Abstract
Background
To evaluate the efficacy and tolerability of the addition of two monoclonal antibodies, bevacizumab and cetuximab, to two cycles of high-dose cisplatin administered concurrently with IMRT for HNSCC.
Methods
Patients with newly diagnosed stage III/IVB (M0) HNSCC received cetuximab (400 mg/m2 loading dose, followed by 250 mg/m2 weekly), bevacizumab (15 mg/kg, days 1 and 22), and cisplatin (50 mg/m2, days 1, 2, 22, and 23) concurrently with IMRT (70 Gy). The primary endpoint was progression free survival (PFS). Secondary endpoints were overall survival (OS) and safety and tolerability.
Results
Among 30 patients enrolled, the primary tumor site was oropharynx in 25 patients (p16 immunohistochemistry was positive in 17, negative in 1, and not done in 6 of the oropharynx tumors). Median age was 57 years (range, 38–77 years) and 27 patients had clinical stage IVA disease. All patients completed the full planned dose of radiation therapy. The most common ≥ grade 3 adverse events were lymphopenia, mucositis (functional), and dysphagia. With a median follow up of 33.8 months, 2 year PFS was 88.5% (95% CI, 68.1–96.1) and 2 year OS was 92.8% (95% CI, 74.2–98.1%)
Conclusion
The addition of bevacizumab and cetuximab to two cycles of cisplatin, given concurrently with IMRT, was well tolerated and was associated with favorable efficacy outcomes in this patient population.
INTRODUCTION
Large randomized studies established the regimen of three cycles of bolus cisplatin administered concurrently with radiation therapy for locally/regionally advanced HNSCC.1,2 The only molecularly targeted agent which has surpassed investigational status in the treatment of HNSCC is cetuximab, a chimeric monoclonal antibody that targets the extracellular domain of epidermal growth factor receptor (EGFR). Cetuximab modestly improves efficacy when combined with radiation therapy for locally/regionally advanced HNSCC,3,4 and modestly improves overall survival when added to platinum + 5-fluorouracil chemotherapy for recurrent/metastatic disease.5
Vascular endothelial growth factor-A (VEGF-A), a cental regulator of tumor angiogenesis, is targeted by the monoclonal antibody bevacizumab. Increased VEGF-A expression is associated with poor prognosis in HNSCC,6 and inhibition of angiogenesis is a potent radiosensitization strategy pre-clinically.7 However, in a phase II combined modality study that called for three cycles of bevacizumab and cisplatin administered concurrently with radiation therapy for stage II/IVB HNSCC, we found only half of patients were able to receive the full planned 3 cycles of both drugs due to cumulative adverse events.8 To improve tolerability in HNSCC combined modality clinical trials that incorporate bevacizumab, a heuristic next step would be to reduce exposure to the cytotoxic agent cisplatin.
Preclinical and early clinical studies support a model in which cross-talk between EGFR and VEGF pathways enhances tumor growth and angiogenesis in HNSCC.9 In phase II clinical study for 46 patients with advanced HNSCC, the combination of cetuximab and bevacizumab yielded an objective response rate of 16% and a disease control rate of 73%.9 These data suggest that the combined inhibition of VEGF and EGFR may yield additive efficacy in HNSCC.
During the protocol-writing phase, we proposed that the combination of cetuximab and bevacizumab may improve efficacy, and that the third cycle of cisplatin may be omitted without compromising efficacy. Here we report the mature results of a non-randomized phase II study in which patients with locally/regionally advanced HNSCC received two cycles of bolus cisplatin, plus two cycles of bevacizumab, and weekly cetuximab,4,3 administered concurrently with radiation therapy.
METHODS
Patient Eligibility and Baseline Assessment
The study was approved by the local Institutional Review Board, and all subjects provided written informed consent. Patient selection criteria and pre-treatment evaluation, including computed tomography (CT) or magnetic resonance imaging (MRI) scan of tumor site and whole-body fluorodeoxyglucose-positron emission tomography (FDG-PET) scan, were essentially as described in our prior phase II study of bevacizumab plus chemoradiation.8 A significant difference is that the current study did not mandate pre-treatment placement of a percutaneous gastrostomy (PEG) tube, but this was allowed at the discretion of the investigator.
Study Objectives and Treatment Plan
The primary endpoint of the study was to determine the 2-year progression-free survival (PFS) for patients with locally or regionally advanced HNSCC treated with concurrent IMRT + cisplatin + bevacizumab + cetuximab. Secondary endpoints were to determine median overall survival (OS) and to describe the safety and tolerability of the regimen.
The cetuximab schedule was a loading dose (400 mg/m2 on day minus 7) followed by weekly dosing (250 mg/m2 on days 1, 8, 15, 22, 29, 36, and 43).3 Two cycles of cisplatin (50 mg/m2 on days 1, 2, 22, 23) and bevacizumab (15 mg/kg on days 1 and 22) were planned. Day 1 refers to the first day of radiation therapy. Guidelines for cisplatin and bevacizumab treatment modifications were as previously described.8 Bevacizumab and/or cetuximab doses could be delayed do to adverse events, but dose reductions for bevacizumab and/or cetuximab were not allowed. Concurrent intensity-modulated radiation therapy (IMRT) was delivered on a standard schedule of one fraction per day (excluding weekends and holidays) to a total dose of 7000 cGy over 33 to 35 treatment days. High risk subclinical disease received 5940 to 6300 cGy while the lower risk subclinical disease received 5400 to 5600 cGy.
Evaluation During Study and Response Assessment
Adverse events were graded using the NCI Common Terminology Criteria for Adverse Events, Version 3.0. Acute toxicities were defined as those occurring from date of registration until 30 days after completion of therapy. Routine laboratory studies (complete blood cell count, basic metabolic panel with magnesium) were obtained weekly during the treatment period. CT or MRI scan of tumor site and whole-body FDG-PET scan were repeated approximately 3 – 4 months after the completion of chemoradiation. Neck surgery was not offered to patients who had a complete clinical and radiographic response, but could be offered to patients without complete response. For patients who maintained active follow-up, planned surveillance included clinic visits approximately every 3 months for 2 years, and every 6 months thereafter.
Statistical Considerations
Time endpoints were measured from the start of treatment until the first reported distant or local/regional failure or until death from any cause. Second malignancies that were not upper aerodigestive squamous cancers were not scored as events. Patients with persistent local-regional persistent disease at the time of post-treatment imaging could undergo salvage surgery. Patients who underwent complete resection of persistent disease at salvage surgery, and thereby achieved status of no evidence of disease, were considered to be progression-free. Patients with any unresectable disease after chemoradiation would be considered to have experienced disease progression. PFS data was censored at the time of last follow-up for patients without distant or local/regional failure. The protocol contained an early stopping rule for unanticipated toxicity. The regimen would be deemed worthy of further study if 2-year PFS was 70%. With a sample size of 30 subjects, the statistical design yields a 3% type 1 error rate and 84% power.
RESULTS
Patient Characteristics and Treatment Delivery
30 subjects were enrolled between December 14, 2009 and June 18, 2013. Patient characteristics are summarized in Table 1. It was a predominantly male population with median age of 57 years (range, 38–77 years). The most common clinical stage was IVA, and the most primary tumor site was oropharynx. Positive p16 immunohistochemistry, a surrogate marker for HPV-related oropharynx cancer, was detected in 71% (17/24) of oropharynx cancers. Only 23% (7/30) of the study subjects had tobacco histories of more than 10 pack-years. Twenty patients were treated at MSKCC Main Campus in New York City, and 10 patients were treated at regional network sites.
Table 1.
Patient characteristics
| Patient Characteristic | Summary |
|---|---|
| Age, median (range) | 57 years (38–77 years) |
| Gender (M/F) | 27/3 |
| Karnofsky Performance Status, median (range) | 90 (80–100) |
| Subsite, N subjects (%) | |
| Oropharynx | 24 (80) |
| Tonsil | 11 |
| Base of tongue | 13 |
| Hypopharynx | 3 (10) |
| Larynx | 2 (7) |
| Oral cavity | 1 (3) |
| Tumor (T) classification, N subjects (%) | |
| 1 | 7 (23) |
| 2 | 14 (47) |
| 3 | 7 (23) |
| 4 | 2 (7) |
| Node (N) classification, N subjects (%) | |
| 0 | 2 (7) |
| 1 | 2 (7) |
| 2a | 3 (10) |
| 2b | 19 (63) |
| 2c | 4 (13) |
| 3 | 0 |
| Overall Stage, N subjects (%) | |
| III | 3 (10) |
| IVA | 27 (90) |
| p16 status, N subjects (%), oropharynx only | |
| Positive | 17/24 (71) |
| Negative | 1/24 (4) |
| Unknown/insufficient material for assay | 6/24 (25) |
| Tobacco Pack-Years, N subjects (%) | |
| 0 to ≤ 1 pack year | 14 (47) |
| > 1 to ≤ 10 pack years | 8 (27) |
| >10 to ≤ 20 pack years | 1 (3) |
| >20 pack years | 6 (20) |
| Unknown | 1 (3) |
All 30 patients completed the prescribed course of radiation therapy (70 Gy). Both planned cycles of bevacizumab were received by 28 patients; two patients receive only 1 cycle of bevacizumab. Both cycles of cisplatin were administered at full dose for 24 patients. Three patients received cycle 2 of cisplatin with 50% dose reduction, and three patients received only one cycle of cisplatin. Eighteen patients received all 8 planned cetuximab infusions, including the loading dose. Cetuximab infusions were administered for 7 or 6 weeks for 5 and 4 patients, respectively. One patient each received 5, 4, and 0 weeks of cetuximab. The latter patient experienced a cetuximab hypersensitivity reaction during the loading dose attempt, but remained on study to receive both cycles of bevacizumab and cisplatin at full dose.
Acute Toxicities and Late Functional Outcomes
Table 2 lists acute adverse events, regardless of attribution, that occurred in ≥ one-third of patients at any grade, and/or were ≥ grade 3 in at least 2 patients. The most common adverse events of any grade, occurring in 29 patients each, were mucositis (clinical exam), fatigue, and low hemoglobin. The most common adverse events ≥ grade 3 were lymphopenia, mucositis (functional), and dysphagia. There were 3 episodes of neutropenic fever, but there were no grade 5 events. Fifteen patients underwent placement of percutaneous gastrostomy tube.
Table 2.
Summary of Adverse Events (N= 30 subjects)
| Adverse Event | Number of patients (%) | ||
|---|---|---|---|
| Any Grade | Grade | Grade 4 | |
| Mucositis- clinical exam | 29 (97) | 6 (20) | 1 (3) |
| Fatigue | 29 (97) | 3 (10) | 0 |
| Low hemoglobin | 29 (97) | 1 (3) | 0 |
| Nausea | 28 (93) | 6 (20) | 0 |
| Constipation | 28 (93) | 0 | 0 |
| Lymphopenia | 27 (90) | 16 (53) | 11 (37) |
| Weight loss | 27 (90) | 4 (13) | 0 |
| Dysphagia | 26 (87) | 9 (30) | 1 (3) |
| Leukopenia | 26 (87) | 7 (23) | 1 (3) |
| Rash, acne/acneiform | 26 (87) | 3 (10) | 0 |
| Hypoalbuminemia | 25 (83) | 0 | 0 |
| Pain- throat/pharynx/larynx | 24 (80) | 4 (13) | 0 |
| Mucositis- functional | 23 (77) | 11 (37) | 0 |
| Hyponatremia | 23 (77) | 3 (10) | 0 |
| ALT elevation | 22 (73) | 2 (7) | 0 |
| Thrombocytopenia | 19 (63) | 1 (3) | 0 |
| Vomiting | 17 (57) | 2 (7) | 0 |
| Hypomagnesemia | 17 (57) | 0 | 0 |
| AST elevation | 15 (50) | 0 | 0 |
| Pain - headache | 14 (47) | 0 | 0 |
| Diarrhea | 13 (43) | 0 | 0 |
| Neutropenia | 12 (40) | 2 (7) | 1 (3) |
| Creatinine elevation | 12 (40) | 2 (7) | 0 |
| Cough | 12 (40) | 0 | 0 |
| Hyperkalemia | 12 (40) | 0 | 0 |
| Hypokalemia | 11 (37) | 3 (10) | 0 |
| Fever (without neutropenia) | 10 (33) | 0 | 0 |
| Epistaxis | 10 (33) | 0 | 0 |
| Thrombosis/embolism | 6 (20) | 5 (17) | 0 |
| Hypophosphatemia | 6 (20) | 3 (10) | 0 |
| Febrile neutropenia | 3 (10) | 3 (10) | 0 |
| Pain – abdomen | 3 (10) | 3 (10) | 0 |
| Allergic reaction/hypersensitivity | 3 (10) | 2 (7) | 0 |
The Performance Status Scale for Head and Neck Cancer Patients (PSS-HN)10 was used to evaluate post-treatment speech and swallowing function for 23 patients. PSS-HN scores for these patients were obtained at a median of 3.1 months after completion of treatment (range, 1.0 to 14.8 months). PSS-HN scores are summarized in Table 3. Median scores for eating in public, understandability of speech, and normalcy of diet were 100, 100, and 80, respectively.
Table 3.
Summary of results obtained after chemoradiation with the Performance Status Scale for Head and Neck Cancer Patients (N=23 patients)
| Function | Median | Range | No. patients with score of 100 (%) |
|---|---|---|---|
| Eating, median (range) | 100 | 25 – 100 | 14/23 (61) |
| Speech, median (range) | 100 | 75 – 100 | 18/23 (78) |
| Diet, median (range) | 80 | 30 – 80 | 8/23 (35) |
Efficacy
Three patients experienced recurrent disease. One was a 63 year-old male never-smoker with base of tongue squamous cell cancer, stage IVA, p16 positive. Cycle one of cisplatin was complicated by neutropenic fever, and cycle two of cisplatin was given with a 50% dose reduction. Approximately 12 months after completion of treatment, he was found to have recurrent neck disease, and this was cytologically confirmed. A second patient with disease recurrence was a 57 year-old man with hypopharynx squamous cell cancer, stage IVA. Approximately 7 months after completion of treatment, imaging studies and physical exam demonstrated evidence of local/regional disease recurrence; tissue confirmation was not obtained due to rapid clinical decline. The third patient to experience treatment failure was a 66 year-old woman with right tonsil squamous cancer, stage IVA. She had a 25 pack-year tobacco history; p16 status of tumor was not obtained. She developed cytologically confirmed lung metastases approximately 3 months after completion of treatment.
One patient with hypopharynx carcinoma underwent neck surgery 3.2 months after the final radiation treatment, and surgical pathology findings were notable for one level 2 neck node with persistent squamous carcinoma. Because he was rendered disease-free by this salvage surgery, this did not meet criteria for a progression event per the statistical methods. A patient with base of tongue carcinoma underwent resection of persistent level 2 neck node 3.7 months after completion of radiation therapy, and pathology finding were non-malignant cells only.
Regarding the primary endpoint of the study, 2 year PFS was 88.5% (95% CI: 68.1–96.1%) and was unchanged after 3 years (Figure 1A). At a median follow up of 33.8 months (range, 4.8 to 55.5 months), median PFS was not reached. Overall survival at 2 years was 92.8% (95% CI: 74.2–98.1%) and was stable after 3 years (Figure 1B). Median overall survival was not reached.
Figure 1.
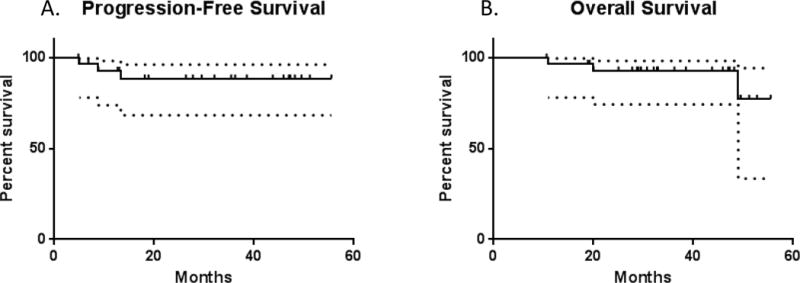
The Kaplan-Meier plots illustrate (A) Progression Free Survival and (B) Overall Survival. The dotted lines indicate 95% confidence intervals.
DISCUSSION
This phase II study describes safety and encouraging efficacy with the addition of two monoclonal antibodies, bevacizumab and cetuximab, to two cycles of cisplatin administered concurrently with intensity-modulated radiation therapy in locally/regionally advanced HNSCC. Both planned cycles of bevacizumab were completed by 93% of study subjects, and all completed the planned radiation therapy. The most common ≥ grade 3 adverse events were lymphopenia, functional mucositis, and dysphagia. Regarding the primary endpoint of the study, 2 year PFS was 88.5%.
The current study adds to the published experience of numerous non-randomized phase II studies in which bevacizumab was added to various combined modality regimens for locally/regionally advanced HNSCC.8,11–14 Key results from five of these studies have recently been summarized recently.14 Two studies combined bevacizumab with erlotinib, an orally administered EGFR inhibitor, during radiation therapy for locally/regionally advanced HNSCC. Yoo and colleagues combined bevacizumab, erlotinib, and weekly cisplatin with concurrent twice-daily radiation therapy; 3- year estimated PFS was 82%.13 Hainsworth and colleagues conducted a prospective trial of bevacizumab plus induction chemotherapy, followed by radiation therapy given concurrently with weekly paclitaxel, bevacizumab, and erlotinib. Estimated 3 year PFS was 71%.12 A central limitation of these studies, and the current study, is the lack of a randomized design to test the null hypothesis regarding the efficacy of bevacizumab. A single institution non-randomized trial with a relatively small sample size, such as the current study, does not provide a direct comparison with the efficacy of standard of care treatment.
The favorable efficacy outcomes in the current study are probably influenced, at least in part, by the favorable prognostic features of the study population. 80% of the patients on this study had oropharynx cancer, and the oropharynx cancers that were available for testing were predominantly HPV-related. The study population had relatively few patients (23%) with greater than 10 pack-years of tobacco history. After the design of the study, data from RTOG 0129 demonstrated that the combination of HPV-related cancer and ≤ 10 pack-year tobacco history defines a group of patients with a favorable prognosis in oropharynx cancer studies.15 RTOG 0129 also demonstrated no significant efficacy advantage for three cycles of cisplatin (concurrent with standard fractionation) versus two cycles of cisplatin (concurrent with accelerated fractionation),15 which is consistent with the results of this study in which high efficacy was achieved with only two planned cycles of concurrent cisplatin.
As this study was nearing completion of accrual, negative data regarding the combination of cisplatin + cetuximab, given concurrently with radiation therapy, emerged from the RTOG 0522 trial.16 The negative RTOG 0522 data do not address the hypothesis of potential added activity with the addition of bevacizumab to the cisplatin/cetuximab/radiation combination. There are signals of potentially useful clinical efficacy with combined EGFR and VEGF blockade from several HNSCC studies in the locally/regionally advanced setting12,13 and in the recurrent/metastatic setting,9,17 albeit in non-randomized studies. The negative RTOG 0522 data underscore the need for predictive biomarkers to guide the development of targeted therapy regimens. Potential predictive biomarkers for combined EGFR and VEGF inhibition have been described.17 Further interrogation of potential biomarkers would be an essential part of any future development effort regarding combined EGFR and VEGF inhibition in HNSCC.
The role of bevacizumab in HPV-related tumors warrants further study. In pre-clinical studies with cervical cancer cell lines, transfection with constructs expressing HPV E6 and E7 upregulates VEGF expression and enhances angiogenesis.18 The clinical benefit of bevacizumab in HPV-related cancers was established in a randomized phase III trial for patients with advanced cervical cancer, in which the addition of bevacizumab to standard chemotherapy increased overall survival by 3.7 months.19 In recurrent/metastatic HNSCC, the results of ECOG 1305 are awaited regarding standard chemotherapy with our without bevacizumab.20 The impact of bevacizumab among patients with HPV-related cancers in ECOG 1305 could help elucidate if sensitivity to bevacizumab may be a general characteristic of HPV-related malignancies.
In conclusion, the current study adds to a growing body of literature which demonstrates that bevacizumab is well tolerated when added to radiation-based therapy for locally/regionally advanced HNSCC. Favorable efficacy results were observed in this single-arm study, but a larger randomized study would be needed to assess the efficacy of this regimen conclusively. Targeting angiogenesis with bevacizumab may be appropriate for de-intensification trials that seek to reduce exposure to conventional cytotoxic agents for patients with HPV-related oropharynx cancer.
References
- 1.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced larynx cancer. New Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival from a phase 3 randomized trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 4.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous cell carcinoma of the head and neck. New Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 5.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. New Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 6.Kyzas PA, Cunha IW, Ionnidis JPA. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11:1434–1440. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 7.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of action. Clin Cancer Res. 2003;9:1957–1971. [PubMed] [Google Scholar]
- 8.Fury MG, Lee NY, Sherman E, et al. A phase 2 study of bevacizumab with cisplatin plus intensity-modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer. 2012;118:5008–5014. doi: 10.1002/cncr.27498. [DOI] [PubMed] [Google Scholar]
- 9.Argiris A, Kotsakis AP, Hoang T, et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2013;24:220–225. doi: 10.1093/annonc/mds245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.List MA, Ritter-Sterr C, Lansky SB. A Performance Status Scale for Head and Neck Cancer Patients. Cancer. 1990;66:564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Salama JK, Haraf DJ, Stenson KM, et al. A randomized phase II study of 5-fluoruracil, hydroxyurea, and twice-daily radiotherapy compared with bevacizumab plus 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy for intermediate-stage and T4N0-1 head and neck cancers. Ann Oncol. 2011 Feb 17; doi: 10.1093/annonc/mdq736. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Hainsworth JD, Spigel DR, Greco FA, et al. Combined modality treatment with chemotherapy, radiation therapy, bevacizumab, and erlotinib in patients wtih locally advanced squamous carcinoma of the head and neck. Cancer J. 2011;17:267–272. doi: 10.1097/PPO.0b013e3182329791. [DOI] [PubMed] [Google Scholar]
- 13.Yoo DS, Kirkpatrick JP, Craciunescu O, et al. Prospective trial of synchronous bevacizumab, erlotinib, and concurrent chemoradiation in locally advanced head and neck cancer. Clin Cancer Res. 2012;18:1404–1414. doi: 10.1158/1078-0432.CCR-11-1982. [DOI] [PubMed] [Google Scholar]
- 14.Yao M, Galanopoulos N, Lavertu P, et al. A phase II study of bevacizumab in combination with docetaxel and radiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck. 2014 Jun 21; doi: 10.1002/hed.23813. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ang KK, Harris J, Wheeler R, Weber R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. New Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III and IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen EEW, Davis DW, Karrison TG, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10:247–257. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang X, Zhang Q, Nishitani J, Brown J, Shi S, Le AD. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin Cancer Res. 2007;13:2568–2576. doi: 10.1158/1078-0432.CCR-06-2704. [DOI] [PubMed] [Google Scholar]
- 19.Tewari KS, Sill MW, Long HJ, et al. Improved survival with bevacizumab in advanced cervical cancer. New Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chemotherapy with or without bevacizumab in treating pateints with recurrent or metastatic head and neck cancer. clinicaltrialsgov: NCT00588770.


