Abstract
Background
Lung cancer is among the most common causes of cancer-related deaths worldwide, but its tumorigenic mechanisms are largely unknown. Long non-coding RNAs (LncRNAs) have been shown to have significant roles in multiple cancers. Herein, we aimed to elucidate the detailed effects of a newly-discovered LncRNA, termed PRAL, on cell proliferation in lung cancer.
Material/Methods
A total of 100 lung cancer patients were subjected to RT-PCR analysis to detect the expressions of PRAL. Western blot analysis was performed to examine P53 protein levels. PRAL plasmid and specific siRNA against P53 was transfected into lung cancer cell lines NCI-H929 and A549. Cell viability assay was conducted in the presence or absence of siP53.
Results
The transcript level of PRAL in human lung cancer was remarkably decreased in vivo compared with their adjacent non-cancerous counterparts, and the protein levels of P53 were accordingly suppressed. Moreover, the expression of PRAL was also decreased in all of the 5 lung cancer cell lines. Transfection of PRAL plasmid inhibited cell proliferation in NCI-H929 and A549 cells and promoted the transcription of P53; however, knockdown of P53 caused no notable effects on PRAL transcription, but it retarded the inhibitory effects mediated by PRAL.
Conclusions
The transcript level of PRAL was decreased in lung cancer in vivo and in vitro. Overexpression of PRAL inhibited cell proliferation by upregulating the expression of P53. Our results indicate that PRAL might be a tumor suppressor in lung cancer and thus provides novel clues for the diagnosis and treatment for lung cancer in clinical practice.
MeSH Keywords: Cell Proliferation; Genes, p53; Lung Neoplasms; RNA, Long Noncoding
Background
Lung cancer is among the most common causes of cancer-related deaths worldwide, accounting for 25%, exceeding deaths from breast cancer, prostate cancer, and colon cancer combined [1]. Although great efforts have been made to improve the diagnosis and treatment of lung cancer patients, more than 80% of cases with lung cancer are diagnosed with locally advanced or metastatic disease [2]. Thus, it is a high priority to find novel clues to diagnose lung cancer in an early stage.
Long non-coding RNAs (LncRNAs) are a class of RNAs with a length of more than 200 nucleotides and without the ability to encode proteins due to the lack of open reading frame (ORF) [3,4]. Accumulating evidence have been shown that LncRNAs play significant roles in tumorigenesis of lung cancer. For example, Liu et al. found LncRNA MALAT1 promoted cancer progression and inhibited cell apoptosis in human lung cancer cells [5]. Overexpression of LncRNA AGAP2-AS1 interacted with EZH2 and LSD1 and inhibited LATS2 and KLF2 expression in human non-small-cell lung cancer cell [6], indicating the pro-oncogenic potential of this new-discovered LncRNA.
P53 protein, which is a well-known tumor suppressor, plays essential roles in the process of tumorigenesis through multiple mechanisms, such as DNA damage repair, cell cycle regulation, and cell death control [7,8]. The pivotal effects of P53 have been further underscored by the high frequency of its somatic mutations in cancer patients, as high as 36.1% [9]. P53 regulation-association LncRNA (LncRNA PRAL) showed its essential role in human hepatocellular carcinoma (HCC) [10]. PRAL genomic alterations were related with poor prognosis of HCC patients and involved in P53-mediated HCC growth and apoptosis. However, the detailed mechanisms underlying the regulatory role of PRAL remain largely unknown.
In this study, we aimed to explore the role of PRAL in human lung cancer. To this end, we examined the expression of PRAL in vivo and in vitro by RT-PCR analysis. Cell viability assay was performed in lung cancer cell lines NCI-H929 and A549. Overexpression of PRAL inhibited cell proliferation in both cell lines. The relative expression of P53 was also detected with RT-PCR and Western blot analysis. Our data indicated that PRAL functioned as a tumor suppressor by interaction with P53 in human lung cancer, which might provide novel clues for the diagnosis and treatment of lung cancer in clinical practice.
Material and Methods
Human samples
This study was approved by the Ethics Committee of Xi’an Red Cross Hospital. A total of 100 patients diagnosed with lung cancer were admitted to this study and all patients showed their full intentions to participate in our study. Prior to the surgery, no chemotherapy or radiotherapy was received by any patient. The tumor tissues and their adjacent non-cancerous counterparts were frozen in liquid nitrogen once dissected from patients and subjected to subsequent analysis.
Cell culture and transfection
Normal lung epithelial cell MRC-5 and 5 lung cancer cell lines (H-125, A549, 95D, NCI-H929, and H1975) were all purchased from the American Type Culture Collection (ATCC, Massachusetts, USA). Cells were cultured with recommended media supplied with 10% fetal bovine serum (FBS, Gibco, NY, USA) at 37°C in a humidified 5% CO2 incubator. Cell transfection was performed with lipofectamine 2000 (Invitrogen, NY, USA) according to the manufactures’ instructions.
Plasmids and siRNAs
PRAL-expressing plasmid was treated with pcDNA3.0 vector obtained from GenePharma Co. (Shanghai, China). Specific siRNA against P53 (5′-CUACUUCCUGAAAACAACGdTdT-3′) and control siRNA ((5′-UUCUCCGAACGUGUCACGUTT-3′) were synthesized by Shengong (Shanghai, China). Both plasmid and siRNAs were dissolved into distilled water at a concentration of 20 μM as a stocking solution.
RNA isolation and real-time polymerase chain reaction
Total RNAs from clinical samples and cultured cells were extracted by TRIzol reagent (TaKaRa, Dalian, China) with a dose of 1 ml/well for 6-well plates and quantified by Nanodrop 2000 by measuring the absorbance of 260 nm and 280 nm. cDNAs were reversely transcribed by use of a synthesis kit (TaKaRa). RT-PCR was performed with the SYBR Premiers Ex Taq Kit (TaKaRa) in an ABI PRISM 7900 real-time system (ABI Co., NY, USA). The procedure is briefly described below: denaturation (95°C for 2 min), annealing (40 repetition of 95°C for 30 s and 60°C for 60 s) and extension (72°C for 10 min). The primers used were synthesized by Shengong Co. (Shanghai, China). PRAL: forward: 5′-GGCAGAGTCTCGCTTGGT-3 and reverse: 5′-GAAACTCC GTCTCCGCTAA-3′. P53: forward: 5′-TTGAGGTGCGTGTTTGTG-3′ and reverse: 5′-CTGGGCATCCTTGAGTTC-3′. GAPDH: forward: 5′-CGGATTTGGTCGTATTGGG-3′ and reverse: 5′-CTGGAAGATGG TGATGGGATT-3′. GAPDH was included as an internal control.
Western blot analysis
Western blotting analysis was performed as per the manufacturer’s protocols. Total proteins from human samples were extracted by lysis buffer (Beyotime, Nanjing, China). Protein quality and quantity were determined with the Bradford dye-binding protein kit (Bio-Rad Laboratories, CA, USA). Afterward, a total of 40 μg protein was loaded onto a 10% SDS polyacrylamide gel and transferred to a PVDF membrane. The primary antibodies against P53 were purchased from Cell Signal Technology (Danvers, MA, USA). Primary antibody against GAPDH and secondary antibodies were obtained from Santa Cruz Biotech (Santa Cruz, CA, USA). Immunoreactivity of proteins of each sample was determined by enhanced chemoluminescent autoradiography (Thermo Scientific) using a FluorChem Q system (Proteinsimple, Santa Clara, CA, USA). Blots were quantified using Quantity One (Bio-Rad Laboratories, CA, USA).
Cell viability assays
Cell viability was assessed by cell counts and Bromodeoxyuridine (BrdU) incorporation (Millipore, USA). Briefly, NCI-H929 and A549 cells were transfected with PRAL-expressing plasmid with or without siP53 and allowed to grow for 48 h. Then, cells were replaced with serum-free media for 24 h, trypsinized and collected by low-speed centrifugation (1000 rpm). Equal numbers (5000 cells) of NCI-H929 or A549 cells from each group were seeded into 96-well plates and incubated in culture medium (10% FBS) supplemented with 10 μM BrdU. BrdU incorporation was detected with additional peroxidase substrates. Spectrophotometric detection was performed at the wave length of 450 nm with a microplate reader (TECAN, NY, USA).
Statistically analysis
All data are presented as means ± standard derivation (SD) unless otherwise stated. The Student’s t-test was included between variables. All statistical analyses were assessed with SPSS PASW Statistics 18.0 software (Chicago, IL, USA) and values of p<0.05 were considered as statistically significant.
Results
The transcript level of PRAL was decreased in human lung cancer and associated with cancer progression
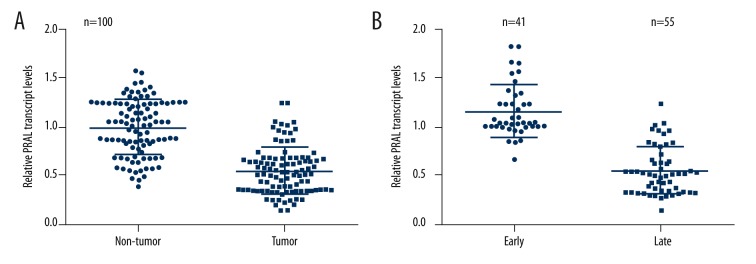
First, we examined the relative expressions of PRAL in clinical lung cancer patients. A total of 100 patients diagnosed with lung cancer were admitted into the study, and their tumor tissues and adjacent non-cancerous tissues were subjected to RNA extraction and RT-PCR analysis. As shown in Figure 1A, the relative transcript level of PRAL was significantly decreased, of which 92% of lung cancer cases showed lower expressions compared with the average PRAL level in their adjacent non-cancerous counterparts. Afterwards, the patients were classified into 2 groups after excluding 4 cases without essential information: early stage (n=41) and late stage (n=55), the latter of which are those patients diagnosed with metastatic tumors. RT-PCR analysis revealed that the relative transcript level of PRAL was further suppressed in the tissues from advanced lung cancer patients compared with those in early stage (Figure 1B).
Figure 1.
The transcript level of PRAL was decreased in human lung cancer and was associated with cancer progression. (A) Tumor tissues and their adjacent non-cancerous tissues from 100 lung cancer patients were subjected to RT-PCR analysis to examine the transcript level of PRAL. (B) Ninety-six of the 100 cancer patients were classified into 2 groups: early stage (n=41, without tumor metastasis) and late stage (n=55, with tumor metastasis) and subjected to RT-PCR analysis. The other 4 cases were excluded due to missing information.
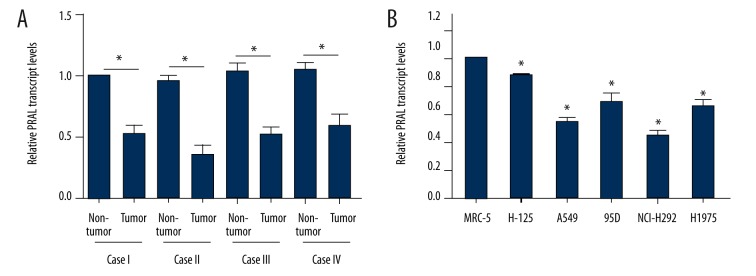
Next, 4 cases from advanced lung cancer patients were randomly selected and RT-PCR assays showed that the transcript level of PRAL was lower in each case compared to their counterparts (Figure 2A). MRC-5 cells are derived from human healthy lung epithelial tissues and used here as a control. Figure 2B shows that all of the 5 lung cancer cell lines exhibited lower PRAL transcript levels compared with that in MRC-5 cells, of which the expression of PRAL in A549 and NCI-H929 cells was the lowest. Of note, these 2 cell lines originated from advanced lung cancer patients. Thus, A549 and NCI-H929 cells were selected for subsequent analysis for the detailed roles of PRAL in human lung cancer. These data suggest that the expression of PRAL is remarkably decreased in human lung cancer and is associated with cancer progression.
Figure 2.
The transcript level of PRAL was suppressed in vivo and in vitro. (A) Four cases from late-stage patients were randomly selected and subjected to RT-PCR analysis. In each patient, the relative transcript level of PRAL in tumor tissues was significantly lower than in their adjacent non-tumor counterparts. * P<0.05, as indicated. (B) Normal lung epithelial cell line MRC-5 and 5 lung cancer cell lines (H-125, A549, 95D, NCI-H929, and H1975) were included to assess the expression of PRAL. * P<0.05, vs. MRC-5.
Overexpression of PRAL inhibited cell proliferation in lung cancer cell lines
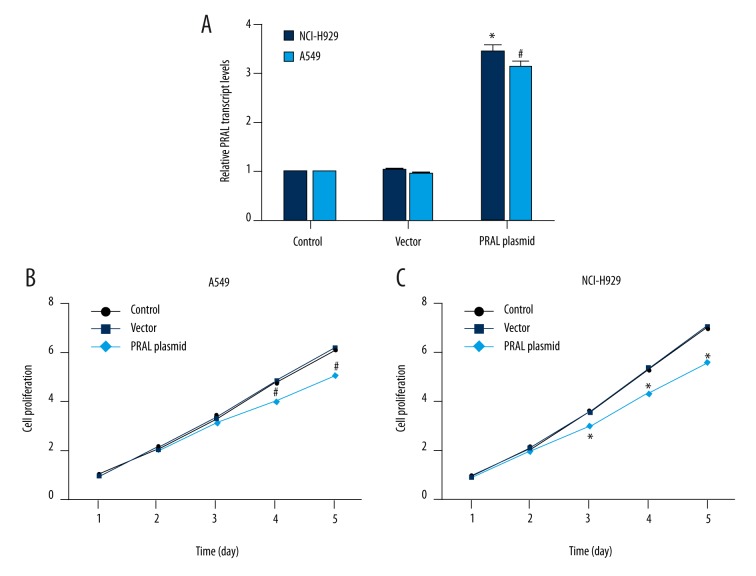
We aimed to elucidate the role of PRAL in lung cancer; to this end, PRAL-expressing plasmid was constructed and transfected into A549 and NCI-H929 cells. The relative transcript level of PRAL was upregulated by 3.3-fold in NCI-H929 cells and by 3-fold in A549 cells upon transfection of PRAL plasmid compared to the control cells, indicating the high efficiency of the expressing plasmid and transfection process (Figure 3A). Afterwards, cell proliferation assay showed that in the previous 2 days, no notable difference was observed in the 3 experimental groups. However, the cell proliferative rate was remarkably decreased by 17.3% on the third day, 18.3% on the fourth day, and 20% on the fifth day in NCI-H929 cells (Figure 3B). Likewise, cell proliferation of A549 cells was also retarded on the fourth and fifth day by approximately 15% upon overexpression of PRAL compared with the control A549 cells (Figure 3C). These results revealed that overexpression of PRAL inhibited cell proliferation in lung cancer cell lines NCI-H929 and A549.
Figure 3.
Overexpression of PRAL inhibited cell proliferation in lung cancer cell lines. (A) PRAL-expressing plasmid cloned into pcDNA3.0 backbone vector was transfected into NCI-H929 and A549 cells and cell lysate was used for RT-PCR analysis. * P<0.05, vs. Control in NCI-H929 cells. # P<0.05, vs. Control in A549 cells. (B) Cell proliferative rate was explored upon overexpression of PRAL in NCI-H929 cells during 5 consecutive days. The cell proliferative rate in the control group on the first day was set as 1. * P<0.05, vs. Control in NCI-H929 cells. (C) Cell proliferative rate was explored upon overexpression of PRAL in A549 cells during 5 consecutive days. The cell proliferative rate in the control group on the first day was set as 1. # P<0.05, vs. Control in A549 cells.
The expression of P53 was accordingly decreased by PRAL overexpression in lung cancer patients
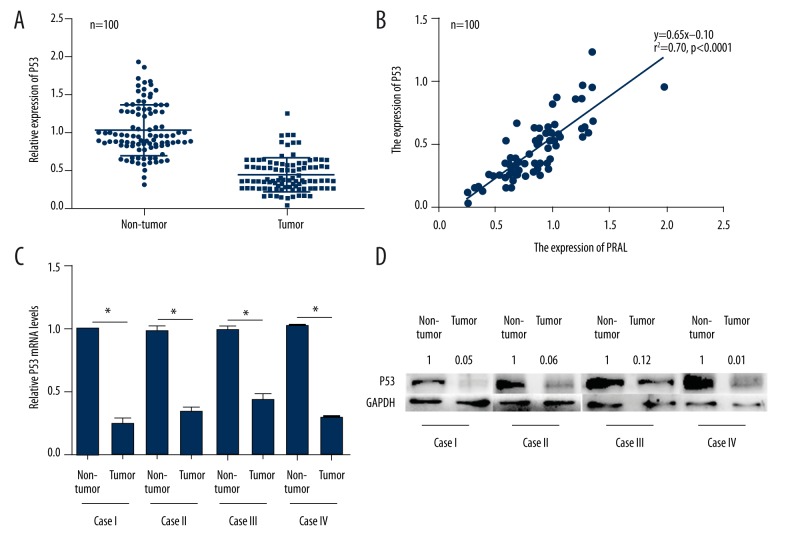
The potential association between PRAL and P53 has been previously shown in human hepatocellular carcinoma [10], so we next explored the detailed mechanism of PRAL concerning the inhibitory effects on lung cancer cell proliferation. First, we examined the relative expressions of P53 in 100 lung cancer patients. As shown in Figure 4A, the P53 expression was significantly decreased in tumor tissues compared with their non-cancerous counterparts, and it was positively related with that of PRAL in these lung cancer patients (r2=070, P<0.0001, Figure 4B). The 4 cases mentioned above (Figure 2A) were also used to explore the expression of P53. Figure 4C shows that the relative mRNA levels of P53 in tumor tissues were significantly lower compared with their adjacent non-cancerous counterparts, the tendency of which was consistent with those of PRAL transcript levels. Afterwards, Western blot analysis showed that the protein levels of P53 in the 4 advanced lung cancer patients were also suppressed, consistent with transcriptional levels (Figure 4D). We also examined the expression of P53 in NCI-H929 and A549 cells, but no detectable P53 protein was observed with Western blot assays (data not shown). These results suggested that the expression of P53 was associated with that of PRAL in lung cancer cells.
Figure 4.
The expression of P53 was accordingly decreased with PRAL in lung cancer patients. (A) The relative expression of P53 was examined in 100 clinical lung cancer patients by qRT-PCR. (B) The expression of P53 was positively correlated with that of PRAL in the 100 lung cancer patients. r2=070, P<0.0001. (C) RNAs from 4 randomly selected lung cancer patients in late stage were extracted and subjected to RT-PCR analysis to explore the expression of P53 in lung cancer. * P<0.05, as indicated. (D) Total proteins from the 4 lung cancer patients were extracted and subjected to Western blot analysis to assess the protein level of P53 in lung cancer patients. The numbers nearby the blots of P53 were quantified values for each lane, with the value in each non-tumor group set as 1.
Knockdown of P53 in NCI-H929 and A549 cells relieved the inhibitory effects of PRAL on cell proliferation
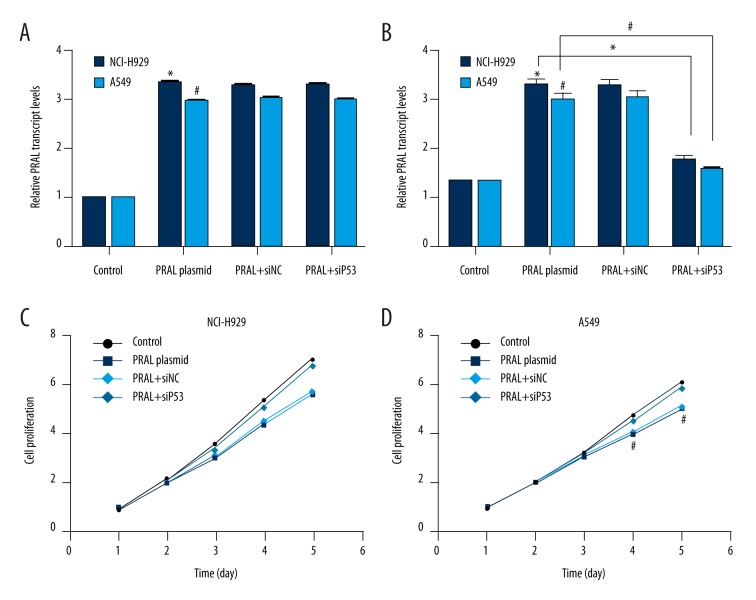
We examined the detailed role of P53 in the inhibitory effects of PRAL on cell proliferation with specific siRNA against P53. Transfection of PRAL-expressing plasmid upregulated the transcript level of PRAL in both cell lines, and transfection of siP53 caused no notable changes in PRAL transcript levels (Figure 5A). However, P53 mRNA levels were significantly increased upon overexpression of PRAL in NCI-H929 and A549 cells, indicating that LncRNA PRAL promoted the expression of P53 in lung cancer (Figure 5B). Moreover, transfection of siP53 significantly blunted the upregulation of P53 by PRAL overexpression, while control siRNA caused no changes in P53 transcription in either cell line (Figure 5B).
Figure 5.
Knockdown of P53 in NCI-H929 and A549 cells relieved the inhibitory effects of PRAL on cell proliferation. (A) Both NCI-H929 and A549 cells were transfected with PRAL-expressing plasmid with or without siNC/siP53 and the relative transcript level of PRAL was explored in these experimental cells.* P<0.05, vs. Control in NCI-H929 cells. # P<0.05, vs. Control in A549 cells. (B) Both NCI-H929 and A549 cells were transfected with PRAL-expressing plasmid with or without siNC/siP53 and the mRNA levels of P53 were explored in these experimental cells. * P<0.05, vs. Control in NCI-H929 cells. # P<0.05, vs. Control in A549 cells. (C) NCI-H929 cells were transfected with PRAL-expressing plasmid with or without siNC/siP53 and the proliferative rate was explored in these experimental cells. * P<0.05, vs. Control. (D) A549 cells were transfected with PRAL-expressing plasmid with or without siNC/siP53 and the proliferative rate was explored in these experimental cells. # P<0.05, vs. Control.
Subsequently, cell proliferation assay revealed that transfection of PRAL-expressing plasmid suppressed cell proliferative rate, which was remarkably blunted by knockdown of P53 in NCI-H929 cells (Figure 5C). Similarly, in A549 cells, the proliferative rate was slightly and insignificantly lower in the PRAL+siP53 group than that in the control group, and that in the PRAL plasmid group was almost the same as in the PRAL+siNC group. However, the proliferative rate was significantly inhibited after knockdown of P53 in PRAL-overexpressed A549 cells (Figure 5D). All of these data suggest that PRAL promotes the expression of P53 in lung cancer cells. Knockdown of P53 relieves the inhibitory effects of PRAL on cell proliferation, which means that P53 is essential in this process.
Discussion
Lung cancer is characterized by uncontrolled cell growth in the tissues of the lungs [11,12], the most common symptoms of which are coughing, weight loss, shortness of breath, and chest pains [13,14]. Researchers have focused on finding novel molecular biomarkers for the early diagnosis of lung cancer in the past decades; however, until now, no reliable therapeutic targets are clinically used. Therefore, this area requires additional research.
LncRNAs have been shown to have regulatory roles in gene transcription, gene translation, posttranscriptional modification, and tumorigenesis, working as either oncogenic gene or tumor suppressors in various cancers. Ma et al. showed LncRNA ATB promoted glioma malignancy by negatively regulating miR-200a, indicating the oncogenic potential of this LncRNA [15]. Zhu et al. demonstrated that LncRNA LOC572558 inhibited cell proliferation and tumor growth in human bladder cancer through regulation of the AKT-MDM2-P53 signaling axis [16]; thus, this LncRNA works as a tumor suppressor.
In our study, LncRNA PRAL also worked as a tumor suppressor in human lung cancer. PRAL locates on chromosome 17p13.1 and is often deleted in HCC patients. Our results also showed that the transcript level of PRAL was decreased in lung cancer in vivo and in vitro. Overexpression of PRAL inhibited cell proliferation in lung cancer cell lines NCI-H929 and A549 (Figure 3). However, these inhibitory effects of PRAL depended on the expression of P53. Overexpression of PRAL upregulated the mRNA level of P53 in NCI-H929 and A549 cells, whereas knockdown of P53 caused no effects on the transcript level of PRAL. Of note, depletion of P53 blunted PRAL-mediated inhibitory effects on cell proliferation (Figure 5).
The tumor-suppressor protein P53 is a widely studied transcription factor that induces cell growth arrest or apoptosis in response to multiple stress signals. Downregulation or inactivation of P53 is the most common event observed in human cancers [17,18]. Our data also revealed that P53 was downregulated in human lung cancer and was associated with the PRAL-mediated inhibitory effects on cell proliferation. Other molecules might also be involved in the process, such as HSP90, and the potential interaction of HSP90 and P53 was explored previously [19]. P53 was demonstrated to utilize a HSP90-denpendent movement system to translocate to the nucleus [20]. Thus, our next step is to identify the specific mechanism involved in PRAL, P53, and HSP90.
Conclusions
Our study showed that PRAL might work as a tumor suppressor in human lung cancer and provided novel clues for the diagnosis and treatment of lung cancer patients in clinical practice.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Stiles BM, Pua B, Altorki NK. Screening for Lung Cancer. Surg Oncol Clin N Am. 2016;25(3):469–79. doi: 10.1016/j.soc.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Stiles BM, Altorki NK. Screening for lung cancer: Challenges for the thoracic surgeon. Surg Oncol Clin N Am. 2011;20(4):619–35. doi: 10.1016/j.soc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Huang X, Luo YL, Mao YS, Ji JL. The link between long noncoding RNAs and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016 doi: 10.1016/j.pnpbp.2016.06.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Xu Q, Deng F, Qin Y, et al. Long non-coding RNA regulation of epithelial-mesenchymal transition in cancer metastasis. Cell Death Dis. 2016;7(6):e2254. doi: 10.1038/cddis.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Jiang X, Li W, et al. Inhibition of the long non-coding RNA MALAT1 suppresses tumorigenicity and induces apoptosis in the human ovarian cancer SKOV3 cell line. Oncol Lett. 2016;11(6):3686–92. doi: 10.3892/ol.2016.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Sun M, Zang C, et al. Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells. Cell Death Dis. 2016;7:e2225. doi: 10.1038/cddis.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sengupta S, Harris CC. p53: Traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol. 2005;6(1):44–55. doi: 10.1038/nrm1546. [DOI] [PubMed] [Google Scholar]
- 8.Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science. 2013;339(6127):1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science. 2015;349(6255):1483–89. doi: 10.1126/science.aab4082. [DOI] [PubMed] [Google Scholar]
- 10.Zhou CC, Yang F, Yuan SX, et al. Systemic genome screening identifies the outcome associated focal loss of long noncoding RNA PRAL in hepatocellular carcinoma. Hepatology. 2016;63(3):850–63. doi: 10.1002/hep.28393. [DOI] [PubMed] [Google Scholar]
- 11.Romaszko A, Świetlik E, Doboszyńska A, et al. Lung cancer and multiple neoplasms: A retrospective analysis. Adv Exp Med Biol. 2016;911:53–58. doi: 10.1007/5584_2016_224. [DOI] [PubMed] [Google Scholar]
- 12.Wey SL, Chang KM. Tumor-to-tumor metastasis: Lung carcinoma metastasizing to thyroid neoplasms. Case Rep Pathol. 2015;2015:153932. doi: 10.1155/2015/153932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.William J, Variakojis D, Yeldandi A, Raparia K. Lymphoproliferative neoplasms of the lung: A review. Arch Pathol Lab Med. 2013;137(3):382–91. doi: 10.5858/arpa.2012-0202-RA. [DOI] [PubMed] [Google Scholar]
- 14.Current world literature. Neoplasms of the lung. Curr Opin Pulm Med. 2013;19(4):399–401. doi: 10.1097/MCP.0b013e3283628b1f. [DOI] [PubMed] [Google Scholar]
- 15.Ma CC, Xiong Z, Zhu GN, et al. Long non-coding RNA ATB promotes glioma malignancy by negatively regulating miR-200a. J Exp Clin Cancer Res. 2016;35(1):90. doi: 10.1186/s13046-016-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Dai B, Zhang H, et al. Long non-coding RNA LOC572558 inhibits bladder cancer cell proliferation and tumor growth by regulating the AKT-MDM2-p53 signaling axis. Cancer Lett. 2016;380(2):369–74. doi: 10.1016/j.canlet.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15(1):2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 18.Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13(2):83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagn F, Lagleder S, Retzlaff M, et al. Structural analysis of the interaction between Hsp90 and the tumor suppressor protein p53. Nat Struct Mol Biol. 2011;18(10):1086–93. doi: 10.1038/nsmb.2114. [DOI] [PubMed] [Google Scholar]
- 20.Galigniana MD, Harrell JM, O’Hagen HM, et al. Hsp90-binding immunophilins link p53 to dynein during p53 transport to the nucleus. J Biol Chem. 2004;279(21):22483–89. doi: 10.1074/jbc.M402223200. [DOI] [PubMed] [Google Scholar]