Abstract
Mesyl Salvinorin B (MSB) is a potent selective kappa opioid receptor (KOP-r) agonist that has potential for development as an anti-psychostimulant agent with fewer side-effects (e.g., sedation, depression and dysphoria) than classic KOP-r agonists. However, no such study has been done on alcohol. We investigated whether MSB alone or in combination with naltrexone (mu-opioid receptor antagonist) altered voluntary alcohol drinking in both male and female mice. Mice, subjected to 3 weeks of chronic escalation drinking (CED) in a two-bottle choice paradigm with 24-h access every other day, developed rapid escalation of alcohol intake and high preference. We found that single, acute administration of MSB dose-dependently reduced alcohol intake and preference in mice after 3-week CED. The effect was specific to alcohol, as shown by the lack of any effect of MSB on sucrose or saccharin intake. We also used the drinking-in-the-dark (DID) model with limited access (4 h/day) to evaluate the pharmacological effect of MSB after 3 weeks of DID. However, MSB had no effect on alcohol drinking after 3-week DID. Upon investigation of potential synergistic effects between naltrexone and MSB, we found that acute administration of a combination of MSB and naltrexone reduced alcohol intake profoundly after 3-week CED at doses lower than those individual effective doses. Repeated administrations of this combination showed less tolerance development than repeated MSB alone. Our study suggests that the novel KOP-r agonist MSB both alone and in combination with naltrexone shows potential in alcoholism treatment models.
Keywords: Mesyl Salvinorin B, KOP-r, alcohol escalation drinking, naltrexone, combined therapy
1. INTRODUCTION
Alcohol consumption affects multiple neurobiological systems including the endogenous opioid systems. Specifically, activation of the kappa opioid receptor (KOP-r) system has been implicated in the negative reinforcing aspects of alcohol, opiate, and psychostimulant addictions [Herz 1997; Koob and Kreek 2007]. In rats and mice, acute administration of KOP-r agonists attenuates alcohol drinking [Sandi et al 1988; Lindholm et al 2001; Henderson-Redmond and Czachowski 2014], increases alcohol drinking [Anderson et al 2016; Rose et al 2016], and alters alcohol-induced conditioned place preference [Logrip et al 2009; Sperling et al 2010], while the selective KOP-r antagonist nor-binaltorphimine (nor-BNI) increases alcohol drinking in Lewis rats with relatively high intake [Mitchell et al 2005]. Recently KOP-r antagonists have been reported to attenuate stress-induced alcohol-seeking behavior in mice and rats [Sperling et al 2010; Deehan et al 2012; Funk et al 2014] and to reduce alcohol consumption in alcohol “dependent” rats [Walker and Koob 2008]. These findings provide support for the importance of the KOP-r systems in the processes of alcohol consumption and addiction.
Alcohol may activate the endogenous dynorphin neurons involved in neuronal structures related to alcohol’s reinforcing and motivational behaviors. Microdialysis studies have found that acute alcohol administration increases the extracellular dynorphin A1-8 concentrations in the nucleus accumbens (NAc) and the central nucleus of the amygdala (CeA), the two brain regions known to be involved in the regulation of alcohol consumption [Marinelli et al 2006; Lam and Gianoulakis 2011]. In support of this concept, dynorphin mRNA and peptide levels in the CeA have been found to be increased after acute withdrawal from multiple alcohol “binge” administrations in Sprague-Dawley rats [D'Addario et al 2013], voluntary alcohol drinking in Sardinian alcohol-preferring rats [Zhou et al 2013a] or in alcohol-dependent Wistar rats [Kissler et al 2014]. This enhanced dynorphin/KOP-r expression and/or activity in the CeA may be involved in the homeostatic adaptations of the brain after chronic alcohol exposure and in the negative affective state during withdrawal.
It has been found that activation of KOP-r by the novel neoclerodane diterpene salvinorin A (Sal A) and classic agonists such as U69,593 and U50,488H have anti-psychostimulant (including cocaine and amphetamine) effects in preclinical models of drug addiction [Negus et al 1997; Schenk et al 1999; Morani et al 2009]. However, most “classic” KOP-r agonists produce significant sedation and dysphoria, and those side effects limit their clinical use potential. Mesyl Salvinorin B (MSB) is a potent and selective KOP-r agonist, with a longer duration of action in mice than the structurally similar Sal A. As a novel KOP-r agonist with few side effects, MSB has been developed as a potential anti-cocaine compound, as acute administration of MSB significantly attenuates cocaine seeking in a rat self-administration model [Simonson et al 2015]. However, there is no report on the effect of MSB, Sal A or its analogues on alcohol related behaviors in rodent models. In this study, therefore, we investigated whether MSB alters voluntary alcohol drinking in mice to explore its potential for development as a therapeutic agent for alcoholism.
In the present study, we evaluated the pharmacological effect of MSB in both male and female mice using both chronic escalation drinking (CED) and drinking-in-the-dark (DID) models. In the CED model, the mice had access to alcohol drinking for 3 weeks in a two-bottle choice paradigm with voluntary alcohol drinking every other day, and the mice exposed to alcohol rapidly developed high alcohol consumption (15–25 g/kg/day). Thus, the CED constitutes an appropriate animal model for studying escalation of alcohol drinking [Hwa et al 2011]. We further determined the pharmacological effect of MSB in the DID model, which allows for limited access (4 h/day) to a single alcohol bottle after the beginning of the dark period [Rhodes et al 2005], and the DID appears to be more suited to model “binge” drinking to the point of intoxication.
Numerous pharmacological studies provide consistent evidence that the opioid antagonist naltrexone (NTN) decreases alcohol reward, consumption, craving, and relapse episodes in human alcoholics [O’Malley et al 1992; Volpicelli et al 1992]. Preclinical investigations have consistently found that NTN decreases alcohol intake and alcohol’s reinforcing and motivational properties [Zhou and Kreek 2014]. In select experiments, therefore, we used the well-known MOP-r antagonist NTN as a reference compound to compare its effects on mouse alcohol drinking behaviors with those of the KOP-r agonist MSB.
Finally, an investigation into the combination of these two compounds could be particularly intriguing, given that these drugs have distinctly different mechanisms of actions (MOP-r antagonism and full KOP-r agonism). Therefore, we specifically tested a combination of MSB and NTN using doses of each compound low enough that no effect on alcohol consumption was seen with either drug alone. These doses were also chosen to minimize potential side effects of both drugs and avoid tolerance to the KOP-r agonist after repeated administration, in order to maximize its effect on alcohol drinking. We hypothesized that this combination would be more effective in reducing alcohol escalation drinking than either drug alone.
2. RESULTS
2.1. Single, acute administration of MSB alone dose-dependently reduced alcohol (but not sucrose or saccharin) intake and preference after CED
2.1.1. CED model and blood ethanol concentration (BEC) levels
Both male and female mice exposed to the 2-bottle “15% alcohol vs. water” choice regimen every other day rapidly acquired alcohol drinking behaviour, and daily alcohol intake subsequently rose to levels averaging approximately 15–20 g/kg/day in males and 20–25 g/kg/day in females after 3 weeks with 15% alcohol, with a high preference ratio above 0.8–0.9 in both sexes (Table 1). In a separate experiment, mice had alcohol intake of 5.3±0.8 g/kg in males (n=10) and 8.1±1.3 g/kg in females (n=11) after 4 hours of alcohol access following 3 weeks of CED. Blood samples were taken at the 4-hour time point (2 males and 1 female did not show reliable alcohol intake and were excluded from the BEC measurement), and this 3-week voluntary CED exposure was found to give rise to BECs of 0.50±0.08 mg/ml in males (n=8) and 0.63±0.07 mg/ml in females (n=10). These BEC values were within the range required to produce specific psycho-pharmacological effects [e.g., Rhodes et al 2005; Hwa et al 2011].
Table 1.
Alcohol intake and alcohol preference in a 3-week CED model in male and female mice. The mice were exposed to the 2-bottle “alcohol (15%) vs. water” choice regimen every other day for 3 weeks. Data are presented after 4, 8 and 24 hours of alcohol access in session 1 on the first day and in session 10 after 3 weeks of CED.
| male (n=10) | female (n=11) | ||||
|---|---|---|---|---|---|
| Session 1 | Session 10 | Session 1 | Session 10 | ||
| Intake g/kg | 0–4h | 1.34 ± 0.65 | 5.48 ± 0.56 ** | 1.92 ± 0.23 | 8.17 ± 1.15 ** |
| 5–8h | 4.22 ± 0.51 | 5.00 ± 1.03 | 5.34 ± 0.97 | 3.36 ± 0.73 | |
| 9–24h | 5.83 ± 1.02 | 8.73 ± 0.93 | 10.9 ± 2.00 && | 14.1 ± 1.47 && | |
| Preference | 0–4h | 0.46 ± 0.08 | 0.88 ± 0.03 ** | 0.44 ± 0.07 | 0.86 ± 0.05 ** |
| 5–8h | 0.43 ± 0.07 | 0.58 ± 0.05 | 0.60 ± 0.04 | 0.50 ± 0.07 | |
| 9–24h | 0.55 ± 0.06 | 0.64 ± 0.06 | 0.52 ± 0.08 | 0.72 ± 0.05 * | |
Session difference: *p<0.05, **p<0.01 vs. the same sex at the same hour in session 1; and Sex difference: &&p<0.01 vs. the male at the same hour in the same session (see the statistical analysis in Supporting Information section).
2.1.2. Single, acute MSB dose-dependently reduced 15% alcohol intake and preference
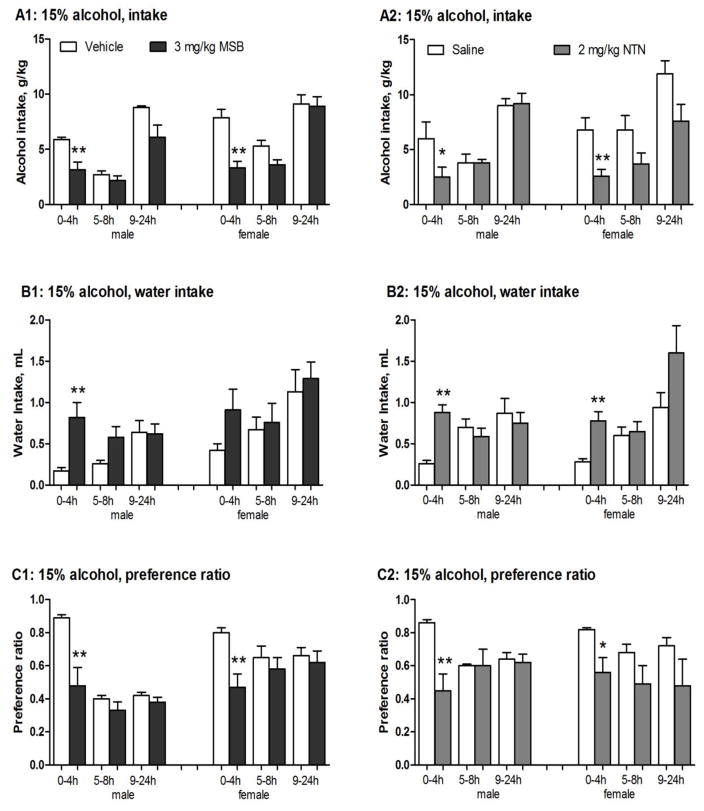
After 0.3 or 1 mg/kg MSB, there was no significant effect on alcohol intake, water intake or alcohol preference ratio in males or females. At 3 mg/kg, MSB significantly reduced alcohol intake in males [2-way ANOVA, F(1,13)=5.5, p<0.05] at 4 hours [post-hoc test p<0.01] (Fig 1A1, left) and in females [2-way ANOVA, F(1,26)=11.2, p<0.005] at 4 hours [post-hoc test p<0.01] (Fig 1A1, right). In comparison to the vehicle controls, 3 mg/kg MSB reduced mean alcohol intake by 46% in males and 68% in females. This was associated with a compensatory increase in water intake in males [2-way ANOVA, F(1,13)=5.4, p<0.05] at 4 hours [post-hoc test p<0.01] (Fig 1B1, left) and in females [an apparent but not statistically significant increase at 4 hours] (Fig 1B1, right), resulting in virtually unchanged total fluid intake in both sexes (Table 2). At this dose, MSB also significantly reduced preference ratio in males [2-way ANOVA, F(1,13)=7.8, p<0.05] at 4 hours [post-hoc test p<0.01] and in females [2-way ANOVA, F(1,26)=7.2, p<0.05] at 4 hours [post-hoc test p<0.01] (Fig 1C1).
Figure 1.
Effects of single, acute administration of Mesyl Salvinorin B (MSB, 3 mg/kg) or naltrexone (NTN, 2mg/kg) on 15% alcohol intake, water intake, and preference ratio in male and female mice after 3-week chronic escalation drinking (CED). (1) Control groups (n=6–8): males and females received one vehicle injection (1% DMSO for MSB control, or saline for NTN control, i.p.) before the drinking test; (2) MSB groups: males (n=6–8) and females (n=14) received one MSB injection (3 mg/kg in 1% DMSO, i.p.) 30 min before the drinking test; and (3) NTN group: males (n=6) and females (n=8) received one NTN injection (2 mg/kg in saline, i.p.) 10 min before the drinking test. On the test day, alcohol (15%) and water intake values were recorded after 4, 8 and 24 hours of alcohol access. * p<0.05, **p<0.01 and ***p<0.005 vs. control at the same time point (see the statistical analysis in Supporting Information section).
Table 2.
No effects of single, acute administration of Mesyl Salvinorin B (MSB, 3mg/kg) (A), naltrexone (NTN, 2mg/kg) (B), or their combinations (0.3mg/kg MSB+1mg/kg NTN or 1mg/kg MSB+1mg/kg NTN) (C) on total fluid intake in male and female mice after 3-week chronic escalation drinking (CED).
| A. | ||||
|---|---|---|---|---|
| Total fluid intake, ml | male (n=6–8) | female (n=8–14) | ||
| Vehicle | 3mg/kg MSB | Vehicle | 3mg/kg MSB | |
| 0–4h | 1.6 ± 0.1 | 1.6 ± 0.2 | 1.9 ± 0.1 | 1.6 ± 0.2 |
| 5–8h | 1.0 ± 0.1 | 1.2 ± 0.3 | 1.7 ± 0.2 | 1.5 ± 0.3 |
| 9–24h | 2.8 ± 0.3 | 2.8 ± 0.4 | 3.0 ± 0.4 | 3.1 ± 0.5 |
| B. | ||||
|---|---|---|---|---|
| Total fluid intake, ml | male (n=6–8) | female (n=6–8) | ||
| Vehicle | 2mg/kg NTN | Vehicle | 2mg/kg NTN | |
| 0–4h | 1.9 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.1 | 1.3 ± 0.1 |
| 5–8h | 1.4 ± 0.2 | 1.5 ± 0.3 | 1.9 ± 0.2 | 1.4 ± 0.4 |
| 9–24h | 3.0 ± 0.4 | 3.1 ± 0.5 | 3.2 ± 0.3 | 3.2 ± 0.5 |
| C. | ||||
|---|---|---|---|---|
| Total fluid intake, ml | female (n=6–8) | female (n=6–8) | ||
| Vehicle | 0.3 mg/kg MSB+ 1mg/kg NTN | Vehicle | 0.3 mg/kg MSB+ 1mg/kg NTN | |
| 0–4h | 1.5 ± 0.1 | 1.2 ± 0.2 | 1.5 ± 0.1 | 1.3 ± 0.2 |
| 5–8h | 1.2 ± 0.3 | 1.9 ± 0.4 | 1.5 ± 0.2 | 1.8 ± 0.5 |
| 9–24h | 3.5 ± 0.5 | 3.4 ± 0.5 | 3.7 ± 0.6 | 3.2 ± 0.3 |
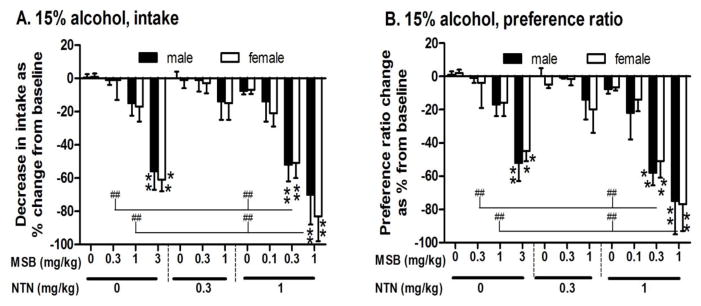
The full-dose response of single, acute MSB administration (0, 0.3, 1 and 3 mg/kg) in terms of 15% alcohol intake and preference at the 4-hour time point is presented in Fig 2. In comparison to the control group, mean alcohol intake in MSB-treated mice was reduced by 15% and 56% in males and 7% and 61% in females at 1 and 3 mg/kg MSB doses, respectively. For alcohol intake (Fig 2A, left), there was a main effect of MSB [2-way ANOVA, F(10,124)=28, p<0.0000001] with significance at 3 mg/kg in both males and females [post-hoc test p<0.005 for both]. For preference ratio (Fig 2B, left), there was a main effect of MSB [2-way ANOVA, F(10,124)=27, p<0.0000001] with significance at 3 mg/kg in both males and females [post-hoc test p<0.005 for both].
Figure 2.
Dose responses of single, acute administration of Mesyl Salvinorin B (MSB, 0, 0.1, 0.3, 1 or 3 mg/kg) alone or combined with naltrexone (NTN, 0, 0.3 or 1 mg/kg) on reducing 15% alcohol intake (A) and alcohol preference (B) in both male and female mice (n=5–8) after 3-week chronic escalation drinking. Data were collected at the 4-hour time point on the baseline and testing day (24 hours later) and are expressed as a percentage of baseline alcohol intake to account for the differences in baseline that contribute to variation between experiments. **p<0.01 vs. control (both MSB and NTN at 0 mg/kg); ## p<0.01 between treatment groups (see the statistical analysis in Supporting Information section).
2.1.3. Single, acute MSB slightly reduced 7.5% alcohol intake and preference
In males, preference ratio in 3 mg/kg MSB-treated mice was significantly reduced [2-way ANOVA, F(1,10)=7.9, p<0.05] at 4 hours [post-hoc test p<0.05] (Fig S2C1, left). In female mice, MSB (3 mg/kg) marginally reduced alcohol intake [2-way ANOVA, F(1,12)=5.9, p<0.05] at 4 hours [post-hoc test p=0.05] (Fig S2A1, right).
2.1.4. Single, acute MSB reduced 30% alcohol preference in male only
In males, preference ratio in 3 mg/kg MSB-treated mice was significantly reduced [2-way ANOVA, F(1,10)=5.9, p<0.05] at 4 hours [post-hoc test p<0.05] (Fig S2C2).
2.1.5. Pretreatment with the selective KOP-r antagonist nor-BNI blocked the reducing effect of acute MSB on 15% alcohol drinking
For intake (Table 3), 2-way ANOVA revealed: (1) a significant effect of 3 mg/kg MSB treatment [F(1,24)=8.5, p<0.01]; (2) a significant effect of 5 mg/kg nor-BNI pretreatment [F(1,24)=7.2, p<0.05]; and (3) a significant interaction between the nor-BNI pretreatment and the MSB treatment [F(1,24)=7.1, p<0.05]. For preference ratio (Table 3), 2-way ANOVA revealed: (1) a significant effect of MSB treatment [F(1,24)=23, p<0.001]; (2) a significant effect of nor-BNI pretreatment [F(1,24)=16, p<0.001]; and (3) a significant interaction between the nor-BNI pretreatment and the MSB treatment [F(1,24)=22, p<0.001]. MSB at 3 mg/kg significantly reduced alcohol intake and preference ratio at 4 hours [post-hoc test p<0.01 for both]. Though pretreatment with nor-BNI at 5 mg/kg alone had no effect, it blunted the effect of 3 mg/kg MSB on alcohol drinking at 4 hours.
Table 3.
Pretreatment with selective KOP-r antagonist nor-BNI (5 mg/kg) blocks the effect of single, acute Mesyl Salvinorin B (MSB, 3 mg/kg) on reducing 15% alcohol drinking in female mice (n=7).
| Pretreatment | Saline | Saline | Nor-BNI | Nor-BNI |
|---|---|---|---|---|
| Treatment | Vehicle | MSB | Vehicle | MSB |
| Alcohol intake (g/kg/4h) | 7.8 ± 0.79 | 4.2 ± 0.82 ** | 7.8 ± 0.92 | 7.7 ± 0.60 |
| Water intake (ml/4h) | 0.30 ± 0.05 | 0.66 ± 0.26 | 0.32 ± 0.09 | 0.26 ± 0.09 |
| alcohol preference | 0.84 ± 0.04 | 0.34 ± 0.11 ** | 0.80 ± 0.05 | 0.79 ± 0.04 |
p<0.01 vs. vehicle control with the same pretreatment (see the statistical analysis in Supporting Information section).
2.1.6. No effects of single, acute MSB on sucrose or saccharin intake and preference
As alcohol is a caloric reinforcer, the specificity of the action of MSB on alcohol intake was verified by testing the effects of acute administration of 3 mg/kg MSB on sucrose (caloric reinforcer) or saccharin (non-caloric reinforcer) intake in mice after CED. In these experiments, the chronic 15% alcohol exposure procedures were identical to those in the above experiments. After 3 weeks of CED, the alcohol tube was switched to sucrose or saccharin for 3 sessions, during which stable intake was observed. The mice assigned to the vehicle-treated or MSB-treated groups had similar sucrose or saccharin intake 24 hours before the test day. On the test day, no significant effect of 3 mg/kg MSB on 4% sucrose (Table 4A) or 0.1% saccharin (Table 4C) drinking was found after 4 hours in either males (left) or females (right). There was no effect on sucrose or saccharin drinking observed after 8 or 24 hours (data not shown). Effects of MSB on consumption of other concentrations of sucrose (8% or 16%) or saccharin (0.2% or 0.4%) were also tested in males and females (n = 3–4) and no significant differences were found. Similarly, there was no effect of acute MSB at 3 mg/kg on sucrose or saccharin drinking in alcohol-naïve males and females (data not shown).
Table 4.
No effect of single, acute administration of Mesyl Salvinorin B (MSB, 3 mg/kg) alone or the combination of MSB (0.3 mg/kg) and naltrexone (NTN, 1 mg/kg) on 4% sucrose (A and B) or 0.1% saccharin (C and D) intake, water intake and their preference ratio in male or female mice (n=6–7).
| A | male | female | ||
|---|---|---|---|---|
| Treatment | Vehicle | 3 mg/kg MSB | Vehicle | 3 mg/kg MSB |
| Sucrose (4%) intake (g/kg/4h) | 11.8 ± 0.81 | 9.5 ± 1.3 | 12.1 ± 1.75 | 13.7 ± 1.1 |
| Water intake (ml/4h) | 0.17 ± 0.06 | 0.23 ± 0.08 | 0.11 ± 0.02 | 0.19 ± 0.10 |
| Preference ratio | 0.98 ± 0.02 | 0.95 ± 0.02 | 0.98 ± 0.01 | 0.97 ± 0.02 |
| B | male | female | ||
|---|---|---|---|---|
| Treatment | Vehicle + Saline | 0.3 mg/kg MSB + 1 mg/kg NTN | Vehicle + Saline | 0.3 mg/kg MSB + 1 mg/kg NTN |
| Sucrose (4%) intake (g/kg/4h) | 9.9 ± 1.2 | 9.1 ± 1.0 | 11.5 ± 0.95 | 10.1 ± 1.10 |
| Water intake (ml/4h) | 0.13 ± 0.06 | 0.20 ± 0.05 | 0.15 ± 0.05 | 0.19 ± 0.11 |
| Preference ratio | 0.98 ± 0.02 | 0.97 ± 0.01 | 0.98 ± 0.01 | 0.98 ± 0.02 |
| C | male | female | ||
|---|---|---|---|---|
| Treatment | Vehicle | 3 mg/kg MSB | Vehicle | 3 mg/kg MSB |
| Saccharin (0.1%) intake (g/kg/4h) | 0.13 ± 0.03 | 0.11 ± 0.03 | 0.17 ± 0.02 | 0.19 ± 0.01 |
| Water intake (ml/4h) | 0.19 ± 0.10 | 0.26 ± 0.09 | 0.10 ± 0.04 | 0.16 ± 0.06 |
| Preference ratio | 0.95 ± 0.05 | 0.98 ± 0.05 | 0.97 ± 0.01 | 0.99 ± 0.01 |
| D | male | female | ||
|---|---|---|---|---|
| Treatment | Vehicle + Saline | 0.3 mg/kg MSB + 1 mg/kg NTN | Vehicle + Saline | 0.3 mg/kg MSB + 1 mg/kg NTN |
| Saccharin (0.1%) intake (g/kg/4h) | 0.17 ± 0.02 | 0.14 ± 0.07 | 0.19 ± 0.02 | 0.15 ± 0.06 |
| Water intake (ml/4h) | 0.15 ± 0.04 | 0.20 ± 0.09 | 0.14 ± 0.03 | 0.19 ± 0.06 |
| Preference ratio | 0.98 ± 0.02 | 0.98 ± 0.03 | 0.97 ± 0.01 | 0.98 ± 0.04 |
2.2. Single, acute administration of NTN alone dose-dependently reduced alcohol (but not sucrose or saccharin) consumption after CED
At lower doses (0.3 or 1 mg/kg), there was no significant effect of NTN on 15% alcohol drinking in either males or females, as shown in Fig 2. At 2 mg/kg, NTN significantly reduced alcohol intake in males [2-way ANOVA, F(1,10)=8.7, p<0.05] after 4 hours [post-hoc test p<0.05] (Fig 1A2, left) and in females [2-way ANOVA, F(1,14)=8.9, p<0.05] after 4 hours [post-hoc test p<0.01] (Fig 1A2, right). In contrast to the saline controls, the reduced alcohol intake in the NTN-treated mice was associated with a compensatory increase in water intake in males and females after 4 hours [post-hoc test p<0.01 for both] (Fig 1B2), resulting in virtually unchanged total fluid intake (Table 2). For preference ratio, two-way ANOVA revealed a significant interaction between the 2 mg/kg NTN treatment and the time interval in males [F(2,20)=12, p<0.0005], and NTN significantly reduced preference ratio at 4 hours [p<0.01] [post-hoc test p<0.05] (Fig 1C2, left). Also, NTN significantly reduced preference ratio in females [2-way ANOVA, F(1,14)=11, p<0.01] at 4 hours [post-hoc test p<0.05] (Fig 1C2, right). At this dose range (0.3–2 mg/kg), NTN had no effect on sucrose (4–16%) or saccharin (0.1–0.4%) consumption in either male or female mice (data not shown).
2.3. Single, acute administration of MSB combined with NTN dose-dependently reduced alcohol (but not sucrose or saccharin) consumption after CED
2.3.1. Effect of single, acute administration of MSB combined with NTN on alcohol drinking
In both males and females, single, acute administration of MSB (0.1, 0.3 or 1 mg/kg) combined with NTN (0, 0.3 or 1 mg/kg) reduced 15% alcohol intake and preference in a dose-dependent manner (data at the 4-hour time point are analyzed together and presented in Fig 2). Acute administration of MSB at two lower doses (0.3 or 1 mg/kg) combined with NTN at 0 or 0.3 mg/kg did not reduce alcohol intake or preference in either males or females (Fig 2A, 2B). Combined with a higher dose of 1 mg/kg NTN, however, the MSB at 0.3 mg/kg and 1 mg/kg significantly reduced alcohol intake [post-hoc test p<0.001 for both] (Fig 2A, right) and preference ratio [post-hoc test p<0.005 for both] (Fig 2B, right) in both sexes. Of note, MSB at 0.3 or 1 mg/kg combined with 1 mg/kg NTN showed more reductions than MSB alone at either dose in males or females [post-hoc test p<0.01 for all]. Similarly, the combinations showed greater reductions than 1 mg/kg NTN alone [post-hoc test p<0.01 for all] (Fig 2A, 2B). However, there was no significant effect of 0.1 mg/kg MSB combined with 1 mg/kg NTN.
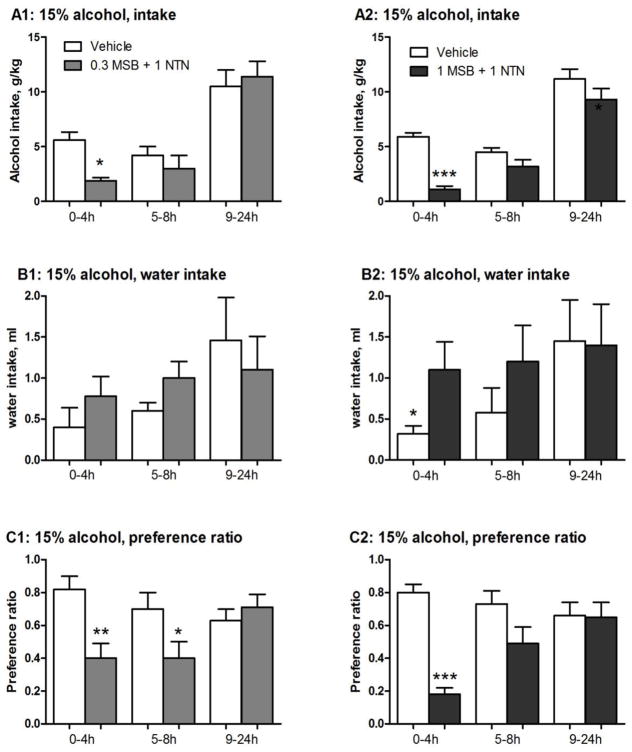
Figure 3 presents 15% alcohol and water intake values recorded at all three time points (4, 8 and 24 hours) following the two combination doses in females. Combined with 1 mg/kg NTN, 0.3 mg/kg MSB significantly reduced alcohol intake [2-way ANOVA, F(1,10)=5.4, p<0.05] at 4 hours [post-hoc test p<0.05] (Fig 3A1), with a 66% reduction compared to the controls. This combination significantly also reduced preference ratio [2-way ANOVA, F(1,10)=5.7, p<0.05] after 4 hours and between 5–8 hours [post-hoc test p<0.01 and p<0.05, respectively] (Fig 3C1). With 1 mg/kg NTN, 1 mg/kg MSB further reduced alcohol intake [2-way ANOVA, F(1,14)=18, p<0.001] after 4 hours [post-hoc test p<0.001] (Fig 3A2), with a 82% reduction compared to the controls. This was associated with a compensatory increase in water intake at 4 hours [post-hoc test p<0.05] (Fig 3B2). This combination also significantly reduced preference ratio [2-way ANOVA, F(1,14)=10, p<0.01] after 4 hours [post-hoc test p<0.001] (Fig 3C2). Neither combination dose had an effect on total fluid intake (Table 2).
Figure 3.
Effects of single, acute Mesyl Salvinorin B (MSB) administration at 0.3 mg/kg (n=6) or 1 mg/kg (n=8) combined with naltrexone (NTN, 1 mg/kg) on 15% alcohol intake, water intake and preference ratio in female mice after 3-week chronic escalation drinking. Control groups: females received one vehicle (1% DMSO, i.p.) followed by saline before the drinking test in two separate experiments with two different MSB+NTN combinations (Vehicle 1 and Vehicle 2). Test groups: females received one MSB injection (0.3 or 1 mg/kg in 1% DMSO, i.p.) followed by one NTN injection (1 mg/kg, i.p.) before the drinking test. On the test day, 15% alcohol (A) and water intake (B) values were recorded after 4, 8 and 24 hours of alcohol access. Preference ratio is shown (C). * p<0.05; ** p<0.01 and ***p<0.005 vs. control at the same time point (see the statistical analysis in Supporting Information section).
2.3.2. No effect of single, acute administration of MSB combined with NTN on sucrose or saccharin drinking
The specificity of the effect of the MSB+NTN combination on alcohol intake was ascertained by testing its effect on sucrose and saccharin drinking after CED. After 4 hours, no significant effect of 0.3 mg/kg MSB+1 mg/kg NTN (the most effective combination for reducing alcohol) on 4% sucrose or 0.1% saccharin drinking was found in either males (Table 4B, 4D, left) or females (Table 4B, 4D, right). Similarly, no significant effects of MSB+NTN on other concentrations of sucrose (8% or 16%) or saccharin (0.2% or 0.4%) intake were observed in either males or females (n=3–4). There was no effect of MSB+NTN on sucrose or saccharin drinking in alcohol-naïve males or females (data not shown).
2.4. Development of tolerance to repeated administrations of MSB alone on 15% alcohol drinking after CED
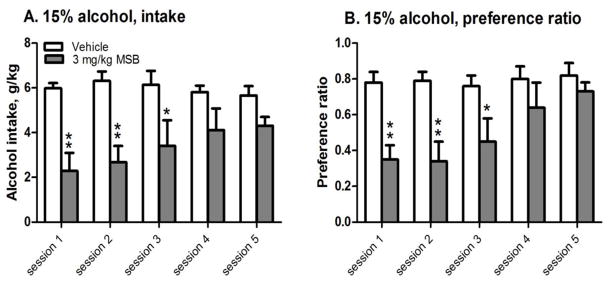
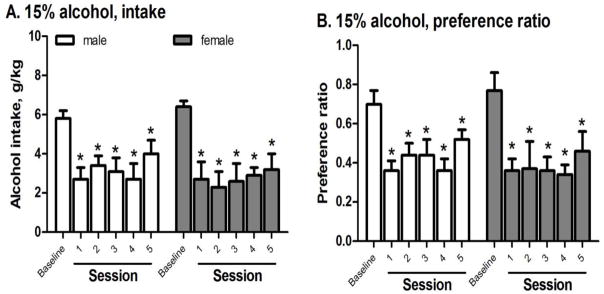
To assess the effect of repeated MSB on alcohol drinking after 3 weeks of CED, one group of females were given 5 repeated administrations of 3 mg/kg MSB and the effects on alcohol intake and preference were compared among the 5 sessions. For intake after 4 hours (Fig 4A), 2-way ANOVA with repeated measure revealed: (1) a significant effect of MSB [F(1,12)=29, p<0.0005]; and (2) a significant interaction between MSB and sessions [F(4,48)=4.4, p<0.005]. MSB significantly reduced intake in sessions 1, 2, and 3 [post-hoc test p<0.01, p<0.01 and p<0.05, respectively], but not sessions 4 or 5. For preference ratio (Fig 4B), 2-way ANOVA with repeated measure revealed: (1) a significant effect of MSB [F(1,12)=18, p<0.005]; and (2) a significant interaction between MSB and sessions [F(4,48)=2.9, p<0.005]. MSB significantly reduced preference ratio in sessions 1, 2, and 3 [post-hoc test p<0.01, p<0.01 and p<0.05, respectively], but not sessions 4 or 5.
Figure 4.
Effects of repeated administration of Mesyl Salvinorin B (MSB, 3 mg/kg) on 15% alcohol intake (A) and preference ratio (B) after 3-week chronic escalation drinking in females (n=6–8). *p<0.05 or **p<0.01 vs. control in the same session (see the statistical analysis in Supporting Information section).
2.5. Reduced development of tolerance after repeated administrations of MSB combined with NTN on 15% alcohol drinking after CED
The effect of the 0.3 mg/kg MSB+1 mg/kg NTN combination on alcohol drinking was assessed in all 5 consecutive sessions in both males and females (Fig 5). For intake, 1-way ANOVA revealed a significant effect of MSB+NTN in males (Fig 5A, left) and females (Fig 5A, right) [F(5,24)=3.9, p<0.01 and F(5,30)=3.8, p<0.01, respectively], and the combination significantly reduced intake in all the sessions [post-hoc test p<0.05 for all] in both sexes, with no tolerance development. For preference ratio, 1-way ANOVA revealed a significant effect of MSB+NTN in males (Fig 5B, left) and females (Fig 5B, right) [F(5,30)=4.6, p<0.005 and F(5,24)=3.2, p<0.05, respectively], and the combination significantly reduced alcohol preference in all the sessions [post-hoc test p<0.05 for all] in both sexes, with no tolerance development.
Figure 5.
Effects of repeated administration of Mesyl Salvinorin B (0.3 mg/kg) combined with naltrexone (NTN, 1 mg/kg) on 15% alcohol intake (A) and preference ratio (B) after 3-week CED in male (left, n=6) and female mice (right, n=5). *p<0.05 vs. the baseline (see the statistical analysis in Supporting Information section).
2.6. No effect of single, acute administration of MSB on 15% alcohol intake after chronic 3-week DID in males or females
To assess the effect of MSB on short-access “binge” alcohol drinking, mice received MSB at 1 or 3 mg/kg after 3 weeks of DID. There was no effect of either dose on alcohol intake in either sex (Table 5).
Table 5.
No Effect of single, acute administration of Mesyl Salvinorin B (1 or 3 mg/kg) on 15% alcohol intake after chronic 3-week drinking-in-the-dark (DID) in male and female mice.
| Sex | Male (n = 6) | Female (n = 6) | ||||
|---|---|---|---|---|---|---|
| Treatment | vehicle | 1 mg/kg | 3 mg/kg | vehicle | 1 mg/kg | 3 mg/kg |
| Alcohol intake (g/kg/4h) | 5.1 ± 0.52 | 4.9 ± 0.55 | 4.5 ± 0.51 | 6.1 ± 0.53 | 5.9 ± 0.71 | 5.6 ± 0.75 |
2.7. No effect of single, acute administration of MSB on locomotor activity in male or female alcohol-naïve mice
We further tested whether MSB could induce sedation in mice at the dose required to attenuate alcohol drinking. The dose of 3 mg/kg and injection schedule (30 min before the test) were based on the above alcohol drinking experiments. There was no effect of 3 mg/kg MSB on locomotor activity in either sex (Table 6).
Table 6.
No effect of single, acute administration of Mesyl Salvinorin B (MSB, 3 mg/kg) on locomotor activity in alcohol-naïve male and female mice. Locomotor activity is assessed as the number of “crossovers,” defined as breaking the light beams at either end of the conditioning chamber.
| Sex | Male (n = 8) | Female (n = 8) | ||
|---|---|---|---|---|
| Treatment | vehicle | MSB | vehicle | MSB |
| Crossover (30 min) | 231 ± 53 | 187 ± 28 | 277 ± 59 | 234 ± 42 |
3. DISCUSSION
Our main objective in the present study was to investigate the potential of KOP-r activation in reducing alcohol consumption in mice after chronic escalation alcohol drinking. We used the systemically active, potent KOP-r agonist MSB to study these effects because of its resistance to metabolism and thereby longer-lasting effects relative to its parent compound Sal A (Fig S4) as well as recent reports of its anti-addiction effects in cocaine-seeking behavior [Simonson et al 2015]. Acute administration of MSB significantly reduced alcohol intake in both males and females in a dose-dependent manner (0.3–3 mg/kg) (Fig 2A). It is unlikely that the effect of MSB in reducing alcohol intake was secondary to a general suppression of appetitive (anhedonic effect) or consumption behaviors, since no tested doses of MSB affected – even minimally – sucrose (caloric reinforcer) or saccharin (non-caloric reinforcer) consumption or preference (Table 4). Further, the MSB-induced reduction of alcohol intake was coupled with a full compensatory increase in water intake, resulting in no change in total fluid intake. Consistently, MSB (0.3–3 mg/kg) produced a dose-dependent reduction in alcohol preference in both sexes (Fig 2B). In the present study, we further confirmed that MSB reduced alcohol drinking in a KOP-r dependent manner, as the selective KOP-r antagonist nor-BNI blocked the effects of MSB on alcohol drinking in female mice (Table 3). Together, these results clearly demonstrate that the MSB-induced activation of KOP-r may play a role in modulating alcohol drinking in mice. This finding is in line with results showing that MSB, acting through the KOP-r, blocked drug-induced reinstatement of cocaine-seeking behavior in rats [Simonson et al 2015].
Acute administration of MSB at 3 mg/kg suppressed alcohol drinking, without inducing sedation (as reflected by spontaneous locomotor activity) (Table 6). Our result is similar to that of a recent study using a functionally selective KOP-r agonist [Brust et al 2016], but different from many traditional KOP-r agonists with sedative effects. This result in mice is also consistent with the study by Simonson et al in 2015 showing no alteration of locomotor activity in rats after acute administration of MSB. In this report, a tail-flick assay performed in mice after intrathecal administration of MSB displayed a signi cant analgesic effect with a longer duration than that of Sal A. The present study showed a relatively long duration (4 hours) of the effect of MSB on alcohol drinking behavior (Fig 1A1), which is likely due to its increased metabolic stability and bioavailability in vivo (half-life is approximately 4 h) compared to that of Sal A [Simonson et al 2015]. Of note, MSB signi cantly reduced alcohol drinking with a similar profile to NTN (Fig 1A2), a reference compound with a relatively long duration of action (half-life is approximately 8 h) in reducing alcohol drinking in our mouse model. Therefore, the development of new KOP-r agonists with reduced side effects and improved pharmacokinetics may have the potential to yield useful compounds for the treatment of cocaine addiction, pain and alcoholism [Prisinzano 2005; Prisinzano and Rothman 2008; Chavkin 2011; Brust et al 2016].
In contrast to animals in the 4-hour limited-access DID paradigm, which tended to have rather stable alcohol intake at 5–6 g/kg-day, animals in the 24-hour intermittent access CED paradigm started off with higher levels of intake (~10 g/kg/day) before almost doubling their intake over 3 weeks to 15–20 g/kg/day in males and 20–25 g/kg/day in females (Table 1). This rapid escalation of intake over the course of several days, paired with a progressive elevation of alcohol preference, is consistent with previous studies in rats and mice [Sillaber et al 2002; Simms et al 2008; Hwa et al 2011] and analogous to the loss of control over drug-taking following long-term access to voluntary alcohol drinking in human alcoholics [Koob and Kreek 2007]. For this reason we purposely examined effects of MSB (1 or 3 mg/kg) in both sexes after CED and found that it reduced intake (Fig 1A1), while it had no effect after DID (Table 5). Our results suggest that the KOP-r agonism of MSB modulates the escalation of alcohol drinking in mice with chronic exposure to alcohol and high levels of intake (~20 g/kg/day), but not in mice with limited access to alcohol and low levels of intake (5–6 g/kg/day). Escalation of drug intake is widely considered a hallmark of the transition from alcohol abuse to addiction in humans. MSB reliably lowered intake in mice following a rapid escalation in their drinking, with an improved pharmacokinetic profile and fewer side effects compared to Sal A. Thus, our rodent study provides new information about the medical potential of KOP-r agonist MSB in the treatment of alcoholism.
Chronic high levels of alcohol consumption may activate endogenous dynorphin/KOP-r systems in neuronal structures related to alcohol dependence or compulsivity. For example, it has been shown that mice lacking dynorphin consume more alcohol [Rácz et al 2013]. Pharmacological studies also support this notion: the selective KOP-r antagonist nor-BNI enhances voluntary alcohol consumption in a continuous two-bottle choice paradigm [Mitchell et al 2005], and more recent work has further demonstrated that KOP-r activation inhibits GABAergic synaptic responses and alcohol effects in the CeA [Kang-Park et al 2013]. Additionally, classic KOP-r agonists, such as U50,488H, U69,593 and Sal A, have all been shown to cause a decrease in dopamine levels in the NAc [e.g, Spanagel et al 1990]. Acute alcohol exposure leads to increased dopamine levels in the NAc, leading to rewarding effects, and KOP-r agonists oppose these effects by modulating the dopamine system [Lindholm et al 2007], suggesting a potential interaction between dopamine in the NAc and KOP-r activation by MSB in alcohol-related behavior. However, there are a number of studies that have found that classic KOP-r agonists increase alcohol drinking and reward [Anderson et al 2016; Rose et al 2016; Sperling et al 2010], and induce alcohol-seeking behavior in a reinstatement model [Funk et al 2014]. Therefore, our new data that the KOP-r agonist MSB reduces alcohol intake presents a scenario that is opposite to the results of previous studies using classic KOP-r agonists, thus warranting further study.
The expression of anhedonia in mice after exposure to chronic stress can be measured by sucrose consumption [Lim et al 2012]. Our results showed that MSB at 3 mg/kg (the most effective dose tested for reducing alcohol intake) did not affect sucrose preference or consumption after chronic alcohol exposure (Table 4), and MSB did not produce conditioned place aversion in rats (unpublished data). Together, these support the notion that MSB exhibits different signaling properties than traditional KOP-r agonists with anhedonic, dysphoric or drug-seeking effects (like U50,488H and U69,593) [e.g., Funk et al 2014]. Our present study is in line with growing research into the identification of functionally selective (i.e. biased) KOP-r ligands for the development of anti-addictive compounds with few side effects [Simonson et al 2015; White et al 2015; Brust et al 2016].
It is well documented that selective KOP-r antagonists attenuate alcohol consumption and alcohol-seeking behavior in male rodents. Indeed, we observed a reduction of alcohol drinking with nor-BNI in male mice, which replicated the above findings (Fig S1). Of note, there are sex differences in alcohol drinking behavior [Becker and Koob 2016] and in dynorphin/KOP-r systems [Chartoff and Mavrikaki 2015], as the nor-BNI treatment had no effect on alcohol drinking in female mice observed in the present study (Fig S1). It is intriguing that both male and female mice had similar responses to MSB (Fig 2). To our knowledge, this is the first description of using KOP-r agonist MSB with NTN and antagonist nor-BNI on alcohol drinking in both sexes side by side.
MSB is a highly selective KOP-r agonist, with a binding affinity of 2 nM at KOP-r and 6800 nM at MOP-r [Simonson et al 2015]. One concern about the use of MSB is that its KOP-r agonist activity after repeated administration could result in tolerance (specifically a gradual reduction of its effect on decreasing alcohol intake). Indeed, while acute administration of MSB at 3 mg/kg reduced alcohol drinking in the first drinking test session, repeated treatments of MSB at this high dose during 5 consecutive sessions showed a blunted effect in the 4th and 5th drinking sessions (Fig 4). By lowering the dose of MSB while co-administering a MOP-r antagonist such as NTN, the development of tolerance may be minimized. Given that NTN therapies have been used extensively in the treatment of alcoholism [Müller et al 2014], NTN and the potent KOP-r agonist MSB are ideal candidates for investigating the potential benefit of combined treatments. NTN is a selective MOP-r antagonist, with binding affinity of 0.89 nM at MOP-r versus 20 nM at KOP-r. Due to its high selectivity for the MOP-r and the low doses used in our studies, we predicted that NTN’s activity at the KOP-r would not interfere with MSB’s agonism at KOP-r. In fact, our results clearly demonstrated that the combination of NTN and MSB together could have a synergistic effect on reducing alcohol intake and preference. Indications that this MSB + NTN combination is more effective and potentially more beneficial in reducing alcohol intake than either drug alone include: (1) the effects of these combined, low-dose administrations of MSB + NTN on alcohol drinking were greater than those of either drug alone (Fig 2); and (2) the combination showed persistent effects after repeated administrations, with less tolerance than the MSB alone (Fig 5, Fig S3). Finally, the specific effect of the combination on alcohol was supported by the lack of any effect on sucrose or saccharin consumption (Table 4).
As the effectiveness of this MSB + NTN combination could involve multiple neuro-pharmacological mechanisms (at least KOP-r and MOP-r), it is possible that the two drugs are synergistic when administered together. Indeed, neurobiological studies have found supportive observations, given the multiple actions of alcohol in the CNS and that both the MOP-r and KOP-r systems are profoundly altered by chronic alcohol exposure [Zhou and Kreek 2014]. NTN's actions are suggested to be mediated through blockade of MOP-r in the NAc that may prevent alcohol-induced dopamine release, as well as through a dopamine-independent mechanism [Unterwald 2008; Marchant et al 2016]. Therefore, the enhanced KOP-r activity by MSB may synergistically enhance NTN’s effects on either dopamine or other neurotransmitters (e.g., GABA as discussed above briefly).
Although NTN is more effective in people with alcoholism who have MOP-r variant A118G [Anton et al 2008], this and other single-pathway targeted pharmacotherapies (e.g., acamprosate on NMDA receptors) have shown modest therapeutic value over placebo, indicating a need for greater efficacy [Müller et al 2014]. By targeting multiple neurotransmitter pathways implicated in different components of alcohol addiction, combination medications are likely to have enhanced efficacy over the traditional single-medication approach. Indeed, our study in rodent alcohol escalation drinking models suggests that the combination of MSB with NTN may be more efficacious in treating alcoholism than NTN alone. There are several precedents to test the combinations of NTN with other compounds, like acamprosate [Heyser et al 2003] or prazosin [Froehlich et al 2013]. In comparison with those combinations, this new combination with MSB showed strong synergistic effects on reducing alcohol consumption, although this could be attributed to different animal models used in different laboratories. Together, consistent with a recent study on cocaine seeking in rats [Simonson et al 2015], our findings have shown further promising in vivo data indicating that the KOP-r agonist MSB, in combination with NTN, may offer novel strategies to treat alcoholism, without traditional dysphoric properties of classic KOP-r agonists.
4. EXPERIMENTAL PROCEDURE
4.1. Animals
Male and female adult C57BL/6J (B6) mice (8 weeks of age) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in a temperature-controlled room (21 ºC). Mice were placed on a 12-hour reverse light-dark cycle (lights off at 7:00 am) upon arrival, and acclimated for a week prior to testing. During the first day of testing (i.e., session 1), all mice were 9 weeks of age. Mice were individually housed in ventilated cages fitted with steel lids and filter tops and given ad libitum access to food and water. Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences 1996), and were approved by the Institutional Animal Care and Use Committee of the Rockefeller University.
4.2. Apparatus for locomotor study
The experiments were performed in a place conditioning apparatus (model ENV-3013, Med Associate), which is a PVC plastic rectangular chamber that consists of dimly lit and sound attenuated chambers (16.8×12.7×12.7 cm).
4.3. Materials
The KOP receptor agonist Mesyl Salvinorin B (MSB) was synthesized from Sal A as described previously [Harding et al 2005], and dissolved in 1% DMSO. Ethanol solutions (7.5%, 15% and 30% v/v) were prepared from 190 proof absolute ethyl alcohol (Pharmco-AAPER, Brookfield, CT, USA) and dissolved in tap water. Sucrose and saccharin were purchased from Sigma-Aldrich Inc. (St. Louis, MO) and diluted in tap water. Naltrexone was purchased from Sigma-Aldrich Inc. and dissolved in physiological saline. Nor-BNI was obtained from the NIDA Division of Drug Supply and Analytical Services and dissolved in saline.
4.4. Procedures
4.4.1. Chronic Escalation Drinking (CED)
This model of alcohol drinking in C57BL/6J mice has been widely used for several years by many laboratories [e.g., Hwa et al 2011].
4.4.1.1. The 3-week CED model
Mice had access to alcohol drinking in their home cages for 3 weeks. Food and water were available at all times in this two-bottle choice paradigm with chronic alcohol exposure every other day. This CED model was similar to earlier protocols [e.g., Hwa et al 2011], with our modifications: At the time when the mice started individual housing (1 week before the experiments), the original water bottles were exchanged for those with sipper tubes to acclimate the mice to the sipper tubes (without ball bearings). Starting at 3 hours after lights off (10:00 am), both the water and alcohol (7.5%, 15% or 30%) solution sipper tubes were placed on their home cages. These sipper tubes were 10-ml pipettes that were cut at both ends, sealed with a rubber stopper, and fitted with a stainless steel straight sipper tubing. The sipper tubing contained a ball bearing at the end to prevent alcohol leakage. The left right position of the tubes was randomly set to avoid the development of side preference. The alcohol pipettes were refilled with fresh alcohol solution, and left for 24 hours before being replaced with the water tubes. Alcohol solutions were prepared fresh every 48 hours by mixing alcohol with tap water to reach the appropriate (v v) alcohol concentration. Body weights were recorded every 2 days. Alcohol and water intake values were recorded after 4, 8 and 24 hours of alcohol access in the drinking days (to the nearest 0.1 ml). These data were used to calculate consumed alcohol intake (i.e., g kg) and relative preference for alcohol (i.e., alcohol intake total fluid intake).
The mice randomized as the vehicle-treated and drug-treated groups in each sex had matched body weight and similar alcohol intake 24 hours before the test day. The drug dissolved in vehicle was administered by an experimenter, blinded to the treatments given to the experimental groups.
4.4.1.2. Single, acute administration in the 3-week CED model
On the test day, alcohol (7.5%, 15% or 30% concentrations) was presented 30 min after a single injection of MSB (0.3, 1, or 3 mg/kg in 1% DMSO, i.p.) or vehicle, and then alcohol and water intake values were recorded. Similarly, the effect of naltrexone (NTN, 0, 0.3, 1 or 2 mg/kg) on alcohol drinking was evaluated after CED. On the test day, alcohol (only 15% concentration) was presented 10 min after a single injection of NTN or vehicle (saline). The combined effects of MSB (0.1, 0.3 or 1 mg/kg) and NTN (0, 0.3 or 1 mg/kg) were evaluated on alcohol drinking after CED. On the test day, the mice received an i.p. injection of MSB or vehicle (1% DMSO) followed by the second i.p. injection of NTN or saline 20 min later. Then 15% alcohol was presented 10 min after NTN or vehicle.
Lastly, we used selective KOP-r antagonist nor-BNI to pharmacologically block the KOP-r to confirm that the MSB effects were mediated via KOP-r. Female mice were pretreated with nor-BNI (5 mg/kg) in saline (i.p.) 1 day before the 24-h drinking test, followed by one MSB (3 mg/kg) or vehicle (1% DMSO) injection 30 min before the drinking test. The rationale behind the exclusive use of females in this experiment came from our pilot study, in which 5 mg/kg nor-BNI had no effect on alcohol drinking in females, while it significantly decreased alcohol drinking in males (see the effect of nor-BNI at 5 mg/kg on drinking behavior in both males and females after 3-week CED in Supporting Information, Results and Fig S1).
4.4.1.3. Repeated administration in the 3-week CED model
After 3 weeks of CED (15% alcohol vs. water), the males and females were assigned to test or vehicle groups. In this experiment, males or females received 5 consecutive administrations of vehicle, MSB (3 mg/kg), or the MSB + NTN combination (0.3 mg/kg MSB + 1 mg/kg NTN) every other day immediately before drinking sessions (total 5 sessions). In each session, alcohol (15%) was presented after i.p. injections of test compound(s) or vehicle. Alcohol and water intake values were recorded after 4, 8 and 24 hours of alcohol access.
We did not observe any sex differences in the dose-response effects of MSB alone or in combination with NTN after either single or repeated administration in the above experiments, suggesting that the estrous cycle and associated hormones might not be important factors in the response to these treatments in females. As females achieved a high alcohol intake of 15% alcohol more easily than males, the efficacy of repeated administration of MSB was tested in females only after 3 weeks of CED.
4.4.2. Chronic (3-week) drinking-in-the-dark (DID)
This model of alcohol drinking in C57BL/6J mice has been widely in use by many laboratories [e.g., Rhodes et al 2005]. In this one-bottle paradigm, alcohol exposure was every day. One recording was taken per day (after 4 hours of alcohol access in the dark cycle). This chronic DID model was similar to earlier protocols [Rhodes et al 2005], with our modifications; The procedures were identical to the above CED with the following exceptions: Starting at 3 hours after lights off (10:00 am), the water bottle was replaced with one 10-ml alcohol (15%) pipette, and left for 4 hours before being replaced with the water bottle. Alcohol intake values were recorded after 4 hours of alcohol access every day (to the nearest 0.1 ml). These data were used to calculate alcohol intake (i.e., g kg). After 3 weeks of DID, the mice assigned to the vehicle-treated and MSB-treated groups with matched body weight had similar 15% alcohol intake 1 day before the test day. The MSB doses (1 and 3 mg/kg) were chosen based on the results of the above CED alcohol study. On the test day, alcohol (15% concentration) was presented 30 min after a single injection of MSB or vehicle. Control groups: males or females experienced DID for 3 weeks, and received one vehicle injection (1% DMSO, i.p.) before the 4-h drinking test. Test groups: males and females experienced DID for 3 weeks, and received one MSB injection (1 or 3 mg/kg in 1% DMSO, i.p.) before the test.
4.4.3. Sucrose (caloric reinforcer) and saccharin (non-caloric reinforcer) drinking
Using the same doses, the specificity of the action of MSB combined with NTN on alcohol intake was further tested using sucrose or saccharin drinking behavior after single, acute administration of the combination following CED. The sucrose preference test in mice is sensitive to the function of brain aversion systems and is widely used to measure the expression of anhedonia after chronic stress [Lim et al 2012]. In the following experiments, 15% alcohol CED exposure was identical to those in the above experiment as described in section 4.4.1.1. After 3 weeks of CED, the alcohol tube was switched to sucrose for 3 sessions with stable intakes. The mice assigned to the vehicle-treated or MSB-treated groups had similar sucrose intake 24 hours before the test day. On the test day, sucrose (4%, 8% or 16%) and water intake values were recorded after 4, 8 and 24 hours of sucrose access. In parallel separate experiments, saccharin drinking (0.1%, 0.2% or 0.4%) was tested after 3 weeks of CED with an identical procedure.
4.4.3.1. Single, acute administration of MSB (3 mg/kg) on sucrose or saccharin drinking after 3-week CED
A single i.p. injection of MSB (3 mg/kg) in 1% DMSO or vehicle was given 30 min before the sucrose and saccharin solutions were presented. Mice were assigned to one of four treatment groups: males or females with vehicle as control; and males or females with MSB.
4.4.3.2. Single, acute administration of MSB (0.3 mg/kg) combined with NTN (1 mg/kg) on sucrose or saccharin drinking after 3-week CED
On the test day, the mice received the first i.p. injection of MSB in 1% DMSO followed by the second i.p. injection of NTN in saline 20 min later. Mice were assigned to one of four treatment groups: males or females with vehicle as control; males or females with MSB + NTN
4.4.3.3. Single, acute administration of MSB (3 mg/kg) on sucrose or saccharin drinking in alcohol-naïve mice
The procedures were identical to the above acute experiment, except the mice were exposed to 4% sucrose or 0.1% saccharin only.
4.4.4. Single, acute administration of MSB (3 mg/kg) on locomotor activity in alcohol-naïve mice
Two groups of mice (n = 8) in each sex were studied and one group at each dose: 0 or 3 mg/kg MSB. The dose and injection schedule chosen were based on the above alcohol experiments. Thirty min after MSB or saline injection, mice were placed into the appropriate chamber (white or black) for 30 min, and locomotor activity was assessed as the number of “crossovers,” defined as breaking the beams at either end of the conditioning chamber. Half the animals from each group were assigned to the white chamber and half to the black ones.
4.4.5. Blood ethanol concentration (BEC) measurement
At the time of decapitation (after 4-hour access to alcohol), trunk blood from each mouse was collected in EDTA-containing tubes, placed on ice, and spun in a centrifuge at 4 °C. Plasma alcohol levels were assayed with the EnzyChrom kit (BioAssay Systems).
4.5. Data analysis
We performed power analyses to determine the number of animals required to provide statistically significant results, based on the levels of differences seen previously [Zhou et al 2011, 2013b], and predicted that these studies require 6–8 animals per group. In CED experiments, group differences in intake and preference ratio were analyzed using 3-way ANOVA with repeated measures for session (session 1, session 10), time interval (0–4, 5–8 vs. 9–24), and sex (male, female) followed by post-hoc tests. In the experiments with single MSB, NTN, their combinations or nor-BNI, alcohol (or sucrose, saccharin) intake, water intake, total fluid and preference ratio differences in each sex across the different groups were analyzed using 2-way ANOVA with repeated measures for treatment (vehicle vs drug) and for time interval (0–4, 5–8 vs. 9–24) followed by post-hoc tests. In the experiments with nor-BNI + MSB, alcohol intake, water intake, total fluid and preference ratio differences in each sex across the different groups were analyzed using 2-way ANOVA for pretreatment (vehicle vs nor-BNI) and for treatment (vehicle vs MSB) followed by Newman-Keuls post-hoc tests. For dose response analysis on MSB alone and MSB + NTN combinations, group differences for alcohol intake and preference ratios at the 4-hour recording time were analyzed using 2-way ANOVA for treatments with different doses and for sex (male vs. female) followed by Newman-Keuls post-hoc tests. In experiments with repeated administrations of MSB, group differences on alcohol intake and preference ratios were analyzed using 2-way ANOVA with repeated measures for treatment (vehicle vs drug) and for session (1, 2, 3, 4 vs 5, or 1 vs 5) followed by post-hoc tests. In experiments with repeated administrations of MSB + NTN, group differences on alcohol intake and preference ratios were analyzed using 1-way ANOVA for treatment (baseline, session 1, 2, 3, 4 vs 5) followed by Newman-Keuls post-hoc tests. The accepted level of significance for all tests was p < 0.05. All statistical analyses were performed using Statistica (version 5.5, StatSoft Inc, Tulsa, OK).
Supplementary Material
Acknowledgments
This work was supported by NIH AA021970 (YZ), DA018151 (TEP), GM008545 (RSC) and AFPE Pre-Doctoral Fellowship in Pharmaceutical Sciences (to RSC), and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (MJK). The authors would like to thank Benjamin Neuenswander for his technical assistance in the microsomal stability studies. Special thanks to Dr. R. Schaefer for providing his comments and corrections on the manuscript.
Footnotes
Contributors: YZ designed the study, wrote the protocol, managed the literature searches and analyses, undertook the behavioral study, conducted statistical analysis, and wrote the manuscript. RSC, TEP synthesized Mesyl Sal B and wrote the manuscript. KB, MJK contributed to the final versions of the manuscript. All authors have approved the final manuscript.
Conflict of interest: All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RI, Lopez MF, Becker HC. Stress-Induced enhancement of ethanol intake in C57BL/6J mice with a history of chronic ethanol exposure: involvement of kappa opioid receptors. Front Cell Neurosci. 2016;10:e45. doi: 10.3389/fncel.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aubé J, Jones SR, Martin TJ, Bohn LM. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal. 2016;9:ra117. doi: 10.1126/scisignal.aai8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Mavrikaki M. Sex Differences in Kappa Opioid Receptor Function and Their Potential Impact on Addiction. Front Neurosci. 2015;9:466. doi: 10.3389/fnins.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., JR Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C. The therapeutic potential of kappa-opioids for treatment of pain and addiction. Neuropsychopharmacology. 2011;36:369–370. doi: 10.1038/npp.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario C, Caputi FF, Rimondini R, Gandolfi O, Del Borrello E, Candeletti S, Romualdi P. Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict Biol. 2013;13:425–433. doi: 10.1111/j.1369-1600.2011.00326.x. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr, McKinzie DL, Carroll FI, McBride WJ, Rodd ZA. The long-lasting effects of JDTic, a kappa opioid receptor antagonist, on the expression of ethanol-seeking behavior and the relapse drinking of female alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2012;101:581–587. doi: 10.1016/j.pbb.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Rasmussen DD. Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcohol Clin Exp Res. 2013;37:1763–1770. doi: 10.1111/acer.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Lê AD. The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav. 2014;4:356–367. doi: 10.1002/brb3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. Neoclerodane diterpenes as a novel scaffold for mu opioid receptor ligands. J Med Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- Henderson-Redmond A, Czachowski C. Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology (Berl) 2014;231:4309–4321. doi: 10.1007/s00213-014-3571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on alcohol deprivation effect in rats. Neuropsychopharmacology. 2003;28:1463–1471. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% alcohol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Kappa opioid receptors in the central amygdala regulate ethanol actions at presynaptic GABAergic sites. J Pharmacol Exp Ther. 2013;346:130–137. doi: 10.1124/jpet.112.202903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM. The one-two punch of alcoholism: role of central amygdala dynorphin/kappa-opioid receptors. Biol Psychiatry. 2014;75:774–782. doi: 10.1016/j.biopsych.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MP, Gianoulakis C. Effects of corticotropin-releasing hormone receptor antagonists on the ethanol-induced increase of dynorphin A1-8 release in the rat central amygdala. Alcohol. 2011;45:621–630. doi: 10.1016/j.alcohol.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Frank J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120:137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Rosin A, Dahlin I, Georgieva J, Franck J. Ethanol alters the effect of kappa receptor ligands on dopamine release in the nucleus accumbens. Physiol Behav. 2007;92:167–171. doi: 10.1016/j.physbeh.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Blockade of ethanol reward by the kappa opioid receptor agonist U50,488. Alcohol. 2009;43:359–365. doi: 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, Hope BT, Heins RC, Prisinzano TE, Vardy E, Bonci A, Bossert JM, Shaham Y. Role of ventral subiculum in context-induced relapse to alcohol seeking after punishment-imposed abstinence. J Neurosci. 2016;36:3281–3294. doi: 10.1523/JNEUROSCI.4299-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30:982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182:384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Morani AS, Kivell B, Prisinzano TE, Schenk S. Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav. 2009;94:244–249. doi: 10.1016/j.pbb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CA, Geisel O, Banas R, Heinz A. Current pharmacological treatment approaches for alcohol dependence. Expert Opin Pharmacother. 2014;15:471–481. doi: 10.1517/14656566.2014.876008. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- O’Malley SS, Jaffe A, Chang G, Schottenfeld RS, Meyer RE, Rounsaville BJ. Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley S, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamic-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Prisinzano TE. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci. 2005;78:527–531. doi: 10.1016/j.lfs.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Prisinzano TE, Rothman RB. Salvinorin A analogs as probes in opioid pharmacology. Chem Rev. 2008;108:1732–1743. doi: 10.1021/cr0782269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rácz I, Markert A, Mauer D, Stoffel-Wagner B, Zimmer A. Long-term ethanol effects on acute stress responses: modulation by dynorphin. Addict Biol. 2013;18:678–688. doi: 10.1111/j.1369-1600.2012.00494.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR. Supersensitive kappa opioid receptors promotes ethanol withdrawal-related behaviors and reduce dopamine signaling in the nucleus accumbens. Int J Neuropsychopharmacol. 2016;19(5):1–10. doi: 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Borrell J, Guaza C. Involvement of kappa type opioids on ethanol drinking. Life Sci. 1988;42:1067–1075. doi: 10.1016/0024-3205(88)90562-0. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek MJ. Nalmefene causes greater hypothalamic–pituitary–adrenal axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcohol Clin Exp Res. 1998;22:1430–1436. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgänsberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson B, Morani AS, Ewald AW, Walker L, Kumar N, Simpson D, Miller JH, Prisinzano TE, Kivell B. Pharmacology and anti-addiction effects of the novel κ opioid receptor agonist Mesyl Sal B, a potent and long-acting analogue of salvinorin A. Br. J Pharmacol. 2015;172:515–531. doi: 10.1111/bph.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Unterwald EM. Naltrexone in the treatment of alcohol dependence. J Addict Med. 2008;2:121–127. doi: 10.1097/ADM.0b013e318182b20f. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL. The G protein-biased κ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352:98–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Carai MA, Ho A, Gessa GL, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcohol Clin Exp Res. 2011;35:1876–1883. doi: 10.1111/j.1530-0277.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Gessa GL, Kreek MJ. Effects of voluntary alcohol drinking on corticotropin-releasing factor and preprodynorphin mRNA levels in the central amygdala of Sardinian alcohol-preferring rats. Neurosci Lett. 2013a;554:110–114. doi: 10.1016/j.neulet.2013.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Grella S, Aldrich J, Kreek MJ. Involvement of dynorphin and kappa opioid receptor in yohimbine-induced reinstatement of heroin seeking in rats. Synapse. 2013b;67:358–361. doi: 10.1002/syn.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kreek MJ. Alcohol: a stimulant activating brain stress responsive systems with persistent neuroadaptation. Neuropharmacology. 2014;87:51–58. doi: 10.1016/j.neuropharm.2014.05.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.