Abstract
Regulatory and functional aspects of the kynurenine (K) pathway (KP) of tryptophan (Trp) degradation are reviewed. The KP accounts for ~95% of dietary Trp degradation, of which 90% is attributed to the hepatic KP. During immune activation, the minor extrahepatic KP plays a more active role. The KP is rate-limited by its first enzyme, Trp 2,3-dioxygenase (TDO), in liver and indoleamine 2,3-dioxygenase (IDO) elsewhere. TDO is regulated by glucocorticoid induction, substrate activation and stabilization by Trp, cofactor activation by heme, and end-product inhibition by reduced nicotinamide adenine dinucleotide (phosphate). IDO is regulated by IFN-γ and other cytokines and by nitric oxide. The KP disposes of excess Trp, controls hepatic heme synthesis and Trp availability for cerebral serotonin synthesis, and produces immunoregulatory and neuroactive metabolites, the B3 “vitamin” nicotinic acid, and oxidized nicotinamide adenine dinucleotide. Various KP enzymes are undermined in disease and are targeted for therapy of conditions ranging from immunological, neurological, and neurodegenerative conditions to cancer.
Keywords: kynureninase; kynurenine aminotransferase; indoleamine 2,3-dioxygenase; tryptophan 2,3-dioxygenase; tryptophan disposition; nicotinamide
Introduction
In addition to its indispensable role in protein synthesis, the essential amino acid l-tryptophan (Trp) is the precursor of many physiologically important metabolites produced during the course of its degradation along 4 pathways, 3 of which are of quantitatively minor significance, with the fourth, the kynurenine (K) pathway (KP), accounting for ~95% of overall Trp degradation.1,2 The 3 minor pathways (and their important products) are (1) hydroxylation (serotonin, 5-hydroxytryptamine or 5-HT in brain, and melatonin in the pineal); (2) decarboxylation (tryptamine); and (3) transamination (indolepyruvic acid [IPA]). The KP produces many biologically active metabolites, including the important redox cofactors oxidized nicotinamide adenine dinucleotide (phosphate) [NAD+(P+)] and their reduced forms NAD(P)H [reduced nicotinamide adenine dinucleotide (phosphate)], pellagra-preventing factor, niacin (collectively referring the to nicotinamide and nicotinic acid) or vitamin B3, Zn-binding compound picolinic acid (PA), N-methyl-d-aspartate (NMDA) receptor agonist quinolinic acid (QA) and antagonist kynurenic acid (KA), neuroactive K, and immunosuppressive K metabolites 3-hydroxykynurenine (3-HK) and 3-hydroxyanthranilic acid (3-HAA), with QA and PA also similarly active. The KP has received greater attention in recent years with the discovery of new and important roles its products play in health and disease and, in particular, conditions associated with immune dysfunction and central nervous system disorders. This article will be concerned mainly with the enzymatic, regulatory, and functional features of the KP with brief reference to clinical consequences of disturbances in activity of the pathway enzymes. This review is not intended to be exhaustive, but will cover the main features of the KP and, where appropriate, will refer to original sources.
General Description of the KP
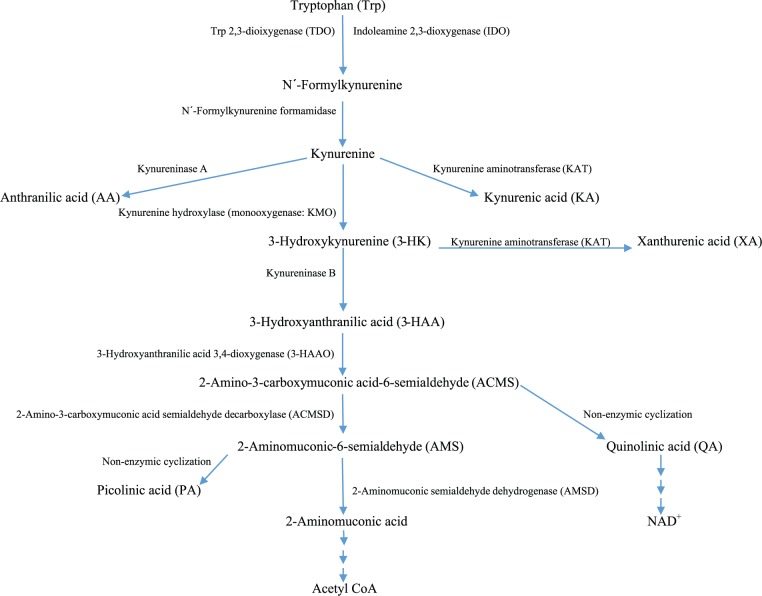
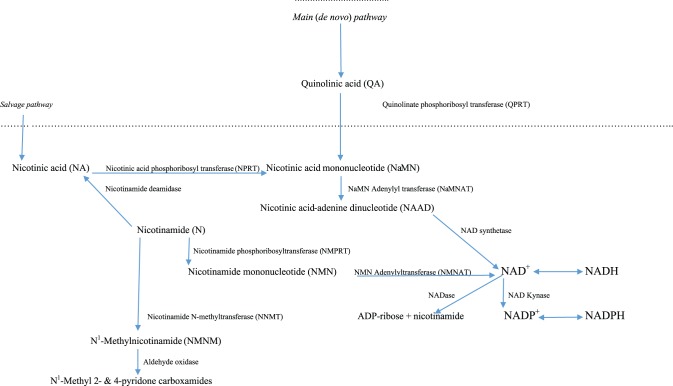
The KP exists mainly in the liver, which contains all the enzymes necessary for NAD+ synthesis from Trp and is responsible for ~90% of overall Trp degradation under normal physiologic conditions. The KP also exists extrahepatically, but its contribution to Trp degradation is normally minimal (5%-10%) but becomes quantitatively more significant under conditions of immune activation.3 The extrahepatic KP does not include all enzymes of the pathway, and this determines which intermediates are produced and hence the type of functional modulation that results. An outline of the KP is given in Figure 14 and of the NAD+ biosynthetic pathway from QA and via the salvage pathway in Figure 2.4
Figure 1.
The kynurenine pathway of tryptophan degradation.
Figure 2.
NAD+(P+) synthesis from quinolinic acid (main pathway) and nicotinic acid (salvage pathway).
Tryptophan is converted to N′-formylkynurenine (NFK) by the action of either Trp 2,3-dioxygenase (TDO, formerly Trp pyrrolase; EC 1.13.11.11) mainly in liver or indoleamine 2,3-dioxygenase (IDO; EC 1.13.11.17) extrahepatically. N′-formylkynurenine is then hydrolyzed to kynurenine (K) by NFK formamidase (FAM). Kynurenine is metabolized mainly by hydroxylation to 3-HK by K hydroxylase (K monooxygenase [KMO]) followed by hydrolysis of 3-HK to 3-HAA by kynureninase. This latter enzyme can also hydrolyze K to anthranilic acid (AA). Both K and 3-HK can also be transaminated to KA and xanthurenic acid (XA) by K aminotransferase (KAT). For the sake of simplicity, KAT will be described as KAT A (K → KA) and KAT B (3-HK → XA), although 4 isoforms exist (KAT I, II, III, and IV). The KAT reactions are normally of minor significance because of the high Km for its 2 substrates, compared with those of KMO and kynureninase, but can be enhanced after loading with Trp and/or K.1 KAT A can also be enhanced after inhibition of KMO.5 The most active among KP enzymes is 3-HAA 3,4-dioxygenase (oxidase: 3-HAAO).6,7 The unstable product of this reaction, acroleyl aminofumarate (2-amino-3-carboxymuconate-6-semialdehyde [ACMS]), occupies a central position at 2 junctions in the KP. The pathway favors its nonenzymic cyclization to QA and hence production of NMN and then NAD+. Picolinic acid is also formed by nonenzymic cyclization of aminomuconic acid semialdehyde. However, PA formation depends on extent of the substrate saturation of the enzyme (2-aminomuconic acid semialdehyde dehydrogenase) competing with this cyclization. Only when such saturation is maximal, PA production can proceed at a faster rate.
Substrates, Cofactors, and Tissue Distribution of Enzymes of the KP
These are outlined in Table 1, the information of which is derived from literature data referred to in the text and also the BRENDA (BRaunschweig ENzyme DAtabase) database for enzymes.8 The l-Trp-specific TDO and mainly liver-specific TDO use O2 as cosubstrate and heme as cofactor. The extrahepatic IDO, by contrast, can oxidize a broad range of substrates, including d-Trp, indoles, and indoleamines. It also uses molecular O2 as cosubstrate and not superoxide as was previously assumed9 and, although it is also a hemoprotein, it exists in the active holoenzyme form that cannot be further activated by heme. Some of the properties of TDO and IDO have been compared.10 Affinity for Trp is one important difference. Although TDO has a much lower affinity but higher capacity for Trp, with a Km of 100 µM in rat hepatocytes, 400 µM in human liver, and 190 µM in the purified human enzyme, IDO has a much greater affinity and lower capacity, with Km values of 3 to 50 µM in various sources.10 Of important relevance to functional differences is that IDO activity, unlike that of TDO, can be substrate inhibited by high [Trp], with a Ki of ⩾200 µM10 (see below).
Table 1.
Enzymes of the kynurenine pathway.
| Enzyme | EC number | Substrate(s) | Product(s) | Cofactor | Km, µM | Main sources |
|---|---|---|---|---|---|---|
| TDO | 1.13.11.11 | l-Trp, O2 | NFK | Heme | 190 | Liver, CNS |
| IDO | 1.13.11.17 | l-Trp, various, O2 | NFK | Heme | 20–50 | Placenta, lung, intestine, others |
| FAM | 3.5.1.9 | NFK | K + formate | None | 50–180 | Liver, kidney, brain |
| KMO | 1.14.13.9 | K + O2 | 3-HK | NADPH | 14–25 | Liver, kidney, CNS, placenta |
| Kynase A | 3.7.1.3 | K | AA | PLP | 1000 | Liver, kidney, wide tissue distribution |
| Kynase B | 3.7.1.3 | 3-HK | 3-HAA | PLP | 77 | Same as above |
| KAT A | 2.6.1.7 | K | KA | PLP | 960–4700 | Liver, kidney, brain, heart |
| KAT B | 2.6.1.7 | 3-HK | XA | PLP | 3800–5700 | Liver, kidney, brain |
| 3-HAAO | 1.13.11.6 | 3-HAA + O2 | ACMS | Fe2+ | 2-3.6 | Liver, kidney, brain |
| ACMSD | 4.1.1.45 | ACMS | AMS + CO2 | Nil | 6.5-14 | Kidney, liver, various organs |
| AMSD | 1.2.1.32 | 2-AMSD + H2O | 2-AMA + 2 H+ | NAD+ | 11–26 | Liver |
| QPRT | 2.4.2.19 | QA + PRPP | NaMN + PPi + CO2 | Nil | 21.6 | Liver, kidney |
| NPRT | 2.4.2.11 | NA + PRPP | NaMN + PPi | Nil | 1 | Liver. kidney, pancreas, heart |
| NMPRT | 2.4.2.12 | NM + PRPP | NMN + PPi | Nil | 0.855–42 | Liver, kidney, spleen, heart, brain |
| NMD | 3.5.1.19 | NM + H2O | NA + NH3 | Nil | 0.2–50 | Liver, small intestine |
| NMNAT | 2.7.7.18 | NMN (NaMN) + ATP | NAD+ (NaAD+) + PPi | Nil | 21–209 | Liver, kidney, spleen, pancreas, brain |
| NADS2 | 6.3.1.5 | NaAD+ + ATP + NH3 | NAD+ + AMP + PPi | Nil | 130–490 | Brain, human cells, kidney |
| NADS1 | 6.3.1.5 | NaAD+ + ATP + Gln/NH3 | Same as above + Glu | Nil | 130–490 | Small intestine, kidney, liver, testis |
| NAD kynase | 2.7.1.23 | NAD+ + ATP | NADP+ + ADP | Nil | 110–6500 | Various (Km: 0.22 mM in brain) |
| NADase | 3.2.2.5 | NAD+ + H2O | ADP-ribose + nicotinamide | Nil | 28–260 | Lung, serum, blood, and other cells |
| NNMT | 2.1.1.1 | Nicotinamide + SAM | NMNM + SAC | Nil | 105–400 | Liver, lung, muscle, kidney, heart, adipose |
| NMNO | 1.2.3.1 | NMN + H2O + O2 | 4-Py + 2-Py + H2O2 | FAD+ | 355–1895 | Liver, kidney, brain, adrenals, etc |
Abbreviations: 3-HAA, 3-hydroxyanthranilic acid; 3-HAAO, 3-hydroxyanthranilic acid oxygenase; 3-HK, 3-hydroxykynurenine; AA, anthranilic acid; ACMS, 2-amino-3-carboxymuconate-6-semialdehyde; ACMSD, 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase or picolinate carboxylase; ADP, adenosine diphosphate; AMS, alpha-aminomuconate semialdehyde; AMSD, 2-aminomuconic-6-semialdehyde dehydrogenase; CNS, central nervous system; EC, Enzyme Commission; FAD+, oxidized flavin adenine dinucleotide; FAM, formylkynurenine formamidase; IDO, indoleamine 2,3-dioxygenase; KA, kynurenic acid; KAT, kynurenine aminotransferase; KMO, kynurenine monooxygenase or kynurenine hydroxylase; Kynase, kynureninase; NADase, NAD+ glycohydrolase; NADP+, oxidized nicotinamide adenine dinucleotide phosphate; NADS, NAD+ synthetase; NaMN, nicotinic acid mononucleotide; NFK, N′-formylkynurenine; NM, nicotinamide; NMD, nicotinamide deamidase; NMN, nicotinamide mononucleotide; NMNAT, nicotinamide/nicotinic acid mononucleotide adenylyltransferase; NMNO, N-methylnicotinamide oxidase or aldehyde oxidases; NMPRT, nicotinamide phosphoribosyltransferase; NNMT, nicotinamide N-methyltransferase; NPRT, nicotinate phosphoribosyltransferase; PLP, pyridoxal 5′-phosphate; QPRT, quinolinate phosphoribosyltransferase; SAC, S-adenosyl cysteine; SAM, S-adenosyl methionine; TDO, Trp 2,3-dioxygenase;XA, xanthurenic acid.
Data were obtained from the general literature and www.brenda-enzymes.org.8
Although TDO is mainly liver specific, it also exists in brain, with 2 variants having been identified at relatively different expression levels in various mouse brain structures and suggested to play important roles during postnatal developent.11 Trp 2,3-dioxygenase is also actively expressed in brain tumors12 (see further below). Indoleamine 2,3-dioxygenase has a much wider tissue distribution, including peripheral blood and other immune cells. Indoleamine 2,3-dioxygenase exists in 2 forms: the traditional IDO 1 and the more recently discovered IDO 2. The latter is expressed in murine kidney, liver, and the male and female reproductive systems.13,14 The human IDO 2 is expressed in kidney, liver, and brain and has a much higher Km for l-Trp than IDO 1 and a much reduced catalytic activity.15 However, as will be described below, IDO 2 exerts important immunoregulatory activity under certain conditions.
N-formylkynurenine formamidase exists in liver, kidney, and brain and is very active in hydrolyzing NFK to K and formate, with the recombinant mouse liver enzyme exhibiting a catalytic rate of 42 µmol/min/mg of protein and a high capacity for NFK (Km: 180-190 µM).16
Kynurenine monooxygenase (or kynurenine hydroxylase) is a mitochondrial flavoprotein that uses O2 as cosubstrate and NADPH as cofactor.17 As a flavin adenine dinucleotide–dependent enzyme, its activity can be expected to be decreased in riboflavin (vitamin B2) deficiency, as suggested by a 10-fold decrease in 3-HK, a 3-fold decrease in both 3-HAA and N-methyl nicotinamide, and a 2-fold increase in KA urinary excretion in riboflavin-deficient baboons.18 Decreased KMO in a 9-year-old girl with pellagra with colitis is accompanied with increased KA and K and decreased XA excretion.19 The decreased KMO activity in B2 deficiency has been suggested to involve decreased cofactor availability because of inhibition of NAD+(P+) synthesis.20
As kynureninase (kynase) is a pyridoxal 5′-phosphate (PLP)–dependent enzyme, its activity is impaired by vitamin B6 deficiency,21–23 whether induced by malnutrition or functionally. Malnutrition-induced inhibition of kynase activity can occur in B6 deficiency or with Leu-rich diets, such as maize or sorghum.24 With Leu, it has been suggested that PLP stores may also become depleted by increased consumption during transamination of Leu by branched-chain amino acid aminotransferase.24 The pellagragenic effect of Leu additionally involves activation of liver TDO and enhancement of the flux of Trp through TDO and possibly also 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase or picolinate carboxylase (ACMSD) of renal origin24 (see further below). Drugs can also inhibit kynase activity by inducing a functional B6 deficiency through binding and thereby inactivating PLP. Examples include estrogens25,26 and hydrazine drugs, such as isoniazid, benserazide, and carbidopa.7 It is noteworthy that incidence of pellagra during the first half of the 20th century was twice as high in women than men, suggesting that estrogens may be a precipitating factor in the absence of adequate Trp or niacin intake.7 The purified recombinant human Kynase appears to selectively use 3-HK as substrate, with a Km value of 3 µM and is inhibited by l-K and d-K with Ki values of 20 and 12 µM, respectively.27 Earlier, it has been reported28 that the human liver enzyme shows a much greater preference to 3-HK (Km = 77 µM) than to K (Km = 1 mM), with a 15:1 ratio of activity toward these 2 substrates. In our recent study29 in normal US subjects of various ethnic backgrounds, we observed ratios of Kynase B:Kynase A (estimated from the fasting plasma [3-HAA]/[3-HK] and [AA]/[K] ratios, respectively) consistent with a much greater Kynase B activity, with ratios of 28:1, 20:1, and 40:1 for the whole group (n = 114), men (n = 54), and women (n = 60), respectively.
The other PLP-dependent enzyme of the KP, KAT, is also subject to inhibition in B6 deficiency21,22 and by benserazide and carbidopa.7 As stated above, the KAT reaction is of minor quantitative significance under normal conditions because of the much higher Km for its 2 substrates, K and 3-HK, compared with those for Kynase. Various aminotransferases capable of catalyzing the conversion of K to KA (and also of 3-HK to XA) exist in different organs and across species and can use a wide range of substrates. There appears to be 4 KATs (KAT I, II, III, and IV) in humans, mice, and rats. KAT I, II, III, and IV are identical to (1) glutamine aminotransferase and cysteine conjugate β lyase (CCBL), (2) α-ketoadipate aminotransferase, (3) CCBL, and (4) aspartate aminotransferase and glutamate oxaloacetate aminotransferase. In the mouse, these KATs show different thermal stabilities, pH dependence, and responses to inhibitors.30 The biochemical behaviors of KAT I and KAT III appear very similar and could reflect the same enzyme.31 In the brain, KAT II appears particularly responsible for transamination of K to KA and can almost equally act on 3-HK as on K.32 Although its Km for K and 3-HK is very high (4.7 and 3.8 mM, respectively), the irreversible nature of the transamination reaction allows for the accumulation of KA. As will be discussed below, KAT II inhibition is a strategy for lowering KA under certain neurological conditions.
3-HAA 3,4-dioxygenase (oxidase: 3-HAAO) is by far the most active among KP enzymes,6,7 which explains the rapid conversion of 3-HAA into the unstable ACMS and nonenzymic cyclization of the latter to QA. The enzyme is a nonheme iron-dependent dioxygenase that uses molecular O2 as cosubstrate and Fe2+ as cofactor. Its activity in human, mouse, and rat brain is stimulated by the addition of Fe2+ and inhibited by iron chelators.33 The important role of QA in neurodegenerative diseases emphasizes the critical role of 3-HAAO activity and its control by fluctuations in endogenous iron and proteins that regulate iron bioavailability.33 The enzyme exhibits a high affinity for its 3-HAA substrate, with a Km of 3 µM for the purified rat liver and brain enzyme34 and 2 µM for the cloned human enzyme.35
2-Amino-3-carboxymuconate-6-semialdehyde decarboxylase or picolinate carboxylase is expressed in the rat only in liver and kidney, but not in 10 other tissues and organs, with the kidney enzyme content being 2.52-fold that in liver.36 The Km values of the 2 purified enzymes toward the ACMS substrate are, however, close (14 and 13 µM, respectively).36 In humans, 2 alternatively spliced ACMSD transcripts (ACMSD I and II) have been examined in brain, liver, and kidney.37 Although both transcripts are present in kidney and liver, no ACMSD II expression is detected in brain. However, the enzymatically active ACMSD I is present at very low levels in brain.37 The enzyme exhibits a Km of 6.5 µM and is inhibited by QA, PA, and KA.37 As is the case in rats, the highest activity of ACMSD is present in the human kidney. As stated earlier, enhancement of ACMSD by Leu explains in part the pellagragenic effect of high Leu diets.38
2-Aminomuconate semialdehyde dehydrogenase catalyzes the conversion of AMS to 2-aminomuconic acid, which is further degraded to acetyl CoA. 2-Aminomuconate semialdehyde dehydrogenase has been widely studied in bacteria. In Pseudomonas pseudoalcaligenes, the enzyme can act on several analogues and shows a great identity to 2-hydroxymuconic semialdehyde,39 which is often used in kinetic studies. Its Km for the latter substrate is 11 to 26 µM. In cat (Felis catus), the Km of the enzyme for the 2-hydroxy substrate and for NAD+ is 16 and 19 µM, respectively.8
Quinolinate phosphoribosyltransferase (QPRT) is a key enzyme in the synthesis of NAD+. Quinolinate phosphoribosyltransferase uses QA and 5-phosphoribosyl-1-pyrophosphate (PRPP) as cosubstrates and produces nicotinic acid mononucleotide (NaMN), inorganic pyrophosphate (PPi), and CO2. The human enzyme exhibits a Km value of 21.6 µM for QA and of 23 µM for PRPP.40 The PRPP exerts substrate inhibition at concentrations greater than 300 µM.41 In the Wistar rat, the enzyme is localized in liver and kidney, with an activity ratio of 6:1.41
Nicotinic acid mononucleotide can also be formed by the action of nicotinate phosphoribosyltransferase (NPRT). Like QPRT, NPRT also uses PRPP as cosubstrate along with nicotinic acid. The enzyme from beef liver cytosols has a Km for nicotinic acid of 1 µM and for PRPP of 50 µM and undergoes product inhibition by NaMN.42 Nicotinate phosphoribosyltransferase is concentrated in liver, kidney, and small intestine of rats and mice but is also expressed to a lesser extent in 7 other organs, including the brain41,42 and in cultured human hepatoma HepG2, kidney epithelia HEK293, and cervical carcinoma HeLa cells.43 Nicotinamide is a poor substrate for NPRT, with a Km of 100 mM.44 Also, the recombinant human NPRT does not catalyze the formation of the mononucleotides of NMN or nicotinic acid from NMN or QA, respectively.45
In the study by Ikeda et al,41 the activity of NMN phosphoribosyltransferase (NMPRT), assayed under the same experimental conditions as those for NPRT but using NMN as substrate, was found to be lower than that of NPRT, with the activity in rat liver, kidney, brain, and heart being only 23%, 48%, 49%, and 45%, respectively,41 of that of NPRT. The NMPRT from rat liver has a Km for NMN of 42 µM,44 whereas the affinity of the human enzyme to NMN (Km = 0.855 µM) is enhanced 10-fold by adenosine triphosphate (ATP) (Km = 0.057 µM).45 Similarly, the Km of the enzyme for PRPP of 7 to 12 µM is decreased to 0.63 µby ATP.45
Nicotinamide deamidase (NMD) converts NMN to nicotinic acid, releasing ammonia in the process. The Km of the enzyme has been widely measured in bacteria and varies between 0.2 and 0.65 mM.8 In mouse liver, the Km is 1 µM,46 whereas that in rat liver is considerably higher, possibly because of the presence of an endogenous inhibitor.47
Nicotinamide/nicotinic acid mononucleotide adenylyltransferases are a group of isoenzymes, collectively referred to here as NMNATs, that transfer the adenylate moiety of ATP to the mononucleotides of nicotinamide (NMN) and nicotinic acid (NaMN) to produce NAD+ and NaAD+, respectively, plus PPi. These NMNATs are critical for NAD+ synthesis. Although the transfer reaction is reversible, net NAD+ formation is likely to be due to substrate availability. In humans, 3 nicotinate/nicotinamide mononucleotide adenylyltransferase (NMNAT) isoforms have been characterized—NMNAT 1, 2, and 3—and an excellent review of their structure, properties, and biology has been published.48 NMNAT 1 in humans has a wide tissue distribution and a dominant activity. It is most highly expressed in skeletal muscle and heart and strongly expressed in liver and kidney. NMNAT 2 is strongly expressed in brain but is also expressed in heart, skeletal muscle, and pancreas. NMNAT 3 is strongly expressed in lung and spleen and generally in tissues in which NMNAT 2 expression is weak. Subcellular localization of NMNATs also differs. Thus, NMNAT 1 activity is present in the nucleus, whereas that of NMNAT 2 and 3 is located in Golgi apparatus and mitochondrion, respectively. With NMN as substrate, the Km of NMNAT 1 and 2 is similar (21-34 µM), whereas that of NMNAT 3 is higher (66-209 µM).48 With NaMN, the Km values of the 3 isoforms are 68 to 116, 15, and 111 µM, respectively.48
NAD synthetase converts nicotinic acid adenine dinucleotide (NaAD+) to NAD+ using as cosubstrates either ATP and NH3 or ATP and glutamine (Gln) and producing, along with NAD+, either AMP and PPi or AMP + PPi + glutamate (Glu). Most studies of NAD synthetase have been performed with enzymes from various bacteria. However, the human enzyme has been studied in detail,49 and 2 species have been identified with different tissue distributions and substrate activities. Nicotinamide adenine dinucleotide synthetase 1 can use as an amide donor either NH3 or Gln, whereas NAD synthetase 2 uses only NH3. The former is strongly expressed in small intestine, kidney, and liver, whereas the latter is predominant in brain and human cells such as HepG2 and Huh7.49 The tissue distribution of 6 enzymes of NAD+ synthesis (from QPRT to NAD synthetase) in rats has been reported.50
NAD kinase catalyzes the phosphorylation of NAD+ to oxidized nicotinamide adenine dinucleotide phosphate (NADP+) using ATP and releasing adenosine diphosphate (ADP). The enzyme is widely distributed throughout organs and tissues of rats and humans.8
NAD glycohydrolase (NADase) hydrolyzes NAD+ to NMN and ADP-ribose. It occurs in many tissues, including erythrocytes and other blood cells,8 and, as will be described below, its activity can be enhanced in infections and cancers.
Nicotinamide N-methyltransferase catalyzes the methylation of nicotinamide to N1-methylnicotinamide by S-adenosyl-l-methionine (SAM), producing, along with NMN, S-adenosyl-l-cysteine. It is strongly expressed in liver, but also occurs in lung, muscle, kidney, heart, and adipose tissue. The Km of the human enzyme varies between 105 and 200 µM (mutant protein), 380 µM (recombinant protein), 400 µM (wild type), and 1360 µM (liver homogenates).8 The enzyme activity can lead to depletion of both NAD+ and SAM and thus plays very important roles in development and disease (see below).
N1-methylnicotinamide oxidases (aldehyde oxidases) oxidize this nicotinamide metabolite to N1-methyl-4-pyridone-3-carboxamide (4-Py) and N1-methyl-2-pyridone-5-carboxamide (2-Py). Little information is available on kinetics of the human enzymes toward N1-methylnicotinamide, but the Km for this substrate is much higher for the rat (0.355-1.895 mM) than the mouse (0.0255-0.1285 mM) enzyme.8 Those of the rabbit and hog liver enzymes are 0.66 and 0.36 mM, respectively.51 The enzyme has a wide human tissue distribution, including liver, kidney, brain, adrenals, lung, spleen, stomach, and testis. Urinary excretion of these 2 metabolites is used as a measure of dietary niacin intake.52 This aldehyde oxidase acts on a wide range of substrates, and it appears that the unusual characteristics of the enzyme enable it to produce 2 oxidation products (2-Py and 4-Py) from 1 substrate.51 The ratio of 2-Py to 4-Py is 100 for the rabbit, and only 3.8 for the hog, enzyme.51 The ratio in humans, determined from urinary excretion data, is ~91.52
Species Differences in KP Enzymes and Choice of Experimental Models of Trp-Related Human Diseases
There are wide species differences in the activity of the KP and its enzymes, necessitating the importance of choice of the most suitable animal model to study human diseases related to Trp metabolism. In human liver and that of rat, mouse and some, but not all, other animal species, TDO exists in both the heme-free apoenzyme and heme-containing holoenzyme in roughly equal proportions.53 Some species (including cat, gerbil, golden hamster, and guinea pig) lack the free apoenzyme and are therefore TDO deficient. They also lack the glucocorticoid induction mechanism (see below) and suffer from toxicity of an excess of Trp.53 For example, a comparative study of cat and rat showed marked differences in several enzymes of the KP other than TDO.54 Thus, cat to rat ratios of 0.4, 0.8, 0.1, 0.03, and 1.7 for liver TDO, liver kynureninase, liver KAT, kidney KAT, and liver KMO, respectively, were observed. The most remarkable difference between cat and rat is the much greater activity of ACMSD, the cat/rat ratio of which is 32.17 in liver and 4.06 in kidney.41 This high ACMSD activity in the cat renders it vulnerable to niacin and NAD+ deficiency, with the conversion of 3-HK to niacin ribonucleotides being only 11% of that in the rat.41
Significant differences in up to 6 enzymes of the KP in liver, kidney, lung, and brain have also been reported in more recent comparative studies in rats, mice, rabbits, gerbils, and guinea pigs.55,56 The distinction between these 2 groups of species based on TDO differences does not apply to IDO.56 The above TDO-deficient species are therefore unsuitable as animal models of human diseases involving Trp metabolism, although they would be valid models in studies addressing issues related to their specific KP characteristics. Rats and mice are the most common animal models in Trp-related studies. The author recommends the Wistar rat as the most suitable animal model in this regard. Significant mouse strain differences in Trp metabolism exist,3 which necessitates careful choice of the mouse strain model.
Regulation of the KP
Most previous studies on KP regulation have focused on the first and most rate-limiting enzyme, hepatic TDO. Because of its short half-life (~2 hours for the apoenzyme) and hence rapid inducibility, TDO served as a model for enzyme regulation during the 1950s and 1960s established by the pioneering work, in the United States, of W. Eugene Knox, Olga Greengard, and Philip Feigelson.57–59 Similarly, the pioneering work in Japan of Osamu Hayaishi,60 discoverer of IDO and its induction by IFN-γ, deserves recognition. The half-lives of other KP enzymes are unknown, but, even if they exhibit slower turnover rates, their important roles in regulating the pathway activity is nevertheless equally important and are best described below from a functional perspective. In this section, the main emphasis will therefore be on regulation of TDO and IDO and the flux of Trp down the pathway.
TDO regulation
Four mechanisms exist for broad regulation of TDO activity (Table 2). These are hormonal induction by glucocorticoid and some other hormones, substrate activation by Trp, cofactor activation by heme, and feedback inhibition by NAD(P)H. The main features of the first 3 mechanisms are induction of new apoenzyme synthesis by glucocorticoids, activation of preexisting apoenzyme and its stabilization by Trp, and activation of preexisting apoenzyme by heme. Glucocorticoid induction involves de novo synthesis of new apoenzyme, whereas substrate activation by Trp involves enhanced conjugation of the apoenzyme with its heme cofactor and stabilization of the holoenzyme.57–59 The increase in TDO activity after Trp administration therefore results from a combination of activation, stabilization, and normal rate of synthesis. Only Trp stabilizes TDO. Estimates of the half-life of the basal enzyme in rat liver following inhibition of further enzyme synthesis by cycloheximide showed61 that the basal holoenzyme and apoenzyme exhibit half-lives of 7.7 and 2.3 hours, respectively, and administration of a 500 mg/kg dose of Trp increases these values to 11.4 and 6.7 hours, respectively. Neither cortisol nor sources of heme prolong the TDO T1/2. Administration of a 50 mg/kg Trp dose either alone or with cortisol doubles the half-life of the apoenzyme.61 Thus, increased TDO activity can be expected in situations involving long-term provision of Trp either via nutritional or therapeutic interventions. Increased Trp breakdown along the hepatic KP leading to decreased availability of Trp for cerebral serotonin synthesis may explain in part the moderate and variable antidepressant activity of this serotonin precursor.62 The increased saturation of TDO with heme is thought to involve enhancement of heme biosynthesis by Trp at the 5-aminolevulinate (5-ALA) dehydratase and possibly also subsequent steps in the heme biosynthetic pathway.63 Although heme itself only activates TDO by virtue of being its cofactor, evidence suggests that it mediates glucocorticoid induction of TDO messenger RNA (mRNA) transcription and translation.64 Furthermore, it appears that heme regulates the TDO gene posttranslationally through enhanced phosphorylation of the α subunit of the eukaryotic initiation factor, eIF2α.65
Table 2.
Regulatory mechanisms of TDO and IDO.
| Mechanism and main feature | TDO | IDO1 |
|---|---|---|
| Glucocorticoid induction (de novo enzyme synthesis at transcription level) | + | − |
| Substrate (Trp) activation (enhanced heme conjugation + stabilization + normal synthesis) | + | (Inhibition by excess Trp through reverse binding sequence) |
| Cofactor (heme) activation (enhanced heme conjugation) | + | − |
| Feedback inhibition by NAD(P)H (allosteric modulation) | + | (Unknown) |
| Cytokine induction (IFN-γ) (enhanced transcription) | − | + |
| Inhibition by nitric oxide (by binding to heme) | (Unknown) | + |
Abbreviations: IDO, indoleamine 2,3-dioxygenase; NAD(P)H, reduced nicotinamide adenine dinucleotide (phosphate); TDO, Trp 2,3-dioxygenase; Trp, tryptophan.
Hormonal induction of TDO by glucocorticoids involves de novo enzyme synthesis effected at the transcriptional level.66,67 The TDO gene from rat liver has earlier been isolated and characterized.68 Induction of the TDO gene is mediated by glucocorticoid-responsive elements, and glucocorticoid receptors play an important role in TDO induction. In response to experimental stress in rats, a new glucocorticoid receptor, termed receptor C, appears and is associated specifically with induction of TDO, but not with that of another glucocorticoid-inducible enzyme, tyrosine aminotransferase.69,70 A potential appearance of this new receptor in humans in response to stress can be assumed to enhance TDO induction by the stress hormone cortisol and may thus undermine cerebral serotonin synthesis.62
Glucocorticoid induction of TDO in primary cultures of adult rat hepatocytes is potentiated by glucagon but inhibited by insulin and adrenaline, all of which act at the transcriptional level.67 In cultured hepatocytes, however, glucagon enhances TDO activity at the translational step provided Trp is present to restore the decreased activity to levels observed in freshly isolated cells, and this enhancement appears to be synergistic to that by glucocorticoids, thus suggesting different mechanisms of action.71 Thus, glucagon seems to exert a permissive effect on glucocorticoid induction of TDO and to act additionally in the presence of Trp. Glucagon can be replaced by cyclic adenosine monophosphate (c-AMP).71
The effects of insulin on TDO activity are controversial. Thus, although insulin inhibits TDO induction by glucocorticoids in cultured rat hepatocytes,67,71 its administration to rats does not inhibit TDO, whether administered alone72,73 or jointly with glucagon or additionally with cortisol.73 Others74 have, however, reported that insulin, as well as glucagon and dibutyryl c-AMP, increases the heme saturation of TDO by enhancing heme synthesis. Dibutyryl c-AMP and glucagon can do so via Trp following stimulation of lipolysis75,76 (see below), whereas insulin may act on TDO by enhancing heme synthesis.77 Studies on experimental (streptozotocin-induced) diabetes have resulted in greater agreement. Thus, TDO activity is enhanced78,79 and the flux of Trp through TDO is increased80 in this experimental model. It is possible that the TDO enhancement in this model is mediated by the decreases in [NAD(P)H],78 resulting in removal of the negative feedback inhibition by these end products of the KP (see below).
Although adrenaline inhibits glucocorticoid induction of TDO in hepatocytes,67 it activates TDO after administration.81,82 Noradrenaline administration also activates TDO.82 Both act via Trp by enhancing lipolysis, leading to increased release of serum albumin–bound Trp. As catecholamine release is increased in stress, a TDO enhancement can be expected in stressed individuals. The effects on TDO activity of catecholamines and cortisol in stress will be additive,61 thereby potentiating the serotonin deficiency under these conditions.
The effect of thyroxine on TDO activity is also controversial, with reports suggesting enhancement81 or no change.73,83
TDO is also regulated by a feedback mechanism involving allosteric inhibition by NAD(P)H, as has been demonstrated in vitro84,85 and after administration of agents causing increases in the hepatic levels of these reduced dinucleotides, eg, glucose and nicotinamide86 and chronic ethanol intake.2 It has been suggested1,84 that 3-HK and 3-HAA may also exert feedback inhibition of TDO activity at (in vitro) concentrations near the physiologic levels or moderately higher. However, administration of these K metabolites to rats resulting in hepatic concentrations within the in vitro inhibitory range does not cause TDO inhibition.87 In fact, 3-HK exerts no effect on TDO, whereas 3-HAA causes an enhancement. A similar effect mediated by 3-HAA is also observed after KA administration.87 These and other changes induced by the above metabolites may be important in situations where levels of immunosuppressive kynurenines are raised in certain clinical conditions.
IDO regulation
Unlike TDO, IDO in rats or mice does not require heme for its activation nor is it inducible by glucocorticoids88,89 (Table 2). Consequently, neither adrenalectomy nor starvation influence IDO activity,88 unlike the case with TDO where the former inhibits, whereas the latter enhances, TDO synthesis.88,90 Also, unlike the several-fold activation of rat liver TDO,86 administration of a large dose of Trp (750 mg/kg) enhances IDO activity in rat intestine (the largest source) by only 50%.89 This may be explained in part by a possible substrate inhibition, reported for the mouse epididymal enzyme to occur at [Trp] above 50 µM.91 The mechanism of this inhibition involves a reversed sequence of binding to the enzyme of Trp and O2.92 At low [Trp], Trp is bound first followed by O2, whereas at high [Trp], this order is reversed, with the heme reduction potential playing an important role.
The principal effector of IDO is IFN-γ,93 and a less efficient inducer is IFN-α.94 Other cytokines and mediators (both proinflammatory and anti-inflammatory) exert various effects on IDO.62 Thus, anti-inflammatory cytokines (interleukin [IL]-4, IL-10, and transforming growth factor β) inhibit IDO induction by IFN-γ. The proinflammatory IL-1β and tumor necrosis factor α potentiate this induction and IL-2 acts via IFN-γ. Thus, the IDO status can be assumed to be determined by the balance between proinflammatory and anti-inflammatory cytokines. Interestingly, IFN-γ induction of IDO is potentiated by the synthetic glucocorticoid dexamethasone, which exerts no effect by itself.94
Nitric oxide (NO) may play an important role in the control of IDO activity. It inhibits the human enzyme.95 Activity of the recombinant human IDO is reversibly inhibited by NO by binding to heme, with the inactivated enzyme complex being the Fe2+-NO-Trp adduct.96 This inhibition may represent an important mechanism of regulating the immune function of IDO.96
Regulation of the flux of tryptophan down the KP
The flux of Trp down the KP is determined primarily by plasma free Trp and secondarily by activities of TDO in liver and IDO elsewhere in the body. Generally, 5% to 10% of plasma Trp exists in the unbound (free) state, with the remaining 90% to 95% being albumin bound. Equilibration between the free and bound fractions is rapid. Free Trp is a labile parameter that can be influenced by many physiological and pharmacological factors, and it is important that Trp binding, usually expressed as the percentage free Trp (100 × [free Trp]/[total Trp]), is assessed at baseline and following experimental treatments or interventions, as it can establish the Trp metabolic status of an individual and its biological determinants and also facilitate accurate interpretation of clinically or experimentally induced changes.97 Failure to consider the free Trp status in pregnancy led to the widely accepted concept of “Trp depletion as a defence mechanism against fetal rejection” being invalid.98 It is also important that free Trp is determined in freshly isolated serum or plasma to avoid the increased albumin binding caused by frozen storage leading to erroneously lower [free Trp] values.97
The flux of Trp down the KP was studied in isolated rat hepatocytes under a variety of conditions, including adrenalectomy, experimental diabetes, fasting, glucocorticoid treatment, and manipulation of Trp binding by albumin and nonesterified fatty acids (NEFA).80,99–102 The results of these hepatocyte studies have generally confirmed the observations described above for these treatments and established that Trp availability to the liver and, in particular, free Trp, is the major determinant of TDO activity in vivo. Tryptophan oxidation by TDO in hepatocytes is enhanced 5-fold by increasing the [Trp] from the physiological level of 0.1 to 0.5 mM, with a further increase to 2.5 mM exerting no additional effect. From a recent study in intact rats,87 the author has now calculated that liver Trp was significantly correlated (Pearson product moment) with liver K (r = 0.616; P = .0381) and liver total kynurenines (r = −0.711; P = .0211; n = 10). After acute Trp loading (50 mg/kg intraperitoneally), liver Trp correlated only with total Trp oxidation (r= −0.472; P = .0171; n = 25). Thus, as in hepatocytes, liver Trp (derived from plasma free Trp) appears to play an important role in flux down the hepatic KP.
In parallel agreement with the above findings in hepatocytes with various Trp concentrations,80,98–101 our recent human study29 showed that TDO activity (expressed as the [K]/[Trp] ratio %) was increased maximally by a 5.15 g Trp dose (~74 mg/kg in a 70 kg human). However, flux of Trp through the KP continued to increase dose dependently beyond maximum TDO activation, further suggesting that flux is primarily determined by Trp availability. In this human study,29 the combined TDO/IDO activity in fasting subjects (n = 114) was estimated to account for a maximum of ~70% of total Trp oxidation, and a similar value was obtained for the [kynurenine]/[total kynurenines] ratio. By taking into account quinolinate formation, which was not measured, this value can be revised to 63%. A similar value (of 60%) can be calculated for the contribution of TDO to Trp oxidation to CO2 in rat hepatocytes.100
Further analysis of the data from our previous 2 studies in humans24,29 was performed to establish the potential role of plasma free and total Trp in the flux down the KP under the conditions listed in Table 3. Here, Pearson product moment correlations were examined for free and total plasma Trp and a number of parameters indicative of the Trp flux, namely, K, TDO, TDOF (TDO relative to free Trp), total Trp oxidation (TTOX), and TTOXF (TTOX relative to free Trp). Under basal fasting conditions, the only significant correlation was between free Trp and K. Significance was then extended to most of the other parameters, as shown, when Trp was administered at 3 dose levels. In the 2 groups (F3 and FO) receiving a small Trp dose (1.15 g) with a, respectively, minimal and greater contribution to the Trp flux from a small and a larger Leu (branched-chain amino acids) dose, there were significant correlations between both free and total Trp and most of the above parameters. Only total kynurenines did not correlate, which may reflect the contribution of extrahepatic tissues to further metabolism of K. After loading with larger doses of Trp, both free and total Trp correlated with total kynurenines in addition to other parameters. From the data in Table 3, it appears that total Trp is as important as free Trp in the flux down the KP. This is not surprising given the rapid equilibration between the free and bound fractions. However, this does not minimize the importance of free Trp as it can only enter tissues after being released from albumin.
Table 3.
Correlations between plasma-free or total tryptophan and parameters of tryptophan oxidation.
| Treatment groups | Parameter |
|||||
|---|---|---|---|---|---|---|
| K |
TDO |
TDOF |
Ks |
TTOX |
TTOXF |
|
| Correlation coefficient r and SIGNIFICANCE (P) | ||||||
| Baseline (n = 111) | ||||||
| Free | 0.22 (.019) | |||||
| Total | ||||||
| F3 (n = 96) | ||||||
| Free | 0.27 (.008) | −0.35 (.000) | −0.62 (.000) | −0.32 (.002) | 0.50 (.000) | |
| Total | 0.56 (.000) | −0.46 (.000) | 0.35 (.000) | −0.45 (.000) | ||
| F0 (n = 96) | ||||||
| Free | 0.20 (.05) | −0.43 (.000) | −0.53 (.000) | −0.39 (.000) | −0.43 (.000) | |
| Total | 0.24 (.02) | −0.58 (.000) | −0.46 (.000) | −0.54 (.000) | −0.42 (.000) | |
| ATL 5.15 (n = 199) | ||||||
| Free | 0.36 (.000) | −0.32 (.000) | 0.44 (.000) | −0.49 (.000) | ||
| Total | 0.35 (.000) | −0.27 (.000) | −0.17 (.015) | 0.37 (.000) | −0.31 (.000) | −0.47 (.000) |
| ATL 10.30 (n = 160) | ||||||
| Free | −0.18 (.021) | −0.38 (.000) | 0.26 (.001) | −0.17 (.029) | −0.42 (.000) | |
| Total | 0.27 (.000) | −0.27 (.000) | 0.45 (.000) | −0.29 (.000) | ||
Abbreviations: K, kynurenine; Ks, sum of total kynurenines; TDO, Trp dioxygenase: 100 × [K]/[total Trp]; TDOF, Trp dioxygenase relative to free Trp: 100 × [K]/[free Trp]; TTOX, total Trp oxidation: 100 × [Ks]/[total Trp]; TTOXF, total Trp oxidation relative to free Trp: 100 × [Ks]/[free Trp].
Definition of treatment groups: baseline, fasting plasma; F3, a small Trp load of 1.15 g with minimal contribution to the Trp flux from a small dose of leucine; F0, same as F3, but with a larger dose of leucine; ATL 5.15, acute Trp loading with a 5.15 g Trp dose and the same dose of leucine as in F0; ATL 10.30, a larger Trp dose with double the leucine content as that in ATL 5.15. Only the significant Pearson product moment correlations are given.
Functions of the KP
The KP performs a number of functions (outlined in Table 4) both within the liver and other organs/tissues and at the general body level. These functions will be discussed with special reference to KP enzymes at the physiological level, their disturbances in disease conditions, and their targeting for therapeutic purposes. It is, however, important to emphasize that all enzymes of the pathway are important, irrespective of their catalytic activities or turnover rates, and that the term “regulatory” should be used with caution when the expression or function of a particular enzyme is undermined with resultant negative health consequences.
Table 4.
Functions of the kynurenine pathway.
| Function | Enzyme(s) responsible |
|---|---|
| 1. Detoxification of excess Trp | TDO |
| 2. Control of hepatic heme biosynthesis | TDO |
| 3. Control of plasma Trp availability | TDO (physiological conditions) |
| IDO (immune activation) | |
| 4. Production of kynurenines | TDO/IDO → 3-HAAO |
| 5. Production of picolinic acid | ACMSD |
| 6. Production of NAD+ | QPRT → NAD synthetase |
| 7. Production of niacin | QPRT → NADase and PARP |
Abbreviations: ACMSD, 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase or picolinate carboxylase; HAAO, hydroxyanthranilic acid oxygenase; IDO, indoleamine 2,3-dioxygenase; NAD+, oxidized nicotinamide adenine dinucleotide; NADase, NAD+ glycohydrolase; PARP, poly-(ADP-ribose) polymerase; QPRT, quinolinate phosphoribosyl transferase; Trp, tryptophan; TDO, Trp 2,3-dioxygenase.
Detoxification of excess tryptophan
As stated above, species lacking the TDO free apoenzyme and its glucocorticoid induction mechanism are sensitive to toxicity of an excess of Trp.53 When adrenalectomized, the rat becomes vulnerable to this toxicity as it no longer possesses the glucocorticoid induction mechanism necessary to process the excess of Trp down the KP.103 Administration of cortisol to adrenalectomized rats confers protection,103 and it is thought that toxicity arises from diversion of Trp degradation toward the indole(amine) pathways.104 This function is exclusive to liver TDO and so is unlikely to involve IDO because although species lacking the TDO apoenzyme, eg, gerbil and rabbit, possess a higher IDO activity than rats, they are still vulnerable to Trp toxicity. To (partially) mitigate this vulnerability, these species metabolize Trp at a faster rate and a lower dosage than does the rat (see Badawy105 for reference). Also, as Trp inhibits IDO activity at concentrations greater than 50 µM,91 it is unlikely that IDO will be able to process any excess Trp. Another mechanism through which Trp-sensitive species process Trp is its oxidation to acetyl CoA along the second arm of the KP. As stated above, the cat possesses a greatly elevated ACMSD activity,41 and a study in isolated hepatocytes106 showed that although the rate of Trp oxidation and QA formation is lower than in rats, guinea pigs, gerbils, and sheep metabolize a much larger part of Trp through the citric acid cycle.
Control of plasma tryptophan availability
Under normal conditions, this control is exerted mainly, if not exclusively, by hepatic TDO. Under conditions of immune activation, IDO assumes the major role, although TDO may also play a part. This major contribution of TDO, which has a much greater capacity for Trp than IDO, is best illustrated by the observation107,108 that deletion of the mouse TDO gene increases plasma [Trp] by 9.3- to 12.7-fold. Plasma [Trp] was not measured in a study involving deletion of the mouse IDO1 gene,109 but brain [Trp] was not altered after mouse IDO1 and IDO2 gene deletion, in contrast to a ~10.6-fold elevation of brain [Trp] by TDO2 gene deletion.110 Thus, IDO under normal physiological conditions does not influence plasma or brain Trp. Earlier work has clearly demonstrated the impact of the TDO control of plasma Trp availability on tissue Trp levels. In relation to CNS function, an inverse relationship exists between TDO activity and brain [Trp] and 5-HT synthesis.111 A potential enhancement of TDO activity by the elevated cortisol levels may explain the serotonin deficiency in major depressive disorder.62 The TDO inhibition by chronic administration to rats of drugs of dependence increases brain [Trp] and enhances 5-HT synthesis, whereas TDO induction by corticosterone during subsequent drug withdrawal exerts the opposite effects.111 The dramatic increase in plasma [Trp] induced by deletion of the mouse TDO gene results in an equally dramatic increase in circulating Trp availability to the brain (expressed as the [Trp]/[competing amino acids] ratio) and consequently in brain Trp, 5-HT, and 5-hydroxyindoleacetic acid (5-HIAA).107 The control of Trp availability to the brain by TDO has also been suggested107 as a modulator of anxiety through changes in brain 5-HT. The deletion of TDO gene also increases Trp availability for the decarboxylation and transamination of Trp,107 and it is of interest that the Trp transamination product IPA possesses anxiolytic properties.112
Although the extrahepatic IDO plays a minimal role in the control of plasma Trp availability, it can influence KP activity even in the absence of TDO, as suggested by the finding107 that plasma [K] and [KA] are maintained at wild-type levels in TDO knockout mice. In the absence of preformed niacin, synthesis of K and its metabolites KA, XA, and 3-HAA is increased, whereas that of QA is decreased, in these mice,108 and it was suggested that K formed by IDO extrahepatically can be used by the liver to form adequate amounts of nicotinamide and NAD+ nucleotides to maintain growth. Also, the hepatic KP can contribute to extrahepatic kynurenine metabolite formation through the availability of the kynurenine precursor. In some situations, this is likely to be quantitatively more important than the modest contribution of IDO itself. The role of IDO in control of plasma Trp availability takes center stage when the immune system is activated. The induction of IDO by IFN-γ and agents acting through it leads to depletion of [Trp] and increased K formation in cultures of monocytes94 and serum.113,114
Production of kynurenine metabolites
Kynurenine metabolites have been the subject of intense research activity over the past 2.5 decades with the discovery of their important roles in neuronal and immune function. In this section, a brief discussion of the functions of KP metabolites from K to QA and PA and the roles of the relevant enzymes will be presented with reference to disease states. Functions of enzymes leading to NAD+ synthesis from QA or nicotinamide will be discussed in the next section and subsequently in relation to their targeting for therapeutic purposes.
A landmark discovery was that of identification of QA and KA, respectively, as agonist and antagonist of the NMDA receptors of the excitatory amino acid glutamate,115 thus heralding a new era of research in neuroscience and neuropsychopharmacology. Another equally important landmark discovery is that of identification of a number of K metabolites as immunomodulators, in particular, 3-HK, 3-HAA, QA, and PA.
In the minor transamination step in the KP, KA formation is controlled by availability of K through either K or Trp loading or KMO inhibition. KAT inhibition is a new strategy for the treatment of schizophrenia,116–118 which is now considered a disease of glutamatergic hypoactivity induced by elevation of the NMDA receptor antagonist and the KAT A reaction product KA.119 Preliminary preclinical results show that inhibition of the brain KAT II isoenzyme by BFF-816 improves performance in spatial and contextual memory tests116 and that by PF-04859989 improves other cognitive functions.117 The NMDA receptors are activated by glycine, but clinical trials of chemicals that inhibit the glycine transporter 1 have given disappointing results.120 A more promising approach is to address the status of KA in schizophrenia with KAT inhibitors. Benserazide inhibits KAT A87 and therefore has the potential to influence cognition in schizophrenia. Two previous studies, however, did not demonstrate an antipsychotic action of benserazide administered either alone or with Trp.121,122 The rationale here was to correct a potential serotonin deficiency by Trp and benserazide based on the ability of (large doses of) the latter to inhibit liver TDO activity. A more likely rationale is KAT A inhibition, and perhaps, efficacy could be demonstrated with doses larger than those used in the above clinical studies.
Another important aspect of KA and KAT A that requires further investigation is that of the greater incidence of schizophrenia in female, compared with male, whites. A potential explanation is that of estrogens inhibiting the KAT A reaction leading to a lower [KA] in women.29 The limitation of this gender difference to white women, as opposed to African American or American Hispanic women, may be explained by the greater use of estrogen contraception by white women.29
KA may also modulate immune function. It has been shown to exert anti-inflammatory effects in mice and to lower levels of proinflammatory cytokines.123 This is likely to influence IDO activity. KA also protects against lipopolysaccharide toxicity in mice.124 A novel feature of relevance to these properties is that KA may modulate levels of AA and thereby lower the [3-HAA]/[AA] ratio. A decrease in this ratio has been reported125 in a wide range of neurological conditions and was suggested as a protective mechanism limiting further damage, particularly in brain. In nonsurviving patients with acute stroke, this decrease is associated with elevated plasma [KA].126 In agreement with these findings in humans, a recent study in rats87 reported that KA administration raises [AA] in liver by enhancing kynase A activity (K → AA) and thus lowers the [3-HAA]/[AA] ratio. These effects of KA have also been established from fasting baseline data in humans.29 In patients with schizophrenia, raised [AA] has recently been reported.127 Thus, apart from its NMDA antagonistic function, KA possesses anti-inflammatory properties, and the potential role of AA in these latter effects is worthy of investigation. The role of AA analogues and derivatives as anti-inflammatory agents is currently the subject of active investigation.
Modulation of immune function has been established for 3-HK, 3-HAA, QA, and PA. Thus, during the 1990s, many observations have demonstrated the immunomodulatory effects of the above K metabolites, namely, (1) PA acting as a second signal in the activation of IFN-γ–primed macrophages and a co-stimulus for induction of reactive nitrogen intermediate, (2) QA as an immune system signaling agent, and (3) 3-HAA as inhibitor of NO synthase expression and activity (for reference, see Badawy et al98). Subsequently, the immunomodulatory effects of kynurenines were extended to include (1) suppression of allogeneic T-cell proliferation by 3-HK and 3-HAA additively by an apoptotic mechanism, (2) apoptosis undermining T-helper type 1 (Th1) cells by 3-HAA and QA shifting the Th1/Th2 balance toward Th2, and (3) prolonged survival of cerebral malaria in mice by KMO inhibition.98
Plasma Trp depletion caused by IFN-γ induction of IDO was originally proposed to underpin the antibacterial, antiparasitic, and antiviral effects of this cytokine128–130 and protection against fetal rejection.131 However, with the demonstration of the immunomodulatory effects of K metabolites, it became necessary to modify the depletion hypothesis in infection to a combination of Trp depletion and K metabolite elevation. Even so, because bacteria, fungi, and parasites can synthesize Trp and do not suffer from host Trp depletion, an alternative Trp utilization concept in infection was proposed based on immunomodulation by K metabolites and the importance of NAD+ synthesis.132 Regarding pregnancy, it would be a physiological disadvantage if Trp were to be depleted, as the Trp requirements in pregnancy are dramatically increased, with the need for increased protein synthesis by mother and fetus, serotonin for signaling pathways, KA for neuronal protection, QA for NAD+ synthesis, and immunosuppressive kynurenines for fetal tolerance. A Trp utilization concept in pregnancy has been proposed satisfying these requirements.3,133 The Trp depletion concept in pregnancy131 did not take into consideration the elevation of plasma free [Trp] during pregnancy but relied on the decrease in total (free + albumin-bound) [Trp], which occurs as a result of increased uptake and utilization of the free fraction. As stated above, accurate interpretation of changes in Trp disposition requires measurement of both free and total plasma [Trp].97 It seems that changes in Trp metabolism and disposition during pregnancy also occur in infections of various types, including cancer, and a hypothesis proposing future Trp-related studies in these areas of public health has been published.98
As the NMDA agonist, QA exerts excitotoxic effects and has been implicated in many neurological conditions, including epilepsy, Huntington disease, trauma, and hypoxia, and in inflammatory conditions, such as HIV infection (for reference, see Malherbe et al35). QA levels are increased in plasma, cerebrospinal fluid (CSF), and/or brain in some of these conditions,134 with activity of 3-HAAO being vital for QA synthesis. QA levels in schizophrenia are not decreased,135 and so, the glutamatergic hypoactivity can be attributed solely to the raised [KA]. Strategies for lowering QA synthesis involve inhibition of IDO, kynureninase, or 3-HAAO. However, this should be accompanied with provision of nicotinic acid to ensure adequate NAD+ synthesis. Although excitotoxic, QA is a very important precursor of NAD+, as will be described below.
Although emphasis on immunosuppression by K metabolites has focused on their ability to suppress T-cell reactivity, less emphasis is given to their effects on innate immunity and in particular cerebral microglial reactivity. It has been known for some time that 3-HK and 3-HAA possess the highest antioxidant properties among all Trp and serotonin metabolites.136 Despite the widely reported pro-oxidant property of 3-HK and 3-HAA in peripheral blood cells and rodent neurons, an anti-inflammatory and neuroprotective role for 3-HAA in brain during inflammation has been suggested.137 A possible explanation of these opposing views is that of species differences (rodents vs humans) and composition of cultures (pure vs mixed neuronal-glial137). Microglia are the major sites of innate immunity, and the above effects of 3-HAA have been shown to be mediated by induction in astrocytes and microglia of heme oxygenase 1, an enzyme of heme degradation that possesses anti-inflammatory and cytoprotective properties.137
Other effects of XA, QA, and PA
Some KP metabolites can influence carbohydrate metabolism in various ways. Activity of the key gluconeogenic enzyme, phosphoenol pyruvate carboxykinase, is inhibited by QA, and Trp administration to rat, but not gerbil, guinea pig, or sheep, inhibits gluconeogenesis,100,106,138 presumably via QA in rats, as QA production from Trp in the 3 other species is poor. As the human KP resembles that of rats in its preference for QA formation, it is likely that under conditions of excessive QA production, gluconeogenesis could be inhibited in humans. It is of interest that animal models of diabetes are mainly those of rodents and pigs, but not species in which QA production is limited. Carbohydrate metabolism could also be influenced via insulin by XA and PA. Thus, XA has been known for some time to be diabetogenic through binding and thus inactivating insulin. A high plasma [XA] is associated with high insulin resistance and higher odds of having diabetes.139 Urinary and plasma [XA] is elevated in patients with diabetes and in experimental diabetes.140,141 In the urinary study,140 the increased XA excretion is accompanied by that of Zn in the form of an XA-Zn complex. Zn is essential for insulin at many levels,142 and its absorption and bioavailability are controlled by PA. As far as the author could ascertain, no information on urinary or plasma [PA] in diabetes is available. The ACMSD activity and mRNA expression are increased in experimental diabetes.143–145 However, hepatocyte PA production is not impaired.145 ACMSD activity is, however, greater in kidney than in liver. In hepatitis C viral infection, plasma [PA] is elevated and more so if diabetes is present.146 Thus, whether the increased urinary excretion of Zn in diabetes is due in part to a potential defect in binding to PA in addition to complex formation with XA remains to be established.
Both PA and XA appear to be the least studied metabolites of the KP. As stated above, PA exerts immunomodulatory effects,98 and its plasma levels are increased in hepatitis C viral infection and hepatic cirrhosis.146 Other than the increase in CSF [PA] in cerebral malaria,147 little is known about the status of this KP metabolite in other CNS conditions.148 Similarly, XA is poorly studied. Other than its insulin and Zn binding, described above, and activation of the malaria gametocyte,149 evidence exists for its role in the brain in synaptic signaling and hence neurotransmission.150 Clearly, more work on these 2 KP metabolites is required.
An important feature of K and its metabolites is their acting as ligands of the aryl hydrocarbon receptor (AhR), the significance of which will be described below.
Regulation of hepatic heme biosynthesis
Induction of heme oxygenase causes an initial decline in hepatic [heme], thereby removing the negative feedback repression of the rate-limiting enzyme of heme biosynthesis, 5-aminolevulinate synthase (5-ALAS). This results in enhancement of heme biosynthesis and consequently heme availability for hemoproteins under normal physiological conditions. This feedback mechanism is achieved by a small pool of heme, so-called free, unassigned, or readily exchangeable, which exists in the hepatic cytosol at a 10−7 M concentration.151 TDO utilizes this pool and exhibits an exquisite response to changes in its level under various conditions involving altered heme synthesis and degradation.151 TDO (in rat and presumably also human liver) thus serves as a sensitive marker of changes in the regulatory heme pool, and this property forms the basis of a screening test for exacerbation of hepatic porphyrias by drugs and other chemicals.152 Thus, by using this regulatory heme pool, TDO can control heme biosynthesis at the 5-ALAS step. Although unaltered in hepatic porphyrias, 5-ALAS is induced during acute porphyric attacks with a resultant accumulation of 5-ALA and intermediates of the pathway proximal to 1 of 6 points of the genetic defect and occurrence of neurological and bodily symptoms. Disturbed Trp metabolism could also contribute to these symptoms. Thus, in experimental hepatic porphyria, the relative heme deficiency decreases the heme saturation of TDO and hence its activity, thereby causing elevation of plasma [Trp] and brain [5-HT].153 Evidence for similar changes during acute attacks in porphyric patients includes elevation of plasma [Trp] and urinary excretion of the major serotonin metabolite 5-HIAA (for reference, see Badawy10). The increased serotonin synthesis and turnover may explain the gastrointestinal disturbances of the acute attacks. In porphyria, urinary excretion of K, 3-HK, KA, and XA is increased after an acute Trp load.154 Although this resembles the excretion pattern in functional vitamin B6 deficiency (defined as decreased availability of the PLP cofactor), it is not corrected by B6 supplementation, and it is thought that the large excretion of K may involve KMO inhibition.154 The 5-ALAS induction during acute attacks could deplete PLP.
Therapy of acute attacks involves intravenous administration of heme arginate or oral glucose, the mechanisms of which involve downregulating 5-ALAS transcription and its mitochondrial import posttranslationally by heme, and downregulation by glucose of the peroxisome proliferator–activated receptor gamma coactivator 1α (PGC-1α).10 A third mechanism could involve TDO participation, based on its utilization of heme during starvation (as fasting precipitates acute attacks) and the ability of glucocorticoids to reverse the 5-ALAS induction through increased heme conjugation with newly synthesized apo-TDO.10 Inhibition of TDO could therefore represent a novel strategy for the treatment of acute hepatic porphyrias.
Niacin synthesis and pellagra prevention
In the absence of adequate intake of niacin, its synthesis is achieved by Trp through the QA arm of the pathway. Niacin deficiency is the central feature of pellagra, usually referred to as the disease of the 3 Ds (dermatitis, diarrhea, and dementia, though more appropriately delirium). The relevant tissues (skin, gastrointestinal tract, and the nervous system) suffer most from the resultant NAD+ deficiency because of the greater demand for this dinucleotide to satisfy their rapid cellular turnover.
Nutritional pellagra therefore occurs only if diets are deficient in both niacin and Trp. With marginal niacin, but adequate Trp, intake, clinical or subclinical pellagra can be induced by drugs interfering with 1 or more steps during the conversion of Trp to nicotinamide dinucleotides, eg, by TDO inhibition by some antibiotics and antiviral drug or kynureninase inhibition by hydrazine compounds or estrogens.1,4 The widespread incidence of pellagra in Southern Europe during the 18th century and in the United States following the American Civil War was due to subsistence of a largely maize staple.1,4 Although maize (and sorghum, widely used in India) are Trp-deficient, they contain sufficient amounts of niacin, but in a polysaccharide-bound form (niacytin), they cannot be hydrolyzed by mammalian digestive enzymes. It was unfortunate in Southern Europe that those who introduced it ignored the need to follow the liming process, a procedure used for millennia by the peasants of Central America in the preparation of tortillas that causes the release of niacin from niacytin.4 The presence of high levels of leucine in maize and sorghum aggravates the pellagra by actions at a number of steps in the KP, namely, activation of TDO and ACMSD and inhibition of kynureninase and QPRT.1,4,24 Although pellagra continues to be caused by malnutrition in certain parts of the world, it appears occasionally in developed countries in association with alcoholism, and it is noteworthy that among other effects, chronic ethanol consumption inhibits TDO activity.2,4
NAD+ synthesis
The final main function of the hepatic KP is production of the all-important redox cofactor NAD+, from which NADP+ is formed by the action of NAD+ kinase. Both oxidized dinucleotides and their reduced forms play vital roles in metabolism at multiple levels and in other cell functions and are therefore essential to life. On this basis, it could be argued that NAD+ synthesis is the most important function of the KP. As the KP favors the arm leading to QA formation (Figures 1 and 2), it could be concluded that NAD+ synthesis from QA and hence Trp is quantitatively more important than that from nicotinamide or nicotinic acid. This is illustrated by the findings44,155,156 that dietary Trp is more effective than dietary nicotinamide or nicotinic acid in elevating liver nicotinamide dinucleotides and urinary levels of N1-methylnicotinamide. The group of Bender1,44,156 investigated activities of the relevant enzymes and concluded that (1) incorporation of nicotinamide into the dinucleotides is limited by the activities of NMD and NMPRT, both of which are substrate saturated at normal (steady-state) levels of liver nicotinamide. (2) Although activities of the above 2 enzymes show a significant correlation with hepatic nicotinamide dinucleotide levels, this is not case with NPRT, which functions normally just below its Vmax. (3) Quinolinate phosphoribosyltransferase, whose activity also does not correlate with liver dinucleotides, by contrast, operates at [QA] well below its Km, thus suggesting that increased availability of QA from Trp should result in greater formation of NaMN and hence NAD+. (4) Although liver dinucleotide levels are increased after a single large dose of nicotinamide, this is more likely to result from decreased NAD+ catabolism, rather than increased synthesis, because both nicotinamide and its N1-methyl metabolite inhibit the NAD+-degrading (depleting) enzyme poly-(ADP-ribose) polymerase (PARP; EC 2.4.2.30). The significance of PARP will be discussed below.
Although QA is the quantitatively most important NAD+ precursor, it does not accumulate in liver after Trp loading, presumably because of its rapid metabolism to NAD+. By contrast, activated cells of the immune system accumulate relatively large amounts of QA, and it has been suggested that this is to provide the substrate for NAD+ synthesis and the PARP reaction in response to immune-related oxidative damage.132
It is generally accepted that 1 mg of niacin arises from intake of 60 mg of Trp,157,158 although this ratio can vary among individuals and by factors, including nutrients, hormones, pregnancy, drugs, and diseases, with some nutrients and hormones enhancing and others suppressing the conversion of Trp to nicotinamide.159 The niacin status is generally determined by measuring urinary excretion of N1-methylnicotinamide and its 2 oxidation products 2-PY and 4-PY.52,160 The correlations between daily niacin intake and urinary excretion of 4-PY and 2-PY are comparable and more superior to those between niacin intake and N1-methylnicotinamide excretion.52 The deletion of TDO gene in mice, however, still allows sufficient synthesis of nicotinamide and NAD+.108
Disturbances in KP Enzymes in Disease and Their Inhibition in Therapeutic Strategies
Disturbed KP functions could have clinical and therapeutic implications, and targeting various KP enzymes is now a strategy for addressing a variety of immune, cognitive, and neurodegenerative diseases116,118,161–163 (Table 5).
Table 5.
Targeting enzymes of the kynurenine pathway for therapeutic purposes.
| Enzyme | Identified clinical condition(s) for targeting |
|---|---|
| TDO | Cancer, major depressive disorder, porphyria |
| IDO | Cancer, immune diseases, neurological disorders, neurodegenerative diseases |
| KAT | Schizophrenia, |
| KMO | Schizophrenia, drug dependence, infectious diseases |
| Kynase | Neurodegenerative conditions |
| 3-HAAO | Neurological disorders |
| QPRT | Inflammatory diseases |
| NMPRT | Cancer |
| NNMT | Cancer, diabetes, schizophrenia |
| NADase | Infectious diseases |
| PARP | Cancer, inflammatory, metabolic, and neurological disorders |
Abbreviations: 3-HAAO, 3-hydroxyanthranilic acid oxygenase; IDO, indoleamine 2,3-dioxygenase; KAT, kynurenine aminotransferase; KMO, kynurenine monooxygenase or kynurenine hydroxylase; Kynase, kynureninase; NADase, NAD+ glycohydrolase; NMPRT, nicotinamide phosphoribosyltransferase; NNMT, nicotinamide N-methyltransferase; QPRT, quinolinate phosphoribosyltransferase; PARP, poly-(ADP-ribose) polymerase; TDO, Trp 2,3-dioxygenase.
The disturbance of TDO in the hepatic porphyrias suggests that its inhibition could form a therapeutic strategy to increase the availability of free heme to block 5-ALAS synthesis during acute attacks. As discussed above, current therapy with glucose may involve TDO inhibition. Similarly, accelerated Trp degradation through TDO induction by cortisol or activation by catecholamines in major depressive disorder62 suggests that TDO inhibition may be a useful therapeutic strategy to correct the serotonin deficiency in this illness and, in fact, a large number of antidepressant drugs of various chemical structures and pharmacologic profiles also inhibit TDO.62 TDO is constitutively expressed in some types of cancers and is capable of suppressing antitumor immune responses,164 hence the need to develop TDO inhibitors as antitumor agents. Pantouris and Mowat165 identified 7 TDO inhibitors among 2800 compounds from the library of the US National Cancer Institute, all of which possess antitumor activity, one of which, dihydroquercetin or taxifolin, is a specific TDO inhibitor, with the other 6 also being IDO inhibitors. A specific TDO inhibitor, LM10 (a 6-fluoroindole substituted in the 3-position by a tetrazolyl-vinyl side chain), was developed and shown166 to reverse tumoral immune resistance in mice.
Targeting IDO has preceded that of TDO, as the former has been well established as a modulator of immune responses, and clinical trials of IDO1 inhibitors are currently in progress.167 Controversy surrounded one such inhibitor, 1-methyltryptophan. Thus, although the d isomer is superior to the l isomer as an antitumor inhibitor in preclinical studies, it possesses little IDO inhibitory activity compared with the l isomer.168,169 The controversy has been resolved in part by the finding that the isoenzyme IDO2 is the preferred target for inhibition by the d isomer.170 IDO2 appears to play important roles in cellular immunity. For example, its gene deletion causes defects in immune responses and the ability of IDO1 to influence the generation of T regulatory cells, and it has been suggested that IDO2 is uniquely regulated by the AhR and involved in shaping immune tolerance in humans.171 In contrast, the absence of IDO1 induces upregulation of IDO2, as shown in epididymis.14 Many other IDO1 inhibitors have been developed, some of which are currently under investigation in phases 1 and 2 trials (for reference, see Frėdėrick162).
The discovery that K and some of its metabolites are endogenous ligands of the AhR is an important landmark in KP research. The AhR is a ligand-activated transcription factor mediating the toxicity of environmental chemicals, such as the dioxins. On binding of these chemicals to the cytosolic AhR, the AhR-ligand complex translocates to the nucleus, where it dimerizes with the AhR nuclear translocator and binds to the ligand response elements in the promoter of target genes. Activation of the AhR then elicits toxic responses, including cell damage and carcinogenesis.172 In addition to modulating xenobiotic metabolism by activating the cytochrome P450 family, the AhR also plays an important role in the control of immune responses.173 Here, the AhR, however, plays both a protective and a destructive role. Thus, although endogenous ligands of the AhR, such as kynurenine, facilitate a dampening of the immune response to prevent excessive inflammation and autoimmunity, exogenous ligands act as signals to enhance inflammatory responses to infection and resistance of cancer to its own destruction.174 In this latter scenario, the overwhelming immune response may represent a state of “pathological immunosuppression,” the mechanism(s) of which is currently unclear. A potential explanation has been proposed98 based on a possible effect of an excess of Trp. The flux of this excess Trp can lead to increased production of immunosuppressive K metabolites that can be further augmented by an upregulated IDO or TDO. Conditions favoring this possibility are met in the case of 2,3,7,8-tetrachlorodibenzo p-dioxin (TCDD), which has been shown to upregulate mouse IDO1 and IDO2175 and to induce a strong elevation of rat plasma free [Trp] by a combination of NEFA elevation and liver TDO inhibition.176 It is important to emphasize here the existence of rat and mouse strain differences in sensitivity to TCDD and the free Trp elevation.176,177
The Trp metabolites with the highest ligand activity for the AhR are KA, XA, and K. In HepG2 reporter cell lines, 10 µM KA activates AhR to the same extent as TCDD, and the relative activation by a similar concentration of XA is 20%.178 By contrast, in mouse Hepa1 cells, K activates by 55% at 2 µM and by 72% at 50 µM.179 It would appear that KA is the strongest KP metabolite ligand of the AhR. Two observations with KA further illustrate the dual (harmful and protective) role of AhR activation. Activation of the AhR by KA may allow certain tumor cells to escape immune surveillance mechanisms by secreting large amounts of IL-6,178 whereas deletion of the mouse AhR gene increases production of KA and expression of KAT II in mouse cortex and striatum, thereby protecting the brain against excitotoxicity and oxidative stress.180
It should be emphasized that targeting TDO, IDO, or subsequent enzymes of the KP will limit the formation of various metabolites, which could result in niacin and NAD+ deficiencies, unless adequate niacin intake is assured. Targeting KMO, kynureninase, or 3-HAAO has been proposed to limit the formation of QA, an excitotoxic K metabolite implicated in a variety of neurological conditions.33,35 Various KMO inhibitors have been developed, the most potent of which is Ro61-8048.162 This compound prolongs survival of a murine model of cerebral malaria.181 Theoretically, KMO inhibition should result in elevation of K (and consequently KA and possibly also AA: see below) and a decrease in 3-HK and subsequent metabolites. Indeed, deletion of the KMO gene in mice increases the concentrations of K, KA, and AA and decreases those of 3-HK and QA in liver, plasma, and brain.182 The deletion of KMO gene, however, does not influence activities of other KP enzymes or tissue [NAD+] and causes a modest decrease in brain [QA], and Giorgini et al182 suggest that brain QA levels may be preserved through increased formation of its precursor 3-HAA by the action of AA oxidase. Similarly, in the study of the murine model of cerebral malaria,181 normal levels of brain QA are maintained in control mice treated with the KMO inhibitor Ro61-8048, possibly because of the strong elevation of [AA]. However, when brain [AA] is further increased by Ro61-8048 in mice infected with Plasmodium berghei, no additional increase in [QA] is caused by this KMO inhibitor.
Recently, KMO inhibition by Ro61-8048 has been shown to inhibit alcohol- and cocaine-seeking behavior and relapse in rat models: an effect attributed to increased cerebral KA.183 Possible mechanisms of these KA-dependent novel effects of KMO inhibition include decreased release of glutamate and/or dopamine by antagonism at the glycine site of NMDA receptors, negative modulation of the α-7-nicotinic acetylcholine receptors, and aversion to alcohol by aldehyde dehydrogenase inhibition.183,184 Although an increase in cerebral KA by KMO inhibition may be desirable for combating drug dependence, the opposite is true in schizophrenia, wherein decreasing the elevated KA levels requires KAT inhibition. As discussed in detail above, KAT inhibition is actively pursued as a strategy for treatment of schizophrenia.116–120
Kynureninase inhibition can also be a strategy to lower QA levels in neurodegenerative conditions, such as the Huntingdon and Alzheimer diseases and AIDS dementia. Various compounds with moderately potent inhibitory potencies have been developed.162 The strongest inhibition is observed with 3-hydroxydesaminokynurenine, with Ki value of 5 nM with recombinant human kynureninase.185 As discussed above, the kynureninase inhibitor benserazide has shown little efficacy in the treatment of schizophrenia.121,122 Inhibitors of 3-HAAO have been developed, the most potent of which are derivatives of AA, with Ki of 0.3 to 6 nM.162 4-Chloro-3-hydroxyanthranilate (Ki = 6 nM)186 has been shown to improve functional recovery and to preserve white matter after spinal cord injury in guinea pigs.187
The KP enzymes closer to NAD+ synthesis have also been targeted for therapeutic purposes on the basis of a variety of observations related to the wide range of NAD+ functions. Thus, QPRT inhibits spontaneous cell death by decreasing the overproduction of active caspase 3.188 Another key enzyme of NAD+ synthesis, NMPRT, possesses multiple functions in health and disease.189 For example, the overexpression of NMPRT in a number of cancers suggests that its inhibition can form a therapeutic strategy, the basis of which involves inhibition of glycolysis at the glyceraldehyde 3-dehydrogenase step leading to ATP depletion.190 In colorectal cancer, NMPRT overexpression is inversely related to microRNA-26b, which targets the enzyme.191 The NMNAT protects against acute neurodegeneration in developing CNS by inhibiting excitotoxic-necrotic cell death.192
The KP enzymes involved in NAD+ degradation perform equally important functions in health and disease and are therefore also targeted for therapeutic intervention. Thus, NADase secreted by Group A streptococci enhances their virulence193 and its targeting can reduce infection.194 Various inhibitors of intrinsic NADase exist, but their efficacy is species dependent.195 Poly-(ADP-ribose) polymerase is another important enzyme that plays important roles in various cellular processes, including DNA repair, and targeting it offers an opportunity for therapeutic intervention in cancer and other conditions, including inflammatory, metabolic, and neurological disorders.196–199 Evidence from preclinical studies and current clinical trials of PARP inhibitors promises a fruitful outcome of these interventions.199
Nicotinamide N-methyltransferase also plays important roles in development and disease. These include participation in regulating the epigenetic landscape during early embryonic development200 and potential female-specific association (in Han Chinese) of its gene with schizophrenia,201 of its mRNA with insulin resistance,202 and of its protein expression with renal cell cancer,203 thus rendering it a target for therapeutic intervention strategies.
General Conclusions and Comments
From the account given in this review, it is clear that the KP of Trp degradation is an important vehicle and fertile ground for basic and clinical research. The KP performs many functions, the most important of which is provision of NAD+, and has been the source of much valuable information over the past 76 years starting with the concept of enzyme physiology and metabolic regulation in the 1950s to 1960s and reaching the current status as a major player in infectious, neurological, and neurodegenerative diseases. Through its wide range of enzymes and intermediates, it uniquely controls many important physiological processes throughout the body and provides multiple opportunities to address various disease states. The KP is currently at the forefront of biomedical research, and experience has proven that it never disappoints those who study it.
Acknowledgments
The author held an honorary professorial appointment at Cardiff Metropolitan University during the period September 2006 to September 2016.
Footnotes
Peer review:Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 611 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: The author is the sole contributor to this article.
Disclosures and Ethics: The author has provided to the publisher signed confirmation of compliance with legal and ethical obligations including, but not limited to, the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The author has read and confirmed his agreement with the ICMJE authorship and conflict of interest criteria. The author has also confirmed that this article is unique and not under consideration or published in any other publication, and that he has permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1. Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6:1–97. [DOI] [PubMed] [Google Scholar]
- 2. Badawy AA-B. Tryptophan metabolism in alcoholism. Nutr Res Rev. 2002;1:123–152. [DOI] [PubMed] [Google Scholar]
- 3. Badawy AA-B. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep. 2015;35:e00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badawy AA-B. Pellagra and alcoholism: a biochemical perspective. Alcohol Alcohol. 2014;49:238–250. [DOI] [PubMed] [Google Scholar]
- 5. Chiarugi A, Carpenedo R, Molina MT, Mattoli L, Pellicciari R, Moroni F. Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and/or kynureninase. J Neurochem. 1995;65:1176–1183. [DOI] [PubMed] [Google Scholar]
- 6. Nishizuka Y, Hayaishi O. Studies on the biosynthesis of nicotinamide adenine nucleotides I. Enzymatic synthesis of niacin ribonucleotides from 3-hydroxyanthranilic acid in mammalian tissue. J Biol Chem. 1963;238:3369–3377. [PubMed] [Google Scholar]
- 7. Bender DA. Inhibition in vitro of the enzymes of the oxidative pathway of tryptophan metabolism and of nicotinamide nucleotide synthesis by benserazide, carbidopa and isoniazid. Biochem Pharmacol. 1980;29:707–712. [DOI] [PubMed] [Google Scholar]
- 8.www.brenda-enzymes.org.
- 9. Werner ER, Werner-Felmayer G. Substrate and cofactor requirements of indoleamine 2,3-dioxygenase in interferon-gamma-treated cells: utilization of oxygen rather than superoxide. Curr Drug Metab. 2007;8:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badawy AA-B. Tryptophan availability for kynurenine pathway metabolism across the life span: control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology. 2015;112:248–263. [DOI] [PubMed] [Google Scholar]
- 11. Kanai M, Nakamura T, Funakoshi H. Identification and characterization of novel variants of the tryptophan 2,3-dioxygenase gene: differential regulation in the mouse nervous system during development. Neurosci Res. 2009;64:111–117. [DOI] [PubMed] [Google Scholar]
- 12. Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. [DOI] [PubMed] [Google Scholar]
- 13. Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467–471. [DOI] [PubMed] [Google Scholar]
- 14. Fukunaga M, Yamamoto Y, Kawasoe M, et al. Studies on tissue and cellular distribution of indoleamine 2,3-dioxygenase 2: the absence of IDO1 upregulates IDO2 expression in the epididymis. J Histochem Cytochem. 2012;60:854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meininger D, Zalameda L, Liu Y, et al. Purification and kinetic characterization of human indoleamine 2,3-dioxygenase 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim Biophys Acta. 2011;1814:1947–1954. [DOI] [PubMed] [Google Scholar]
- 16. Pabarcus MK, Casida JE. Cloning, expression, and catalytic triad of recombinant arylformamidase. Protein Expr Purif. 2005;44:39–44. [DOI] [PubMed] [Google Scholar]
- 17. Müller F. Flavin-dependent hydroxylases. Biochem Soc Trans. 1985;13:443–447. [DOI] [PubMed] [Google Scholar]
- 18. Verjee ZHM. Tryptophan metabolism in baboons: effect of riboflavin and pyridoxine deficiency. Acta Vitaminol Enzymol (Milano). 1975;29:198–201. [PubMed] [Google Scholar]
- 19. Clayton PT, Bridges NA, Atherton DJ, Milla PJ, Malone M, Bender DA. Pellagra with colitis due to a defect in tryptophan metabolism. Eur J Pediatr. 1991;150:498–502. [DOI] [PubMed] [Google Scholar]
- 20. Bender DA. Tryptophan and niacin nutrition—is there a problem? Adv Exp Med Biol. 1996;398:565–567. [PubMed] [Google Scholar]
- 21. Knox WE. The relation of liver kynureninase to tryptophan metabolism in pyridoxine deficiency. Biochem J. 1953;53:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeuchi F, Shibata Y. Kynurenine metabolism in vitamin-B-6-deficient rat liver after tryptophan injection. Biochem J. 1984;220:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bender DA, Njagi ENM, Danielian PS. Tryptophan metabolism in vitamin B6-deficient mice. Br J Nutr. 1990;63:27–36. [DOI] [PubMed] [Google Scholar]
- 24. Badawy AA-B, Lake SL, Dougherty DM. Mechanisms of the pellagragenic effect of leucine: stimulation of hepatic tryptophan oxidation by administration of branched-chain amino acids to healthy human volunteers and the role of plasma free tryptophan and total kynurenines. Int J Tryptophan Res. 2014;7:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bender DA, Laing AE, Vale JA, Papadaki J, Pugh M. The effects of oestrogen administration on tryptophan metabolism in rats and in menopausal women receiving hormone replacement therapy. Biochem Pharmacol. 1983;32:843–848. [DOI] [PubMed] [Google Scholar]
- 26. Bender DA, Totoe L. Inhibition of tryptophan metabolism by oestrogens in the rat: a factor in the aetiology of pellagra. Br J Nutr. 1984;51:219–224. [DOI] [PubMed] [Google Scholar]
- 27. Walsh HA, Botting NP. Purification and biochemical characterization of some of the properties of recombinant human kynureninase. Eur J Biochem. 2002;269:2069–2074. [DOI] [PubMed] [Google Scholar]
- 28. Inada J, Okuno E, Kimura M, Kido R. Intracellular localization and characterization of 3-hydroxykynureninase in human liver. Int J Biochem. 1984;16:623–628. [DOI] [PubMed] [Google Scholar]
- 29. Badawy AA-B, Dougherty DM. Assessment of the human kynurenine pathway: comparisons and clinical implications of ethnic and gender differences in plasma tryptophan, kynurenine metabolites, and enzyme expressions at baseline and after acute tryptophan loading and depletion. Int J Tryptophan Res. 2016;9:31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han Q, Cai T, Tagle DA, Li J. Thermal stability, pH dependence and inhibition of four murine kynurenine aminotransferases. BMC Biochem. 2010;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han Q, Robinson H, Cai T, Tagle DA, Li J. Biochemical and structural properties of mouse kynurenine aminotransferase III. Mol Cell Biol. 2009;29:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han Q, Cai T, Tagle DA, Robinson H, Li J. Substrate specificity and structure of human aminoadipate aminotransferase/kynurenine aminotransferase II. Biosci Rep. 2008;28:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stachowski EK, Schwarcz R. Regulation of quinolinic acid neosynthesis in mouse, rat and human brain by iron and iron chelators in vitro. J Neural Transm. 2012;119:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okuno E, Köhler C, Schwarcz R. Rat 3-hydroxyanthranilic acid oxygenase: purification from the liver and immunocytochemical localization in the brain. J Neurochem. 1987;49:771–780. [DOI] [PubMed] [Google Scholar]
- 35. Malherbe P, Kohler C, Da Prada M, et al. Molecular cloning and functional expression of human 3-hydroxyanthranilic-acid dioxygenase. J Biol Chem. 1994;269:13792–13797. [PubMed] [Google Scholar]
- 36. Tanabe A, Egashira Y, Fukuoka S-I, Shibata K, Sanada H. Purification and molecular cloning of rat 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase. Biochem J. 2002;361:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pucci L, Perozzi S, Cimadamore F, Orsomando G, Raffaelli N. Tissue expression and biochemical characterization of human 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase, a key enzyme in tryptophan catabolism. FEBS J. 2007;274:82740. [DOI] [PubMed] [Google Scholar]
- 38. Bender DA. Effects of a dietary excess of leucine on the metabolism of tryptophan in the rat: a mechanism for the pellagragenic action of leucine. Br J Nutr. 1983;50:25–32. [DOI] [PubMed] [Google Scholar]
- 39. He Z, Davis JK, Spain JC. Purification, characterization, and sequence analysis of 2-aminomuconic 6-semialdehyde dehydrogenase from Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1998;180:4591–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu H, Woznica K, Catton G, Crawford A, Botting N, Naismith JH. Structural and kinetic characterization of quinolinate phosphoribosyltransferase (hQPRTase) from Homo sapiens. J Mol Biol. 2007;373:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, Hayaishi O. Studies on the biosynthesis of nicotinamide adenine dinucleotide. II. A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals. J Biol Chem. 1965;240:1395–1401. [PubMed] [Google Scholar]
- 42. Imsande J, Handler P. Biosynthesis of diphosphopyridine nucleotide III. Nicotinic acid mononucleotide pyrophosphorylase. J Biol Chem. 1961;236:525–530. [PubMed] [Google Scholar]
- 43. Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282:24574–24582. [DOI] [PubMed] [Google Scholar]
- 44. Bender DA, Magboul BI, Wynick D. Probable mechanisms of regulation of the utilization of dietary tryptophan, nicotinamide and nicotinic acid as precursors of nicotinamide nucleotides in the rat. Br J Nutr. 1982;48:119–127. [DOI] [PubMed] [Google Scholar]
- 45. Burgos ES, Schramm VL. Weak coupling of ATP hydrolysis to the chemical equilibrium of human nicotinamide phosphoribosyltransferase. Biochemistry. 2008;47:11086–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wintzerith M, Dierich A, Mandel P. Purification and characterization of a nicotinamide deamidase released into the growth medium of neuroblastoma in vitro. Biochim Biophys Acta. 1980;613:191–202. [DOI] [PubMed] [Google Scholar]
- 47. Petrack B, Greengard P, Craston A, Sheppy F. Nicotinamide deamidase from mammalian liver. J Biol Chem. 1965;240:1725–1730. [PubMed] [Google Scholar]
- 48. Lau C, Niere M, Ziegler M. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front Biosci. 2009;14:410–431. [DOI] [PubMed] [Google Scholar]
- 49. Hara N, Yamada K, Terashima M, Osago H, Shimoyama M, Tsuchiya M. Molecular identification of human glutamine- and ammonia-dependent NAD synthetases. Carbon-nitrogen hydrolase domain confers glutamine dependency. J Biol Chem. 2003;278:10914–10921. [DOI] [PubMed] [Google Scholar]
- 50. Shibata K, Hayakawa T, Iwai K. Tissue distribution of the enzymes concerned with the biosynthesis of NAD in rats. Agr Biol Chem. 1986;50:3037–3041. [Google Scholar]
- 51. Felsted R, Chu AE-Y, Chaykin S. Purification and properties of the aldehyde oxidases from hog and rabbit livers. J Biol Chem. 1973;248:2580–2587. [PubMed] [Google Scholar]
- 52. Shibata K, Matsuo H. Correlation between niacin equivalent intake and urinary excretion of its metabolites, N’-methylnicotinamide, N’-methyl-2- pyridone-5-carboxamide, and N’-methyl-4-pyridone-3-carboxamide, in humans consuming a self-selected food. Am J Clin Nutr. 1989;50:114–119. [DOI] [PubMed] [Google Scholar]
- 53. Badawy AA-B, Evans M. Animal liver tryptophan pyrrolases: absence of hormonal induction mechanism from species sensitive to tryptophan toxicity. Biochem J. 1976;158:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Castro FT, Brown RR, Price JM. The intermediary metabolism of tryptophan by cat and rat tissue preparations. J Biol Chem. 1957;228:777–784. [PubMed] [Google Scholar]
- 55. Allegri G, Bertazzo A, Biasiolo M, Costa CVL, Ragazzi E. Kynurenine pathway enzymes in different species of animals. Adv Exp Med Biol. 2003;527:455–463. [DOI] [PubMed] [Google Scholar]
- 56. Murakami Y, Saito K. Species and cell type difference in tryptophan metabolism. Int J Tryptophan Res. 2013;6:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Knox WE, Greengard O. The regulation of some enzymes of nitrogen metabolism—an introduction to enzyme physiology. Adv Enzyme Regul. 1965;3:247–313. [Google Scholar]
- 58. Feigelson P, Greengard O. Metabolic effects of glucocorticoids as related to enzyme induction. Adv Enzyme Regul. 1965;3:11–27. [Google Scholar]
- 59. Schimke RT, Sweeney EW, Berlin CM. The role of synthesis and degradation in the control of rat liver tryptophan pyrrolase. J Biol Chem. 1965;240:322–331. [PubMed] [Google Scholar]
- 60. Hayaishi O. My life with tryptophan—never a dull moment. Protein Sci. 1993;2:472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Badawy AA-B, Evans M. Regulation of rat liver tryptophan pyrrolase by its cofactor haem: experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975;150:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Badawy AAB. Tryptophan: the key to boosting brain serotonin synthesis in depressive illness. J Psychopharmacol. 2013;27:878–893. [DOI] [PubMed] [Google Scholar]
- 63. Badawy AA-B, Welch AN, Morgan CJ. Tryptophan pyrrolase in haem regulation. The mechanism of the opposite effects of tryptophan on rat liver 5-aminolaevulinate synthase activity and the haem saturation of tryptophan pyrrolase. Biochem J. 1981;198:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ren S, Correia MA. Heme: a regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch Biochem Biophys. 2000;377:195–203. [DOI] [PubMed] [Google Scholar]
- 65. Liao M, Pabarcus MK, Wang YQ, et al. Impaired dexamethasone-mediated induction of tryptophan 2,3-dioxygenase in heme-deficient rat hepatocytes: translational control by a hepatic eIF2alpha kinase, the heme-regulated inhibitor. J Pharmacol Exp Ther. 2007;323:979–989. [DOI] [PubMed] [Google Scholar]
- 66. Danesch U, Hashimoto S, Renkawitz R, Schutz G. Transcriptional regulation of the tryptophan oxygenase gene in rat liver by glucocorticoids. J Biol Chem. 1983;258:4750–4753. [PubMed] [Google Scholar]
- 67. Nakamura T, Niimi S, Nawa K, et al. Multihormonal regulation of transcription of the tryptophan 2,3-dioxygenase gene in primary cultures of adult rat hepatocytes with special reference to the presence of a transcriptional protein mediating the action of glucocorticoids. J Biol Chem. 1987;262:723–732. [PubMed] [Google Scholar]
- 68. Schmid W, Scherer G, Danesch U, et al. Isolation and characterization of the rat tryptophan oxygenase gene. EMBO J. 1982;1:1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hirota T, Hirota K, Sanno Y, Tanaka T. Appearance of new hepatic glucocorticoid binding proteins under various stressful conditions: relation to endogenous glucocorticoid secretion. J Biochem (Tokyo). 1985;97:1371–1376. [DOI] [PubMed] [Google Scholar]
- 70. Hirota T, Hirota K, Sanno Y, Tanaka T. A new glucocorticoid receptor species: relation to induction of tryptophan dioxygenase by glucocorticoids. Endocrinology. 1985;117:1788–1795. [DOI] [PubMed] [Google Scholar]
- 71. Nakamura T, Shinno H, Ichihara A. Insulin and glucagon as a new regulator system for tryptophan oxygenase activity demonstrated in primary cultured rat hepatocytes. J Biol Chem. 1980;255:7533–7535. [PubMed] [Google Scholar]
- 72. Broqua P, Baudrie V, Laude D, Guezennec Y, Chaouloff F. In vivo evidence that insulin does not inhibit hepatic tryptophan pyrrolase activity in rats. Biochem Pharmacol. 1990;40:759–763. [DOI] [PubMed] [Google Scholar]
- 73. Labrie F, Korner A. Effect of glucagon, insulin, and thyroxine on tyrosine transaminase and tryptophan pyrrolase of rat liver. Arch Biochem Biophys. 1969;129:75–78. [DOI] [PubMed] [Google Scholar]
- 74. Yamamoto M, Hayashi N, Kikuchi G. Increase in liver heme concentration after the administration of insulin, glucagon and dibutyryl cyclic AMP to rats and its relevance to regulation of δ-aminolevulinate synthase. Biochem Int. 1982;5:747–754. [Google Scholar]
- 75. Hagen JH. Effect of glucagon on the metabolism of adipose tissue. J Biol Chem. 1961;236:1023–1027. [PubMed] [Google Scholar]
- 76. Carlson MG, Snead WL, Campbell PJ. Regulation of free fatty acid metabolism by glucagon. J Clin Endocrinol Metab. 1993;77:11–15. [DOI] [PubMed] [Google Scholar]
- 77. Granick S, Sinclair P, Sassa S, Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975;250:9215–9225. [PubMed] [Google Scholar]
- 78. Badawy AA-B, Evans M. Effects of streptozotocin on the concentrations of rat liver nicotinamide-adenine dinucleotides (phosphates) and the activity of tryptophan pyrrolase. Biochem Soc Trans. 1977;5:1314–1316. [DOI] [PubMed] [Google Scholar]
- 79. Bitar M, Weiner M. Diabetes-induced metabolic alterations in heme synthesis and degradation and various heme-containing enzymes in female rats. Diabetes. 1984;33:37–44. [DOI] [PubMed] [Google Scholar]
- 80. Salter M, Pogson CI. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem J. 1985;229:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chiancone FM. Enzyme activities in the tryptophan → nicotinic acid path in physiopathology. Ital J Biochem. 1964;13:1–30. [Google Scholar]
- 82. Badawy AA-B, Evans M. The role of free serum tryptophan in the biphasic effect of acute ethanol administration on the concentrations of rat brain tryptophan, 5-hydroxytryptamine and 5-hydroxyindol-3-ylacetic acid. Biochem J. 1976;160:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ku Y, Rogers QR, Harper AE. Effects of thyroxine and cortisol on liver threonine dehydratase and tryptophan pyrrolase in rats fed a high protein diet. Proc Soc Exp Biol Med. 1969;130:556–563. [DOI] [PubMed] [Google Scholar]
- 84. Wagner C. Regulation of the tryptophan-nicotinic acid-DPN pathway in the rat. Biochem Bioph Res Co. 1964;17:668–673. [Google Scholar]
- 85. Cho-Chung YS, Pitot HC. Feedback control of rat liver tryptophan pyrrolase. J Biol Chem. 1967;242:1192–1198. [PubMed] [Google Scholar]
- 86. Badawy AA-B, Evans M. The regulation of rat liver tryptophan pyrrolase activity by reduced nicotinamide-adenine dinucleotide (phosphate). Experiments with glucose and nicotinamide. Biochem J. 1976;156:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Badawy AA-B, Bano S. Tryptophan metabolism in rat liver after administration of tryptophan, kynurenine metabolites, and kynureninase inhibitors. Int J Tryptophan Res. 2016;9:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yoshida R, Nukiwa T, Watanabe Y, Fujiwara M, Hirata F, Hayaishi O. Regulation of indoleamine 2,3-dioxygenase activity in the small intestine and the epididymis of mice. Arch Biochem Biophys. 1980;203:343–351. [DOI] [PubMed] [Google Scholar]
- 89. Cook JS, Pogson CI, Smith SA. Indoleamine 2,3-dioxygenase. A new, rapid, sensitive radiometric assay and its application to the study of the enzyme in rat tissues. Biochem J. 1980;189:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Badawy AA-B. Possible involvement of the enhanced tryptophan pyrrolase activity in the corticosterone- and starvation-induced increases in concentrations of nicotinamide-adenine dinucleotides (phosphates) in rat liver. Biochem J. 1981;196:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ozaki Y, Reinhard JF, Jr, Nichol CA. Cofactor activity of dihydroflavin mononucleotide and tetrahydrobiopterin for murine epididymal indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 1986;137:1106–1111. [DOI] [PubMed] [Google Scholar]
- 92. Efimov I, Basran J, Sun X, et al. The mechanism of substrate inhibition in human indoleamine 2,3-dioxygenase. J Am Chem Soc. 2012;134:3034–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pfefferkorn ER, Rebhun S, Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J Interferon Res. 1986;6:267–279. [DOI] [PubMed] [Google Scholar]
- 94. Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents on induction of indoleamine 2,3-dioxygenase in human peripheral blood monocytes. Biochem Biophys Res Commun. 1987;144:1147–1153. [DOI] [PubMed] [Google Scholar]
- 95. Hucke C, MacKenzie CR, Adjogble KD, Takikawa O, Daubener W. Nitric oxide-mediated regulation of gamma interferon-induced bacteriostasis: inhibition and degradation of human indoleamine 2,3-dioxygenase. Infect Immun. 2004;72:2723–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Thomas SR, Terentis AC, Cai H, et al. Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem. 2007;282:23778–23787. [DOI] [PubMed] [Google Scholar]
- 97. Badawy AA-B. Plasma free tryptophan revisited: what you need to know and do before measuring it. J Psychopharmacol. 2010;24:809–815. [DOI] [PubMed] [Google Scholar]
- 98. Badawy AA-B, Namboodiri AM, Moffett JR. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin Sci. 2016;130:1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Smith SA, Pogson CI. The metabolism of L-tryptophan by isolated rat liver cells. Effect of albumin binding and amino acid competition on oxidation of tryptophan by tryptophan 2,3-dioxygenase. Biochem J. 1980;186:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Smith SA, Carr FPA, Podson CI. The metabolism of L-tryptophan by isolated rat liver cells. Quantification of the relative importance of, and the effect of nutritional status on, the individual pathways of tryptophan metabolism. Biochem J. 1980;192:673–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Smith SA, Pogson CI. The metabolism of L-tryptophan by liver cells prepared from adrenalectomized and streptozotocin-diabetic rats. Biochem J. 1981;200:605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Salter M, Stanley JC, Fisher MJ, Pogson CI. The influence of starvation and tryptophan administration on the metabolism of phenylalanine, tyrosine and tryptophan in isolated rat liver cells. Biochem J. 1984;221:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Knox WE. The regulation of tryptophan pyrrolase activity by tryptophan. Adv Enzyme Regul. 1966;4:287–297. [DOI] [PubMed] [Google Scholar]
- 104. Horita A, Carino MA. Nodifiction of the toxic actions of L=tryptophan by pargyline and p-chlorophenylalanine. Biochem Pharmacol. 1970;19:1521–1524. [DOI] [PubMed] [Google Scholar]
- 105. Badawy AA-B. Tryptophan and inhibitors of tryptophan 2,3-dioxygenase as antidepressants: reply. J Psychopharmacol. 2014;28:169–172. [DOI] [PubMed] [Google Scholar]
- 106. Muñoz-Clares RA, Lloyd P, Lomax MA, Smith SA, Pogson CI. Tryptophan metabolism and its interaction with gluconeogenesis in mammals: studies with the guinea pig, Mongolian gerbil, and sheep. Arch Biochem Biophys. 1981;209:713–717. [DOI] [PubMed] [Google Scholar]
- 107. Kanai M, Funakoshi H, Takahashi H, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behaviour in mice. Mol Brain. 2009;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Terakata M, Fukuwatari T, Kadota E, et al. The niacin required for optimum growth can be synthesized from L-tryptophan in growing mice lacking tryptophan-2,3-dioxygenase. J Nutr. 2013;143:1046–1051. [DOI] [PubMed] [Google Scholar]
- 109. Santillan MK, Pelham CJ, Gibson-Corley KN, Scroggins SM. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol Rep. 2015;3:e12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Too LK, Li KM, Suarna C, et al. Deletion of TDO2, IDO-1 and IDO-2 differentially affects mouse behavior and cognitive function. Behav Brain Res. 2016;312:102–117. [DOI] [PubMed] [Google Scholar]
- 111. Badawy AA-B, Evans M. Opposite effects of chronic administration and subsequent withdrawal of drugs of dependence on the metabolism sand disposition of endogenous and exogenous tryptophan in the rat. Alcohol Alcohol. 1983;18:369–382. [Google Scholar]
- 112. Politi V, D’Alessioi S, Stazio G, De Luca G. Antioxidant properties of indole-3-pyruvic acid. Adv Exp Med Biol. 1996;398:291–298. [DOI] [PubMed] [Google Scholar]
- 113. Werner ER, Fuchs D, Hausen A, et al. Tryptophan degradation in patients infected by human immunodeficiency virus. Biol Chem Hoppe Seyler. 1988;369:337–340. [DOI] [PubMed] [Google Scholar]
- 114. Fuchs D, Forsman A, Hagberg L, et al. Immune activation and decreased tryptophan in patients with HIV-1 infection. J Interferon Res. 1990;10:599–603. [DOI] [PubMed] [Google Scholar]
- 115. Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–385. [PubMed] [Google Scholar]
- 116. Wu H-Q, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effects of an orally active enzyme inhibitor. Schizophr Bull. 2014;40:S152–S158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cherian AK, Gritton H, Johnson DE, Young D, Koza R, Sarter M. A systemically-available kynurenine aminotransferase II (KAT II) inhibitor restores nicotine-evoked glutamatergic activity in the cortex of rats. Neuropharmacology. 2014;82:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jayawickrama GS, Sadig RR, Sun G, et al. Kynurenine aminotransferases and the prospects of inhibitors for the treatment of schizophrenia. Curr Med Chem. 2015;22:29202–29218. [DOI] [PubMed] [Google Scholar]
- 119. Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;3:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Beck K, Javitt DC, Howes OD. Targeting glutamate to treat schizophrenia: lessons from recent clinical studies. Psychopharmacol. 2016;233:2425–2428. [DOI] [PubMed] [Google Scholar]
- 121. Chouinard G, Annable L, Serrano M, Charette R. A controlled study of a dopa decarboxylase inhibitor (benserazide) in the treatment of schizophrenic patients. Int Pharmacopsychiatry. 1977;12:1–8. [DOI] [PubMed] [Google Scholar]
- 122. Chouinard G, Annable L, Young SN, Sourkes TL. A controlled study of tryptophan-benserazide in schizophrenia. Commun Psychopharmacol. 1978;2:21–31. [PubMed] [Google Scholar]
- 123. Małaczewska J, Siwicki AK, Wójcik RM, Turski WA, Kaczorek E. The effect of kynurenic acid on the synthesis of selected cytokines by murine splenocytes—in vitro and ex vivo studies. Cent Eur J Immunol. 2016;41:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Moroni F, Cozzi A, Sili M, Mannaioni G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm. 2012;119:133–139. [DOI] [PubMed] [Google Scholar]
- 125. Darlington LG, Forrest CM, Mackay GM, Smith RA, Smith AJ, Stone TW. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res. 2010;3:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Darlington LG, Mackay GM, Forrest CM, Stoy N, George C, Stone TW. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur J Neurosci. 2007;26:2211–2221. [DOI] [PubMed] [Google Scholar]
- 127. Oxenkrug G, van der Hart M, Roeser J, Summergrad P. Anthranilic acid: a potential biomarker and treatment target for schizophrenia. Ann Psychiatry Ment Health. 2016;4:1059. [PMC free article] [PubMed] [Google Scholar]
- 128. Murray HW, Szuro-Sudol A, Wellner D, et al. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pfefferkorn ER, Eckel M, Rebhun S. Interferon-gamma suppresses the growth of Toxoplasma gondii in human fibroblasts through starvation for tryptophan. Mol Biochem Parasitol. 1986;20:215–224. [DOI] [PubMed] [Google Scholar]
- 130. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 131. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. [DOI] [PubMed] [Google Scholar]
- 132. Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–265. [DOI] [PubMed] [Google Scholar]
- 133. Badawy AA-B. The tryptophan utilization concept in pregnancy. Obstet Gynecol Sci. 2014;5:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chen Y, Guillemin GL. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;2:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schwarcz R, Tamminga CA, Kurlan R, Shoulson I. Cerebrospinal fluid levels of quinolinic acid in Huntington’s disease and schizophrenia. Ann Neurol. 1988;24:580–582. [DOI] [PubMed] [Google Scholar]
- 136. Goda K, Hamane Y, Kishimoto R, Ogishi Y. Radical scavenging properties of tryptophan metabolites. Adv Exp Med Biol. 1999;467:397–402. [DOI] [PubMed] [Google Scholar]
- 137. Krause D, Suh H-S, Tarassishin L, et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase-1. Am J Pathol. 2011;179:1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Smith SA, Pogson CI. Tryptophan and the control of plasma tryptophan concentration in the rat. Biochem J. 1977;168:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Reginaldo C, Jacques P, Scott T, Oxenkrug G, Selhub J, Paul L. Xanthurenic acid is associated with higher insulin resistance and higher odds of diabetes. FASEB J. 2015;29:20. [Google Scholar]
- 140. Ikeda S, Kotake Y. Urinary excretion of xanthurenic acid and zinc in diabetes: (3). Occurrence of xanthurenic acid-Zn2+ complex in urine of diabetic patients and of experimentally-diabetic rats. Ital J Biochem. 1986;35:232–241. [PubMed] [Google Scholar]
- 141. Oxenkrug GF. Increased plasma levels of xanthurenic and kynurenic acids in type 2 diabetes. Mol Neurobiol. 2015;52:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17:109–115. [DOI] [PubMed] [Google Scholar]
- 143. Mehler AH, McDaniel EG, Hundley JM. Changes in the enzymatic composition of liver. I. Increase of picolinic carboxylase in diabetes. J Biol Chem. 1958;232:323–330. [PubMed] [Google Scholar]
- 144. Tanabe A, Egashira Y, Fukuoka S-I, Shibata K, Sanada H. Expression of rat hepatic 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase is affected by a high protein diet and by streptozotocin-induced diabetes. J Nutr. 2002;132:1153–1159. [DOI] [PubMed] [Google Scholar]
- 145. Sasaki N, Egashira Y, Sanada H. Production of L-tryptophan-derived catabolites in hepatocytes from streptozotocin-induced diabetic rats. Eur J Nutr. 2009;48:145–153. [DOI] [PubMed] [Google Scholar]
- 146. Zuwała-Jagiello J, Pazgan-Simon M, Simon K, Warwas M. Picolinic acid in patients with chronic hepatitis C infection: a preliminary report. Mediat Inflamm. 2012;2012:762863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Medana IM, Day NPJ, Salahifar-Sabet H, et al. Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis. 2003;188:844–849. [DOI] [PubMed] [Google Scholar]
- 148. Grant RS, Coggan SE, Smythe GA. The physiological action of picolinic acid in the human brain. Int J Tryptophan Res. 2009;2:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Billker O, Lindo V, Panico M, et al. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. [DOI] [PubMed] [Google Scholar]
- 150. Gobaille S, Kemmel V, Brumaru D, Dugave C, Aunis D, Maitre M. Xanthurenic acid distribution, transport, accumulation and release in the rat brain. J Neurochem. 2008;105:982–993. [DOI] [PubMed] [Google Scholar]
- 151. Badawy AA-B. Central role of tryptophan pyrrolase in haem metabolism. Biochem Soc Trans. 1979;7:575–583. [DOI] [PubMed] [Google Scholar]
- 152. Badawy AAB. Heme utilization by rat liver tryptophan pyrrolase as a screening test for exacerbation of hepatic porphyrias by drugs. J Pharmacol Methods. 1981;6:77–85. [DOI] [PubMed] [Google Scholar]
- 153. Litman DA, Correia MA. L-tryptophan: a common denominator of biochemical and neurological events of acute hepatic porphyria? Science. 1983;222:1031–1033. [DOI] [PubMed] [Google Scholar]
- 154. Price JM, Brown RR, Peters HA. Tryptophan metabolism in porphyria, schizophrenia, and a variety of neurologic and psychiatric diseases. Neurology. 1959;9:456–468. [DOI] [PubMed] [Google Scholar]
- 155. Williams JN, Jr, Feigelson P, Elvehjem CA. Relation of tryptophan and niacin to pyridine nucleotides of tissues. J Biol Chem. 1950;187:597–604. [PubMed] [Google Scholar]
- 156. McCreanor GM, Bender DA. The metabolism of high intakes of tryptophan, nicotinamide and nicotinic acid in the rat. Br J Nutr. 1986;56:577–586. [DOI] [PubMed] [Google Scholar]
- 157. Horwitt MK, Harvey CC, Rothwell WS, Cutler JL, Haffron D. Tryptophan: niacin relationships in man. J Nutr. 1956;60:1–43.13385712 [Google Scholar]
- 158. Goldsmith GA. Niacin-tryptophan relationships in man and niacin requirement. Am J Clin Nutr. 1958;6:479–486. [DOI] [PubMed] [Google Scholar]
- 159. Fukuwatari T, Shibata K. Nutritional aspects of tryptophan metabolism. Int J Tryptophan Res. 2013;6:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Jacob RA, Swendseid ME, McKee RW, Fu CS, Clemens RA. Biochemical markers for assessment of niacin status in young men: urinary and blood levels of niacin metabolites. J Nutr. 1989;119:591–598. [DOI] [PubMed] [Google Scholar]
- 161. Stone T, Darlington LG. Review: the kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol. 2013;169:1211–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Frėdėrick R. Inhibition of the kynurenine pathway of tryptophan metabolism. In: Mittal S. ed. Targeting the Broadly Pathogenic Kynurenine Pathway. Cham: Springer; 2015:393–406. [Google Scholar]
- 163. Sheen M, Soliman H. Clinical trials targeting the kynurenine pathway. In: Mittal S. ed. Targeting the Broadly Pathogenic Kynurenine Pathway. Cham: Springer; 2015:407–417. [Google Scholar]
- 164. Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72:1–6. [DOI] [PubMed] [Google Scholar]
- 165. Pantouris G, Mowat CG. Antitumour agents as inhibitors of tryptophan 2,3-dioxygenase. Biochem Biophys Res Commun. 2014;443:28–31. [DOI] [PubMed] [Google Scholar]
- 166. Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA. 2012;109:2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Platten M, von Knebel Doeberitz N, Oesen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2015;5:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hou DY, Muller AJ, Sharma MD, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. [DOI] [PubMed] [Google Scholar]
- 169. Lob S, Konigsrainer A, Schafer R, Rammensee H-G, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;1111:2152–2154. [DOI] [PubMed] [Google Scholar]
- 170. Metz R, DuHadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. [DOI] [PubMed] [Google Scholar]
- 171. Prendergast GC, Metz E, Muller AJ, Merlo LMF, Mandik-Nayak L. IDO2 in immunomodulation and autoimmune disease. Front Immunol. 2014;5:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Nguyena NT, Kimura A, Nakahama T, Chinen E, Masuda K, Nohara K. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA. 2010;107:19961–19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Julliard W, Fechner JH, Mezrich JD. The aryl hydrocarbon receptor meets immunology: friend or foe? A little of both. Front Immunol. 2014;5:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Vogel CAF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Unkila M, Pohjanvirta R, Yuomisto J. Dioxin-induced perturbations in tryptophan homeostasis in laboratory animals. Adv Exp Med Biol. 1999;467:433–442. [DOI] [PubMed] [Google Scholar]
- 177. Pohjanvirta R, Miettinen H, Sankari S, Hegde N, Lindén J. Unexpected gender difference in sensitivity to the acute toxicity of dioxin in mice. Toxicol Appl Pharmacol. 2012;262:167–176. [DOI] [PubMed] [Google Scholar]
- 178. DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. García-Lara L, Pėrez-Severiano F, González-Esquivel D, Elizondo G, Segovia J. Absence of aryl hydrocarbon receptors increases Endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J Neurosci Res. 2015;93:1423–1433. [DOI] [PubMed] [Google Scholar]
- 181. Clark CJ, Mackay GM, Smythe GA, Bustamante S, Stone TW, Phillips RS. Prolonged survival of a murine model of cerebral malaria by kynurenine pathway inhibition. Infect Immun. 2005;73:5249–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Giorgini F, Huang S-Y, Sathyasaikumar KV, et al. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem. 2013;288:36554–36566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Vengeliene V, Cannella N, Takahashi T, Spanagel R. Metabolic shift of the kynurenine pathway impairs alcohol and cocaine seeking and relapse. Psychopharmacology. 2016;233:3449–3459. [DOI] [PubMed] [Google Scholar]
- 184. Badawy AA-B. Kynurenic acid and alcohol and cocaine dependence: novel effects and multiple mechanisms? Psychopharmacology. 2017;234:169–171. [DOI] [PubMed] [Google Scholar]
- 185. Walsh HA, O’Shea KC, Botting NP. Comparative inhibition by substrate analogues 3-methoxy- and 3-hydroxydesaminokynurenine and an improved 3 step purification of recombinant human kynureninase. BMC Biochem. 2003;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Walsh JL, Todd WP, Carpenter BK, Schwarcz R. 4-Halo-3-hydroxyanthranilic acids: potent competitive inhibitors of 3-hydroxy-anthranilic acid oxygenase in vitro. Biochem Pharmacol. 1991;42:985–990. [DOI] [PubMed] [Google Scholar]
- 187. Yates JR, Heyes MP, Blight AR. 4-Chloro-3-hydroxyanthranilate reduces local quinolinic acid synthesis, improves functional recovery, and preserves white matter after spinal cord injury. J Neurotrauma. 2006;23:866–881. [DOI] [PubMed] [Google Scholar]
- 188. Ishido K, Kamemura N, Imagawa T, Oda M, Sakurai J, Katunuma N. Quinolinate phosphoribosyl transferase, a key enzyme in de novo NAD+ synthesis, suppresses spontaneous cell death by inhibiting overproduction of active-caspase-3. Biochim Biophys Acta. 2010;1803:527–533. [DOI] [PubMed] [Google Scholar]
- 189. Zhang LQ, Heruth DP, Ye SQ. Nicotinamide phosphoribosyltransferase in human diseases. J Bioanal Biomed. 2011;7:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Tan B, Young DA, Lu Z-H, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, in human cancer cells: metabolic basis and potential clinical implications. J Biol Chem. 2013;288:3500–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zhang C, Tong J, Huang G. Nicotinamide phosphoribosyl transferase (Nampt) is a target of microRNA-26b in colorectal cancer cells. PloS ONE 2013;8:e69963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Verghese PB, Sasaki Y, Yang D, et al. Nicotinamide mononucleotide adenylyl transferase 1 protects against acute neurodegeneration in developing CNS by inhibiting excitotoxic-necrotic cell death. Proc Natl Acad Sci USA. 2011;108:19054–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Bricker AL, Carey VJ, Wessels MR. Role of NADase in virulence in experimental invasive group A streptococcal infection. Infect Immun. 2005;73:6562–6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Tatsuno I, Isaka M, Minami M, Hasegawa T. NADase as a target molecule of in vivo suppression of the toxicity in the invasive M-1 group A streptococcal isolates. BMC Microbiol. 2010;10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wall KA, Klis M, Kornet J, et al. Inhibition of the intrinsic NAD+ glycohydrolase activity of CD38 by carbocyclic NAD analogues. Biochem J. 1998;335:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Boulares AH, Yakovlev AG, Ivanova V, et al. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–22940. [DOI] [PubMed] [Google Scholar]
- 197. Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003;31:446–454. [DOI] [PubMed] [Google Scholar]
- 198. Morales JC, Li L, Fattah FJ, Dong Y, Bey EA, Patel M, et al. Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit Rev Eukaryot Gene Expr. 2014;24:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Curtin C, Szabo C. Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol Aspects Med. 2013;34:1217–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sperber H, Mathieu J, Wang Y, et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat Cell Biol. 2015;17:1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Wang G-X, Zhang Y, Lv Z-W, et al. Female specific association between NNMT gene and schizophrenia in a Han Chinese population. Int J Med Sci. 2014;11:1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kannt A, Pfenninger A, Teichert L, et al. Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. Diabetologia. 2015;58:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Zhang J, Xie X-Y, Yang S-W, Wang J, He C. Nicotinamide N-methyltransferase protein expression in renal cell cancer. J Zhejiang Univ: Sci B. 2010;11:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]