Neuromyelitis optica spectrum disorders (NMOSDs) are relapsing inflammatory demyelinating disorders with optic neuritis (ON) as the hallmark. ON causes neuroaxonal damage to the optic nerve and retina, regularly leading to severely impaired visual acuity (VA).1
Peripapillary retinal nerve fiber layer (pRNFL) thickness measured by optical coherence tomography (OCT) has been increasingly recognized as a marker for neuroaxonal damage and correlate of visual dysfunction.1 As such, pRNFL is implemented as an outcome in clinical trials of ON-associated disorders. Blood vessels (BVs) running within the pRNFL contribute approximately 13% to an average RNFL thickness2 and could present an important confounder when tracking small pRNFL changes or in diseases with severe thinning such as NMOSD.1 Against this background, the objective of this study was to investigate the influence of retinal BVs on pRNFL measurements in an NMOSD cohort.
Methods.
Forty patients from a prospective observational cohort study at the NCRC at Charité–Universitätsmedizin Berlin were enrolled (women/men: 39/1, age: 44.7 ± 15.4 years, 42 ON eyes). Inclusion criteria were a minimum age of 18 years and diagnosis of NMOSD according to the 2015 IPND criteria3 (n = 37, aquaporin-4 antibody seropositive n = 28) or myelin oligodendrocyte glycoprotein-IgG–associated encephalomyelitis (n = 3).4 Exclusion criteria were any other diseases which could influence OCT results.
All patients were examined with a Spectralis SD-OCT (Heidelberg Engineering, Heidelberg, Germany) using automatic real time (ART) function for image averaging. pRNFL was measured with a 3.4 mm ring scan around the optic nerve head (12°, 1536 A scans 16 ≤ ART ≤ 100) and segmented semiautomatically (Eye Explorer 1.9.10.0 with viewing module 6.0.9.0) and manually corrected by an experienced grader. BV positions were automatically detected by OCTSEG5 (figure, A and B) and manually corrected. Three eyes were excluded because of insufficient image quality based on OSCAR-IB criteria.
Figure. Contribution of blood vessels to retinal nerve fiber layer thickness.
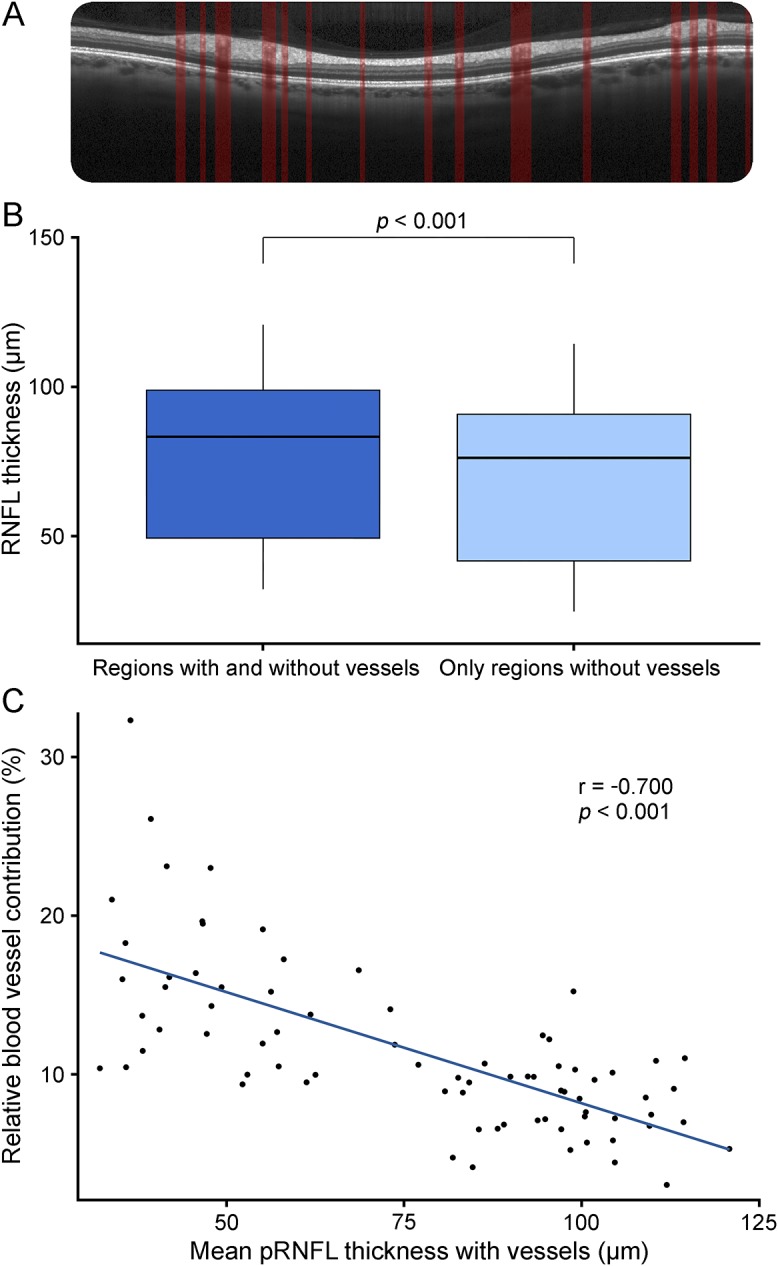
(A) pRNFL scanned by optical coherence tomography, segmented with vessel detection. (B) Mean pRNFL thickness in μm; all regions including vessels and only regions without vessels. (C) Correlation of the relative blood vessel contribution to the pRNFL including vessels. pRNFL = peripapillary retinal nerve fiber layer.
High-contrast VA was examined monocularly under habitual correction and photopic conditions with ETDRS charts at a simulated 20 ft distance using the Optec 6500 P System (Stereo Optical, Chicago, IL).
pRNFL without vessels was calculated as mean thickness of all ring scan positions not marked as part of vessels and was compared with pRNFL with vessels using paired t tests. We performed Pearson correlation analyses for evaluation of the relationship between pRNFL and VA and Fisher z test for correlation comparison. The relative BV contribution in percentage was calculated as ([pRNFLwith vessels − pRNFLwithout vessels]/pRNFLwith vessels × 100%). All statistical tests were performed using R 3.1 with significance established at p < 0.05. The study was approved by the local Ethics Committee at Charité–Universitätsmedizin Berlin and was conducted in accordance with the Declaration of Helsinki.
Results.
pRNFL measurements were thinner without including BVs (76.1 ± 26.6 μm with, 68.3 ± 26.2 μm without, p < 2e−16; figure, C). Relative BV contribution increased with lower pRNFL (r = −0.700, p = 1e−12) (figure, D). When only considering eyes with pRNFL thickness below 60 μm, the mean relative BV contribution was significantly higher with 16% ± 5% compared with 9% ± 3% in eyes with RNFL >60 μm (p = 8e−8).
VA (36 ± 19 ETDRS letters) was associated with pRNFL including BV (r = 0.621, p = 2e−9) and without BV (r = 0.618, p = 2e−9). In eyes with pRNFL measurements below 60 μm, pRNFL-VA correlation was numerically higher for pRNFL excluding BV (r = 0.495, p = 0.007) than pRNFL including BV (r = 0.482, p = 0.009), but the difference was not significant (p = 0.476). There were no influences of antibody status, disease duration and therapy on pRNFL, relative BV contribution, or enlargement of BV areas with pRNFL thinning (data not shown).6
Discussion.
BV contribution to average pRNFL measurements is higher in thin compared with normal/high pRNFL measurements.
A previous study reported an average BV contribution of 13% to pRNFL measurements.2 Our study expands these findings by showing that BV contribution is increased in low pRNFL measurements like the ones regularly found in NMOSD patients with severe ON.
A relevant contribution of BV artifacts to measurement noise has been reported.7 Although our results did not show a structure-function correlation improvement for vessel-corrected measurements, they suggest a downgrade in pRNFL measurement sensitivity. In NMOSD cohorts, a wide range of pRNFL thickness measurements are seen, including those lower than 60 μm.1 Typically, pRNFL differences of only a few micrometers are used to evaluate drug efficacy in ON trials.8 Thus, in longitudinal studies, vessel artifacts potentially interfere with the comparability of an absolute thickness change because the relative vessel contribution increases with thinner pRNFL.
We propose analyzing OCT data in studies including NMOSD and other conditions with low pRNFL measurements in addition to vessel correction. Further studies of retrospective and prospective data and larger cohorts are required to confirm and specify BV influence and to identify reliable surrogates for tracking ON-related damage in NMOSD.
Footnotes
Author contributions: F.C.O. and H.Z.: analysis/interpretation of data, statistical analysis, and drafting of the manuscript. J.M.: analysis/interpretation of data and acquisition of data. M.W. and E.M.K.: analysis/interpretation of data. T.O.: drafting/revising of the manuscript and statistical analysis. F. Pache: study concept/design and acquisition of data. J.B.-S.: study concept/design, acquisition of data, and study coordination. K.R.: drafting/revising of the manuscript for content and study supervision/coordination. F. Paul: drafting/revising of the manuscript and study supervision/coordination. A.U.B.: drafting/revising of the manuscript, study concept/design, analysis/interpretation of data, statistical analysis, and study supervision/coordination.
Study funding: No targeted funding reported.
Disclosure: F.C. Oertel reports no disclosures. H. Zimmermann received speaker honoraria from Teva and Bayer. J. Mikolajczak received travel funding and/or speaker honoraria from TEVA GmbH Germany, Biogen Idec Germany, and Bayer Vital GmbH Germany. M. Weinhold received speaker honoraria from TEVA GmbH Germany. E.M. Kadas has a patent application describing the optic nerve head volume analysis used in this study. T. Oberwahrenbrock received speaker honoraria from TEVA GmbH Germany and Bayer Germany and received research support from Federal Ministry of Education and Research. F. Pache received travel funding and/or speaker honoraria from Genzyme, a Sanofi Company, Bayer, Biogen Idec, and ECTRIMS; received research support from Charité–Universitätsmedizin Berlin, Berlin Institute of Health, KKNMS-Bundesministerium für Bildung und Forschung, and Novartis. J. Bellmann-Strobl received travel funding and/or speaker honoraria from Bayer Healthcare, Sanofi-Aventis/Genzyme, and Teva Pharmaceuticals. K. Ruprecht served on the scientific advisory board for Sanofi-Aventis/Genzyme, Novartis, and Roche; received travel funding and/or speaker honoraria from Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi-Aventis/Genzyme, Teva Pharmaceuticals, Novartis, and Guthy Jackson Charitable Foundation; is an academic editor for PLoS One; receives publishing royalties from Elsevier; and received research support from Novartis and German Ministry of Education and Research. F. Paul serves on the scientific advisory board for Novartis; received speaker honoraria and travel funding from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire; is an academic editor for PLoS One, is an associate editor for Neurology® Neuroimmunology & Neuroinflammation; consulted for Sanofi-Genzyme, Biogen Idec, MedImmune, Shire, and Alexion; received research support from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Arthur Arnstein Foundation Berlin, Guthy Jackson Charitable Foundation, and National Multiple Sclerosis of the USA. A.U. Brandt served on the scientific advisory board for Biogen; received travel funding and/or speaker honoraria from Novartis and Biogen; has patents pending from Method and System for Optic Nerve Head Shape Quantification, perceptive visual computing based postural control analysis, multiple sclerosis biomarker, and perceptive sleep motion analysis; consulted for Nexus and Motognosis; and received research support from Novartis Pharma, Biogen Idec, BMWi, BMBF, and Guthy Jackson Charitable Foundation. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was funded by the authors.
References
- 1.Bennett JL, de Seze J, Lana-Peixoto M, et al. . Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult Scler 2015;21:678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood DC, Fortune B, Arthur SN, et al. . Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography. J Glaucoma 2008;17:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pache F, Zimmermann H, Mikolajczak J, et al. . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients: part 4: afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation 2016;13:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer MA, Hornegger J, Mardin CY, Tornow RP. Retinal nerve fiber layer segmentation on FD-OCT scans of normal subjects and glaucoma patients. Biomed Opt Express 2010;1:1358–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green AJ, Cree BAC. Distinctive retinal nerve fibre layer and vascular changes in neuromyelitis optica following optic neuritis. J Neurol Neurosurg Psychiatry 2009;80:1002–1005. [DOI] [PubMed] [Google Scholar]
- 7.Balk LJ, Mayer M, Uitdehaag BMJ, Petzold A. Retinal hyperaemia-related blood vessel artifacts are relevant to automated OCT layer segmentation. J Neurol 2014;261:511–517. [DOI] [PubMed] [Google Scholar]
- 8.Sühs KW, Hein K, Sättler MB, et al. . A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann Neurol 2012;72:199–210. [DOI] [PubMed] [Google Scholar]


