ABSTRACT
Neutrophil extracellular trap (NET) formation represents a unique effector function of neutrophils (PMN). The mechanism of NET release in response to bacteria is largely unknown. We studied the process by which Pseudomonas aeruginosa, an opportunistic pathogen, interacts with primary PMNs, and found that flagellar swimming motility of the bacterium is essential for inducing NET extrusion. Cystic fibrosis (CF) lung disease is associated with P. aeruginosa infection and PMN-dominated inflammation. Although NETs are abundant in CF airways, the main factors triggering NET release in CF remain unclear. Our study implicates that motile P. aeruginosa is a strong NET-inducer in CF. In early stages of CF lung disease flagellated, motile isolates of P. aeruginosa are characteristic and their interactions with PMNs could lead to NET formation. In chronic CF, P. aeruginosa down-regulates its flagellum expression to avoid recognition by the immune system and forms biofilms. Flagellated bacteria, however, are released from biofilms and could interact with PMNs to form NETs. Although flagellated forms likely represent only a small fraction of the total P. aeruginosa load in chronic CF, NET release induced by them could have a significant impact on inflammation and lung function since flagellated forms trigger the most robust response of the immune system including PMNs.
Overall, we speculate that NET formation driven by motile P. aeruginosa could be a novel, significant contributor to pathogenesis at both, early and late stages of CF lung disease.
KEYWORDS: cystic fibrosis, flagellum, motility, NETs, neutrophil, Pseudomonas aeruginosa
CF airways contain large numbers of PMNs. CF sputum PMN counts, levels of extracellular DNA, myeloperoxidase and human neutrophil elastase all correlate with CF lung disease severity.1-6 PMNs are the clinically most important leukocyte in chronic CF airways and PMN-mediated inflammation contributes to lung disease. Extracellular DNA is derived from the host,7 mainly PMNs.5,7,8 Although PMNs were thought to die by necrosis in CF airways, recently other mechanisms have been proposed. NET formation provides an attractive alternative explanation since PMNs simultaneously release lung-damaging extracellular DNA and primary granule components by forming NETs, and NETs are abundant in the airways of adult CF patients. We and others reported robust NET release from human PMNs induced by laboratory strains and CF clinical isolates of P. aeruginosa.9-14 Since NETs could mediate the release of lung-damaging PMN cargo but could also be important in fighting pathogens, their exact, potentially complex, role in CF airway disease remains to be elucidated (Fig. 1).
Figure 1.
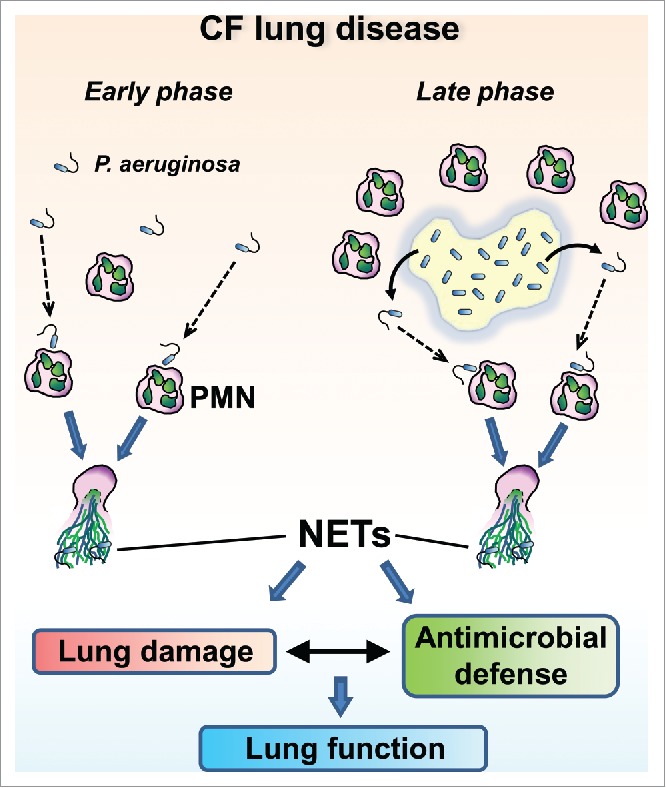
Potential contributions of motile P. aeruginosa-driven NET formation to CF lung disease.
Flagellar motility drives P. aeruginosa-induced NET release
In our current study, we found that early exponential growth phase cultures of P. aeruginosa elicited the most robust NET release and presence of a functional flagellum was essential for this process.14 Immotile bacterial mutants without flagellum or with nonfunctional flagellum are weak NET-inducers.14 Forced contact of immotile P. aeruginosa with PMNs restored their ability to trigger maximal NET extrusion.14 P. aeruginosa flagellin alone was unable to induce NET release.14 In a genetic complementation study we found that both, motAB and motCD loci of P. aeruginosa flagellar motor genes are needed for maximal NET induction in human PMNs.14 Thus, we identified flagellar swimming motility as a novel microbial factor crucial to PMN activation and NET formation.
Flagellated P. aeruginosa in CF
Although it is undocumented whether NETs are present at early stages of CF lung disease, bacterial motility-fueled NET formation likely occurs at this initial phase because early CF clinical isolates of P. aeruginosa typically express flagellum15,16 and PMNs are also present17 (Fig. 1). The interaction of PMNs with early forms of P. aeruginosa must be critical to determine later progression of CF lung disease. Important questions to be answered are why NETs released at this early stage would be incapable of clearing P. aeruginosa infection and instead drive bacterial adaption toward an aflagellated, biofilm-forming phenotype.
Over the course of CF lung disease, P. aeruginosa down-regulates its flagellum expression.15,16,18-20 In chronic CF airways, P. aeruginosa mainly exist in 3-dimensional, “suspension biofilms” also called non-attached aggregates (Fig. 1).21-23 These suspension biofilms surrounded by PMNs represent the characteristic clinical picture in chronic CF airways.21-24 Biofilms are dynamic structures, and motile, flagellated bacteria likely break free from biofilms in chronic CF, possibly interacting with PMNs (Fig. 1). This is supported by recent data showing that P. aeruginosa flagellin is detected in sputa of chronic CF patients.25 PMNs phagocytosing planktonic P. aeruginosa have also been observed in chronic CF.26,27 Nonmucoid revertant cells of P. aeruginosa have also been documented in chronic CF airways.15,18 A minor population of flagellated P. aeruginosa in chronic CF airways could also have been marginalized so far because this topic has not been intensely investigated yet, and this population is hard to study and could have been overlooked in the presence of much more abundant biofilm-bound bacteria accumulating well-characterized mutations (mucA, lasR).28,29 Conclusions with respect to the population structure of P. aeruginosa in CF have largely been generalized based on results obtained on single bacterial isolates, although high levels of phenotypic diversity among P. aeruginosa isolates within individual CF patients have already been noted.30 A small population of flagellated P. aeruginosa could be found in CF airways while most P. aeruginosa are present in form of alginate-producing, elastase-negative bacteria. MucA mutations drive the mucoid, biofilm-forming phenotype, lasR mutations contribute to PMN recruitment28 while outbreaks of flagellated bacteria from biofilms could be mainly responsible for PMN activation and NET release.14 PMNs quickly and easily recognize motile, flagellated forms of P. aeruginosa and launch their robust effector mechanisms including NET release in response to them.14 On the other hand, bacterial biofilms likely provide a much weaker stimulus for PMN activation. Therefore, the way motile P. aeruginosa interacts with PMNs, the cell type representing most of cells found in chronic CF airways, is likely an important factor in influencing the progression of CF lung disease despite the fact that planktonic forms of P. aeruginosa are outnumbered by those found in biofilms (Fig. 1).
Overall, we speculate that P. aeruginosa motility-driven PMN activation has clinical relevance not only at initial but also later stages of CF airway disease.14
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Carlsson M, Eriksson L, Erwander I, Wieslander J, Segelmark M. Pseudomonas-induced lung damage in cystic fibrosis correlates to bactericidal-permeability increasing protein (BPI)-autoantibodies. Clin Exp Rheumatol 2003; 21:S95-100; PMID:14740434 [PubMed] [Google Scholar]
- [2].Garner HP, Phillips JR, Herron JG, Severson SJ, Milla CE, Regelmann WE. Peroxidase activity within circulating neutrophils correlates with pulmonary phenotype in cystic fibrosis. J Lab Clin Med 2004; 144:127-33; PMID:15454881; http://dx.doi.org/ 10.1016/j.lab.2004.04.010 [DOI] [PubMed] [Google Scholar]
- [3].Kim JS, Okamoto K, Rubin BK. Pulmonary function is negatively correlated with sputum inflammatory markers and cough clearability in subjects with cystic fibrosis but not those with chronic bronchitis. Chest 2006; 129:1148-54; PMID:16685004; http://dx.doi.org/ 10.1378/chest.129.5.1148 [DOI] [PubMed] [Google Scholar]
- [4].Gibson RL, Emerson J, Mayer-Hamblett N, Burns JL, McNamara S, Accurso FJ, Konstan MW, Chatfield BA, Retsch-Bogart G, Waltz DA, et al.. Duration of treatment effect after tobramycin solution for inhalation in young children with cystic fibrosis. Pediatr Pulmonol 2007;42:610-23; PMID:17534969; http://dx.doi.org/ 10.1002/ppul.20625 [DOI] [PubMed] [Google Scholar]
- [5].Regelmann WE, Siefferman CM, Herron JM, Elliott GR, Clawson CC, Gray BH. Sputum peroxidase activity correlates with the severity of lung disease in cystic fibrosis. Pediatr Pulmonol 1995; 19:1-9; PMID:7675551; http://dx.doi.org/ 10.1002/ppul.1950190102 [DOI] [PubMed] [Google Scholar]
- [6].Mayer-Hamblett N, Kronmal RA, Gibson RL, Rosenfeld M, Retsch-Bogart G, Treggiari MM, Burns JL, Khan U, Ramsey BW, Investigators E. Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatr Pulmonol 2012; 47:125-34; PMID:21830317; http://dx.doi.org/ 10.1002/ppul.21525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lethem MI, James SL, Marriott C, Burke JF. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur Resp J 1990; 3:19-23; PMID:2107097 [PubMed] [Google Scholar]
- [8].Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med 2012; 186:857-65; PMID:22904182; http://dx.doi.org/ 10.1164/rccm.201203-0507OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo DG, Park JJ, Moskowitz SM, Malech HL, Leto TL. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PloS one 2013; 8:e54205; PMID:23342104; http://dx.doi.org/ 10.1371/journal.pone.0054205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yoo DG, Floyd M, Winn M, Moskowitz SM, Rada B. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes. Immunol Lett 2014; 160:186-94; PMID:24670966; http://dx.doi.org/ 10.1016/j.imlet.2014.03.003 [DOI] [PubMed] [Google Scholar]
- [11].Yoo DG, Winn M, Pang L, Moskowitz SM, Malech HL, Leto TL, Rada B. Release of cystic fibrosis airway inflammatory markers from Pseudomonas aeruginosa-stimulated human neutrophils involves NADPH oxidase-dependent extracellular DNA trap formation. Journal of immunology 2014; 192:4728-38; http://dx.doi.org/ 10.4049/jimmunol.1301589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol 2012; 92:841-9; PMID:22802447; http://dx.doi.org/ 10.1189/jlb.1211601 [DOI] [PubMed] [Google Scholar]
- [13].Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, et al.. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PloS one 2011; 6:e23637; PMID:21909403; http://dx.doi.org/ 10.1371/journal.pone.0023637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Floyd M, Winn M, Cullen C, Sil P, Chassaing B, Yoo DG, Gewirtz AT, Goldberg JB, McCarter LL, Rada B. Swimming Motility Mediates the Formation of Neutrophil Extracellular Traps Induced by Flagellated Pseudomonas aeruginosa. PLoS Pathog 2016; 12:e1005987; PMID:27855208; http://dx.doi.org/ 10.1371/journal.ppat.1005987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mahenthiralingam E, Campbell ME, Speert DP. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun 1994; 62:596-605; PMID:8300217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wolfgang MC, Jyot J, Goodman AL, Ramphal R, Lory S. Pseudomonas aeruginosa regulates flagellin expression as part of a global response to airway fluid from cystic fibrosis patients. Proc Natl Acad Sci U S A 2004; 101:6664-8; PMID:15084751; http://dx.doi.org/ 10.1073/pnas.0307553101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM, Investigators AC. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med 2013; 368:1963-70; PMID:23692169; http://dx.doi.org/ 10.1056/NEJMoa1301725 [DOI] [PubMed] [Google Scholar]
- [18].Luzar MA, Thomassen MJ, Montie TC. Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect Immun 1985; 50:577-82; PMID:3932214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mahenthiralingam E, Speert DP. Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect Immun 1995; 63:4519-23; PMID:7591095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Boyce JR, Miller RV. Motility as a selective force in the reversion of cystic fibrosis-associated mucoid Pseudomonas aeruginosa to the nonmucoid phenotype in culture. Infect Immun 1982; 37:840-4; PMID:6811443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pritt B, O'Brien L, Winn W. Mucoid Pseudomonas in cystic fibrosis. Am J Clin Pathol 2007; 128:32-34; PMID:17580270; http://dx.doi.org/ 10.1309/KJRPC7DD5TR9NTDM [DOI] [PubMed] [Google Scholar]
- [22].Caceres SM, Malcolm KC, Taylor-Cousar JL, Nichols DP, Saavedra MT, Bratton DL, Moskowitz SM, Burns JL, Nick JA. Enhanced in vitro formation and antibiotic resistance of nonattached Pseudomonas aeruginosa aggregates through incorporation of neutrophil products. Antimicrob Agents Chemother 2014; 58:6851-60; PMID:25182651; http://dx.doi.org/ 10.1128/AAC.03514-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sorensen SR, Moser C, Kuhl M, Jensen PO, Hoiby N. The in vivo biofilm. Trends Microbiol 2013; 21:466-74; PMID:23827084; http://dx.doi.org/ 10.1016/j.tim.2013.06.002 [DOI] [PubMed] [Google Scholar]
- [24].Kragh KN, Alhede M, Jensen PO, Moser C, Scheike T, Jacobsen CS, Seier Poulsen S, Eickhardt-Sorensen SR, Trostrup H, Christoffersen L, et al.. Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Infect Immun 2014; 82:4477-86; PMID:25114118; http://dx.doi.org/ 10.1128/IAI.01969-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Balloy V, Thevenot G, Bienvenu T, Morand P, Corvol H, Clement A, Ramphal R, Hubert D, Chignard M. Flagellin concentrations in expectorations from cystic fibrosis patients. BMC Pulm Med 2014; 14:100; PMID:24909229; http://dx.doi.org/ 10.1186/1471-2466-14-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Williams BJ, Dehnbostel J, Blackwell TS. Pseudomonas aeruginosa: host defence in lung diseases. Respirology 2010; 15:1037-56; PMID:20723140; http://dx.doi.org/ 10.1111/j.1440-1843.2010.01819.x [DOI] [PubMed] [Google Scholar]
- [27].Hoiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 2010; 5:1663-74; PMID:21133688; http://dx.doi.org/ 10.2217/fmb.10.125 [DOI] [PubMed] [Google Scholar]
- [28].LaFayette SL, Houle D, Beaudoin T, Wojewodka G, Radzioch D, Hoffman LR, Burns JL, Dandekar AA, Smalley NE, Chandler JR, et al.. Cystic fibrosis-adapted Pseudomonas aeruginosa quorum sensing lasR mutants cause hyperinflammatory responses. Sci Adv 2015; 1(6); pii:e1500199; PMID:26457326; http://dx.doi.org/ 10.1126/sciadv.1500199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hogardt M, Heesemann J. 2013. Microevolution of Pseudomonas aeruginosa to a chronic pathogen of the cystic fibrosis lung. Curr Top Microbiol Immunol 2015; 358:91-118; PMID:22311171 [DOI] [PubMed] [Google Scholar]
- [30].Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Hoiby N, Molin S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol 2012; 10:841-51; PMID:23147702; http://dx.doi.org/ 10.1038/nrmicro2907 [DOI] [PubMed] [Google Scholar]


