Abstract
Neurological deficits in the offspring caused by human maternal hypothyroxinemia are thought to be irreversible. To understand the mechanism responsible for these neurological alterations, we induced maternal hypothyroxinemia in pregnant rats. Behavior and synapse function were evaluated in the offspring of thyroid hormone-deficient rats. Our data indicate that, when compared with controls, hypothyroxinemic mothers bear litters that, in adulthood, show prolonged latencies during the learning process in the water maze test. Impaired learning capacity caused by hypothyroxinemia was consistent with cellular and molecular alterations, including: 1) lack of increase of phosphorylated c-fos on the second day of the water maze test; 2) impaired induction of long-term potentiation in response to theta-burst stimulation to the Schaffer collateral pathway in the area 1 of the hippocampus Ammon’s horn stratum radiatum, despite normal responses for input/output experiments; 3) increase of postsynaptic density protein 95 (PSD-95), n-methyl-d-aspartic acid receptor subunit 1, and tyrosine receptor kinase B levels in brain extracts; and 4) significant increase of PSD-95 at the PSDs and failure of this molecule to colocalize with n-methyl-d-aspartic acid receptor subunit 1, as it was shown by control rats. Our findings suggest that maternal hypothyroxinemia is a harmful condition for the offspring that can affect key molecular components for synaptic function and spatial learning.
MATERNAL HYPOTHYROXINEMIA is a pathological condition that induces cognitive impairment in the progeny. A number of reports, derived from human studies, have shown that there is a strong correlation between maternal hypothyroxinemia and cognitive damage in the offspring. These studies reported that 50% of the offspring gestated in hypothyroxinemic mothers show neuronal damage (1, 2, 3, 4, 5). A hypothyroxinemic patient is characterized by having low serum levels of T4, and normal levels of T3 and TSH (1). Maternal hypothyroxinemia is an asymptomatic condition for the mother, but it is extremely harmful for the fetus. Because the fetus’s thyroid is still too immature to synthesize sufficient T3 and T4, the fetus relies on the maternal thyroid hormone for its optimal brain development. Brain damage in the offspring caused by maternal hypothyroxinemia is considered to be irreversible and characterized by attention deficit, low intelligence quotient, and mental retardation (6, 7). However, the molecular mechanisms responsible for the cognitive damage caused by hypothyroxinemia remain unknown. Recent studies have shown that: 1) maternal hypothyroxinemia can alter the cellular architecture of the somatosensory cortex and hippocampus of the offspring (8), 2) 83% of the offspring had deficient radial migration of projection neurons (9), and 3) maternal hypothyroxinemia also alters tangential migration of medial ganglionic eminence-derived neurons of the progeny (10). Nevertheless, the previously described alterations caused by maternal hypothyroxinemia failed to explain all the features of cognitive deficiency caused by hypothyroxinemia in the offspring. Given that maternal hypothyroxinemia is highly prevalent in humans (1), here, we have evaluated some of the cellular and molecular alterations caused by this disorder in the offspring’s brain. As a model, we used methimazole (MMI) treatment to develop hypothyroxinemia in pregnant rats (9) and followed up the offspring to adulthood. To assess cognitive deficiencies in the progeny gestated in hypothyroxinemic mothers (MMIo), their behavior was evaluated and compared with that of control animals. We found that maternal hypothyroxinemia caused significant cognitive damage to the offspring, manifested as impaired spatial learning behavior. These animals showed impaired responses of collateral-Schaffer in the area 1 of hippocampus Ammon’s horn (CA1) synapsis of the hippocampus to theta-burst stimulus (TBS), which are required for efficient learning and memory processes. Contrarily to what was observed in control rats, the content of phosphorylated c-fos failed to increase in the MMI offspring subjected to spatial learning tests. c-fos is an immediate early gene (IEG) essential for appropriate spatial learning behavior in these animals (11, 12, 13, 14). Finally, analyses of the protein content of excitatory neurons postsynaptic densities (PSDs) revealed an abnormal increase of proteins that are critical for synaptic function. Our data suggest that maternal hypothyroxinemia in rats can severely impair the learning capacity of their progeny, and that this cognitive damage could be due to physiological and molecular alterations at glutamatergic synapses.
Materials and Methods
Animals and treatments
Female adult Sprague Dawley rats weighing approximately 250 g were maintained at 22 C in a room with automatic 12-h light, 12-h dark cycles. These rats were mated, and the next day a vaginal smear was obtained and analyzed under a microscope to search for spermatozoa. Rats with smear positive for spermatozoa were considered mated, and 1 d after mating was referred to as embryonic d 0 (E0). Pregnant rats were separated randomly into two groups. A group of pregnant rats (n = 6) was transiently treated with 2-mercapto-1 methylimidazole (MMI) as described previously (9). Briefly, 0.02% MMI was added to the drinking water during E12–E15. Control animals (n = 6) received the same drinking water but without MMI. After MMI treatment (E15), blood samples were collected from the tail, and sera were analyzed for hormone levels. The day of birth was referred to as postnatal d 0 (P0). The offspring from MMI treated (MMIo) and controls were weaned at P30 and culled to perform the experiments at P60. Pregnant dams used for hormone determination were six both for MMI and controls. TSH determination was performed on MMI (n = 3) and control (n = 2) animals. Rats used for the water maze test were 16 for MMIo and 17 for controls. Rats used for the visible-platform test were three for MMIo and three for controls. Dams used for visible-platform tests had been subjected to the water maze test 2 wk before. The following experiments were done with new dams. Rats used for extracellular field recording and long-term potentiation (LTP) induction assays were: nine for MMIo and six for controls. Western blot analyses were performed for three independent experiments, both for PSDs and brain homogenates. The number of dams used for each experiment was 10 for MMIo and 10 for controls. The number of rats used for Western blots analysis of c-fos and phosphorylated c-fos was three for MMIo and three for controls. The number of rats used for brain-derived neurotrophic factor (BDNF) determination was: 10 MMIo and 10 controls. The number of rats used for immunofluorescence analyses was three for MMIo and three for controls. Dams used for BDNF determination and c-fos analyses were used as well for Western blot analyses of PSDs and brain homogenates.
Hormone determination
Plasma levels of TSH were measured by RIA at the Instituto de Biofísica Carlos Chagas Filho of Federal University of Rio de Janeiro, Brazil (15). Plasma levels of total T3 (tT3) and free T4 (fT4) were measured by chemiluminescence in the laboratory of Instituto de Estudios Médicos Avanzados, Santiago, Chile (16). Thyroid hormone levels resulting from our assays on rat sera were equivalent to those previously reported in the literature (9, 17).
Water maze test
The water maze test used in our experiments was described by Morris in 1984 (18). This method is used to assess spatial learning and memory in rodents. The apparatus consists of a black circular swimming pool (150 cm diameter, 50 cm depth) that was filled with water (22 C temperature) to 20 cm below the rim. The pool was divided into four quadrants of equal area. Each area was arbitrarily called north, south, east, and west. A circular Plexiglas platform (10 cm diameter) was located 2 cm below the water surface in the middle of the north quadrant. This platform had the same color as the swimming pool in order not to be visualized by the rat. The procedure room, where the swimming pool was located, had spatial cues on the walls for spatial orientation.
The water maze test was performed at P60. The entire procedure took 5 d, and rats were moved to the procedure room 30 min before testing. Each rat had five training trials per day, with 20-min intertrial intervals. The two investigators that worked with the water maze procedure were always the same and located always at the same position in the room. On each trial the rat was placed into the water, immediately facing the perimeter of the pool at one of the cardinal compass points (north, south, east, or west), and was allowed to locate the platform with a maximal time of 120 sec. The time spent by the rat to find the platform was recorded and assigned as latency. After staying on the platform for 20 sec, the rat was gently picked up, returned to its home cage, and allotted to warm up and dry off under a heat lamp. If the rat failed to find the platform in the allowed time, it was placed onto the platform for 30 sec and assigned a latency of 120 sec. Cue task training test, a control to assess the motor and visual capacity of rodents, was performed 2 wk after the water maze test. This test is a visible-platform test, in which the platform was 2 cm above the water, and the rest of the procedure was exactly the same as in the aforementioned invisible platform. Results were expressed as the mean + sem of the latencies measured on each day for each group. Statistical analyses for the water maze tests were performed on each day latencies were derived from control and MMIo animals. Analyses were performed using the Mann-Whitney U test. Statistical analyses for the data from the visible platform test were analyzed by the unpaired t test. The SigmaPlot 9 plus SigmaStat 3.5 program (Systat Software, Inc., San Jose, CA) was used for the analyses. Data were considered statistically significant when P was equal to or less than 0.05.
Extracellular field recording and LTP induction
Transverse hippocampal slices of MMIo and control rats were prepared for extracellular field recording and LTP assays. Animals were anesthetized with halothane gas and decapitated soon after the disappearance of any corneal reflexes. Brains were rapidly removed and immersed in ice-cold dissection buffer containing 212.7 mm sucrose, 5 mm KCl, 1.25 mm NaH2PO4, 3 mm MgSO4, 1 mm CaCl2, 26 mm NaHCO3, and 10 mm glucose (pH 7.4). Hippocampi were dissected free, and transverse slices (400 μm thick) were obtained from the middle-third portion using a vibratome (Model HA752; Campden Instruments, Leicester, UK). Slices were transferred to an interface storage chamber containing artificial cerebrospinal fluid (ACSF) saturated with 95% O2/5% CO2 and left for at least 1 h at 37 C before recording. ACSF contained 124 mm NaCl, 5 mm KCl, 1.25 mm NaH2PO4, 1.0 mm MgCl2, 2.0 mm CaCl2, 26 mm NaHCO3, and 10 mm glucose (pH 7.4). Single slices were then transferred to a recording chamber, where they were kept completely submerged in ACSF and continually perfused (2 ml/min).
Field excitatory postsynaptic potentials (fEPSPs) were evoked with electrical stimulation delivered every 15 sec to the Schaffer collateral pathway using bipolar electrodes and recorded in the stratum radiatum (for measuring fEPSP slopes) of the CA1 hippocampal area. Recording electrodes were glass micropipettes (1–3 mΩ) filled with ACSF. At the beginning of each experiment, stimulus/response (input/output) curves were obtained by increasing the intensity of the stimulus to adjust it to elicit 50% of the maximal response. LTP was elicited after 10 min of a stable baseline by TBS consisting of three trains with an intertrain interval of 10 sec. Each train of 10 was made up by bursts at 5 Hz, and each burst had four pulses at 100 Hz separated by 200 msec. After TBS, data acquisition continued for 30 min. Data were acquired using a differential alternating current amplifier (Model 1800; A-M Systems, Inc., Carlsborg, WA) and a data acquisition card (National Instruments, Austin, TX) controlled through IGOR software (WaveMetrics, Inc., Lake Oswego, OR).
Determination of proteins from homogenate and PSD
PSDs and homogenate were isolated from the telencephalon to analyze the presence and amounts of some specific proteins in this structure and fraction, respectively. The PSD fraction was isolated from the rest of the homogenate, as previously described (19). Briefly, MMIo and control male rats at P60 were decapitated and their brains isolated. The cerebral cortex and hippocampus (telencephalon) were dissected, cut, and homogenized on ice in 8 ml homogenization buffer [0.32 m sucrose, 0.5 mm EDTA, and 5 mm Tris (pH 7.4)], using 12 strokes with a 40 ml Tissue Grind Potter with Teflon Pestle (Thomas Scientific, Swedesboro, NJ). One milliliter of the homogenate was saved to be analyzed for total protein concentration. The telencephalon homogenate was then centrifuged at 1000 × g for 10 min at 4 C (Sorvall 5B-plus, SS-34 rotor; Thomas Scientific). The supernatant (S1) was saved. The pellet (P1) was washed, manually homogenized, and centrifuged at 1000 × g for 10 min at 4 C. The pellet (P2) was discarded, and the supernatant (S2) was mixed with S1. S1 plus S2 were centrifuged at 12,000 × g (Sorvall 5B-plus, SS-34 rotor) for 20 min at 4 C. The pellet (P3) was saved and rinsed with solution A [0.32 sucrose, 5 mm Tris-HCl (pH 8.1), 0.5 mm EGTA, and 1 mm dithiothreitol]. The P3 was then manually homogenized with a 17-ml Tissue Grind Potter with Teflon Pestle, and layered on a discontinuous sucrose step gradient [0.32 m, 1 m, 1.2 m sucrose in 5 mm Tris-HCl (pH 8.1)] and centrifuged at 150,000 × g using the AH629 rotor (Sorvall; Thomas Scientific) for 2 h at 4 C. The synaptosome 1 fraction (Syn1) was isolated from 1–1.2 m sucrose gradient and diluted 10 times its volume with the lysis buffer [5 mm Tris-HCl (pH 8.1) and 0.5 mm EGTA]. The lysis was performed by incubating and gently mixing the lysis buffer with Syn1 in ice for 30 min. The Syn1 was then centrifuged at 33,000 × g for 30 min (Sorvall RC5B plus rotor SS34; Thomas Scientific). The pellet (P4) was saved, resuspended in 3 ml solution A, and manually homogenized. P4 was then layered on a discontinuous sucrose step gradient [0.32 m, 1 m, 1.2 m sucrose in 5 mm Tris-HCl (pH 8.1)] and centrifuged at 250,000 × g using the TH660 rotor (Sorvall; Thomas Scientific) for 1 h at 4 C. The fraction of synaptosome 2 (Syn2) was obtained from 1 and 1.2-m fractions. Syn2 was saved and diluted with eight volumes of 0.32 m sucrose, 0.025 mm CaCl2, 1% Triton X-100, 2 mm dithiothreitol, and 10 mm Tris-HCl (pH 8.1). The Syn2 was then gently mixed in this buffer and centrifuged at 33,000 × g (Sorvall RC5B plus rotor SS34) for 30 min at 4 C. The pellet (P5) is the PSD fraction. It was saved and washed with 50 mm HEPES (pH 7.4). The PSDs were centrifuged at 250,000 × g (Beckman Optima TLX, rotor TLA110; Beckman Coulter, Inc., Fullerton, CA) for 10 min at 4 C, and the pellet was resuspended in 50 mm HEPES (pH 7.4). The PSD fraction was frozen in liquid nitrogen and stored at −80 C.
Protein concentration in the PSD fraction and total telencephalon homogenate samples was determined by the bicinchoninic acid method (20). The PSD and total homogenate samples were diluted 1:1 with loading buffer and heated at 100 C for 5 min. Then, 20 and 40 μg protein, respectively, were loaded on a 10% SDS-PAGE, separated by electrophoresis, and electrotransferred to nitrocellulose sheets that were incubated one at a time with: 1) anti-n-methyl-d-aspartate (NMDA) receptor subunit 1 (NR1) antibody (catalog no. AB1516; CHEMICON International, Inc., Temecula, CA) 1:1000 dilution incubated for 1 h (21); 2) anti-NMDA receptor subunit 2 A/B (NR2 A/B) antibody (catalog no. AB1548W; CHEMICON International) 1:2000 dilution incubated for 1.5 h (21); 3) anti-P75 antibody (catalog no. 07-476; Upstate Biotechnology Inc., Lake Placid, NY) 1:1000 dilution incubated for 1 h (22); 4) anti-PSD 95 (PSD-95) antibody (catalog no. MAB377; CHEMICON International) 1:1000 dilution incubated for 16 h (23); 5) anti-tyrosine receptor kinase (Trk) B antibody (catalog no. 610102; Transduction Laboratories, Lexington, KY) 1:1000 dilution incubated for 16 h (24); 6) TrkA antibody (catalog no. SC-14024; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) 1:200 dilution incubated for 16 h (25); 7) anti-calmodulin kinase II (CAMKII) antibody (catalog no. 05-533; Upstate Biotechnology) 1:2000 dilution incubated for 16 h (26); 8) anti-phosphorylated c-fos antibody (catalog no. ab17933; Abcam, Inc., Cambridge, MA) 1:300 dilution incubated for 16 h, which is directed to phospho T232 of c-fos (27); 9) anti-c-fos antibody (catalog no. PC05L; Calbiochem, San Diego, CA) 1:100 dilution incubated for 16 h (28); and 10) antiactin antibody (catalog no. ab1428; Abcam) 1:1000 dilution incubated for 16 h (data sheet; Abcam). A horseradish peroxidase-linked goat antirabbit IgG (catalog no. 401215; Calbiochem) or horseradish peroxidase-linked goat antimouse IgG (catalog no. 401315; Calbiochem) was used as secondary antibody. Both of these secondary antibodies were diluted to 1:1500 and incubated with the nitrocellulose for 1 h. For c-fos and phosphorylated c-fos, the secondary antibody was peroxidase-linked horseradish antirabbit (catalog no. 115-035-003; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted to 1:25,000 in Tween 20 10%, BSA 5%, 150 mm NaCl, 10 mm Tris (pH 8.0) (TBST). The nitrocellulose was washed and then incubated with the enhanced chemiluminescence Western blot detection system (Amersham Biosciences Inc., Piscataway, NJ) to visualize the proteins.
BDNF determination
The content of BDNF was analyzed using 7 mg of the total telencephalon homogenate. The homogenate was centrifuged at 14,000 rpm in a MIKRO 22R Hettich Zentrifuge (Andreas Hettich GmbH & control KG, Tuttlingen, Germany) at 4 C for 30 min. The supernatant was discarded, and the pellet was lysed in 137 mm NaCl, 20 mm Tris-HCl (pH 8.0), 1% NP40, 10% glycerol, 1 mm phenylmethylsulfonylfluoride, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.5 mm sodium vanadate. The samples were sonicated with three pulses for 15 sec at intensity 4 with 5-sec intervals in a Misonix Sonicator Ultrasonic processor XL (Misonix, Inc., Farmingdale, NY). After sonication, samples were centrifuged at 16,000 rpm for 30 min at 4 C in a MIKRO 22R Hettich Zentrifuge. Aliquots of 100 μl of the supernatants were isolated and mixed with 40 μl 137 mm NaCl, 2.68 mm KCl, 1.47 mm KH2PO4, 8.1 mm Na2HPO4 (pH 7.35), 0.9 mm CaCl2, and 0.5 mm MgCl2. These were then treated with 20 μl HCl 1 n for 15 min and neutralized with 20 μl 1 n NaOH. The samples were kept at −80 C. The content of BDNF in the extracts was analyzed by the BDNF Emax Immunoassay System (Promega Corp., Madison, WI) as described (29). Briefly, a 96-well plate was covered with anti-BDNF monoclonal antibody. To allow the antibody to stick to the well, 100 μl 1:1000 diluted antibodies was added to the well and left overnight at 4 C. The plate was then washed with 150 mm NaCl, 0.05% Tween 20, and 20 mm Tris-HCl (pH 7.6), and was blocked with 200 μl Promega 1× blocking solution and sample buffer for 1 h. Plates were then washed with the TBST buffer, and 100 μl sample or BDNF standard was added in triplicate to each well and incubated for 2 h at 4 C. The plate was then washed five times using TBST buffer, and 100 μl antihuman BDNF polyclonal antibody diluted to 1:500 was added to each well of the plate and incubated for 2 h. The plate was again washed with TBST buffer and incubated with 100 μl 1:200 dilution of anti-IgY horseradish peroxidase conjugate for 1 h at room temperature. The plate was washed with TBST buffer and developed with 100 μl Promega 3,3′, 5,5′-tetramethylbenzidine solution. This reaction was stopped with 100 μl 1 n HCl. The absorbance was measured at 450 nm. The BDNF concentration was expressed as pg of BDNF/mg of protein.
Immunofluorescence
Telencephalons obtained from decapitated rats were covered with OCT, then frozen with cold isopentane (Merck & Co., Inc., Whitehouse Station, NJ) and immediately treated with liquid nitrogen for 2 min. Using a cryostat (CM 1510; Leica Microsystems GmbH, Wetzlar, Germany), 30-μm coronal sections were obtained from these telencephalons. The sections were fixed and permeabilized in 70% ethanol for 20 min at −20 C. Sections were incubated in blocking solution (5 mm EDTA, 1% fish gelatin, 2% horse serum, and 1% essentially immunoglobulin-free BSA) for 30 min at room temperature. Sections were then incubated in primary antibody overnight at 4 C at the following concentrations: anti-NR1 (rabbit polyclonal, 1:50; Sigma-Aldrich, St. Louis, MO); and anti-PSD-95 (goat polyclonal, 1:300; Santa Cruz Biotechnology). Fixed sections were washed four times with PBS, incubated with fluorescein isothiocyanate (FITC)-conjugated goat antirabbit IgG and Cy3-conjugated sheep antigoat IgG (1:300) for 1 h at room temperature, followed by another wash in PBS for 1 h. Coverslips were then mounted using Prolong Gold with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Inc., Eugene OR) and examined by confocal microscopy using a Leica microscope. Specific immunoreactivity was confirmed by replacing the primary antibody with a nonimmune polyclonal reagent (30). To analyze colocalization of both antigens by confocal microscopy, serial Z sections from the sections were obtained (0.8 μm each section), and all sections were integrated using ImageJ (Institutes of Health, Bethesda, MD), Voxx (Voxx Technologies, Inc., San Clemente, CA), and Adobe Photoshop (Adobe Systems, Inc., San Jose, CA) programs.
Statistical analyses
Unpaired two-tailed t tests were performed on the experimental data shown here. The SigmaPlot 9 plus SigmaStat 3.5 program was used for the analyses. The results were considered significantly different when P < 0.05.
Results
MMI treatment causes hypothyroxinemia in pregnant rats
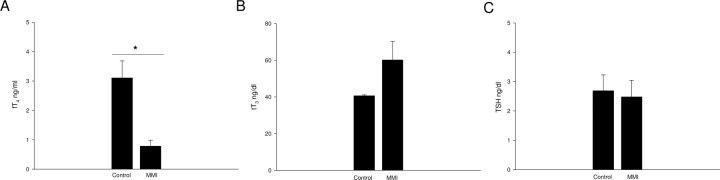
Pregnant rats were treated with MMI during E12–E15 to induce transient and mild hypothyroxinemia, as described previously (9, 10) and detailed in Materials and Methods. Hypothyroxinemia was confirmed by determining plasma levels of fT4, tT3, and TSH at the end of the MMI treatment (E15). The MMI treatment showed a significant reduction in fT4 compared with control rats (MMI 0.788 ± 0.201 ng/ml; control 3.107 ± 0.578 ng/ml; sem n = 6; P < 0.05; Fig. 1A). No significant differences were observed for tT3 (MMI 60 ± 10.062 ng/dl; control 40.7 ± 0.5 ng/dl; sem n = 6; Fig. 1B) or TSH (MMI 2.48 ± 0.5378 ng/dl n = 3; control 2.8 ± 0.5672 ng/dl; sem n = 4; Fig. 1C). The reduction observed only for the fT4 plasma level indicated that pregnant rats treated with MMI developed hypothyroxinemia.
Fig. 1.
MMI treatment induced hypothyroxinemia in pregnant rats. Plasmatic levels of fT4, tT3, and TSH in pregnant rats treated with MMI or not (Co). Rats at E12 were treated with MMI for 4 d, and on the last day of treatment (E15), a blood sample was taken from these animals and the control group to measure the TSH and thyroid hormone levels (see Materials and Methods). A, Mean values for fT4 levels (ng/dl) ± sem of MMI and control rats (n = 6) were plotted. B, Mean values for tT3 levels (ng/dl) ± sem of MMI and control rats (n = 6) were plotted. C, Mean values for TSH levels ± sem of MMI (n = 3) and control rats (n = 4) were plotted. Significant differences were observed only at the levels of fT4 among control and MMI rats (P < 0.05).
Maternal hypothyroxinemia leads to spatial learning impairment in adult offspring
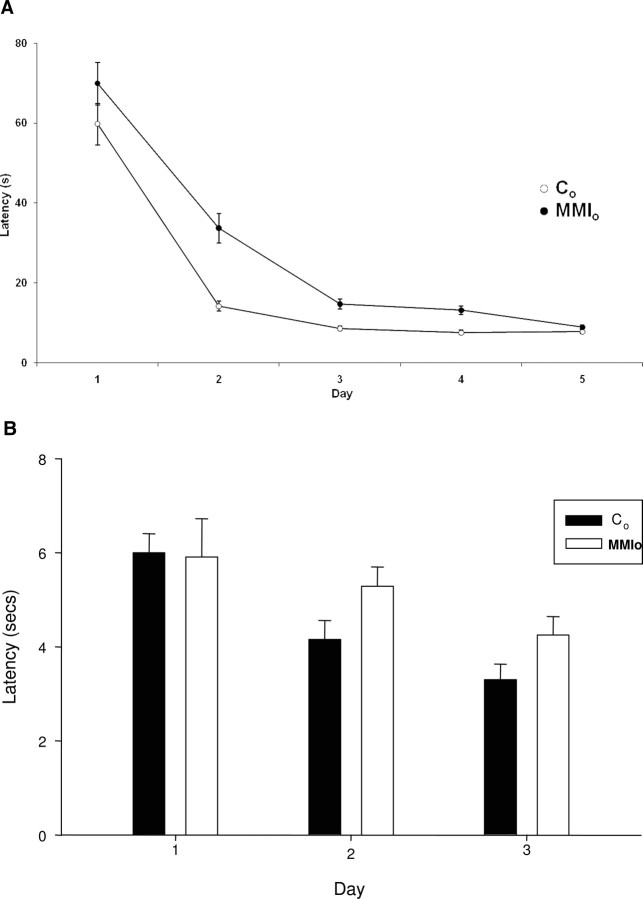
To evaluate whether maternal hypothyroxinemia could cause cognitive and behavioral alterations in the offspring, a water maze test was performed on its progeny (MMIo) or control dams. Controls consisted of male progeny from dams that were not exposed to MMI treatment, and that had normal levels of thyroid hormones and TSH (Fig. 1). Significant differences were observed between the MMIo and control animals in the water maze test (Fig. 2). This test was performed during 5 consecutive days with five trials per day. In each trial the rat was left in the water, and the time (latency) needed for the rat to reach the hidden platform was recorded. On the first day, MMIo and control rats had latencies between 60 and 70 sec, indicating that none of the groups had swimming impediments (Fig. 2). Importantly, from d 2–4, significantly higher latencies were observed for the MMIo group, compared with controls (Fig. 2A). However, on d 5, latencies of MMIo and control animals showed no significant differences, suggesting that MMIo rats needed more time and/or experience to learn the spatial cues required to find the hidden platform than the controls (Fig. 2A). It seems unlikely that the learning delay shown by MMIo rats could be due to visual perception defects because MMIo and control rats showed equivalent latencies during a 3-d water maze test with a visible platform (cued task training) (Fig. 2B). Therefore, because MMIo rats had no apparent visual perception defects, our data suggest that maternal hypothyroxinemia during gestation may have impaired the inner capacity of the offspring to learn the location of the hidden platform using spatial orientation.
Fig. 2.
Mean escape latency in the water maze test. A, Rats gestated in hypothyroxinemic mothers (MMIo) and control (Co) rats were subjected for 5 d to a water maze test. Each of the rats performed five trials. Each trial consisted of positioning the rat in the pool and recording the time that the rat required to reach the hidden platform (see Materials and Methods). The day of each trial is indicated on the x-axis, and the mean ± se escape latency for every group is indicated on the y-axis. The mean escape latency is the average of five trials performed in that day for all rats of the same experimental group. Empty circles (○) show the mean ± se escape latency for the Co (n = 17); black circles (•) show the mean ± se latencies for MMIo (n = 16). Statistical analysis (Mann-Whitney U test) shows significant differences on d 2, 3, and 4 with a P < 0.05. B, control (n = 3) and MMIo (n = 3) where subjected for 3 d consecutive to the water maze test with a visible platform. The day of each trial is indicated on the x-axis, and the mean ± se escape latency for every group is indicated on the y-axis. The mean escape latency is the average of five trials performed in 1 d for all rats of the same experimental group. Statistical analysis (unpaired t test) shows no significant differences between the control and MMIo groups.
Content of phosphorylated c-fos after the water maze test was unaffected in adult offspring gestated by hypothyroxinemic mothers
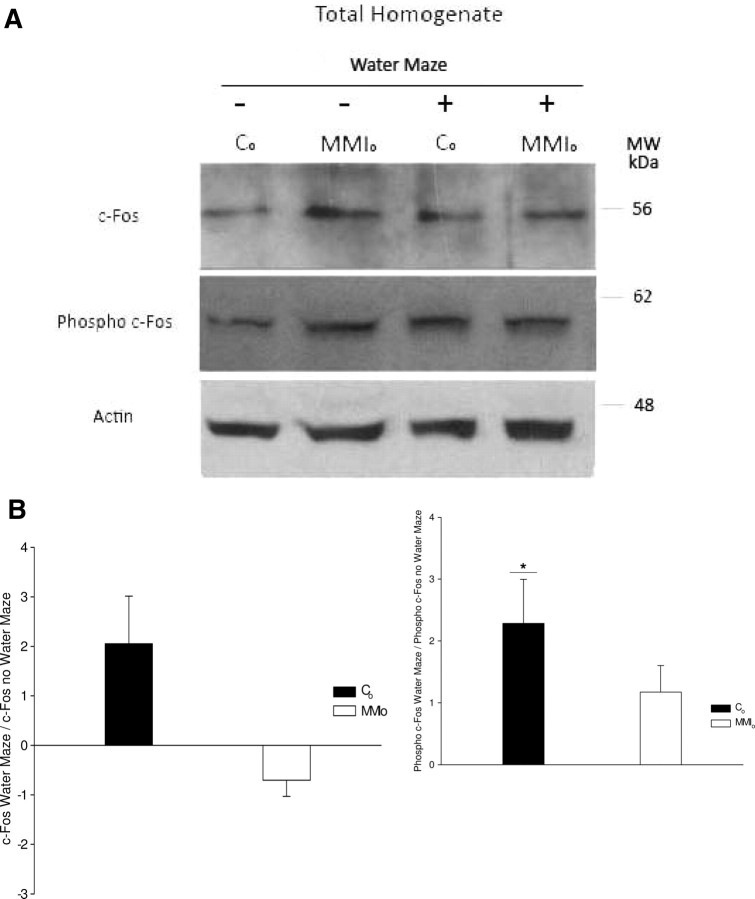
c-fos is an IEG required for spatial learning (11, 12, 13, 14), whose expression in rat brain increases during water maze test training (14). An important posttranscriptional regulatory mechanism for c-fos activity is phosphorylation, which is required to keep active c-fos in the nucleus and for the assembly of a stable activator protein-1 complex (AP-1) (31, 32, 33). Thus, we tested whether the telencephalon content of c-fos and phosphorylated c-fos could increase in the MMIo group compared with controls on d 2 of the water maze test (time when MMIo latencies were significantly longer than those of the controls). In addition, the content of c-fos and phosphorylated c-fos was also evaluated for MMIo and control rats not subjected to the water maze test.
Western blot analyses showed an increase in the relative content of phosphorylated c-fos in control rats on d 2 of the water maze test (normalized for actin content) (Fig. 3A). In striking contrast, MMIo rats showed no significant changes for content of either c-fos or phosphorylated c-fos after 2 d of water maze testing (Fig. 3, A and B). These data are consistent with the longer latencies shown by MMIo rats at d 2 of the water maze test.
Fig. 3.
Content of c-fos and phosphorylated c-fos in the telencephalon of rats gestated in hypothyroxinemic mothers. A, Representative pictures of Western blots for c-fos and phosphorylated c-fos (phospho c-fos) from total telencephalon homogenates. These samples corresponded to total telencephalon homogenate of the MMIo or control (Co) group subjected (+) or not (−) to a water maze test on the second day. To analyze that the same amount of protein was loaded in each well, a Western blot analysis of actin was performed with the same nitrocellulose used for c-fos and phosphorylated c-fos antibodies. B, Ratio of c-fos or phosphorylated c-fos (phosphor c-fos) of rats subjected to the water maze test over those that were not. The intensities of c-fos or phosphorylated c-fos bands from the Western blots were measured by densitometry. The ratios between the intensity of c-fos or phosphorylated c-fos bands of rats subjected to the water maze test over those that were not were plotted. The statistical analysis indicated that the Co increased the content of phosphorylated c-fos after the second day of the water maze test (n = 3; *, P < 0.05). MW, Molecular weight.
MMI treatment alters LTP in adult offspring
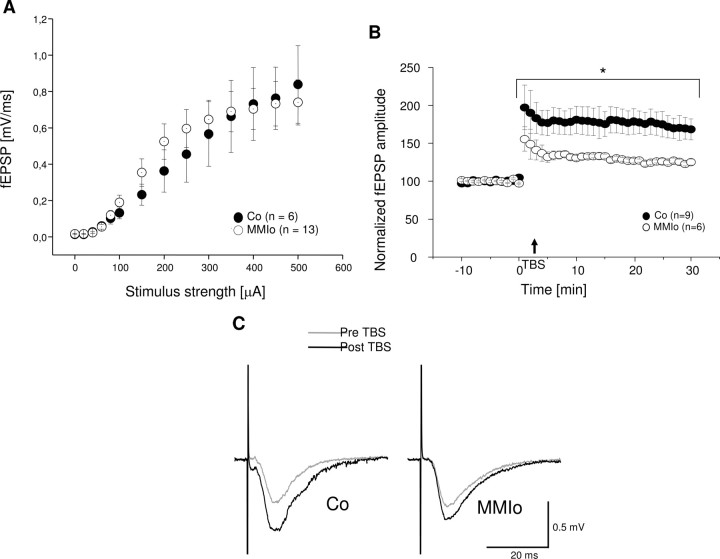
Because LTP at the hippocampus is required for cognitive processes, such as spatial learning (34, 35), we tested whether long latencies shown by MMIo rats during water maze tests could be due to LTP alterations caused by hypothyroxinemia. Furthermore, to establish if MMI treatment affects the synaptic efficacy of the Schaffer collateral → CA1 pathway, input to output ratios were measured in response to single electrical stimulation, comparing between control and MMIo. fEPSP responses elicited by stimulus strength intervals of 20 μA were measured at the CA1 stratum radiatum area. When stimulus strength reached 100 μA, increments were added on steps of 50 μA up to a 500 μA maximum. Three responses were collected and averaged for each stimulus increment. To construct input-output curves (Fig. 4A), data were averaged across all slices measured for each group (control and MMIo). These assays showed no significant differences between control and MMIo groups, suggesting that these two groups had similar synapse densities at the stratum radiatum of CA1 and displayed equivalent excitability in response to single electrical stimulation.
Fig. 4.
Effect of maternal hypothyroxinemia over Schaffer collateral → CA1 synaptic function of their progeny. A, Input-output (I/O) curves display the mean fEPSP amplitude + sem for control (Co) (•) and MMIo (○) rat hippocampal slices. At least three different animals were used for each experimental condition. B, Effect of maternal hypothyroxinemia over LTP induction of their progeny. Mean fEPSP amplitude + sem obtained from slices of control (•) and MMIo rats (○). *, P < 0.05; control (n = 9) and MMIo (n = 6). C, The traces correspond to fEPSP measured in the stratum radiatum of representative experiments for each condition (control and MMIo) before TBS (gray line) and 30 min after TBS (black line). ms, msec; Pre, before; Post, after.
To evaluate a possible effect of MMI treatment on the mechanisms of synaptic plasticity, we performed LTP experiments consisting of recordings of stimulus-evoked fEPSPs in the stratum radiatum of CA1 of hippocampal slices obtained from control and MMIo rats. A 10-min stable baseline transmission was obtained and tested every 15 sec. After baseline acquisition, TBS was applied as described in Materials and Methods. After stimulation, data were acquired once every 15 sec for at least 30 min. Data from several experiments were aligned relative to the time of TBS stimulation for control and MMIo animals (Fig. 4B). For control rats, 178 ± 16% (sem, n = 9) potentiation was achieved with respect to the baseline. In contrast, for MMIo rats, potentiation was significantly smaller (130 ± 3%, sem, n = 6). Representative traces of fEPSPs observed before and after LTP induction for control and MMIo animals are shown in Fig. 4C. These results indicate that MMI treatment during gestation significantly impairs the LTP response of the offspring.
Maternal hypothyroxinemia promotes an increase of PSD proteins
Considering the MMIo group had impaired LTP and that hippocampus glutamatergic synapses play an essential role during this process (36), their PSD protein composition was analyzed. The following PSD proteins fundamental for the transmission of glutamatergic synapses were studied: 1) the subunits of the NMDA receptor NR1 and NR2A/B; 2) PSD-95; 3) CaMKII; 4) TrkB and its ligand, BDNF; 5) TrkA; and 6) receptor P75.
The content of all these proteins was analyzed both in the PSD fraction and total telencephalon homogenates. Representative Western blots for each protein are shown in Fig. 5A. To evaluate the unspecific binding of the secondary antibodies, all membranes were incubated with only the second antibodies. No bands were detected, indicating that all the bands shown in Fig. 5A correspond to the first antibody reaction and not to nonspecific binding of the secondary antibody. Standardization of the loaded amount of protein per well was performed using actin as a loading control both for PSD and total telencephalon homogenate. The antiactin antibody recognized a 43-kDa band. No significant differences were found between the content of actin from the MMIo group compared with the control group, both in the PSD and total telencephalon homogenate, indicating that the same amount of total protein was loaded for all samples (Fig. 5A). For PSD-95 and TrkB, densitometry analyses showed a significant increase in the content of these proteins in both PSD and total telencephalon homogenate of MMIo relative to control group. For PSD-95 [band (23)], densitometry analyses showed a 40 ± 0.7% increase in total MMIo telencephalon homogenates, compared with control animals (P < 0.05; n = 3). An equivalent PSD-95 increase was also observed in the PSD samples of MMIo rats, relative to controls (P < 0.05; n = 3). TrkB [145-kDa band (24)] in the PSDs of the MMIo showed an increase of 10 ± 1.73% (P < 0.01) compared with control. TrkB in the total telencephalon homogenate showed an increase of 15 ± 5.29% (P < 0.05) in the MMI relative to control. The total levels of BDNF (TrkB ligand) from telencephalon were analyzed by ELISA (Fig. 5B). Although the control group had a larger BDNF content (108 ± 72 pg/mg protein) than the MMIo group (47 ± 15 pg/mg protein), this disparity was not statistically significant. For NR1 [120 kDa band (21)], densitometry analyses showed no significant differences between control and MMIo rats in the NR1 content at PSD (1 ± 4.62%, n = 3). However, a significant increase (3.5-fold) of NR1 content was observed in total telencephalon homogenates from MMIo rats (35 ± 10%; P < 0.05; n = 3) compared with control rats (Fig. 5A). For NR2A/B [180-kDa band (21)], CaMKII [33-kDa band (26)], TrkA [140-kDa band (25)], and p75 [75-kDa band (22)], densitometry analyses showed no significant differences either in the PSD or total telencephalon homogenates. We observed an increase in the content of p75 in the PSD and total telencephalon homogenate of the MMIo compared with controls (10 ± 29.9% and 10 ± 61.5%, respectively); however, these increases were not statistically significant.
Fig. 5.
A, Expression of PSD proteins in the total telencephalon homogenate and PSD fractions of rats gestated in hypothyroxinemic mothers. Total telencephalon homogenate and PSD were prepared from the MMIo or control (Co) group. About 20 μg PSD and 40 μg homogenate were loaded in a SDS-PAGE gel, separated by electrophoresis, were electrotransferred to nitrocellulose, and analyzed by Western blots. The figure shows representative Westerns blots performed for six different proteins of the PSD. Each Western blot was repeated three times, each time with three independent samples. Every sample used each time corresponding to a pool of 10 animals. A Western blot for actin was used as loaded Co. B, BDNF content in the telencephalon of rats gestated in hypothyroxinemic mothers. The telencephalon was isolated, homogenized, and subjected to BDNF determination (see Materials and Methods). BDNF was quantified by ELISA, and its concentration was normalized against the total amount of protein in the sample. BDNF from rats gestated in hypothyroxinemic mothers was termed “MMIo,” and BDNF from control rats was termed “control” The values represent the means ± se of six independent experiments. The Student’s t test (unpaired) yielded P > 0.05.
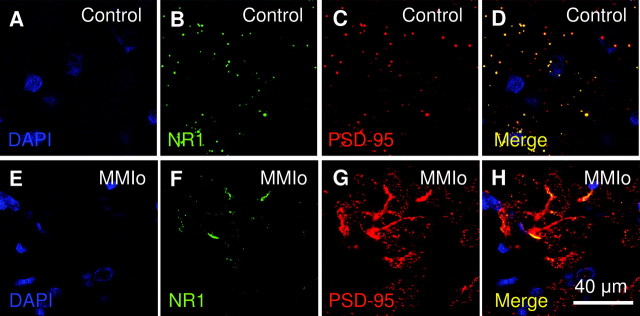
Offspring of hypothyroxinemic dams showed a different distribution of PSD-95 and NR1 in the CA1 hippocampus
PSD-95 and NR1 are two key players in PSDs because they contribute significantly to cognitive processes, such as synaptic plasticity and learning. Considering that Schaffer collateral-CA1 synapses showed an impaired LTP response, and that expression of PSD-95 and NR1 was elevated in total telencephalon homogenates, it was important to evaluate the localization of these two proteins in the hippocampus CA1 area. Thus, localization of NR1 and PSD-95 was analyzed by confocal microscopy in coronal brain sections of 30 μm obtained from control and MMIo rats (Fig. 6). NR1 and PSD-95 showed significant colocalization and a punctuated pattern in hippocampi of control rats. However, a different NR1 and PSD-95 distribution was observed in the hippocampuses of MMIo rats. The hippocampus of these animals showed a stronger PSD-95 staining than control rats. Furthermore, PSD-95 staining appeared more concentrated, forming large aggregated structures in the hippocampus. Although a punctuated pattern was also observed for PSD-95 in MMIo rats, this was very different from that in control rats. PSD-95 spots in MMIo hippocampi were clustered, and looked more diffused and less bright than in controls. In addition, the NR1 stain in MMIo rats was concentrated mainly in big aggregated structures with few bright spots, which was very different from the pattern observed in control animals. Finally, in MMIo rats, PSD-95 and NR1 colocalization was restricted to the aggregated structures detected in the hippocampus.
Fig. 6.
NR1 and PSD-95 localization in the hippocampus of rats gestated in hypothyroxinemic mothers. The NR1 and PSD-95 colocalization were analyzed in CA1 area of the hippocampus of MMIo or Co groups by confocal microscopy. A and E show DAPI staining (blue) for nucleus identification at the CA1 area. B and F, NR1 staining (FITC, green). C and G, PSD-95 staining (Cy3, red). D and H, The merger of the three fluorochromes DAPI, FITC, and Cy3, to analyze the colocalization of NR1 and PSD-95. Bar, 40 μm. A–D correspond to confocal microscopy pictures of the Co group. E–H correspond to confocal microscopy pictures of the MMIo group.
Discussion
It has been reported that maternal hypothyroxinemia in humans can cause irreversible cognitive damage to the progeny (1, 6, 7). To understand further the alterations that are responsible for these cognitive impairments, we analyzed several molecular and functional parameters in the neurons from the telencephalon and characterized the cognitive capacity of the offspring from rats with hypothyroxinemia (9). Two studies had previously reported that effects induced in the offspring of hypothyroxinemic dams can be reversed by the administration of T4 to the pregnant rats during the MMI treatment (9, 10). Here, mild maternal hypothyroxinemia was induced in pregnant rats by a 4-d treatment with MMI, which led to reduced plasmatic levels of fT4 without affecting significantly the plasmatic levels of tT3 and TSH. Neither the outcome of the pregnancy nor the survival and weight of the offspring was affected by the MMI treatment. All experimental procedures were performed on offspring at the age of P60 because at this age, rats are considered to be adults, and their brain synapses are mature and functional. Our data show for the first time that maternal hypothyroxinemia can cause in the offspring a significant reduction in the capacity of the brain for spatial learning. MMIo rats systematically showed impaired performance in water maze tests, as demonstrated by significantly extended latencies during testing on the second, third, and fourth days (Fig. 2). However, the observation that by d 5 MMIo rats reached the platform with the same latencies as the controls, suggests that MMIo rats need to experience the event more times for establishing or reinforcing the appropriate neuronal connections required for spatial learning. Considering that no significant differences were observed between MMIo and control rats in other behavior tests, such as the elevated plus maze test and the passive avoidance test, it seems likely that maternal hypothyroxinemia leads specifically to a deficiency in spatial learning, rather than other types of behavioral alterations.
To understand better the molecular alterations in the brain of MMIo rats that could lead to an impaired performance in water maze tests, the content of c-fos and phosphorylated c-fos was analyzed. c-fos is an IEG involved in spatial learning (11, 12, 13, 14). Although experiencing the water maze test led to a significant increase in the telencephalon content of phosphorylated c-fos in control rats, no significant increase was seen in MMIo rats exposed to the test (Fig. 3, A and B). Our data agree with findings suggesting a relationship between spatial learning and phosphorylated c-fos content in the telencephalon.
Given that hippocampus synapses play an important role during water maze training (37, 38, 39, 40) and are required for a proper spatial learning and memory formation (38, 39, 41), LTP responses were analyzed in hippocampuses of control and MMIo rats. Our data showed a significant decrease in LTP magnitudes for rats gestated from hypothyroxinemic mothers, compared with controls (Fig. 4B). Because input/output curves are consistent with an operative pathway for synaptic excitability of the Schaffer collateral (Fig. 4A), it seems unlikely that hypothyroxinemia impairs directly the LTP mechanism. Instead, it is possible that hypothyroxinemia could prevent MMIo rats from reaching an LTP level equivalent to that observed for control animals. These results suggest that the diminished LTP could cause low cognitive performance in rats gestated from hypothyroxinemic mothers.
We found significant differences in the content of some proteins that are important for LTP, and are located at the PSDs of glutamatergic synapses, such as PSD-95, NR1, and TrkB. In contrast, the content of actin, P75, TrkA, CAMKII, NR2A, and/or NR2B showed no significant differences between MMIo and control animals (Fig. 5A). A striking increase in the expression and aggregation of the scaffolding protein PSD-95 was observed in the MMIo group. We tested whether abnormal PSD-95 content could alter the NMDA receptor location and the content at the PSD. Although the content of NR1 and NR2A/B subunits at the PSDs were similar between MMIo and control animals, an accumulation of NR1 was observed in the hippocampus (Fig. 6). In addition, an excess of PSD-95 could alter the signal transduction pathway triggered by NMDA activation. This alteration in the signal transduction pathway could impair the increase of phosphorylated c-fos required for the assembly of a stable activator protein-1 complex and for the learning process. Finally, it is likely that overexpression of PSD-95 in the MMI group could alter the dendrite stability, as described for immature neurons (42). Dendrite stability and synapses are modulated both by PSD-95 and TrkB (42, 43, 44). The small but significant increase of TrkB at the PSD and total telencephalon homogenate supports the notion that synapse stability could be impaired in the offspring of hypothyroxinemic mothers.
In conclusion, we found an increase of PSD-95 and TrkB in the PSD of MMIo. Alterations in the protein composition of the PSD in the telencephalon could be responsible for reduced synaptic function. Because the PSD is responsible for neurotransmitter reception and for postsynaptic response, changes in PSD protein composition could affect the synaptic function of these neurons. Consequently, mechanisms like LTP could be deteriorated, affecting cognitive processes like spatial memory.
Here, we present new and novel evidence supporting the notion that maternal hypothyroxinemia affects the central nervous system of the offspring, which is in conceptual agreement with previous studies (6, 7, 8, 9, 10). We have shown that cognition in rats was affected in the same manner as in humans and that this damage persisted to adulthood. We reported detrimental changes in the LTP performance of this offspring and observed changes of protein composition in the PSD of these animals. These cellular and molecular findings underscore the reduced spatial learning abilities of the MMI offspring. Better knowledge of the molecular process that underlies the cognitive damage caused by maternal hypothyroxinemia can encourage clinicians to search early for this condition in pregnant women.
Understanding the molecular deficiencies underlying learning disabilities could contribute to ultimately design new therapy alternatives for this condition. Considering that commonly maternal hypothyroxinemia is caused by an insufficient iodine intake by pregnant or lactating women, it is feasible that alterations caused by this condition could be prevented by daily administration of KI supplement (200 μg iodine) during pregnancy. This harmless procedure might possibly contribute to the full potential neurodevelopment of the fetus and newborn (45).
Acknowledgments
We thank Drs. Robert J. Wenthold for anti-n-methyl-d-aspartate receptors 1 and 2 A/B subunit antibodies, and Francisca Bronfman for the anti-tyrosine receptor kinase A antibody. We also thank Drs. Nancy Carrasco and Diane O’Dwod, as well as Susannah Volpe for critically reading the manuscript.
Footnotes
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico Grants (FONDECYT) 1040349, 1070352, and ICM P04/030-F. P.A.G. is a fellow from Comisión Nacional de Investigación Científica y Tecnológica Chile.
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 19, 2008
Abbreviations: ACSF, Artificial cerebrospinal fluid; AP-1, activator protein-1 complex; BDNF, brain-derived neurotrophic factor; CaMKII, calmodulin kinase II; CA1, collateral-Schaffer in the area 1 of hippocampus Ammon’s horn; DAPI, 4′,6-diamidino-2-phenylindole; E, embryonic d; fEPSP, field excitatory postsynaptic potential; FITC, fluorescein isothiocyanate; fT4, free T4; IEG, immediate early gene; LTP, long-term potentiation; MMI, methimazole; MMIo, offspring from methimazole treated; NMDA, n-methyl-d-aspartate; NR, n-methyl-d-aspartate receptor; P, postnatal d; PSD, postsynaptic density; PSD-95, postsynaptic density protein 95; Syn1, synaptosome 1 fraction; Syn2, fraction of synaptosome 2; TBS, theta-burst stimulus; TBST, Tween 20 10%, BSA 5%, 150 mm NaCl, 10 mm Tris (pH 8.0); Trk, tyrosine receptor kinase; tT3, total T3.
References
- 1.Morreale de Escobar G, Obregon MJ, Escobar del Rey F 2000. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab 85:3975–3987 [DOI] [PubMed] [Google Scholar]
- 2.Glinoer D, Delange F 2000. The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid 10:871–887 [DOI] [PubMed] [Google Scholar]
- 3.Vermiglio F, Lo Presti VP, Moleti M, Sidoti M, Tortorella G, Scaffidi G, Castagna MG, Mattina F, Violi MA, Crisa A, Artemisia A, Trimarchi F 2004. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab 89:6054–6060 [DOI] [PubMed] [Google Scholar]
- 4.Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ 2006. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics 117:161–167 [DOI] [PubMed] [Google Scholar]
- 5.Kasatkina EP, Samsonova LN, Ivakhnenko VN, Ibragimova GV, Ryabykh AV, Naumenko LL, Evdokimova YA 2006. Gestational hypothyroxinemia and cognitive function in offspring. Neurosci Behav Physiol 36:619–624 [DOI] [PubMed] [Google Scholar]
- 6.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL 1999. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 50:149–155 [DOI] [PubMed] [Google Scholar]
- 7.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ 2003. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 59:282–288 [DOI] [PubMed] [Google Scholar]
- 8.Lavado-Autric R, Auso E, Garcia-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, Morreale de Escobar G 2003. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest 111:1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P 2004. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145:4037–4047 [DOI] [PubMed] [Google Scholar]
- 10.Cuevas E, Auso E, Telefont M, Morreale de Escobar G, Sotelo C, Berbel P 2005. Transient maternal hypothyroxinemia at onset of corticogenesis alters tangential migration of medial ganglionic eminence-derived neurons. Eur J Neurosci 22:541–551 [DOI] [PubMed] [Google Scholar]
- 11.Guzowski JF 2002. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus 12:86–104 [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, Layer LE, Kvello A, Reschke M, Spanagel R, Sprengel R, Wagner EF, Gass P 2003. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci 23:9116–9122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gass P, Fleischmann A, Hvalby O, Jensen V, Zacher C, Strekalova T, Kvello A, Wagner EF, Sprengel R 2004. Mice with a fra-1 knock-in into the c-fos locus show impaired spatial but regular contextual learning and normal LTP. Brain Res Mol Brain Res 130:16–22 [DOI] [PubMed] [Google Scholar]
- 14.Teather LA, Packard MG, Smith DE, Ellis-Behnke RG, Bazan NG 2005. Differential induction of c-Jun and Fos-like proteins in rat hippocampus and dorsal striatum after training in two water maze tasks. Neurobiol Learn Mem 84:75–84 [DOI] [PubMed] [Google Scholar]
- 15.Correa da Costa VM, Rosenthal D 1996. Effect of aging on thyroidal and pituitary T4–5′-deiodinase activity in female rats. Life Sci 59:1515–1520 [DOI] [PubMed] [Google Scholar]
- 16.Biebinger R, Arnold M, Langhans W, Hurrell RF, Zimmermann MB 2007. Vitamin A repletion in rats with concurrent vitamin A and iodine deficiency affects pituitary TSHβ gene expression and reduces thyroid hyperstimulation and thyroid size. J Nutr 137:573–577 [DOI] [PubMed] [Google Scholar]
- 17.Gorski JR, Rozman K 1987. Dose-response and time course of hypothyroxinemia and hypoinsulinemia and characterization of insulin hypersensitivity in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated rats. Toxicology 44:297–307 [DOI] [PubMed] [Google Scholar]
- 18.Morris R 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60 [DOI] [PubMed] [Google Scholar]
- 19.Carlin R, Grab D, Cohen R, Siekevitz P 1980. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol 86:831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC 1985. Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85 [DOI] [PubMed] [Google Scholar]
- 21.Blahos J, Wenthold RJ 1996. Relationship between N-methyl-D-aspartate receptor NR1 splice variants and NR2 subunits. J Biol Chem 271:15669–15674 [DOI] [PubMed] [Google Scholar]
- 22.Roux PP, Colicos MA, Barker PA, Kennedy TE 1999. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci 19:6887–6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH 1995. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269:1737–1740 [DOI] [PubMed] [Google Scholar]
- 24.Muller D, Djebbara-Hannas Z, Jourdain P, Vutskits L, Durbec P, Rougon G, Kiss JZ 2000. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule-deficient hippocampus. Proc Natl Acad Sci USA 97:4315–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray SS, Perez P, Lee R, Hempstead BL, Chao MV 2004. A novel p75 neurotrophin receptor-related protein, NRH2, regulates nerve growth factor binding to the TrkA receptor. J Neurosci 24:2742–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patton BL, Molloy SS, Kennedy MB 1993. Autophosphorylation of type II CaM kinase in hippocampal neurons: localization of phospho- and dephosphokinase with complementary phosphorylation site-specific antibodies. Mol Biol Cell 4:159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pezet S, Marchand F, D'Mello R, Grist J, Clark AK, Malcangio M, Dickenson AH, Williams RJ, McMahon SB 2008. Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions. J Neurosci 28:4261–4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg ME, Ziff EB 1984. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature 311:433–438 [DOI] [PubMed] [Google Scholar]
- 29.Szapacs ME, Mathews TA, Tessarollo L, Ernest Lyons W, Mamounas LA, Andrews AM 2004. Exploring the relationship between serotonin and brain-derived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J Neurosci Methods 140:81–92 [DOI] [PubMed] [Google Scholar]
- 30.Eugenin EA, Berman JW 2003. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods 29:351–361 [DOI] [PubMed] [Google Scholar]
- 31.Deng T, Karin M 1994. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature 371:171–175 [DOI] [PubMed] [Google Scholar]
- 32.Bannister AJ, Brown HJ, Sutherland JA, Kouzarides T 1994. Phosphorylation of the c-Fos and c-Jun HOB1 motif stimulates its activation capacity. Nucleic Acids Res 22:5173–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treisman R 1996. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol 8:205–215 [DOI] [PubMed] [Google Scholar]
- 34.Morris RG, Anderson E, Lynch GS, Baudry M 1986. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319:774–776 [DOI] [PubMed] [Google Scholar]
- 35.Lynch MA 2004. Long-term potentiation and memory. Physiol Rev 84:87–136 [DOI] [PubMed] [Google Scholar]
- 36.Collingridge GL, Kehl SJ, McLennan H 1983. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol 334:33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T 2002. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 113:607–615 [DOI] [PubMed] [Google Scholar]
- 38.Yaka R, Salomon S, Matzner H, Weinstock M 2007. Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behav Brain Res 179:126–132 [DOI] [PubMed] [Google Scholar]
- 39.Wozniak DF, Xiao M, Xu L, Yamada KA, Ornitz DM 2007. Impaired spatial learning and defective θ burst induced LTP in mice lacking fibroblast growth factor 14. Neurobiol Dis 26:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris RG, Garrud P, Rawlins JN, O'Keefe J 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683 [DOI] [PubMed] [Google Scholar]
- 41.Barco A, Bailey CH, Kandel ER 2006. Common molecular mechanisms in explicit and implicit memory. J Neurochem 97:1520–1533 [DOI] [PubMed] [Google Scholar]
- 42.Charych EI, Akum BF, Goldberg JS, Jornsten RJ, Rongo C, Zheng JQ, Firestein BL 2006. Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD-95. J Neurosci 26:10164–10176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehrlich I, Klein M, Rumpel S, Malinow R 2007. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci USA 104:4176–4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji Y, Pang PT, Feng L, Lu B 2005. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci 8:164–172 [DOI] [PubMed] [Google Scholar]
- 45.Berbel P, Obregon MJ, Bernal J, Escobar del Rey F, Morreale de Escobar G 2007. Iodine supplementation during pregnancy: a public health challenge. Trends Endocrinol Metab 18:338–343 [DOI] [PubMed] [Google Scholar]