Abstract
Background
Epipactis helleborine is an Eurasian orchid species which prefers woodland environments but it may also spontaneously and successfully colonise human-made artificial and disturbed habitats such as roadsides, town parks and gardens. It is suggested that orchids colonising anthropogenic habitats are characterised by a specific set of features (e.g., large plant size, fast flower production). However, as it is not well known how pollinator diversity and reproductive success of E. helleborine differs in populations in anthropogenic habitats compared to populations from natural habitats, we wanted to compare pollinator diversity and reproductive success of this orchid species between natural and anthropogenic habitat types.
Methods
Pollination biology, reproductive success and autogamy in populations of E. helleborine from anthropogenic (roadside) and natural (forest) habitats were compared. Eight populations (four natural and four human-disturbed ones) in two seasons were studied according to height of plants, length of inflorescences, as well as numbers of juvenile shoots, flowering shoots, flowers, and fruits. The number and diversity of insect pollinators were studied in one natural and two human-disturbed populations.
Results
Reproductive success (the ratio of the number of flowers to the number of fruits) in the populations from anthropogenic habitats was significantly higher than in the natural habitats. Moreover, plants from anthropogenic habitats were larger than those from natural ones. In both types of populations, the main insect pollinators were Syrphidae, Culicidae, Vespidae, Apidae and Formicidae. With respect to the type of pollinators’ mouth-parts, chewing (39%), sponging (34%) and chewing-sucking (20%) pollinators prevailed in anthropogenic habitats. In natural habitats, pollinators with sponging (55%) and chewing mouth-parts (32%) dominated, while chewing-sucking and piercing-sucking insects accounted for 9% and 4% respectively.
Discussion
We suggest that higher reproductive success of E. helleborine in the populations from anthropogenic habitats than in the populations from natural habitats may result from a higher number of visits by pollinators and their greater species diversity, but also from the larger size of plants growing in such habitats. Moreover, our data clearly show that E. helleborine is an opportunistic species with respect to pollinators, with a wide spectrum of pollinating insects. Summarising, E. helleborine is a rare example of orchid species whose current range is not declining. Its ability to make use of anthropogenically altered habitats has allowed its significant spatial range expansion, and even successful colonisation of North America.
Keywords: Apophytes, Anthropogenic habitats, Natural habitats, Autogamy, Insect pollinators
Introduction
Orchidaceae is one of the most diverse and species-rich (20,000–30,000 species) plant families (Baumann, Kunkele & Lorenz, 2010; Djordjević et al., 2016a), with many species that are seriously endangered and require conservation efforts to maintain their populations.
Destruction of natural habitats is causing extinction of many orchid species (Swarts & Dixon, 2009). However, some orchid species, especially in temperate regions of Europe and North America, found anthropogenic habitats as suitable as natural ones (Pedersen, Watthana & Srimuang, 2013). A recent study of orchids in Turkey has indicated that graveyards are places where orchid species occur frequently (Löki et al., 2015). In addition, Djordjević et al. (2016b) have noted that Himantoglossum calcaratum, Anacamptis pyramidalis and Ophrys species often grow in habitats along the roads in western Serbia. Moreover, the same authors have shown that Orchis purpurea can be an indicator of ruderal habitat type. The most common colonisers of secondary habitats in Central Europe are Epipactis and Dactylorhiza species (Adamowski, 2004; Adamowski, 2006; Esfeld et al., 2008; Rewicz, Kołodziejek & Jakubska-Busse, 2016). Moreover, Jurkiewicz et al. (2001) noted that populations of Epipactis atrorubens, E. helleborine and Dactylorhiza majalis were observed on several mine tailings in southern Poland. Colonisation of such habitats is fuelled by disturbances of surface soil layers and exposure of deeper layers including bedrock (i.e., quarries where limestone or chalk are exposed). Additionally, the surface layer of soil and vegetation could be destroyed, which weakens the competitive potential of original vegetation (Adamowski, 2006). Orchids that colonize anthropogenic habitats are characterized by a specific set of features: fast growth resulting in large plant size and fast flower production (Forman et al., 2009).
An important aspect of orchid population biology is its unique reproductive system (Machaka-Houri et al., 2012), which in this case means mass production of very small and light seeds (from 0.31 µg to 24 µg, depending on the species) (Arditti, 1967). However, the high number of seeds does not lead to high recruitment of seedlings. Low reproductive success, which is defined as the ratio of the number of flowers to the number of fruits (Doust & Doust, 1988), may be caused by high level of morphological adaptation of flowers to particular pollinators. More than 60% of all orchid species are pollinated by a single pollinator (Tremblay, 1992; Tremblay et al., 2005).
Human disturbance, especially habitat transformation (including its impact on soil, moisture conditions, and changes of the floristic composition of plant communities), is regarded as a principal cause of pollinator decline on a global scale (Goulson, Lye & Darvill, 2008), as well as an important factor directly and indirectly changing the species structure of pollinating insects (Clemente, 2009). Deficiency of suitable pollinators may also be a reason for low reproductive success. Thus, autogamy (self-autonomy) is an alternative way of seed production. A question arises which reproductive system is preferred by orchids rapidly and successfully colonising anthropogenic habitats (Light & MacConaill, 2006). Epipactis helleborine can be a suitable model species, as it occurs in both natural and disturbed habitats, is able to undergo both auto- and allogamy and, furthermore, has been successfully established in North America. Ehlers, Olesen & Gren (2002) have investigated the reproductive success of E. helleborine in natural habitats; however, there is a lack of knowledge concerning the reproductive success and pollinator diversity of E. helleborine in relation to certain types of natural and anthropogenic habitats. Moreover, in the literature there are no detailed data about the diversity of insect pollinators of E. helleborine in relation to the type of their mouth-parts. Such information is important as some types of mouth-parts allow insects to collect food from morphologically different types of flowers, as a result such insects can be pollinators of many taxonomically different plant groups. In addition, insects with specialised mouth-parts can collect food only from one or two morphologically different types of flowers, which means that they can only be pollinators of a narrow number of plants (Gillott, 2005). Some orchid species have flowers which are morphologically adapted for nectar and pollen collecting by insects with different types of mouth-parts, while other can be pollinated only by insects with one, sometimes strongly specialised, type of mouth-parts.
In this study, we compared the reproductive success and pollinator diversity of E. helleborine from natural and anthropogenic habitats. Specifically, we addressed the following questions: (a) what is the composition of and the differences in the pollinator fauna of E. helleborine from anthropogenic and natural habitats in terms of insect diversity and diversity of insect mouth-parts; (b) does the number of capsules produced through autogamy and natural pollination differ between populations from anthropogenic and natural habitats; (c) is the reproductive success of E. helleborine different in populations from anthropogenic and natural habitats?
Materials and Methods
Studied species and study area
Epipactis helleborine (L.) Crantz (broad-leaved helleborine) is a Eurasian species (Kolanowska, 2013) introduced in the 19th century (Owen, 1879) to several regions of North America (Procházka & Velísek, 1983). It usually grows in deciduous and coniferous forest communities, on edges and in clearings in woodland, up to 2,000 m a.s.l (Delforge, 2006). Furthermore, this species inhabits different types of anthropogenic habitats such as: roadsides, cemeteries, poplar plantations, gravel pits, quarries, railway embankments, and mine tailings (Świercz, 2004; Świercz, 2006; Kiedrzyński & Stefaniak, 2011), and may also appear spontaneously in urban areas such as town parks and gardens (Kolanowska, 2013). Some studies have shown that E. helleborine is a species with a broad ecological tolerance, which is not highly specialised, and often acts as a pioneer in human-disturbed areas (Piękoś-Mirkowa & Mirek, 2006; Tsiftsis et al., 2008). Epipactis helleborine is thus a fine example of apophytism—i.e., a native species growing in disturbed or human-made habitats (Sukopp, 2006; Rewicz et al., 2015). Orchids that colonise anthropogenic habitats are characterised by a specific set of features: fast growth resulting in large plant size and fast flower production (Forman et al., 2009).
We accepted the definition of reproductive success after Doust & Doust (1988) as the ratio of the number of flowers to the number of fruits. All shoots found in each population were measured. The following parameters were recorded for each plant in a population: number of juvenile shoots (JS), number of flowering shoots (NFS), number of flowers (NF), number of fruits (capsules) (NFR), and height of plants (HP). Population size includes the total number of shoots (juvenile and flowering). The area occupied by a given population was determined by the most remote ramets of E. helleborine. Border posts indicating the surface area of the population were placed at intervals of one meter from each other, and then the edges of the study area were delineated using a piece of string. The population area measured using a square net was approximately 1 m2 (Table 1). Density of each population was also measured as shoots/m2. The consent to conduct the research was issued by the Regional Directorate of Environmental Protection in Białystok, permit no. WPN6400.74.2013.MW and the Ministry of the Environment—permit no. 35/17258/12/RS.
Table 1. Studied populations of Epipactis helleborine.
| Population code | Locality | Coordinates | Altitude (m asl) | Area (m2) | Population size (number of shoots) | Density (shoots/m2) |
|---|---|---|---|---|---|---|
| Anthropogenic habitats | ||||||
| A1* | roadside (Guszczewina) | N52.831600 E23.794836 | 148 | 36 | 127 | 3.53 |
| A2* | roadside (Hajnówka) | N52.734217 E23.603314 | 181 | 108 | 102 | 0.94 |
| A3 | roadside (Sulejów) | N51.353793 E19.883155 | 166 | 460 | 80 | 0.17 |
| A4 | roadside (Sulejów) | N51.349757 E19.882484 | 167 | 46 | 152 | 3.30 |
| Natural habitats | ||||||
| N1 | mixed forest (Kotowice) | N51.041241 E17.176701 | 128 | 100 | 300 | 3.00 |
| N2 | mixed forest (Kaczawskie Mts) | N50.963255 E15.963255 | 480 | 40 | 150 | 3.75 |
| N3* | mixed forest(Białowieża Primeval Forest) | N52.800743 E23.914125 | 51 | 120 | 34 | 0.28 |
| N4 | mixed forest (Białowieża Primeval Forest) | N52.7650022 E23.884316 | 44 | 400 | 41 | 0.10 |
Notes.
Asterix indicates populations where pollinator fauna were analyzed.
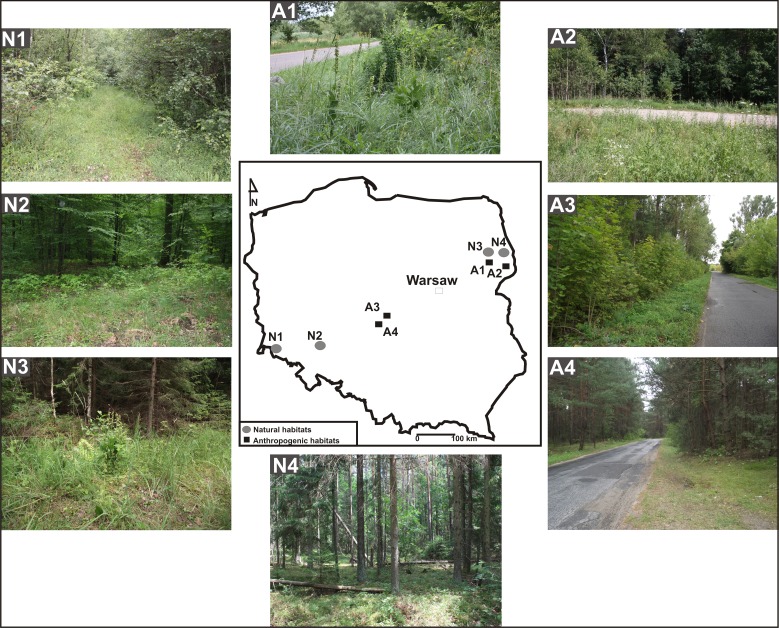
Eight populations of E. helleborine occurring in Poland were studied in two seasons, 2011 and 2012 (July—flowering and pollinators, September—seed capsules collection). The identified habitat types were divided in to two categories. One included human-disturbed habitats, such as roadsides (population A1—between a road and a wooden fence in the village of Guszczewina; A2—close to a car park in Hajnówka; A3—in a thicket by a roadside in Sulejów; A4—on a roadside bordering Pinus sylvestris forest in Sulejów) (Fig. 1). The other one grouped natural habitats: population N1—in a forest of Galio sylvatici-Carpinetum betuli Oberd. 1957 in Kotowice (Jakubska & Orlowski, 2003); N2—in a forest of Galio sylvatici-Carpinetum betuli Oberd. 1957 in Kaczawskie Mts. (Kwiatkowski, 2006); N3, N4—Galio sylvatici-Carpinetum betuli Oberd. 1957 in the Strict Reserve of the Białowieża Primeval Forest (Faliński, 2001) (Fig. 1, Table 1).
Figure 1. Habitats of E. helleborine.
A —anthropogenic habitat, N—natural habitat, A1—between a road and a wooden fence in the village of Guszczewina, A2—close to a car park in Hajnówka, A3—in a thicket by a roadside in Sulejów, A4— on a roadside bordering a Pinus sylvestris forest in Sulejów, N1—a forest of Galio sylvatici-Carpinetum betuli Oberd. 1957 in Kotowice, N2—a forest of Galio sylvatici-Carpinetum betuli Oberd. 1957 in Kaczawskie Mts., N3 and N4—Galio sylvatici-Carpinetum betuli Oberd. 1957 in the natural habitat (Strict Reserve of the Białowieża Primeval Forest).
The detailed review of the vascular flora of the studied habitats (the sample area was 30 m2) is presented in Table S1 . For each species recorded in this study, its relative frequency of occurrence was calculated (Brower & Zar, 1984). Frequency (Fi) was calculated according to the formula: Fi = (ji∕k) × 100%, where: j—the number of populations in which species ‘i’ was recorded, and k—the total number of populations (Table S1).
In total, 68 species of vascular plant species were recorded; 38 (ten species of trees) in natural and 38 (five species of trees) in anthropogenic habitats. The most frequently occurring plants in natural habitats were: Carpinus betulus, Veronica chamaedrys and Rubus sp., registered in all the study plots (Fi = 100%). The most frequently occurring plants in populations in anthropogenic habitats were: Achillea millefolium, Conyza canadensis, Dactylis glomerata, Galium aparine, Medicago lupulina, Poa annua, P. nemoralis and Vicia cracca.
Pollinators of E. helleborine
Pollinators were both caught and observed in two populations of E. helleborine in two anthropogenic habitats: a roadside in the village of Guszczewina (A1), and close to a car parking in the city of Hajnówka (A2), and in one population in a natural habitat—in the Białowieża Primeval Forest (N3) (Fig. 1, Table 1).
Insects from fresh orchid flowers were caught using entomological hand nets in two periods: 15.–23.07.2011 and 13.–22.07.2012. The fieldwork was carried out during days with sunny weather. In the anthropogenic (A1, A2) E. helleborine populations, the insects were caught by two two-people teams between 9 a.m. and 7 p.m. In the population (N3) from natural habitat, insects were caught by four people to get comparable hours effort between populations from anthropogenic and natural habitat. Insects were collected from 10 shoots (20 in the natural habitat) growing close to each other. Specimens were collected until the transfer of pollinia by pollinators was observed. Insects were killed using ethyl acetate and preserved in 75% ethanol (except bumble-bees Bombus spp. which are protected by Polish law—these specimens were only photographed). Insects which arrived with attached pollinia or departed the flower with pollinia were recognised as pollinators. The number of captured insects corresponded to the number of visits, with the exception of the Bombus species, for which only the number of visits was counted. Identification of the insects to order/family levels was done based on Gillott (2005), Hůrka (2005) and Oosterbroek (2006), while entomological nomenclature of insect mouth-parts was provided according to Gillott (2005).
The ability of E. helleborine to undergo autogamy
The autogamy experiment was carried out from July to September 2012. Ten shoots in the early stage of flowering (closed buds) were selected in each population for the autogamy experiment. Flowers on each shoot were counted and inflorescences were covered by bags made from a mosquito net. We also used ten control plants (not covered by mosquito-net bags). After three months, the isolators were removed and the number of fruit sets was counted. Viability of seed was examined by the tetrazolium test (live seed with stained embryos and dead seed with unstained embryos) (Van Waes & Debergh, 1986).
Data analysis
The software package STATISTICA PL. ver. 10 (StatSoft Inc, 2011) was used for all the statistical analyses (Van Emden, 2008). Diversity of pollinator fauna in natural and anthropogenic habitats was evaluated using the chi-squared test. To compare reproductive success between habitats, we used the Student’s t-test (we used values of individuals). Correlation between the number of flowers and the number of fruits (we used average) in inflorescence in different habitats was evaluated using the Spearman’s correlation The relationship between reproductive success and the height of plant and number of flowers was investigated by linear regression model (Meissner, 2010). To compare the number of fruits (capsules) produced by autogamy in different habitats (we used average) we used the Mann–Whitney Utest.
Results
Pollinators of E. helleborine
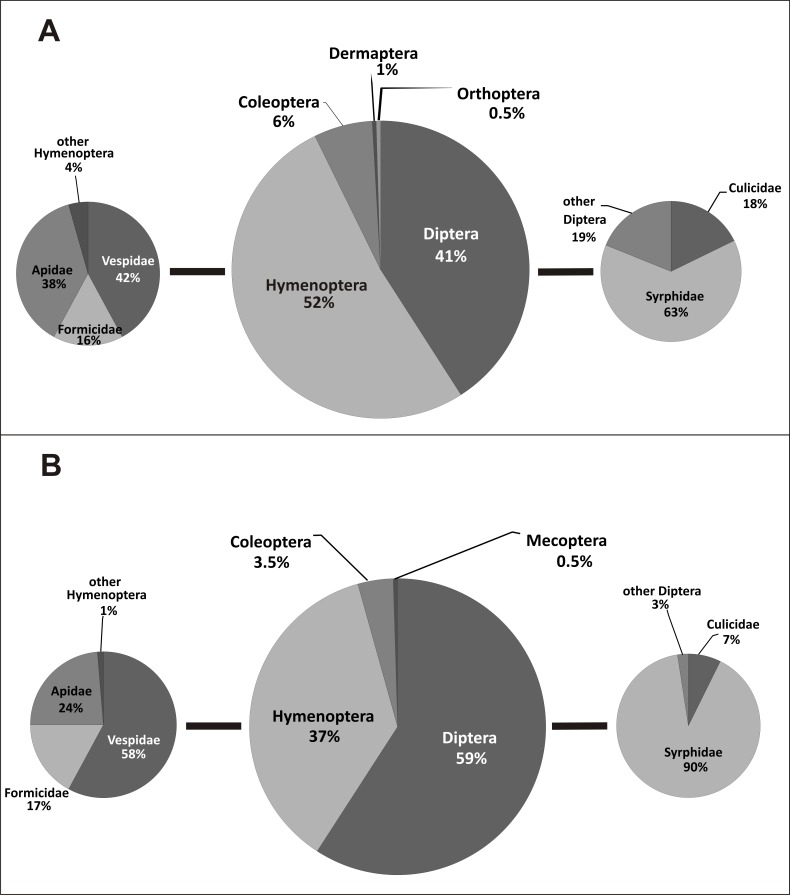
Pollinators of E. helleborine collected during this study belonged to six orders and 24 families of insects (Table 2, Fig. 2). In the case of the populations from the anthropogenic habitats, taxonomic diversity of pollinators was higher, with 19 families grouped in five orders, while in the natural habitats we noted only 14 families from four orders (statistically significant values, chi-squared test, χ2 = 0.001161, df = 6, p = 0.05). In the population from the natural habitat, the most frequent families were Syrphidae (111 visits) and Vespidae (44). In the populations from the anthropogenic habitats, the most frequent families were Syrphidae (57), Vespidae (48) and Apidae (43). In both types of habitats, Diptera and Hymenoptera clearly dominated, with 41% and 52% of all the pollinators observed in the populations from the anthropogenic habitats, and with 59% and 37% observed in the population from the natural habitat (Fig. 3). Coleoptera were the third main group of pollinators making up 6% of the populations from the anthropogenic habitats and 4% of the population from the natural habitat. Occasionally, single individuals of grasshoppers (Orthoptera) and earwigs (Dermaptera) were also noted as pollinators of E. helleborine in the populations from the anthropogenic habitats and scorpion flies (Mecoptera) in the population from the natural habitat.
Table 2. Pollinators of Epipactis helleborine in natural and anthropogenic habitats.
| Taxon of pollinator | Type of mouthparts | Habitat type | ||
|---|---|---|---|---|
| Order | Family | Natural | Anthropogenic | |
| Orthoptera | Acrididae | C | 0 | 1 |
| Dermaptera | Forficulidae | C | 0 | 1 |
| Diptera | Calliphoridae | S | 1 | 7 |
| Culicidae | PS | 9 | 16 | |
| Lauxanidae | S | 0 | 1 | |
| Muscidae | S | 1 | 3 | |
| Scathopagidae | S | 0 | 1 | |
| Sepsidae | S | 0 | 1 | |
| Syrphidae | S | 111 | 57 | |
| Tachinidae | S | 1 | 2 | |
| Tephritidae | S | 0 | 1 | |
| Tipulidae | S | 0 | 1 | |
| Mecoptera | Planorpidae | C | 4 | 0 |
| Hymenoptera | Apidae | CS | 18 | 43 |
| Formicidae | C | 13 | 18 | |
| Ichneumonidae | C | 1 | 4 | |
| Pamphiliidae | C | 0 | 1 | |
| Vespidae | C | 44 | 48 | |
| Coleoptera | Cantharidae | C | 0 | 10 |
| Cerambycidae | C | 1 | 0 | |
| Coccinellidae | C | 0 | 4 | |
| Elateridae | C | 2 | 0 | |
| Melyridae | C | 4 | 0 | |
| Nitidulidae | C | 1 | 0 | |
| Total | 208 | 220 | ||
Notes.
- C
- chewing
- S
- sponging
- PS
- piercing and sucking
- CS
- chewing and sucking
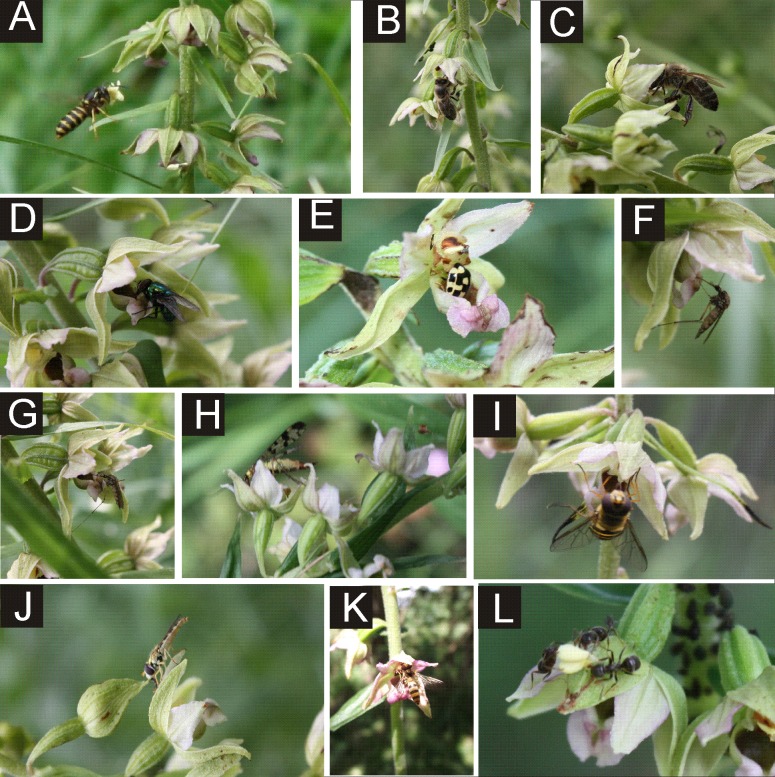
Figure 2. Pollinators of E. helleborine.
(A) wasp (Vespidae) with pollinia attached to its head, (B–C) –honeybees (Apidae), (D) carrion fly (Calliphoridae), (E) ladybird (Coccinellidae), (F–G) mosquito (Culicidae), (H) scorpionfly (Panorpidae), (I–K) hoverflies (Syrphidae), (L) ants (Formicidae) (photo: A Rewicz 2011/2012).
Figure 3. Taxonomic diversity of E. helleborine pollinators in anthropogenic (A) and natural (B) habitats.
In the populations from the anthropogenic habitats, the main dipteran pollinators were hoverflies (Syrphidae), making up 63% of dipteran pollinators and 26% of all the observed pollinators, with the most frequent species Meliscaeva cinctella and Episyrphus balteatus, followed by mosquitoes (Culicidae) (18% of dipteran and 7% of all the pollinators). The main hymenopteran pollinators were wasps (Vespidae—42% of hymenopteran and 22% of all the pollinators, with the most frequent species Dolichovespula saxonica), bees (Apidae—38% and 20%, respectively, with the main pollinator Apis mellifera), and ants (Formicidae—16% and 8% respectively) (Fig. 3). In the population from the natural habitat, true flies (Syrphidae) accounted for 90% (53% of all the pollinators; with Meliscaeva cinctella as the most frequent species), and mosquitoes (Culicidae) made up 7% of pollinators (4% of all the pollinators), while the main hymenopteran pollinators were wasps (Vespidae—58% of hymenopterans and 21% of all the pollinators; with Dolichovespula saxonica as the most frequent pollinator), bees (Apidae—24% and 9%, respectively), and ants (Formicidae—17% and 6%, respectively) (Fig. 3).
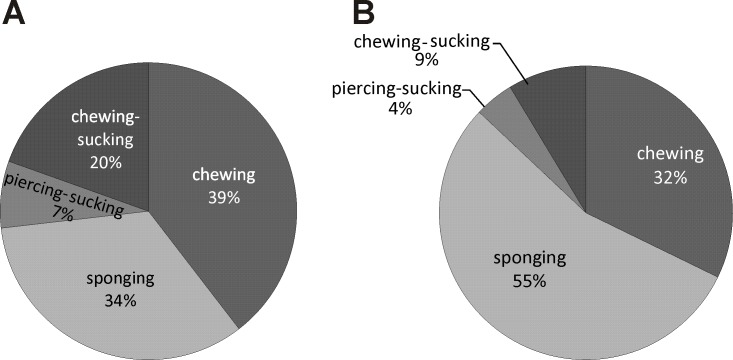
According to the type of mouth-parts, the pollinators of E. helleborine can be ascribed to four groups: 1/sponging insects (Diptera excluding Culicidae—44% of all the noted pollinators), 2/ chewing (= mandibulate) insects (Hymenoptera excluding Apidae, Coleoptera, Dermaptera, Orthoptera and Mecoptera—36%), 3/chewing-sucking insects (Apidae—14%), and 4/piercing and sucking insects (Culicidae—6%) (Fig. 4).
Figure 4. Diversity of E. helleborine pollinators in anthropogenic (A) and natural (B) habitats based on insect mouthparts.
In the population from the natural habitat, the main groups of pollinators of E. helleborine were sponging (55%) and chewing insects (32%), while the chewing-sucking and piercing and sucking insects made up respectively 9% and 4% of all the pollinators. In the populations from the anthropogenic habitats, the most frequent pollinators belonged to the groups of chewing (39%), sponging (34%), and chewing-sucking (20%) insects, and only 7% of the noted insects were characterised by piercing and sucking mouth-parts.
Reproductive success
Reproductive success in the populations from anthropogenic habitats (average from 2011 to 2012—87.1%) was significantly higher than in the populations from the natural habitats (average from 2011 to 2012—72.3%) (Student’s t-test, p = 0.02, df = 14). In the populations from the anthropogenic habitats, reproductive success ranged from 77.8% (A4—in 2011) to 100% (A2—2012), while in the populations from the natural habitats, it ranged from 44.4% (N1—2011) to 85.0.3% (N1—2012) (Table 3). The number of flowers in the populations from the natural habitats ranged from 20 (in 2011) to 22 (in 2012), while in the populations from the anthropogenic habitats from 15 (2011) to 14 (2012). Differences between habitats of the number of flowers were statistically significant (Student’s t-test, p = 0.03).
Table 3. Reproductive success of Epipactis helleborine in natural and anthropogenic habitats.
| Anthropogenic habitats | Natural habitats | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | D (n/m2) | HP | NFS | NF | NFR | RS | Site | D (n/m2) | HP | NFS | NF | NFR | RS |
| 2011 | 2011 | ||||||||||||
| A1 | 0.28 | 84.79 ± 24.04 | 39 | 20 ± 9.20 | 19 ± 9.48 | 95.00 ± 7.20 | N1 | 0.83 | 61.96 ± 17.91 | 25 | 18 ± 11.8 | 8 ± 7.80 | 44.40 ± 5.13 |
| A2 | 0.93 | 54.08 ± 15.84 | 26 | 19 ± 8.63 | 16 ± 7.83 | 84.20 ± 8.30 | N2 | 0.77 | 57.36 ± 15.99 | 30 | 18 ± 10.8 | 13 ± 8.36 | 72.20 ± 8.78 |
| A3 | 0.9 | 56.29 ± 11.80 | 22 | 12 ± 6.20 | 10 ± 5.92 | 83.30 ± 10.4 | N3 | 0.21 | 34.53 ± 15.27 | 28 | 18 ± 12.5 | 14 ± 11.99 | 77.80 ± 9.54 |
| A4 | 0.4 | 41.98 ± 12.64 | 38 | 9 ± 9.25 | 7 ± 9.21 | 77.80 ± 12.34 | N4 | 0.88 | 48.36 ± 23.60 | 33 | 24 ± 14.8 | 20 ± 13.15 | 83.30 ± 11.40 |
| Average | 0.63 | 59.28 | 31.25 | 15 | 13 | 85.08 | Average | 0.67 | 50.55 | 29 | 20 | 14 | 69.42 |
| 2012 | 2012 | ||||||||||||
| A1 | 0.38 | 87.65 ± 22.48 | 43 | 19 ± 8.37 | 18 ± 8.28 | 94.70 ± 6.54 | N1 | 1.24 | 66.67 ± 13.60 | 15 | 20 ± 6.34 | 17 ± 5.19 | 85.00 ± 7.45 |
| A2 | 0.77 | 64.3 ± 19.51 | 45 | 20 ± 14.1 | 20 ± 13.87 | 100.00 ± 10.30 | N2 | 0.97 | 62.15 ± 16.58 | 24 | 19 ± 11.4 | 14 ± 10.00 | 73.70 ± 6.78 |
| A3 | 2.39 | 56.0 ± 19.07 | 13 | 6 ± 6.20 | 5 ± 5.81 | 83.30 ± 8.56 | N3 | 0.22 | 55.22 ± 20.32 | 25 | 24 ± 13.4 | 15 ± 10.45 | 62.50 ± 5.45 |
| A4 | 0.33 | 40.24 ± 15.97 | 46 | 9 ± 8.73 | 7 ± 8.66 | 77.80 ± 8.34 | N4 | 0.81 | 46.07 ± 23.91 | 32 | 25 ± 14.3 | 20 ± 14.00 | 80.00 ± 9.45 |
| Average | 0.97 | 62.05 | 36.75 | 14 | 12 | 88.95 | Average | 0.81 | 57.53 | 24 | 22 | 17 | 75.30 |
Notes.
- D(n/m2)
- density of population
- HP
- height of plants
- NFS
- number of flowering shoots
- NF
- number of flowers
- NFR
- number of fruits
- RS
- reproductive success, ±standard deviation
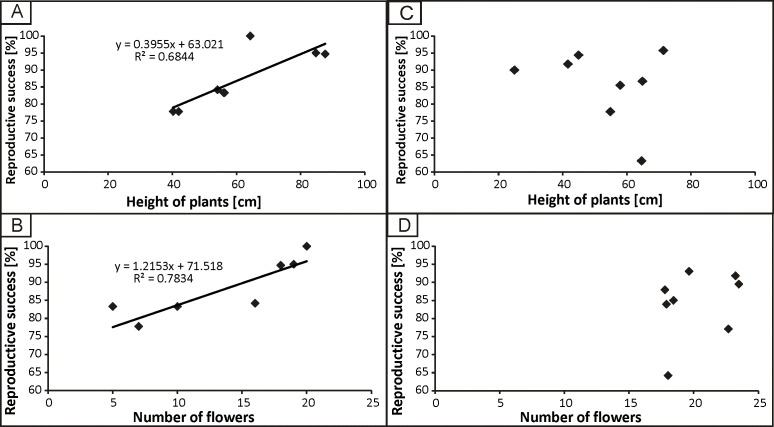
The strongest correlation was found between the reproductive success and height of plants (r = 0.82, p < 0.05) in the populations from the anthropogenic habitats. No significant correlation was found between reproductive success and population density. In the populations from natural habitats, a weak correlation was found between reproductive success and density of populations (r = 0.40, p < 0.05). The regression analysis was significant only in the case of populations from the anthropogenic habitats between reproductive success and height of plants, as well as between reproductive success and the number of flowers (Fig. 5).
Figure 5. The dependence of reproductive success from height of plants and number of flowers in populations from anthropogenic (A, B) and natural (C, D) habitats.
Autogamy
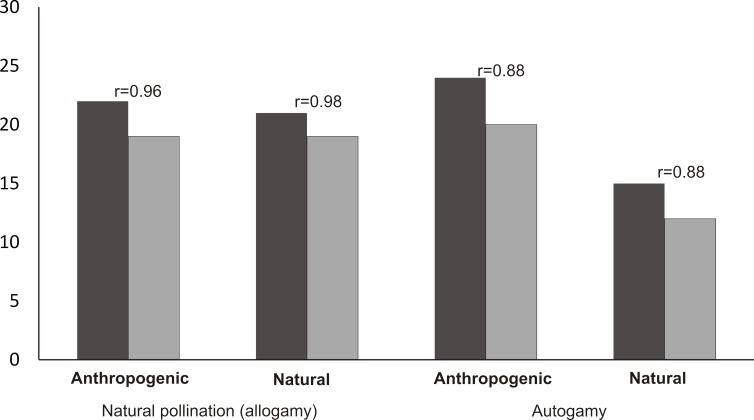
The mean number of capsules (20) produced in the autogamy treatment in the populations from the anthropogenic habitats was significantly higher than the number of capsules produced in the population from the natural habitat (12 capsules) (Mann–Whitney U test, Z = 3.30, p = 0.0008). In the case of autogamy in the populations from the natural and anthropogenic habitats, the number of capsules was strongly positively correlated with the number of flowers per inflorescence (Spearman’s correlation, r = 88, p < 0.05) (Fig. 6). In the case of natural pollination, in both the populations from anthropogenic and natural habitats, the number of fruits was the same (19) and the number of capsules was also strongly positively correlated (Spearman’s correlation, respectively: r = 96 and r = 98, p < 0.05) with the number of flowers in the inflorescence.
Figure 6. Mean number of flowers and fruits in analyzed populations and Spearman correlation between number of flowers and fruits.
In the population from the natural habitat, the number of fruits produced by open-pollination was slightly higher than the number of fruits produced by autogamy (Mann–Whitney U test, Z = 1.30, p = 0.48). The proportion of dead seeds (with unstained embryo) resulting from autogamy varied from 70.5% to 75.4%, and was higher compared to natural pollination (which varied from 48.8% to 50.3%) (Table 4).
Table 4. Ratio of dead and alive seeds developed in autogamy and natural pollination in analyzed populations.
| Trait | Populations | Mann–Whitney U test, p < 0.05 | ||
|---|---|---|---|---|
| Anthropogenic | Natural | |||
| Natural pollination (allogamy) | ||||
| Live seed (%) | 49.7 ± 0.5 | 51.2 ± 2.1 | ns | |
| Dead seeds (%) | 50.3 ± 0.7 | 48.8 ± 2.6 | ns | |
| Autogamy | ||||
| Live seed (%) | 24.6 ± 2.9 | 29.5 ± 3.7 | p < 0.05 | |
| Dead seeds (%) | 75.4 ± 10.2 | 70.5 ± 8.6 | p < 0.05 | |
Notes.
- ns
- Non-significant results
Discussion
Pollinators of E. helleborine
Pollinator availability is one of the key aspects of reproductive success of angiosperm species including orchids. According to Kearns, Inouye & Waser (1998) and Potts et al. (2003), in approximately 90% of all angiosperm species, insect pollinators are involved in sexual reproduction of these plants. In orchids, approximately 70% of the species are closely related to specific insect pollinators (Neiland & Wilcock, 1998). Although our knowledge of how human-induced habitat disturbance affects diversity and composition of pollinator fauna is still fragmentary (Aizen & Vázquez, 2006); it is suggested that pollinator diversity decline is mainly due to the transformation of the environment associated with urbanisation, agricultural development and transformation of the area. Numerous studies have confirmed declining diversity and changes in species composition in cities and other anthropogenic habitats, especially in the case of bees (Apidae) and hoverflies (Syrphidae) (e.g.: Schweiger et al., 2007; Banaszak-Cibicka & Zmihorski, 2012). Regarding these two insect groups, we have found that only hoverflies occurred in much smaller numbers in anthropogenic habitats compared to natural ones, while in the case of bees, these values were very similar (Table 2). In contrast, the effect of small-scale disturbance can be positive for pollinator fauna, especially in forest habitats. Quintero, Morales & Aizen (2010) have noted that local species richness, abundance, and diversity of insect pollinators were higher in the case of disturbed areas than in natural ones. Our results clearly correspond with those latter observations, as in our studies in natural habitats members of only 14 insect families were noted, while in anthropogenic areas 19 families of insect pollinators occurred. Many authors suggest that higher insolation can provide an explanation of such a phenomenon (e.g., Herrera, 1995; Thompson, 2000; Hegland & Boeke, 2006). Herrera (1995) and Quintero, Morales & Aizen (2010) have noted that small patches and other forest gaps with high insolation can be characterised by higher pollinator activity, particularly of small-sized insects, which may increase local richness and abundance of day-active pollinators. Moreover, forest areas exposed to higher insolation are often characterised by higher flower diversity and, as a result, by higher abundance of insect pollinators (Thompson, 2000; Hegland & Boeke, 2006). Sometimes it is even suggested that small-scale disturbed forest areas might act as “diversity oases” for flowering plants (Romey et al., 2007). The higher pollinator diversity in anthropogenic habitats of the studied sites may be explained by the higher insolation of anthropogenic habitats as the most common species were small and medium-size flowering plants, whereas the tree species dominated in natural habitats (Table S1). The anthropogenic habitats probably were located near the natural habitats, so the edge effects maybe also had an important role influencing the high pollinator diversity.
Traditionally, the orchid E. helleborine demonstrates different morphological and physiological adaptations to attract social wasps as pollinators (e.g., Judd, 1971; Müller, 1988; Claessens & Kleynen, 2011). According to the literature, its main pollinators are wasps belonging to the following genera: Vespula, Vespa, and Dolichovespula (Claessens & Kleynen, 2011). However, at least in some regions of the orchid distributional range, additional insect groups, such as flies and beetles, may play an important role in pollination (Jakubska et al., 2005; Claessens & Kleynen, 2014). As shown above, the pollinators of E. helleborine noted during our studies belonged to six orders and 24 insect families (Fig. 3). All these insects could be characterised by four different types of mouth-parts adapted to collect food in different ways (Fig. 4). Similar results were noted by Jakubska et al. (2005) who observed five coleopteran, four hymenopteran, two dipteran, and one lepidopteran family acting as pollinators of this orchid species and also belonging to four groups according to the type of mouth-parts. In comparison with our results, only piercing and sucking insects were not recorded as pollinators of E. helleborine by the above-mentioned authors, while, on the other hand, they noted sucking insects (Lepidoptera), which we did not observe. Such high taxonomical diversity of insects, as well as their diverse morphological adaptations of mouth-parts (all five main types of insect mouth-parts) used for collecting nectar and pollen, clearly suggest that E. helleborine is an opportunistic species according to pollinators. As it was shown by Jacquemyn, Brys & Hutchings (2014), such a strategy can be also used in some other species belonging to this orchid genus. In their summary of knowledge concerning pollinators of E. palustris, the authors have noted members of six families of Coleoptera, 22 of Diptera, 12 of Hymenoptera, and one of Heteroptera (wrongly placed among Hymenoptera). However, it seems that a few insect groups play a much more important role in pollination biology of E. helleborine than the others. In our studies, both in natural and anthropogenic sites, sponging (flies, mainly Syrphidae) and chewing insects (mainly Vespidae and Formicidae but also Coleoptera) dominated, with the chewing-sucking insects (Apidae) as the third group according to the frequency of occurrence (Table 2, Figs. 3 and 4). Such differences may result from: geographical location, diversity of pollinator fauna, weather conditions, and especially air temperature, which may change emission of attractants contained in the nectar of Epipactis (Ehlers & Olesen, 1997). The important role of Vespidae, as well as of Syrphidae and Apidae, was observed not only in other Polish populations of E. helleborine (Jakubska et al., 2005), but also in other regions of Europe (e.g., Claessens & Kleynen, 2014). Similar patterns were also observed in other species of Epipactis such as E. palustris (Vöth, 1988; Jacquemyn, Brys & Hutchings, 2014), E. atrorubens (Jakubska-Busse & Kadej, 2011), E. consimilis (Ivri & Dafni, 1977), E. turcica (Fateryga, 2012) and E. veratrifolia (Jin et al., 2014). As a result, at least some insect species visit Epipactis not only for its highly energetic pollen and/or nectar, but also to look for prey, i.e., other insects attracted by the flowers (Rico-Gray & Oliveira, 2007). For example, Vespidae, Crabronidae or Ichneumonidae, but also some Syrphidae flies, can be classified as such predators. As was shown by Turlings, Tumlinson & Lewis (1990) and Brodmann et al. (2008), at least some Epipactis species (including E. helleborine and E. purpurata), produce green-leaf volatiles (GLVs) whose chemical composition is very similar to those emitted by damaged plant tissues when the plant is attacked by caterpillars. The latter are known as one of the most important prey for wasps. Surprisingly, Jin et al. (2014) have noted that females of some Syrphidae can lay their eggs on orchids attacked by aphids (Aphidoidea), which are the main food for their hatched larvae. The explanation of this phenomenon was provided by Stökl et al. (2010), who noted that flowers of E. veratrifolia are visited by some aphidophagous Syrhipidae as the orchid produces α- and β-pinene, β-myrcene and β-phellandrene. These substances are very similar to aphid-derived kairomones, which normally are emitted as alarm pheromones by several aphid species. Hoverflies were also noted as important pollinators of E. helleborine, both during our studies (Table 2, Fig. 3) and by Jakubska et al. (2005). Moreover, aphids are regularly noted as feeding on this orchid species (A Rewicz, pers. obs., 2011–2013). Thus, we can suppose that such chemical mimicry is more common among Epipactis species than was shown until now. Overall, these results indicate that E. helleborine has a diverse group of pollinators, which may promote this species in very rapidly changing areas transformed by man and which is one of the key features of apophytes.
Reproductive success and effect of autogamy of E. helleborine seeds
Autogamy in E. helleborine was observed by many authors, some of them (Richards & Porter, 1982; Robatsch, 1983) have claimed that this species shows optional autogamy (mixed-mating) (Ehlers & Pedersen, 2000; Claessens & Kleynen, 2011). However, Ehlers, Olesen & Gren (2002) have suggested that autogamy in E. helleborine is rare and that this phenomenon occurs only in specific conditions, i.e., when suitable pollinators are lacking. Our results provide evidence that autogamy occurs in populations from both anthropogenic and natural habitats of E. helleborine (Table 4). Despite some differences in the number of fruits between the populations of the two habitats, we have found no significant differences in the number of fruits formed between autogamy and natural pollination, which is congruent with work of Weijer (1952). In our opinion, autogamy is a common phenomenon in the life cycle of E. helleborine.
According to Grime’s (1979) theory, some species can tolerate environmental disturbances. Hágsater & Dumont (1996) have suggested that orchids belong to the group between ruderal and stress-tolerant plants. Recent studies of Rewicz, Kołodziejek & Jakubska-Busse (2016) have highlighted a positive impact of disturbed anthropogenic habitats on occurrence of some orchids species, even against the general thesis that orchids are competitively weaker than other plant species. In this particular case, it may be caused by reduction in the vigour of other plants by some management practices. Djordjević et al. (2016a) suggested that disturbed habitats can be preferred by orchids. It is possible that E. helleborine from the anthropogenic habitats has more space with a favourable light regime. Furthermore, some ecological conditions, such as soil moisture, soil pH, and organic matter could also be conducive to the growth of E. helleborine, which results in its larger size in anthropogenic habitats.
The relationship between plant height and reproductive success was confirmed by Machaka-Houri et al. (2012) in their studies on Orchis galilea, as well as on Ferocactus cylindraceus, F. wislizeni, and Lotus corniculatus (Ollerton & Lack, 1998; McIntosh, 2002). Our results also suggest that there is no association between reproductive success and the number of flowers on a sprout in populations from both anthropogenic and natural habitats. It appears that the height of the plant and the number of flowers in orchids enhances attractiveness for insect pollinators (Kindlmann & Jersakova, 2005). Specimens from the populations from the anthropogenic habitats we studied were higher and had more diverse pollinating fauna. Plants with bigger shoots and more flowers are more tempting for pollinators, which results in more efficient transport of pollinia (Van der Piper & Waite, 1988). Moreover, no significant correlation between density of plants and reproductive success in populations from the anthropogenic habitats was noted and in populations from the natural habitats such correlation was weak. Similar results were obtained in other studies (Sih & Baltus, 1987; Ågren, 1989; Alexanderson & Agren, 1996; Ehlers, Olesen & Gren, 2002).
In conclusion, our research demonstrates that the spectrum of insects pollinating E. helleborine is much wider than it has been suggested in the literature. Increased variety of possible pollinators allows faster and better adaptation to the human-changed environment. The reproductive success of E. helleborine was higher in anthropogenic habitats, which might have been a higher number of visits and greater species diversity of pollinators, as well as by a larger size of the plants. Moreover, autogamy was not uncommon as the reproductive strategy, and we found no significant differences between the number of fruits formed by autogamy and by natural pollination. In addition, this study contributes to a better understanding of why E. helleborine is one of the few Eurasian orchid species that has been naturalised in North America. The study confirms the general thesis that orchid species which are not highly specialised in relation to the type of pollinator have wider distribution ranges and are less rare than orchid species that have a high level of pollinator specialisation (Swarts & Dixon, 2009).
Summarising, our study helps to explain why E. helleborine is one of the (few) orchid species that manages to successfully use anthropogenic habitats in a manner comparable to that of natural ones. The question how wide the tolerance range of this species is still remains open. To answer the question, we plan further research on pollinator diversity and reproductive success of E. helleborine in other types of anthropogenic habitats (the surroundings of industrial facilities, fly ash, mine tailing, highway, railway, urban parks, etc.).
Supplemental Information
(A) In anthropogenic habitats (A1–A4), (B) Vascular flora in natural habitats (N1–N4).
Abbreviations: population code (Pop), height of plants (HP), number of flowers (NF), number of fruit (capsules) (NFR).
Explanations: population (Pop), density (D), number of flowers (NF), number of fruit (capsules) (NFR), reproductive success (RS).
Acknowledgments
The authors wish to thank Małgorzata Karczewska, Alicja Klejps, Damian Kanioski, Jakub Kierzkowski and Iwona Stefaniak for their assistance in the fieldwork. We also thank Michał Grabowski and Marta Koniarek for improving the final English version of the manuscript. We are also grateful to Vladan Djordjević and an anonymous reviewer for constructive comments on the manuscript. The second author would like to dedicate this paper to Barbara Szmalenberg.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Agnieszka Rewicz conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Radomir Jaskuła and Tomasz Rewicz performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Grzegorz Tończyk contributed reagents/materials/analysis tools.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Regional Directoriate of Environmental Protection in Białystok (Regionalna Dyrekcja Ochrony Środowiska w Białymstoku) - permission no. WPN6400.74.2013.MW.
Ministry of the Environment (Ministerstwo Środowiska) - permission no.
35/17258/12/RS.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary Files.
References
- Adamowski (2004).Adamowski W. Population expansion of native orchids in anthropogenous habitats . Abstract 8Neobiota. 2004;3 [Google Scholar]
- Adamowski (2006).Adamowski W. Expansion in native orchids in anthropogenous habitats. Polish Botanical Studies. 2006;22:35–44. [Google Scholar]
- Ågren (1989).Ågren J. Seed size and number in Rubus chamaemorus: between habitat variation, and effects of defoliation and supplemental pollination. Journal of Ecology. 1989;77:1080–1092. doi: 10.2307/2260824. [DOI] [Google Scholar]
- Aizen & Vázquez (2006).Aizen MA, Vázquez DP. Ecology and evolution of flowers. Oxford University Press; Oxford: 2006. Flower performance in human-altered habitats; pp. 159–179. [Google Scholar]
- Alexanderson & Agren (1996).Alexanderson R, Agren J. Population size, pollinator visitation rate and fruit production in the deceptive orchid Calypso bulbosa. Oecologia. 1996;107:533–540. doi: 10.1007/BF00333945. [DOI] [PubMed] [Google Scholar]
- Arditti (1967).Arditti J. Factors affecting the germination of orchid seeds. Botanical Review. 1967;33:1–97. doi: 10.1007/BF02858656. [DOI] [Google Scholar]
- Banaszak-Cibicka & Zmihorski (2012).Banaszak-Cibicka W, Zmihorski M. Wild bees along an urban gradient: winners and losers. Journal of Insect Conservation. 2012;16:331–343. doi: 10.1007/s10841-011-9419-2. [DOI] [Google Scholar]
- Baumann, Kunkele & Lorenz (2010).Baumann H, Kunkele S, Lorenz R. Storczyki Europy i obszarów sąsiednich. Multico; Warszawa: 2010. [Google Scholar]
- Brodmann et al. (2008).Brodmann J, Twele R, Francke W, Hölzler G, Zhang QH, Ayasse M. Orchids mimic green-leaf volatiles to attract prey-hunting wasps for pollination. Current Biology. 2008;18:740–744. doi: 10.1016/j.cub.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Brower & Zar (1984).Brower JE, Zar JH. Field and laboratory methods for general ecology. Dubuque: Wm. C. Brown Publisher; 1984. [Google Scholar]
- Claessens & Kleynen (2011).Claessens J, Kleynen J. The flower of the European orchid. Form and function. Schrijen-Lippertz; Venlo: 2011. [Google Scholar]
- Claessens & Kleynen (2014).Claessens J, Kleynen J. The pollination of European Orchids Part 3: Limodorum and Epipactis. Journal of the Hardy Orchid Society. 2014;11(2):64–71. [Google Scholar]
- Clemente (2009).Clemente M. Orchid conservation and trade: are these concepts incompatible? In: Pridgeon AM, Suarez JP, editors. Proceedings of the second scientific conference on andean orchids. Universidad Técnica Particular de Loja; Loja: 2009. pp. 46–55. [Google Scholar]
- Delforge (2006).Delforge P. Orchids of Europe, North Africa and the Middle East. Timber Press Inc; Portland: 2006. [Google Scholar]
- Djordjević et al. (2016b).Djordjević V, Tsiftsis S, Lakušić D, Jovanović S, Stevanović V. Factors affecting the distribution and abundance of orchids in grasslands and herbaceous wetlands. Systematics and Biodiversity. 2016b;14(4):355–370. doi: 10.1080/14772000.2016.1151468. [DOI] [Google Scholar]
- Djordjević et al. (2016a).Djordjević V, Tsiftsis S, Lakušić D, Stevanović V. Niche analysis of orchids of serpentine and non-serpentine areas: implications for conservation. Plant Biosystems. 2016a;150(4):710–719. doi: 10.1080/11263504.2014.990534. [DOI] [Google Scholar]
- Doust & Doust (1988).Doust JL, Doust L. Plant reproductive ecology: patterns and strategies. Oxford University Press; New York: 1988. [Google Scholar]
- Ehlers & Olesen (1997).Ehlers BK, Olesen JM. The fruit-wasp route to toxic nectar in Epipactis orchids? Flora. 1997;192:223–229. doi: 10.1016/S0367-2530(17)30787-9. [DOI] [Google Scholar]
- Ehlers, Olesen & Gren (2002).Ehlers BK, Olesen JM, Gren JA. Floral morphology and reproductive success in the orchid Epipactis helleborine: regional and local across-habitat variation. Plant Systematic and Evolution. 2002;236(1/2):9–32. doi: 10.1007/s00606-002-0197-x. [DOI] [Google Scholar]
- Ehlers & Pedersen (2000).Ehlers BK, Pedersen H. Genetic variation in three species of Epipactis (Orchidaceae): geographic scale and evolutionary inferences. Biological Journal of the Linnean Society. 2000;69:411–430. doi: 10.1111/j.1095-8312.2000.tb01214.x. [DOI] [Google Scholar]
- Esfeld et al. (2008).Esfeld K, Hensen I, Wesche K, Jakob SS, Tischew S, Blattner FR. Molecular data indicate multiple colonizations of former lignite mining areas in Eastern Germany by Epipactus palustris (Orchidaceae) Biodiversity and Conservation. 2008;17:2441–2453. doi: 10.1007/s10531-008-9391-7. [DOI] [Google Scholar]
- Faliński (2001).Faliński JB. Phytophenological atlas of the forest communities and species of Białowieża National Park, Phytocoenosis N.S. 13. Archivio Geobotanico. 2001;8:1–176. [Google Scholar]
- Fateryga (2012).Fateryga AV. Pollination ecology of the species from the genus Epipactis (Orchidaceae) in the Crimea. Ekosistemy, Ikh Optimizatziya I Okhrana. 2012;6:136–150. [Google Scholar]
- Forman et al. (2009).Forman RTT, Sperling D, Bissonette J, Clevenger AP, Cutshall C, Dale V, Fahrig L, France R, Goldman C, Heanue K, Jones J, Swanson F, Turrentine T, Winter T. Science and solutions. Island Press; Washington, D.C.: 2009. Road ecology. [Google Scholar]
- Gillott (2005).Gillott C. Entomology. 3rd edition Springer; Dordrecht: 2005. [Google Scholar]
- Goulson, Lye & Darvill (2008).Goulson D, Lye GC, Darvill B. Decline and conservation of bumble bees. Annual Review of Entomology. 2008;53:191–208. doi: 10.1146/annurev.ento.53.103106.093454. [DOI] [PubMed] [Google Scholar]
- Grime (1979).Grime JP. Plant strategies and vegetation processes. John Wiley & Son; Chichester: 1979. [Google Scholar]
- Hágsater & Dumont (1996).Hágsater E, Dumont V. Orchids: status, survey and conservation action plan. IUCN; Gland: 1996. [Google Scholar]
- Hegland & Boeke (2006).Hegland SJ, Boeke L. Relationships between the density and diversity of floral resources and flower visitor activity in a temperate grassland community. Ecological Entomology. 2006;31:532–538. doi: 10.1111/j.1365-2311.2006.00812.x. [DOI] [Google Scholar]
- Herrera (1995).Herrera CM. Microclimate and individual variation in pollinators: flowering plants are more than their flowers. Ecology. 1995;76:1516–1524. doi: 10.2307/1938153. [DOI] [Google Scholar]
- Hůrka (2005).Hůrka K. Beetles of the Czech and Slovak Republics. Nakladatelstvi Kabourek; Zlin: 2005. [Google Scholar]
- Ivri & Dafni (1977).Ivri Y, Dafni A. The pollination ecology of Epipactis consimilis Don (Orchidaceae) in Israel. New Phytologist. 1977;79:173–177. doi: 10.1111/j.1469-8137.1977.tb02193.x. [DOI] [Google Scholar]
- Jacquemyn, Brys & Hutchings (2014).Jacquemyn H, Brys R, Hutchings MJ. Biological Flora of the British Isles: Epipactis palustris. Journal of Ecology. 2014;102:1341–1355. doi: 10.1111/1365-2745.12287. [DOI] [Google Scholar]
- Jakubska et al. (2005).Jakubska A, Kadej M, Prządo D, Steininger M. Pollination ecology of Epipactis helleborine (L.) Crantz (Orchidaceae, Neottieae) in the south-western Poland. Acta Botanica Silesiaca. 2005;2:131–144. [Google Scholar]
- Jakubska & Orlowski (2003).Jakubska A, Orlowski G. Naturalnie występujące gatunki storczyków na terenie Wrocławia i okolic. In: Korczyński M, editor. Flora miast. Bydgoszcz: Kujawsko-pomorskie centrum edukacji ekologicznej; 2003. pp. 87–91. [Google Scholar]
- Jakubska-Busse & Kadej (2011).Jakubska-Busse A, Kadej M. The pollination of Epipactis Zinn, 1757 (Orchidaceae) species in central Europe—the significance of chemical attractants, floral morphology and concomitant insects. Acta Societatis Botonicorum Poloniae. 2011;80:49–57. doi: 10.5586/asbp.2011.007. [DOI] [Google Scholar]
- Jin et al. (2014).Jin XH, Ren ZX, Xu SZ, Wang H, Li DZ, Li ZY. The evolution of floral deception in Epipactis veratrifolia (Orchidaceae): from indirect defense to pollination. BMC Plant Biology. 2014;14(1):63. doi: 10.1186/1471-2229-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd (1971).Judd WW. Wasps (Vespoidea) pollinating Helleborine Epipactis helleborine (L.) Crantz, at Owen Sound, Ontario. Proceedings of the Entomological Society of Ontario. 1971;102:115–118. [Google Scholar]
- Jurkiewicz et al. (2001).Jurkiewicz A, Turnau K, Mesjasz-Przybylowicz J, Przybylowicz W, Godzik B. Heavy metal localisation in mycorrhizas of Epipactis atrorubens (Hoffm.) Besser (Orchidaceae) from zink mine tailings. Protoplasma. 2001;218:117–124. doi: 10.1007/BF01306601. [DOI] [PubMed] [Google Scholar]
- Kearns, Inouye & Waser (1998).Kearns CA, Inouye DW, Waser NM. Endangered mutualisms: the conservation of plant–pollinator interactions. The Annual Review of Ecology, Evolution, and Systematics. 1998;29:83–112. doi: 10.1146/annurev.ecolsys.29.1.83. [DOI] [Google Scholar]
- Kiedrzyński & Stefaniak (2011).Kiedrzyński M, Stefaniak A. Występowanie rodzaju Epipactis Zinn 1757 w Puszczy Pilickiej na stanowiskach naturalnych i antropogenicznych. In: Brzosko E, Wróblewska A, Jarmakowicz E, editors. Storczykowate w Polsce. Białystok: Biologia i Ochrona; 2011. pp. 86–96. [Google Scholar]
- Kindlmann & Jersakova (2005).Kindlmann P, Jersakova J. Floral display, reproductive success, and conservation of terrestrial orchids. Selbyana. 2005;26:136–144. [Google Scholar]
- Kolanowska (2013).Kolanowska M. Niche conservatism and the future potential range of Epipactis helleborine (Orchidaceae) PLOS ONE. 2013;8(10):e77352. doi: 10.1371/journal.pone.0077352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski (2006).Kwiatkowski P. Current state, separateness and dynamics of vascular floraof the Góry Kaczawskie (Kaczawa Mountains) and Pogórze Kaczawskie (KaczawaPlateau). I. Distribution atlas of vascular plants. W. Szafer Institute of Botany, Polish Academy of Sciences; Kraków: 2006. pp. 1–468. [Google Scholar]
- Light & MacConaill (2006).Light MHS, MacConaill M. Appearance and disappearance of a weedy orchid, Epipactis helleborine. Folia Geobotanica. 2006;41:77–93. doi: 10.1007/BF02805263. [DOI] [Google Scholar]
- Löki et al. (2015).Löki V, Tökölyi J, Süveges K, Lovas-Kiss A, Hürkan K, Gábor S, Molnár AV. The orchid flora of Turkish graveyards: a comprehensive field survey. Willdenowia. 2015;45:231–243. doi: 10.3372/wi.45.45209. [DOI] [Google Scholar]
- Machaka-Houri et al. (2012).Machaka-Houri N, Al-Zein MS, Westbury DB, Talhouk SN. Reproductive success of the rare endemic Orchis galilaea (Orchidaceae) in Lebanon. Turkish Journal of Botany. 2012;36:677–682. doi: 10.3906/bot-1104-4. [DOI] [Google Scholar]
- McIntosh (2002).McIntosh EM. Plant size breeding system and limits to reproductive success in two sister species of Ferocactus (Cactaceae) Plant Ecology. 2002;162:273–288. doi: 10.1023/A:1020329718917. [DOI] [Google Scholar]
- Meissner (2010).Meissner W. Przewodnik do ćwiczeń z przedmiotu metody statystyczne w biologii. Wydawnictwo Uniwersytetu Gdańskiego; Sopot: 2010. [Google Scholar]
- Müller (1988).Müller I. Vergleichende blütenökologische Untersuchungen an der Orchideengattung Epipactis. Mitteilungen des Arbeitskreises Heimische Orchideen Baden-Württemberg. 1988;20(4):701–803. [Google Scholar]
- Neiland & Wilcock (1998).Neiland MM, Wilcock CC. Fruit set nectar reward and rarity in the Orchidaceae. American Journal of Botany. 1998;85:1657–1671. doi: 10.2307/2446499. [DOI] [PubMed] [Google Scholar]
- Ollerton & Lack (1998).Ollerton J, Lack A. Relationships between flowering phenology, plant size and reproductive success in lotus corniculatus (Fabaceae) Plant Ecology. 1998;139(1):35–47. doi: 10.1023/A:1009798320049. [DOI] [Google Scholar]
- Oosterbroek (2006).Oosterbroek P. The European families of Diptera. Identification, diagnosis, biology. Utrecht: KNNV Publishing; 2006. [Google Scholar]
- Owen (1879).Owen ML. An orchid new to America. Bulletin of the Torrey Botanical Club. 1879;6:329–330. [Google Scholar]
- Pedersen, Watthana & Srimuang (2013).Pedersen HAE, Watthana S, Srimuang K. Orchids in the torrent: on the circumscription, conservation and rheophytic habit of Epipactis flava. Botanical Journal of the Linnaean Society. 2013;172:358–370. doi: 10.1111/boj.12023. [DOI] [Google Scholar]
- Piękoś-Mirkowa & Mirek (2006).Piękoś-Mirkowa H, Mirek Z. Atlas roślin chronionych. Seria Flora Polski; Warszawa: 2006. [Google Scholar]
- Potts et al. (2003).Potts SG, Vulliamy B, Ne’eman G, O’Toole C, Roberts S, Willmeret P. Response of plant–pollinator communities to fire: changes in diversity, abundance and floral reward structure. Oikos. 2003;101:103–112. [Google Scholar]
- Procházka & Velísek (1983).Procházka F, Velísek V. Orchideje nasi prirody. Ceskoslovenska Akademie; Prague: 1983. [Google Scholar]
- Quintero, Morales & Aizen (2010).Quintero C, Morales CL, Aizen MA. Effects of anthropogenic habitat disturbance on local pollinator diversity and species turnover across a precipitation gradient. Biodiversity and Conservation. 2010;19(10):257–274. doi: 10.1007/s10531-009-9720-5. [DOI] [Google Scholar]
- Rewicz, Kołodziejek & Jakubska-Busse (2016).Rewicz A, Kołodziejek J, Jakubska-Busse A. The role of anthropogenic habitats as substitutes for natural habitats: a case study on Epipactis helleborine (L.) Crantz (Orchidaceae, Neottieae). Variations in size and nutrient composition of seeds. Turkish Journal of Botany. 2016;40(3):258–268. doi: 10.3906/bot-1404-69. [DOI] [Google Scholar]
- Rewicz et al. (2015).Rewicz A, Zielińska KM, Kiedrzyński M, Kucharski L. Orchidaceae in the anthropogenic landscape of Central Poland: diversity, extinction and conservation perspectives. Archives of Biological Sciences. 2015;67:119–130. doi: 10.2298/ABS140428014R. [DOI] [Google Scholar]
- Richards & Porter (1982).Richards AJ, Porter AF. On the identity of a Northumberland Epipactis. Watsonia. 1982;14:121–128. [Google Scholar]
- Rico-Gray & Oliveira (2007).Rico-Gray V, Oliveira PS. The ecology and evolution of ant–plant interactions. University of Chicago Press; Chicago: 2007. [Google Scholar]
- Robatsch (1983).Robatsch K. Beiträge zur Blütenbiologie und Autogamie der Gattung Epipactis. Jahresber Naturwiss Ver Wuppertal. 1983;36:25–32. [Google Scholar]
- Romey et al. (2007).Romey WL, Ascher JS, Powell DA, Yanek M. Impacts of logging on midsummer diversity of native bees (Apoidea) in a northern hardwood forest. Journal of the Kansas Entomological Society. 2007;80:327–338. doi: 10.2317/0022-8567(2007)80[327:IOLOMD]2.0.CO;2. [DOI] [Google Scholar]
- Schweiger et al. (2007).Schweiger O, Musche M, Bailey D, Billeter R, Diekötter T, Hendrickx F, Herzog F, Liira J, Maelfait JP, Speelmans M, Dziock F. Functional richness of local hoverfly communities (Diptera, Syrphidae) in response to land use across temperate Europe. Oikos. 2007;116:461–472. doi: 10.1111/j.2007.0030-1299.15372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih & Baltus (1987).Sih A, Baltus M. Patch size, pollinator behavior and pollinator limitation in Catnip. Ecology. 1987;68:1679–1690. doi: 10.2307/1939860. [DOI] [PubMed] [Google Scholar]
- StatSoft Inc (2011).StatSoft Inc STATISTICA (data analysis software system), version 10. 2011. http://www.statsoft.com http://www.statsoft.com
- Stökl et al. (2010).Stökl J, Brodmann J, Dafni A, Ayasse M, Hansson BH. Smells like aphids: orchid flowers mimic aphid alarm pheromones to attract hoverflies for pollination. Proceedings of the Royal Society B Biological Science. 2010;278:1216–1222. doi: 10.1098/rspb.2010.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukopp (2006).Sukopp H. Apophyts in the flora of Central Europe. Polish Botanical Studies. 2006;22:473–485. [Google Scholar]
- Swarts & Dixon (2009).Swarts DN, Dixon WD. Terrestrial orchid conservation in the age of extinction. Annals of Botany. 2009;104:543–556. doi: 10.1093/aob/mcp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świercz (2004).Świercz A. The cement and lime industry and the occurrence of selected species of orchids. Przegląd Przyrodniczy. 2004;15:117–123. [Google Scholar]
- Świercz (2006).Świercz A. Przegląd stanowisk wybranych gatunków z rodziny Orchideaceae w sąsiedztwie cementowni regionu świętokrzyskiego. Regionalne Studia Ekologiczno-Krajobrazowe. Problemy Ekologii Krajobrazu. 2006;16:433–440. [Google Scholar]
- Thompson (2000).Thompson JD. How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia. 2000;126:386–394. doi: 10.1007/s004420000531. [DOI] [PubMed] [Google Scholar]
- Tremblay (1992).Tremblay RL. Trends in the pollination ecology of the Orchidaceae: evolution and systematics. Canadian Journal of Botany. 1992;70:642–650. doi: 10.1139/b92-083. [DOI] [Google Scholar]
- Tremblay et al. (2005).Tremblay RL, Ackerman JD, Zimmermann JK, Calvo RN. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnaean Society. 2005;84:1–54. doi: 10.1111/j.1095-8312.2004.00400.x. [DOI] [Google Scholar]
- Tsiftsis et al. (2008).Tsiftsis S, Tsiripidis I, Karagiannakidou V, Alifragis D. Niche analysis and conservation of the orchids of east Macedonia (NE Greece) Acta Oecologica. 2008;33:27–35. doi: 10.1016/j.actao.2007.08.001. [DOI] [Google Scholar]
- Turlings, Tumlinson & Lewis (1990).Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- Van der Piper & Waite (1988).Van der Piper J, Waite S. The gender role of flowers of broad leaved Helleborine Epipactis helleborine (L) Functional Ecology. 1988;2:35–40. doi: 10.2307/2389457. [DOI] [Google Scholar]
- Van Emden (2008).Van Emden H. Statistics for terrified biologists. Oxford: Blackwell Publishing; 2008. [Google Scholar]
- Van Waes & Debergh (1986).Van Waes JM, Debergh PC. Adaptation of the tetrazolium method for testing the seed viability, and scanning electron microscopy study of some Western European orchids. Physiologia Plantarum. 1986;66:435–442. doi: 10.1111/j.1399-3054.1986.tb05947.x. [DOI] [Google Scholar]
- Vöth (1988).Vöth W. Die Honigbiene, Apis mellifera, L. (Apidae), erwiest sich als der effektive Bestaüber von Epipactis palustris (L.) Crantz. Mitteilungen des Arbeitskreises Heimische Orchideen Baden-Württemberg. 1988;19:112–118. [Google Scholar]
- Weijer (1952).Weijer J. The colour differences in Epipactis helleborine (L.) Cr. Wats. & Coult. and the selection of the genetical varieties by environment. Genetica. 1952;26:1–32. doi: 10.1007/BF01690613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) In anthropogenic habitats (A1–A4), (B) Vascular flora in natural habitats (N1–N4).
Abbreviations: population code (Pop), height of plants (HP), number of flowers (NF), number of fruit (capsules) (NFR).
Explanations: population (Pop), density (D), number of flowers (NF), number of fruit (capsules) (NFR), reproductive success (RS).
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as Supplementary Files.