Abstract
Myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) suppress normal hematopoietic activity in part by enabling a pathogenic inflammatory milieu in the bone marrow. In this report, we show that elevation of angiopoietin-1 in myelodysplastic CD34+ stem-like cells is associated with higher risk disease and reduced overall survival in MDS and AML patients. Increased angiopoietin-1 expression was associated with a transcriptomic signature similar to known MDS/AML stem-like cell profiles. In seeking a small molecule inhibitor of this pathway, we discovered and validated pexmetinib (ARRY-614), an inhibitor of the angiopoietin-1 receptor Tie-2, which was also found to inhibit the pro-inflammatory kinase p38 MAPK (which is overactivated in MDS). Pexmetinib inhibited leukemic proliferation, prevented activation of downstream effector kinases and abrogated the effects of TNF-alpha on healthy hematopoietic stem cells. Notably, treatment of primary MDS specimens with this compound stimulated hematopoiesis. Our results provide preclinical proof of concept for pexmetinib as a Tie-2/p38 MAPK dual inhibitor applicable to the treatment of MDS/AML.
Introduction
Acute Myeloid Leukemia (AML) and myelodysplastic syndromes (MDS) are generally incurable hematologic neoplasms that are characterized by malignant clonal expansions in the bone marrow. Due to the lack of curative conventional chemotherapies, there is a need to identify newer therapeutic agents that can target malignant cell survival pathways. Most of the morbidity and mortality experienced by these patients are due to low blood counts (1). Cytopenias such as anemia, neutropenia and thrombocytopenia lead to fatigue, immune deficiencies and bleeding, respectively, and are a major source of complications in this patient population (2, 3). These cytopenias are in part due to suppression of normal hematopoietic activity due to the inflammatory milieu that is seen in bone marrow. It has been shown that various inflammatory cytokines such as TNF, TGF, IFNs are overexpressed in the marrow microenvironment and lead to suppression of normal hematopoietic stem and progenitor cells (4). Over the course of disease, the malignant cells that are relatively resistant to these inflammatory myelosuppressive cytokines expand, while the numbers of normal stem cell clones gradually decrease. Thus, strategies to increase normal hematopoietic activity would be useful in the resolution of cytopenias experienced by patients with MDS and AML.
In the present study we have identified the Angiopoietin-1/TIE2 pathway as a novel therapeutic target in AML and MDS. Angiopoietin-1 (Angpt-1) is a cytokine that is implicated in vascular development and angiogenesis and binds to the receptor tyrosine kinase Tie-2. In murine studies Tie-2 has been shown to promote quiescence and self-renewal in hematopoietic stem cells (HSCs) (5). HSCs expressing Tie2 were shown to be quiescent and anti-apoptotic, and comprised a side-population of cells that adhered to osteoblasts in the bone marrow niche. Since leukemic cells and AML/MDS initiating stem cells are also associated with high rates of self-renewal and associated with a differentiation block (6), we investigated the role of Angpt-1/Tie2 pathway in these malignancies.
We determined that Angpt-1 is overexpressed in MDS/AML samples and that inhibition/knockdown of Tie-2 can inhibit leukemic proliferation and lead to enhanced hematopoietic differentiation. We demonstrate the synthesis and validation of a novel inhibitor of both Tie-2 and p38 MAPK and show that pexmetinib (ARRY-614) can lead to inhibition of malignant cells while also reversing cytokine induced suppression on hematopoietic stem cells. The p38 mitogen activated protein kinase (MAPK) is an evolutionary conserved serine-threonine kinase, originally discovered as a stress-activated kinase, that has now been shown to be involved in controlling cell cycle or regulating apoptosis, with its effects being cell and context specific (7). Our previous studies demonstrated that myelosuppressive actions of TNF-alpha, Interferons and TGF-beta are regulated via activation of p38 MAPK (8-10). We demonstrated that treatment of hematopoietic cells with small molecule inhibitors of p38 MAPK lead to a reversal of the growth inhibitory effects of these cytokines on hematopoietic cells. These studies demonstrate the preclinical efficacy of combined inhibition of Tie-2 and p38 MAPK by pexmetinib in MDS and support ongoing efforts to test this in clinical trials in these diseases.
Materials and Methods
Patient samples, cell lines and reagents
Specimens were obtained from patients diagnosed with MDS and AML after IRB approval by the Albert Einstein College of Medicine. The AML cell lines KG1 and CMK were obtained from ATCC and were grown in RPMI supplemented with 10% FBS and 1% Penicillin/Streptomycin. Cell line authentication was done by ATCC by STR profiling. ARRY-614 was obtained from Array BioPharma, dissolved in DMSO and stored at −20C at a concentration of 100mM.
Cell Viability assay
Cell lines and primary samples were incubated at indicated doses of of TIE2/p38 inhibitor ARRY-614. Viability was assessed by addition of Cell Titer Blue (Promega) and measured via Fluostar Omega Microplate reader (BMG Labtech).
siRNAs and nucleofection
For knockdown studies, siRNAs against TIE2 were purchased from Dharmacon. Nucleofection with AMAXA was used for knockdown studies as performed previously (11). Efficiency of Tie2 knockdown efficiency was measured by real time PCR.
Clonogenic assays
For clonogenic assays, primary patient samples and healthy controls were plated in methylcellulose (Stem Cell Technologies H4435) in 35mm dishes and incubated with the ARRY-614 at indicated doses. Colonies were counted after 14-17 days in culture. Clonogenic assays with siRNA mediated Tie2 knockdown were performed after nucelofection of primary human CD34+ cells with specific siRNAs and controls, as in our previous studies (8, 12). Hematopoietic cells were extracted for flow-cytometric analysis by careful aspiration of the semi-liquid medium after 14 days. Cells from triplicate wells were pooled, washed 3 times with ice-cold PBS, and stained with the following antibody-fluorophore conjugates: CD34 - PE, CD45 - PE-Cy7, CD14 - PB, CD11b - APC, Glycophorin A - PE-Cy5, CD71 - FITC.
Database and survival data
Gene expression data from 183 MDS CD34+ samples and 17 controls were obtained from GEO (GSE19429)(13). Data on 200 AML samples was obtained from TCGA (14). Multivariate analysis with Cox Proportional Hazard testing was done using IPSS scores as a variable for the MDS dataset. Multivariate analysis for post day 200 survival using cytogenetics as a variable was done for the AML TCGA dataset.
In vitro assays for determination of IC50
Human Umbilical Vein Endothelial Cells (HUVEC) and HEK-293 human embryonic kidney cells (American Type Culture Collection, Manassas, VA) engineered to express constitutively active Tie-2 (HEK-Tie2) were used to assess p38 and Tie2 phosphorylation in vitro. To create HEK-Tie2 cells, a synthetic cDNA was generated (Blue Heron Biotechnology, Bothell, WA) that directs the synthesis of a hybrid form of the Tie-2 receptor engineered to contain a FLAG epitope tag just downstream of a prolactin signal peptide at the extreme amino terminus of the polypeptide. In addition, this receptor construct was engineered with an arginine to tryptophan substitution at amino acid position 849 which has confers constitutive activation of the receptor. The cDNA was subcloned into the retroviral vector pLNCX2 using the HindIII and NotI sites in the polylinker and contains a doxycyclin-inducible promoter. HEK-Tie2 cells were treated with 1 μg/mL doxycyclin 24 hours prior to drug treatment to induce Tie-2 expression, and HUVECs were pretreated for 1 hr with 1 μg/mL anisomycin or 0.1 μg/mL recombinant angpt-1 (R&D Systems #923-AN-025) to activate p38 or Tie-2, respectively. Cells were treated with varying concentrations of pexmetinib for 2hr (0.25% BSA, 0.2% DMSO), lysed in RIPA buffer then subjected to Western blot analysis. Effect on phosphorylation was detected by near-infrared(NIR) fluorescence using primary antibodies directed to p-p38 (CST#9211s), pHsp27 (CST#2401s), pTie2 (CST#4226s) or pAkt (CST#4060) followed by infrared labeled secondary antibodies Alexa Fluor 680 donkey anti-rabbit (Invitrogen #A10043) or donkey anti-mouse IR800 (Rockland #610-732-124), normalized to GAPDH (Sigma T6199) using LICOR Odyssey software. Data are presented relative to DMSO-treated control.
Correction for protein binding
LPS-induced TNFα was determined in undiluted whole blood (WB) and peripheral blood mononuclear cells (PBMCs) from matched donors (N=9 healthy human subjects). Blood was collected by venipuncture into heparinized tubes (BD 367874) for WB samples and into CPT tubes (BD, 362753) for PBMCs. PBMCs were isolated according to manufacturer's directions and resuspended at 1 million/mL in RPMI-1640 supplemented with 2% heat-inactivated fetal bovine serum (HyClone). WB and PBMC's were pre-incubated with varying concentrations of pexmetinib for 1 hr, 37°C, 5% CO2 then stimulated with 100 ng/ml LPS (Escherichia coli K-235, Sigma #L2018) for 16 hours at 37°C, 5% CO2. TNF-α levels in the cell-free supernatants were quantified by ELISA (R&D systems, DTA00C) as a measure of p-p38 inhibition.
In vivo assays for target inhibition of p-p38 and pTie2 in vivo
Naïve male CD-1 (Charles River) or female nu/nu NCr mice (Taconic Labs) inoculated with 5×10^6 HEK-Tie2 cells subcutaneously near the axillary region on the right flank were utilized to assess the relationship between plasma concentration and target inhibition in lung (p-p38) or tumor (p-p38 and pTie2). To induce Tie2 expression in tumor-bearing animals, a single dose of 30 mg/kg doxycycline in 5% sucrose was administered 16 hours prior to administration of pexmetinib (amorphous free base) as a suspension in 1% CMC/0.02%SDS. All treatments were administered by oral gavage in a dosing volume of 10 mL/kg. At predetermined time points, plasma was collected for analysis of drug concentrations by LC-MS/MS and tissue (lung or tumor) harvested for analysis of target inhibition by Western blot. Lung and tumor tissue were homogenized (FastPrep) in RIPA buffer then subjected to immunoblot analysis. Effect on phosphorylation was detected by near-infrared (NIR) fluorescence as above, normalized to GAPDH and data analyzed relative to vehicle-treated control.
Functional inhibition of p-p38 in vivo
The lipopolysaccharide (LPS) challenge or endotoxemia model evaluates the ability of an animal to mount an acute phase response to an inflammatory stimulus. A hallmark of this response is production of TNFα which is mediated by p38 pathway activation, thus, inhibition of TNFα provides a functional readout of p38 inhibition. Naïve Swiss Webster mice were orally administered increasing doses of pexmetinib as a single agent 30 minutes prior to intraperitoneal challenge with 2 mg/kg LPS (serotype 0127:B8, Sigma). Ninety minutes after LPS injection, whole blood was collected to process for serum and TNFα measured by ELISA (#MTA00, R&D Systems).
Results
Angiopoietin-1 (ANGPT1) is overexpressed in MDS stem and progenitor cells and is associated with worse prognosis in MDS and AML
We assessed the expression of Angiopoietins in a large cohort of MDS patient derived bone marrow stem cells and compared it to healthy controls. Angiopoietin-1 (Angpt1) was found to be significantly overexpressed in MDS CD34+ hematopoietic stem and progenitor cells (HSPCs) (n=183) when compared to healthy controls (N=17) (TTest, P Value<0.05) (Fig 1A). Additionally, the receptor for Angiopoietin-1, Tie2, was also found to be overexpressed in MDS HSPC samples when compared to controls (Fig 1B). Since MDS is a heterogeneous collection of low and high risk disease subtypes, we next evaluated which pathological subgroups had the highest expression of Angpt1. Angpt1 expression was found to be the highest in the RAEB (Refractory anemia with excess of blasts) subtype of MDS, that is associated with the highest risk of transformation to AML (Fig 1C) (TTest, P Value<0.05). Furthermore, when we divided the MDS cohort on the basis of Apgpt1 expression using median expression value as a cutoff for high and low expressors, we observed that percentage of leukemic blast cells in the bone marrow was also significantly higher in patients with high Angpt1 expression (Fig 1D) (TTest, P Value <0.05). Finally, we determined that high Angpt1 expressers had a significantly reduced overall survival with median survival of 2.6 years vs 5.8 years for low expressers (log rank P value =0.001) (Fig 1E). This association was significant after multivariate analysis using the IPSS score as a variable (P=0.004). In addition to MDS, we also evaluated the relationship of overall survival with Angpt1 expression levels in a large acute myeloid leukemia (AML) cohort (TCGA, N=200) (15). Kaplan Meier curves demonstrated reduced overall survival in subjects with high Angpt1 expression (Log Rank P Value=0.0009) that was significant after multivariate analysis using cytogenetics as a variable (P=0.01). (Fig 1F).
Fig 1. ANGPT1 is overexpressed in MDS stem and progenitor cells and is associated with worse prognosis in MDS and AML.
ANGPT1 expression in 183 MDS bone marrow derived CD34+ cells and 17 healthy control CD34+ cells is shown. (A)(TTest, P Value<0.05). Tie-2 expression in 183 MDS bone marrow derived CD34+ cells and 17 healthy control CD34s is shown (B)(TTest, P Value<0.05). ANGPT1 expression for different subtypes of MDS are shown. RA (Refractory anemia), RARS (Refractory anemia with ringed sideroblasts) and RAEB (Refractory anemia with excess of blasts).(C) (Ttest). Mean percentage of leukemic blast cells in the bone marrow of patients with low and high ANGPT1 expression is shown (D) (Ttest, P Value <0.05). Kaplan Meir curves for patients with AML (N=200)(F) and MDS (N=183)(E) are shown. Log rank P value was 0.001 for MDS and 0.057 for AML cohorts.
Important functional pathways are dysregulated in MDS cases with high expression of Angiopoietin-1
Having demonstrated that MDS cases with higher Angpt1 in HSPCs have a worse prognosis, we next wanted to determine whether these cases also had a distinct molecular profile. Gene expression profiles from MDS stem and progenitor cells with low and high Angpt1 were compared and differentially expressed transcripts were identified (FDR<0.1) (Supp Fig 1A, Supp Table 1). We observed that important functional pathways controlling Cell Cycle, Hematopoiesis, Cell signaling were selectively dysregulated in high ANGPT-1 samples (Fig 2A). Furthermore, the gene signature of high Angpt1 expressers was found to be significantly similar to known hematopoietic stem cell and MDS/AML signatures (Supp Fig 1B). Top 3 GSEA profiles that were similar to the Angpt1 high gene signature included 2 stem cell signatures and 1 related to the NUP98/HOX gene fusion that is seen in MDS and AML (Supp Fig 1) (16). Next, we tested whether the high Angpt-1 expression signature had any overlap with known stem cell gene expression profiles. Gene set enrichment analysis with recently published preleukemic stem cell signatures (GSE10754 and GSE303774) (17, 18) revealed highly significant enrichment, demonstrating that HSPCs from Angpt-1 high MDS patients have a similar transcriptomic profile to known leukemia-initiating cell populations (Fig 2B). Similarity to healthy stem cell signature was also observed (Fig 2B), suggesting that high Angpt1 is associated with a distinct hematopoietic stem cell related molecular signature characterized by dysregulation of many important genetic pathways.
Fig 2. Important functional pathways are dysregulated in MDS cases with high expression of Angiopoietin-1.
Gene expression profiles from samples with low and high ANGPT-1 were compared and differentially expressed transcripts were identified (FDR<0.1). Genetic pathways controlling Cell Cycle, Hematopoiesis, Cell signaling were dysregulated in high ANGPT-1 cases (A). Gene signature of high ANGPT-1 is similar to known leukemia and healthy stem cell signatures (B).
Knockdown of Tie-2 inhibits proliferation in malignant cells and leads to enhanced differentiation from healthy human CD34+ cells
Since we saw overexpression of Angpt1 and its receptor Tie2 in MDS/AML, we next wanted to assess the functional significance of this pathway in leukemic cells. Specific knockdown of Tie2 with siRNAs was achieved in leukemic cell lines (Fig 3A) and these cells were used for functional studies. Proliferation of two different leukemic cell lines (KT-1 and CMK) was assessed after transfection with Tie2 and control siRNAs and demonstrated that Tie2 knockdown led to significantly decreased viability (Fig 3B,C) (TTest, P Value<0.05) while no significant inhibition was observed with knockdown of Angpt1 (Supp Fig 2). Next, we wanted to study the effects of Tie2 knockdown on normal human hematopoiesis. Specific knockdown of Tie2 was achieved in primary healthy CD34+ cells (TTest, P value<0.05)(Fig 3D) and primary CD34+ stem cells transfected with Tie2 and control siRNAs were grown in methylcellulose supplemented with cytokines for 14 days. Increased erythroid colony formation (Blast Forming Unit- Erythroid, BFU-E) was seen after Tie2 knockdown (TTest, P Value<0.05)(Fig 3E). Increased erythroid differentiation was also demonstrated by FACS analysis done on colonies that were selected and analyzed for erythroid differentiation markers. We observed an increase in total percentage of erythroid cells (Glycophorin A positivity, Fig 3F), along with increased maturation as evident by higher percentage of mature erythroid forms after Tie2 knockdown (Fig 3G).
Fig 3. Knockdown of Tie-2 inhibits proliferation in leukemic cells and leads to enhanced differentiation in healthy human CD34+ cells.
Specific knockdown of Tie2 with two different siRNAs was achieved in leukemic KT-1 cell line as demonstrated by qRTPCR (Ttest, P alue<0.05)(A). Proliferation of leukemic cells KT-1 and CMK was assessed by MTT assay after transfection with TIE2 and control siRNAs. Tie2 knockdown led to decreased numbers of viable cells (Ttest, P Value<0.05)(B). Specific knockdown of TIE2 with siRNAs was achieved in primary healthy CD34+ cells as demonstrated by qRTPCR (Ttest, P alue<0.05)(C). CD34+ cells transfected with Tie2 and control siRNAs were grown in methylcellulose for 14 days and demonstrated increased erythroid colony formation (Ttest, P Value<0.05)(D). Colonies were picked and analyzed for differentiation by multiparameter flow cytometry. Increased erythroid differentiation was seen (higher Glycophorin A positivity) after Tie2 knockdown. Increased maturation of erythroid cells was also demonstrated (increased percentages of late erythroid)(E) after specific knockdown of Tie2.
Discovery and synthesis of Tie-2 inhibitor that also inhibits p38 MAPK inhibitor
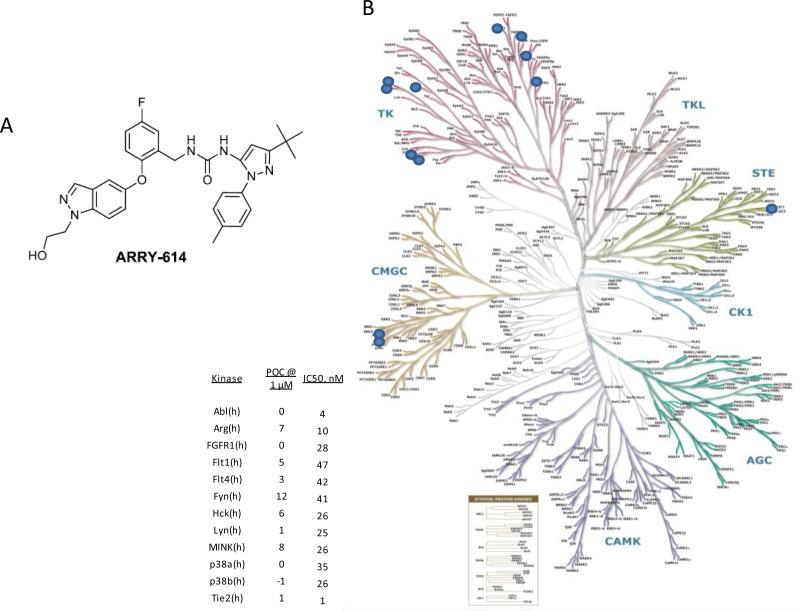
A small molecule orally bioavailable inhibitor of Tie-2 was synthesized (pexmetinib, ARRY-614) also known as 1-(3-(tert-butyl)-1-(p-tolyl)-1H-pyrazol-5-yl)-3-(5-fluoro-2-((1-(2-hydroxyethyl)-1H-indazol-5-yl)oxy)benzyl)urea (Fig 4A)(15). When tested against a panel of 220 kinases, pexmetinib was found to be highly potent against Tie-2 as well as the p38 MAPK α and β (Fig 4B). The compound was also determined to be a type 2 kinase inhibitor that binds both p38 MAP kinase and Tie-2 kinase in the “DFG-out” conformation.
Fig 4. Kinome dendrogram illustrating the selectivity of ARRY-614.
The chemical structure of pexmetinib (ARRY-614) is shown (A). Kinome dendrogram illustrating the selectivity of ARRY-614 (B).
Next, we determined the efficacy of inhibition using cell-based systems including HUVEC stimulated with appropriate ligands for p38 and Tie2 and HEK-293 human embryonic kidney cells (American Type Culture Collection, Manassas, VA) engineered to express phosphorylated Tie-2 (HEK-Tie2). Cells were treated with varying concentrations of pexmetinib and evaluated for efficacy in inhibiting p-Tie-2 and p-p38 MAPK directly or proximally (e.g. pHsp27 for p38 or pAkt for Tie2). We determined that pexmetinib was able to inhibit both kinases in nano-molar ranges (Fig 5A,B).
Fig 5. Pexmetinib is a potent in vitro and in vivo inhibitor of p38 and Tie-2.
HEK-Tie2 cells express high basal levels of p-p38 and were induced to express constitutively active p-Tie-2 by 24 hr preincubation with doxycyclin. Following 2 hr treatment with varying concentrations of pexmetinib, samples were immunoblotted for p-Tie2 and p-p38 then normalized to GAPDH (A). The IC50s for p-Tie-2 and p-p38 inhibition in this mechanistic assay were 16 and 1 nM, respectively (B). Data were combined with results from other in vitro mechanistic models (Angpt-1 or anisomycin-stimulated HUVEC) as well as functional readouts (LPS-induced TNF-alpha release from human PBMCs) and corrected for human protein binding from HWB functional assays. The protein-corrected in vitro IC50s were 2282 nM and 172 nM for Tie2 and p38, respectively (C). To test the prediction, in vivo IC50s were generated from mechanistic (Tie2 and p38 inhibition in HEK-Tie2 xenografts) or functional (inhibition of LPS-induced TNF-alpha release) murine models following a single oral dose of pexmetinib. The in vivo IC50s were 2066 nM and 203 nM for Tie2 and p38, respectively (C).
In order to predict the inhibition that could occur in clinical studies, it was necessary to evaluate protein binding in human samples. To do this, we used an LPS stimulated TNFα production assay which provides a functional readout of p38 inhibition to provide an accurate correction factor. Whole blood (WB) and isolated peripheral blood mononuclear cells (PBMCs) from matched healthy subjects (N=9) were pre-incubated with varying concentrations of pexmetinib for 1 hr and then stimulated with 100 ng/ml LPS and assessed for TNF-α levels. The IC50s for whole blood and isolated PBMCs was 313 ± 176 nM and 4.5 ± 4.1 nM, respectively, providing a human protein binding correction factor of 93X. Based on in vitro IC50 values corrected for protein binding, the predicted plasma concentrations to achieve 50% inhibition for pTie2 and p-p38 in human samples were 2282 nM and 172 nM, respectively.
Due to similar protein binding between mouse and human plasma, we were able to test this prediction by performing murine in vivo studies with HEK-Tie2 xenografts to assess the relationship between plasma concentration and target inhibition in lung (p-p38) or tumor (p-p38 and pTie-2). Tie-2 expression was induced by doxycline in tumor-bearing animals 16 hours prior to administration of ARRY-614 (amorphous free base) as a suspension in 1% CMC/0.02% SDS. At predetermined time points, plasma was collected for analysis of drug concentrations by LC-MS/MS and tissue (lung or tumor) harvested for analysis of target inhibition by immunoblot. Additionally, we tested functional inhibition of p-p38 by monitoring the inhibition of LPS-induced TNFα in an in vivo model of endotoxemia. There was good concordance between plasma concentrations required to inhibit the p-p38 as assessed by immunoblot and the functional readout of LPS-induced TNFα, so data for this target were combined to generate an in vivo inhibitory value (Fig 5C). We determined that murine plasma concentrations required to achieve 50% inhibition for pTie2 and p-p38 were 2066 nM and 203 nM, respectively, confirming that our predictions from protein binding-corrected in vitro inhibition were highly accurate.
ARRY-614 inhibits p38 MAPK, abrogates cytokine mediated myelosuppressive effects in hematopoietic cells and can stimulate MDS hematopoiesis
To determine the efficacy of pexmetinib in human hematopoietic cells, leukemic KG1 cells were treated with TNF-α with and without pexmetinib for indicated times and immunoblotted for phospho/activated p38 MAPK. TNF-α led to activation of p38 MAPK that was completely abrogated by pexmetinib treatment (Fig 6A). Downstream mediators of p38 MAPK, MAPKAPK2 and EIF4E were also evaluated by immunoblotting and pexmetinib was able to inhibit the activation of these effector kinases after TNF exposure. (Fig 6B,C). To assess the functional role of pexmetinib in human hematopoiesis, primary CD34+ stem cells were grown in methylcellulose media in the presence and absence of TNF-α (5ng/ml) and pexmetinib (0.1μM). Erythroid (BFU-E) and myeloid (CFU-GM) colonies were assessed after 14 days and revealed that TNF led myelosuppressive effects were significantly reversed by pexmetinib treatment (Fig 6D)(N=4, TTest, P Value<0.05). Finally, the effects of pexmetinib were assessed on leukemic cell proliferation and revealed the ability to significantly inhibit proliferation in vitro for two different AML derived cell lines (Fig 6E,F). Having established the efficacy of pexmetinib in cell lines, we next wanted to determine its effects on primary MDS samples. Mononuclear cells were collected from 5 patients with a variety of MDS subtypes (Supp Table 2). Treatment with the pexmetinib resulted in an increase in erythroid (BFU-E) and myeloid (CFU-GM) colony numbers in all the cases (Fig 6G). An increase in the size of colonies was also seen demonstrating increased hematopoietic differentiation from these samples (Fig 6F).
Fig 6. ARRY-614 abrogates cytokine mediated myelosuppresive effects in hematopoietic cells; inhibits leukemic cell proliferation and stimulates hematopoietic activity in MDS.
Leukemic KG1 cells were treated with TNF-α (10ng/ml) with and without ARRY-614 (10uM) for indicated times and immunblotted for phospho/activated p38 MAPK (A). Downstream mediators of p38 MAPK, MAPKAPK2 and EIF4E were also evaluated by immunoblotting (B,C). Human CD34+ cells were grown in methylcellulose media in the presence and absence of TNF-α (5ng/ml) and ARRY-614 (0.1uM). Erythroid (BFU-E) and Myeloid (CFU-GM) colonies were assessed after 14 days. (N=4, TTest). Proliferation of leukemic cells KT-1 and KG-1 was assessed by MTT assay after treatment with ARRY-614. (Ttest, P Value<0.05)(E,F). Primary MDS mononuclear cells (n=6) were grown in methylcellulose with and without ARRY-614. Erythroid (BFU-E) and Myeloid (CFU-GM) colonies were evaluated after 14 days of culture (G). Representative picture of colonies from one sample are shown (H).
Discussion
Myelodysplastic syndromes and acute myeloid leukemias are hematologic malignancies characterized by malignant cell expansions coupled with ineffective hematopoiesis that results in reduced blood counts. We demonstrate that the Angpt1/Tie2 pathway is overexpressed in MDS and is a marker of adverse prognosis. We have also demonstrated previously that the p38 MAPK is overactivated in these diseases and regulates the myelo-suppressive effects of inhibitory cytokines in the marrow microenvironment. In the present study, we have developed a novel clinically useful inhibitor of Tie2 that also inhibits p38 MAPK. We demonstrate that combined blockade of Tie2 and p38 MAP kinases by pexmetinib leads to inhibition of malignant cell growth while stimulating hematopoietic activity in MDS/AML cell lines and primary samples (Supp Fig 3).
Our data implicate the Angiopoietin-1/Tie2 pathway as a novel therapeutic target in AML and MDS. These myeloid malignancies are associated with disease initiating stem cells that are not eliminated by conventional therapies. We have demonstrated that hematopoietic stem cells (HSC) are aberrantly expanded in MDS/AML and can persist during phenotypic remissions and can predict relapse. Since approved therapies are frequently associated with refractory and relapsed disease, novel therapeutic targets against such pre-leukemic / MDS stem cells need to be identified for potentially curative strategies. Tie2 has been shown to regulate self-renewal and promote symmetric cell division in murine healthy hematopoietic stem cells (5) and our work also implicates this pathway in malignant myeloid neoplasms.
The p38 MAPK is also an attractive therapeutic target in MDS. Although MDS can transform into leukemia, most of the morbidity experienced by these patients is due to chronically low blood counts. Approved agents such as lenalidomide and conventional cytotoxic agents used to treat MDS have yielded some encouraging results but are characterized by many adverse effects including worsening cytopenias in the predominantly elderly patient population. Thus therapies aimed at reversing the bone marrow failure and increasing the peripheral blood counts would be advantageous in these patients. Our previous studies have demonstrated over-activated signaling of p38 MAPK by myelo-suppressive cytokines such as TNF-α, TGF-β, and Interferons in MDS hematopoietic stem cells (10, 19). Overactivation of the p38 MAP kinase pathway promotes aberrant apoptosis of stem and progenitor cells in MDS and leads to ineffective hematopoiesis. Thus, inhibition of p38 MAPK may be an attractive therapeutic strategy in combination with an agent that targets malignant cell growth also. Our data provides preclinical rationale for testing pexmetinib in trials in MDS and AML.
Supplementary Material
Footnotes
Conflict of Interest Statement: SLW, JR, JG, SB, LW, GH, DW, MM, MR, SG, DC and LEB are employees of Array BioPharma.
References
- 1.Bachegowda L, Gligich O, Mantzaris I, Schinke C, Wyville D, Carrillo T, et al. Signal transduction inhibitors in treatment of myelodysplastic syndromes. Journal of hematology & oncology. 2013;6:50. doi: 10.1186/1756-8722-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Msaouel P, Lam AP, Gundabolu K, Chrysofakis G, Yu Y, Mantzaris I, et al. Abnormal platelet count is an independent predictor of mortality in the elderly and is influenced by ethnicity. Haematologica. 2014;99:930–6. doi: 10.3324/haematol.2013.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam AP, Gundabolu K, Sridharan A, Jain R, Msaouel P, Chrysofakis G, et al. Multiplicative interaction between mean corpuscular volume and red cell distribution width in predicting mortality of elderly patients with and without anemia. American journal of hematology. 2013;88:E245–9. doi: 10.1002/ajh.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma A, List A. Cytokine targets in Myelodysplastic syndromes. Curr Hematol Rep. 2005 In Print. [PubMed] [Google Scholar]
- 5.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Will B, Zhou L, Vogler TO, Ben-Neriah S, Schinke C, Tamari R, et al. Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations. Blood. 2012;120:2076–86. doi: 10.1182/blood-2011-12-399683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platanias LC. The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacology & therapeutics. 2003;98:129–42. doi: 10.1016/s0163-7258(03)00016-0. [DOI] [PubMed] [Google Scholar]
- 8.Verma A, Deb DK, Sassano A, Uddin S, Varga J, Wickrema A, et al. Activation of the p38 mitogen-activated protein kinase mediates the suppressive effects of type I interferons and transforming growth factor-beta on normal hematopoiesis. The Journal of biological chemistry. 2002;277:7726–35. doi: 10.1074/jbc.M106640200. [DOI] [PubMed] [Google Scholar]
- 9.Verma A, Mohindru M, Deb DK, Sassano A, Kambhampati S, Ravandi F, et al. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to arsenic trioxide. The Journal of biological chemistry. 2002;277:44988–95. doi: 10.1074/jbc.M207176200. [DOI] [PubMed] [Google Scholar]
- 10.Navas TA, Mohindru M, Estes M, Ma JY, Sokol L, Pahanish P, et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood. 2006;108:4170–7. doi: 10.1182/blood-2006-05-023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schinke C, Giricz O, Li W, Shastri A, Gordon S, Barreyro L, et al. IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood. 2015 doi: 10.1182/blood-2015-01-621631. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma A, Deb DK, Sassano A, Kambhampati S, Wickrema A, Uddin S, et al. Cutting edge: activation of the p38 mitogen-activated protein kinase signaling pathway mediates cytokine-induced hemopoietic suppression in aplastic anemia. J Immunol. 2002;168:5984–8. doi: 10.4049/jimmunol.168.12.5984. [DOI] [PubMed] [Google Scholar]
- 13.Pellagatti A, Cazzola M, Giagounidis A, Perry J, Malcovati L, Della Porta MG, et al. Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24:756–64. doi: 10.1038/leu.2010.31. [DOI] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research N Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. The New England journal of medicine. 2013;368:2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al. DNMT3A mutations in acute myeloid leukemia. The New England journal of medicine. 2010;363:2424–33. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam DH, Aplan PD. NUP98 gene fusions in hematologic malignancies. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2001;15:1689–95. doi: 10.1038/sj.leu.2402269. [DOI] [PubMed] [Google Scholar]
- 17.Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, Quake SR, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Science translational medicine. 2012;4:149ra18. doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of preleukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–33. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, Nguyen AN, Sohal D, Ying Ma J, Pahanish P, Gundabolu K, et al. Inhibition of the TGF-beta receptor I kinase promotes hematopoiesis in MDS. Blood. 2008;112:3434–43. doi: 10.1182/blood-2008-02-139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.