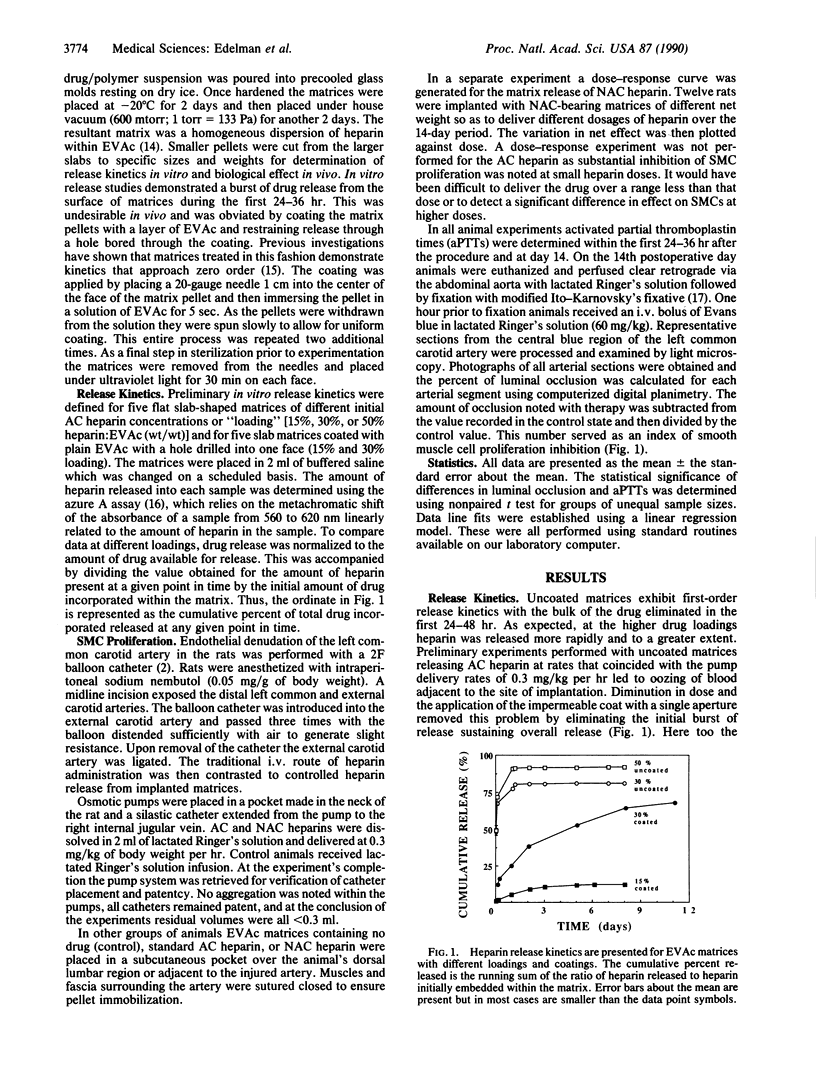
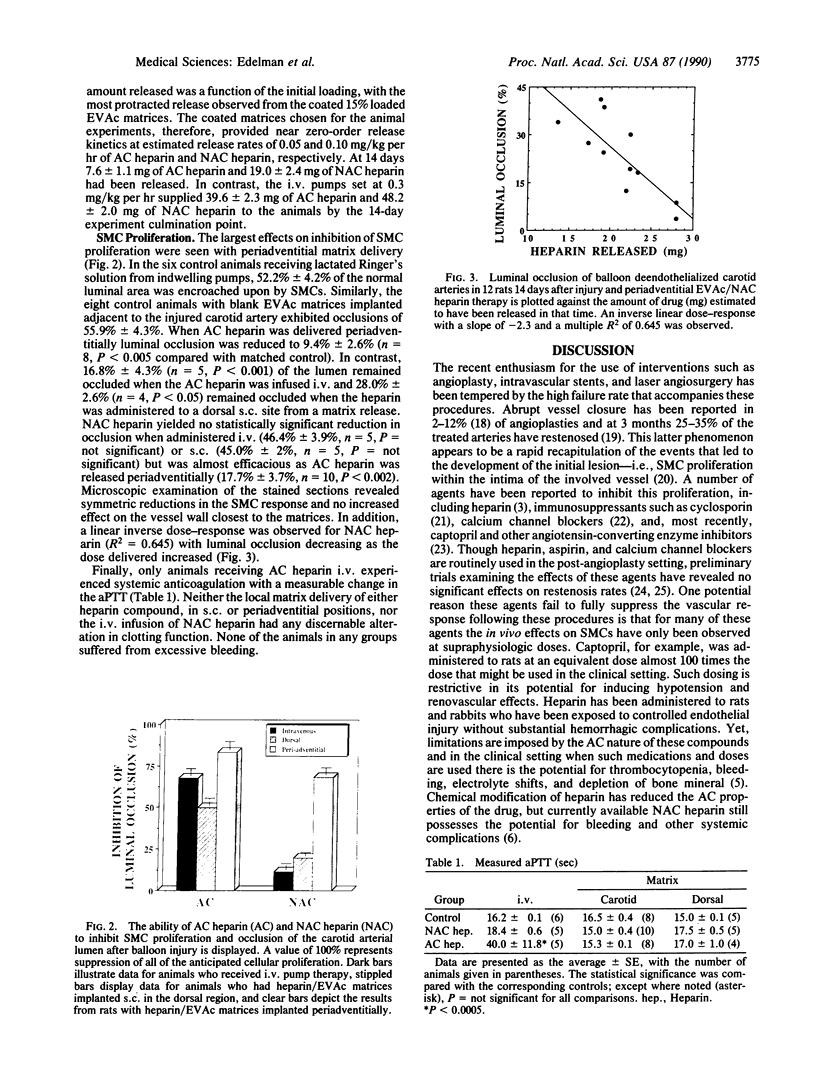
Abstract
Continuous intravenous infusion of heparin suppresses smooth muscle cell proliferation in rats after endothelial injury but may lead to hemorrhage and other complications. The anticoagulant property has been removed from chemically modified heparin without loss of antiproliferative effect but use of such compounds is still limited. In this study ethylene-vinyl acetate copolymer matrices containing standard and modified heparin were placed adjacent to rat carotid arteries at the time of balloon dendothelialization. After 14 days arterial occlusion by smooth muscle cell proliferation was defined. Matrix delivery of both heparin compounds effectively diminished this proliferation in comparison to controls without producing systemic anticoagulation or side effects. In addition, this mode of therapy appeared more effective than the administration of the same agents by either intravenous pumps or heparin/polymer matrices placed in a subcutaneous site distant from the injured carotid artery. Thus, heparin's inhibition of smooth muscle cell proliferation after vascular injury might be most effective within the microenvironment of the injured vessel wall, and the accelerated atherosclerosis or restenosis that often follows angioplasty and other vascular interventions might best be treated with site-specific therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin G. E., Ratliff N. B., Hollman J., Tabei S., Phillips D. F. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985 Aug;6(2):369–375. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- Bick R. L., Ross E. S. Clinical use of intrapulmonary heparin. Semin Thromb Hemost. 1985 Apr;11(2):213–217. doi: 10.1055/s-2007-1004377. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Wei C. L., Langer R. In vivo and in vitro release of macromolecules from polymeric drug delivery systems. J Pharm Sci. 1983 Oct;72(10):1181–1185. doi: 10.1002/jps.2600721019. [DOI] [PubMed] [Google Scholar]
- Brown L., Munoz C., Siemer L., Edelman E., Langer R. Controlled release of insulin from polymer matrices. Control of diabetes in rats. Diabetes. 1986 Jun;35(6):692–697. doi: 10.2337/diab.35.6.692. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Beeler D. L., Rosenberg R. D., Karnovsky M. J. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth muscle cells. J Cell Physiol. 1984 Sep;120(3):315–320. doi: 10.1002/jcp.1041200309. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Wright T. C., Karnovsky M. J. Regulation of vascular smooth muscle cell growth by heparin and heparan sulfates. Semin Thromb Hemost. 1987 Oct;13(4):489–503. doi: 10.1055/s-2007-1003525. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Dawes J., Prowse C. V., Pepper D. S. Absorption of heparin, LMW heparin and SP54 after subcutaneous injection, assessed by competitive binding assay. Thromb Res. 1986 Dec 1;44(5):683–693. doi: 10.1016/0049-3848(86)90169-6. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L., Dominguez C. Relation between arterial intimal thickening and the vasa-vasorum. Virchows Arch A Pathol Anat Histopathol. 1985;406(2):165–177. doi: 10.1007/BF00737083. [DOI] [PubMed] [Google Scholar]
- Ellis S. G., Roubin G. S., Wilentz J., Douglas J. S., Jr, King S. B., 3rd Effect of 18- to 24-hour heparin administration for prevention of restenosis after uncomplicated coronary angioplasty. Am Heart J. 1989 Apr;117(4):777–782. doi: 10.1016/0002-8703(89)90612-1. [DOI] [PubMed] [Google Scholar]
- Fareed J., Walenga J. M., Hoppensteadt D., Huan X., Nonn R. Biochemical and pharmacologic inequivalence of low molecular weight heparins. Ann N Y Acad Sci. 1989;556:333–353. doi: 10.1111/j.1749-6632.1989.tb22515.x. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Gundry S. R., Klein M. D., Drongowski R. A., Kirsh M. M. Clinical evaluation of a new rapid heparin assay using the dye azure A. Am J Surg. 1984 Aug;148(2):191–194. doi: 10.1016/0002-9610(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Habib J. B., Bossaller C., Wells S., Williams C., Morrisett J. D., Henry P. D. Preservation of endothelium-dependent vascular relaxation in cholesterol-fed rabbit by treatment with the calcium blocker PN 200110. Circ Res. 1986 Feb;58(2):305–309. doi: 10.1161/01.res.58.2.305. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Hansson G. K. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2303–2306. doi: 10.1073/pnas.85.7.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R., Brown L., Edelman E. Controlled release and magnetically modulated release systems for macromolecules. Methods Enzymol. 1985;112:399–422. doi: 10.1016/s0076-6879(85)12032-x. [DOI] [PubMed] [Google Scholar]
- Langer R., Siegel R., Brown L., Leong K., Kost J., Edelman E. Controlled release and magnetically modulated systems for macromolecular drugs. Ann N Y Acad Sci. 1985;446:1–13. doi: 10.1111/j.1749-6632.1985.tb18386.x. [DOI] [PubMed] [Google Scholar]
- Larsen A. K., Lund D. P., Langer R., Folkman J. Oral heparin results in the appearance of heparin fragments in the plasma of rats. Proc Natl Acad Sci U S A. 1986 May;83(9):2964–2968. doi: 10.1073/pnas.83.9.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W., Lange R. A., Hillis L. D. Restenosis after successful coronary angioplasty. Pathophysiology and prevention. N Engl J Med. 1988 Jun 30;318(26):1734–1737. doi: 10.1056/NEJM198806303182606. [DOI] [PubMed] [Google Scholar]
- Powell J. S., Clozel J. P., Müller R. K., Kuhn H., Hefti F., Hosang M., Baumgartner H. R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989 Jul 14;245(4914):186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Takagi H. A note on the use of picric acid-paraformaldehyde-glutaraldehyde fixative for correlated light and electron microscopic immunocytochemistry. Neuroscience. 1982 Jul;7(7):1779–1783. doi: 10.1016/0306-4522(82)90035-5. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth H. B., Roubin G. S., Hollman J., Meier B., Leimgruber P. P., Douglas J. S., Jr, King S. B., 3rd, Gruentzig A. R. Effect of nifedipine on recurrent stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1986 Dec;8(6):1271–1276. doi: 10.1016/s0735-1097(86)80296-0. [DOI] [PubMed] [Google Scholar]



