Abstract
Recent work indicates an intimate interaction of the tryptophan catabolite (TRYCAT) pathways with the melatonergic pathways, primarily via TRYCAT pathway induction taking tryptophan away from the production of serotonin, which is a necessary precursor for the melatonergic pathways. The alpha 7 nicotinic receptor may be significantly modulated by this interaction, given its inactivation by the TRYCAT, kynurenic acid, and its induction by melatonin. Similarly, the aryl hydrocarbon receptor is activated by both kynurenic acid and kynurenine, leading to CYP1A2 and melatonin metabolism, whereas melatonin may act to inhibit the aryl hydrocarbon receptor. These 2 receptors and pathways may therefore be intimately linked, with relevance to a host of intracellular processes of clinical relevance. In this article, these interactions are reviewed. Interestingly, mitochondria may be a site for direct interactions of these pathways and receptors, suggesting that their differential induction may not only be modulating neuronal, glia, and immune cell processes and activity but also be directly acting to regulate mitochondrial functioning. This is likely to have significant consequences as to how an array of diverse central nervous system and psychiatric conditions are conceptualized and treated.
Keywords: tryptophan catabolites, melatonin, aryl hydrocarbon, alpha 7 nicotinic, mitochondria, CNS, psychiatry, sirtuins, glia, gut-brain axis
Introduction
There is a growing interest in the role of tryptophan catabolites (TRYCATs), such as kynurenic acid (KYNA) and quinolinic acid (QUIN), in the etiology, course, and treatment of a wide array of central nervous system (CNS) disorders, including Alzheimer disease and Parkinson disease,1,2 and psychiatric disorders, including depression and schizophrenia.3,4 This work has been complemented by the appreciation of the influence of TRYCAT induction on the levels of tryptophan available for serotonin synthesis and therefore for the synthesis and release of N-acetylserotonin (NAS) and melatonin.5 Although primarily known for its release by the pineal gland in modulating circadian rhythms, accumulating data show that melatonin is produced by a host of different cells, including astrocytes, macrophages, T cells, gut enterochromaffin cells, and fibroblasts.6 It has recently been proposed that melatonin may be produced by all mitochondria-containing cells,7 including within mitochondria.8 Such synchronized interactions of the TRYCAT pathway with levels of serotonin and melatonin synthesis are likely to have significant consequences for an array of CNS and psychiatric disorders, including via the regulation of glia and immune cell reactivity levels. Given that melatonin modulates the levels and activity of the alpha 7 nicotinic acetylcholine receptor (a7nAChR)9 and that the a7nAChR may mediate some of melatonin’s effects,10 it is likely that alterations in a7nAChR levels and activity may also covary with TRYCAT induction.
In this article, the effects of pro-inflammatory cytokine–induced indoleamine 2,3-dioxygenase (IDO) and stress/cortisol-induced tryptophan 2,3-dioxygenease (TDO), which drive tryptophan to TRYCAT synthesis, are reviewed. This is looked at in the context of coordinated alterations in levels and activation of serotonin and the melatonergic pathways. The interactions of these pathways have significant consequences for the levels and activity of the a7nAChR and aryl hydrocarbon receptor. The interactions of these pathways and receptors may not only be by the regulation of intracellular neuronal, glia, and immune cell pathways and activity but also directly within mitochondria. Such interactions have implications for a host of CNS and psychiatric conditions. First, the TRYCAT pathways are overviewed.
Tryptophan Catabolites
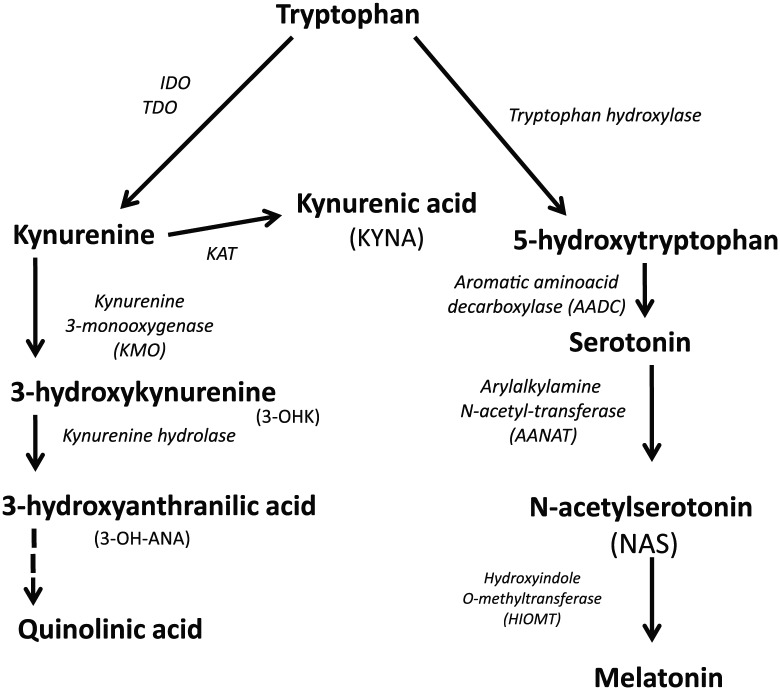
Indoleamine 2,3-dioxygenase is induced by the pro-inflammatory cytokines, interleukin (IL)-1B, IL-6, IL-18, and tumor necrosis factor α (TNF-α), but especially interferon-γ.11 Stress, and the stress hormone cortisol, can also lead to TRYCAT induction via TDO activation.12 By driving tryptophan down the TRYCAT pathways, IDO and TDO lead to the production of a number of neuroregulatory products, including KYNA, which has protective effects via its inhibition of glutamatergic receptor activation, including the N-methyl-d-aspartate receptor. As such, KYNA induction has been proposed as a significant treatment target under conditions associated with excessive excitatory glutamatergic activity, including for epilepsy and multiple sclerosis (MS). Another commonly researched TRYCAT is QUIN, which is excitotoxic13 and increased in a number of CNS and psychiatric conditions, where it contributes to neuronal and cognitive losses.14 Another frequently investigated TRYCAT is 3-hydroxykunurenine (3-OHK), which is increased in Alzheimer disease and major depressive disorder (MDD), as well as other CNS and psychiatric disorders.15,16 As indicated in Figure 1, any inhibition of kynurenine aminotransferase (KAT) will decrease KYNA synthesis and drive an increased synthesis of 3-OHK, which may further be enzymatically converted to QUIN and oxidized nicotinamide adenine dinucleotide (NAD+). As such, any KAT inhibition, by decreasing KYNA and increasing 3-OHK and QUIN, will contribute to cellular damage and/or inflammatory processes.
Figure 1.
The major components of the kynurenine and serotonergic/melatonergic pathways and their interactions. The initial conversion of tryptophan by tryptophan hydroxylase leads to the synthesis of serotonin and melatonin. When stress and pro-inflammatory cytokines are increased, inducing TDO and IDO, respectively, tryptophan is driven down the kynurenine pathway leading to the increased synthesis of tryptophan catabolites. IDO indicates indoleamine 2,3-dioxygenase; TDO, tryptophan 2,3-dioxygenease.
Recently, we showed that an increase in the kynurenine/KYNA ratio is evident in MDD and chronic fatigue syndrome/myalgic encephalomyelitis, but only in association with the somatization that is dramatically increased under these conditions.17 As to whether this is mediated by an increased availability of kynurenine for conversion to 3-OHK and QUIN requires further investigation. However, it does indicate how the classical association of MDD with an array of different neurodegenerative conditions, such as Alzheimer disease, MS, and Parkinson disease, may be mediated by particular aspects of MDD, namely, somatization, that are intimately associated with TRYCAT pathway alterations. Most CNS and psychiatric conditions show an increase in the kynurenine/KYNA ratio, with increased levels in the kynurenine/tryptophan ratio also evident in many neuropsychiatric conditions, including MDD, bipolar disorder, schizophrenia, Alzheimer disease, and MS.18-20 Given that the biological underpinnings of MDD may act on processes that biologically prime or accelerate the pathophysiology of such CNS disorders, it requires investigation as to whether it is the somatization aspect of MDD, via increased kynurenine/KYNA ratio, that is mediating the MDD influence on the etiology and course of these CNS and psychiatric conditions. It is of note that more than 60% of central kynurenine is peripherally derived, indicating the influence that peripheral inflammatory processes may have on levels of neuroregulatory TRYCATs.21
Overall, TRYCATs may be significant effectors of how oxidative and nitrosative stress and pro-inflammatory cytokines mediate their influence on CNS and psychiatric conditions. However, in addition to increasing TRYCATs, the activation of IDO and TDO drives down serotonin levels and therefore melatonergic pathway activity. This is reviewed in the next section.
Melatonergic Pathways
The methoxyindole N-acetyl-5-methoxytryptamine (melatonin) is primarily known for its nighttime release by the pineal gland, by which means it has a significant role in circadian rhythm entrainment. This is of some importance as circadian dysregulation is a significant clinical aspect of many CNS and psychiatric conditions, including Alzheimer disease and bipolar disorder.18,19 However, accumulating data show melatonin to be released by many different cell types, including enterochromaffin cells of the gut, glia, and immune cells.6 In the gut, melatonin release can be up to 400-fold greater than its maximal pineal gland release levels, with gut melatonin contributing to the maintenance of the gut barrier. In immune cells, the autocrine effects of melatonin lead to an anti-inflammatory phenotype that acts to prevent excessive pro-inflammatory activity.22
On the basis of a growing body of evidence, melatonin has been proposed to be released by all mitochondria-containing cells, including possibly from within mitochondria.8 Consequently, pro-inflammatory cytokine–induced IDO or stress-induced TDO, by increasing TRYCAT and decreasing serotonin for the melatonergic pathways, may have significant consequences for a wide array of physiologic and pathophysiologic processes that melatonin acts to regulate.6 Alterations in the levels of melatonergic pathway activity may also have significant and direct impacts on mitochondrial functioning.
Melatonin has powerful effects on many fundamental cellular processes, including being a powerful antioxidant and driver of nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2)-induced endogenous antioxidants; increasing sirtuin-1, the longevity protein, which regulates mitochondrial functioning and aging-associated processes; being an anti-inflammatory, including from its autocrine release by immune cells; being a clinically relevant antinociceptive; and being an optimizer of mitochondrial functioning and enhancing neurogenesis levels.23 Such an array of effects highlights the relevance of altered melatonergic pathway activation under conditions of TRYCAT induction. Consequently, it is worth looking at the melatonergic pathways in more detail.
Serotonin is enzymatically converted by arylalkylamine-N-acetyltransferase (AANAT) to NAS, with NAS then enzymatically converted by hydroxyindole o-methyltransferase (HIOMT) (also known as acetylserotonin methyltransferase) to melatonin. Melatonin synthesis is therefore highly dependent on the levels of tryptophan availability for serotonin production and on the levels of serotonin degradation by monoamine oxidase (MAO). As such, many of the protective and clinical effects of different types of antidepressants, such as selective serotonin reuptake inhibitors and MAO inhibitors, may ultimately be mediated by their regulation of local melatonin synthesis. Notably, it is not only increased central melatonin and NAS that is mediating such effects but also melatonergic pathway activation in a variety of systemic systems and peripheral processes, including in the regulation of gut microbiota and gut permeability.24 It should also be noted that melatonergic pathway activation leads to the synthesis and efflux of both NAS and melatonin.
NAS and melatonin are amphiphilic, allowing their ready diffusion across cell membranes, as well as intercellularly. Although far less investigated than melatonin, NAS has many similar effects, including as an antioxidant, neurogenesis inducer, and mitochondria regulator. N-acetylserotonin also mimics the effects of brain-derived neurotrophic factor (BDNF) via the activation of tropomyosin receptor kinase B (TrKB), a BDNF receptor.25 Some melatonin is also converted back to NAS, whereas other factors, such as increased adenosine triphosphate, may inactivate HIOMT,26 leading to only NAS synthesis and release. Consequently, an array of factors can modulate the NAS/melatonin ratio, which may be of relevance to the cause and course of the pathophysiologic processes that underlie glioblastoma27 and bipolar disorder18 which may be primarily mediated by the differential effects of NAS and melatonin on the activation of TrkB and a7nAChR, respectively.
It should also be noted that many of melatonin’s metabolites, including N1-acetyl-N2-formyl-5-methoxykynuramine and N1-acetyl-5-methoxykynuramine, also have significant anti-inflammatory, antioxidant, and immune regulatory effects.23 As such, melatonergic pathway activation leads to the synthesis of products with antioxidant and generally beneficial effects that are relevant to all CNS and psychiatric disorders.2-6,19,20 It is also clear that the products of the melatonergic pathway also have some quite specific effects that are likely to be of clinical relevance across different CNS conditions, including via melatonin’s regulation of the a7nAChR and the NAS activation of TrkB. It remains to be determined as to whether there are other differential effects of the various products of the melatonergic pathways that would be relevant to CNS and psychiatric disorders.
Although many of melatonin’s effects can be via the activation of the melatonin receptors (MT1 and MT2),28,29 melatonin also has powerful nonreceptor effects, aided by its amphiphilic nature. Melatonin frequently accumulates around mitochondria, where it may have an important role in the regulation of membrane fluidity, as recent data indicate.30 Structural membrane regulation may be another aspect of how melatonin can act to modulate and synchronize wider cellular processes.
The 14-3-3 protein is a crucial determinant of pineal gland melatonergic pathway activity, due to its stabilization of AANAT,31 indicating that alterations in 14-3-3 levels will significantly modulate NAS and melatonin synthesis in other cell types. This role of 14-3-3 is likely to have significance in a number of CNS and psychiatric disorders. In motor neuron disease, for example, 14-3-3 levels are often increased. However, in this medical condition, 14-3-3 is mostly in a complex with mutant superoxide dismutase, thereby preventing its stabilization of AANAT. This suggests that decreased NAS and melatonin synthesis may be relevant to the array of pathophysiologic changes that are evident in a number of cell types in this poorly conceptualized and poorly managed condition.
It is also of note that antidepressants increase a number of 14-3-3 isoforms, suggesting that some of their efficacy is mediated via effects on melatonergic pathway regulation, as well as increasing serotonin availability.32 Tumor necrosis factor α and other pro-inflammatory cytokines inhibit pineal gland NAS and melatonin synthesis,6,23 whereas increased adenosine triphosphate prevents the conversion of NAS to melatonin, suggesting a significant impact on the NAS/melatonin ratio.26 As such, the melatonergic pathways may be regulated by a variety of cellular and systemic processes, in turn acting to modulate the reactivity of glia and immune cells.
Interestingly, the regulation of inflammatory processes may be intimately linked to the synchronized regulation of the pineal gland and immune/glia reactivity. The work of Regina Markus and colleagues33 indicates the presence of an immune-pineal axis. By pro-inflammatory cytokines switching off pineal gland melatonin synthesis, the generally immune-dampening effects of pineal gland melatonin are prevented during the course of infection and inflammation, allowing an appropriate immune response to develop.33 However, when the immune response is no longer required, the autocrine effects of melatonin on activated immune cells lead to a dampening of the immune response and therefore the reinstatement of pineal gland melatonin synthesis. As such, alterations in melatonin synthesis, in the pineal gland and immune cells, may be intimately associated with consequences for alterations in circadian regulation and the temporal coordination of the immune response.
Melatonergic pathway regulation is therefore intimately linked to an array of core cellular and systemic processes. Melatonin effects contrast with many TRYCAT effects, including in the levels and activity of the a7nAChR, when compared with KYNA effects on this receptor. The a7nAChR is looked at next.
The a7nAChR
The a7nAChR is expressed in most CNS cell types. The a7nAChR is also expressed in many immune cells and gut cells, as well as in the vagal nerve and cells of the enteric nervous system.34,35 Activation of the neuronal presynaptic a7nAChR increases the influx of calcium and sodium ions, increasing neurotransmitter release. The a7nAChR is also postsynaptically expressed, indicating a wider role in synaptic plasticity.36 Activation of the a7nAChR in astrocytes and microglia decreases inflammatory responses.37,38 Activation of the a7nAChR also regulates endothelial cells,39 neurogenesis,40 macrophages,41 T cells, and immature dendritic cell maturation,42 indicating a wide array of non-neuronal effects. The a7nAChR also inhibits the immune-activating consequences arising from increases in gut permeability,43 possibly via the regulation of vagal nerve activity,34 as well as having analgesic and cognitive-enhancing effects.44-46
Melatonin and a7nAChR Interactions
Given the wide expression of the a7nAChR and the possibly ubiquitous expression of melatonin, it is likely that interactions of the a7nAChR and melatonin will occur in many central and peripheral sites, including in immune cells, glia, and the gut-brain axis. Mitochondria may be an important site for these interactions. For example, melatonin’s protection against rodent ischemia is a7nAChR dependent.47 Both melatonin and a7nAChR activation optimize mitochondrial functioning.48 The interactions of melatonin and the a7nAChR may therefore affect a number of processes, including glia reactivity, macrophage reactivity, autophagy, and gut-brain axis. This will be looked at in more detail below, including in the context of interactions within mitochondria.
The Aryl Hydrocarbon Receptor
The aryl hydrocarbon receptor (AhR), commonly referred to as the dioxin receptor, is widely expressed, both centrally and systemically. Investigations of AhR effects have widely used 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). However, different TRYCATs can also activate the AhR, suggesting that this could be an important consequence of TRYCAT induction.
The AhR is a ligand-activated receptor of the Per-Arnt-Sim family of basic helix-loop-helix transcription factors. In addition to cigarette smoke–associated TCDD, a growing number of endogenous AhR ligands have emerged, including kynurenine, KYNA, and formylindolo(3,2-b)carbazole. Exogenous ligands include an array of environmental toxins, such as many of the particulate matter components of air pollution,49 which can alter the gut microbiome and increase gut permeability.50 When ligand bound, the AhR translocates to the nucleus, where it binds to the AhR nuclear translocator (Arnt), with this AhR-Arnt heterodimer binding to dioxin-responsive elements in the promoters of target genes. Target genes include the cytochrome P450 family (including CYP1A1, CYP1A2, and CYP1B1),51 with these target genes then acting to regulate an array of factors, including many pharmaceuticals, but also melatonin, suggesting a role for the AhR in the modulation of the circadian rhythm,52 as well as levels of local melatonin availability in many cell types.
A number of pathways can interact with the AhR, especially in the formation of the AhR-Arnt complex.53 The AhR has recently been reconceptualized, from a xenobiotic receptor to one where an array of endogenous ligands may have a variety of effects on AhR-induced genes.53 The AhR can therefore have complex effects, with different AhR ligands seemingly having differential effects on the patterning of the specific genes induced. The AhR activation, via increased IDO, can induce the TRYCAT pathway, as well as increase immune-suppressive regulatory T cells. However, the AhR can also increase the levels of autoimmune-associated pro-inflammatory T-helper 17 cells.54 Consequently, the AhR has complex effects, seemingly determined by specific ligands and local microenvironmental conditions, in part via the differential regulation of xenobiotic or dioxin response elements (X/DRE) as well as non-X/DRE-mediated pathways.54 As the AhR repressor (AhRR) is induced by AhR binding to X/DRE in the AhRR promoter, the AhR negatively feeds back on itself. This also indicates that the non-X/DRE effects of the AhR will not affect AhRR expression, suggesting that variations in AhRR induction may contribute to the complex effects and inductions of the AhR.54 Recent data support a role for the AhRR in the regulation of specific pro-inflammatory cytokines, including IL-1B, but not all pro-inflammatory factors are induced by the AhR.55
The AhR, via IDO induction, can directly regulate TRYCAT production, with kynurenine and KYNA also activating the AhR, suggesting a possible positive feedback loop that would be inhibited by the AhRR. AhR activation, via CYP1A2, also degrades melatonin, with melatonin inhibiting some AhR effects,56 indicating interactions of the AhR with the melatonergic pathways. Such data indicate a role for the AhR in the interactions of the melatonergic and TRYCAT pathways and therefore with the effects of the a7nAChR. Although all of these factors may differentially interact in different cell types and local microenvironments, it is their expression and interactions directly in mitochondria that this article will now focus on.
Interactions Within Mitochondria
Recent data show that the AhR is expressed in mitochondria, including in the mitochondrial intermembrane space.57 With the a7nAChR and the melatonergic pathway synthesis enzyme (AANAT) also expressed in mitochondria, coupled with the direct effects of the TRYCATs, 3-OHK, and 3-hydroxyanthranillic acid (3-OH-ANA), on mitochondrial functioning,58 mitochondria may be important sites for the interactions of these pathway and receptors. We review the data pertaining to these receptors and pathways in mitochondria, before looking at how they may interact in this crucial organelle.
Some of the effects of kynurenine and KYNA are mediated via its activation of the AhR, although at physiologic levels kynurenine and KYNA can have quite distinct effects.59 As to whether kynurenine or KYNA can directly activate the AhR in mitochondria is an area of current investigation, including in the gut where the AhR can modulate the IgA mucosal response,60 indicating a possible AhR role in the gut that would be relevant to levels of mucosal immune-inflammatory activity that are increasingly appreciated as having a role in a host of CNS and psychiatric conditions.61 There is a growing appreciation of the role of early developmental processes in an array of CNS and psychiatric conditions, as well as recognition of a role for suboptimal vascular functioning and cardiovascular disease in such classically conceived brain disorders.62,63 In this context, it is notable that the AhR has significant early developmental impacts in cardiomyocytes that increase cardiovascular disease (CVD) risk,64 with cardiomyocytes being densely packed within mitochondria. As such, alterations in the early developmental regulation of the AhR may play a role in a host of medical conditions that can modulate many CNS and psychiatric disorders, as well as having direct impacts on cells classically linked to brain disorders. Some of these effects may occur directly in mitochondria.
It is also important to emphasize that TRYCAT pathway activation may lead to other factors that directly modulate mitochondrial functioning, with 3-OH-Kyn and 3-OH-ANA directly affecting the respiratory control index of brain mitochondrial functioning.58 These authors also showed that both of these TRYCATs lowered the adenosine diphosphate/oxygen ratio in brain mitochondria, as well as having impacts on hepatic and cardiac mitochondria.58 Such data indicate that the classical role attributed to TRYCATs in the regulation of synaptic activity of CNS and psychiatric disorders may be overly simplistic. Rather, some TRYCATs may more directly modulate mitochondrial functioning across a host of cell types. TCDD, the classical AhR ligand, has a plethora of pathophysiologic effects in different cell types, including changes in mitochondrial functioning and increasing the risk of metabolic syndrome.57 As to whether changes in specific TRYCATs, and their direct effects in mitochondria, are relevant to how metabolic syndrome increases the risk of CNS and psychiatric disorders, such as Alzheimer disease65 and MDD,66 requires further information.
As to how such 3-OHK and 3-OH-ANA effects in mitochondria would modulate levels of mitochondrial melatonergic pathway activity and melatonin production requires investigation. As AANAT needs to be stabilized by 14-3-3, it may be of note that proteomic analysis of hippocampal cells of patients with schizophrenia indicates coordinated changes in the levels of 14-3-3 and the AhR.67 Inhibition of 14-3-3 is used as a preclinical model of schizophrenia.68 Desynchronization of 14-3-3 and the AhR may therefore be of some importance in psychiatric conditions. It requires investigation as to whether the TRYCAT pathway, including via direct effects in mitochondria, modulates the melatonergic pathway, including within mitochondria, and as to whether alterations in 14-3-3 levels are relevant to this. In addition to decreasing serotonin availability as a necessary precursor for the melatonergic pathways, TRYCAT effects in mitochondria, including possibly in mitochondrial AhR, may also act to regulate the melatonergic pathways.
As indicated above, emerging data now indicate melatonergic pathway activity in mitochondria, with levels of mitochondrial melatonin synthesis dependent on serotonin availability.8 As such, the regulation of MAO, which can bind to the mitochondrial membrane, may be another target for the regulation of melatonergic pathway activation in mitochondria. Alterations in MAO function have been associated with most CNS and psychiatric disorders, possibly as a consequence of chronic stress increasing MAO and therefore decreasing the availability of monoamines, including serotonin.69 These authors also showed that exogenous melatonin prevented chronic stress–induced MAO in preclinical models, implicating alterations in melatonin in the increase in MAO that is induced by chronic stress.69 Activation of the AhR by tryptamine requires MAO,70 being another route by which chronic stress may act to modulate AhR activity, including possibly in mitochondria, and thereby increase melatonin metabolism. It is also of note that melatonin has impacts on the structural organization of the lipids that form the cell and organelle membranes, including mitochondrial membranes.30 As to whether alterations in mitochondrial melatonin synthesis have any impacts on wider mitochondrial functions via such membrane lipid changes requires further investigation. Overall, melatonin is a significant regulator of mitochondrial functioning, suggesting that factors that modulate melatonin synthesis in mitochondria are likely to affect an array of fundamental cellular processes.
Mitochondria express the a7nAChR, with the a7nAChR acting to regulate Ca2+ accumulation and cytochrome c release in isolated mitochondria.38 As noted, melatonin can increase the levels and activation of the a7nAChR,9 suggesting that the interactions of the TRYCAT and melatonergic pathways will affect the levels and activity of the a7nAChR, including in mitochondria. It also important to note that the specific TRYCATs produced may be of some relevance, as KYNA inhibits the a7nAChr. Given that the a7nAChR may mediate some of melatonin’s effects, IDO induction may not only decrease melatonergic pathway activity but also increase KYNA, thereby inhibiting melatonin’s effects via the a7nAChR. This indicates that there may be a number of means by which the TRYCAT and melatonergic pathways engage in mutual inhibition. However, this may be primarily under conditions when the KYNA wing of the TRYCAT pathway is predominantly activated, which is likely to be considerably different to the activation of the 3-OHK, 3-OH-ANA, and QUIN wing. The relevance of the a7nAChR in mediating the interactions of wider TRYCATs with the melatonergic pathways awaits investigation. This is likely to be of some importance, given the role of the a7nAChR in the regulation of reactivity levels in glia, macrophages, and other immune cells.41 This also suggests that the biological underpinnings in the recent phase III trials of a7nAChR agonists44,45 may be considerably more complicated than simple alterations in synaptic activity.
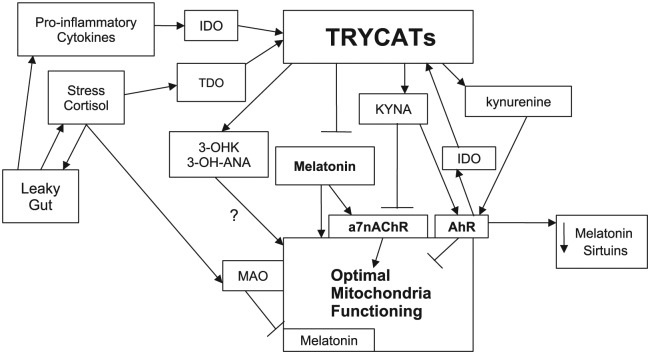
Such an array of data indicates that mitochondria may be a crucial hub for the interactions of the AhR and a7nAChR with the TRYCAT and melatonergic pathways. The AhR activation, including when activated by KYNA and kynurenine, can also decrease NAD+ levels, thereby decreasing levels of sirtuins, as has been shown for sirtuin-3 in hepatic cells.71 Although sirtuin-1 is commonly referred to as the longevity protein, data in humans, to date, only show alleles in sirtuin-3 to modulate human longevity.72 As such, the interacting pathways and receptors described herein, by acting on NAD+ induced sirtuins, may affect aging processes. This is of obvious relevance to almost all medical conditions, including cancers and CVD, as well as to CNS and psychiatric disorders. Figure 2 summarizes these mitochondrial interactions.
Figure 2.
The interactions of the AhR and a7nAChR with the TRYCAT and melatonergic pathways. Pro-inflammatory cytokines and chronic stress increase IDO and TDO, respectively, leading to TRYCAT induction, with differential effects on mitochondrial functioning. TRYCAT activation and stress-induced MAO, by decreasing serotonin availability, decrease melatonergic pathway activation. Increased KYNA may also compete with melatonin via the opposing regulation of the a7nAChR. AhR activation, via IDO induction, may positively feedback on TRYCAT synthesis, as well as decrease the availability of melatonin by increasing its metabolism. Some data indicate that the AhR decreases sirtuins, which may also be mediated by decreased melatonin. Both 3-OHK and 3-OH-ANA can modulate mitochondrial functioning. 3-OH-ANA indicates 3-hydroxyanthranillic acid; 3-OHK, 3-hydroxykynurenine; a7nAChR, alpha 7 nicotinic receptor; AhR, aryl hydrocarbon receptor; IDO, indoleamine 2,3-dioxygenase; KYNA, kynurenic acid; MAO, monoamine oxidase; TDO, tryptophan 2,3-dioxygenase; TRYCATs, tryptophan catabolites.
Conclusions
The overlapping benefits of melatonin and the a7nAChR over an array of diverse medical presentations and pathophysiologic processes have been primarily attributed to their antioxidant effects and neuronal regulation, respectively. The TRYCATs have been portrayed as neuroregulatory effectors of pro-inflammatory processes, whereas the AhR has primarily been viewed as a modulator of specific immune activators. The data presented here suggest that these diverse processes and effects may be mediated by the interactions of these receptors and pathways directly in mitochondria. Such a perspective dramatically changes the understanding of the consequences of alterations in tryptophan utilization in the body and suggests readily achievable future experiments, with significant clinical implications. Mitochondria have long been recognized as important sites of pathophysiological change across most medical conditions, including cancers and CVD, as well as CNS and psychiatric disorders. The mitochondrial interactions of the receptors and pathways described above are likely to be important determinants of an array of medical pathophysiological processes, including via alterations in the levels of aging-associated sirtuins within mitochondria. It should be noted that such mitochondrial interactions, which are relevant to the cause and course of CNS and psychiatric disorders, will not all be occurring centrally, but also in systemic systems, including the immune system, and peripheral organs and tissues, including the gut and the gut-brain axis.
Footnotes
Peer review:Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 2096 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosure: This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
Author Contributions: Both authors contributed to this article.
References
- 1. Lim CK, Fernández-Gomez FJ, Braidy N, et al. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease [published online ahead of print April 9, 2016]. Prog Neurobiol. doi: 10.1016/j.pneurobio.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 2. Anderson G, Ojala J. Alzheimer’s and seizures: interleukin-18, indoleamine 2,3-dioxygenase and quinolinic acid. Int J Tryptophan Res. 2010;3:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson G, Maes M. Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: treatment implications. Curr Pharm Des. 2014;20: 3812–3847. [DOI] [PubMed] [Google Scholar]
- 4. de Bie J, Lim CK, Guillemin GJ. Kynurenines, gender and neuroinflammation; showcase schizophrenia. Neurotox Res. 2016;30:285–294. [DOI] [PubMed] [Google Scholar]
- 5. Anderson G, Kubera M, Duda W, Lasoń W, Berk M, Maes M. Increased IL-6 trans-signaling in depression: focus on the tryptophan catabolite pathway, melatonin and neuroprogression. Pharmacol Rep. 2013;65:1647–1654. [DOI] [PubMed] [Google Scholar]
- 6. Anderson G, Maes M. Local melatonin regulates inflammation resolution: a common factor in neurodegenerative, psychiatric and systemic inflammatory disorders. CNS Neurol Disord Drug Targets. 2014;13:817–827. [DOI] [PubMed] [Google Scholar]
- 7. Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res. 2013;54:127–138. [DOI] [PubMed] [Google Scholar]
- 8. He C, Wang J, Zhang Z, et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int J Mol Sci. 2016;17:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Markus RP, Silva CL, Franco DG, Barbosa EM, Jr, Ferreira ZS. Is modulation of nicotinic acetylcholine receptors by melatonin relevant for therapy with cholinergic drugs? Pharmacol Ther. 2010;126:251–262. [DOI] [PubMed] [Google Scholar]
- 10. Sommansson A, Nylander O, Sjöblom M. Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor-dependent pathway in rats in vivo. J Pineal Res. 2013;54:282–291. [DOI] [PubMed] [Google Scholar]
- 11. Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. [DOI] [PubMed] [Google Scholar]
- 12. Ren S, Correia MA. Heme: a regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch Biochem Biophys. 2000;377:195–203. [DOI] [PubMed] [Google Scholar]
- 13. Foster AC, Collins JF, Schwarcz R. On the excitotoxic properties of quinolinic acid, 2,3-piperidine dicarboxylic acids and structurally related compounds. Neuropharmacology. 1983;22:1331–1342. [DOI] [PubMed] [Google Scholar]
- 14. Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2013;34:136–143. [DOI] [PubMed] [Google Scholar]
- 15. Schwarz MJ, Guillemin GJ, Teipel SJ, Buerger K, Hampel H. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer’s disease patients from controls. Eur Arch Psychiatry Clin Neurosci. 2013;263:345–352. [DOI] [PubMed] [Google Scholar]
- 16. Young KD, Drevets WC, Dantzer R, Teague TK, Bodurka J, Savitz J. Kynurenine pathway metabolites are associated with hippocampal activity during autobiographical memory recall in patients with depression. Brain Behav Immun. 2016;56:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson G, Berk M, Maes M. Biological phenotypes underpin the physio-somatic symptoms of somatization, depression, and chronic fatigue syndrome. Acta Psychiatr Scand. 2014;129:83–97. [DOI] [PubMed] [Google Scholar]
- 18. Anderson G, Jacob A, Bellivier F, Geoffroy PA. Bipolar disorder: the role of the kynurenine and melatonergic pathways. Curr Pharm Des. 2016;22:987–1012. [DOI] [PubMed] [Google Scholar]
- 19. Maes M, Anderson G. Overlapping the tryptophan catabolite (TRYCAT) and melatoninergic pathways in Alzheimer’s disease. Curr Pharm Des. 2016;22:1074–1085. [DOI] [PubMed] [Google Scholar]
- 20. Anderson G, Rodriguez M. Multiple sclerosis: the role of melatonin and N-acetylserotonin. Mult Scler Relat Disord. 2015;4:112–123. [DOI] [PubMed] [Google Scholar]
- 21. Anderson G, Maes M, Berk M. Inflammation-related disorders in the tryptophan catabolite pathway in depression and somatization. Adv Protein Chem Struct Biol. 2012;88:27–48. [DOI] [PubMed] [Google Scholar]
- 22. Muxel SM, Pires-Lapa MA, Monteiro AW, et al. NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS ONE. 2012;7:e52010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–384. [DOI] [PubMed] [Google Scholar]
- 24. Anderson G, Maes M. The gut-brain axis: the role of melatonin in linking psychiatric, inflammatory and neurodegenerative conditions. Adv Integr Med. 2015;2:31–37. [Google Scholar]
- 25. Jang SW, Liu X, Pradoldej S, et al. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci U S A. 2010;107:3876–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Souza-Teodoro LH, Dargenio-Garcia L, Petrilli-Lapa CL, et al. Adenosine triphosphate inhibits melatonin synthesis in the rat pineal gland. J Pineal Res. 2016;60:242–249. [DOI] [PubMed] [Google Scholar]
- 27. Beischlag TV, Anderson G, Mazzoccoli G. Glioma: tryptophan catabolite and melatoninergic pathways link microRNA, 14-3-3, chromosome 4q35, epigenetic processes and other glioma biochemical changes. Curr Pharm Des. 2016;22:1033–1048. [DOI] [PubMed] [Google Scholar]
- 28. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jockers R, Delagrange P, Dubocovich ML, et al. Update on melatonin receptors: IUPHAR review 20. Br J Pharmacol. 2016;173:2702–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drolle E, Kučerka N, Hoopes MI, et al. Effect of melatonin and cholesterol on the structure of DOPC and DPPC membranes. Biochim Biophys Acta. 2013;1828:2247–2254. [DOI] [PubMed] [Google Scholar]
- 31. Obsil T, Ghirlando R, Klein DC, Ganguly S, Dyda F. Crystal structure of the 14-3-3zeta:serotonin N-acetyltransferase complex. A role for scaffolding in enzyme regulation. Cell. 2001;105:257–267. [DOI] [PubMed] [Google Scholar]
- 32. Choi MR, Hwang S, Park GM, et al. Effect of fluoxetine on the expression of tryptophan hydroxylase and 14-3-3 protein in the dorsal raphe nucleus and hippocampus of rat. J Chem Neuroanat. 2012;43:96–102. [DOI] [PubMed] [Google Scholar]
- 33. Markus RP, Cecon E, Pires-Lapa MA. Immune-pineal axis: nuclear factor κB (NF-kB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int J Mol Sci. 2013;14:10979–10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalkman HO, Feuerbach D. Modulatory effects of α7 nAChRs on the immune system and its relevance for CNS disorders. Cell Mol Life Sci. 2016;73:2511–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Costantini TW, Krzyzaniak M, Cheadle GA, et al. Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol. 2012;181:478–486. [DOI] [PubMed] [Google Scholar]
- 36. Yakel JL. Nicotinic ACh receptors in the hippocampal circuit; functional expression and role in synaptic plasticity. J Physiol. 2014;592:4147–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci U S A. 2001;98:4148–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noda M, Kobayashi AI. Nicotine inhibits activation of microglial proton currents via interactions with α7 acetylcholine receptors. J Physiol Sci. 2017;67:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peña VB, Bonini IC, Antollini SS, Kobayashi T, Barrantes FJ. Alpha 7-type acetylcholine receptor localization and its modulation by nicotine and cholesterol in vascular endothelial cells. J Cell Biochem. 2011;112:3276–3288. [DOI] [PubMed] [Google Scholar]
- 40. Anderson G, Maes M. Reconceptualizing adult neurogenesis: role for sphingosine-1-phosphate and fibroblast growth factor-1 in co-ordinating astrocyte-neuronal precursor interactions. CNS Neurol Disord Drug Targets. 2014;13:126–136. [DOI] [PubMed] [Google Scholar]
- 41. Han Z, Shen F, He Y, et al. Activation of α-7 nicotinic acetylcholine receptor reduces ischemic stroke injury through reduction of pro-inflammatory macrophages and oxidative stress. PLoS ONE. 2014;9:e105711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. John D, Shelukhina I, Yanagawa Y, Deuchars J, Henderson Z. Functional alpha7 nicotinic receptors are expressed on immature granule cells of the postnatal dentate gyrus. Brain Res. 2015;1601:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson G, Seo M, Carvalho A, Berk M, Maes M. Gut permeability and Parkinson’s disease. Curr Pharm Des. 2016;22(40):6142–6151. [DOI] [PubMed] [Google Scholar]
- 44. Deardorff WJ, Shobassy A, Grossberg GT. Safety and clinical effects of EVP-6124 in subjects with Alzheimer’s disease currently or previously receiving an acetylcholinesterase inhibitor medication. Expert Rev Neurother. 2015;15:7–17. [DOI] [PubMed] [Google Scholar]
- 45. Marder SR. Alpha-7 nicotinic agonist improves cognition in schizophrenia. Evid Based Ment Health. 2016;19:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. [DOI] [PubMed] [Google Scholar]
- 47. Parada E, Buendia I, León R, et al. Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J Pineal Res. 2014;56:204–212. [DOI] [PubMed] [Google Scholar]
- 48. Gergalova G, Lykhmus O, Kalashnyk O, et al. Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria. PLoS ONE. 2012;7:e31361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pálková L, Vondráček J, Trilecová L, et al. The aryl hydrocarbon receptor-mediated and genotoxic effects of fractionated extract of standard reference diesel exhaust particle material in pulmonary, liver and prostate cells. Toxicol In Vitro. 2015;29:438–448. [DOI] [PubMed] [Google Scholar]
- 50. Salim SY, Kaplan GG, Madsen KL. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes. 2014;5:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sánchez-Martín FJ, Fernández-Salguero PM, Merino JM. Aryl hydrocarbon receptor-dependent induction of apoptosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin in cerebellar granule cells from mouse. J Neurochem. 2011;118:153–162. [DOI] [PubMed] [Google Scholar]
- 52. Anderson G, Beischlag TV, Vinciguerra M, Mazzoccoli G. The circadian clock circuitry and the AHR signaling pathway in physiology and pathology. Biochem Pharmacol. 2013;85:1405–1416. [DOI] [PubMed] [Google Scholar]
- 53. Wu WL, Adams CE, Stevens KE, Chow KH, Freedman R, Patterson PH. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav Immun. 2015;46:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mohinta S, Kannan AK, Gowda K, Amin SG, Perdew GH, August A. Differential regulation of Th17 and T regulatory cell differentiation by aryl hydrocarbon receptor dependent xenobiotic response element dependent and independent pathways. Toxicol Sci. 2015;145:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vogel CF, Chang WL, Kado S, et al. Transgenic overexpression of aryl hydrocarbon receptor repressor (AhRR) and AhR-mediated induction of CYP1A1, cytokines, and acute toxicity. Environ Health Perspect. 2016;124:1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chang TK, Chen J, Yang G, Yeung EY. Inhibition of procarcinogen-bioactivating human CYP1A1, CYP1A2 and CYP1B1 enzymes by melatonin. J Pineal Res. 2010;48:55–64. [DOI] [PubMed] [Google Scholar]
- 57. Hwang HJ, Dornbos P, Steidemann M, Dunivin TK, Rizzo M, LaPres JJ. Mitochondrial-targeted aryl hydrocarbon receptor and the impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on cellular respiration and the mitochondrial proteome. Toxicol Appl Pharmacol. 2016;304:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baran H, Staniek K, Bertignol-Spörr M, Attam M, Kronsteiner C, Kepplinger B. Effects of various kynurenine metabolites on respiratory parameters of rat brain, liver and heart mitochondria. Int J Tryptophan Res. 2016;9:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moyer BJ, Rojas IY, Kerley-Hamilton JS, et al. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGFβ and IDO1. Toxicol Appl Pharmacol. 2016;300:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chmill S, Kadow S, Winter M, Weighardt H, Esser C. 2,3,7,8-Tetrachlorodibenzo-p-dioxin impairs stable establishment of oral tolerance in mice. Toxicol Sci. 2010;118:98–107. [DOI] [PubMed] [Google Scholar]
- 61. Roomruangwong C, Kanchanatawan B, Sirivichayakul S, et al. IgA/IgM responses to tryptophan and tryptophan catabolites (TRYCATs) are differently associated with prenatal depression, physio-somatic symptoms at the end of term and premenstrual syndrome [published online ahead of print April 1, 2016]. Mol Neurobiol. doi: 10.1007/s12035-016-9877-3. [DOI] [PubMed] [Google Scholar]
- 62. Ford DE. Zeroing in on depression as a cardiovascular risk factor. Can lifting mood improve outcomes? Postgrad Med. 2003;114:6–13. [DOI] [PubMed] [Google Scholar]
- 63. Hazar N, Seddigh L, Rampisheh Z, Nojomi M. Population attributable fraction of modifiable risk factors for Alzheimer disease: a systematic review of systematic reviews. Iran J Neurol. 2016;15:164–172. [PMC free article] [PubMed] [Google Scholar]
- 64. Carreira VS, Fan Y, Kurita H, et al. Disruption of Ah receptor signaling during mouse development leads to abnormal cardiac structure and function in the adult. PLoS ONE. 2015;10:e0142440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Campos-Peña V, Toral-Rios D, Becerril-Pérez F, et al. Metabolic syndrome as a risk factor for Alzheimer’s disease: is Aβ a crucial factor in both pathologies? [published online ahead of print August 16, 2016]. Antioxid Redox Signal. doi: 10.1089/ars.2016.6768. [DOI] [PubMed] [Google Scholar]
- 66. Chen YJ, Lin CL, Li CR, et al. Associations among integrated psychoneuroimmunological factors and metabolic syndrome. Psychoneuroendocrinology. 2016;74:342–349. [DOI] [PubMed] [Google Scholar]
- 67. Schubert KO, Föcking M, Cotter DR. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: potential roles in GABAergic interneuron pathology. Schizophr Res. 2015;167:64–72. [DOI] [PubMed] [Google Scholar]
- 68. Foote M, Qiao H, Graham K, Wu Y, Zhou Y. Inhibition of 14-3-3 proteins leads to schizophrenia-related behavioral phenotypes and synaptic defects in mice. Biol Psychiatry. 2015;78:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stefanovic B, Spasojevic N, Jovanovic P, Jasnic N, Djordjevic J, Dronjak S. Melatonin mediated antidepressant-like effect in the hippocampus of chronic stress-induced depression rats: regulating vesicular monoamine transporter 2 and monoamine oxidase A levels. Eur Neuropsychopharmacol. 2016;26:1629–1637. [DOI] [PubMed] [Google Scholar]
- 70. Vikström Bergander L, Cai W, Klocke B, Seifert M, Pongratz I. Tryptamine serves as a proligand of the AhR transcriptional pathway whose activation is dependent of monoamine oxidases. Mol Endocrinol. 2012;26:1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. He J, Hu B, Shi X, et al. Activation of the aryl hydrocarbon receptor sensitizes mice to nonalcoholic steatohepatitis by deactivating mitochondrial sirtuin deacetylase Sirt3. Mol Cell Biol. 2013;33:2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Albani D, Ateri E, Mazzuco S, et al. Modulation of human longevity by SIRT3 single nucleotide polymorphisms in the prospective study “Treviso Longeva (TRELONG).” Age (Dordr). 2014;36:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]