ABSTRACT
Chloroplasts have evolved from a cyanobacterial endosymbiont and multiply by dividing. Chloroplast division is performed by constriction of the ring-like protein complex (the PD machinery), which forms at the division site. The PD machinery is composed of cyanobacteria-descended components such as FtsZ and eukaryote-derived proteins such as the dynamin-related protein, DRP5B. In the red alga Cyanidioschyzon merolae, FtsZ ring formation on the stromal side precedes PDR1 and DRP5B ring formation on the cytosolic side. In this study, we impaired FtsZ ring formation in C. merolae by overexpressing FtsZ just before FtsZ ring formation. As a result, PDR1 and DRP5B failed to localize at the chloroplast division site, suggesting that FtsZ ring formation is required for the PDR1 and DRP5B rings. We further found, by expressing a dominant negative form of DRP5B, that DRP5B ring formation begins on the nuclear side of the chloroplast division site. These findings provide insight into how the PD machinery forms in red algae.
KEYWORDS: chloroplast division, Cyanidioschyzon merolae, DRP5B, FtsZ, PDR1, Plastid division machinery
Chloroplasts evolved from a cyanobacterial endosymbiont and have been retained for more than 1 billion years by coordinated chloroplast division in multiplying eukaryotic cells (reviewed in 1). Chloroplast division is performed by a ring-like protein complex (the PD machinery) at the division site, encompassing both the inside and the outside of the 2 envelope membranes (reviewed in refs. 2, 3, 4).
The PD machinery consists of proteins of cyanobacterial and eukaryotic origin. FtsZ, which is structurally similar to eukaryotic tubulin, is descended from the cytokinetic machinery of a cyanobacterial endosymbiont. It self-assembles into a ring structure on the stromal side of the inner envelope at the chloroplast division site.5,6,7 The inner and the outer PD rings are detectable by transmission electron microscopy on the stromal side of the inner envelope and the cytosolic side of the outer envelope at the division site.8 The inner PD ring locates between the FtsZ ring and the inner envelope membrane,9 but the components are still unknown. The outer PD ring consists of glucan filaments which are synthesized by PDR1 of host eukaryotic origin.10 The dynamin-related protein DRP5B/ARC5 is of eukaryotic host origin and self-assembles on the cytosolic side of the outer envelope membrane.11,12
In Arabidopsis, molecular genetic studies have suggested the following scheme in the formation of the chloroplast division machinery. The inner-envelope spanning protein ARC6 (of cyanobacterial origin) facilitates FtsZ ring formation on the stromal side by direct interaction. Then ARC6 recruits the outer-envelope-spanning protein PDV2 (of host eukaryotic origin) to the division site thorough direct interaction in the intermembrane space. Finally, PDV2 and PDV1 (a paralog of PDV2) recruit DRP5B through a direct interaction between PDV2 and DRP5B.13,14
In a similar manner, in the red alga C. merolae, morphological observations using synchronous culture showed that FtsZ, the inner and the outer PD rings, and DRP5B ring form in that order.11 In addition, knockdown of PDR1 impaired DRP5B localization at the chloroplast division site, suggesting that the PDR1 or outer PD ring is required for recruitment of DRP5B to the division site.10 However, PDV proteins are unique to land plants and streptophyte algae (for example, GenBank: GAQ78465 in Klebsormidium flaccidum) and the C. merolae genome does not encode ARC6 of cyanobacterial origin (it probably lost ARC6 during evolution). Thus, it has remained unclear whether the formation of cytosolic PD machinery (PD and DRP5B rings) depends on the FtsZ ring on the stromal side in red algae as in land plants.
To examine the effect of impairment of FtsZ ring formation on the assembly of the cytosolic components of the PD machinery, we overexpressed nucleus-encoded chloroplast FtsZ in C. merolae. It is well known that overexpression of FtsZ impairs FtsZ ring formation and subsequent chloroplast division in Arabidopsis15 and cell division in bacteria.16 To this end, we integrated a heat-shock promoter17,18 and FTSZ2–1 (CMS004C) orf fusion into a C. merolae chromosomal neutral locus.19
The cell/chloroplast division cycle of the resultant transformant was synchronized by a 12-h light/12-h dark cycle at 42°C (optimal temperature for the hot-spring red alga C. merolae) and then FtsZ2–1 was overexpressed by heat shock at 50°C at hour 8 (around the onset of FtsZ ring formation) for 2 h (Fig. 1A). As a control, we used a GFP heat-inducible strain.18 Then, we examined the localization of FtsZ2–1, PDR1 and DRP5B by immunofluorescence microscopy with respective antibodies. The specificity of the antibodies was previously confirmed (the detail is described in the Materials and Methods). At hour 16, the FtsZ2–1, PDR1 and DRP5B rings formed in the control cell (Fig. 1B). In contrast, in the FtsZ2–1 overexpressing cells, FtsZ2–1 appeared as aggregates between the thylakoid and envelope membranes (Fig. 1B).19 In these cells, the PDR1 and DRP5B rings did not form (Fig. 1B) at hour 16, although the cellular PDR1 and DRP5B level, which was detected by immunoblotting, was comparable to the level in control cells.19 These results suggest that FtsZ ring formation is required for the recruitment of PDR1 and DRP5B to the provisional division site.
Figure 1.
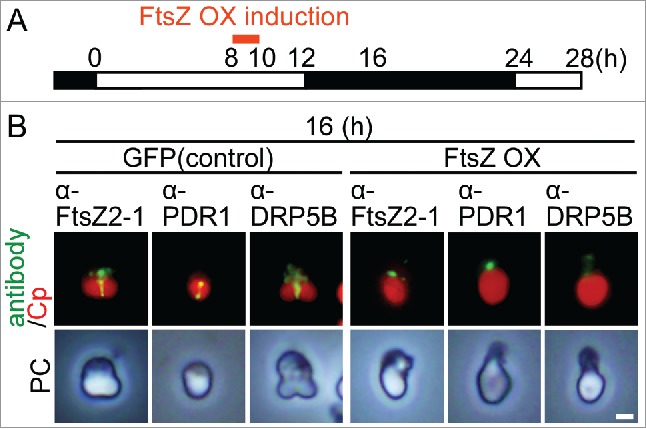
Effect of FtsZ2–1 overexpression before the onset of chloroplast division on the localization of chloroplast division proteins in synchronously cultured C. merolae. (A) Schematic diagram of the culture conditions. The control GFP heat-inducible (GFP) or FtsZ heat-inducible (FtsZ OX) cells were synchronized by a 12-h light/12-h dark cycle at 42°C and heat-shocked at 50°C at hour 8 (before the beginning of chloroplast division) for 2 h. (B) Immunofluorescent images of the control GFP and FtsZ OX cells showing the localization of FtsZ2–1, PDR1, and DRP5B at hour 16 in the synchronous culture. Green, immunostained FtsZ2–1, PDR1, or DRP5B; red, autofluorescence of the chloroplast; PC, phase-contrast. Scale bar = 1 μm. Two independent experiments produced similar results and the results from one experiment are shown.
In the course of our studies,19 we also obtained information on how DRP5B ring forms in C. merolae. We previously found that the expression of the dominant negative DRP5B K135A blocks chloroplast division in C. merolae.18,19 This mutation, DRP5B K135A, corresponds with the K44A mutation in human dynamin 1, which results in a defect in GTP binding and hydrolysis, has been widely used to inhibit the function of endogenous dynamin.20 The expression of DRP5B K135A inhibits the constriction or final scission of the chloroplast depending on the timing of expression in terms of the stage of chloroplast division.18,19
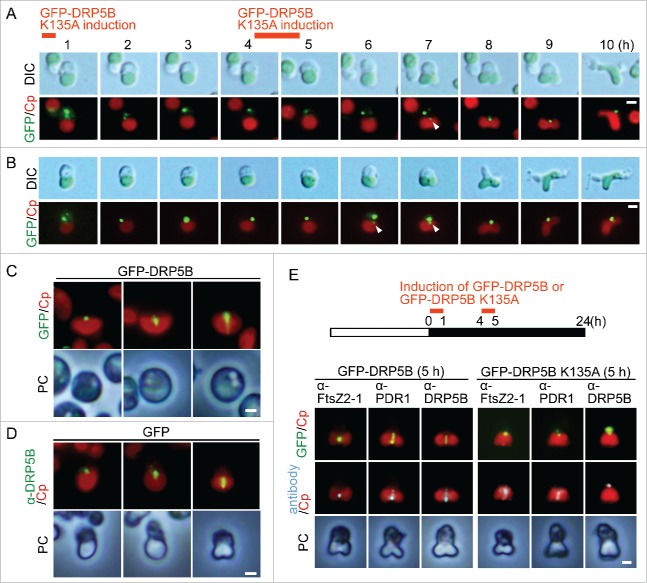
To investigate how DRP5B K135A is recruited to the chloroplast division site and how it inhibits the division site constriction, we integrated a heat-shock promoter and GFP-DRP5B K135A into a C. merolae chromosomal neutral locus.18 Then GFP-DRP5B K135A was overexpressed by heat shock twice (50˚C for 1h each) in cells before recruitment of endogenous DRP5B to the chloroplast division site. The heat-shocked cell was observed every hour after the first heat shock treatment (Fig. 2). GFP-DRP5B K135A localized in the cytoplasm as aggregates just after the expression by heat-shock (Fig. 2A, hour 1–6; Fig. 2B, hour 1–5). Then a portion of GFP-DRP5B K135A migrated to the nuclear side (upper side) of the provisional chloroplast division site (Fig. 2A, the arrowhead in hour 7; Fig. 2B, hour 6–7). The chloroplast constricted slightly at the site where GFP-DRP5B K135A localized (Fig. 2A, hour 8; Fig. 2B, hour 7). However, the chloroplast division did not progress further, although cytokinesis, which occurs after the completion of chloroplast division in normal cells, did start in these cells (Fig. 2A, hour 10; Fig. 2B, hour 9). These results raised the possibility that DRP5B ring formation starts from a certain specific point at the nuclear side of the chloroplast division site and that formation of the DRP5B ring from this specific site requires GTP binding or hydrolysis by DRP5B. When GFP-DRP5B was expressed by heat shock, in addition to the localization of GFP-DRP5B as a dot at the nuclear side, the GFP-DRP5B arc and ring were observed at the chloroplast division site in contrast to GFP-DRP5B K135A (Fig. 2C). In addition, also in the control GFP cell, the DRP5B dot, arc and ring were observed by immunostaining with the anti-DRP5B antibody (Fig. 2D). Thus the DRP5B ring probably forms from a specific point at the nuclear side.
Figure 2.
DRP5B ring formation and effect of GFP-DRP5B K135A expression before the onset of chloroplast division on the localization of chloroplast division proteins. (A, B) GFP-DRP5B K135A was expressed before the onset of chloroplast division site constriction by heat-shock. Two independent results obtained by differential interference contrast (DIC) and fluorescence microscopy are shown. Green, GFP-DRP5B K135A; red, autofluorescence of the chloroplast. Scale bars = 1 μm. The arrowheads indicate the GFP-DRP5B K135A signal at the nuclear side of the chloroplast division site. (C) GFP-DRP5B was expressed before the onset of chloroplast division site constriction by heat-shock. The DRP5B dot, arc and ring are shown. Green, GFP-DRP5B; red, autofluorescence of the chloroplast. Scale bar = 1 μm. (D) Immunofluorescent images showing the DRP5B dot, arc and ring in the control GFP cells that were detected with the anti-DRP5B antibody. Green, DRP5B detected with the DRP5B antibody; red, autofluorescence of the chloroplast; PC, phase-contrast. Scale bar = 1 μm. (E) Immunofluorescent images showing FtsZ2–1, PDR1, and DRP5B localization in the GFP-DRP5B- or GFP-DRP5B K135A-expressing cells. GFP-DRP5B K135A cells cultured under light were transferred to dark and heat-shocked twice at 50°C to express GFP-DRP5B K135A. Green, GFP fluorescence of GFP-DRP5B or GFP-DRP5B K135A; cyan, immunostained FtsZ2–1, PDR1, or DRP5B (the anti-DRP5B antibody detects both GFP-tagged and endogenous DRP5B); red, autofluorescence of the chloroplast; PC, phase-contrast. Scale bar = 1 μm. Two independent experiments produced similar results and the results from one experiment are shown.
Immunofluorescence microscopy showed that, in GFP-DRP5B K135A-expressing cells, both the FtsZ and PDR1 rings formed at the chloroplast division site as in the case in GFP-DRP5B-expressing cells (Fig. 2E). In contrast, endogenous DRP5B did not form a ring in GFP-DRP5B K135A-expressing cells, although localization as a small dot on the nuclear side of the chloroplast division site was observed (Fig. 2E). These results suggest (1) that DRP5B ring formation is not required for the recruitment of PDR1 to the division site, (2) that DRP5B ring formation starts from a certain specific point (on the nuclear side of the chloroplast division site), and (3) that GTP binding and/or GTP hydrolysis by DRP5B is required for extension of DRP5B localization to whole division site as a ring.
In spite of the differences in molecular composition of the PD machinery between C. merolae and A. thaliana especially in terms of the absence of the ARC6 and PDV proteins in C. merolae, our present study showed that FtsZ ring formation is required for recruitment of PDR1 and DRP5B to the chloroplast division site in C. merolae. Thus, as-yet-unidentified proteins probably relay the topological information of the FtsZ ring from the stromal side of the inner envelope to the cytosolic side of the outer envelope membrane to recruit cytosolic PDR1 and DRP5B. The present study also suggests that DRP5B ring formation starts from a certain specific point and that formation of the DRP5B ring from this specific site requires GTP binding or hydrolysis by DRP5B. However, it is still unclear how this specific site is produced depending on the formation of the FtsZ ring. In a manner similar to chloroplast division, mitochondrial division in C. merolae is performed by the FtsZ ring of α-proteobacterial origin (mitochondria evolved from an α-proteobacterial endosymbiont), the MD ring (which is a structure similar to the outer PD ring) and another dynamin-related protein.21 In C. merolae, the mitochondrion locates between the nucleus and the chloroplast, and the PD and MD machinery touch each other. In C. merolae mitochondrial division, recent study showed that the C-terminal kinesin-like protein TOP localizes on the nuclear side of the mitochondrial division site as a dot and that TOP activates both the MD and PD machineries through Aurora kinase.22 Thus, both the PD and MD machinery possess a specific point on the nuclear side, which is important for the formation and activation of the MD and PD machinery.
Because red algae and Viridiplantae (the green algae, streptophyte algae and land plants) diverged relatively soon after the establishment of chloroplasts (reviewed in ref. 1), further study on the linker proteins in the PD machinery in C. merolae should yield important insights into the evolution of the PD machinery.
Materials and methods
Algal culture
Cyanidioschyzon merolae 10D and its stable transformants (18,19) were grown in 300 mL 2×Allen's medium in a 500-mL flat bottle (60 mm thick) with 5 L·min−1 aeration by ambient air at 100 μE·m−2·s−1.
Immunofluorescence microscopy
Immunofluorescence staining for C. merolae was performed as described (19). Rabbit polyclonal anti-FtsZ2–1 antibody (1:1,000, 19), rat polyclonal anti-PDR1 antibody (1:500, 10), or rabbit polyclonal anti-DRP5B antibody (1:500, 11) was used as the primary antibody and the Alexa Fluor 488 goat anti-rabbit IgG antibody, Alexa Fluor 555 goat anti-rabbit IgG antibody, or Alexa Fluor 555 goat anti-rat IgG antibody (Thermo Fisher Scientific) was used as the secondary antibody at a dilution of 1:1,000. The cells were examined using an epifluorescence microscope (BX51; Olympus) equipped with a CCD camera (DP70; Olympus). The specificity of the anti-PDR1, anti-FtsZ2–1, and anti-DRP5B antibodies in C. merolae was previously confirmed as follows. The immunofluorescent signal of PDR1 antibody was detected at the chloroplast division site in the wild type but not in PDR1 knockdown cells.10 When FtsZ2–1 was overexpressed, FtsZ2–1 aggregates were detected in the chloroplast with the FtsZ2–1 antibody by immunofluorescence microscopy.19 In addition, by the immunoblotting, the density of the band detected by this antibody was higher in the FtsZ2–1 overexpresser than that in the wild type.19 The localization pattern of the immunofluorescence with the DRP5B antibody overlapped with GFP-DRP5B and GFP-DRP5B K135A.19 In addition, by the immunoblotting, the antibody detected both endogenous DRP5B and GFP-DRP5B and the difference in the size of the 2 bands corresponded to the size of GFP.19
Time-lapse imaging
Time lapse imaging of C. merolae was performed as described in ref. 19.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Kuroiwa (Japan Women's University) for the PDR1 antibody, and Ms. Yamashita and Ms. Hashimoto (National Institute of Genetics) for technical assistance.
Funding
This study was supported by Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research 25251039 (to S.-y.M.) and by the Core Research for Evolutional Science and Technology Program of the Japan Science and Technology Agency (S.-y.M.).
References
- [1].Archibald JM. Endosymbiosis and eukaryotic cell evolution. Curr Biol 2015; 25:R911-21; PMID:26439354; http://dx.doi.org/ 10.1016/j.cub.2015.07.055 [DOI] [PubMed] [Google Scholar]
- [2].Miyagishima S-y. Mechanism of plastid division: from a bacterium to an organelle. Plant Physiol 2011; 155:1533-44; PMID:21311032; http://dx.doi.org/ 10.1104/pp.110.170688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yoshida Y, Miyagishima S-y, Kuroiwa H, Kuroiwa T. The plastid-dividing machinery: formation, constriction and fission. Curr Opin Plant Biol 2012; 15:714-21; PMID: 22824141; http://dx.doi.org/ 10.1016/j.pbi.2012.07.002 [DOI] [PubMed] [Google Scholar]
- [4].Osteryoung KW, Pyke KA. Division and dynamic morphology of plastids. Ann Rev Plant Biol 2014; 65:443-72; http://dx.doi.org/ 10.1146/annurev-arplant-050213-035748 [DOI] [PubMed] [Google Scholar]
- [5].Vitha S, McAndrew R, Osteryoung K. FtsZ ring formation at the chloroplast division site in plants. J Cell Biol 2001; 153:111-9; PMID:11285278; http://dx.doi.org/ 10.1083/jcb.153.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mori T, Kuroiwa H, Takahara M, Miyagishima S-y, Kuroiwa T. Visualization of an FtsZ ring in chloroplasts of Lilium longiflorum leaves. Plant Cell Physiol 2001; 42:555-9; PMID:11427673; http://dx.doi.org/ 10.1093/pcp/pce095 [DOI] [PubMed] [Google Scholar]
- [7].Kuroiwa H, Mori T, Takahara M, Miyagishima S-y, Kuroiwa T. Chloroplast division machinery as revealed by immunofluorescence and electron microscopy. Planta 2002; 215:185-90; PMID:12029466; http://dx.doi.org/ 10.1007/s00425-002-0734-4 [DOI] [PubMed] [Google Scholar]
- [8].Mita T, Kanbe T, Tanaka K, Kuroiwa T. A ring structure around the dividing plane of the Cyanidium caldarium chloroplast. Protoplasma 1986; 130:211-3; http://dx.doi.org/ 10.1007/BF01276603 [DOI] [Google Scholar]
- [9].S-y Miyagishima, Kuroiwa H, Kuroiwa T. The timing and manner of disassembly of the apparatuses for chloroplast and mitochondrial division in the red alga Cyanidioschyzon merolae. Planta 2001; 212:517-28; PMID:11525508; http://dx.doi.org/ 10.1007/s004250000426 [DOI] [PubMed] [Google Scholar]
- [10].Yoshida Y, Kuroiwa H, Misumi O, Yoshida M, Ohnuma M, Fujiwara T, Yagisawa F, Hirooka S, Imoto Y, Matsushita K, et al.. Chloroplasts divide by contraction of a bundle of nanofilaments consisting of polyglucan. Science 2010; 329:949-53; PMID:20724635; http://dx.doi.org/ 10.1126/science.1190791 [DOI] [PubMed] [Google Scholar]
- [11].S-y Miyagishima, Nishida K, Mori T, Matsuzaki M, Higashiyama T, Kuroiwa H, Kuroiwa T. A plant-specific dynamin-related protein forms a ring at the chloroplast division site. Plant Cell 2003; 15:655-65; PMID:12615939; http://dx.doi.org/ 10.1105/tpc.009373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gao H, Kadirjan-Kalbach D, Froehlich JE, Osteryoung KW. ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proc Natl Acad Sci USA 2003; 100:4328-33; PMID: 12642673; http://dx.doi.org/ 10.1073/pnas.0530206100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Glynn J, Froehlich J, Osteryoung KW. Arabidopsis ARC6 coordinates the division machineries of the inner and outer chloroplast membranes through interaction with PDV2 in the intermembrane space. Plant Cell 2008; 20:2460-70; PMID:18812496; http://dx.doi.org/ 10.1105/tpc.108.061440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Glynn J, Yang Y, Vitha S, Schmitz A, Hemmes M, Miyagishima S-y, Osteryoung KW. PARC6, a novel chloroplast division factor, influences FtsZ assembly and is required for recruitment of PDV1 during chloroplast division in Arabidopsis. Plant J 2009; 59:700-11; PMID: 19453460; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03905.x [DOI] [PubMed] [Google Scholar]
- [15].Stokes KD, McAndrew RS, Figueroa R, Vitha S, Osteryoung KW. Chloroplast division and morphology are differentially affected by overexpression of FtsZ1 and FtsZ2 genes in Arabidopsis. Plant Physiol 2000; 124:1668-77; PMID: 11115884; http://dx.doi.org/ 10.1104/pp.124.4.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dai K, Lutkenhaus J. The proper ratio of FtsZ to FtsA is required for cell division to occur in Escherichia coli. J Bacteriol 1992; 174:6145-51; PMID: 1400163; http://dx.doi.org/ 10.1128/jb.174.19.6145-6151.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Miyagishima S-y, Fujiwara T, Sumiya N, Hirooka S, Nakano A, Kabeya Y, Nakamura M. Translation-independent circadian control of the cell cycle in a unicellular photosynthetic eukaryote. Nat Commun 2014; 5:3807; PMID: 24806410; http://dx.doi.org/ 10.1038/ncomms4807 [DOI] [PubMed] [Google Scholar]
- [18].Sumiya N, Fujiwara T, Kobayashi Y, Misumi O, Miyagishima S-y. Development of a heat-shock inducible gene expression system in the red alga Cyanidioschyzon merolae. PLoS ONE 2014; 9:e111261; PMID: 25337786; http://dx.doi.org/ 10.1371/journal.pone.0111261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sumiya N, Fujiwara T, Era A, Miyagishima S-y. Chloroplast division checkpoint in eukaryotic algae. Proc Natl Acad Sci USA 2016; 113:E7629-38; http://dx.doi.org/ 10.1073/pnas.1612872113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol 1993; 122:553-63; PMID: 8101525; http://dx.doi.org/ 10.1083/jcb.122.3.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nishida K, Takahara M, Miyagishima S-y, Kuroiwa H, Matsuzaki M, Kuroiwa T. Dynamic recruitment of dynamin for final mitochondrial severance in a primitive red alga. Proc Natl Acad Sci USA 2003; 100:2146-51; PMID: 12566569; http://dx.doi.org/ 10.1073/pnas.0436886100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yoshida Y, Fujiwara T, Imoto Y, Yoshida M, Ohnuma M, Hirooka S, Misumi O, Kuroiwa H, Kato S, Matsunaga S, et al.. The kinesin-like protein TOP promotes Aurora localisation and induces mitochondrial, chloroplast and nuclear division. J Cell Sci 2013; 126:2392-400; PMID: 23549784; http://dx.doi.org/ 10.1242/jcs.116798 [DOI] [PubMed] [Google Scholar]