higher A1C values as well as significantly lower self-reported levels of self-care behaviors, self-efficacy, and medication adherence.
Table 1. Comparison of baseline demographic and clinical characteristics by baseline distress level.
| Baseline parameter | Normal distress at baseline, n=84 | Elevated distress at baseline, n=45 | P |
| Mean age ± SD, yrs | 54.9 ± 10.7 | 48.8 ± 10.7 | .003 |
| Annual income ≤ $40,000 | 85% | 86% | .8 |
| ≤High school education | 48% | 42% | .7 |
| Employed | 37% | 30% | .2 |
| Using or has used a food assistance program | 68% | 71% | .8 |
| Diabetes duration ± SD, yrs | 10.8 ± 9.2 | 9.1 ± 6.4 | .30 |
| Intervention arm | 55% | 56% | 1.0 |
| Doctor visits in last year | 4.6 | 5.2 | .4 |
| Mean BMI ± SEM | 36.5 ± .7 | 38.5 ± 1.2 | .14 |
| On daily insulin injections | 52% | 67% | .13 |
| Mean med. adherence score ± SEM | 6.1 ± .2 | 4.1 ± .3 | ≤.001 |
| Mean A1C ± SEM, % | 8.8 ± .2 | 9.4 ± .3 | .05 |
| Mean self-care activities score ± SEM | 3.8 ± .1 | 2.5 ± .2 | ≤.001 |
| Mean self-efficacy score ± SEM | 7.3 ± 2.3 | 5.4 ± .3 | ≤.001 |
| Mean duration of diabetes ± SEM, yrs | 10.8 ± 1.0 | 9.1 ± 1.0 | .30 |
| Mean number of provider visits in the last one year ± SEM | 4.6 ± .4 | 5.2 ± .7 | .40 |
BMI, body mass index; SD, standard deviation; SEM, standard error of the mean.
Changes in Distress and Corresponding Changes in Clinical and Behavioral Parameters
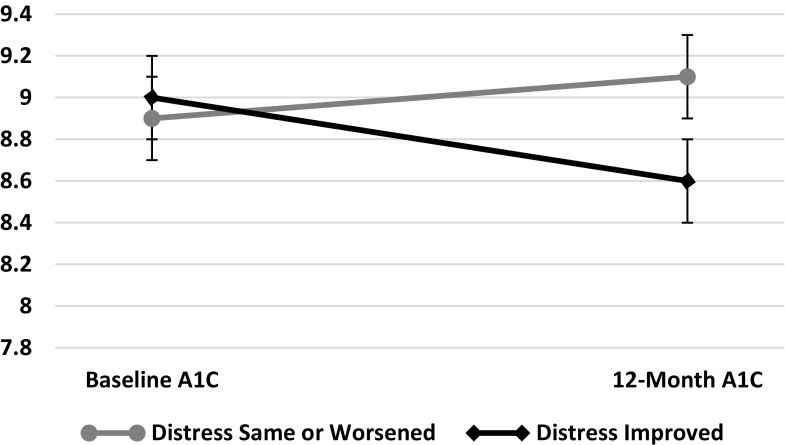
Seventy-nine of 129 (61%) women across both treatment arms improved their mean diabetes-related distress level between baseline and 12-month follow-up while 50 (39%) women had unchanged or worsened distress levels. There were no significant differences in baseline characteristics including mean A1C, age, duration of diabetes, education level, employment status, and annual income between those whose distress level improved vs those whose distress level stayed the same or worsened (data not shown). However, there was a significant difference in the change in A1C as shown in Figure 1. In women whose distress declined, there was a mean reduction in A1C of .34 while in those whose distress stayed the same or worsened, A1C increased by an average of .2, leaving the 12-month endpoints an average of .55 apart (P=.05). This change was accompanied by significant changes in mean self-efficacy, self-care activities, empowerment, and medication adherence scores (Table 2) from baseline to 12-month follow-up in the two groups. Further, the change in diabetes-distress level from baseline to 12-month follow-up was significantly and inversely correlated with the changes in self-efficacy (Pearson correlation=-.45), self-care behaviors (Pearson correlation=-.45), and medication adherence (Pearson correlation=-.40) scores (all P=.0001).
Figure 1. Change in A1C (± S.E.M.) in Patients by Change in Distress Level from Baseline to 12-Month Follow-up.
Table 2. Comparison of mean changes in clinical outcomes ± SD across 12 months by change in distress level.
| Baseline parameter | Distress level same or increased, n=50 | Distress level decreased, n=79 | P |
| Mean change in weight, lbs | -2.1 ± 15.0 | -2.7 ± 11.2 | .81 |
| Mean change in systolic BP, mm Hg | +2.1 ± 22.1 | -3.1 ± 22.3 | .20 |
| Mean change in med. adherence score | -0.21 ± 1.62 | +.81 ± 1.80 | .001 |
| Mean change in A1C, % | +.21 ± 1.7 | -.34 ± 1.5 | .05 |
| Mean change in self-care activities score | 3.8 ± .1 | 2.5 ± .2 | .001 |
| Mean change in self-efficacy score | 7.3 ± 2.3 | 5.4 ± .3 | .001 |
| Mean change in empowerment score | -.15 ± .7 | +.25 ± 1.1 | .014 |
BMI, body mass index; SD, standard deviation.
Discussion
Our present study highlights the critical nature of behavioral co-morbidities in chronic disease in a high-risk subset of African American women, a group with important disparities in diabetes-related morbidity and mortality. Further, this study is among the first to suggest that reduction in emotional distress in this high-risk population may be associated with important changes in A1C and the behavioral correlates of self-care. The study was conducted in a rural area in the southeastern United States with a sample of African American women with limited education and income and with a history of uncontrolled T2D; this group that has been inadequately studied in many reports. At baseline, elevated levels of diabetes-related distress were associated with higher A1C values and with lower levels of self-care behaviors, self-efficacy, and medication adherence, as we have previously reported.6 The findings across 12 months of follow-up suggest a strong relationship between reduction in diabetes-related distress and improvements in glycemic control, regardless of the patient’s randomly assigned intervention arm. Of note, patients whose diabetes-related distress stayed the same or worsened actually had a modest worsening of A1C at 12-month follow-up. The importance of addressing behavioral co-morbidities, such as diabetes-related distress, are illustrated in our recent article in which patients with diabetes and elevated levels of stress or depressive symptoms or both in the national REGARDS study were at substantially increased risk for adverse cardiovascular outcomes.8 Of note, African American women represented the highest risk demographic group.
The exact mechanisms whereby elevated levels of diabetes-related distress are associated with higher A1C values and whereby reduction in diabetes-related distress is associated with improvements in A1C remain to be fully elucidated. However, previous data have shown that elevated stress in diabetes results in increased cortisol levels that are associated with higher glucose concentrations.18 Our present findings in a high-risk population of women suggest an important relationship between the reduction in diabetes-related distress and improvements in self-care activities, self-efficacy, and medication adherence. This suggests the possibility that therapeutic strategies to reduce diabetes-related distress may improve glycemic control in part through improving the patient’s self-care behaviors, self-efficacy, and medication adherence. These findings add to those of Hessler et al10 who also found a relationship between behavioral correlates of improved glycemic control in the context of reducing elevated levels of regimen-related distress. However, we acknowledge the potential for bi-directionality and that improvements in glycemic control may be followed by reductions in diabetes-related distress.
Our present study has some limitations. The study was undertaken in rural African American women in the southeastern United States and generalizability to other racial or geographic groups is not possible. The study was not powered to test the relationship between changes in distress and A1C using multivariate statistical analyses that might control for the independent effects of selected demographic and/or clinical variables. Despite this, our study suggests that diabetes-related emotional distress may be an important therapeutic target and suggests the need for further research in high-risk African American women with uncontrolled T2D.
These findings have important implications for the delivery of diabetes care in rural African American women. Specifically, these results suggest the need to not only screen for diabetes-related distress in rural primary care but to pursue additional trials regarding whether lowering elevated levels of diabetes-related distress might represent an important therapeutic target that is associated with not only improving behavioral outcomes but also glycemic control.
Conclusion
In conclusion, our study in rural African American women with uncontrolled T2D demonstrates that the lowering of diabetes-related distress over a period of one year may be associated with significant improvements in A1C as well as self-efficacy, self-care behaviors, and medication adherence. Additional research should specifically explore interventions designed to reduce elevated levels of diabetes-related distress, examining its impact on glycemic control, while carefully controlling for other potential covariates. Further, research should examine the effect of changes in diabetes-related distress on cortisol and other biological mechanisms as well as on behavioral correlates of improved glycemic control such as lifestyle behaviors and medication adherence.
Acknowledgments
The authors acknowledge the generous financial support of Bristol-Myers Squibb Foundation’s Together on Diabetes Initiative that funded this study. The authors also acknowledge the substantial contributions of our peer advisors: Ms. J. Royal-Burgess; Ms. J. Jordan; Ms. F. Parker;, Ms. E. Roberson-Morning; and Ms. S. Taylor; as well as staff member Ms. T. Kono; research assistants: Ms. J. King; Mr. K. Quinn; Mr. E. Soto; Ms. L. Vines; and Ms. S. Williamson; our clinical lab assistants Mr. B. Gilpin; Ms. T. Davis; Ms. B. Whitehurst; Ms. N. Johnson; and Ms. J. Sanderson; our Research Division supporters: Ms. C. Kennedy and Ms. W. Wynne; and our community participants.
Ethical Standards and Disclosure
The authors have no competing financial interests. As noted herein, this study was approved by the East Carolina University Institutional Review Board and all subjects signed a statement of informed consent. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants included in the study.
References
- 1. American Diabetes Association Approaches to glycemic treatment. Diabetes Care. 2016;39(suppl 1):S52-S59. 10.2337/dc16-S010 [DOI] [PubMed] [Google Scholar]
- 2. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33(5):1034-1036. 10.2337/dc09-2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. 10.2337/dc09-1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. 10.2337/diacare.28.3.626 [DOI] [PubMed] [Google Scholar]
- 5. Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6(3):246-252. 10.1370/afm.842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings DM, Lutes L, Littlewood K, et al. Regimen-related distress, medication adherence, and glycemic control in rural African American Women with type 2 diabetes mellitus. Ann Pharmacother. 2014;48(8):970-977. 10.1177/1060028014536532 [DOI] [PubMed] [Google Scholar]
- 7. Ehrmann D, Kulzer B, Haak T, Hermanns N. Longitudinal relationship of diabetes-related distress and depressive symptoms: analysing incidence and persistence. Diabet Med. 2015;32(10):1264-1271. 10.1111/dme.12861 [DOI] [PubMed] [Google Scholar]
- 8. Cummings DM, Kirian K, Howard G, et al. Consequences of comorbidity of elevated stress and/or depressive symptoms and incident cardiovascular outcomes in diabetes: Results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Diabetes Care. 2016;39(1):101-109. 10.2337/dc15-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36(9):2551-2558. 10.2337/dc12-2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hessler D, Fisher L, Glasgow RE, et al. Reductions in regimen distress are associated with improved management and glycemic control over time. Diabetes Care. 2014;37(3):617-624. 10.2337/dc13-0762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38(4):551-560. [DOI] [PubMed] [Google Scholar]
- 12. Cummings DM, Lutes LD, Littlewood K, Dinatale E, Hambidge B, Schulman K. EMPOWER: a randomized trial using community health workers to deliver a lifestyle intervention program in African American women with Type 2 diabetes: design, rationale, and baseline characteristics. Contemp Clin Trials. 2013;36(1):147-153. 10.1016/j.cct.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 13. Littlewood K, Cummings DM, Lutes L, Solar C. Psychometric properties of the family support scale adapted for African American women with type 2 diabetes mellitus. Ethn Dis. 2015;25(2):193-199. [PubMed] [Google Scholar]
- 14. Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10(5):348-354. 10.1111/j.1751-7176.2008.07572.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943-950. 10.2337/diacare.23.7.943 [DOI] [PubMed] [Google Scholar]
- 16. Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. 10.2337/diacare.26.5.1641-a [DOI] [PubMed] [Google Scholar]
- 17. Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J, eds. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks, CA: Sage Publications; 1996. [Google Scholar]
- 18. Faulenbach M, Uthoff H, Schwegler K, Spinas GA, Schmid C, Wiesli P. Effect of psychological stress on glucose control in patients with Type 2 diabetes. Diabet Med. 2012;29(1):128-131. 10.1111/j.1464-5491.2011.03431.x [DOI] [PubMed] [Google Scholar]