Abstract
Smoking is the most common route of administration for cannabis; however, vaping cannabis extracts and synthetic cannabinoids (“fake marijuana”) in electronic cigarette devices has become increasingly popular. Yet, most animal models used to investigate biological mechanisms underlying cannabis use employ injection as the route of administration. This study evaluated a novel e-cigarette device that delivers aerosolized cannabinoids to mice. The effects of aerosolized and injected synthetic cannabinoids (CP 55,940, AB-CHMINACA, XLR-11, and JWH-018) in mice were compared in a battery of bioassays in which psychoactive cannabinoids produce characteristic effects. The most potent cannabinoids (CP 55,940 and AB-CHMINACA) produced the full cannabinoid profile (ie, hypothermia, hypolocomotion, and analgesia), regardless of the route of administration. In contrast, aerosolized JWH-018 and XLR-11 did not produce the full profile of cannabimimetic effects. Results of time course analysis for hypothermia showed that aerosol exposure to CP 55,940 and AB-CHMINACA produced faster onset of effects and shorter duration of action than injection. The ability to administer cannabinoids to rodents using the most common route of administration among humans provides a method for collecting preclinical data with enhanced translational relevance.
Keywords: Animal models, e-cigarettes, synthetic cannabinoids, vaping
Introduction
Cannabis is the most frequently used illicit substance of abuse in the United States.1 Increased availability due to legalization efforts and decreased perceptions of harm have been correlated with increasing use.1-3 Although smoking cannabis in the form of a cigarette (joint) or in a pipe remains the most common methods of use,4 adaptation of electronic cigarette (e-cigarette) devices to vape e-liquids infused with cannabis extracts has been gaining popularity, particularly with youth and young adults who may vape cannabis oil or wax containing high concentrations of Δ9-tetrahydrocannabinol (THC),5 the primary psychoactive constituent of the cannabis plant. This upward trend in vaping cannabis products is likely to continue because almost half of the states in the United States have passed laws that allow legal medicinal and/or recreational use of cannabis. In addition, use of e-cigarettes to deliver synthetic cannabinoids (sometimes mistakenly called “fake marijuana,” “spice,” or “herbal incense”) in aerosol has been reported.6,7 Despite evidence that synthetic cannabinoids may be associated with serious toxicities or even death in human users,7-10 the behavioral and toxicologic consequences of using these compounds remain sparsely studied.11
Because investigation of the effects of uncharacterized synthetic cannabinoids in humans is not possible, much of the research examining the pharmacology of these compounds has been conducted in animals. Yet, despite smoking being the most common route of administration in humans, most preclinical research to date has employed the use of injected THC or other cannabinoids, with only a few exceptions.12-17 Some studies have used intravenous administration,18 which may allow for rapid speed of onset, as is observed after smoking synthetic cannabinoid products; however, most behavioral studies have used intraperitoneal or subcutaneous injections.19,20 Hence, first-pass metabolism (intraperitoneal) and delayed onset (intraperitoneal and subcutaneous) may play a greater role in animal models than in human users. During metabolism, chemical alterations occur, with some metabolites of synthetic cannabinoids producing different in vivo effects than their parent compounds.8,21,22 In addition, heating or burning synthetic cannabinoids through vaping or smoking may change the chemicals themselves, as can degradation during storage.23,24 Animal models using inhalation as a more translationally relevant route of administration may be useful in delineating these factors which may, in turn, facilitate more accurate predictions of the effects of synthetic cannabinoids in humans.
The primary goal of this study was to evaluate a novel device that allows administration of aerosolized cannabinoids to mice via the e-cigarette technology used by humans. For comparison purposes, other mice were exposed to the same cannabinoids via the more typical intraperitoneal injection route of administration. In this study, a set of 3 endpoints were used as a type of behavioral phenotype to detect the extent to which aerosolized compounds reached the brain and produced a profile of pharmacological effects that is characteristic of THC and other psychoactive cannabinoids in rodents following systemic injection, including suppression of locomotor activity, analgesia, and decrease in body temperature.25,26 Because this study represents the first assessment of cannabinoids in this device, we chose to examine the effects of selected synthetic cannabinoids (CP 55,940, AB-CHMINACA, XLR-11, and JWH-018) that had higher affinity and, in some cases, higher potency compared with THC. AB-CHMINACA, XLR-11, and JWH-018 have been reported to be present in “fake marijuana” products.27-29 CP 55,940 was chosen for testing because it is a high-potency synthetic cannabinoid that has often been used as a standard in preclinical and in vitro research with cannabinoids.30-32 The ability to employ inhalation is important for cannabinoid research for several reasons: (1) state-level legalization of recreational and medicinal marijuana has increased potential exposure, (2) greater equivalence in route of administration between humans and in preclinical models may enhance translational relevance of results, and (3) some of the toxicological or other health effects (pulmonary) of drug abuse are likely to be specific to inhalation and may not be detected in studies using other routes of administration.
Methods
Subjects
Adult male ICR mice (25-35 g; Envigo Laboratories, Frederick, MD, USA) were single housed in polycarbonate cages under temperature-controlled conditions (20°C-24°C) with a 12-hour light-dark cycle (lights on at 6:00 AM). The mice had ad lib access to food and water and were left undisturbed in their home cages, except during testing. Separate mice were tested at each dose/concentration of each compound, with the exception that the temperature time course experiment was conducted in a subset of mice that had been tested previously in the entire test battery. The studies were conducted in accordance with federal regulatory guidelines and were approved by our Institutional Animal Care and Use Committee.
Drugs
JWH-018 (National Institute on Drug Abuse; NIDA, Rockville, MD, USA), CP 55,940 (NIDA), XLR-11 (Drug Enforcement Administration [DEA] Special Testing and Research Laboratory, Dulles, VA, USA), and AB-CHMINACA (DEA) were dissolved in 7.8% polysorbate 80 NF (Fisher Scientific, Pittsburgh, PA, USA) and 92.2% saline USP (Patterson Veterinary, Devens, MA, USA) for injection or in 50% propylene glycol USP (Fisher) + 50% glycerin USP (Fisher) for aerosol administration. Compounds were injected intraperitoneally at a volume of 10 mL/kg. Concentrations for aerosol administration (aerosol 0, 0.72, 7.2, and 72 mg/mL) are expressed as milligram per milliliter of solution that was contained in the e-cigarette tank and do not represent the actual amount of drug inhaled. Doses for intraperitoneal injection (0, 0.03, 0.3, and 3 mg/kg) are expressed as milligram per kilogram. Vehicles (concentration/dose 0) of 7.8% polysorbate 80 + 92.2% saline and 50% propylene glycol + 50% glycerin were used as comparisons for injected and aerosolized compounds, respectively.
Aerosol delivery apparatus
Aerosol was generated using a modified e-cigarette device to deliver aerosol to mouse-sized chambers (Figure 1), as described previously.33,34 An iStick 30 W Variable Wattage (ELeaf, Irvine, CA, USA) supplied power (7 W) to a CE5-S (tank/clearomizer) with bottom dual coil atomizer (1.8Ω) (Aspire, Kent, WA, USA). Air/aerosol was pumped through the bottom of the tank at 1 L/min and into an EZ-177 Sure-Seal mouse induction anesthesia chamber (10 cm × 10 cm × 10 cm) (E-Z-Anesthesia, Palmer, PA, USA) via Tygon tubing (Fisher Scientific, Pittsburgh, PA, USA) and controlled by 3-way stopcocks (Grainger, Raleigh, NC, USA). Mice were placed individually into the anesthesia chambers and aerosol was generated for 10 seconds, filling the chamber. The stopcocks then held the aerosol in the chamber for 5 minutes while the mouse remained in the chamber. After 5 minutes, mice were removed from the chambers and placed back into their home cages for the pretreatment interval (see in vivo test battery section). The 5-minute exposure period was based on preliminary experiments and on previous work with stimulants and nicotine in the aerosol delivery apparatus.33,34
Figure 1.
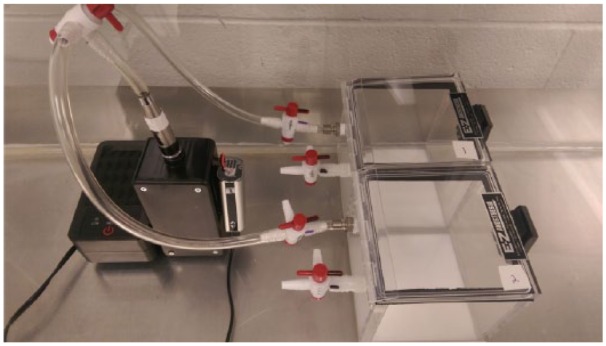
Modified electronic cigarette exposure apparatus that was used to deliver aerosolized synthetic cannabinoids to mice.
Apparatus
Locomotor activity was assessed in clear Plexiglas activity chambers (47 cm × 25.5 cm × 22 cm). Each chamber was surrounded by 2 arrays of 4 × 8 photocell infrared beams, interfaced with software for automated data collection (San Diego Instruments, San Diego, CA, USA). Temperature readings were taken using a BAT-12 Microprobe Thermometer with RET-3 Rectal Probe (PhysiTemp Instruments Inc., Clifton, NJ, USA). Analgesia was measured with a Tail Flick Analgesia Meter (IITC Inc. Life Science, Woodland Hills, CA, USA).
In vivo test battery
A group of ICR mice (n = 208) was tested for pharmacological effects of aerosolized or intraperitoneally injected CP 55,940, AB-CHMINACA, JWH-018, and XLR-11 (n = 8 per dose). Prior to drug administration, baseline rectal temperature and tail flick latency were measured. For tail flick analgesia assessment, a beam of light was focused on the tail, and the latency to remove the tail from the light was recorded. The system was configured to shut off automatically at 10 seconds to prevent tissue damage to the tail. Mice were then administered their assigned concentration/dose via aerosol (0, 0.72, 7.2, or 72 mg/mL) or intraperitoneal injection (0, 0.03, 0.3, or 3 mg/kg) and returned to their home cages. Injected doses were chosen based on previous research,35-37 and concentrations for aerosol were determined by applying ICR mouse respiration data38 and aerosol delivery system settings to injection doses. Fifteen minutes after the end of aerosol exposure or 30 minutes after injection, temperature and tail flick latency were measured again, after which mice were immediately placed into locomotor chambers for a 10-minute session. Temperature was then measured a third time 25 or 40 minutes after the end of exposure or injection, respectively. The choice of initial post-session time point for measurement of temperature and tail flick for injected compounds was based on our previous behavioral research with cannabinoids administered via intraperitoneal injection.35,37 Because inhaled compounds were expected to reach the brain quicker, the initial time point for measurement with this route of administration was shortened by half (ie, from 30 to 15 minutes). For both routes, temperature was measured again immediately after the mice were removed from the locomotor chambers. In summary, baseline temperature and tail flick latency were measured in separate mice for each concentration/dose of each compound. Then, each mouse was exposed to its assigned concentration/dose of compound, either through intraperitoneal injection or placement in aerosol exposure chamber for 5 minutes. Thirty minutes after injection or 15 minutes after removal from the aerosol exposure chamber, temperature and tail flick latency were measured for the second time. Immediately afterward, mice were placed in locomotor chambers where their activity was measured for 10 minutes. On removal from the locomotor chambers at the end of this session (ie, 25 or 40 minutes after aerosol exposure or injection, respectively), temperature was again measured for each mouse.
Temperature time course
After a 1-week washout, a subset of mice (n = 36) previously exposed to vehicle or a dose/concentration of CP 55,940 and AB-CHMINACA were given an additional drug administration to determine the time course of their hypothermic effects. Of the 3 original measures, temperature was chosen because it is the least invasive and does not habituate with repeated assessment (ie, like locomotor activity). CP 55,940 and AB-CHMINACA were chosen for time course testing as they produced the most robust effects on temperature for both routes during the concentration/dose effect curve determinations. Aerosol (72 mg/mL) and injected (3 mg/kg) compounds and their vehicles were administered as described above, and temperature data were collected 5, 15, 30, 60, 120, 180, 240, and 300 minutes after administration.
Data analysis
Temperature was analyzed as change in degrees from baseline to degrees after compound administration (Δ°C). Tail flick analgesia was analyzed as percent of maximum possible effect (%MPE) with a 10-second maximum latency. Analgesia was calculated as follows: 100 × [(test − baseline)/(10 − baseline)]. Locomotor activity was measured as total beam breaks in the 10-minute session. Between-subjects analyses of variance (ANOVAs) were used to analyze all dependent measures, with each compound and route of administration analyzed separately. Mixed-model ANOVAs (time × concentration) were used to analyze time course data. Significant ANOVAs were followed by Tukey post hoc tests (α = 0.05) as appropriate. Analgesia data from 1 subject injected with 0.3 mg/kg CP 55,940 were excluded from all graphs and analyses because this subject did not flick its tail during baseline assessment.
Results
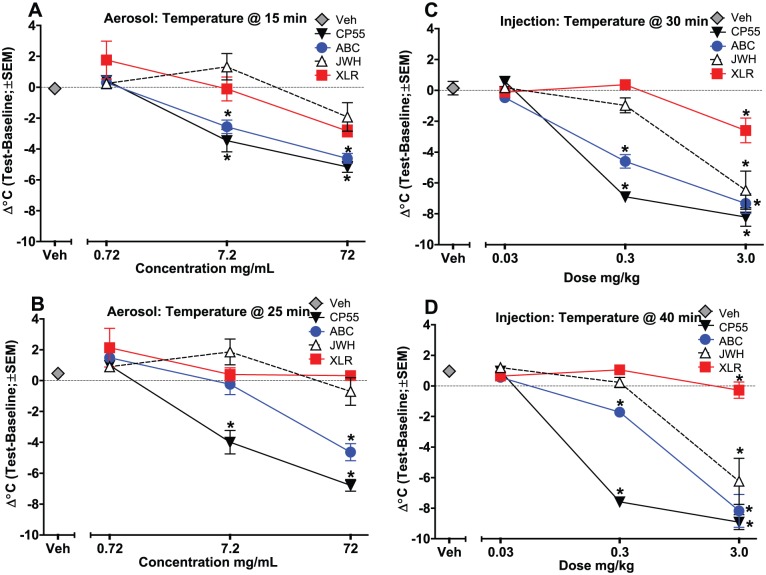
Figure 2 shows the effects of cannabinoid administration on change in temperature at 2 time points. For the first temperature measurement at 15 or 30 minutes after aerosol or injection administration, respectively, all 4 cannabinoids produced hypothermia when injected, but only CP 55,940 and AB-CHMINACA produced hypothermia when administered via aerosol (Figure 2, panels A and C) (CP 55,940 aerosol: F3,28 = 38.35, P < .001; AB-CHMINACA aerosol: F3,28 = 41.16, P < .001; CP 55,940 inject: F3,28 = 119.91, P < .001; AB-CHMINACA inject: F3,28 = 57.31, P < .001; JWH-018 inject: F3,28 = 20.15, P < .001; XLR-11 inject: F3,28 = 8.19, P < .001). The second temperature measurement at 25 or 40 minutes after administration (aerosol or injection, respectively) showed the same differentiation between the cannabinoids based on the route of administration (Figure 2, panels B and D) (CP 55,940 aerosol: F3,28 = 70.31, P < .001; AB-CHMINACA aerosol: F3,28 = 36.63, P < .001; CP 55,940 inject: F3,28 = 270.84, P < .001; AB-CHMINACA inject: F3,28 = 53.11, P < .001; JWH-018 inject: F3,28 = 20.29, P < .001; XLR-11 inject: F3,28 = 3.69, P < .05). At both time points, injection tended to produce greater decreases in temperature than did aerosol administration. Interestingly, 7.2 mg/mL AB-CHMINACA aerosol produced a hypothermic response at the first time point (15 minutes), but temperature had renormalized 10 minutes later at this concentration (Figure 2, panel B).
Figure 2.
Effects of cannabinoids on change in temperature (test − baseline), plotted as a function of dose for intraperitoneal injection and concentration for aerosol exposure. (Limitations of the system did not allow for determination of exact dose of aerosolized compound.) Panel A: 15 minutes after aerosol exposure; panel B: 25 minutes after aerosol exposure; panel C: 30 minutes after injection; and panel D: 40 minutes after injection (n = 8/group). *Significant temperature change compared with vehicle (main effect). ABC indicates AB-CHMINACA; CP55, CP 55,940; JWH, JWH-018; SEM, standard error of the mean; Veh, vehicle; XLR, XLR-11.
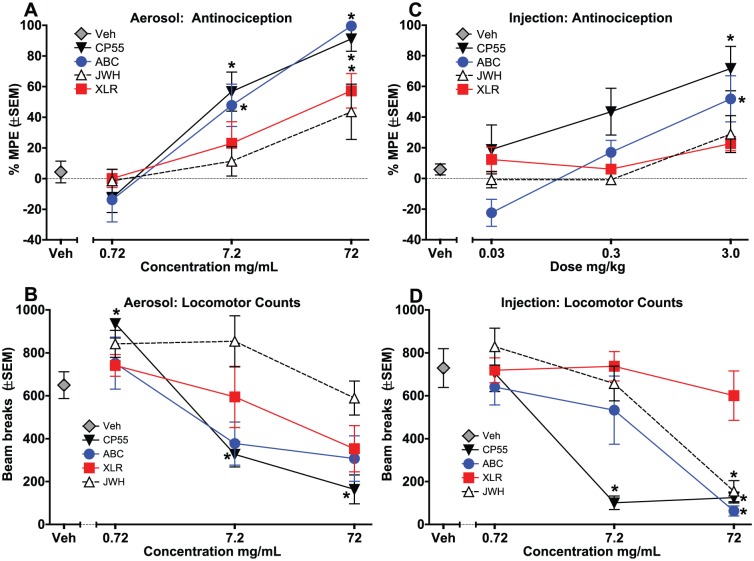
Figure 3 shows the %MPE for tail flick analgesia after aerosol exposure (panel A) and injection (panel C), and percent inhibition of locomotor activity for aerosol exposure (panel B) and injection (panel D). CP 55,940 and AB-CHMINACA produced dose-dependent analgesia for both routes of administration (Figure 3, panels A and C) (CP 55,940 aerosol: F3,28 = 24.65, P < .001; AB-CHMINACA aerosol: F3,28 = 22.73, P < .001; CP 55,940 inject: F3,28 = 4.95, P < .01; AB-CHMINACA inject: F3,28 = 10.05, P < .001), whereas XLR-11 only produced analgesia via aerosol (Figure 3, panel A) (aerosol: F3, 28 = 6.66, P < .01), and JWH-018 did not produce analgesia through either route. Visual inspection of the data revealed that the analgesic effects of CP 55,940, AB-CHMINACA, and XLR-11 were more robust after aerosol exposure than following injection. Aerosol exposure produced biphasic effects on locomotor activity, with locomotor increases at low concentrations and decreases at high concentrations, although effects were only significant for CP 55,940 (Figure 3, panel B) (CP 55,940: F3,28 = 28.74, P < .001). In contrast, injection produced only decreases in activity for 3 of the 4 compounds (Figure 2, panel D) (CP 55,940: F3,28 = 28.24, P < .001; AB-CHMINACA: F3,28 = 8.60, P < .001; JWH-018: F3,28 = 14.68, P < .001).
Figure 3.
Effects of cannabinoids on tail flick analgesia (panels A and C) and locomotor activity (panels B and D), plotted as a function of dose for intraperitoneal injection and concentration for aerosol exposure. (Limitations of the system did not allow for determination of exact dose of aerosolized compound.) Aerosol effects are shown in panels A and B, and injection effects are shown in panels C and D (n = 8/group except n = 7 for 0.3 mg/kg CP55 in panel C). *Significant effect compared with vehicle (main effect). ABC indicates AB-CHMINACA; CP55, CP 55,940; JWH, JWH-018; SEM, standard error of the mean; Veh, vehicle; XLR, XLR-11.
Figure 4 shows the effects of aerosolized (panel A) and injected (panel B) CP 55,940 and AB-CHMINACA on temperature over the course of 5 hours. As expected, both cannabinoids produced hypothermia compared with vehicle, regardless of the route of administration; however, the time course of this effect differed for both compounds across the route of administration (concentration × time interactions: CP 55,940 aerosol: F3,28 = 97.36, P < .001; AB-CHMINACA aerosol: F3,28 = 28.74, P < .001; CP 55,940 inject: F3,28 = 35.63, P < .001; AB-CHMINACA inject: F3,28 = 94.82, P < .001). Although aerosolized vehicle did not decrease temperature compared with its own pre-exposure baseline over the 5-hour period, both cannabinoids produced significant hypothermia within 5 minutes (compared with vehicle), with maximal hypothermic effect occurring at 15 minutes for AB-CHMINACA and 30 minutes for CP 55,940 (Figure 4, panel A). Visual inspection revealed that the magnitude of the temperature drop for the 2 compounds differed substantially: −3.69°C for AB-CHMINACA and −5.8°C for CP 55,940. Furthermore, mice exposed to aerosolized AB-CHMINACA showed rapid recovery. Their body temperatures had returned to baseline by 60 minutes after exposure. In contrast, mice exposed to aerosolized CP 55,940 showed more gradual recovery, with body temperatures not returning to baseline until 240 minutes after exposure (Figure 4, panel A).
Figure 4.
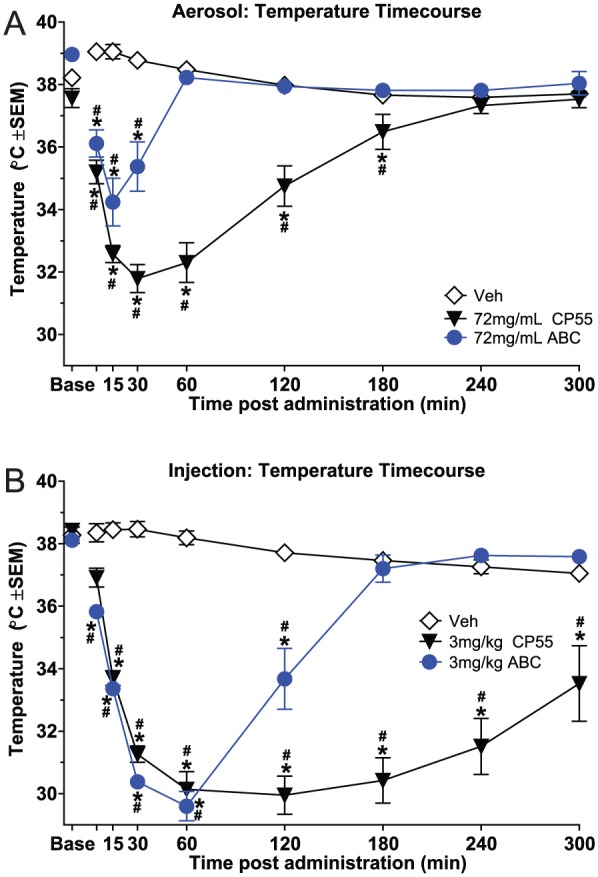
Time course of drug effects on body temperature (°C), plotted as a function of minutes after administration (n = 8/group). Panel A shows aerosol effects for vehicle and 72 mg/mL CP 55,940 and AB-CHMINACA, and panel B shows intraperitoneal injection effects for vehicle and 3 mg/kg CP 55,940 and AB-CHMINACA. (Limitations of the system did not allow for determination of exact dose of aerosolized compound.) *Significant temperature change compared with vehicle at the same time point (concentration × time interaction); #significant temperature change compared with baseline for the same dose (concentration × time interaction). ABC indicates AB-CHMINACA; Base, baseline; CP55, CP 55,940; SEM, standard error of the mean; Veh, vehicle.
Both synthetic cannabinoids also produced hypothermia following injection (Figure 4, panel B). Although injected vehicle did not affect temperature over the 5-hour time course, CP 55,940 and AB-CHMINACA produced comparable drops in temperature over the first 60 minutes after injection. Maximal magnitude of this effect was observed at 60 minutes and was similar for both compounds (−8.5°C); however, the duration of hypothermia differed. Mice injected with AB-CHMINACA exhibited a return to baseline temperature by 180 minutes after injection. In contrast, mice injected with CP 55,940 remained profoundly hypothermic at 180 minutes and showed only a gradual increase in body temperature over the next 2 hours. Body temperature was still not at baseline by the end of the 5-hour period of measurement.
Discussion
The first step in validation of the novel preclinical mouse model of vaping cannabinoids presented here was to demonstrate that the selected set of bioassays were sensitive to the test compounds delivered via the typical injected route of administration. To this end, results showed that systemic (intraperitoneal) injection with the 4 synthetic cannabinoids dose dependently produced 1 or more overt effects that have been previously associated with psychoactive cannabinoids, including THC25: CP 55,940 and AB-CHMINACA suppressed locomotor activity, induced analgesia, and decreased body temperature; JWH-018 decreased locomotor activity and body temperature without effect on the analgesia measure; and XLR-11 produced mild hypothermia without affecting the other 2 measures. Unlike CP 55,940 and AB-CHMINACA, JWH-018 and XLR-11 did not elicit the entire profile of cannabinoid effects. The most likely explanation for this finding is that the doses of these 2 compounds tested in this study were not high enough, as previous research has shown that all 4 of these synthetic cannabinoids produced potent cannabimimetic effects in the mouse test battery and in THC drug discrimination,18,32,35-37,39 an animal model of cannabis intoxication.40
Furthermore, the rank-order potencies of the compounds in producing in vivo cannabinoid effects following intraperitoneal injection were generally in accordance with their binding affinities for the CB1 receptor in this study and in previous studies (CP 55,940 > AB-CHMINACA > JWH-018 > XLR-11),18,35,37 ie, compounds with greater affinity showed greater potency, one of the hallmarks of a receptor-mediated event. Previous research showed that the pharmacological effects of THC and these synthetic cannabinoids were reversed by coadministration of the selective CB1 receptor antagonist, rimonabant,32,35-37 another hallmark of a receptor-mediated event. Together, these findings confirm that the pharmacological effects (ie, behavioral phenotype) produced by systemic injection of the 4 synthetic cannabinoids were sufficiently robust to serve as comparison controls for evaluation of the e-cigarette cannabinoid vaping model.
When aerosolized, CP 55,940 and AB-CHMINACA again produced cannabimimetic effects in the 3 bioassays, suggesting that the novel e-cigarette device delivered pharmacologically relevant doses of compound to the mice. Furthermore, the concentration-dependent nature of the responses demonstrated a degree of control by dose. Interestingly, the lowest concentration of CP 55,940 stimulated activity, whereas the 2 higher concentrations inhibited it, a biphasic effect on motor activity that has been reported previously following cannabinoid injection.41 Although these 2 cannabinoids have not previously been administered via inhalation, nose-only or whole-body exposure to the smoke of combusted JWH-018 and another indole-derived synthetic cannabinoid (JWH-073) elicited cannabimimetic effects in mice,16,17 as did marijuana smoke.17 The present results extend these previous findings by demonstrating that 2 other synthetic cannabinoids that are structurally distinct from JWH-018, and that are highly potent when injected, also produce cannabimimetic effects when inhaled. In addition, the present results represent the first report of effective delivery of synthetic cannabinoids to mice via e-cigarette technology (ie, aerosol vs smoke), although others have reported that aerosolized THC induced cannabimimetic effects in rats12 and mice.42
In contrast to AB-CHMINACA and CP 55,940, aerosolized JWH-018 and XLR-11 did not produce the full profile of cannabimimetic effects typically observed following injection with these compounds. These 2 compounds have lower affinity for the CB1 receptor and are less potent in vivo than CP 55,940 and AB-CHMINACA,18,37 suggesting that one issue may be failure to assess high enough concentrations. Indeed, aerosolized THC (10 mg/mL) itself did not produce hypothermia or suppression of locomotor activity when administered to mice via “nose-only” exposure.42 In addition, the concentrations of aerosolized drugs of several classes (eg, nicotine, cannabinoids, and stimulants) required to produce overt pharmacological effects in rodents usually exceed amounts needed for systemic injection.12,33,34,43 In this study, the need for higher concentrations of JWH-018 and XLR-11 was hampered by their limited solubility in the vehicle of propylene glycol and glycerin, a vehicle that is also commonly used in e-liquids formulated for human use. Nevertheless, aerosolized JWH-018 and XLR-11 did produce mild hypothermia and analgesia, with XLR-11 also suppressing activity to a limited extent. In addition, although the magnitude of hypothermia observed with aerosolized JWH-018, XLR-11, CP 55,940, and AB-CHMINACA was less than or equal to that seen following injection, aerosol exposure enhanced the analgesic efficacy of all of the synthetic cannabinoids except JWH-018. Although beyond the scope of this study, further research is warranted to uncover reasons for this difference.
Not surprisingly, the time course for hypothermia conducted with CP 55,940 and AB-CHMINACA revealed that maximal temperature drop occurred faster with aerosol exposure (15 and 30 minutes, respectively) vs injection (60 minutes for both). Furthermore, duration of effect differed across route, with CP 55,940–induced hypothermia lasting approximately 180 minutes after aerosol exposure, but over 300 minutes following injection. Similarly, the duration of hypothermia for aerosolized AB-CHMINACA was 30 minutes compared with 120 minutes after injection. In humans, data are not yet available on time course of synthetic cannabinoid effects; however, the latency to onset of effects after smoking synthetic cannabinoids and cannabis was rapid and comparable with the findings in mice reported here, with peak effect reported at 5 minutes for synthetic cannabinoids and 6 to 10 minutes for cannabis.44 Humans also reported variability in the duration of intoxication after smoking products containing synthetic cannabinoids, with a range from 1 to more than 5 hours.45 This variable duration of action across synthetic cannabinoids was represented here through differences in the hypothermia time course for AB-CHMINACA vs CP 55,940. One particular concern with quick-onset and short-acting synthetic cannabinoids is that users will smoke or vape more frequently to maintain their high, thereby also exposing themselves to greater potential for toxicities of these compounds.
In summary, results of the present experiment demonstrate the feasibility of using e-cigarette technology to deliver synthetic cannabinoid aerosol to mice for the purpose of investigating the pharmacological and toxicological effects of these substances of abuse. Although determination of the actual dose to which the mouse was exposed remains a limitation of the current apparatus, variation in concentrations was associated with systematic changes in the measures (ie, concentration dependence), suggesting that mice in the different concentration groups were exposed to different doses. To address this limitation in a previous similar study with nicotine, plasma and brain levels of nicotine and its metabolite cotinine were measured in an attempt to equate exposure levels across intraperitoneal injection and aerosol inhalation.34 This step would be more difficult with synthetic cannabinoids, for which metabolites may not yet have been identified. Despite this limitation, the ability to administer cannabinoids to rodents using the most common route of administration among humans provides a method for collecting preclinical data with enhanced translational relevance.
Acknowledgments
The authors thank Ricardo Cortes and Nikita Pulley for their excellent technical assistance.
Footnotes
Peer review:Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 2089 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by RTI International internal research and development funds and NIH/NIDA grants DA-003672 and DA-040460. The funding sources had no role other than financial support.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed in a significant way to the manuscript and have read and approved the final manuscript.
References
- 1. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey Results on Drug Use, 1975-2015: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2016. [Google Scholar]
- 2. Okaneku J, Vearrier D, McKeever RG, LaSala GS, Greenberg MI. Change in perceived risk associated with marijuana use in the United States from 2002 to 2012. Clin Toxicol (Phila). 2015;53:151-155. [DOI] [PubMed] [Google Scholar]
- 3. Cerda M, Wall M, Keyes KM, Galea S, Hasin D. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, vaping, and eating for health or fun: marijuana use patterns in adults, U.S., 2014. Am J Prev Med. 2016;50:1-8. [DOI] [PubMed] [Google Scholar]
- 5. Morean ME, Kong G, Camenga DR, Cavallo DA, Krishnan-Sarin S. High school students’ use of electronic cigarettes to vaporize cannabis. Pediatrics. 2015;136:611-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castellanos D, Gralnik LM. Synthetic cannabinoids 2015: an update for pediatricians in clinical practice. World J Clin Pediatr. 2016;5:16-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trecki J, Gerona RR, Schwartz MD. Synthetic cannabinoid-related illnesses and deaths. N Engl J Med. 2015;373:103-107. [DOI] [PubMed] [Google Scholar]
- 8. Tyndall JA, Gerona R, De Portu G, et al. An outbreak of acute delirium from exposure to the synthetic cannabinoid AB-CHMINACA. Clin Toxicol (Phila). 2015;53:950-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Center for Disease Control and Prevention. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93-98. [PMC free article] [PubMed] [Google Scholar]
- 10. Ghosh T, Herlihy R, Van Dyke M, et al. Notes from the field: severe illness associated with reported use of synthetic marijuana—Colorado, August-September 2013. MMWR Morb Mortal Wkly Rep. 2013;62:1016-1017. [PMC free article] [PubMed] [Google Scholar]
- 11. Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen JD, Aarde SM, Vandewater SA, et al. Inhaled delivery of Δ(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropsychopharmacology. 2016;109:112-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruijnzeel AW, Qi X, Guzhva LV, et al. Behavioral characterization of the effects of cannabis smoke and anandamide in rats. PLoS ONE. 2016;11:e0153327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE. A vapourized Δ(9)-tetrahydrocannabinol (Δ(9)-THC) delivery system part I: development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J Pharmacol Toxicol Methods. 2014;70:120-127. [DOI] [PubMed] [Google Scholar]
- 15. Manwell LA, Ford B, Matthews BA, Heipel H, Mallet PE. A vapourized Δ(9)-tetrahydrocannabinol (Δ(9)-THC) delivery system part II: comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J Pharmacol Toxicol Methods. 2014;70:112-119. [DOI] [PubMed] [Google Scholar]
- 16. Marshell R, Kearney-Ramos T, Brents LK, et al. In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Δ9-THC in mice: inhalation versus intraperitoneal injection. Pharmacol Biochem Behav. 2014;124:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE. Inhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in mice. Drug Alcohol Depend. 2012;126:316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiley JL, Compton DR, Dai D, et al. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995-1004. [PubMed] [Google Scholar]
- 19. Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behav Pharmacol. 2014;25:750-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jarbe TU, Gifford RS. “Herbal incense”: designer drug blends as cannabimimetics and their assessment by drug discrimination and other in vivo bioassays. Life Sci. 2014;97:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brents LK, Gallus-Zawada A, Radominska-Pandya A, et al. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brents LK, Reichard EE, Zimmerman SM, et al. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS ONE. 2011;6:e21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bell S, Nida C. Pyrolysis of drugs of abuse: a comprehensive review. Drug Test Anal. 2015;7:445-456. [DOI] [PubMed] [Google Scholar]
- 24. Thomas BF, Lefever TW, Cortes RA, et al. Thermolytic degradation of synthetic cannabinoids: chemical exposures and pharmacological consequences. J Pharmacol Exp Ther. 2017;361:162-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin BR, Compton DR, Thomas BF, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471-478. [DOI] [PubMed] [Google Scholar]
- 26. Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471:185-193. [DOI] [PubMed] [Google Scholar]
- 27. Uchiyama N, Shimokawa Y, Kikura-Hanajiri R, et al. A synthetic cannabinoid FDU-NNEI, two 2-indazole isomers of synthetic cannabinoids AB-CHMINACA and NNEI indazole analog (MN-18), a phenethylamine derivative -OH-EDMA, and a cathinone derivative dimethoxy-alpha-PHP, newly identified in illegal products. Forensic Toxicol. 2015;33:244-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Louis A, Peterson BL, Couper FJ. XLR-11 and UR-144 in Washington state and state of Alaska driving cases. J Anal Toxicol. 2014;38:563-568. [DOI] [PubMed] [Google Scholar]
- 29. Grabenauer M, Krol WL, Wiley JL, Thomas BF. Analysis of synthetic cannabinoids using high-resolution mass spectrometry and mass defect filtering: implications for nontargeted screening of designer drugs. Anal Chem. 2012;84:5574-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605-613. [PubMed] [Google Scholar]
- 31. Compton DR, Rice KC, De Costa BR, et al. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218-226. [PubMed] [Google Scholar]
- 32. Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR. Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology. 1995;34:669-676. [DOI] [PubMed] [Google Scholar]
- 33. Marusich JA, Lefever TW, Blough BE, Thomas BF, Wiley JL. Pharmacological effects of methamphetamine and alpha-PVP vapor and injection. Neurotoxicology. 2016;55:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lefever TW, Lee YOK, Kovach AL, et al. Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug Alcohol Depend. 2016;172:80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wiley JL, Marusich JA, Lefever TW, et al. AB-CHMINACA, AB-PINACA, and FUBIMINA: affinity and potency of novel synthetic cannabinoids in producing Δ9-tetrahydrocannabinol-like effects in mice. J Pharmacol Exp Ther. 2015;354:328-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiley JL, Marusich JA, Lefever TW, Lefever TW. Cross-substitution of Δ9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol Biochem Behav. 2014;124:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN and, Thomas BF. Cannabinoids in disguise: Δ9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fairchild GA. Measurement of respiratory volume for virus retention studies in mice. Appl Microbiol. 1972;24:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201-209. [PubMed] [Google Scholar]
- 40. Balster RL, Prescott WR. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55-62. [DOI] [PubMed] [Google Scholar]
- 41. Katsidoni V, Kastellakis A, Panagis G. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol. 2013;16:2273-2284. [DOI] [PubMed] [Google Scholar]
- 42. Lichtman AH, Peart J, Poklis JL, et al. Pharmacological evaluation of aerosolized cannabinoids in mice. Eur J Pharmacol. 2000;399:141-149. [DOI] [PubMed] [Google Scholar]
- 43. Nguyen JD, Aarde SM, Cole M, Vandewater SA, Grant Y, Taffe MA. Locomotor stimulant and rewarding effects of inhaling methamphetamine, MDPV, and mephedrone via electronic cigarette-type technology. Neuropsychopharmacology. 2016;41:2759-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winstock AR, Barratt MJ. Synthetic cannabis: a comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend. 2013;131:106-111. [DOI] [PubMed] [Google Scholar]
- 45. Barratt MJ, Cakic V, Lenton S. Patterns of synthetic cannabinoid use in Australia. Drug Alcohol Rev. 2013;32:141-146. [DOI] [PubMed] [Google Scholar]