Abstract
Oxidative stress is considered an important factor in the development of endometriosis, including its malignant transformation. Previous studies have found that AT-rich interactive domain 1A (ARID1A), a tumor suppressor gene, is frequently mutated and inactivated in endometriosis-associated ovarian cancer (EAOC), and such a change in this gene is considered an early event in malignant transformation. We observed oxidative stress status by measuring the activity of the antioxidant enzyme manganese superoxide dismutase (MnSOD), malondialdehyde (MDA), and ARID1A gene expression in tissue samples from patients with endometriosis, EAOC, or non–endometriosis-associated ovarian cancer (non-EAOC). We also induced oxidative stress in the cultured cells from patients with primary endometriosis by adding H2O2 and tested for any alteration of ARID1A gene expression based on different H2O2 concentrations. The results showed that MnSOD activity in endometriosis and EAOC was lower than in non-EAOC, but MDA levels were higher. This study also showed that oxidative stress reduced ARID1A gene expression.
Keywords: endometriosis, ovarian cancer, malignant transformation, oxidative stress, tumor suppressor gene, ARID1A
Introduction
Endometriosis is a benign gynecologic abnormality that signifies the presence of endometrial tissues outside the uterine cavity; these lesions contain glands and stroma that respond to local, exogenous, and endogenous hormones.1 Various theories have been postulated regarding the cause and pathophysiology of endometriosis, such as menstrual blood regurgitation, persistent Müllerian duct abnormality, and coelomic metaplasia. However, these theories remain controversial.2,3
Endometriosis develops in 5% to 10% of women of reproductive age,4 and in Cipto Mangunkusumo Hospital (CMH), Jakarta, in 2010, endometriosis accounted for 42.62% of all benign gynecologic abnormalities.5 The clinical manifestations are varied and range from no symptoms to debilitating pain in about 40% to 60% of patients with endometriosis-associated dysmenorrhea.6 One of the clinical challenges of treating endometriosis is the high recurrence rate, which can necessitate reoperation.7 Furthermore, as a benign gynecologic lesion, this disease is often associated with ovarian cancer. Patients with endometriosis have a 4.2-fold higher risk of developing ovarian cancer than the normal population.8 Studies have identified atypical changes in endometriosis lesions found in patients with endometriosis-associated ovarian cancer (EAOC). These atypical changes are considered as initial malignant transformations of endometriosis that may lead to ovarian cancer. The types of ovarian cancer that are associated with endometriosis are clear cell carcinoma (CCC) and endometrioid ovarian carcinoma (EnOC),9,10 which accounted for 14% and 28%, respectively, of all cases of ovarian malignancy in CMH, Jakarta.5
It is well known that oxidative stress influences carcinogenesis in endometrial lesions, and iron accumulation from cyclic bleeding may promote the formation of reactive oxygen species (ROS). One of the counteracting mechanisms against ROS is the production of the antioxidant manganese superoxide dismutase (MnSOD). When the amount of antioxidants produced in the body cannot overcome the effects of oxidants, oxidative stress occurs.11 At a cellular level, ROS degrades lipids and forms malondialdehyde (MDA), so the MDA level in tissue depicts the level of lipid degradation or peroxidation.12 Oxidative stress can subsequently cause DNA damage that may possibly lead to inactivation of several tumor suppressor genes such as p53, phosphatase and tensin homolog (PTEN), and AT-rich interactive domain 1A (ARID1A).11,13 Furthermore, ARID1A gene mutations have been found in endometriotic lesions, CCC, and EnOC.
In this study, we investigated the effect of oxidative stress, which may promote malignant transformation, on the ARID1A gene by assessing its expression in endometriotic, EAOC, and non-EAOC tissue samples, as well as endometriotic primary cell cultures.
Methods
This study was conducted in the Faculty of Medicine Universitas Indonesia and CMH, Jakarta, from July 2011 to March 2014. The tissue and cell culture analyses were performed in the Molecular Biology Laboratory of the Biochemistry and Molecular Biology Department, Institute of Human Virology and Cancer Biology, Faculty of Medicine, Universitas Indonesia, and Integrated Laboratory of Faculty of Medicine, Universitas Indonesia.
In general, this study comprised 2 parts. In the first part of the study, we measured ARID1A expression, MnSOD activity, and MDA levels in endometriotic and ovarian cancer tissues. In the second part, we measured ARID1A expression in endometriotic cultured cells.
Sample collection
All tissue and primary cultured cell samples were obtained from patients who underwent surgical procedures at the CMH for endometriosis or ovarian cancer diagnoses, with or without hysterectomy. In total, 10 endometriotic, 10 epithelial ovarian cancer, and 3 normal endometrial tissues were obtained for the first part of the study. In the second part, primary cultured cells of normal endometrium (isolated from normal endometrial cells) and endometriosis (isolated from endometriotic cyst wall) were created and used.
Tissue homogenization and obtaining RNA and protein isolates
To examine MnSOD and MDA activity and obtain messenger RNA (mRNA) isolate and protein, tissue homogenates were prepared by washing collected tissue using phosphate-buffered saline (PBS) and chopping the samples. Homogenate for MnSOD and MDA examination was prepared by transferring the tissue into 1.5 mL homogenate tubes and adding 1 mL PBS.
For the mRNA and protein examination, the tissue was placed inside a 1.5-mL homogenate tube. TriPure Isolation Reagent (Roche) was added to the tube, and then the tissue was homogenized. From the homogenate produced, mRNA and protein were isolated by following kit protocol.
Cell culture
Two media for cell culture were used in this study. The first medium was used for endometriotic cells and the second medium was used for normal endometrial cells. The first medium comprised a mixture of Dulbecco’s Modified Eagle Medium (DMEM from Gibco, catalogue number: 12800017, 12800082), 20% fetal bovine serum (FBS, Gibco), 50 µg/mL gentamycin, 100 IU/mL penicillin, 100 µg/mL streptomycin, and 100 µg/mL Fungizone. The second medium was made by mixing DMEM F12 (DMEM F12 from Gibco, catalogue number: 12500062, 12500096), 10% FBS (FBS, Gibco), 50 µg/mL gentamycin, 100 IU/mL penicillin, 100 µg/mL streptomycin, and 100 µg/mL Fungizone. In these media, the tissue samples were stored at 4°C for less than 24 hours.
Around 500 to 700 mg of tissue was taken for cell extraction. The tissue was washed and cleaned using PBS and chopped until it reached a porridge-like consistency. Then, 1 mL of 100 U/mL collagenase was added. The homogenate was divided into two 50 mL homogenate tubes, and 6.5 mL of collagenase was added. The tubes were incubated at 37°C for 2 hours. To stop the reaction, we added DMEM and 5% FBS, 2 mL each. The tubes were centrifuged at 1250 rpm, and then 4 mL of PBS and 6 mL of red blood cell buffer were added during the process. After confluence was obtained, the sample was transferred to 24-well culture plates. Either the first or second medium for cell culture, as described above, was put in each well. The wells were reincubated at 37°C for around 2 days.
Subcultures
Around 80% to 90% of confluenced cells were harvested and then transferred to new wells. Previous growth mediums were removed, and the wells were washed using PBS; then 500 µL of trypsin (1 mg/mL) was added and the wells were incubated for 3 to 5 minutes until the cells were released microscopically. Later, 750 µL of medium was added to stop the trypsin reaction. The cells were relocated to 15 mL tubes and centrifuged for 5 minutes at 1500 rpm twice. Afterward, the pellets were divided into 12 wells, and 750 µL of either first or second growth medium was added.
mRNA and protein measurement
Messenger RNA concentration was measured using NanoDrop 2000 (Thermo Scientific) at a wavelength of 260 nm, and both mRNA and protein concentrations were measured at a wavelength of 280 nm. The mRNA and protein concentrations were expressed as relative expression and milligram per milliliter, respectively. We performed double measurements using duplicated samples and took the mean value.
RT-qPCR for ARID1A mRNA expression
Messenger RNA quantification was performed using a 1-step reverse transcription polymerase chain reaction (RT-qPCR) technique. We used LightCycler 480A (Roche Molecular Systems Inc., Branchburg, NJ, USA) and MiniOpticon (Bio-Rad Laboratories, Inc., Watford, United Kingdom) for tissue and cell culture, respectively. Homo sapiens SWI-like ARID1A with 178 bp length, transcript variant 1, was used for ARID1A primary sequence. The primary forward sequence was 5′-AACCCAGACTCGGGGATGTA-3′ with 20 bp length, and the primary reverse sequence was 5′-GCCGCTTGTAATTCTGCTGTT-3′ with 21 bp length. The master mix used in this study was KAPA SYBR FAST One-Step RT-qPCR Kit (Kapa Biosystems, Wilmington, MA,USA).
Each 50 ng of mRNA isolate from tissue samples and cell culture was used for double RT-qPCR measurement. Cycle threshold results were measured using Livak formula 2−ΔΔCt to gain mRNA relative expression of ARID1A.
ELISA for ARID1A protein expression
Protein from tissue samples and cell culture, which was extracted using TriPure Isolation Reagent, was analyzed using enzyme-linked immunosorbent assay (ELISA) kit (Cusabio, catalogue number: CSB-E13529h) based on the kit protocol. Well mapping (depicting standard and sample wells) was conducted, and 100 µL of either the standard or sample was added to each well. The wells were incubated at 37°C for 2 hours. The liquid was removed from the wells and changed with 100 µL of biotin antibody, and then the wells were reincubated at 37°C for 1 hour. Afterward, the wells were warmed in room temperature, the biotin antibody was aspired, and the wells were washed using buffer solution. The wells were shaken and left for 2 minutes, and then the process was repeated 3 times.
Later, 100-µL of horseradish peroxidase-avidin was added and the wells were reincubated at 37°C for 1 hour. The wells were washed with buffer solution and 90 µL of 3,3′,5,5′-tetramethylbenzidine substrate was added; then, the wells were reincubated at 37°C for 15 to 30 minutes by covering the wells with aluminum foil to protect them from light. As soon as the solution on the wells turned light blue, stop solution from the kit was added. Each well’s optical density was measured in less than 5 minutes using a microplate reader at 450 nm length.
MnSOD activity measurement
Manganese superoxide dismutase activity was measured using an MnSOD activity assay kit (Randox, Crumlin, County Antrim, United Kingdom.), which used xanthine and xanthine oxidase (XOD). The oxidation process produced superoxide radicals that reacted with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. The SOD activity was expressed as the degree of inhibition of this reaction. Tissue samples and standard samples from the kit were used. On each sample, 1.7 mL of mixed substrate (containing xanthine and INT) was added and then mixed evenly. Later, 5 µL of 5 mM NaCN was added, and then the samples were incubated at 37°C for 5 minutes. Afterward, 250 µL of XOD (8 U/L) was added, and then the absorbance was measured under 505 nm wavelength. The measurement was done twice, after 30 seconds and after 3 minutes.
MDA measurement
Malondialdehyde concentration was measured using the thiobarbituric acid reactive substances method. Blank solution was composed of a mixture of 2000 µL Aqua Dest and 1000 µL of 20% trichloroacetic acid (TCA). Four standard solutions were prepared (concentration of 0.625, 1.25, 2.5, and 5 nmol/mL) with various concentrations of Aqua Dest and TCA. Standard solution tubes were homogenized until confluence was obtained. Sample solution was also mixed with 1000 µL of 20% TCA and homogenized. Then, 2000 µL of TBA 0.67% was added to both standard and sample solutions. Blank, standard, and sample solutions were warmed in a 95°C to 100°C water bath and then cooled with cold water and ice packs. Afterward, the absorbance was read under 530 nm wavelength.
H2O2 induction and viable cell counting
H2O2 induction was performed using various concentrations of H2O2 (100 and 1000 nM of H2O2 and 0 nM as control). Around 10 M H2O2 was diluted in both mediums for cell culture until 100 and 1000 nM H2O2 concentrations were reached. Both 100 and 1000 nM wells and a control well were made in triplicate, so 9 wells in total were obtained. Induction was performed in a 96-well culture plate. Normal endometrial and endometriotic cells were added into wells with a density of 25 000 cells/mL. Cells were incubated on the growth medium for 24 hours. On the following day, the growth medium was changed with medium containing H2O2. Induction was performed for 48 hours, and then the cell viability and level of ROS were analyzed.14
The viable cells were manually counted using a slide-improved Neubauer Hemocytometer under microscope with ×40 magnitude. Afterward, 500 µL of trypsin (1 mg/mL) was added to release the cells from the wells.
Dichlorodihydrofluorescein diacetate assay for ROS measurement
Reactive oxygen species level was measured using a dichlorodihydrofluorescein diacetate (DCFH-DA) assay. This assay was performed by diluting 150 µL of 20 nM DCFH-DA in 1.5 mL PBS to obtain 2 µM DCFH-DA. Trypsin was added to each well that would be measured later (as described in the “Subcultures” section), and then the samples were transferred to 1.5 mL tubes. To each tube, 250 µL of 2 µM DCFH-DA was added, and then the sample was incubated at 37°C for 30 minutes. The tubes were taken out, washed with PBS, recentrifuged for 1 minute, and rewashed with PBS. From each tube, a 25-µL sample was taken and placed inside a slide chamber to be read with a Tali Image-Based Cytometer (Life Technologies, Carlsbad, CA, United States.). Double measurements of fluorescence absorbance reading were conducted.
Results
ARID1A expression was lower in endometriosis and EAOC tissues and correlated with MnSOD activity but negatively correlated with MDA
Serous adenocarcinoma and mucinous adenocarcinoma tissue samples (5 and 1, respectively, representing non-EAOC), CCC and endometrioid ovarian cancer samples (3 and 1, respectively, representing EAOC), and endometriotic and normal endometrial tissue samples (10 and 3, respectively) as controls were collected and analyzed.
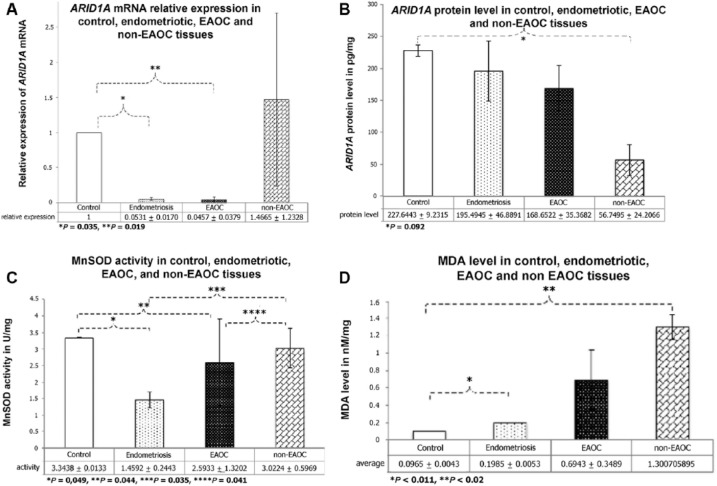
The qRT-PCR revealed that the relative mRNA expression of ARID1A was lowest in the EAOC (CCC) and endometriotic tissues and highest in non-EAOC tissues (Figure 1A). Furthermore, the endometrial and ovarian cancer tissue samples exhibited lower protein expression levels than the control sample of normal endometrial tissue (Figure 1B).
Figure 1.
Endometriotic tissues exhibited decreased ARID1A mRNA relative expression, protein level, and MnSOD activity but increased MDA level. Normal endometrial tissues were used as control. (A) ARID1A mRNA relative expression was significantly lower in the EAOC and endometriotic tissues. Relative expression shown in y-axis is average of gene expression, measured by Livak formula (2−ΔΔCt). mRNA quantification was done using a 1-step reverse transcription polymerase chain reaction. Statistical significance difference was determined using 1-way ANOVA (*P = .035, **P = .019). (B) Endometriosis and ovarian cancer tissue samples had lower protein level. Extracted protein was analyzed using ELISA and optical density was measured at 450 nm length (expressed in pg/mg). Statistical significance difference was determined using 1-way ANOVA (*P = .092). (C) MnSOD activity in endometriotic tissues was the lowest. MnSOD activity, depicting antioxidant enzyme, was measured using xanthine and xanthine oxidase. The wells were incubated at 37°C for 5 minutes, and their absorbance was read at 505 nm wavelength. Statistical significance difference was determined using 1-way ANOVA (*P = .049, **P = .035, ***P = .044, ****P = .041). (D) Increased MDA level in endometriotic and ovarian cancer tissues. MDA level, reflecting the extent of oxidative stress, was measured using thiobarbituric acid reactive substances method with various concentrations of Aqua Dest and trichloroacetic acid. Its absorbance was read at 530 nm wavelength. Statistical significance difference was determined using Mann-Whitney test (*P < .011 and **P < .02). ANOVA indicates analysis of variance; ARID1A, AT-rich interactive domain 1A; EAOC, endometriosis-associated ovarian cancer; ELISA, enzyme-linked immunosorbent assay; MDA, malondialdehyde; MnSOD, manganese superoxide dismutase; mRNA, messenger RNA.
The activity of MnSOD was lower in endometriotic tissues than it was in the normal endometrial, EAOC, and non-EAOC tissues (Figure 1C). Furthermore, MDA levels, which reflect the extent of oxidative stress in tissues, were lowest in the control group (Figure 1D).
ARID1A expression was affected by the level of oxidative stress in cultured endometriotic cells
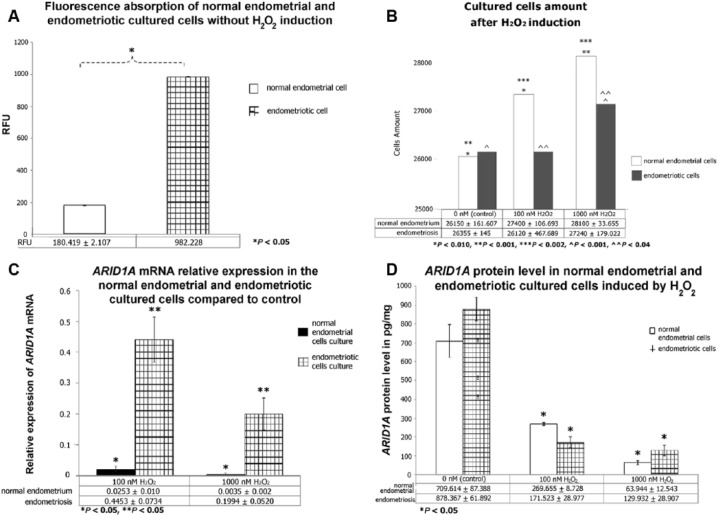
In the second part of the study, endometriotic and normal endometrial cells were cultured and subsequently exposed to H2O2 at various concentrations (0, 100, and 1000 nM) for 48 hours. Exposure to H2O2 increased the number of eutopic endometrial cells, dependent on concentration. In contrast, the number of viable endometriotic cells was decreased after exposure to 100 nM H2O2 and increased after exposure to 1000 nM H2O2. Furthermore, a significant increase in cell number was observed after exposure to 1000 nM H2O2 in both groups.
Reactive oxygen species levels were measured immediately after the cultured cells were exposed to various H2O2 concentrations (0, 100, and 1000 nM) for 48 hours using a Tali Cytometer (Invitrogen, Carlsbad, CA, United States.). Reactive oxygen species level reflects the ability of cells to accumulate fluorescence. Normal endometrial cell culture showed higher ROS levels after being exposed to higher concentrations of H2O2 than when exposed to lower concentrations. The endometriotic cell culture ROS levels were already extremely high compared with those of the normal endometrial cell culture before exposure to H2O2 and therefore could not be estimated following the addition of H2O2. A comparison of the ROS content of the normal endometrial and endometriotic cells before H2O2 exposure is shown in Figure 2A, and cell viability after H2O2 exposure is shown in Figure 2B.
Figure 2.
Oxidative stress suppressed ARID1A mRNA relative expression and protein level. Normal endometrial and endometriotic cells were cultured using Dulbecco’s Modified Eagle Medium with a density of 25 000 cells/mL. Tissue samples were stored in those media at 4°C for less than 24 hours. (A) Fluorescence absorption was significantly higher in endometriotic cells, even with the absence of H2O2 induction. Fluorescence absorption, depicting reactive oxygen species level, was measured using a dichlorodihydrofluorescein diacetate assay. Samples were incubated at 37°C for 30 minutes and read by cytometer. Statistical significance difference was determined using t-test (*P < .05; RFU, relative fluorescence unit). (B) Both normal endometrial and endometriotic cells responded to increased concentration of H2O2 induction by increasing cell proliferation. H2O2 induction was done using various concentrations of H2O2 (100 and 1000 nM; 0 nM as control) for 48 hours. Viable cells were manually counted using hemocytometer under microscope with ×40 magnitude. Statistical significance difference was determined using paired t-test (*P = .010, **P = .001, ***P = .002, ^P = .001, ^^P = .04). (C) H2O2 suppressed relative mRNA expression of ARID1A with dramatic reduction in endometriotic cultured cells. Relative expression was measured by Livak formula (2−ΔΔCt) and mRNA quantification was performed using a 1-step reverse transcription polymerase chain reaction. Statistical significance difference was determined using t-test (*P < .05). (D) ARID1A protein level was dose dependently suppressed by increasing amount of H2O2. Extracted protein was analyzed using enzyme-linked immunosorbent assay, and optical density was measured at 450 nm length (expressed in picogram per milligram). Statistical significance difference was determined using 1-way analysis of variance (*P < .05). ARID1A indicates AT-rich interactive domain 1A; EAOC, endometriosis-associated ovarian cancer; mRNA, messenger RNA.
In this study, higher concentrations of H2O2 were associated with a lower relative mRNA expression of ARID1A in both cell groups relative to the control (cell culture without H2O2). Furthermore, H2O2 suppressed the relative mRNA expression of ARID1A (Figure 2C), although the decrease was much higher in the normal endometrial tissue group (100 and 1000 nM, 0.025 and 0.0035, respectively) than in the endometriotic tissue group (100 and 1000 nM, 0.45 and 0.2, respectively). In addition, the protein expression of ARID1A was dose dependently decreased by H2O2 (Figure 2D).
Discussion
Oxidative stress has been hypothesized to be involved in the development of endometriotic lesions,15,16 whereas decreased expression of the tumor suppressor gene ARID1A is often found in EAOC.17 In this study, we explored the effect of oxidative stress on ARID1A gene expression in endometriotic and normal endometrial cell cultures. This is in contrast to previous studies that investigated its expression in cancer tissues or endometriotic tissues and found atypical changes in both tissues18,19 or those that investigated endometriosis without analyzing the effect of oxidative stress.20
Expression of the ARID1A tumor suppressor gene
In the first part of this study, we found that ARID1A mRNA expression was lower in endometriotic tissue than it was in the control (normal endometrial tissue). These findings are important because they demonstrate that low expression levels of the ARID1A gene in endometriotic tissues may be one of the characteristics contributing to its tendency to transform malignantly.10
ARID1A protein expression was lower in endometriotic tissues than it was in normal endometrial tissues. This could have been caused by higher oxidative stress levels in the endometriotic tissues than in normal endometrial tissues. Reactive oxygen species as free radicals may cause protein damage.21 Compared with the EAOC and non-EAOC tissues, the ARID1A protein expression in endometriotic tissues was higher, which could be more attributable to the inactivation of the ARID1A gene protein expression than its mRNA expression. Furthermore, a loss of ARID1A expression was found in endometriotic tissue that had already exhibited atypical cell changes.19,22
Interestingly, we found that levels of ARID1A mRNA expression were dramatically different in some samples. However, there was only a subtle difference in protein level in exactly the same 4 samples. Through our results, we noticed that damage in mRNA level does not correlate with damage in protein level. As the mRNA was damaged, numerous factors at the gene level would affect the process of mRNA transformation into protein (eg, mutation, loss of heterozygosity, and ARID1A gene haploinsufficiency).
One limitation of our study was that for some tissue types we used, there was only 1 available sample (mucinous adenocarcinoma tissue and endometrioid ovarian cancer). Therefore, the data might be too small for statistical analysis. Further study with larger sample sizes should be considered in future.
Oxidative stress in endometriosis and ovarian cancer
In the first part of this study, the activity of MnSOD in the EAOC, non-EAOC, and endometriotic tissues was lower than that in control tissues. This finding indicates that low levels of MnSOD may permit the growth of tumors, particularly malignancies.23 This tumor growth may also be associated with the role of MnSOD as a tumor suppressor.24 In our study, the low levels of MnSOD also suggested a greater utilization of MnSOD in endometriotic lesions than in normal endometrial tissues.
In this study, we also found that MnSOD activity tended to be lower in the ovarian cancer tissues group (EAOC and non-EAOC) than it was in the normal endometrial tissues. This may be associated with the growth requirements of cancer cells, which include high levels of oxidative stress.25
Moreover, the difference in MnSOD activity between the EAOC and non-EAOC tissues may be explained by the difference in the characteristics of both malignancies. Endometriosis-associated ovarian cancer lesions tend to grow more slowly than non-EAOC lesions. Furthermore, the fast-growing characteristic of non-EAOC lesions indicates that these tumors consume more energy during growth, thereby producing greater oxidative stress.26 Tumor cell growth requires both oxidative stress and antioxidants to neutralize the deleterious effects of oxidative stress.14 We found that MDA levels were highest in the non-EAOC tissues, followed by EAOC and endometriotic tissues, whereas control tissues showed the lowest levels. This finding is consistent with previous reports that non-EAOC lesions tend to grow faster than EAOC lesions and therefore cause greater oxidative stress. Moreover, cancer cells tend to produce more oxidants than normal cells,26 which explains why MDA levels were the lowest in control tissues.27
Role of oxidative stress in cell proliferation and ARID1A gene expression
We observed increased cell viability after H2O2 exposure. Previous studies determined that increased ROS production can be accompanied by an increase in cell proliferation.14,27 Theoretically, this may be because of the role of ROS in activating the Raf/mitogen-activated protein kinase/extracellular signal–regulated kinase pathways, resulting in increased cell proliferation.28 A similar finding of increased cell numbers in response to exposure to high concentrations of H2O2 was noted in this study. Oxidative stress production in this study was assessed by the ability of cells to accumulate fluorescence, and the results showed that both endometriotic and normal endometrial cells withstood increasing levels of oxidative stress. However, we noticed a difference in cell viability between both endometriotic and normal endometrial cells, which we believe was caused by different molecular responses toward oxidative stress, although the exact mechanism remained unknown.
In endometriotic and normal endometrial cell cultures, it was observed that H2O2 levels were inversely related to tumor suppressor protein and mRNA expression. The implication of this result is that iron, which is released by cyclic bleeding of endometriotic tissues, functions as an oxidant and therefore causes oxidative stress in endometriotic lesions. Therefore, ARID1A expression was lower in endometriotic tissues than it was in normal endometrial tissues.
As shown in Figure 1B, we found that normal endometrial cells had higher protein levels than the other samples; however, a contradictive result is shown in Figure 2D. This finding might be caused by the different samples (tissues collected directly from gynecologic surgeries for Figure 1B and culture of those tissues for Figure 2D). We have not yet determined the cause of this difference. Therefore, we cannot conclude that protein levels of normal endometrial cells will always be higher than that of endometriotic cells. This could be investigated further in future studies.
Conclusions
In endometriotic tissue, the expression of ARID1A mRNA was lower than that in the normal endometrial (control) and non-EAOC tissues, whereas its protein expression was lower than that of the control but higher than EAOC and non-EAOC tissues. These findings demonstrate the early decreased expression of ARID1A, especially its mRNA in endometriotic tissue. Moreover, oxidative stress appeared to have a role in decreasing the expression of ARID1A protein and mRNA levels in endometriotic cells. Oxidative stress appeared to suppress ARID1A expression in endometriotic cells, whereas low ARID1A gene activity in endometriosis may be a contributing factor to the enhanced susceptibility of these lesions to malignant transformation.
Footnotes
Peer Review:Seven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1935 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: HW, MIT, MS, and SIW conceived and designed the experiments, analyzed the data, wrote the first draft of the manuscript, contributed to the writing of the manuscript, agreed with manuscript results and conclusions, jointly developed the structure and arguments for the paper, and made critical revisions and approved final version. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics: As a requirement of publication, author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including, but not limited to, the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1. Baldi A, Campioni M, Signorile PG. Endometriosis: pathogenesis, diagnosis, therapy and association with cancer (review). Oncol Rep. 2008;19:843–846. [PubMed] [Google Scholar]
- 2. Pirdel L, Pirdel M. Role of iron overload-induced macrophage apoptosis in the pathogenesis of peritoneal endometriosis. Reproduction. 2014;147:199–207. [DOI] [PubMed] [Google Scholar]
- 3. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. [DOI] [PubMed] [Google Scholar]
- 4. Leyland N, Casper R, Laberge P, Singh SS; and The Society of Obstetricians and Gynaecologists of Canada. Endometriosis: diagnosis and management. J Obstet Gynaecol (Canada). 2010;32: S1–32. [PubMed] [Google Scholar]
- 5. Winarto H, Laihad BJ, Nuranna L. Modification of cutoff values for HE4, CA125, the risk of malignancy index, and the risk of malignancy algorithm for ovarian cancer detection in Jakarta, Indonesia. Asian Pac J Cancer Prev. 2014;15:1949–1953. [DOI] [PubMed] [Google Scholar]
- 6. Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759–1769. [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi. Molecular pathogenesis of endometriosis-associated clear cell carcinoma of the ovary (review). Oncol Rep. 2009;22:233–240. http://www.spandidos-publications.com/or/22/2/233. [PubMed] [Google Scholar]
- 8. Maeda D, IeM S. Pathogenesis and the role of ARID1A mutation in endometriosis-related ovarian neoplasms. Adv Anat Pathol. 2013;20:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;112:1171–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3483701/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi H, Yamada Y, Kanayama S, et al. The role of iron in the pathogenesis of endometriosis. Gynecol Endocrinol. 2009;25:39–52. [DOI] [PubMed] [Google Scholar]
- 13. Shigetomi H, Higashiura Y, Kajihara H, Kobayashi H. A potential link of oxidative stress and cell cycle regulation for development of endometriosis. Gynecol Endocrinol. 2012;28:897–902. [DOI] [PubMed] [Google Scholar]
- 14. Foyouzi N, Berkkanoglu M, Arici A, Kwintkiewicz J, Izquierdo D, Duleba AJ. Effects of oxidants and antioxidants on proliferation of endometrial stromal cells. Fertil Steril. 2004;82:1019–1022. [DOI] [PubMed] [Google Scholar]
- 15. Wiegand KC, Lee AF, Al-Agha OM, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224:328–333. [DOI] [PubMed] [Google Scholar]
- 16. Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod Pathol. 2012;25:615–624. [DOI] [PubMed] [Google Scholar]
- 18. Samartzis E, Noske A, Dedes K, Fink D, Imesch P. ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas. Int J Mol Sci. 2013;14:18824–18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griffiths HR, Dias IHK, Willetts RS, Devitt A. Redox regulation of protein damage in plasma. Redox Biol. 2014;2:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao W, Awadallah A, Xin W. Loss of ARID1A/BAF250a expression in ovarian endometriosis and clear cell carcinoma. Int J Clin Exp Pathol. 2012;5:642–650. [PMC free article] [PubMed] [Google Scholar]
- 21. Naidu MSK, Suryakar AN, Swami SC, Katkam RV, Kumbar KM. Oxidative stress and antioxidant status in cervical cancer patients. Indian J Clin Biochem. 2007;22:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weydert C, Roling B, Liu J, et al. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol Cancer Ther. 2003;2:361–369. [PubMed] [Google Scholar]
- 23. Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32. [DOI] [PubMed] [Google Scholar]
- 24. Kurman RJ, Shih I-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Hum Pathol. 2011;42:918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. [DOI] [PubMed] [Google Scholar]
- 26. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ngô C, Chéreau C, Nicco C, Weill B, Chapron C, Batteux F. Reactive oxygen species controls endometriosis progression. Am J Pathol. 2009;175:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. [DOI] [PubMed] [Google Scholar]